Abstract
Objective
Probiotics have been hypothesized to mediate inflammation through gut microbiome modulation. Spondyloarthropathies have subclinical gut inflammation associated with inflammatory disease that may benefit from probiotic use. We aimed to evaluate associations between probiotic use and patient‐reported outcomes in patients with psoriatic arthritis (PsA).
Methods
Using FORWARD, The National Databank for Rheumatic Diseases, we examined probiotic use among patients with PsA and rheumatoid arthritis (RA), a comparator in which gut inflammation is less clearly related to pathogenesis. Patient‐reported outcome measures such as pain and physical function were compared for probiotic users and nonusers among patients with PsA and RA. Patients were propensity score–matched for taking a probiotic by demographics and nonmedication supplements.
Results
More patients have reported probiotic use over the past decade, with less than 1% reporting use in 2008 and approximately 7% in 2018. Probiotic users are more likely to be white women with higher education, income, and supplement use. Following propensity score matching, probiotic users with PsA had significantly lower Short Form 36 Physical Component Summary (SF‐36 PCS) scores and higher pain scores than nonusers with PsA (33.11 ± 11.50 vs. 40.82 ± 11.03; P = 0.04 and 4.78 ± 3.09 vs. 3.00 ± 2.58; P = 0.03). There were no significant differences in Patient Activity Scale II, Health Assessment Questionnaire II, SF‐36 PCS, and Short Form 36 Mental Component Summary scores or pain among users with PsA before and after probiotic initiation.
Conclusion
We found increasing probiotic use in patients with PsA and important differences between users and nonusers. After accounting for these differences, we found no statistical difference in health outcomes after probiotic use.
Introduction
Spondyloarthritis (SpA) encompasses closely related inflammatory arthritides, which include psoriatic arthritis (PsA), reactive arthritis, inflammatory bowel disease–associated arthritis, and ankylosing spondylitis. Associated extra‐articular disease manifestations commonly include psoriasis, uveitis, Crohn disease, and ulcerative colitis. SpA disease pathogenesis is currently thought to be the result of a combination of environmental triggers and genetic predisposition; however, the exact mechanism has not been delineated. Growing evidence has suggested that our intestinal gut microbiome may play a role in the development or persistence of SpA diseases (1). New research has even found a difference in the intestinal microbiome composition of patients taking interleukin 17 inhibitors vs. tumor necrosis factor (TNF) inhibitors (2). A healthy gut microbiome is thought to have a protective effect (3).
The list of supplements many patients now take as complementary therapies for their rheumatic disease is long. Complementary and alternative medicine, particularly supplements, is a growing industry. By some estimates, over 20 billion doses of probiotics are sold yearly (3). Compared with the side effects of most disease modifying antirheumatic drugs (DMARDs), many patients believe that the side effects of supplements are minimal, and there are anecdotal reports of benefit. New advances in medical research have highlighted the role that the microbiome may play in inflammatory disease pathways, opening a new potential area for therapeutic research. Probiotic use may mediate inflammation involved in the pathogenesis of many diseases by stabilizing the gut microenvironment, restoring the intestinal barrier, and increasing removal of enteral antigens (4, 5). In this study, we used a national cohort to examine the frequency of probiotic use and disease severity outcomes measured with clinically relevant markers.
Patients and Methods
Study design
We conducted a retrospective observational cohort study.
Study population
Patients were participants in FORWARD, The National Databank for Rheumatic Diseases (6), a longitudinal study of rheumatic disease treatments and outcomes from 2000 through 2017. Participants are recruited primarily from US rheumatologists and answer detailed semiannual questionnaires that provide treatment information and other characteristics (demographics, comorbidities, and clinical status). We focused on patients with PsA, and the larger RA cohort acted as a comparison group. Patients with dual diagnoses were excluded.
Exposure
Patients who reported probiotic use in at least one encounter were classified as probiotic users.
