Abstract
Objective
Immunoglobulin M antibodies against phosphorylcholine (anti‐PCs) may be protective in atherosclerosis, cardiovascular disease (CVD), and systemic lupus erythematosus (SLE). We study immunoglobulin G1 (IgG1) and immunoglobulin G2 (IgG2) anti‐PCs, with a focus on atherosclerosis and SLE.
Methods
We determined anti‐PCs by using the enzyme‐linked immunosorbent assay in 116 patients with SLE and 110 age‐ and sex‐matched controls. For functional studies, we used three in‐house–generated, fully human monoclonal IgG1 anti‐PCs (A01, D05, and E01). Apoptosis was induced in Jurkat T cells and preincubated with A01, D05, E01, or IgG1 isotype control, and effects on efferocytosis by human macrophages were studied. Anti‐PC peptide/protein characterization was determined using a proteomics de novo sequencing approach.
Results
IgG1, but not IgG2, anti‐PC levels were higher among patients with SLE (P = 0.02). IgG1 anti‐PCs were negatively associated with Systemic Lupus International Collaborating Clinics (SLICC) damage index and Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) scores (odds ratio [OR]: 2.978 [confidence interval (CI): 0.876‐10.098] and OR: 5.108 [CI 1.3‐20.067], respectively) and negatively associated with CVD, atherosclerotic plaques, and echolucent plaques (potentially vulnerable plaques), but the association for the two former was not significant after controlling for confounders. D05 had a maximum effect on macrophage efferocytosis efficiency, followed by A01 and E01. The monoclonal antibodies showed differential binding specificity to PC and PC‐associated neoepitopes. A peptide analysis showed a difference in the complementarity‐determining region 3 of the three IgG1 anti‐PC clones that are crucial for recognition of PC on apoptotic cell surfaces and other neoepitopes.
Conclusion
IgG1 anti‐PCs are negatively associated with disease activity and disease damage in SLE, but the negative association with CVD is also dependent on confounding risk factors. One potential underlying mechanism could be increased clearance of dead cells.
Short abstract
What is already known about this subject?
-
•
Low levels of immunoglobulin M antibodies against phosphorylcholine (anti‐PCs) is more common in patients with systemic lupus erythematosus (SLE) compared with controls and is associated with increased prevalence of vulnerable plaque among patients with SLE.
What does this study add?
Immunoglobulin G1 (IgG1) anti‐PCs are negatively associated with disease activity, disease damage, cardiovascular disease, and measures of atherosclerosis in SLE.
We have produced in‐house, fully human monoclonal antibodies of the IgG1 isotype that increase apoptotic cell uptake efficiently and reduce inflammation induced by lipopolysaccharide. Effects varied depending on the clone used.
A peptide analysis showed a difference in the complementarity‐determining region 3 of the three IgG1 anti‐PC clones that are crucial for the recognition of phosphorylcholine (PC) on apoptotic cell surfaces and other neoepitopes.
How might this impact clinical practice or future developments?
Measurement of IgG1 anti‐PCs, along with other autoantibodies, could improve prevention in patients with SLE with vascular implications.
Anti‐PCs could be developed as a novel treatment in SLE, either as monoclonal antibodies or as a vaccine with PC.
INTRODUCTION
Phosphorylcholine (PC) is an important component in cellular membranes and in lipoproteins that is exposed and recognized by the immune system when cells undergo apoptosis or when lipoproteins, such as low‐density lipoprotein (LDL), undergo oxidation. PC is also exposed in some microorganisms, including nematodes and bacteria (non‐self). PC can be exposed on protein, lipid, or carbohydrate carriers, and antibodies against PC (anti‐PCs) of the immunoglobulin M (IgM) isotype are prevalent, constituting as much as 5% to 10% of the circulating IgM pool 1. PC exposed on oxidized phospholipids in oxidized low‐density lipoprotein (OxLDL) contributes to activation of immune cells such as T lymphocytes and macrophages 2.
We reported that IgM anti‐PCs are negatively associated with cardiovascular disease (CVD), including stroke and myocardial infarction (MI), atherosclerosis development, and mortality after MI 1, 3, 4. IgM anti‐PCs could also play a role in systemic lupus erythematosus (SLE) because low levels of IgM anti‐PCs are associated with atherosclerotic plaques, vulnerable plaques in SLE, and disease activity 5, 6. These and similar findings have largely been confirmed and extended into other diseases such as vasculitis and even osteoarthritis 7, 8, 9, 10, 11, 12.
Efficient phagocytosis of dead and dying cells is essential for maintaining tissue homeostasis. If not cleared in the early stages, secondary necrosis and eventual accumulation may contribute to the development of autoimmune diseases such as SLE 13. Of note, atherosclerosis is an inflammatory process in which activated immune‐competent cells, OxLDL, and dead cells in a necrotic core are key elements 1. In both atherosclerosis and SLE, clearance of dead cells is thus a problem, and both CVD and prevalence of atherosclerotic plaques are increased in SLE 5, 6. Extensive studies in the past decades have shown various functions that could explain underlying protective properties of anti‐PCs, especially the IgM isotype 1.
Although there has been extensive focus on the IgM isotype, immunoglobulin G (IgG) anti‐PCs and especially immunoglobulin G1 (IgG1) and immunoglobulin G2 (IgG2) anti‐PCs still have not been elaborately studied. Their putative role was explored in infection 14 but not in chronic inflammatory diseases (such as SLE) and CVD complications, although we determined that IgG1 anti‐PC, in contrast to IgG2 anti‐PC, is a protection marker in atherosclerosis development 15. In this study, we report the protective role of IgG1 anti‐PCs in SLE and a possible mechanism using in‐house–produced anti‐PC IgG1 clones.
