Immunoglobulin A (IgA) is essential for defense of the intestinal mucosa against harmful pathogens. Gut microbiota impact IgA production, but the specific species responsible for IgA production remain largely elusive. Previous studies have shown that IgA and Bacteroidetes, the major phyla of gut microbiota, were increased in soluble high-fiber diet-fed mice. We show here that the levels of IgA in the gut and the expression of activation-induced cytidine deaminase (AID) in the large intestine lamina propria, which is crucial for class switch recombination from IgM to IgA, were correlated with the abundance of Bacteroides fragilis group species such as Bacteroides faecis, Bacteroides caccae, and Bacteroides acidifaciens. B. acidifaciens monoassociated mice increased gut IgA production and AID expression. Soluble dietary fiber may improve gut immune function, thereby protecting against bowel pathogens and reducing inflammatory bowel diseases.
KEYWORDS: dietary fiber, soluble high-fiber diet, hsp60-based profiling analysis, activation induced cytidine deaminase, Bacteroidetes, Bacteroides fragilis group, Bacteroides acidifaciens, immunoglobulin A, no-fiber diet, dextran sulfate sodium-induced colitis, Bacteriodes vulgatus, metabolome analysis, Spearman's rank correlation coefficient, Pearson’s correlation coefficient
ABSTRACT
Immunoglobulin A (IgA) is essential for defense of the intestinal mucosa against harmful pathogens. Previous studies have shown that Bacteroidetes, the major phylum of gut microbiota together with Firmicutes, impact IgA production. However, the relative abundances of species of Bacteroidetes responsible for IgA production were not well understood. In the present study, we identified some specific Bacteroidetes species that were associated with gut IgA induction by hsp60-based profiling of species distribution among Bacteroidetes. The levels of IgA and the expression of the gene encoding activation-induced cytidine deaminase (AID) in the large intestine lamina propria, which is crucial for class switch recombination from IgM to IgA, were increased in soluble high-fiber diet (sHFD)-fed mice. We found that Bacteroides acidifaciens was the most abundant Bacteroidetes species in both sHFD- and normal diet-fed mice. In addition, the gut IgA levels were associated with the relative abundance of Bacteroides fragilis group species such as Bacteroides faecis, Bacteroides caccae, and Bacteroides acidifaciens. Conversely, the ratio of B. acidifaciens to other Bacteroidetes species was reduced in insoluble high-fiber diet fed- and no-fiber diet-fed mice. To investigate whether B. acidifaciens increases IgA production, we generated B. acidifaciens monoassociated mice and found increased gut IgA production and AID expression. Collectively, soluble dietary fiber increases the ratio of gut Bacteroides fragilis group, such as B. acidifaciens, and IgA production. This might improve gut immune function, thereby protecting against bowel pathogens and reducing the incidence of inflammatory bowel diseases.
IMPORTANCE Immunoglobulin A (IgA) is essential for defense of the intestinal mucosa against harmful pathogens. Gut microbiota impact IgA production, but the specific species responsible for IgA production remain largely elusive. Previous studies have shown that IgA and Bacteroidetes, the major phyla of gut microbiota, were increased in soluble high-fiber diet-fed mice. We show here that the levels of IgA in the gut and the expression of activation-induced cytidine deaminase (AID) in the large intestine lamina propria, which is crucial for class switch recombination from IgM to IgA, were correlated with the abundance of Bacteroides fragilis group species such as Bacteroides faecis, Bacteroides caccae, and Bacteroides acidifaciens. B. acidifaciens monoassociated mice increased gut IgA production and AID expression. Soluble dietary fiber may improve gut immune function, thereby protecting against bowel pathogens and reducing inflammatory bowel diseases.
INTRODUCTION
Immunoglobulin A (IgA) is abundant in the intestine and has an essential role in the defense of the intestinal mucosa against harmful pathogens (1, 2). IgA is released from IgA+ cells in the intestinal lamina propria to protect the host mucosal barrier. Previous studies have demonstrated that the T cell-dependent pathway through CD40 ligand and the T cell-independent pathway through dendritic cells (DCs) in Peyer’s patches (PPs) are required for IgA production (3). Additionally, accumulating evidence has suggested that diet and gut microbiota are involved in the regulation of IgA production (4–6). However, the mechanisms by which IgA is increased by diet and gut microbiota remain largely elusive.
The diet impacts the gut microbiota and the immune system (7, 8). Dietary fiber is classified as soluble or insoluble, and these two forms are digested in different ways (9, 10). Soluble dietary fiber is fermented in the intestine by specific bacteria, which results in the production of short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate. In contrast, insoluble dietary fiber is not well fermented by gut microbiota. Soluble dietary fiber intake is associated with the species composition of gut commensal microbiota and has been implicated in immune system modulation. In particular, butyrate is known to act as a histone deacetylase inhibitor and induce regulatory T cells in the gut (11). Also, it has been reported that SCFAs can increase the level of IgA in the intestine (12).
Hundreds of species reside in the intestinal tract. In particular, Firmicutes and Bacteroidetes are major phyla of gut microbiota and are believed to function in both energy metabolism and immune protection (13–15). Notably, recent studies have reported that a soluble high-fiber diet (sHFD) can increase the ratio of gut Bacteroidetes to other phyla (16, 17).
According to the previous studies, the phylum of Bacteroidetes is related to the induction of IgA in the gut. However, the relative abundance of species of Bacteroidetes for IgA production were not well understood.
Based on these observations, we unveiled the species levels of Bacteroidetes using an hsp60-based profiling method of analysis of the gut content samples from mice fed sHFD, a no-fiber diet (NFD), an insoluble high-fiber diet (iHFD), or a normal diet (ND).
Some previous studies using the 16S rRNA amplicon method suggested that Bacteroidetes abundance could be linked with dietary fiber intake. However, the 16S rRNA amplicon method has poor resolving power to identify below the genus level unless taxonomic assignments are performed by using sequences of multiple variable regions or full-length regions (18, 19). Therefore, how soluble or insoluble dietary fiber influences bacterial components at the species level among the Bacteroidetes phylum has remained largely unknown. In order to analyze the species distribution among Bacteroidetes against murine fecal specimens in the present study, we used a method targeting heat shock protein (hsp) 60, utilizing partial sequences widely used in species identification using Sanger sequencing because of its higher diversity than that of 16S rRNA. This hsp60-based method using next-generation sequencing has been used in recent studies (18, 19).
In the present study, we demonstrated the relationship between IgA induction in the gut and the relative abundance of Bacteroidetes species levels by using hsp60-based profiling analysis in mice.
RESULTS
Dietary fiber affects the number of IgA+ B220+ cells in the LILP.
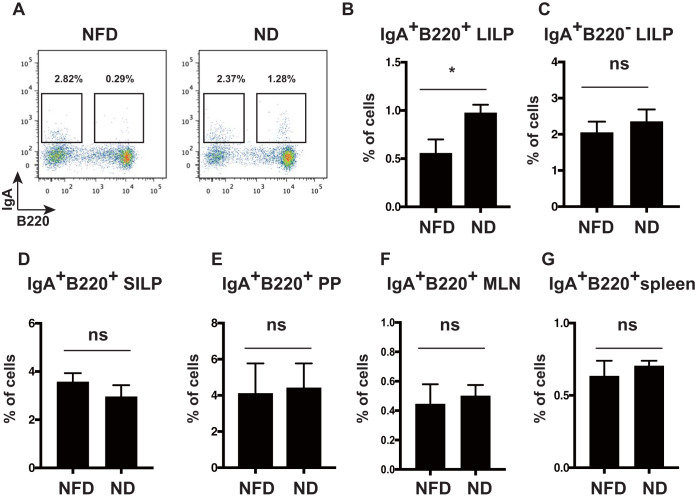
IgA+ cells secreting IgA develop in PPs and then move to the lamina propria (3). We first examined whether dietary fiber affects IgA+ cell induction in the intestinal tract. To address the effect of dietary fiber, we fed wild-type mice the ND, which contains approximately 2% dietary fiber, or the NFD, which contains 0% dietary fiber. We detected IgA+ B220+ cells and IgA+ B220– cells in the large intestine lamina propria (LILP). We observed that the frequency of IgA+ B220+ cells was significantly lower in NFD-fed mice than in ND-fed mice (Fig. 1A and B), whereas the frequency of IgA+ B220− cells in the LILP did not differ between ND- and NFD-fed mice (Fig. 1C). On the other hand, the frequency of IgA+ B220+ cells in the small intestine lamina propria (SILP) did not differ between ND- and NFD-fed mice (Fig. 1D). In addition, the frequency of IgA+ B220+ cells in PPs, mesenteric lymph nodes (MLNs), and the spleen did not differ between ND- and NFD-fed mice (Fig. 1E to G). These results suggest that dietary fiber affects the number of IgA+ B220+ cells but not IgA+ B220− cells in the LILP. Also, there was no effect on the number of IgA+ B220+ and IgA+ B220− cells in the SILP, PPs, spleen, and MLNs. These results raised the possibility that the gut microbiota in the large intestine are involved in IgA+ B220+ cell induction by dietary fiber.
