Abstract
Porcine epidemic diarrhea (PED) virus (PEDV) is a coronavirus that primarily infects porcine intestinal epithelial cells and causes severe diarrhea and high fatality in piglets. A77 1726 is the active metabolite of leflunomide, a clinically approved anti-rheumatoid arthritis (RA) drug. A77 1726 inhibits the activity of protein tyrosine kinases (PTKs), p70 S6 kinase (S6K1), and dihydroorotate dehydrogenase (DHO-DHase). Whether A77 1726 can control coronavirus infections has not been investigated. Here we report that A77 1726 effectively restricted PEDV replication by inhibiting Janus kinases (JAKs) and Src kinase activities but not by inhibiting DHO-DHase and S6K1 activities. Overexpression of Src, JAK2 or its substrate STAT3 enhanced PEDV replication and attenuated the antiviral activity of A77 1726. Our study demonstrates for the first time the ability of A77 1726 to control coronavirus replication by inhibiting PTK activities. Leflunomide has potential therapeutic value for the control of PEDV and other coronavirus infections.
Keywords: Porcine epidemic diarrhea virus, A77 1726, Leflunomide, Protein tyrosine kinases, p70 S6 kinase
1. Introduction
Porcine epidemic diarrhea (PED) virus (PEDV) is an important pathogen that primarily infects the villous gastrointestinal epithelial cells of pigs. The main symptoms of PEDV infection are severe vomiting, watery diarrhea, and dehydration (Chen et al., 2014; Song and Park, 2012). The disease was first reported in Europe in 1960 and in Japan in 1982. PED rapidly spread to its neighboring countries, particularly South Korea and China. PEDV evolved in late 2010 has since caused large scale epizootics in China and many other Asian countries including South Korea, Thailand, Philippines, and Vietnam (Chen et al., 2014; Song and Park, 2012). Newly emerging PEDV strains are highly pathogenic, incurring an 80–100% fatality in piglets (Chen et al., 2014; Song and Park, 2012). CV777,a vaccine used in the 1990s, has been losing its efficacy in preventing newly emerged PEDV strains. PED was exotic in the United States until 2013. PED outbreaks then rapidly spread throughout the country and has caused severe economic losses to the pig industry (Vlasova et al., 2014). The virus initially isolated in the United States shares a great homology with PEDV strains that have reemerged in China. Live attenuated or killed vaccines have been developed in Asia and the United States. These vaccines have provided limited protection and sometimes are not safe. There has been increasing interest in developing antiviral drugs for the control of PEDV infections.
PEDV is a member of the α genera of the Coronaviridae family (Fehr and Perlman, 2015). The virus contains a single-stranded positive-sense RNA genome of approximately 28 kb (Beall et al., 2016). Approximately two-third of its genome encodes two open reading frames (ORF1a and ORF1b) that function as an RNA polymerase for duplicating the virus genome and viral gene transcription (Beall et al., 2016). The remaining portion of the PEDV genome encodes a non-structural protein (ORF3) and four structural proteins, including envelope (E), membrane (M), nucleocapsid (N), and spike-like protein (S) (Beall et al., 2016). These proteins are assembled into virions and play important roles in virus entry into the host cells and the induction of antiviral immunity (Beall et al., 2016). PEDV primarily infects the porcine villous gastrointestinal epithelial cells or enterocytes. PEDV infection leads to activation of the epidermal growth factor receptor (EGFR) and its downstream transcription factor STAT3 (Yang et al., 2018). Inhibition of EGFR or STAT3 by siRNA or their specific inhibitors leads to increased expression of antiviral interferons and decreased PEDV replication (Yang et al., 2018). In addition to being phosphorylated and activated by EGFR, STAT3 is phosphorylated at Tyr 705 by the cytokine receptor-activated Janus kinases (JAKs) and by Src tyrosine kinase (Bowman et al., 2000). Recent studies have shown that JAKs play important roles in promoting virus replication (Eierhoff et al., 2010; Gavegnano et al., 2014, 2017; Han et al., 2018; Watanabe et al., 2014). For example, JAK1 and JAK2 have been recently identified as novel cellular factors that are crucial for influenza A virus and HIV replication (Eierhoff et al., 2010; Gavegnano et al., 2014, 2017; Han et al., 2018; Watanabe et al., 2014). Whether targeting JAK and Src could lead to the suppression of coronavirus replication remains to be investigated.
Leflunomide is an anti-inflammatory drug primarily used for treating rheumatoid arthritis. After ingestion, leflunomide is quickly converted in the liver and gastrointestinal tract (Fig. 1 A) into its active metabolite, A77 1726 (Breedveld and Dayer, 2000). We and others have reported earlier that A77 1726 is able to inhibit the activity of several protein tyrosine kinases (PTKs), including platelet-derived growth factor receptor (PDGF), JAK1, JAK3, and two Src family tyrosine kinases (p56lck and p59fyn) (Elder et al., 1997; Ruckemann et al., 1998; Siemasko et al., 1996, 1998; Xu et al., 1995, 1996, 1997, 1999). Later studies showed that A77 1726 also inhibits the activity of DHO-DHase, a rate-limiting enzyme in de novo pyrimidine nucleotide synthesis (Ruckemann et al., 1998; Williamson et al., 1996; Xu et al., 1996, 1997, 1999). Recently, our lab discovered a third molecular target of A77 1726. It inhibits the activity of p70 S6 kinase 1 (S6K1), a serine/threonine kinase in the PI-3 kinase pathway (Chen et al., 2018; Doscas et al., 2014; Sun et al., 2018; Xu et al., 2017). A77 1726 inhibits the activity of S6K1 and PTKs in an in vitro kinase assay and in cell culture with the IC50 values of 50–75 μM (Doscas et al., 2014; Xu et al., 1995, 1996) but inhibits the activity of DHO-DHase more potently, with an IC50 value in the nano- to micromolar range (Ruckemann et al., 1998; Williamson et al., 1996; Xu et al., 1996).
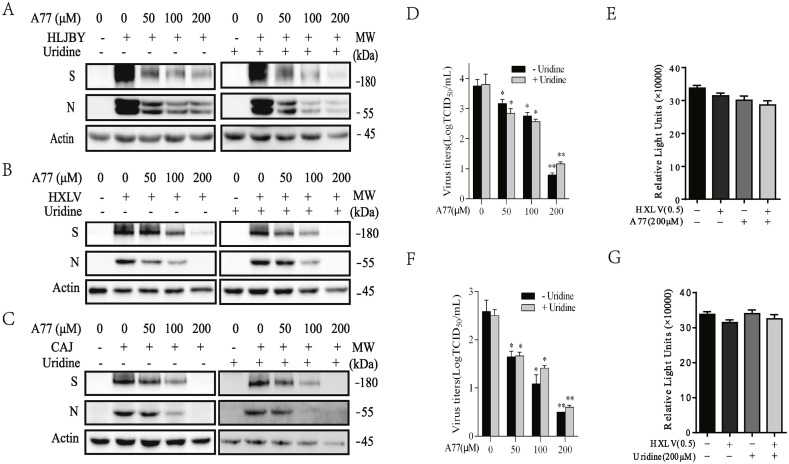
Fig. 1.
Inhibition of PEDV replication by A77 1726. (A) IPEC-DQ cells were infected with the HLJBY strain of PEDV (0.5 MOI) and then incubated in the absence or presence of the indicated concentration of A77 1726 without or with uridine (200 μM) for 24 h. The cell lysates were analyzed for the expression of the S and N proteins by Western blot. Vero cells were infected with HXLV (B & D) or CAJ strain (C & F) of PEDV, 0.5 MOI each, were incubated in the absence or presence of the indicated concentration of A77 1726 without or with uridine (200 μM) for 12 h. The cell lysates were analyzed for the expression of the S and N proteins by Western blot. (D & F) The conditioned media were collected and analyzed for the TCID50 values. The results represent the mean ± standard deviation (SD) of three independent experiments and statistically analyzed by a Student's t-test. *p < 0.05, **p < 0.01. (E & G) Effect of A77 1726 (E) and uridine (G) on Vero cell proliferation. Vero cells seeded in 96-well plates were left uninfected or infected with PEDV virus (0.5 MOI) and then incubated in the absence or presence of A77 1726 or uridine (200 μM each). After incubation for 12 h, the cells were analyzed for proliferation by using a CellTiter-Glo kit. Data are the mean ± SD of the triplicate from one representative of three independent experiments with similar results.