Outcomes
The outcomes of interest were the Patient Activity Scale II (PAS‐II), Health Assessment Questionnaire II (HAQ‐II), pain visual analog scale (VAS), Short Form 36 Physical Component Summary (SF‐36 PCS), and Short Form 36 Mental Component Summary (SF‐36 MCS) scores.
Assessments
Characteristics and clinical data reported included sociodemographics (age, sex, race, education, and income), Rheumatic Disease Comorbidity Index (RDCI), medications (DMARDs, nonsteroidal anti‐inflammatory drugs [NSAIDs], proton pump inhibitors [PPIs]), and additional supplement use (fish oil, vitamin D, and turmeric).
Prevalence of probiotic use
The prevalence of probiotic use was determined for each calendar year since the earliest report on record in the databank. For each cohort, this was defined as the number of probiotic users over the total number of patients contributing data in that calendar year.
Statistical analysis
Descriptive statistics (means/SDs or percentages) were calculated for each of these variables for probiotic users and nonusers (in the PsA and RA cohorts) at baseline, during the most recent encounter, and following propensity score matching. Significance was assessed with Student's t tests for continuous variables and Fisher's exact tests for categorical variables, and P values less than 0.05 were considered significant. Paired t tests and panel regressions with random effects (selected based on Hausman tests and adjusted for age, sex, race, education level, RDCI, fish oil use, vitamin D use, DMARD use, NSAID use, and PPI use) were used to assess differences in PAS‐II, HAQ‐II, pain VAS, SF‐36 PCS, and SF‐36 MCS scores for the PsA cohort from the measurement just before the initiation of probiotics to the first measurement following the initiation of probiotics. Patients who reported probiotic use at baseline were excluded.
Propensity score matching
For comparisons made between probiotic users and nonusers, we used propensity score matching, separately performed for PsA and RA, to balance confounders. The most recent encounter during which probiotic users reported taking a probiotic was compared with the most recent encounter overall for nonusers. The probability of initiating a probiotic was calculated using psmatch2 (7) (caliper of 0.1; common support imposed), including the covariates age, sex, race, education level, income, RDCI, turmeric use, fish oil use, vitamin D use, any DMARD use, NSAID use, and PPI use. We found no differences when separating any DMARD use into csDMARD and bDMARD use.
Results
Prevalence of probiotic use
Probiotic use has increased in recent years. The first report of probiotic supplementation was in 2001, after which there were very few users for several years. In 2006, use began to increase exponentially to approximately 7% of patients reporting probiotic use in 2018 (Figure 1). Of the 782 patients with PsA and the 34 378 patients with RA included in the study, 3.7% and 3.0% of patients reported probiotic use at some point, respectively.
Figure 1.
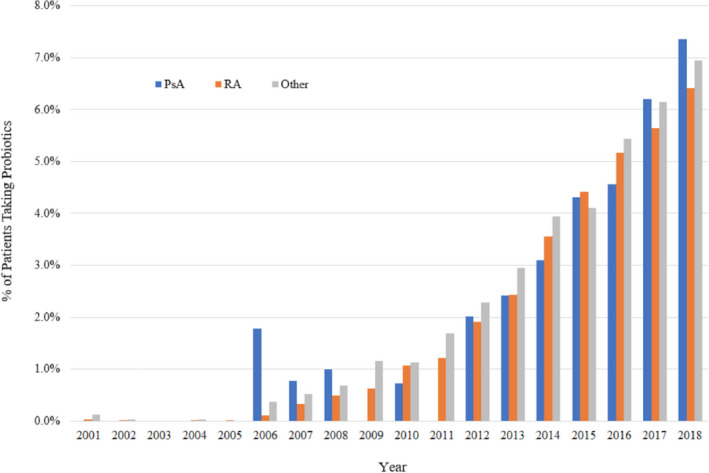
Probiotic use over time by primary rheumatic disease diagnosis in FORWARD. Average N per year was 5 for psoriatic arthritis, 137 for RA, and 81 for other.