MATERIALS AND METHODS
Subjects with SLE
One hundred sixteen patients with SLE and their age‐ and sex‐matched controls were recruited at the Karolinska University Hospital in Huddinge, Sweden. The study, the SLE Vascular Impact Cohort (SLEVIC), has been described elsewhere 5. All patients with SLE fulfilled the 1982 revised criteria of the American College of Rheumatology (ACR) for SLE. The study was approved by the Karolinska Institutet Research Ethics Committee and was done in accordance with the Declaration of Helsinki. All subjects gave informed consent before entering the study.
The SLEVIC study included a written questionnaire, an interview, and a physical examination by a rheumatologist. In addition, there were laboratory determinations of routine and other blood samples and an ultrasound examination of the carotid arteries. SLE activity was determined with the Systemic Lupus Activity Measure (SLAM) and the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) 16. To determine organ damage, we used the Systemic Lupus International Collaborating Clinics (SLICC) damage index 16.
Determination of atherosclerosis and atherosclerotic plaques
We studied characteristics of atherosclerosis among patients and controls using B‐mode carotid ultrasound. We have described the ultrasonographic methods used herein in detail previously 5, 17, 18. Briefly, the carotid arteries were examined with a duplex scanner (ACUSON Sequoia Ultrasound; Siemens Healthineers) using a 6‐MHz linear array transducer 5. The far wall of the common carotid artery (CCA), 0.5 to 1.0 cm proximal to the beginning of the carotid bulb, was used for measurements of the intima‐media thickness (IMT), defined as the distance between the leading edge of the lumen‐intima echo and the leading edge of the media‐adventitia echo. The CCA lumen diameter was defined as the distance between the leading edge of the intima‐lumen echo of the near wall and the leading edge of the lumen‐intima echo of the far wall. The examinations were digitally stored for subsequent computer analyses 19. The mean values of the IMT and lumen diameter within the 10‐mm long section were calculated. When a plaque was observed in the region of the CCA measurements, the IMT was not measured.
A carotid plaque was defined as a localized intima‐media thickening greater than 1 mm and at least a 100% increase in thickness compared with adjacent wall segments. Plaque was screened for in the common, internal, and external carotid arteries. Plaque occurrence was scored as the absence of plaque, the presence of unilateral plaque, and the presence of bilateral plaque. Plaque morphology in terms of echogenicity was assessed in a modified version of the classification proposed by Gray‐Weale et al 20 and graded from 1 to 4: 1) echolucent, 2) predominantly echolucent, 3) predominantly echogenic, and 4) echogenic. Echolucency was defined with the arterial lumen as reference and echogenicity with the far wall adventitia as reference.
IgG1 anti‐PC generation
Monoclonal antibodies (mAbs) were produced as described previously 21. Briefly, the mAbs A01, D05, and E01 against PC were isolated from single PC‐reactive B cells of the healthy human donors. The complementary‐DNA was synthesized and cloned into expression vectors containing human immunoglobulin γ (Igγ), immunoglobulin λ (Igλ), or immunoglobulin κ (Igκ). The antibodies were produced by cotransfection of exponentially growing human embryonic kidney cells. After 7 days of cultures, the proteins were purified by protein G chromatography column. The antibody protein purity and the expression of heavy and light chains were confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. The antibody affinity to PC hapten was measured by surface plasmon resonance on the Biacore X‐100 (GE Healthcare). Isotype control 1 (ET‐901) was purchased from Eureka Therapeutics.
Antigens and generation of oxidation products
To test the antibody binding to different oxidation‐specific epitopes (OSEs), antigens exposing PC were chosen for the study. 1‐palmitoyl‐2‐(5‐oxovaleroyl)‐sn‐glycero‐3‐phosphocholine (POVPC) was purchased from Avanti Polar Lipids and conjugated with bovine serum albumin (BSA), as described elsewhere 22. Phosphatidylcholine (PtC) and BSA were also purchased from Sigma. The capsular polysaccharide (C‐PS) was a kind gift from Professor Birgitta Norrmark Henriques. Lipopolysaccharide (LPS) was purchased from Sigma‐Aldrich (L4391).
Cell culture
Buffy coats from healthy blood donors were bought from the Karolinska University Hospital blood bank. Peripheral blood mononuclear cells were isolated using Ficoll‐Paque Plus (GE Healthcare) and differentiated into M2 macrophages with 50 ng/ml macrophage colony stimulating factor (MCSF) (ImmunoTools) at a density of 5 × 105 cells per ml in Roswell Park Memorial Institute Medium (RPMI) media (Gibco; Life Technologies) in a 24‐well plate. Media were replaced at day 3 and restimulated with 50 ng/ml MSCF again. Cells were subjected to phagocytosis at days 4 to 5 depending on their level of attachment.
For the phagocytosis assay, Jurkat T cells were purchased from the American Type Culture Collection (ATCC) and grown in a continuous culture in RPMI media according to manufacturer's instruction and subjected to apoptosis as described below. THP1 cells were bought from ATCC, cultured according to the manufacturer's instruction, and stimulated with LPS, as indicated, to study anti‐inflammatory effects.
IgG1 and IgG2 isotype measurement in SLEVIC
A standard enzyme‐linked immunosorbent assay (ELISA) was performed to measure IgG1 and IgG2 anti‐PCs in serum samples of patients and controls from SLEVIC. The plates were coated with a 10‐μg/ml concentration of phosphorylcholine conjugated with BSA (PC‐BSA) and incubated at 4°C overnight. The plates were washed and blocked with 2% BSA for at least an hour. Standards and samples were diluted at 1:100 dilution and added at 100 ul per well and incubated for 2 hours at room temperature. After repeated washing, biotin‐conjugated secondary antibodies IgG1 (B6775; Sigma) and IgG2 (I5635; Sigma) were added at 1:1000 and 1:20 000 dilution at 100 ul per well. Color was developed using 3,3',5,5'‐tetramethylbenzidine (TMB) (Life Technologies); the reaction was stopped with stop solution 1N H2SO4, and the reaction was read at 450 nm using an ELISA plate reader (Spectra Max 250; Molecular Devices). Pilot experiments indicate that there is no cross‐reactivity between the secondary antibodies (data not shown).