Numerous studies have shown that A77 1726 possesses some strong antiviral activities. For example, A77 1726 inhibits the replication of BK virus (Bernhoff et al.; Liacini et al., 2010) and cytomegalovirus (CMV) (Chacko and John, 2012; Waldman et al., 1999a, 1999b), two types of viruses that cause graft rejection in immunosuppressed transplant patients. The antiviral activity of leflunomide has been validated in transplant patients infected with CMV and BK (Chacko and John, 2012). A77 1726 also inhibits the replication of several other virus types, including herpes simplex type 1 virus (Knight et al., 2001), and Epstein-Barr virus (EBV) (Bilger et al., 2017), hepatitis E virus (HEV) (Wang et al., 2016), and rotavirus (Chen et al., 2019). Because A77 1726 hits multiple molecular targets, how A77 1726 exerts its antiviral effects remain controversial. Our present study focused on the ability of A77 1726 to control PEDV replication and its underlying mechanisms. Here we report that A77 1726 suppressed PEDV replication by inhibiting the activity of JAK and Src.
2. Materials and methods
Reagents. A77 1726 and Ruxolitinib (Rux) were purchased from Selleck Inc. (Shanghai, China). Brequinar sodium (BQR), a potent inhibitor of DHO-DHase, was kindly provided by Dupont Corporation (Wilmington, DE). Antibodies for phospho-tyrosine (P–Y-1000 #8954), STAT3 (#9139), phosphor-STAT3Y705 (#9145), JAK2 (#3230), phospho-Src (#2101), Src (#2110), S6 (#2217), and phospho-S6S235/236 (#4858) were purchased from Cell Signaling Technology (Danvers, MA). An anti-phospho-JAK1 antibody (#10-P1628-1) was purchased from American Research Products, Inc. (Waltham, MA). An anti-JAK2 antibody (#06–1310) was purchased from Sigma Aldrich (St. Louis, MO). PF-4708671 and antibodies against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and β-actin were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Monoclonal antibodies against the S and N proteins of PEDV were kindly provided by Dr. Ying Fang, College of Veterinary Medicine, the University of Illinois at Urbana-Champaign, Urbana, Illinois. pBABE-JAK2 plasmid was kindly provided by Dr. Eric Chang, (Baylor College of Medicine, Houston, USA). pRc/CMV-STAT3-FLAG and pLNCX-Src (Y527F) (encoding the Src gene of chicken) were purchased from Addgene (Beijing Zhongyuan, Ltd, Beijing China).
Cell culture and viruses. Vero cells (CCL-81) were purchased from the American Tissue Culture Collection (Manassas, VA) and grown in complete MEM media containing 10% fetal bovine serum, streptomycin and penicillin (100 U/ml each), sodium pyruvate (1 mM), and l-glutamine (2 mM). IPEC-DQ cells, a subline derived from the porcine intestinal epithelial cell line IPEC-J2, were kindly provided by Dr. Dongwan Yoo, College of Veterinary Medicine, the University of Illinois at Urbana-Champaign, Urbana, Illinois. IPEC-DQ cells were maintained in RPMI 1640 supplemented with 10% FBS as previously reported (Zhang et al., 2018). HXLV and CAJ, two highly pathogenic PEDV isolates, were kindly provided by Liyuan Biotechnology Inc., Nanning, Guanxi Province, China. HLJBY strain, a PEDV isolate with low pathogenicity, has been recently reported (Huan et al., 2019). The virus was propagated in Vero cells in the presence of 8 μg/ml TPCK trypsin (8 μg/ml) during and after virus inoculation. The virus titers were measured by a 10-fold serial dilution (10−1 to 10−5), each dilution was inoculated into Vero cells. The 50% tissue culture infection dose (TCID50/0.1 ml) was calculated according to the Reed and Muench method. Vero cells infected with 0.5 MOI PEDV virus were incubated in the absence or presence of the indicated inhibitors for 12 h. The virus titers in the conditioned media were determined by measuring the TCID50 values. Data represent the mean ± standard deviation (SD) of three independent experiments.
Western blotting. Vero cells infected with PEDV were incubated for 12 h in the absence or presence of the indicated inhibitors. Cell lysates were prepared and analyzed for the levels of interested proteins or phosphorylation as previously reported (Zhang et al., 2019). The density of the bands was analyzed by using NIH Image-J software and normalized by the arbitrary units of their corresponding total proteins or β-actin. Quantified results were presented as the mean ± standard deviation (SD) from three experiments in bar graphs.
JAK2, STAT3, and Src transfection. Vero and IPEC-DQ cells were transiently transfected with pcDNA3.1, pBABE-JAK2, pRc/CMV-STAT3-FLAG, or pLNCX-Src (Y527F) plasmid DNA. After incubation for 36 h, the cells were left uninfected or infected with the indicated MOI of PEDV and then incubated in the absence or presence of the indicated concentrations of A77 1726 for 12 h.
Cell lysates were prepared and analyzed for JAK2 and STAT3 phosphorylation and the levels of viral NP and M1 proteins. The virus titers in the conditioned media were collected and analyzed for the TCID50 values. The results represent the mean ± SD of three independent experiments.
Cell proliferation assay. Vero cells seeded in 96-well plates (20000 cells/well) were left uninfected or infected with 0.5 MOI PEDV. After incubation for 12 h in the absence or presence of indicated inhibitors, cell proliferation was determined using an ATP-based CellTiter-Glo kit (Promega, Madison, WI) following the manufacturer's instruction.
Statistical analysis. Differences in the virus titers in the conditioned media of virus-infected cells, in cell proliferation, and in the Western blot band density were statistically analyzed by using an unpaired Student's t-test. A p value of <0.05 was considered statistically significant. All statistics were performed with SigmaPlot 11 software (Systat Software, Inc, San Jose, CA).
3. Results
A77 1726 inhibits PEDV replication. We first determined the inhibitory effect of A77 1726 on viral protein synthesis. HLJBY virus is a strain isolated in 2011 from a piglet with diarrhea from Helongjiang province, China. HLJBY virus has low pathogenicity that is comparable to CV777, a PEDV vaccine strain (Huan et al., 2019). This strain can readily replicate in Vero cells and in IPEC-DQ cells, a subline that was derived from IPEC-J2 cells, a porcine intestinal epithelial cell line (Zhang et al., 2018). The other two strains of PEDV (HXLV and CAJ) used in this study, can only be propagated in Vero cells. A77 1726 dose-dependently decreased the levels of viral S and N proteins in IPEC-DQ cells infected with HLJBY (Fig. 1A) and in Vero cells infected with two PEDV isolates, HXLV (Fig. 1B) and CAJ (Fig. 1C). Uridine is converted to UMP, UDP, and UTP by the salvage pathway. Intracellular pyrimidine nucleotide levels in A77 1726-treated cells are restored to normal levels by exogenous uridine (Xu et al., 1996, 1999). To determine if the antiviral activity of A77 1726 was mediated by inhibition of pyrimidine nucleotide synthesis, we tested if excessive uridine added to the culture media of PEDV-infected cells could ablate the antiviral effect of A77 1726. As shown in Fig. 1A–C, A77 1726 was still able to dose-dependently reduce the N and S protein levels in the cells infected with two strains of PEDV in the presence of uridine (200 μM).
The titers of the HLJBY virus in IPEC-DQ cells were too low to be detected (data not shown). A77 1726 lowered the titers of HXLV (Fig. 1D) and CAJ (Fig. 1F) strains in the conditioned media of Vero cells in a dose-dependent manner. The IC50 values of A77 1726 to lower the titers of HXLV and CAJ viruses in the media of virus-infected Vero cells are approximately 25.7 and 11.5 μM, respectively. A77 1726 in combination with uridine was still able to lower the virus titers in the conditioned media of PEDV-infected Vero cells (Fig. 1D and F). The antiviral effect of A77 1726 was not due to its antiproliferative activity since A77 1726 minimally inhibited the proliferation of Vero cells (Fig. 2 E). Uridine had little effect on Vero cell proliferation (Fig. 1G). These observations suggest that A77 1726 inhibits PEDV replication largely independent of its inhibitory effect on pyrimidine nucleotide synthesis.