Demographics and clinical characteristics
In the RA cohort, probiotic users are more likely than nonusers to be white women and to have more education, have a higher income, have a higher comorbidity index, use PPIs, and take other supplements in addition to probiotics. Probiotic users with RA also had higher SF‐36 MCS scores and reported NSAID use less frequently than nonusers. Probiotic users and nonusers in the PsA cohort generally follow these same trends, but the differences do not reach statistical significance except in the case of fish oil use. Compared with probiotic users with RA, probiotic users with PsA had significantly lower SF‐36 MCS scores and higher pain scores, and they were less likely to be taking a DMARD and more likely to be taking fish oil (Table 1).
Table 1.
Baseline demographic and clinical characteristics of patients by primary diagnosis at enrollment
| Characteristics | Patients With PsA (n = 782) | Patients With RA (n = 34378) | P (Users; PsA vs. RA) | ||||
|---|---|---|---|---|---|---|---|
| Probiotic Users (n = 29) | Nonusers (n = 753) | P | Probiotic Users (n = 1019) | Nonusers (n = 33 091) | P | ||
| Probiotic use baseline, % | 0.9 | … | … | 0.7 | … | … | 0.51 |
| Probiotic use ever, % | 3.7 | … | … | 3.0 | … | … | 0.24 |
| Age, mean ± SD, y | 55.30 ± 12.22 | 53.19 ± 12.34 | 0.37 | 59.19 ± 11.75 | 58.43 ± 13.53 | 0.08 | 0.08 |
| Female, % | 86.2 | 70.0 | 0.06 | 93.4 | 79.4 | <0.001 | 0.13 |
| White, % | 96.2 | 91.5 | 0.72 | 94.2 | 86.0 | <0.001 | 1 |
| Education, mean ± SD, y | 14.92 ± 2.23 | 14.52 ± 2.21 | 0.36 | 14.35 ± 2.40 | 13.33 ± 2.87 | <0.001 | 0.23 |
| Household income, mean ±SD, 1000 USD | 61.96 ± 34.81 | 61.42 ± 33.75 | 0.93 | 61.25 ± 34.43 | 49.28 ± 32.45 | <0.001 | 0.91 |
| RDCI, mean ± SD, 0‐9 | 2.17 ± 1.28 | 1.80 ± 1.60 | 0.22 | 1.87 ± 1.65 | 1.70 ± 1.54 | <0.001 | 0.33 |
| Turmeric use, % | 3.4 | 0.7 | 0.20 | 2.2 | 0.3 | <0.001 | 0.48 |
| Fish oil use, % | 34.5 | 8.6 | <0.001 | 16.0 | 4.7 | <0.001 | 0.02 |
| Vitamin D use, % | 20.7 | 10.2 | 0.11 | 17.4 | 6.9 | <0.001 | 0.62 |
| DMARD use, % | 51.7 | 58.6 | 0.57 | 76.5 | 74.4 | 0.13 | <0.01 |
| NSAID use, % | 62.1 | 49.7 | 0.26 | 52.6 | 55.8 | 0.04 | 0.35 |
| PPI use, % | 41.4 | 26.8 | 0.09 | 33.8 | 22.7 | <0.001 | 0.43 |
| Pain VAS, mean ± SD, 0‐10 | 5.07 ± 3.03 | 4.09 ± 2.84 | 0.07 | 4.01 ± 2.74 | 4.15 ± 2.85 | 0.12 | 0.04 |
| SF‐36 PCS, mean ± SD, 0‐100 | 35.26 ± 10.94 | 38.45 ± 11.29 | 0.15 | 36.52 ± 10.76 | 36.24 ± 10.83 | 0.44 | 0.55 |
| SF‐36 MCS, mean ± SD, 0‐100 | 43.99 ± 12.55 | 46.57 ± 11.70 | 0.26 | 48.85 ± 11.19 | 47.77 ± 11.87 | <0.01 | 0.03 |
| HAQ‐II, mean ± SD, 0‐3 | 4.08 ± 2.42 | 3.48 ± 2.29 | 0.18 | 3.78 ± 2.18 | 3.93 ± 2.27 | 0.09 | 0.49 |
| PAS‐II, mean ± SD, 0‐10 | 1.00 ± 0.65 | 0.79 ± 0.65 | 0.09 | 1.00 ± 0.66 | 1.03 ± 0.68 | 0.26 | 0.99 |
Abbreviation: DMARD, disease‐modifying antirheumatic drug; HAQ‐II, Health Assessment Questionnaire II; MCS, Mental Component Summary; NSAID, nonsteroidal anti‐inflammatory drug; PAS‐II, Patient Activity Scale II; PCS, Physical Component Summary; PPI, proton pump inhibitor; PsA, psoriatic arthritis; RA, rheumatoid arthritis; RDCI, Rheumatic Diseases Comorbidity Index; SF‐36; Short Form 36; USD, US dollars; VAS, visual analog scale.