Binding of anti‐PCs to antigens and competition inhibition by ELISA
Binding was assessed using the standard ELISA procedure. A Nunc immunosorbent plate was coated with (PC‐BSA) at a 10‐μg/ml concentration at 4°C overnight. The plate was washed and blocked with 2% BSA for 1 hour. Samples (A01, D05, E01, and an isotype control) at various concentrations (20, 10, 5, 2.5, 1.25, 0.625, and 0.3125 μg) were added at 100 μl per well and incubated for 2 hours. In addition, an IgG secondary antibody (Sigma) conjugated with horseradish peroxidase was added at a concentration of (1:60 000) 100 μl per well and incubated further for 2 hours. The color was developed using TMB (Life Technologies) at 100 μl per well and stopped with 1N H2SO4. The plate was read in an ELISA plate reader (Spectra Max 250; Molecular Devices) at 450 nm. For the competition assay, the plate was coated at a 10‐μg/ml concentration of the mentioned antigen, and the antibody was coated with the different concentration of the competitor or without the competitor. More than 70% of binding to the antigen was inhibited by the competitor. The percentage of inhibition by the competitor was assessed by the following formula: ([optical density {OD} value with competitor − OD value without competitor]/OD value without competitor) × 100.
Antibody binding and complement deposition to apoptotic cells
To assess the binding of anti‐PC clones or the isotype control to the apoptotic Jurkat cells, cells induced apoptosis by incubating with CD95 Fas ligand (catalog No. SY‐001; MBL International) for apoptosis or by heat killing (necrosis). Apoptotic cells without any antibodies are used as experimental controls. Apoptosis was assessed using annexin A5 (fluorescein isothiocyanate) and 7‐aminoactinomycin D (7‐AAD) (catalog No. 559763; BD Biosciences) (not shown). The apoptotic cells were incubated with anti‐PCs A01, D05, or E01 or isotype controls at a 1‐ or 5‐μg/ml concentration for 1 hour, followed by a 30‐minunte incubation with an allophycocyanin‐conjugated anti‐IgG antibody (catalog No. 409305; BioLegend). Binding efficiency was assessed using the BD LSRFortessa (BD Biosciences), and at least 10 000 events were acquired for analysis. Analysis was done using FlowJo 7.5 software. The results are represented as the median fluorescent intensity of the bound antibodies. The experiments were repeated at least thrice.
For complement deposition assays (C1q and C3b), the apoptotic cells were incubated with mAbs A01, D05, and E01 and isotype controls at different concentration (1, 5, or 20 μg/ml) in the presence of 20% IgG‐deficient sera containing RPMI media. The cells were further incubated with anti‐C1q (catalog No. 565327; BD Biosciences) and anti‐C3b antibodies (catalog No. 551531; BD Biosciences) for 30 minutes in fluorescence‐activated cell sorting (FACS) buffer. The cells were acquired in FACS LSRFortessa, with at least 10 000 events, and the acquired events were analyzed through FlowJo.
Phagocytosis assay
Apoptosis was induced in 5‐carboxytetramethylrhodamine (TAMRA)‐labeled (catalog No. C2734; Sigma) Jurkat T cells by CD95 ligand (catalog No. SY‐001; MBL International), which was confirmed by annexin A5 and 7‐AAD (catalog No. 559763; BD Biosciences) staining by flow cytometry (data not shown). Apoptotic Jurkat cells were incubated with A01, D05, E01, or the isotype control IgG1 (1 μg/ml) in IgG‐deficient serum or in a serum‐free condition 1 hour prior to phagocytosis. IgG1 anti‐PC–labeled apoptotic Jurkat cells were fed to mature MCSF‐stimulated M2 macrophages for 60 to 80 minutes in triplicates. For microscopic observation, after 60 minutes, unbound Jurkat cells were discarded. The cells were washed thoroughly with phosphate‐buffered saline with 5 mM EDTA at least five times to completely get rid of loosely bound cells. The washed cells were formalin fixed for microscopic examination. The cells were again washed and counter stained with 4',6‐diamidino‐2‐phenylindole with mounting media before pictures were taken using a Nikon ECLIPSE TE2000‐S fluorescent microscope. The cells with at least 250 to 300 cells per area were chosen, and at least three different regions were chosen for each well. The cells were merged and counted using Image J software (National Institutes of Health). Phagocytosis efficiency was assessed by the number of macrophages up‐taking the TAMRA‐labeled Jurkat cells to the total number of macrophages in the given area. Similarly, for the FACS analysis, the Jurkat cells were labeled with carboxyfluorescein succinimidyl ester and fed to macrophages. The cells were washed, stained with CD11b, PE (BD Biosciences), and acquired in FACS LSRFortessa for analysis. The phagocytosis efficiency is represented in a percentage. The experiments were repeated at least thrice for statistical analysis.
Measurement of cytokines by ELISA
ELISA kits for interleukin 6 (IL‐6) were purchased from R&D Systems, and the method was performed according to the manufacturer's instructions.
Gene sequence analysis
For each single B cell in the 96‐well polymerase chain reaction plate, matching immunoglobulin H (or Igγ), Igκ, and Igλ amplicons were obtained, sequenced (Eurofins MWG Operon), and analyzed for complementarity‐determining region 3 (CDR3) features and the number of V gene somatic hypermutation by IgBLAST comparison (Supplementary Table 1). The immunoglobulin CDR3 length was determined, as indicated previously 23, using multiple sequence alignment software (MUSCLE; European Molecular Biology Laboratory).