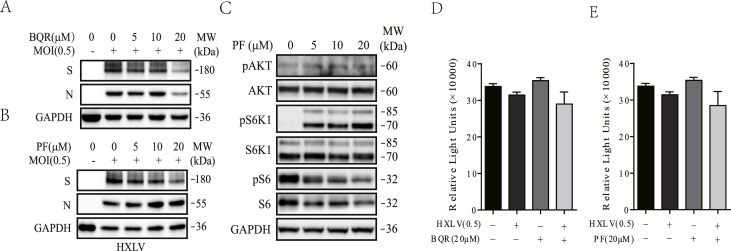
Fig. 2.
The inability of PF-4708671 and BQR to inhibit PEDV replication. Vero cells infected with HXLV strain of PEDV (0.5 MOI) were incubated in the absence or presence of the indicated concentrations of BQR (A) or PF-4708671 (B & C) for 12 h. The cell lysates were analyzed for the expression of the S and N proteins (A & B) or analyzed for the levels of protein phosphorylation (C) by Western blot. (D & E) Effect of BQR (20 μM) and PF-4708671 (20 μM) on Vero cell proliferation. Vero cells seeded in 96-well plates were left uninfected or infected with PEDV virus (0.5 MOI) and then incubated in the absence or presence of BQR (20 μM) (D) and PF-4708671 (20 μM) (E). After incubation for 12 h, the cells were analyzed for proliferation by using a CellTiter-Glo kit. Data are the mean ± SD of the triplicate from one representative of three independent experiments with similar results and statistically analyzed by a Student's t-test.
Evidence that A77 1726 inhibits PEDV replication independent of its activity on DHO-DHase and S6K1. Having shown the inability of uridine to neutralize the antiviral activity of A77 1726, we then tested if BQR, a potent DHO-DHase inhibitor, was also unable to inhibit PEDV replication. As shown in Fig. 2A, BQR used up to 20 μM, a very high concentration since its IC50 value to inhibit DHO-DHase activity is at least 10-fold lower than that of A77 1726, had little effect on viral N and S protein synthesis. We next determined if inhibition of S6K1 activity by A77 1726 could contribute to its antiviral activity by investigating the ability of PF-4708671, a specific and potent inhibitor of S6K1, to inhibit PEDV replication. PF-4708671 up to 20 μM did not significantly decrease viral N and S protein levels (Fig. 2B). PF-4708671 decreased S6 phosphorylation but increased S6K1 and AKT phosphorylation due to feedback activation of the PI-3 kinase pathway (Fig. 2C), a well-established phenomenon for S6K1 inhibitor (Pearce et al., 2010). Neither BQR nor PF-4708671 significantly caused cytotoxicity in Vero cells in the absence or presence of PEDV infection (Fig. 2D and E).
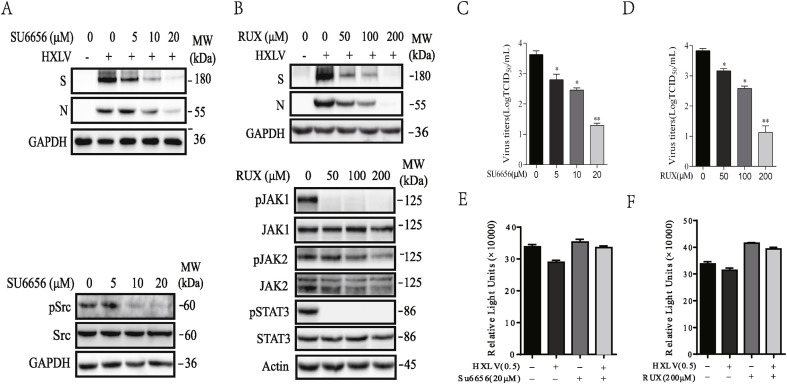
Inhibition of PEDV replication by PTK inhibitors. Since inhibition of DHO-DHase and S6K1 activities by their specific inhibitors did not significantly inhibit PEDV replication, A77 1726 likely inhibited PEDV replication by inhibiting the activity of PTKs. Our prior studies have shown that A77 1726 inhibits the JAK and Src family PTK activities (Elder et al., 1997; Siemasko et al., 1998; Xu et al., 1995). Here we tested if SU6656 and Ruxolitinib (Rux), specific inhibitors of Src and JAK tyrosine kinases, respectively, were able to inhibit PEDV replication. SU6656 decreased the levels of the S and N proteins of PEDV in Vero cells (Fig. 3 A) and virus titers in the conditioned media (Fig. 3C). SU6656 inhibited Src tyrosine phosphorylation in uninfected Vero cells in a dose-dependent manner (Fig. 3A). Rux dose-dependently decreased the levels of the S and N proteins (Fig. 3B) and decreased the titers of PEDV (Fig. 3D) in Vero cells. Rux had a very weak effect on JAK2 tyrosine phosphorylation. Rux ablated JAK1 and STAT3 tyrosine phosphorylation in uninfected Vero cells even when it was used at a low concentration of 50 μM (Fig. 3B). SU6656 and Rux did not significantly decrease Vero cell proliferation (Fig. 3E and F).
Fig. 3.
Inhibition of PEDV replication by PTK inhibitors. Vero cells infected with HXLV strain of PEDV (0.5 MOI) were incubated in the absence or presence of the indicated concentration of SU6656 (A) or Rux (B) for 12 h. The cell lysates were analyzed for the expression of the S and N proteins or the phosphorylation of the indicated proteins by Western blot. (C & D) The conditioned media were collected and analyzed for the TCID50 values. The results represent the mean ± SD of three independent experiments and statistically analyzed by a Student's t-test. *p < 0.05, **p < 0.01. (E & F) Effect of SU6656 and Rux on Vero cell proliferation. Vero cells seeded in a 96-well plate were left uninfected or infected with PEDV virus (0.5 MOI) and then incubated in the absence or presence of SU6656 (20 μM) and Rux (200 μM). After incubation for 12 h, the cells were analyzed for proliferation by using a CellTiter-Glo kit. Data are the mean ± SD of the triplicate from one representative of three independent experiments with similar results.