FIG 1.
Dietary fiber increased IgA+ B220+ cell numbers in the LILP of mice. (A) Flow cytometric analysis of IgA+ cells in the LILP of NFD-fed mice (left) versus ND-fed mice (right). LILP cells from NFD-fed and ND-fed mice were stained with anti-IgA and anti-B220 antibodies. The frequencies of IgA+ B220− (gated in the left square) and IgA+ B220+ cells (gated in the right square) were analyzed using flow cytometry. The percentages of IgA+B220− and IgA+ B220+ cells are indicated in the panels. The figure shows the representative data from at least three independent experiments. n = 8 mice per group. (B and C) The frequencies of IgA+ B220+ cells (B) and IgA+ B220– cells (C) in the LILP of NFD- and ND-fed mice. n = 8 mice per group. Data are presented as the mean ± SD of at least three independent experiments. *, P < 0.05. The graphs were enumerated as in panel A. (D) The frequency of IgA+ B220+ cells in the SILP of NFD- and ND-fed mice. n = 8 mice per group. Data are shown as the mean ± SD. ns, not significant. (E to G) The frequency of IgA+ B220+ cells in PPs (E), MLNs (F), and spleen (G) of NFD- and ND-fed mice. n = 8 mice per group. Data are shown as the mean ± SD. ns, not significant. Results are representative of at least three independent experiments.
Soluble dietary fiber promotes gut IgA production and attenuates dextran sulfate sodium (DSS)-induced colitis.
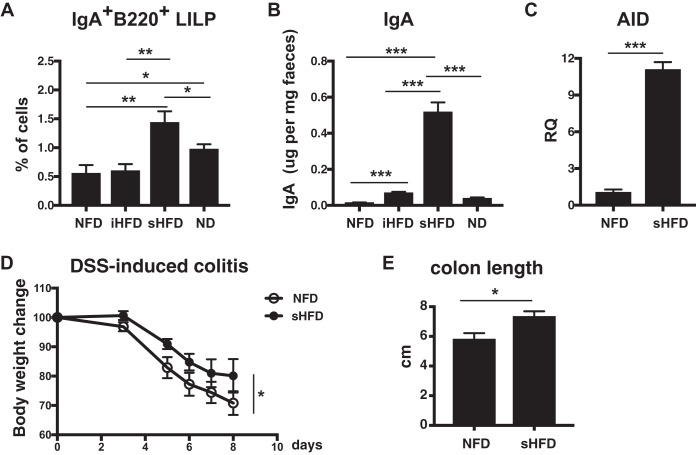
Next, we examined which type of dietary fiber—soluble, insoluble, or both—influences gut IgA production in the LILP. Wild-type mice were fed either sHFD, containing 10% inulin, iHFD, containing 10% cellulose, NFD, or ND for 3 weeks, and then the frequency of IgA+ B220+ cells in the LILP was measured. The frequency of IgA+ B220+ cells was higher in the LILP of sHFD-fed mice than in that of iHFD- and NFD-fed mice (Fig. 2A). In addition, the amount of IgA was considerably higher in the isolated gut contents of sHFD-fed mice than in those of iHFD-, NFD-, and ND-fed mice (Fig. 2B).
FIG 2.
sHFD increased the level of IgA and AID gene expression in the LILP. sHFD intake attenuates DSS-induced colitis. (A) The frequency of IgA+ B220+ cells in the LILP of NFD-, iHFD-, sHFD-, and ND-fed mice. Mice were housed individually and fed either NFD, sHFD, iHFD, or ND for 3 weeks. LILP cells from NFD-, iHFD-, sHFD-, and ND-fed mice were collected and stained with anti-IgA and anti-B220 antibodies. The frequency of IgA+ B220+ cells was estimated as described in Fig. 1A. *, P < 0.05; **, P < 0.01. (B) The level of IgA in the gut contents from NFD-, iHFD-, sHFD-, and ND-fed mice was measured with ELISA. n = 8 mice per group. Data are presented as the mean ± SD from three independent experiments with feces collected in a different animal facility. ***, P < 0.001. (C) AID expression in the LILP B cells of NFD- and sHFD-fed mice. LILP B cells were sorted using autoMACS, and gene expression levels were analyzed using real-time PCR. AID gene expression was determined by relative quantification (RQ) of the mean value of triplicate wells, with the NFD-fed mouse samples arbitrarily set to 1. Data are shown as the mean ± SD. ***, P < 0.001. (D and E) Wild-type mice at 8 weeks old were fed either NFD or sHFD for 3 weeks. Then, 2.5% DSS was orally administered to NFD-fed and sHFD-fed mice for 5 days, and then, DSS was switched to drinking water for the next 3 days. Body weight (D) was measured every day, and colon length (E) was measured on day 8. n = 4 mice per group. Data are presented as the mean ± SD of at least three independent experiments. *, P < 0.05.
Activation-induced cytidine deaminase (AID) is required for class switch recombination (CSR) and somatic hypermutation (SHM) of immunoglobulin from IgM to IgA. AID is a crucial gene for generating IgA in the gut (20, 21). AID-deficient mice developed microbiota disruption caused by the lack of IgA (6, 22). Therefore, we investigated whether AID expression in the LILP is involved in IgA induction in sHFD- and NFD-fed mice. As a result, we observed that AID expression is significantly increased in the LILP B cells of sHFD-fed mice compared with NFD-fed mice (Fig. 2C). Thus, the soluble dietary fiber is related to increased gut IgA and AID expression in the LILP. Next, we performed DSS-induced colitis to examine the physiological relevance of increased IgA levels and AID expression by sHFD intake in mice. We found that there was a significant difference in body weight loss (Fig. 2D) and colon length (Fig. 2E) between NFD- and sHFD-fed mice. NFD-fed mice were more sensitive to DSS-induced colitis than sHFD-fed mice.
Dietary fiber alters the composition of Bacteroidetes species.