Proteomics peptide sequencing, sample preparation, liquid chromatography–mass spectrometry analysis, and data processing
Samples were treated similarly to what has previously been described 24. Briefly, prior to the liquid chromatography (LC)–mass spectrometry (MS/MS) analysis, IgG samples (10 μg per sample) were reduced with 20 mM dithiothreitol for 30 minutes at 56°C and alkylated with 66 mM iodoacetamide for 30 minutes in darkness. Trypsin was added (1∶50 enzyme to protein ratio), and digestion was performed at 37°C overnight. Peptides were then desalted using C18 HyperSep Filer Plates (Thermo Fisher Scientific), dried using SpeedVac, and resuspended in 0.1% formic acid and 0.5% acetonitrile solution. Samples were kept at 10°C and injected on the column in 5‐μl aliquots containing 0.3 μg of digest. The instrument method was similar to what has previously been described 25. Briefly, an EASY‐nLC system connected in line to a Fusion Orbitrap mass spectrometer (–Thermo Fisher Scientific) was used. Reversed‐phase nano‐LC separation of the peptides was performed on a 15‐cm long EASY‐Spray column (PepMap, C18, 2 μm, 10 nm) using a 2‐hour gradient solvent system with 1) water with 2% acetonitrile and 0.1% formic acid and 2) acetonitrile with 2% water and 0.1% formic acid. The flow rate was set at 300 nl/min. The mass spectrometer was operating in the positive DDA mode. A survey mass spectrum was acquired in a mass to charge ratio of200:1700, with a nominal resolution of 120 000. Ion selection for MS/MS was performed for each precursor with both high resolution (15 000) higher‐energy collisional dissociation (HCD) and electron‐transfer dissociation (ETD) fragmentation. Peptide sequences were confirmed using PEAKS Studio software (Bioinformatics Solution Inc.) using a combined Swiss‐Prot protein sequence database (April 2013; 20 242 entries) and the monoclonal IgG genome light and heavy chain data information. Peptide mass error tolerance was set at 10 ppm. MS/MS fragment mass accuracy at 0.05 Da and tryptic digestion was set with a maximum of two missed cleavages. Carbamidomethylation of cysteine was used as a fixed modification, whereas the variable modifications were asparagine and glutamine deamidation and methionine oxidation. Peptide sequence quality was set as 1% false discovery rate (FDR), a post‐translational modification (PTM) score of 50, 2% ion intensity, and de novo–only average local confidence greater than or equal to 80%.
Statistical analysis
For clinical association studies, we employed SPSS version 22 (IBM Corporation). A conditional binary logistic regression was applied for association studies between antibodies, atherosclerotic plaque, and SLICC and SLAM index scores. A normal Student's t test was employed for other clinical comparisons. All experimental statistical analyses were conducted using Prism 7 software. Student's unpaired t test was employed for all experimental data analyses.
RESULTS
IgG1 and IgG2 anti‐PC levels and other baseline characteristics
The baseline characteristics of patients with SLE are shown in Table 1. Briefly, patients with SLE are characterized by raised blood pressure or hypertension (P < 0.001), raised C‐reactive protein levels (P < 0.001), raised homocysteine levels (P < 0.001) and decreased LDL levels (P = 0.03). The patients with SLE possessed higher CVD risk compared with controls, as shown in Table 1. A Significantly higher prevalence of CVD (P < 0.001) was observed in patients with SLE (16.1%) compared with controls (2.3%). Similarly, the prevalence of both atherosclerotic plaque and echolucent plaque was increased among patients with SLE, as described in Supplementary Table 1 (P < 0.05). The IgG1, but not the IgG2, anti‐PC level was significantly higher in patients with SLE (P = 0.02) compared with controls (Table 1). The secondary antibodies did not cross‐react with the other isotypes.
Table 1.
IgG1 and IgG2 anti‐PCs among patients with SLE and controls
| Patients | Controls | P | |
|---|---|---|---|
| No. | 116 | 110 | … |
| Age, mean ± SD, y | 47.85 ± 13.35 | 49.31 ± 12.68 | 0.45 |
| Female sex, n (%) | 102 (87.9) | 97 (88.2) | 0.89 |
| Current smokers, % | 14 | 15.6 | 0.47 |
| LDL, mean ± SD, mmol/L | 2.5 ± 0.9 | 2.8 ± 0.8 | 0.031 |
| Homocysteine, mean ± SD | 13.84 ± 5.93 | 10.95 ± 2.30 | <0.001 |
| HDL, mean ± SD, mmol/L | 1.61 ± 0.47 | 1.68 ± 0.55 | 0.29 |
| CRP, mean ± SD, mg/ml | 4.5 ± 6.4 | 2.1 ± 3.2 | 0.001 |
| Glucose, mean ± SD, mmol/L | 4.7 ± 1.1 | 4.9 ± 0.9 | <0.001 |
| Hypertension, n (%) | 64 (5.6) | 31 (26.3) | <0.001 |
| CVD, % | 16.1 | 2.3 | <0.001 |
| IgG1 anti‐PCs, mean ± SD, AU | 67.97 ± 4.41 | 55.83 ± 2.69 | 0.02 |
| IgG2 anti‐PCs, mean ± SD, AU | 107.1 ± 9.73 | 123.7 ± 10.27 | 0.2432 |
Abbreviation: anti‐PC, antibody against phosphorylcholine; AU, arbitrary units; CRP, C‐reactive protein; CVD, cardiovascular disease; HDL, high‐density lipoprotein; IgG1, immunoglobulin G1; IgG2, immunoglobulin G2; LDL, low‐density lipoprotein; PC, phosphorylcholine; SLE, systemic lupus erythematosus.