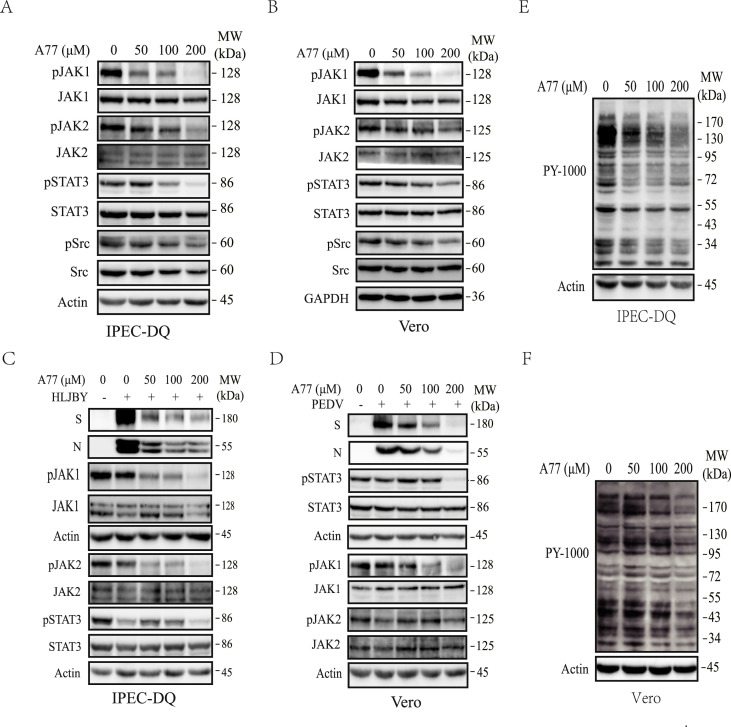
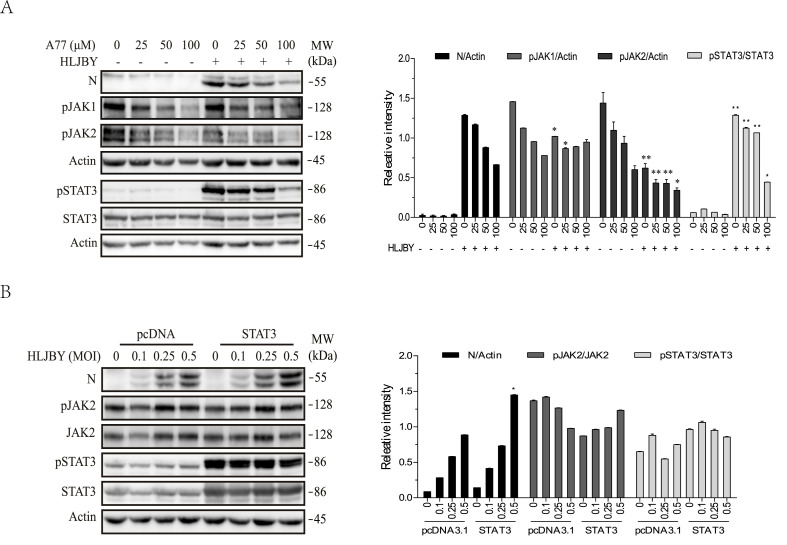
Inhibition of Src and JAK tyrosine phosphorylation by A77 1726. The inability of S6K1 and DHO-DHase inhibitors to inhibit PEDV replication and the ability of Src and JAK tyrosine kinase inhibitors to inhibit PEDV replication strongly suggest that A77 1726 inhibits PEDV replication by inhibiting tyrosine kinase activities. Here we examined the ability of A77 1726 to inhibit the activities of Src and JAK tyrosine kinases. As shown in Fig. 4 A and B, A77 1726 inhibited JAK1, JAK2, STAT3, and Src tyrosine phosphorylation in uninfected IPEC-DQ and Vero cells in a dose-dependent manner. We also determined if A77 1726 inhibited JAK1, JAK2, and STAT3 tyrosine phosphorylation in PEDV-infected cells. IPEC-DQ and Vero cells infected with PEDV were incubated in the absence or presence of the indicated concentrations of A77 1726 for 24 or 12 h, respectively. As shown in Fig. 4C, PEDV infection alone led to the partial inhibition of JAK1, JAK2, and STAT3 phosphorylation in IPEC-DQ and Vero cells. A77 1726 inhibited JAK1 and JAK2 phosphorylation in a dose-dependent manner in PEDV infected IPEC-DQ cells. A77 1726 inhibited STAT3 phosphorylation but not in a dose-dependent manner, probably due to the ability of PEDV itself to inhibit STAT3 phosphorylation (Fig. 4C). A77 1726 inhibited JAK1 phosphorylation in PEDV-infected Vero cells in a dose-dependent manner but inhibited STAT3 and JAK2 tyrosine phosphorylation only when A77 1726 was used at 200 μM (Fig. 4D). A77 1726 dose-dependently inhibited total protein tyrosine phosphorylation in uninfected IPEC-DQ (Fig. 4E) and Vero (Fig. 4F) cells.
Fig. 4.
Inhibition of S6K1 and PTK by A77 1726. IPEC-DQ (A, C, E) and Vero cells (B, D, F) were left uninfected (A, B, E, F) or infected with 0.5 MOI PEDV (C & D). The cells were then incubated in the absence or presence of the indicated concentrations of A77 1726 for 12 h (A, C, E) or 24 h (B, D, F). The cell lysates were analyzed for the levels of JAK1, JAK2, STAT3, and Src phosphorylation by Western blot (A-D) or for total protein tyrosine phosphorylation (E & F).
A77 1726 inhibits PEDV replication by inhibiting STAT3 phosphorylation. Yang et al. (2018) reported that PEDV infection of 293T and IPEC-J2 cells rapidly induces STAT3 tyrosine phosphorylation by EGFR tyrosine kinase activation. Consistent with this observation, PEDV infection rapidly induced STAT3 phosphorylation in IPEC-DQ cells (Fig. 5 A) but did not increase but rather slightly decreased JAK1 and JAK2 phosphorylation, suggesting that STAT3 phosphorylation is mediated by EGFR. A77 1726 inhibited JAK1, JAK2, and STAT3 phosphorylation in a dose-dependent manner in uninfected and virus-infected IPEC-DQ cells (Fig. 5A). STAT3 overexpression significantly increased total and phosphorylated STAT3 in IPEC-DQ cells but did not affect JAK2 phosphorylation and total JAK2 protein levels (Fig. 5B). STAT3 overexpression accelerated viral N protein synthesis (Fig. 5B), suggesting that STAT3 phosphorylation can accelerate PEDV replication.
Fig. 5.
STAT3 overexpression enhances PEDV replication. (A) IPEC-DQ cells were pre-incubated in the absence or presence of the indicated concentrations of A77 1726 for 2 h and then infected with the HLJBY strain of PEDV by incubating the cells at 4 °C for 2 h. After rinse twice, the cells were incubated in serum-free medium and incubated at 37 °C for 30 min. Cell lysates were prepared and analyzed for JAK1, JAK2, and STAT3 tyrosine phosphorylation and the level of the N protein of PEDV by Western blot. (B) STAT3 overexpression enhances viral protein synthesis. IPEC-DQ cells were transfected with the pcDNA3.1 empty vector or the expression vector encoding STAT3. After incubation for 24 h, the cells were infected with the indicated MOI of PEDV and then incubated for 24 h. The cell lysates were prepared and analyzed for the expression of indicated proteins by Western blot. The density of the phosphorylated JAK1, JAK2, and STAT3 bands was analyzed by using NIH Image-J software and normalized by the arbitrary units of β-actin or STAT3. *p < 0.05, **p < 0.01, compared to the corresponding samples in the cells transfected with pcDNA3.1 with a Student's t-test.
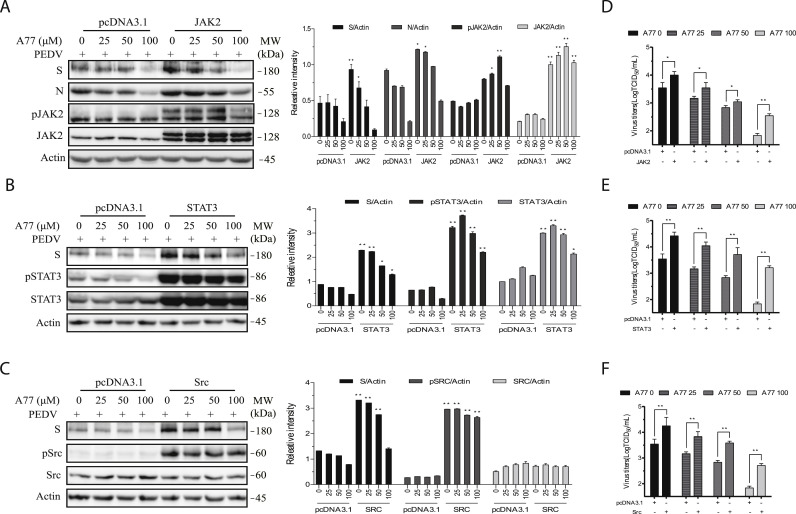
Finally, we determined if overexpression of a constitutively activated Src, JAK2, or STAT3 gene in Vero cells could enhance PEDV replication and compromise the antiviral activity of A77 1726. As shown in Fig. 6 A–C, phosphorylated JAK2, STAT3, and Src proteins were significantly increased in Vero cells transfected with their corresponding expression vectors. Because of the lack of the reactivity of an anti-Src antibody with chicken Src, increased levels of Src protein in Vero cells transfected with the expression vector encoding a Src gene of chicken origin were not detected (Fig. 6C). Src, STAT3, and JAK2 overexpression significantly increased the levels of viral N and M proteins. A77 1726 dose-dependently inhibited viral protein synthesis in Vero cells transfected with the expression vector encoding JAK2, STAT3, and Src at a significantly lower magnitude than in those transfected with the empty vector (Fig. 6A–C). The virus titers in untreated or A77 1726-treated cells overexpressing JAK2, STAT3, or Src were significantly higher than their corresponding counterparts in pcDNA3.1-transfected Vero cells (Fig. 6D–F).