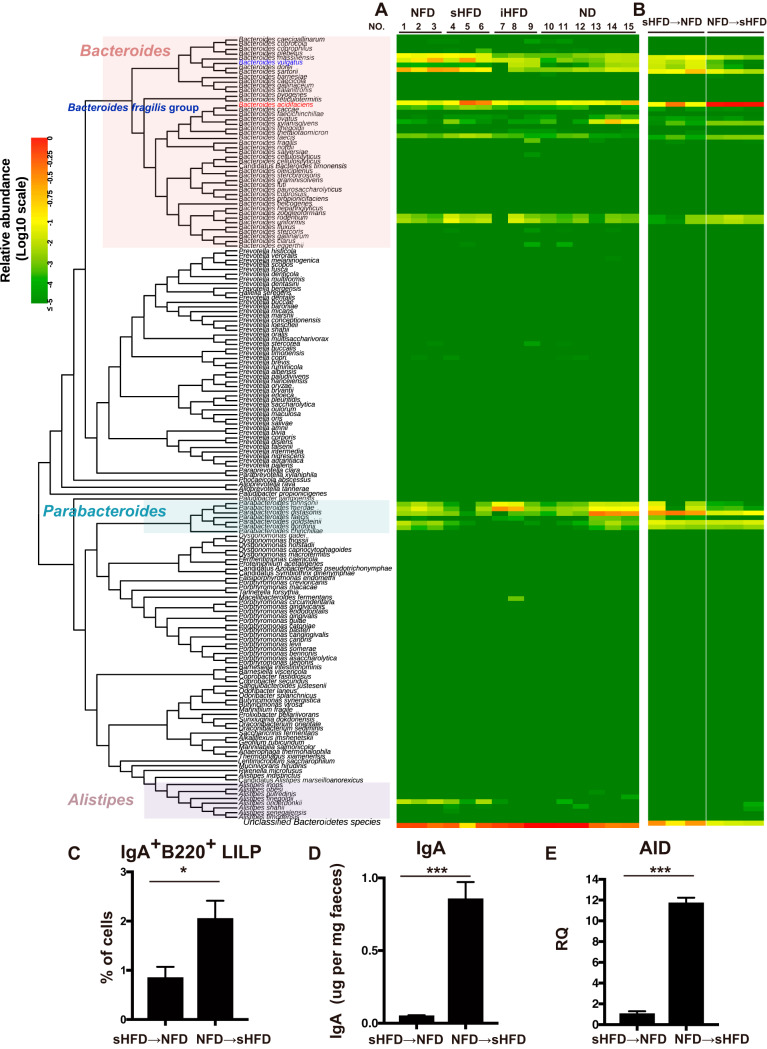
We and other groups have shown that the ratio of Bacteroidetes to other phyla is higher in sHFD-fed mice than in NFD-fed mice (16, 17). Thus, Bacteroidetes may be the major phylum involved in gut IgA induction. We assumed that Bacteroidetes species are induced by sHFD and are involved in gut IgA induction. To identify which bacterial species included in the Bacteroidetes were increased by sHFD, we performed hsp60-based profiling analysis of the gut contents of the large intestine from mice fed different fiber diets (sHFD, iHFD, NFD, and ND) for 3 weeks at the species level by hsp60 amplicon sequencing, as reported previously (23). The hsp60 PCR amplification has been widely used for species identification and taxonomic studies in Bacteroidetes bacteria (24, 25). We analyzed the species component of 167 species among the Bacteroidetes phylum, whose hsp60 sequences were obtained from the NCBI database. From DNA libraries of hsp60 PCR products, we obtained an average of 298,533 reads per sample (see Table S1 in the supplemental material). The PCR primers used in this analysis can amplify the target sequences not only in Bacteroidetes but also in the other phyla. The abundance ratio of the Bacteroidetes phylum among total reads was 21.2% (range, 4.8% to 48.4%). Log10 scales of relative abundance are visualized using a red-yellow-green gradient, with red representing the highest score (0) and green representing the lowest score (≤−5) in terms of the abundance ratios among 167 Bacteroidetes species (Fig. 3).
FIG 3.
Dietary fiber alters the composition of gut Bacteroidetes species. (A and B) Eight-week-old C57BL/6J mice were housed individually and fed either NFD, sHFD, iHFD, or ND for 3 weeks. The gut contents from the large intestine were used to analyze Bacteroidetes species with an hsp60-based profiling method. (A and B) Heatmap showing the relative abundances of 167 species of the Bacteroidetes and the Bacteroidetes subgroup, including the Prevotella cluster and the Porphyromonas cluster identified in the gut contents of NFD-, iHFD-, sHFD-, and ND-fed mice (A) and both sHFD → NFD-fed and NFD → sHFD-fed mice (B) using the hsp60-based profiling analysis. The relative value for each bacterial genus is indicated by color intensity (legend at the top-left corner). The phylogenetic tree was constructed with the neighbor-joining method based on alignment using MAFFT version 6.864 (28). The Bacteroides fragilis group was surrounded by a blue square. n = 3 NFD, sHFD, and iHFD-fed mice. n = 6 ND-fed mice. (C) The frequency of IgA+ B220+ cells in the LILP of sHFD → NFD-fed and NFD → sHFD-fed mice. n = 3 mice per group. Data are presented as the mean ± SD. *, P < 0.05. (D) The level of IgA in the gut contents from sHFD → NFD-fed and NFD → sHFD-fed mice were measured with ELISA. n = 3 mice per group. Data are presented as the mean ± SD from three independent experiments with feces. ***, P < 0.001. (E) AID expression in the LILP B cells in sHFD → NFD-fed and NFD → sHFD-fed mice. LILP B cells were sorted using autoMACS, and gene expression levels were analyzed using real-time PCR. AID gene expression was determined by RQ of the mean value of triplicate wells, with the sHFD → NFD-fed mouse samples arbitrarily set to 1. Data are shown as the mean ± SD. n = 3 mice per group. ***, P < 0.001.
According to hsp60-based profiling of species distribution among the Bacteroidetes phylum at the species level, the predominant Bacteroidetes species in ND-fed mice were Parabacteroides distasonis (average relative abundance, 0.1502 ± 0.1660) and Bacteroides acidifaciens (0.0523 ± 0.0576), followed by Parabacteroides merdae (0.0487 ± 0.0491), Bacteroides xylanisolvens (0.0429 ± 0.0682), and Bacteroides sartorii (0.0114 ± 0.0100) (Fig. 3A).
In a comparison of Bacteroidetes species components between two groups of sHFD and ND-fed mice, the relative abundances of 8 Bacteroides species (B. massiliensis, B. vulgatus, B. sartorii, B. acidifaciens, B. caccae, B. faecis, B. rodentium, and B. uniformis) in sHFD-fed mice were significantly increased (P < 0.05; a Wilcoxon rank sum test) compared with those in ND-fed mice. B. acidifaciens was the predominant species (average relative abundance, 0.2729 ± 0.1523) in sHFD-fed mice, followed by B. sartorii (0.1529 ± 0.0607), B. vulgatus (0.1018 ± 0.1266), B. massiliensis (0.0675 ± 0.0347), and B. uniformis (0.0280 ± 0.0289).
The relative abundances of B. massiliensis, B. vulgatus, and B. sartorii in NFD-fed mice were significantly increased (P < 0.05; a Wilcoxon rank sum test) compared with those in ND-fed mice. B. sartorii was the predominant species (average relative abundance, 0.1991 ± 0.0555) in NFD-fed mice, followed by B. vulgatus (0.1206 ± 0.0864), B. massiliensis (0.0873 ± 0.0255), and B. acidifaciens (0.0598 ± 0.0277). In iHFD-fed mice, only the relative abundance of B. vulgatus was significantly increased (P < 0.05; a Wilcoxon rank sum test) compared with that of ND-fed mice. Parabacteroides merdae was the predominant species (average relative abundance, 0.1600 ± 0.1395) in iHFD-fed mice, followed by B. acidifaciens (0.0428 ± 0.0283), Parabacteroides johnsonii (0.0426 ± 0.0375), and B. vulgatus (0.0173 ± 0.0049).
The switch from NFD to sHFD increases the relative abundance of B. acidifaciens in murine intestine.
To confirm whether B. acidifaciens induction is actually dependent on a soluble fiber diet, mice were fed either sHFD or NFD for the first 2 weeks and then switched to the other diet for the next 2 weeks. B. acidifaciens was increased in NFD → sHFD-fed mice (average relative abundance, 0.8273 ± 0.0198; P = 0.0128) but decreased in sHFD → NFD-fed mice (average relative abundance, 0.1761 ± 0.1240) (Fig. 3B). These results suggest that the composition of the gut microbiota changes rapidly if the fiber type in the diet is altered and that B. acidifaciens induction is dependent on sHFD intake. We detected IgA+ B220+ cells in the LILP using flow cytometry and observed that the frequency of IgA+ B220+ cells in the LILP was significantly lower in sHFD → NFD-fed mice than NFD → sHFD-fed mice (Fig. 3C). The amount of IgA was considerably higher in the isolated gut contents of NFD → sHFD-fed mice than in those of sHFD → NFD-fed mice (Fig. 3D). Next, we examined AID expression of the LILP B cells. AID expression was increased in NFD → sHFD-fed mice compared with sHFD → NFD-fed mice (Fig. 3E).