The P values are as they stand, in bold.
IgG1 and IgG2 anti‐PC levels in relation to atherosclerotic plaque
The IgG1 anti‐PC levels (P = 0.006) were lower in patients with SLE with atherosclerotic plaque compared with patients without atherosclerotic plaque, as shown in Supplementary Table 1. No significant difference in IgG2 anti‐PC levels was observed between the two groups. Among patients with echolucent plaque, the IgG1 anti‐PC levels were significantly lower (P = 0.02) compared with those without echolucent plaque (Supplementary Table 1).
IgG1 and IgG2 anti‐PC levels in relation to clinical outcomes
The association of these antibodies were determined in relation to disease activity and damage. IgG1 anti‐PCs were negatively associated with the SLAM and SLICC index scores, as shown in Table 2 , although only the SLICC index score reached significance. When comparing the SLAM index score and IgG1 anti‐PCs, there was a considerably negative trend that did not reach significance at any given percentile, as shown in Table 2. Similarly, with the SLICC damage index score, the negative trend was seen in the lower quartiles, although the levels were only significant below the 33rd percentile. For the SLEDAI, a strong negative association was established below the 25th percentile, as shown in Figure 2. In contrast to IgG1 anti‐PCs, IgG2 anti‐PCs were not associated with these clinical outcomes.
Table 2.
Association of IgG1 anti‐PCs in relation to disease activity and organ damage scores in patients with SLE: SLAM index, SLICC damage index, and SLEDAI a
| OR | CI | P | |
|---|---|---|---|
| Association between IgG1 anti‐PCs and the SLAM index | |||
| >66th percentile | 1.13 | 0.506‐2.523 | 0.7659 |
| <50th percentile | 1.021 | 0.473‐2.203 | 0.9581 |
| <33rd percentile | 1.425 | 0.638‐3.186 | 0.3876 |
| <25th percentile | 2.418 | 0.818‐4.579 | 0.137 |
| <10th percentile | 2.717 | 0.802‐9.202 | 0.1083 |
| Association between IgG1 anti‐PCs and the SLICC index | |||
| >66th percentile | 0.632 | 0.272‐1.466 | 0.2845 |
| <50th percentile | 1.649 | 0.751‐3.62 | 0.2127 |
| <33rd percentile | 2.7 | 1.193‐6.111 | 0.0172 |
| <25th percentile | 2.165 | 0.911‐5.149 | 0.0805 |
| <10th percentile | 2.975 | 0.876‐10.098 | 0.0804 |
| Association between IgG1 anti‐PCs and the SLEDAI | |||
| >66th percentile | 0.718 | 0.322‐1.599 | 0.4167 |
| <50th percentile | 0.941 | 0.473‐1.99 | 0.8746 |
| <33rd percentile | 1.479 | 0.67‐3.262 | 0.3327 |
| <25th percentile | 3.214 | 1.337‐7.728 | 0.0091 |
| <10th percentile | 5.108 | 1.3‐20.067 | 0.0195 |
Abbreviation: anti‐PC, antibody against phosphorylcholine; CI, confidence interval; IgG1, immunoglobulin G1; OR, odds ratio; SLAM, Systemic Lupus Activity Measure; SLE, systemic lupus erythematosus; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; SLICC, Systemic Lupus International Collaborating Clinics.
A conditional logistic regression above the mean was applied for all the parameters.
The P values are as they stand, in bold.
Similarly, a negative association was observed with atherosclerotic plaque and history of CVD (Supplementary Table 2). IgG1 anti‐PCs were associated with protection above the 66th percentile (odds ratio [OR]: 0.41; confidence interval [CI]: 0.17‐0.95), and the risk increased below 50th percentile, although it did not reach significance after adjustment (Supplementary Table 2). IgG1 anti‐PCs were negatively associated with cerebrovascular events. Although above the 33rd percentile, the OR (2.31 [CI:0.77‐6.97]) did not reach significance after adjustment for age, hypertension, hyperlipidemia, and glucose below the 10th percentile (OR: 4.74; CI: 1.10‐20.53), as shown in Supplementary Table 2. All the percentiles mentioned here refer to the percentiles of antibodies.
Gene and proteomics peptide sequencing results
Multiple‐sequence alignment of the anti‐PC sequences showed similarities in the CDR1 and CDR2 in the heavy chain but revealed differences in the CDR3. Sequence A01 had the most number of peptides compared with D05 or E01. Interestingly E01 had lesser peptides in the CDR3 compared with D05, as shown in Figure 1A. The genetic sequence of the light chain was also similar, except that the CDR1 of A01 had more sequences compared with that of D05 or E01 (Figures 1A and B). Probably, this difference in the CDR3 of the antibodies could be attributed to recognition of the PC moiety conjugated to a carbohydrate, protein or lipid. The characteristics and genetic information also suggest that the mAbs are different regarding the number of nonsilent mutations (Supplementary Table 2). To validate that the gene sequences (most importantly the CDRs) match the protein expression, the antibodies were subjected to a proteomics peptide sequencing analysis. The sequence coverages of A01 (heavy variable (HV): 99%; kappa variable (KV): 100%), D05 (HV: 88%; KV: 96%), and E01 (HV: 98%; KV: 96%) confirmed the genetic mAb sequences (Supplementary Figure 1A).
Figure 1.