Fig. 6.
Overexpression of JAK2, STAT3, and Src enhances PEDV replication and attenuates the antiviral activity of A77 1726. Vero cells were transfected with the pcDNA3.1 empty expression vector or the vector encoding JAK2 (A), STAT3 (B), or a constitutively active Src gene (C). After incubation for 36 h, the cells were infected with HXLV (0.5 MOI) and then incubated in the absence or presence of the indicated concentrations of A77 1726 for 12 h. The cell lysates were analyzed for the levels of the S and N proteins or tyrosine phosphorylation of JAK2, STAT3, and Src by Western blot. The density of the S and N proteins and the phosphorylated JAK2, STAT3, and Src bands was analyzed by using NIH Image-J software and normalized by the arbitrary units of β-actin or their corresponding total proteins. *p < 0.05, **p < 0.01, compared to the corresponding samples in the cells transfected with pcDNA3.1 with a Student's t-test. (D-F) The conditioned media were collected and analyzed for the TCID50 values. The results represent the mean ± standard deviation (SD) of three independent experiments and statistically analyzed by a Student's t-test. *p < 0.05, **p < 0.01.
4. Discussion
Our present study shows that A77 1726 dose-dependently restricted virus replication of three PEDV strains in Vero cells and in an IPEC-DQ porcine intestinal epithelial cell line. We provide several lines of evidence suggesting that A77 1726 inhibits PEDV replication by targeting Src and JAKs: 1) A77 1726 effectively inhibited the autophosphorylation of Src, JAK1, and JAK2; 2) A77 1726 inhibited tyrosine phosphorylation and activation of STAT3, a transcription factor that has been shown to play an important role in PEDV replication (Yang et al., 2018); 3) SU6656 and Rux, a Src and JAK-specific inhibitor, respectively, also inhibited PEDV replication; 4) Overexpression of Src, JAK2, and STAT3 enhanced PEDV replication but attenuated the antiviral activity of A77 1726 in Vero and IPEC-DQ cells; 5) Inhibition of DHO-DHase and S6K1 activities did not contribute to the antiviral activity of A77 1726. These data collectively suggest that inhibition of PTK activity plays an important role in A77 1726-mediated antiviral effects.
Fast replicating viruses propagated in their permissive host cells often require a large quantity of nucleotides as the building blocks for virus gene transcription and genome replication. The depletion of intracellular nucleotide pools by blocking nucleotide biosynthesis should lead to the suppression of virus replication. There has been a growing interest in searching for the inhibitors of pyrimidine nucleotide synthesis as novel antiviral drugs (Hoffmann et al., 2011; Lucas-Hourani et al., 2017; Marschall et al., 2013; Wang et al., 2011). DHO-DHase, a rate-limiting enzyme in pyrimidine nucleotide synthesis, converts dihydroorotate acid to orotic acid (Munier-Lehmann et al., 2013). Several studies have shown that DHO-DHase inhibitors such as brequinar sodium and A3 are capable of inhibiting virus replication in vitro (Hoffmann et al., 2011; Lucas-Hourani et al., 2017; Marschall et al., 2013; Wang et al., 2011). A77 1726, an inhibitor of DHO-DHase with moderate potency, reduced the levels of intracellular pyrimidine nucleotides in fast proliferating lymphocytes (Breedveld and Dayer, 2000; Ruckemann et al., 1998). Numerous studies have shown that A77 1726 inhibits the replication of several types of viruses by inhibiting pyrimidine nucleotide synthesis. For example, A77 1726 suppresses the replication of BK virus (Bernhoff et al., 2010), hepatitis E virus (Wang et al., 2016), and rotavirus (Chen et al., 2019) by inhibiting pyrimidine nucleotide synthesis since its antiviral activity can be readily blocked by the addition of exogenous uridine. In contrast, other studies showed that A77 1726 inhibits the replication of BK virus (Liacini et al., 2010), Epstein-Barr (EB) virus (Bilger et al., 2017), CMV (Chacko and John, 2012; Waldman et al., 1999a, 1999b), and herpes simplex type 1 virus (Knight et al., 2001) independent of its inhibitory effect on pyrimidine nucleotide biosynthesis. Our present study showed that uridine was unable to reverse A77 1726-mediated inhibition of PEDV replication and that BQR, a potent DHO-DHase inhibitor, was unable to suppress PEDV replication. These observations collectively suggest that A77 1726-mediated antiviral activity against PEDV is independent of its inhibitory effect on pyrimidine nucleotide synthesis. The notion that A77 1726 restricted PEDV replication not by inhibiting pyrimidine nucleotide synthesis is strengthened by the observations that leflunomide is the only DHO-DHase inhibitor capable of controlling virus replication in vivo (Waldman et al., 1999a, 1999b). Other DHO-DHase inhibitors do not have any in vivo antiviral activities due to the presence of serum uridine that can be used to synthesize pyrimidine nucleotides by the salvage pathway (Bonavia et al., 2011; Hoffmann et al., 2011; Wang et al., 2011).
It is puzzling why inhibition of pyrimidine nucleotide synthesis is responsible for the antiviral activity of A77 1726 towards certain types of viruses but not others. We speculate that, the ability of A77 1726 to restrict virus replication by inhibiting pyrimidine nucleotide synthesis depends on how fast a virus replicates and how fast the cells used to propagate this virus proliferate. When the fast proliferating tumor cell lines used to grow viruses are plated in a low density, tumor cell proliferation and virus replication consume a large quantity of nucleotides that could quickly deplete intracellular nucleotide pools in the presence of a DHO-DHase inhibitor. However, when the cells used to grow viruses are plated at a high density and are less proliferative, inhibition of DHO-DHase activity will not deplete pyrimidine nucleotide pools, virus replication will not be inhibited. In our experiment, Vero and IPEC-DQ cells, an immortalized noncancerous cell line (Zhang et al., 2018), were plated at a high density. Following virus inoculation, the cells were incubated in the absence of serum. Virus replication was analyzed from 12 to 24 h.
The STAT family transcription factors have been implicated in playing an important role in regulating antiviral innate immunity. Recent studies have shown that coronaviruses evade antiviral immunity by modulating STAT activity and protein stability. Zhu et al. (2017) reported that the nsp5 protease of porcine deltacoronavirus cleaves STAT2 and interferes with the interferon signaling pathway, leading to the suppression of the expression of the interferon stimulating genes (ISGs). Likewise, Guo et al. (2016) reported that PEDV infection induces ubiquitin-proteasome degradation of STAT1, leading to the downregulation of IFN-mediated response. Unlike STAT1 and STAT2, the role of STAT3 in virus replication appears to be very complex (Chang et al., 2018; Kuchipudi, 2015; Roca Suarez et al., 2018). STAT3 can be positively or negatively regulated by different virus types and plays a pro- and antiviral role in virus replication (Kuchipudi, 2015) (Chang et al., 2018). Yang et al. (2018) recently reported that PEDV infection rapidly activates EGFR, leading to STAT3 tyrosine phosphorylation and that inhibition of EGFR and STAT3 by their specific inhibitors decreases PEDV replication in HEK293 and IPEC-J2 cells. These investigators further showed that inhibition of EGFR and STAT3 augments the expression of interferon-stimulating genes including MxA, ISG15, and IFN-β. Consistent with these observations, our present study showed that STAT3 overexpression enhanced PEDV replication and compromised the antiviral activity of A77 1726. Since A77 1726 inhibited PEDV replication in Vero cells, an IFN-deficient cell line, it is highly likely that the antiviral activity of A77 1726 in Vero cells is not due to increased IFN response. STAT3 has been implicated in suppressing apoptosis by inducing Bcl-2 expression and activating NF-κB (Fan et al., 2013). It is not clear if inhibition of STAT3 activation may sensitize virus-infected host cells to undergo apoptosis, thus suppressing PEDV replication.