Identification of the Bacteroidetes species associated with IgA induction in murine intestine.
To identify the Bacteroidetes species associated with gut IgA induction by dietary fiber, we examined correlation coefficients between gut IgA levels as shown in Fig. 2B and the relative abundance of Bacteroidetes species from NFD-, sHFD-, iHFD-, and ND-fed mice as shown in Fig. 3A and Table S1. Eight Bacteroidetes species (B. faecis, B. caccae, B. acidifaciens, B. uniformis, B. rodentium, Alistipes shahii, Bacteroides finegoldii, and Bacteroides plebeius) were initially identified as species correlated with gut IgA level (Table 1). According to the results based on the false-discovery rate, B. faecis, B. caccae, and B. acidifaciens were consequently identified as Bacteroidetes species significantly correlated with gut IgA level (q value = 0.001428, 0.002857, and 0.004285, respectively). These belonged phylogenetically to the B. fragilis group (Fig. 3A). Among 3 species (B. faecis, B. caccae, and B. acidifaciens), B. faecis correlated most significantly with IgA level (correlation coefficient R = 0.8161, P value; two-tailed probability P = 0.0002; 95% confidence interval [CI] = 0.5221 to 0.9367), followed by B. caccae (R = 0.7908, P = 0.0004, 95% CI = 0.4682 to 0.9274) and B. acidifaciens (R = 0.7099, P = 0.0030, 95% CI = 0.3105 to 0.8962) as determined using the Pearson’s correlation coefficient (Table 1). B. vulgatus, B. massiliensis, and B. sartorii, whose relative abundances were increased in the sHFD-fed mice, were not correlated with gut IgA level (R = 0.2378, 0.3865, and 0.3938, respectively, as determined using the Pearson’s correlation coefficient). Additionally, we also calculated by the Spearman’s rank correlation coefficient to reassess these relationships. The relative abundances of B. faecis, B. caccae, and B. acidifaciens remained weakly correlated with gut IgA level (R = 0.2107143, 0.2484362, and 0.2392857, respectively). Thus, the relative abundances of B. faecis, B. caccae, and B. acidifaciens in the murine intestinal microbiota exhibited a certain level of correlation with gut IgA level.
TABLE 1.
List of correlation coefficients between the relative abundance of Bacteroidetes species and the IgA levels in each experimental groupa
| Species | Correlation coefficient R | P value (two-tailed probability) | 95% confidence interval | Avg relative abundance (SD) |
|||
|---|---|---|---|---|---|---|---|
| NFD (n = 3) | sHFD (n = 3) | iHFD (n = 3) | ND (n = 6) | ||||
| Bacteroides faecis | 0.8161 | 0.0002 | 0.5221 to 0.9367 | 0.001403 (0.000518) | 0.006557 (0.003049) | 0.000580 (0.000336) | 0.000451 (0.000509) |
| Bacteroides caccae | 0.7908 | 0.0004 | 0.4682 to 0.9274 | 0.000590 (0.000223) | 0.002838 (0.001495) | 0.000288 (0.000198) | 0.000216 (0.000212) |
| Bacteroides acidifaciens | 0.7099 | 0.0030 | 0.3105 to 0.8962 | 0.059837 (0.027721) | 0.272976 (0.152368) | 0.042822 (0.028310) | 0.074600 (0.057628) |
| Bacteroides uniformis | 0.6510 | 0.0086 | 0.2082 to 0.8724 | 0.003647 (0.003061) | 0.028074 (0.028916) | 0.013628 (0.016874) | 0.001495 (0.001010) |
| Bacteroides rodentium | 0.6229 | 0.0131 | 0.1624 to 0.8605 | 0.002126 (0.001996) | 0.011454 (0.009974) | 0.006069 (0.008947) | 0.001043 (0.000655) |
| Alistipes shahii | 0.6066 | 0.0165 | 0.1368 to 0.8536 | 0 (0.000000) | 0.000009 (0.000015) | 0 (0.000000) | 0 (0.000000) |
| Bacteroides finegoldii | 0.6066 | 0.0165 | 0.0353 to 0.8721 | 0 (0.000000) | 0.000006 (0.000010) | 0 (0.000000) | 0 (0.000000) |
| Bacteroides plebeius | 0.5455 | 0.0355 | 0.0461 to 0.8267 | 0 (0.000000) | 0.000218 (0.000362) | 0 (0.000000) | 0.000012 (0.000009) |
| Bacteroides vulgatus | 0.2378 | 0.3934 | –0.313 to 0.6686 | 0.120671 (0.086473) | 0.101832 (0.126610) | 0.017397 (0.004913) | 0.001521 (0.005477) |
The Pearson’s correlation coefficients with the 95% confidence interval and P value between IgA levels in Fig. 2B and relative abundance of Bacteroidetes species in Fig. 3A and Table S1 were calculated using Excel. The significance levels were evaluated with two-tailed probability in 15 data pairs in 4 experimental groups (sHFD-, iHFD-, NFD- and ND-fed mice).
IgA is elevated in B. acidifaciens monoassociated mice.
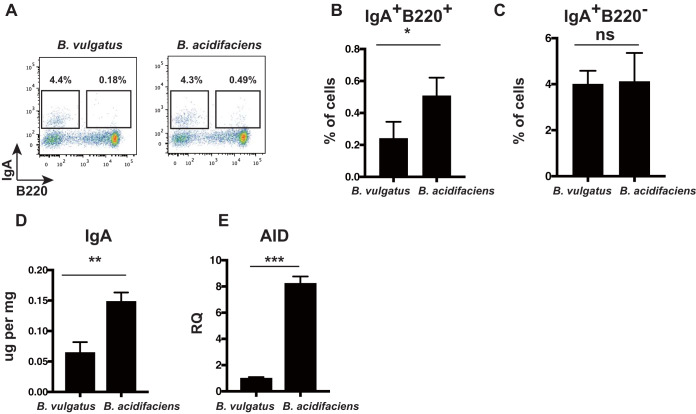
Based on these results, we analyzed the function of the B. fragilis group in gut IgA induction. We focused on B. acidifaciens because this is the predominant species in the B. fragilis group. To address whether B. acidifaciens increases gut IgA production, we generated gnotobiotic mice. B. vulgatus was used as a control of B. acidifaciens because these two species are phylogenetically distinct and their ratios were opposite sHFD-fed and NFD-fed mice (Fig. 3A and B). Additionally, there was no correlation coefficient between IgA levels and relative abundance of B. vulgatus, although B. acidifaciens had correlation coefficients (Table 1). Monoassociated mice were fed a normal diet (CMF) for 3 weeks in a gnotobiotic isolator. B. acidifaciens monoassociated mice had 10.7 to 10.8 CFU/g feces (in log10), and B. vulgatus monoassociated mice had 10.83 to 11.09 CFU/g feces (in log10). These results suggested that colonization levels of B. acidifaciens and B. vulgatus monoassociated mice were equivalent. We detected IgA+ cells in the LILP and IgA in the gut contents of the large intestine of B. acidifaciens and B. vulgatus monoassociated mice. IgA+ B220+ cells and IgA+ B220– cells in the LILP were measured with flow cytometry (Fig. 4A). The frequency of IgA+ B220+ cells was higher in the LILP of B. acidifaciens monoassociated mice than in those of B. vulgatus monoassociated mice (Fig. 4B), whereas the frequency of IgA+ B220− cells did not differ (Fig. 4C). In addition, IgA was higher in the gut contents of B. acidifaciens monoassociated mice than in those of B. vulgatus monoassociated mice (Fig. 4D). Moreover, AID expression in the LILP B cells was higher in B. acidifaciens monoassociated mice than in those of B. vulgatus monoassociated mice (Fig. 4E). B. acidifaciens may have a higher potential role in increasing IgA secretion and upregulating AID expression than B. vulgatus in monoassociated mice. Collectively, we found that there might be a causal association among gut IgA, B. acidifaciens, and AID expression.