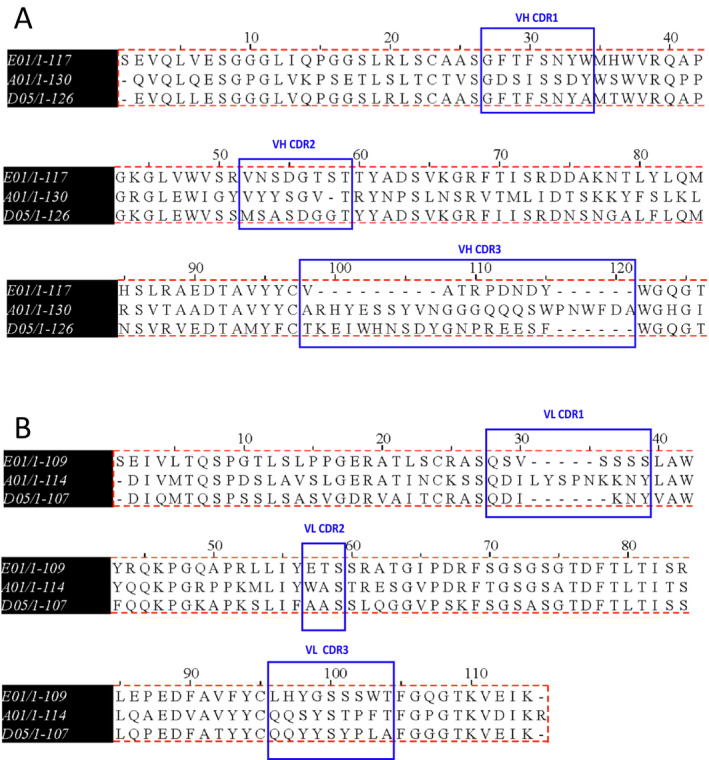
Peptide sequence alignment by multiple sequence alignment (MUSCLE). The figure shows reduced amino acid sequences of monoclonal antibodies A01, D05, and E01. The amino acid sequences were confirmed by de novo sequencing using the liquid chromatography–mass spectrometry approach. The variable region of the heavy (A) and the light (B) chains are shown. Complementarity‐determining regions (CDRs) of both heavy and light chains are indicated by double arrow, and the sequences are underlined. The peptide sequence analysis of the three antibodies indicate differences in the CDR3 of both chains. The low‐affinity monoclonal antibody, A01 (determined by enzyme‐linked immunosorbent assay), has more peptides in the CDR3 of heavy chains and CDR1 of light chains compared with high‐affinity binders, D05 and E01. On the contrary, monoclonal antibody E01, with the highest dissociation constant (Kd value, has the least number of peptides in the CDR3. The sequences were aligned and compared using MUSCLE software. Vh, variable heavy chain; Vl, variable light chain.
Anti‐PC clones express differential binding patterns to OSEs
IgG1 Anti‐PCs were produced as described previously, and the dissociation constant (Kd) of the mAbs to PC was assessed using Biacore. Consistent with the Kd value, anti‐PC (A01, E01, and D05) clones showed differential binding expression to the PC‐exposing molecules. As shown in Figure 2A, antibodies D05 and E01 showed higher binding specificity compared with A01.We further assessed the binding of these antibodies to pneumococcal C‐PS; compared with the other two antibodies, E01 showed the highest affinity, followed by D05 and A01. But these antibodies did not bind to native proteins, such as PtC, or the irrelevant protein BSA, which was conjugated with PC. In addition, isotype controls also did not bind to any of the OSEs or native proteins. To further understand how the mAbs compete with C‐PS, a competition ELISA was performed. The clone E01 inhibited 100% of binding with PC as antigen, but the clone D05 was able to inhibit about 75%, followed by A01 at specified concentrations (Figure 2B).
Figure 2.
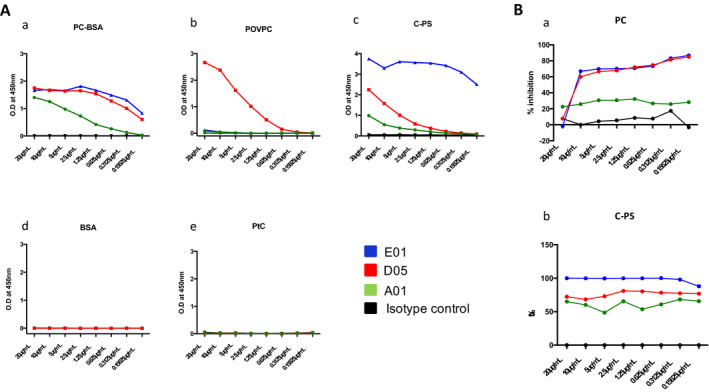
Antibodies against phosphorylcholine (anti‐PCs) binding to different oxidation‐specific neoepitopes and competition inhibition by the antibodies. A, Binding of immunoglobulin G anti‐PCs to different oxidation‐specific epitopes (OSEs) was assessed by enzyme‐linked immunosorbent assay (ELISA). Anti‐PC clones express differential binding patterns to different OSEs. Because phosphorylcholine (PC) is found in cell walls of Gram‐positive bacteria, such as pneumococcus, and also in some altered self‐structures, such as apoptotic cells and 1‐palmitoyl‐2‐(5‐oxovaleroyl)‐sn‐glycero‐3‐phosphocholine (POVPC); the antibody binding is tested for all known antigens that expose PC. Capsular polysaccharide (C‐PS), POVPC conjugated with bovine serum albumin (BSA), and native proteins that do not expose phosphorylcholine phosphatidylcholine (PtC) were coated in 96 well plates. Antibodies A01, D05, and E01 and an isotype control at a different concentration was added to the respective antigen, followed by a secondary antibody (1:70 000). The optical density (OD) for different antigens and different antibody concentrations was obtained. Clones D05 and E01, with high affinity (as determined by the dissociation constant [Kd] value), strongly bind to PC conjugated with BSA (PC‐BSA) with highest capacity, followed by A01 (as determined by ELISA). But D05 binds strongly to POVPC compared with E01 or A01. Contrary to lipid antigens, monoclonal antibodies binding to bacterial C‐PS show a different pattern. Monoclonal antibody E01 binds strongly to bacterial C‐PS, followed by D05 and A01. However, none of the antibodies bind to BSA or PtC. As expected, isotype controls do not bind to any antigens. B, Inhibition of PC to PC or C‐PS. PC (10 μg/ml) was plated on microtiter wells overnight at 4°C. Antibodies were added to wells in the absence or presence of the indicated concentrations of competitors, and the amount of bound E01 was detected by biotin‐conjugated anti‐human immunoglobulin G. The amount of bound antibodies was expressed as the percentage of E01 binding to C‐PS in the absence of a competitor.