Bold values indicates P < 0.05.
After propensity score matching (Figure 2) (Supplementary Tables 1 and 2), probiotic users with PsA had significantly lower SF‐36 PCS scores and significantly higher pain VAS scores than nonusers with PsA (33.11 ± 11.50 vs. 40.82 ± 11.03; P =0.04 and 4.78 ± 3.09 vs. 3.00 ± 2.58; P =0.03). There were no other statistically significant differences between users and nonusers in either diagnosis group.
Figure 2.

Standardized differences before and after propensity score matching.
In the paired comparison of probiotic users with PsA before initiating probiotic use and after probiotic exposure (n = 22), there were no statistically significant differences in PAS‐II, HAQ‐II, pain VAS, SF‐36 PCS, or SF‐36 MCS (Supplementary Table 3). Changes in outcomes among individuals in this group are highly variable (Supplementary Figure 1).
Discussion
Probiotic use is increasingly common among patients in the United States, particularly with increasing lay understanding of the microbiome and how it may contribute to disease and health. In 2012, 1.6% of US adults reported using probiotics or prebiotics, which is four times as many than those who reported use in 2007 (8). In this observational study, we similarly observed increasing use of probiotics among patients with inflammatory arthritis. However, use of probiotics did not result in significant changes in patient‐reported outcomes over the course of 6 months in patients with PsA and RA.
Probiotics are microorganisms that naturally colonize our gut as part of the microbiome and can be used to help patients rebuild the microflora after long‐term antibiotics or gut acid–altering medication use. The goal of probiotic use is to administer a live organism that can help restore the balance in the intestinal microbiome with commensal bacteria—bifidobacterium and lactobacilli are the most common because of their history of safety (3). Using a similar mechanism to fecal transplant for Clostridium difficile infection, probiotics have been found to downregulate inflammatory cytokines (9). As a complement to a healthy diet and medical management, a probiotic supplement may be a low‐cost and low‐side effect way to reduce inflammation and promote a rich microbiome.
Probiotics have been studied in in vitro microbial colony growth assays and mouse experiments in RA and other rheumatic diseases. Metanalysis has shown a decrease in proinflammatory cytokine interleukin 6 (IL‐6) with probiotic initiation. IL‐6 is a critical cytokine in RA and has been associated with joint damage. However, randomized controlled trials have not found any significant benefit of probiotics in RA (10). Potential reasons for the failure of these studies to show a difference from placebo may be the small size of the studies, short length of follow up, the possibility that probiotic initiation was too late in the disease course, or the possibility that probiotic supplementation is not efficacious (11). An oral probiotic, Bifidobacterium infantis 35624, was tested in a small study of patients with psoriasis, and measurement of inflammatory markers following treatment showed a reduction in TNF‐α (12). Based on the presumed pathogenesis and the results of basic science studies, probiotics may theoretically have a higher likelihood of efficacy in treating SpA, particularly given the implications of the microbiome playing a role in triggering immune dysregulation and subsequent inflammatory disease in SpA (13).