FIG 4.
IgA level and AID expression are elevated in B. acidifaciens monoassociated mice compared with B. vulgatus monoassociated mice. (A) Flow cytometric analysis of IgA+ B220+ and IgA+ B220− cells in the LILP of B. acidifaciens and B. vulgatus monoassociated mice. LILP cells of B. acidifaciens and B. vulgatus monoassociated mice were stained with anti-IgA and anti-B220 antibodies. The frequencies of IgA+ B220+ and IgA+ B220− cells are indicated in the square. n = 3 mice per group. (B and C) The frequencies of IgA+ B220+ (B) and IgA+ B220− (C) cells in the LILP of B. acidifaciens and B. vulgatus monoassociated mice. Data are presented as the mean ± SD. *, P < 0.05. (D) The levels of IgA in the gut contents from B. acidifaciens and B. vulgatus monoassociated mice were measured using ELISA. n = 3 mice per group. Data are presented as the mean ± SD. **, P < 0.01. (E) AID expression in the LILP B cells of B. acidifaciens and B. vulgatus monoassociated mice. LILP B cells were sorted using autoMACS, and AID gene expression was determined by RQ of the mean value of triplicate wells, with the untreated samples arbitrarily set to 1. n = 3 mice per group. Data are presented as the mean ± SD. ***, P < 0.001.
Metabolites produced by the gut microbiota are involved in the regulation of immune responses in various ways. Thus, we analyzed the metabolites produced by B. acidifaciens in vivo that could have a role in increasing IgA and upregulating AID expression in the gut. To investigate which metabolites were increased in B. acidifaciens monoassociated mice, we performed metabolome analysis with the gut contents of the large intestine of B. acidifaciens and B. vulgatus monoassociated mice. The hierarchical cluster analysis (HCA) results of B. acidifaciens and B. vulgatus monoassociated mice showed a different profile (Fig. S1A). In addition, principal-component analysis (PCA) results by principal component 1 (PC1) and PC2 were different in B. acidifaciens and B. vulgatus monoassociated mice (Fig. S1B). Factor loading analysis for PC1 revealed metabolites elevated in B. acidifaciens monoassociated mice compared to those of B. vulgatus monoassociated mice among 212 metabolites (Table S2). Metabolites might provide possibilities for addressing the mechanism by which gut IgA is induced.
DISCUSSION
Diet and the microbiota are closely linked to the host immune systems. A previous report suggested that Bacteroidetes levels correlated positively with long-term patterns of fiber intake (26). In this study, we reported that a soluble high-fiber diet increases the ratio of the B. fragilis group, IgA production, and AID expression in the gut. Previous studies have shown that IgA production is influenced by the phylum Bacteroidetes; however, the species levels of Bacteroidetes were not understood. We previously demonstrated that the ratio of Bacteroidetes to Firmicutes is higher in sHFD-fed mice than in NFD-fed mice (16). Therefore, in this study, we demonstrated the Bacteroidetes species profile using the hsp60-based profiling method to analyze the abundances of different species in the Bacteroidetes phylum. Our results showed that a soluble high-fiber diet increased the ratio of some specific species, such as B. acidifaciens, belonging to the B. fragilis group, as well as IgA and AID induction. These results enabled us to investigate whether B. acidifaciens and IgA/AID induction are associated. Thus, we generated monoassociated mice to reveal the association. Monoassociated mice with B. acidifaciens showed the involvement of B. acidifaciens in IgA production and AID expression (Fig. 4).
In this study, we reported that the frequency of IgA+ B220+ cells was selectively increased in the LILP in sHFD- and ND-fed mice and reduced in iHFD- and NFD-fed mice (Fig. 1B and 2A), suggesting that the gut microbiota affect the frequency of IgA+ B220+ cells. Consistent with this result, the population of IgA+ B220+ was higher in B. acidifaciens monoassociated mice than in B. vulgatus monoassociated mice, whereas the population of IgA+ B220– did not differ (Fig. 4B and C). Previous studies demonstrated that B. acidifaciens is associated with IgA induction (27).
According to the previous studies, IgA+ B220+ cells are generated from IgM+ B220+ cells and have just completed CSR, whereas IgA+ B220– cells have already completed CSR (28). Therefore, it has been suggested that increased IgA+ B220+ cells in the LILP of sHFD-fed mice and B. acidifaciens monoassociated mice have just completed CSR and contribute to gut IgA production. AID is a crucial gene to induce CSR from IgM to IgA in the LILP B cells. In this study, we showed that AID expression in the LILP B cells was increased in sHFD-fed mice, NFD → sHFD-fed mice, and B. acidifaciens monoassociated mice (Fig. 2C, 3E, and 4E). Upregulation of AID expression was correlated with the increase in the level of gut IgA (Fig. 2, 3, and 4). Indeed, previous studies have shown that AID-deficient mice fail to generate IgA+ B220+ cells (29). Therefore, upregulation of AID expression by B. acidifaciens may contribute to generating the population of IgA+ B220+ cells in the LILP.
It has been reported that diets such as high-fiber diets and high-fat diets can affect the gastrointestinal immune response (8, 17). In this study, we used inulin as a soluble high-fiber diet and cellulose as an insoluble high-fiber diet. Although there might be a limitation to generalizing the effect of soluble or insoluble fiber using these diets, the composition of the gut microbiota is altered. Notably, we showed the alteration of the Bacteroidetes species profile by the hsp60-based profiling method. The microbiota in the gut contents was altered rapidly. Previous studies have demonstrated that short-term consumption of an animal-based or a plant-based diet altered microbial structure in humans (30). The ratio of members of the B. fragilis group, such as B. acidifaciens, IgA induction, and AID gene expression can be altered in 2 weeks by changes in diet from NFD to sHFD intake (Fig. 3B to D). On the other hand, the B. fragilis group, IgA production, and AID gene expression were decreased by changes in diet from sHFD to NFD intake (Fig. 3B to D).
Our present study revealed that B. acidifaciens is the predominant Bacteroidetes species in murine intestinal microbiota and can be involved in gut IgA production in murine intestine. The other Bacteroidetes species, such as B. faecis and B. caccae, might be involved in IgA production, because these two species also have significant correlation coefficients with IgA levels (Table 1). On the other hand, B. vulgatus was not associated with IgA production. Also, Parabacteroides species such as P. johnsonii and P. merdae, which were induced by iHFD, were not associated with IgA production.
B. acidifaciens has not yet been identified in humans. B. acidifaciens is equivalent to the B. fragilis group and is thought to be a closely related species to Bacteroides ovatus and Bacteroides thetaiotaomicron in humans (31). Accumulating evidence has suggested that the gut microbiota is involved in metabolic diseases and inflammatory diseases. Recent studies have revealed that B. ovatus and B. thetaiotaomicron are involved in immune responses (32, 33). Furthermore, a previous study also reported that B. acidifaciens contributes to inhibiting obesity and type 2 diabetes by improving insulin sensitivity in mice (34). Based on these observations, the B. fragilis group, including B. acidifaciens, mediates immune systems and affects health and diseases. Our results showed that NFD intake tends to be sensitive to DSS-induced colitis (Fig. 2D and E), suggesting that a soluble fiber diet might have a potential role in attenuating ulcerative colitis.
We identified B. fragilis group species, such as B. acidifaciens, as potential inducers of IgA under sHFD. Our study provides a novel insight into the cross talk of a soluble high-fiber diet, the B. fragilis group, and IgA production.
MATERIALS AND METHODS
Mice and diets.