Anti‐PC D05 improves M2 macrophage efferocytosis efficiency in a complement‐independent manner
Apoptosis was induced in TAMRA‐labeled Jurkat cells using CD95 for 3 hours. Apoptosis was confirmed by annexin A5 expression. Apoptotic Jurkat cells labeled with monoclonal anti‐PCs were fed to macrophages for 1 hour, and the phagocytosis efficiency was determined by fluorescent microscopy by counting the number of apoptotic cells absorbed by the macrophages to the total number of macrophages. Jurkat cells were incubated at 1μg in immunoglobulin‐deficient serum or in a serum‐free condition, and the phagocytosis efficiency was determined, compared with untreated and isotype controls. The representative microscopic images are shown in Figure 3A. The representative FACS images are shown in Figure 3B. Antibodies did not bind to the live cells, as shown in Figure 3C. Macrophages in the serum condition also absorbed the apoptotic cells but in lesser efficiency. D05 facilitated macrophage uptake efficiency even in the serum‐free condition Figure 3D. The uptake efficiency was improved with the addition of serum deficient in IgG. Of the three mAbs, D05 improved the efferocytosis efficiency two times (from 19% to 30%), even at a 1‐ug concentration, followed by A01 (23%), as shown in Figure 3E. But E01 and the isotype control did not increase efferocytosis by macrophages in IgG‐deficient serum or in serum free medium. This was in consistent with the binding experiment; E01 and the isotype do not facilitate apoptotic cell uptake by the macrophages. The binding of monoclonal anti‐PCs to the apoptotic cells was assessed by FACS (Supplementary Figure 1), which is in line with the apoptotic cell uptake data. Because IgM anti‐PCs improve phagocytosis efficiency, we next wanted to understand if IgG1 anti‐PCs also facilitate efferocytosis by depositing complement components such as C1q or C3b. As seen in Supplementary Figure 1, compared with the live cell or the cells untreated with IgG1, the deposition is increased from 0.102% to 4.7%. But the addition of anti‐PCs (A01, D05, E01) at three different concentrations (1, 5, and 20 μg/ml) did not improve the complement deposition (Supplementary Figure 2). Similar to C1q, antibody incubation did not facilitate C3b deposition on to the apoptotic cells.
Figure 3.
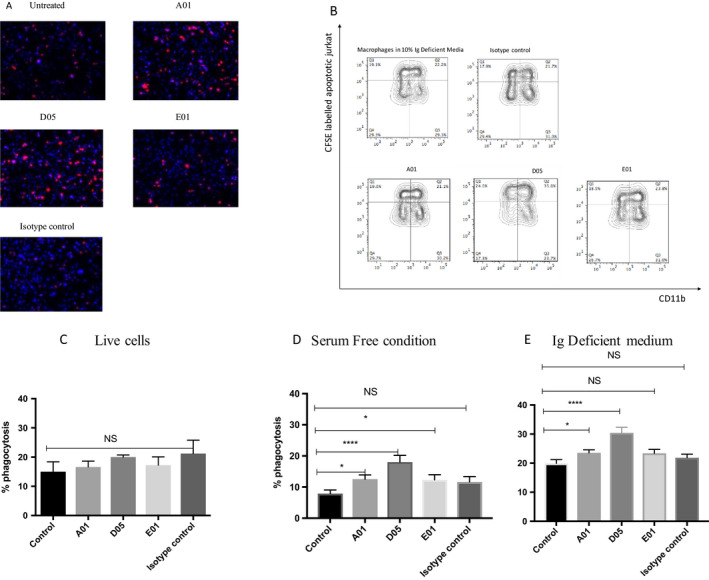
Antibodies against phosphorylcholine (anti‐PCs) improve apoptotic cell uptake by macrophages. Anti‐PCs increase phagocytosis efficiency of the macrophages. Apoptosis was induced in 5‐carboxytetramethylrhodamine (TAMRA)‐labeled Jurkat T cells using CD95 Fas ligand (MBL International Corporation) for 3 hours. The apoptosis was confirmed by annexin A5 and 7‐aminoactinomycin D staining with flow cytometry (not shown). Apoptotic Jurkat cells were incubated with A01, D05, E01, or immunoglobulin G1 isotype control (1μg/ml), 1 hour prior to feeding to M2 macrophages. The anti‐PC–labeled apoptotic Jurkat cells were fed to mature M2 macrophages at a 1:1 ratio in an immunoglobulin G–deficient medium for 60 mins. The cells were washed thoroughly with 5 mM EDTA before formalin fixing for microscopic examination. The cells were counterstained with 4′,6‐diamidino‐2‐phenylindole with mounting media before pictures were taken using a Nikon ECLIPSE TE2000‐S fluorescent microscope. The cells were merged and counted using Image J software (National Institutes of Health). Phagocytosis efficiency was assessed by the number of macrophages up‐taking the TAMRA‐labeled Jurkat cells to the total number of macrophages in the given area. The phagocytosis efficiency is represented in a percentage. A, Representative microscopic picture (×10) of one of the phagocytosis assays. B, fluorescence‐activated cell sorting (FACS) experiment showing uptake of carboxyfluorescein succinimidyl ester‐labeled Jurkat cells by macrophages. C–E, the bar graphs represent the mean ± SEM of at least three independent experiments for live and serum‐free conditions and six independent experiments for an immunoglobulin (Ig)–deficient medium. ****P < 0.0001; *P < 0.05. NS, non‐significant.