In addition to regulating the antiviral innate immune response by activating the STAT transcription factors, PTKs are involved in the multiple steps of the life cycle of virus replication. For example, the Src family PTKs are required for the entry of MERS-CoV into cells (Shin et al., 2018). Saracatinib, a Src family tyrosine kinase inhibitor, inhibits the early stages of the MERS-CoV life cycle (Shin et al., 2018). JAKs may also promote virus endocytosis by regulating the cycling G-associated kinase (GAK) (Richardson et al., 2020). p59Fyn, a member of the Src family tyrosine kinase, participates in viral RNA replication and virion assembly and/or secretion of the dengue virus (de Wispelaere et al., 2013). Recent studies have shown that JAK1 and JAK2 are required for influenza A virus (IAV) replication (Han et al., 2018; Watanabe et al., 2014). JAK1 plays an important role in virion assembly of IAV (Watanabe et al., 2014). Our recent study showed that A77 1726 inhibits IAV replication in vitro and in vivo by targeting JAK2 (Wang et al., 2020). In addition, PTKs may activate the MAP kinase pathway to promotes virus replication (Shin et al., 2018). Our preliminary study showed that addition of A77 1726 to Vero cells 2 h post inoculation and to IPEC-DQ cells 12 post inoculation was able to inhibit PEDV replication (data not shown), suggesting that A77 1726 blocks a step in the late stage of the viral life cycle. How inhibition of JAK and Src tyrosine kinase activity by A77 1726 decreases PEDV replication remains elusive.
Coronavirus infections pose a great threat to human and animal health. Among them, severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) coronaviruses are highly contagious and often fatal (Hilgenfeld and Peiris, 2013; Hui et al., 2020; Lu et al., 2020; Zhang et al., 2020a; Zumla et al., 2016). SARS-CoV2, a novel coronavirus in the β genera of the Coronaviridae family and the etiological agent that causes a worldwide pandemic currently, has infected more than 10 million individuals worldwide by June, 2020 and caused hundreds of thousands of deaths. Currently, there are no effective antiviral drugs for treating coronavirus infections in humans and animals (Zumla et al., 2016). Remdesivir, a monophosphoramidate prodrug of an adenosine analogue with broad spectrum antiviral activities on filoviruses, paramyxoviruses, pneumoviruses, and coronaviruses, has been recently approved by the Food and Drug Administration for emergency use to treat COVID-19 (Ledford, 2020). There has been great interest in developing novel antiviral agents by targeting crucial host factors required for virus replication (Hilgenfeld and Peiris, 2013). In the present study, we provide evidence that A77 1726 effectively suppressed PEDV replication by inhibiting JAK and Src tyrosine kinase activities. Our study uncovers a previously unrecognized mechanism of action of A77 1726-mediated antiviral effects. Leflunomide, a clinically approved anti-inflammatory drug, could be potentially repurposed for the control of coronavirus infections by inhibiting virus replication and by alleviating severe inflammation in virus-infected tissues. Remarkably, JAK inhibitors are currently being tested in clinical trials for the control of the cytokine storm in COVID-19 patients (Mehta et al., 2020; Richardson et al., 2020; Russell et al., 2020; Zhang et al., 2020b). Since the antiviral activity of A77 1726 has been well documented, leflunomide may hold great advantages over other JAK inhibitors. However, great cautions should be taken since leflunomide is an immunosuppressant and has numerous side-effects such as diarrhea and hepatic toxicity. These side-effects may exacerbate the symptoms of COVID-19 patients and complicate its use.
Whether leflunomide can be used as an antiviral drug to treat PEDV infections in piglets remains to be investigated. The concentrations of A77 1726 in the plasma of mice treated with leflunomide (35 mg/kg/day) and in RA patients treated with leflunomide (100 mg per day for three days, 20 mg daily later) reach over 500 and 200 μM, respectively (Chan et al., 2005; Chong et al., 1999). Leflunomide has been shown to be effective in suppressing virus replication in animal models and in transplant patients (Chacko and John, 2012). If leflunomide is indeed capable of suppressing PEDV replication in pigs, it could be potentially useful for PED prophylaxis and treatment. Leflunomide, a small-molecule drug that can be manufactured at a low cost for veterinary use, should be practical for treating PED in piglets.
CRediT authorship contribution statement
Xiaomei Li: Investigation, Data curation, Formal analysis, Visualization, Methodology, Validation. Jing Sun: Visualization, Project administration, Resources. Richard A. Prinz: Writing - review & editing. Xiufan Liu: Resources. Xiulong Xu: Conceptualization, Supervision, Writing - original draft, Project administration.
Acknowledgements
This work was supported in part by the Priority Academic Program Development of Jiangsu Higher Education Institutions to Xiulong Xu, the College Inovation and Enterprenure Program of Jiangsu Higher Education Institutions to Xiulong Xu (201811117011Z), and by China Postdoctoral Science Foundation (2015M581873), Natural Science Foundation of Jiangsu Province (BK20150450), and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (2015311) to Jing Sun. We are very grateful to Dr. Ying Fang (College of Veterinary Medicine, University of Illinois at Urbana-Champaign, Urbana, Illinois) for kindly providing anti-PEDV antibodies, Dr. Dongwan Yoo (College of Veterinary Medicine, The University of Illinois at Urbana-Champaign, Urbana, Illinois) for kindly providing IPEC-DQ cells, Dr. Changchao Huan for kindly providing PEDV HLJBY strain, Dr. Eric Chang (Baylor College of Medicine) for kindly providing pBABE-JAK2 plasmid.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.virol.2020.06.009.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Beall A., Yount B., Lin C.M., Hou Y., Wang Q., Saif L., Baric R. Characterization of a pathogenic full-length cDNA clone and transmission model for porcine epidemic diarrhea virus strain PC22A. mBio. 2016;7 doi: 10.1128/mBio.01451-15. e01451-01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhoff E., Tylden G.D., Kjerpeseth L.J., Gutteberg T.J., Hirsch H.H., Rinaldo C.H. Leflunomide inhibition of BK virus replication in renal tubular epithelial cells. J. Virol. 2010;84:2150–2156. doi: 10.1128/JVI.01737-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilger A., Plowshay J., Ma S., Nawandar D., Barlow E.A., Romero-Masters J.C., Bristol J.A., Li Z., Tsai M.H., Delecluse H.J., Kenney S.C. Leflunomide/teriflunomide inhibit Epstein-Barr virus (EBV)- induced lymphoproliferative disease and lytic viral replication. Oncotarget. 2017;8:44266–44280. doi: 10.18632/oncotarget.17863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonavia A., Franti M., Pusateri Keaney E., Kuhen K., Seepersaud M., Radetich B., Shao J., Honda A., Dewhurst J., Balabanis K., Monroe J., Wolff K., Osborne C., Lanieri L., Hoffmaster K., Amin J., Markovits J., Broome M., Skuba E., Cornella-Taracido I., Joberty G., Bouwmeester T., Hamann L., Tallarico J.A., Tommasi R., Compton T., Bushell S.M. Identification of broad-spectrum antiviral compounds and assessment of the druggability of their target for efficacy against respiratory syncytial virus (RSV) Proc. Natl. Acad. Sci. U.S.A. 2011;108:6739–6744. doi: 10.1073/pnas.1017142108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman T., Garcia R., Turkson J., Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- Breedveld F.C., Dayer J.M. Leflunomide: mode of action in the treatment of rheumatoid arthritis. Ann. Rheum. Dis. 2000;59:841–849. doi: 10.1136/ard.59.11.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko B., John G.T. Leflunomide for cytomegalovirus: bench to bedside. Transpl. Infect. Dis. 2012;14:111–120. doi: 10.1111/j.1399-3062.2011.00682.x. [DOI] [PubMed] [Google Scholar]