C57BL/6J female mice at 8 weeks old were purchased from Japan SLC (Shizuoka, Japan). Specific-pathogen-free (SPF) mice used for the experiment in Fig. 3A were housed at animal facility A (mice 1 through 12) or at animal facility B (mice 13 through 15) of Juntendo University (Tokyo, Japan). C57BL/6J mice were housed individually and fed either a no-fiber diet (0% fiber; product no. D14071801; Research Diets, New Brunswick, NJ, USA), a soluble high-fiber diet (10% inulin; product no. D14071803; Research Diets), an insoluble high-fiber diet (10% cellulose; product no. D14071802; Research Diets), or a normal diet (2.9% fiber) (CRF-1; Charles River, Wilmington, MA, USA). All animal experiments were approved by the Animal Experimentation Committee of Juntendo University. Monoassociated mice were generated from germfree C57BL/6N mice at SLC and fed a sterile normal diet (3.1% fiber) (CMF; Oriental Yeast Co., Tokyo, Japan) in gnotobiotic isolators. B. vulgatus monoassociated mice were used as a control of B. acidifaciens monoassociated mice.
To confirm whether proliferation of any kind of Bacteroidetes species is actually dependent on a soluble fiber diet, mice were fed either sHFD or NFD for the first 2 weeks and then switched to the other diet for the next 2 weeks.
Reagents and antibodies.
Mouse monoclonal antibodies, FITC-IgA (C10-3) and APC-B220 (RA3-6B2), were purchased from BD Bioscience (San Jose, CA, USA) and Miltenyi Biotec (Bergish Gladbach, Germany), respectively.
Preparation of the large intestine and the small intestine lamina propria.
Cells from the large intestine lamina propria (LILP) and small intestine lamina propria (SILP) were prepared using previously reported methods (35). Briefly, isolated sections of large and small intestines were cut longitudinally, and the contents were removed. Tissues were shaken for 40 min at 37°C in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific, Waltham, MA, USA) containing 5 mM EDTA, washed with phosphate-buffered saline (PBS), and cut into smaller pieces. These smaller sections were incubated for 60 min at 37°C in DMEM containing 0.8 mg/ml dispase (Roche, Basel, Switzerland), 1 mg/ml collagenase D (Roche), and 0.1 mg/ml DNase I (Thermo Fisher). Every 15 min, tissues were washed with warm DMEM and reincubated with fresh medium. The supernatant from the washing step was collected, and the debris was removed using Percoll (GE Healthcare, Buckinghamshire, UK) to isolate viable cells.
Isolation of LILP B cells.
Cells from LILP were prepared as described above. LILP B cells were sorted using anti-B220 microbeads with an autoMACS instrument (Milteni Biotec, Bergisch Gladbach, Germany) in accordance with the manufacturer’s instructions. Isolated LILP B cells were used for RNA isolation and quantitative mRNA analysis.
Flow cytometric analysis.
Lamina propria cells were incubated in the indicated combination of fluorescently labeled mouse monoclonal antibodies for 30 min at 4°C in the dark. After washing with 1% fetal bovine serum in PBS, the cells were analyzed using a FACSVerse flow cytometer (BD Bioscience). Dead cells were removed using 7-aminoactinomycin D (7-AAD) labeling (Biolegend, San Jose, CA, USA). All data were analyzed using FlowJo (Tree Star, Ashland, OR, USA).
IgA measurement by ELISA.
IgA was measured using a mouse IgA enzyme-linked immunosorbent assay (ELISA) kit (Immunology Consultants Laboratory, Ashland, OR, USA) in accordance with the manufacturer’s instructions (36). The gut content samples were diluted with PBS to 100 mg/ml and then shaken for 30 min at 4°C. Supernatants were collected and used for ELISA. After centrifugation, supernatants were further diluted at 1/100 and added to the supplied plate in duplicate. Samples were incubated for 60 min at room temperature and then washed with wash solution four times. The diluted enzyme-antibody conjugate was added to each well and incubated for 30 min at room temperature. The plates were washed with wash solution four times. Then, tetramethylbenzidine (TMB) substrate solution was added to each well for 10 min, and developing reactions were stopped with stop solution. The plates were immediately read at 450 nm using the optical density readings by a microplate reader.
DSS-induced colitis.
C57BL/6J female mice at 8 weeks old were fed either NFD or sHFD for 3 weeks. Then, 2.5% dextran sulfate sodium (DSS) (MP Biomedicals, Santa Ana, CA) dissolved in sterile distilled drinking water was orally administered for 5 days. Then, 2.5% DSS was switched to regular drinking water for the next 3 days. The body weight and colon length of each mouse were measured.
Bacteroidetes profiling based on hsp60 amplicon sequencing.
The gut content samples were diluted 10-fold in Tris-EDTA (TE) buffer (10 mM Tris, 1 mM EDTA [pH 8.0]) and frozen at −80°C until use. A 500-μl aliquot of each diluted sample was used for DNA extraction. Samples were pretreated with 50 U of achromopeptidase (Wako, Tokyo, Japan) in TE buffer at 50°C for 30 min; then, DNA was purified using phenol:chloroform:isoamyl alcohol (25:24:1, vol/vol/vol).
We performed Bacteroidetes profiling at the species level using hsp60 amplicon sequencing as reported previously (23). The hsp60 partial sequence (558 bp) was amplified using H729 (5′-CGCCAGGGTTTTCCCAGTCACGACGAIIIIGCIGGIGAYGGIACIAC-3′) and H730 (5′-AGCGGATAACAATTTCACACAGGAYKITCICCRAAICCIGGIGCYTT-3′), which have been widely used for species identification and taxonomic studies of Bacteroidetes bacteria (24, 25). In these sequences, I represents inosine, K represents guanosine or thymidine, R represents adenosine or guanosine, and Y represents cytidine or thymidine. We used KOD Multi and Epi enzyme (Toyobo Co. Ltd., Osaka, Japan) as the polymerase for the hsp60-based profiling method by PCR because of its acceptable high fidelity, low amplification bias, and compatibility with inosine-containing primers. PCR was performed in 25-μl reaction mixtures containing 70 ng of DNA extract, 0.5 μl of KOD polymerase, 50 pmol of each primer, 12.5 μl of the 2× KOD buffer, and molecular-grade water. Reaction conditions were as follows: 1 cycle at 94°C for 2 min, followed by 25 to 30 cycles at 98°C for 20 s, at 50°C for 30 s, and at 68°C for 1 min, and a final cycle at 68°C for 2 min.
DNA fragments of hsp60 were analyzed by electrophoresis in 1× Tris-acetate-EDTA buffer on a 1% agarose gel stained with ethidium bromide. PCR products were purified using AMPure beads (Beckman Coulter, Inc., Brea, CA, USA) according to the manufacturer’s protocol.
DNA libraries were prepared by transposon-based fragmentation of 558-bp hsp60 using a Nextera XT DNA sample prep kit (Illumina, San Diego, CA, USA). NucleoMag NGS Clean-up and Size Select (Macherey-Nagel, Düren, Germany) was used for cleanup and size selection of next-generation sequencing (NGS) libraries according to a protocol for removing adapter dimers. Sequencing was performed using a Miseq reagent kit v3 (600 cycle) and a paired-end 2 × 300-bp cycle run on an Illumina MiSeq sequencing system.
Next-generation sequencing, sequencing read processing, and taxonomic analysis.
After sequencing, Miseq reads 1 and 2 of the hsp60 amplicon were stitched using FLASH (http://ccb.jhu.edu/software/FLASH/) (37) to obtain longer reads. The reads were filtered and trimmed by removing bases with quality value scores of ≤20 and reads of <150 bases. The reads were converted from FASTQ to FASTA format using the FastX-Toolkit version 0.0.13 (http://hannonlab.cshl.edu/fastx_toolkit/index.html).