Probiotics may have a role in supporting DMARD use. Colifant (probiotic bacteria Escherichia coli 083) has been shown to increase the efficacy of methotrexate treatment of arthritis by reducing inflammation (9). A recent study involving treatment of mice with an anti–interleukin 23 monoclonal antibody found suppression of SpA pathology and an absence of overgrowth of bacteria associated with arthritis and irritable bowel syndrome during the treatment course, which is a topic for further investigation (14). Recent research has found superior anti‐TNF treatment response in axial spondyloarthritis in human leukocyte antigen B27 genotype–positive patients with an abundance of Clostridiales species (15). Other studies have associated high levels of Burkholderiales at anti‐TNF initiation with increased efficacy in patients with SpA (16).
In our study, probiotic users with PsA had significantly lower SF‐36 PCS scores and higher VAS pain scores compared with nonusers with PsA after propensity score matching. However, no significant differences were found in the PsA cohort outcomes (PAS‐II, HAQ‐II, pain VAS, SF‐36 PCS, or SF‐36 MCS) before and after using probiotics. Although there are no differences in mean outcomes, some individual patients had worse outcomes following the initiation of probiotics. It is not clear if this is due to probiotic use, if probiotics were initiated in response to worsening disease symptoms, or if the two are unrelated. Given the difference noted in pain scores between our PsA cohort users and nonusers, patients who take probiotics may have a higher baseline pain level.
Our study has limitations. We did not have details on the specification of probiotic species and dose. Additionally, because probiotic use is usually nonprescription, many patients start and stop taking them, making it difficult to precisely measure time on therapy, particularly as it relates to the collected outcomes. Furthermore, our study is limited by a small cohort of patients with PsA compared with patients with RA. Strengths of this study include the length of time our cohort was followed, the number of functional measurements captured, and the overall number of participants in comparison with previously published reports. Additionally, we calculated a propensity score for taking a probiotic because many factors may influence the patient initiating probiotic use.
The microbiome is of increasing interest in modulating inflammatory disease. Overall, little data are available on probiotic use in inflammatory arthritis. Prospective randomized controlled trials would better evaluate the effectiveness of probiotics in inflammatory arthritis and which species have the greatest benefit. In this real‐world observational study, we found no significant change in patient function scores before and after initiation of a probiotic.
Acknowledgements
We would like to thank the many participants in the FORWARD databank that made this study possible.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Michaud had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Grinnell, Wipfler, Michuad.
Acquisition of data
Ogdie, Wipfler.
Analysis and interpretation of data
Ogdie, Wipfler, Michuad.
Supporting information
Dr. Ogdie has received grants to the University of Pennsylvania from Pfizer and Novartis and to FORWARD Databank from Amgen. Dr. Michaud's work is supported by a grant from the Rheumatology Research Foundation.
Madison Grinnell, BA: University of Nebraska Medical Center, Omaha; 2Alexis Ogdie, MD, MSCE: University of Pennsylvania, Philadelphia; 3Kristin Wipfler, PhD: FORWARD, The National Databank for Rheumatic Diseases, Wichita, Kansas; 4Kaleb Michaud, PhD: University of Nebraska Medical Center, Omaha, and FORWARD, The National Databank for Rheumatic Diseases, Wichita, Kansas.
Dr. Ogdie has served as a consultant for Abbvie, Amgen, Bristol Myers Squibb, Celgene, Corrona, Janssen, Lilly, Novartis, and Pfizer, and her husband has received royalties from Novartis. No other disclosures relevant to this article were reported.