- Chan V., Charles B.G., Tett S.E. Population pharmacokinetics and association between A77 1726 plasma concentrations and disease activity measures following administration of leflunomide to people with rheumatoid arthritis. Br. J. Clin. Pharmacol. 2005;60:257–264. doi: 10.1111/j.1365-2125.2005.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Z., Wang Y., Zhou X., Long J.E. STAT3 roles in viral infection: antiviral or proviral? Future Virol. 2018;13:557–574. doi: 10.2217/fvl-2018-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Sun J., Doscas M.E., Ye J., Williamson A.J., Li Y., Li Y., Prinz R.A., Xu X. Control of hyperglycemia in male mice by leflunomide: mechanisms of action. J. Endocrinol. 2018;237:43–58. doi: 10.1530/JOE-17-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Li G., Stasko J., Thomas J.T., Stensland W.R., Pillatzki A.E., Gauger P.C., Schwartz K.J., Madson D., Yoon K.J., Stevenson G.W., Burrough E.R., Harmon K.M., Main R.G., Zhang J. Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States. J. Clin. Microbiol. 2014;52:234–243. doi: 10.1128/JCM.02820-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Ding S., Yin Y., Xu L., Li P., Peppelenbosch M.P., Pan Q., Wang W. Suppression of pyrimidine biosynthesis by targeting DHODH enzyme robustly inhibits rotavirus replication. Antivir. Res. 2019;167:35–44. doi: 10.1016/j.antiviral.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Chong A.S., Huang W., Liu W., Luo J., Shen J., Xu W., Ma L., Blinder L., Xiao F., Xu X., Clardy C., Foster P., Williams J.A. In vivo activity of leflunomide: pharmacokinetic analyses and mechanism of immunosuppression. Transplantation. 1999;68:100–109. doi: 10.1097/00007890-199907150-00020. [DOI] [PubMed] [Google Scholar]
- de Wispelaere M., LaCroix A.J., Yang P.L. The small molecules AZD0530 and dasatinib inhibit dengue virus RNA replication via Fyn kinase. J. Virol. 2013;87:7367–7381. doi: 10.1128/JVI.00632-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doscas M.E., Williamson A.J., Usha L., Bogachkov Y., Rao G.S., Xiao F., Wang Y., Ruby C., Kaufman H., Zhou J., Williams J.W., Li Y., Xu X. Inhibition of p70 S6 kinase (S6K1) activity by A77 1726 and its effect on cell proliferation and cell cycle progress. Neoplasia. 2014;16:824–834. doi: 10.1016/j.neo.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eierhoff T., Hrincius E.R., Rescher U., Ludwig S., Ehrhardt C. The epidermal growth factor receptor (EGFR) promotes uptake of influenza A viruses (IAV) into host cells. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder R.T., Xu X., Williams J.W., Gong H., Finnegan A., Chong A.S. The immunosuppressive metabolite of leflunomide, A77 1726, affects murine T cells through two biochemical mechanisms. J. Immunol. 1997;159:22–27. [PubMed] [Google Scholar]
- Fan Y., Mao R., Yang J. NF-kappaB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell. 2013;4:176–185. doi: 10.1007/s13238-013-2084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavegnano C., Brehm J.H., Dupuy F.P., Talla A., Ribeiro S.P., Kulpa D.A., Cameron C., Santos S., Hurwitz S.J., Marconi V.C., Routy J.P., Sabbagh L., Schinazi R.F., Sekaly R.P. Novel mechanisms to inhibit HIV reservoir seeding using Jak inhibitors. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavegnano C., Detorio M., Montero C., Bosque A., Planelles V., Schinazi R.F. Ruxolitinib and tofacitinib are potent and selective inhibitors of HIV-1 replication and virus reactivation in vitro. Antimicrob. Agents Chemother. 2014;58:1977–1986. doi: 10.1128/AAC.02496-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Luo X., Li R., Xu Y., Zhang J., Ge J., Bu Z., Feng L., Wang Y. Porcine epidemic diarrhea virus infection inhibits interferon signaling by targeted degradation of STAT1. J. Virol. 2016;90:8281–8292. doi: 10.1128/JVI.01091-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Perez J.T., Chen C., Li Y., Benitez A., Kandasamy M., Lee Y., Andrade J., tenOever B., Manicassamy B. Genome-wide CRISPR/Cas9 screen identifies host factors essential for influenza virus replication. Cell Rep. 2018;23:596–607. doi: 10.1016/j.celrep.2018.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgenfeld R., Peiris M. From SARS to MERS: 10 years of research on highly pathogenic human coronaviruses. Antivir. Res. 2013;100:286–295. doi: 10.1016/j.antiviral.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann H.H., Kunz A., Simon V.A., Palese P., Shaw M.L. Broad-spectrum antiviral that interferes with de novo pyrimidine biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 2011;108:5777–5782. doi: 10.1073/pnas.1101143108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huan C., Pan H., Fu S., Xu W., Gao Q., Wang X., Gao S., Chen C., Liu X. Characterization and evolution of the coronavirus porcine epidemic diarrhoea virus HLJBY isolated in China. Transbound. Emerg. Dis. 2019;67:65–79. doi: 10.1111/tbed.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S., E I.A., Madani T.A., Ntoumi F., Kock R., Dar O., Ippolito G., McHugh T.D., Memish Z.A., Drosten C., Zumla A., Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - the latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D.A., Hejmanowski A.Q., Dierksheide J.E., Williams J.W., Chong A.S., Waldman W.J. Inhibition of herpes simplex virus type 1 by the experimental immunosuppressive agent leflunomide. Transplantation. 2001;71:170–174. doi: 10.1097/00007890-200101150-00031. [DOI] [PubMed] [Google Scholar]
- Kuchipudi S.V. The complex role of STAT3 in viral infections. J Immunol Res. 2015;2015:272359. doi: 10.1155/2015/272359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledford H. Hopes rise for coronavirus drug remdesivir. Nature. 2020 doi: 10.1038/d41586-020-01295-8. [DOI] [PubMed] [Google Scholar]
- Liacini A., Seamone M.E., Muruve D.A., Tibbles L.A. Anti-BK virus mechanisms of sirolimus and leflunomide alone and in combination: toward a new therapy for BK virus infection. Transplantation. 2010;90:1450–1457. doi: 10.1097/TP.0b013e3182007be2. [DOI] [PubMed] [Google Scholar]
- Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J. Med. Virol. 2020 doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Hourani M., Dauzonne D., Munier-Lehmann H., Khiar S., Nisole S., Dairou J., Helynck O., Afonso P.V., Tangy F., Vidalain P.O. Original chemical series of pyrimidine biosynthesis inhibitors that boost the antiviral interferon response. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.00383-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschall M., Niemann I., Kosulin K., Bootz A., Wagner S., Dobner T., Herz T., Kramer B., Leban J., Vitt D., Stamminger T., Hutterer C., Strobl S. Assessment of drug candidates for broad-spectrum antiviral therapy targeting cellular pyrimidine biosynthesis. Antivir. Res. 2013;100:640–648. doi: 10.1016/j.antiviral.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., Hlh Across Speciality Collaboration, U.K. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munier-Lehmann H., Vidalain P.O., Tangy F., Janin Y.L. On dihydroorotate dehydrogenases and their inhibitors and uses. J. Med. Chem. 2013;56:3148–3167. doi: 10.1021/jm301848w. [DOI] [PubMed] [Google Scholar]
- Pearce L.R., Alton G.R., Richter D.T., Kath J.C., Lingardo L., Chapman J., Hwang C., Alessi D.R. Characterization of PF-4708671, a novel and highly specific inhibitor of p70 ribosomal S6 kinase (S6K1) Biochem. J. 2010;431:245–255. doi: 10.1042/BJ20101024. [DOI] [PubMed] [Google Scholar]
- Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A., Stebbing J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca Suarez A.A., Van Renne N., Baumert T.F., Lupberger J. Viral manipulation of STAT3: evade, exploit, and injure. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckemann K., Fairbanks L.D., Carrey E.A., Hawrylowicz C.M., Richards D.F., Kirschbaum B., Simmonds H.A. Leflunomide inhibits pyrimidine de novo synthesis in mitogen-stimulated T-lymphocytes from healthy humans. J. Biol. Chem. 1998;273:21682–21691. doi: 10.1074/jbc.273.34.21682. [DOI] [PubMed] [Google Scholar]
- Russell B., Moss C., George G., Santaolalla A., Cope A., Papa S., Van Hemelrijck M. Associations between immune-suppressive and stimulating drugs and novel COVID-19-a systematic review of current evidence. Ecancermedicalscience. 2020;14:1022. doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J.S., Jung E., Kim M., Baric R.S., Go Y.Y. 2018. Saracatinib Inhibits Middle East Respiratory Syndrome-Coronavirus Replication in Vitro. Viruses 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemasko K., Chong A.S., Jack H.M., Gong H., Williams J.W., Finnegan A. Inhibition of JAK3 and STAT6 tyrosine phosphorylation by the immunosuppressive drug leflunomide leads to a block in IgG1 production. J. Immunol. 1998;160:1581–1588. [PubMed] [Google Scholar]
- Siemasko K.F., Chong A.S., Williams J.W., Bremer E.G., Finnegan A. Regulation of B cell function by the immunosuppressive agent leflunomide. Transplantation. 1996;61:635–642. doi: 10.1097/00007890-199602270-00020. [DOI] [PubMed] [Google Scholar]
- Song D., Park B. Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Gene. 2012;44:167–175. doi: 10.1007/s11262-012-0713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Mu Y., Jiang Y., Song R., Yi J., Zhou J., Sun J., Jiao X., Prinz R.A., Li Y., Xu X. Inhibition of p70 S6 kinase activity by A77 1726 induces autophagy and enhances the degradation of superoxide dismutase 1 (SOD1) protein aggregates. Cell Death Dis. 2018;9:407. doi: 10.1038/s41419-018-0441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasova A.N., Marthaler D., Wang Q., Culhane M.R., Rossow K.D., Rovira A., Collins J., Saif L.J. Distinct characteristics and complex evolution of PEDV strains, North America. Emerg. Infect. Dis. 2014;20:1620–1628. doi: 10.3201/eid2010.140491. May 2013-February 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman W.J., Knight D.A., Blinder L., Shen J., Lurain N.S., Miller D.M., Sedmak D.D., Williams J.W., Chong A.S. Inhibition of cytomegalovirus in vitro and in vivo by the experimental immunosuppressive agent leflunomide. Intervirology. 1999;42:412–418. doi: 10.1159/000053979. [DOI] [PubMed] [Google Scholar]
- Waldman W.J., Knight D.A., Lurain N.S., Miller D.M., Sedmak D.D., Williams J.W., Chong A.S. Novel mechanism of inhibition of cytomegalovirus by the experimental immunosuppressive agent leflunomide. Transplantation. 1999;68:814–825. doi: 10.1097/00007890-199909270-00014. [DOI] [PubMed] [Google Scholar]
- Wang J., Sun J., Hu J., Wang C., Prinz R.A., Peng D., Liu X., Xu X. A77 1726, the active metabolite of the anti-rheumatoid arthritis drug leflunomide, inhibits influenza A virus replication in vitro and in vivo by inhibiting the activity of Janus kinases. Faseb. J. 2020 doi: 10.1096/fj.201902793RR. Accepted. [DOI] [PubMed] [Google Scholar]
- Wang Q.Y., Bushell S., Qing M., Xu H.Y., Bonavia A., Nunes S., Zhou J., Poh M.K., Florez de Sessions P., Niyomrattanakit P., Dong H., Hoffmaster K., Goh A., Nilar S., Schul W., Jones S., Kramer L., Compton T., Shi P.Y. Inhibition of dengue virus through suppression of host pyrimidine biosynthesis. J. Virol. 2011;85:6548–6556. doi: 10.1128/JVI.02510-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang W., Xu L., Zhou X., Shokrollahi E., Felczak K., van der Laan L.J., Pankiewicz K.W., Sprengers D., Raat N.J., Metselaar H.J., Peppelenbosch M.P., Pan Q. Cross talk between nucleotide synthesis pathways with cellular immunity in constraining hepatitis E virus replication. Antimicrob. Agents Chemother. 2016;60:2834–2848. doi: 10.1128/AAC.02700-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Kawakami E., Shoemaker J.E., Lopes T.J., Matsuoka Y., Tomita Y., Kozuka-Hata H., Gorai T., Kuwahara T., Takeda E., Nagata A., Takano R., Kiso M., Yamashita M., Sakai-Tagawa Y., Katsura H., Nonaka N., Fujii H., Fujii K., Sugita Y., Noda T., Goto H., Fukuyama S., Watanabe S., Neumann G., Oyama M., Kitano H., Kawaoka Y. Influenza virus-host interactome screen as a platform for antiviral drug development. Cell Host Microbe. 2014;16:795–805. doi: 10.1016/j.chom.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R.A., Yea C.M., Robson P.A., Curnock A.P., Gadher S., Hambleton A.B., Woodward K., Bruneau J.M., Hambleton P., Spinella-Jaegle S., Morand P., Courtin O., Sautes C., Westwood R., Hercend T., Kuo E.A., Ruuth E. Dihydroorotate dehydrogenase is a target for the biological effects of leflunomide. Transplant. Proc. 1996;28:3088–3091. [PubMed] [Google Scholar]
- Xu X., Blinder L., Shen J., Gong H., Finnegan A., Williams J.W., Chong A.S. In vivo mechanism by which leflunomide controls lymphoproliferative and autoimmune disease in MRL/MpJ-lpr/lpr mice. J. Immunol. 1997;159:167–174. [PubMed] [Google Scholar]
- Xu X., Shen J., Mall J.W., Myers J.A., Huang W., Blinder L., Saclarides T.J., Williams J.W., Chong A.S. In vitro and in vivo antitumor activity of a novel immunomodulatory drug, leflunomide: mechanisms of action. Biochem. Pharmacol. 1999;58:1405–1413. doi: 10.1016/s0006-2952(99)00228-2. [DOI] [PubMed] [Google Scholar]
- Xu X., Sun J., Song R., Doscas M.E., Williamson A.J., Zhou J., Sun J., Jiao X., Liu X., Li Y. Inhibition of p70 S6 kinase (S6K1) activity by A77 1726, the active metabolite of leflunomide, induces autophagy through TAK1-mediated AMPK and JNK activation. Oncotarget. 2017;8:30438–30454. doi: 10.18632/oncotarget.16737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Williams J.W., Bremer E.G., Finnegan A., Chong A.S. Inhibition of protein tyrosine phosphorylation in T cells by a novel immunosuppressive agent, leflunomide. J. Biol. Chem. 1995;270:12398–12403. doi: 10.1074/jbc.270.21.12398. [DOI] [PubMed] [Google Scholar]
- Xu X., Williams J.W., Gong H., Finnegan A., Chong A.S. Two activities of the immunosuppressive metabolite of leflunomide, A77 1726. Inhibition of pyrimidine nucleotide synthesis and protein tyrosine phosphorylation. Biochem. Pharmacol. 1996;52:527–534. doi: 10.1016/0006-2952(96)00303-6. [DOI] [PubMed] [Google Scholar]
- Yang L., Xu J., Guo L., Guo T., Zhang L., Feng L., Chen H., Wang Y. Porcine epidemic diarrhea virus-induced epidermal growth factor receptor activation impairs the antiviral activity of type I interferon. J. Virol. 2018;92 doi: 10.1128/JVI.02095-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Ruan T., Sheng T., Wang J., Sun J., Wang J., Prinz R.A., Peng D., Liu X., Xu X. Role of c-Jun terminal kinase (JNK) activation in influenza A virus-induced autophagy and replication. Virology. 2019;526:1–12. doi: 10.1016/j.virol.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Wang L., Deng X., Liang R., Su M., He C., Hu L., Su Y., Ren J., Yu F., Du L., Jiang S. Recent advances in the detection of respiratory virus infection in humans. J. Med. Virol. 2020 doi: 10.1002/jmv.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Ke H., Blikslager A., Fujita T., Yoo D. Type III interferon restriction by porcine epidemic diarrhea virus and the role of viral protein nsp 1 in IRF1 signaling. J. Virol. 2018;92 doi: 10.1128/JVI.01677-17. e01677-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X., Zeng X., Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the Perspectives of clinical immunologists from China. Clin. Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Wang D., Zhou J., Pan T., Chen J., Yang Y., Lv M., Ye X., Peng G., Fang L., Xiao S. Porcine deltacoronavirus nsp5 antagonizes type I interferon signaling by cleaving STAT2. J. Virol. 2017;91 doi: 10.1128/JVI.00003-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.Y. Coronaviruses - drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.