Analyses of the trimmed sequencing reads were performed using BLAST 2.5.0+ with an E value cutoff of e−10 (38). The multi-FASTA file of Bacteroidetes hsp60 reference sequences, which was constructed in the previous study (23), was used for BLAST analysis. Taxonomic assignment based on the best E value scores was performed against trimmed sequencing reads. To consider the existence of unclassified Bacteroidetes species in the intestinal microbiota and the mean pairwise hsp60 gene sequence similarity (73.8 to 97.1%) between species in each Bacteroidetes genus as previously reported (39), query reads exhibiting <95% nucleotide identity relative to any Bacteroidetes species were omitted from species-level identification. Consequently, this cutoff value resulted in reducing the matching of query reads against multiple Bacteroidetes species, and we prevented the erroneous taxonomic assignments in our hsp60-based method. A phylogenetic tree was constructed using the neighbor-joining method based on alignment using MAFFT version 6.864 (40). Log10 scales of relative abundance are visualized using a red-yellow-green gradient, with red representing the highest score (0) and green representing the lowest score (≤−5) in terms of the abundance ratios among 167 Bacteroidetes species, whose hsp60 sequences were obtained from the NCBI database.
Isolation of Bacteroides acidifaciens and Bacteroides vulgatus.
B. acidifaciens was isolated as described previously (31). B. acidifaciens strain A40 was used for colonization. B. vulgatus was isolated from the feces of C57BL/6J wild-type mice. Briefly, fecal contents were diluted in PBS and inoculated in Gifu anaerobic medium (GAM) agar plates (Wako). The plates were incubated anaerobically at 37°C for 24 h. Colonies were then picked and cultured at a large scale under anaerobic conditions. DNA was extracted and analyzed as described above.
Colonization of germfree mice.
Germfree C57BL/6N mice were housed in gnotobiotic isolators at Japan SLC (Shizuoka, Japan) and fed a sterile normal diet. Nine-week-old males were orally inoculated with a single gavage of 108 CFU B. acidifaciens A40 or isolated B. vulgatus strain. Gnotobiotic mice were then housed in each gnotobiotic isolator for an additional 3 weeks, and 12-week-old mice were used for the experiments. The colonization level in monoassociated mice was checked in feces. Feces were diluted at 1/50, and 0.1 ml of diluted feces was cultured in Eggerth-Gagnon (EG) agar for 48 h at 37°C.
Metabolite extraction.
For extracting ionic metabolites, approximately 50 mg of gut content samples was dissolved in MilliQ water containing internal standards (H3304-1002; Human Metabolome Technologies [HMT], Tsuruoka, Yamagata, Japan) at a ratio of 1:9 (wt/vol). The mixture was centrifuged, and the supernatant was passed through a Millipore 5000-Da cutoff filter (Ultrafree MC-PLHCC; HMT) using centrifugation to remove macromolecules (9,100 × g, 4°C, 60 min) for subsequent analysis by capillary electrophoresis–time-of-flight mass spectrometry (CE-TOF-MS).
Metabolome analysis.
Metabolome analysis was conducted with the Basic Scan package of HMT using CE-TOF-MS and liquid chromatography–time-of-flight mass spectrometry (LC-TOF-MS) for ionic and nonionic metabolites, respectively, based on the methods described previously (41, 42). Briefly, CE-TOF-MS analysis was performed using an Agilent CE capillary electrophoresis system equipped with an Agilent 6210 time-of-flight mass spectrometer, Agilent 1100 isocratic HPLC pump, and Agilent G1607A CE-MS sprayer kit (Agilent Technologies, Waldbronn, Germany). The system was controlled by Agilent G2201AA ChemStation software version B.03.01 for CE (Agilent Technologies) and connected by a fused silica capillary (50 μm inside diameter [i.d.] by 80 cm total length) with commercial electrophoresis buffer (H3301-1001 and H3302-1021 for cation and anion analyses, respectively; HMT) as the electrolyte. The spectrometer was scanned from m/z 50 to 1,000 (42). Peaks were extracted using automatic integration software MasterHands (Keio University, Tsuruoka, Yamagata, Japan) to obtain peak information, including m/z, peak area, and migration time (MT) for CE-TOF-MS analysis (43). Signal peaks corresponding to isotopomers, adduct ions, and other product ions of known metabolites were excluded, and the remaining peaks were annotated according to the HMT metabolite database based on m/z values with MTs determined by TOF-MS. Areas of the annotated peaks were then normalized based on internal standard levels and sample amounts to obtain relative concentrations of each metabolite. Hierarchical cluster analysis (HCA) and principal-component analysis (PCA) were performed using the HMT proprietary software packages PeakStat and SampleStat, respectively. Detected metabolites were plotted on metabolic pathway maps using VANTED software (44).
RNA isolation and quantitative mRNA analysis.
Total RNA was isolated using RNeasy micro and minikits (Qiagen, Hilden, Germany) in accordance with the manufacturer’s instructions. cDNA was reverse-transcribed with RivaTra (Toyobo, Osaka, Japan). Real-time PCR was performed using SYBR green (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol with 40 cycles of amplification. Results were derived from the relative quantification of target genes. The housekeeping gene hypoxanthine-guanine phosphoribosyltransferase (Hprt) was used for normalization. All values are presented as the mean ± standard deviation (SD). The primers used were as follows: mouse AID, 5′-AAATTCTGTCCGGCTAACCA-3′ and 5′-CATTCCAGGAGGTTGCTTTC-3′; mouse Hprt, 5′-GCAGTACAGCCCCAAAATGG-3′ and 5′-AACAAAGTCTGGCCTGTATCCAA-3′.
Statistical analysis.
Statistical analysis was performed using the Student’s t test. GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA) was used for all statistical calculations. A P value of <0.05 was considered statistically significant. In the comparison of Bacteroidetes species components between two groups of ND-fed and sHFD-, iHFD-, or NFD-fed mice, we performed a Wilcoxon rank sum test against bacterial species exhibiting an increase or decrease in relative abundance. A P value of <0.05 was considered statistically significant. The Pearson’s correlation coefficient and the false-discovery rate correction were calculated to assess the relationships between IgA and abundance of Bacteroidetes species in 15 data pairs in 4 experimental groups (sHFD-, iHFD-, NFD-, and ND-fed mice). A significant level was evaluated using two-tailed probability and the false-discovery rate, and the 95% confidence interval (CI) was shown for assessment of repeatability in the result of the correlation coefficient. In addition, the Spearman’s rank correlation coefficient was also calculated against the same data sets.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Laboratory of Molecular and Biochemical Research, Research Support Center, Juntendo University Graduate School of Medicine, Tokyo, Japan, for technical assistance.