References
- 1. Norman E, Lefferts A, Kuhn KA. Gut‐joint trafficking in a model of bacteria‐driven murine IBD‐Spa [abstract]. Arthritis Rheumatol 2018;70 Suppl 10 URL: https://acrabstracts.org/abstract/gut-joint-t-cell-trafficking-in-a-model-of-bacteria-driven-murine-ibd-spa/. [Google Scholar]
- 2. Manasson J, Wallach D, Guggino G, Stapylton M, Badri MH, Solomon G, et al. Interleukin‐17 Inhibition in spondyloarthritis is associated with subclinical gut microbiome perturbations and a distinctive interleukin‐25‐driven intestinal inflammation. Arthritis Rheumatol 2020;72:645–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reid G. Safe and efficacious probiotics: what are they? [opinion]. Trends Microbiol 2006;14:348–52. [DOI] [PubMed] [Google Scholar]
- 4. Isolauri E, Kirjavainen PV, Salminen S. Probiotics: a role in the treatment of intestinal infection and inflammation? [review]. Gut 2002;50 Suppl 3:III54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Owaga E, Hsieh RH, Mugendi B, Masuku S, Shih CK, Chang JS. Th17 cells as potential probiotic therapeutic targets in inflammatory bowel diseases. Int J Mol Sci 2015;16:20841–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolfe F, Michaud K. The National Data Bank for rheumatic diseases: a multi‐registry rheumatic disease data bank. Rheumatology (Oxford) 2011;50:16–24. [DOI] [PubMed] [Google Scholar]
- 7. Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Boston: Statistical Software Components, Boston College Department of Economics; 2003. [Google Scholar]
- 8. Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002‐2012. Natl Health Stat Report 2015;1–16. [PMC free article] [PubMed] [Google Scholar]
- 9. Rovensky J, Stancikova M, Svik K, Uteseny J, Bauerova K, Jurcovicova J. Treatment of adjuvant‐induced arthritis with the combination of methotrexate and probiotic bacteria Escherichia coli 083 (Colifant). Folia Microbiol (Praha) 2009;54:359–63. [DOI] [PubMed] [Google Scholar]
- 10. Mohammed AT, Khattab M, Ahmed AM, Turk T, Sakr N, m Khalil A, et al. The therapeutic effect of probiotics on rheumatoid arthritis: a systematic review and meta‐analysis of randomized control trials. Clin Rheumatol 2017;36:2697–707. [DOI] [PubMed] [Google Scholar]
- 11. Aqaeinezhad Rudbane SM, Rahmdel S, Abdollahzadeh SM, Zare M, Bazrafshan A, Mazloomi SM. The efficacy of probiotic supplementation in rheumatoid arthritis: a meta‐analysis of randomized, controlled trials. Inflammopharmacology 2018;26:67–76. [DOI] [PubMed] [Google Scholar]
- 12. Groeger D, O'Mahony L, Murphy E, Bourke J, Dinan T, Kiely B, et al. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes 2013;4:325–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Allen S, Jordan S, Storey M, Thornton C, Gravenor M, Garaiova I, et al. Dietary supplementation with lactobacilli and bifidobacteria is well tolerated and not associated with adverse events during late pregnancy and early infancy. J Nutr 2010;140:483–8. [DOI] [PubMed] [Google Scholar]
- 14. Rehaume L, Matigian N, Kang A, Zbarskaya O, Kikly K, Lachner N, et al. Treatment of ZAP‐70 mutant SKG mice with anti‐IL‐23 antibody alters fecal microbiota composition and prevents outgrowth of bacteria associated with susceptibility to spondyloarthritis and ileitis. Arthritis Rheumatol 2016;68 Suppl 10 URL: https://acrabstracts.org/abstract/treatment-of-zap-70-mutant-skg-mice-with-anti-il-23-antibody-alters-fecal-microbiota-composition-and-prevents-outgrowth-of-bacteria-associated-with-susceptibility-to-spondyloarthritis-and-ileitis/. [Google Scholar]
- 15. Vallier M, Chamaillard M, Ferreira S, Menegatti S, Bianchi E, Rogge L, et al. SAT0265 the response to TNF‐blockers treatment of spa patients is influenced by the interplay between HLA‐B27 and gut microbiota composition at baseline. Ann Rheum Dis 2018;77:996.29453217 [Google Scholar]
- 16. Bazin T, Hooks K, Barnetche T, Truchetet ME, Enaud R, Richez C, et al. Microbiota composition may predict anti‐Tnf alpha response in spondyloarthritis patients: an exploratory study. Sci Rep 2018;8:5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