This work was supported by a Grant-in-Aid for Scientific Research (C) (no. 15K08533) to A. Nakajima from the Japan Society for the Promotion of Science.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Macpherson AJ, Yilmaz B, Limenitakis JP, Ganal-Vonarburg SC. 2018. IgA function in relation to the intestinal microbiota. Annu Rev Immunol 36:359–381. doi: 10.1146/annurev-immunol-042617-053238. [DOI] [PubMed] [Google Scholar]
- 2.Gutzeit C, Magri G, Cerutti A. 2014. Intestinal IgA production and its role in host-microbe interaction. Immunol Rev 260:76–85. doi: 10.1111/imr.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fagarasan S, Honjo T. 2003. Intestinal IgA synthesis: regulation of front-line body defences. Nat Rev Immunol 3:63–72. doi: 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- 4.Pabst O. 2012. New concepts in the generation and functions of IgA. Nat Rev Immunol 12:821–832. doi: 10.1038/nri3322. [DOI] [PubMed] [Google Scholar]
- 5.Petta I, Fraussen J, Somers V, Kleinewietfeld M. 2018. Interrelation of diet, gut microbiome, and autoantibody production. Front Immunol 9:439. doi: 10.3389/fimmu.2018.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubinak JL, Round JL. 2016. Do antibodies select a healthy microbiota? Nat Rev Immunol 16:767–774. doi: 10.1038/nri.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakajima A, Habu S, Kasai M, Okumura K, Ishikawa D, Shibuya T, Kobayashi O, Osada T, Ohkusa T, Watanabe S, Nagahara A. 2020. Impact of maternal dietary gut microbial metabolites on an offspring’s systemic immune response in mouse models. Biosci Microbiota Food Health 39:33–38. doi: 10.12938/bmfh.19-013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maslowski KM, Mackay CR. 2011. Diet, gut microbiota and immune responses. Nat Immunol 12:5–9. doi: 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]
- 9.Dhingra D, Michael M, Rajput H, Patil RT. 2012. Dietary fibre in foods: a review. J Food Sci Technol 49:255–266. doi: 10.1007/s13197-011-0365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai FJ, Chau CF. 2017. Classification and regulatory perspectives of dietary fiber. J Food Drug Anal 25:37–42. doi: 10.1016/j.jfda.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. 2013. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 12.Kim M, Qie Y, Park J, Kim CH. 2016. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 20:202–214. doi: 10.1016/j.chom.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Human Microbiome Project Consortium. 2012. Structure, function and diversity of the healthy human microbiome. Nature 486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honda K, Littman DR. 2016. The microbiota in adaptive immune homeostasis and disease. Nature 535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 15.Stiemsma LT, Michels KB. 2018. The role of the microbiome in the developmental origins of health and disease. Pediatrics 141:e20172437. doi: 10.1542/peds.2017-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakajima A, Kaga N, Nakanishi Y, Ohno H, Miyamoto J, Kimura I, Hori S, Sasaki T, Hiramatsu K, Okumura K, Miyake S, Habu S, Watanabe S. 2017. Maternal high fiber diet during pregnancy and lactation influences regulatory t cell differentiation in offspring in mice. J Immunol 199:3516–3524. doi: 10.4049/jimmunol.1700248. [DOI] [PubMed] [Google Scholar]
- 17.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ. 2014. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 18.Uyaguari-Diaz MI, Chan M, Chaban BL, Croxen MA, Finke JF, Hill JE, Peabody MA, Van Rossum T, Suttle CA, Brinkman FS, Isaac-Renton J, Prystajecky NA, Tang P. 2016. A comprehensive method for amplicon-based and metagenomic characterization of viruses, bacteria, and eukaryotes in freshwater samples. Microbiome 4:20. doi: 10.1186/s40168-016-0166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Links MG, Dumonceaux TJ, Hemmingsen SM, Hill JE. 2012. The chaperonin-60 universal target is a barcode for bacteria that enables de novo assembly of metagenomic sequence data. PLoS One 7:e49755. doi: 10.1371/journal.pone.0049755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stavnezer J, Guikema JE, Schrader CE. 2008. Mechanism and regulation of class switch recombination. Annu Rev Immunol 26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Z, Zan H, Pone EJ, Mai T, Casali P. 2012. Immunoglobulin class-switch DNA recombination: induction, targeting and beyond. Nat Rev Immunol 12:517–531. doi: 10.1038/nri3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honjo T. 2002. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science 298:1424–1427. doi: 10.1126/science.1077336. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa D, Sasaki T, Takahashi M, Kuwahara-Arai K, Haga K, Ito S, Okahara K, Nakajima A, Shibuya T, Osada T, Hiramatsu K, Watanabe S, Nagahara A. 2018. The microbial composition of bacteroidetes species in ulcerative colitis is effectively improved by combination therapy with fecal microbiota transplantation and antibiotics. Inflamm Bowel Dis 24:2590–2598. doi: 10.1093/ibd/izy266. [DOI] [PubMed] [Google Scholar]
- 24.Goh SH, Potter S, Wood JO, Hemmingsen SM, Reynolds RP, Chow AW. 1996. HSP60 gene sequences as universal targets for microbial species identification: studies with coagulase-negative staphylococci. J Clin Microbiol 34:818–823. doi: 10.1128/JCM.34.4.818-823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakamoto M, Ohkuma M. 2010. Usefulness of the hsp60 gene for the identification and classification of Gram-negative anaerobic rods. J Med Microbiol 59:1293–1302. doi: 10.1099/jmm.0.020420-0. [DOI] [PubMed] [Google Scholar]
- 26.Simoes CD, Maukonen J, Kaprio J, Rissanen A, Pietilainen KH, Saarela M. 2013. Habitual dietary intake is associated with stool microbiota composition in monozygotic twins. J Nutr 143:417–423. doi: 10.3945/jn.112.166322. [DOI] [PubMed] [Google Scholar]
- 27.Yanagibashi T, Hosono A, Oyama A, Tsuda M, Suzuki A, Hachimura S, Takahashi Y, Momose Y, Itoh K, Hirayama K, Takahashi K, Kaminogawa S. 2013. IgA production in the large intestine is modulated by a different mechanism than in the small intestine: Bacteroides acidifaciens promotes IgA production in the large intestine by inducing germinal center formation and increasing the number of IgA+ B cells. Immunobiology 218:645–651. doi: 10.1016/j.imbio.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 28.Fagarasan S, Kinoshita K, Muramatsu M, Ikuta K, Honjo T. 2001. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature 413:639–643. doi: 10.1038/35098100. [DOI] [PubMed] [Google Scholar]
- 29.Han JH, Akira S, Calame K, Beutler B, Selsing E, Imanishi-Kari T. 2007. Class switch recombination and somatic hypermutation in early mouse B cells are mediated by B cell and Toll-like receptors. Immunity 27:64–75. doi: 10.1016/j.immuni.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. 2014. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyamoto Y, Itoh K. 2000. Bacteroides acidifaciens sp. nov., isolated from the caecum of mice. Int J Syst Evol Microbiol 50Pt 1:145–148. doi: 10.1099/00207713-50-1-145. [DOI] [PubMed] [Google Scholar]
- 32.Porter NT, Luis AS, Martens EC. 2018. Bacteroides thetaiotaomicron. Trends Microbiol 26:966–967. doi: 10.1016/j.tim.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Clemente JC, Ursell LK, Parfrey LW, Knight R. 2012. The impact of the gut microbiota on human health: an integrative view. Cell 148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang JY, Lee YS, Kim Y, Lee SH, Ryu S, Fukuda S, Hase K, Yang CS, Lim HS, Kim MS, Kim HM, Ahn SH, Kwon BE, Ko HJ, Kweon MN. 2017. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol 10:104–116. doi: 10.1038/mi.2016.42. [DOI] [PubMed] [Google Scholar]
- 35.Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, Di Santo JP, Eberl G. 2010. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science 330:665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 36.Moon C, Baldridge MT, Wallace MA, Burnham CA, Virgin HW, Stappenbeck TS. 2015. Vertically transmitted faecal IgA levels determine extra-chromosomal phenotypic variation. Nature 521:90–93. doi: 10.1038/nature14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magoc T, Salzberg SL. 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. 2009. BLAST+: architecture and applications. BMC Bioinformatics 10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakamoto M, Ohkuma M. 2011. Identification and classification of the genus Bacteroides by multilocus sequence analysis. Microbiology 157:3388–3397. doi: 10.1099/mic.0.052332-0. [DOI] [PubMed] [Google Scholar]
- 40.Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ooga T, Sato H, Nagashima A, Sasaki K, Tomita M, Soga T, Ohashi Y. 2011. Metabolomic anatomy of an animal model revealing homeostatic imbalances in dyslipidaemia. Mol Biosyst 7:1217–1223. doi: 10.1039/c0mb00141d. [DOI] [PubMed] [Google Scholar]
- 42.Ohashi Y, Hirayama A, Ishikawa T, Nakamura S, Shimizu K, Ueno Y, Tomita M, Soga T. 2008. Depiction of metabolome changes in histidine-starved Escherichia coli by CE-TOFMS. Mol Biosyst 4:135–147. doi: 10.1039/b714176a. [DOI] [PubMed] [Google Scholar]
- 43.Sugimoto M, Wong DT, Hirayama A, Soga T, Tomita M. 2010. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 6:78–95. doi: 10.1007/s11306-009-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Junker BH, Klukas C, Schreiber F. 2006. VANTED: a system for advanced data analysis and visualization in the context of biological networks. BMC Bioinformatics 7:109. doi: 10.1186/1471-2105-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.