Anti‐PC clones reduce IL‐6 production by LPS‐stimulated THP1 cells
Antibodies had shown their effect in increasing phagocytosis efficiency. Next, we wanted to understand if the antibodies possess any anti‐inflammatory properties. To study this, THP1 cells were preincubated with or without antibodies at different concentrations from 100 ng/ml to 20 μg/ml and stimulated with 100 ng of LPS overnight. All the clones were able to inhibit IL‐6 production but at different degree, even at the lowest concentration (100 ng/ml), as shown in Figures 4A‐C.
Figure 4.
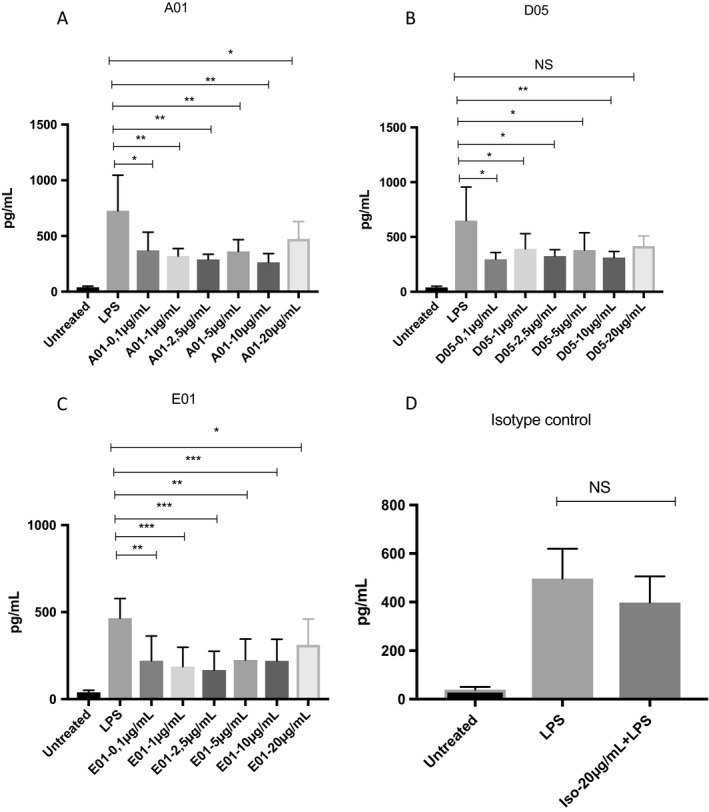
Immunoglobulin G1 (IgG1) antibodies against phosphorylcholine (anti‐PCs) inhibit interleukin 6 (IL‐6) production by lipopolysaccharide (LPS)‐stimulated THP1‐like macrophages. Anti‐PC clones reduce IL‐6 production on LPS‐stimulated THP1 cells. THP1 monocytes were stimulated with 100 ng/ml phorbol 12‐myristate 13‐acetate for 72 hours. The cells were preincubated with IgG1 anti‐PCs at different concentrations (100 ng/ml to 20 μg/ml) prior to LPS (100 ng/ml) stimulation overnight. Supernatants were collected the next day and analyzed for IL‐6 secretion. All three antibodies reduced IL‐6 production even at a low concentration, such as 100 ng/ml. The graphs represent the mean ± SD of three independent experiments. A, A01. B, D05. C, E01. D, Isotype control. NS, non‐significant.
DISCUSSION
Here, we demonstrate that IgG1 anti‐PC, but not IgG2 anti‐PC, levels are negatively associated with atherosclerotic plaque in SLE as well as with potentially vulnerable echolucent atherosclerotic plaque. This finding indicates that IgG1 anti‐PCs, but not IgG2 anti‐PCs, have properties comparable to IgM anti‐PCs, which were also negatively associated with atherosclerotic and echolucent plaque in our previous study in this cohort 5. In contrast to IgM anti‐PCs, in which low levels are more prevalent in SLE, IgG1 and IgG2 anti‐PC levels were increased among patients with SLE, but only IgG1 reached significance. This may reflect that IgG levels are generally raised in SLE, including pathogenic antibodies against cardiolipin and DNA or other nuclear components, which in fact represents the hallmark of the disease pathogenesis.
Relatively little is known about IgG1 anti‐PCs in SLE or atherosclerosis. We previously reported a negative association between atherosclerosis development and the circulating IgG1 anti‐PC, but not IgG2 anti‐PC, level among hypertensive subjects, which is in line with our current findings 15. We have described in detail associations between cardiac risk factors and lupus factors in a previous article 5. Here, we also report that IgG1 anti‐PCs are negatively associated with cardiovascular events, a finding in line with the results in relation to atherosclerosis and prevalence of plaque. Little is known about associations between IgG1 anti‐PCs and CVD in the general population, but such studies are ongoing in our laboratory. Another important finding is that there was a negative association with both organ damage (SLICC) and disease activity as determined by the SLEDAI, whereas associations with the SLAM index score did not reach statistical significance. We think this favors the notion that the antibodies are not a negative factor in SLE even though their levels are raised. It is possible that although the IgG1 anti‐PC level is raised in SLE, it could be beneficial to have higher levels. A caveat is that we cannot rule out immune‐complex formation involving IgG1 and/or IgG2 anti‐PCs, a possibility that deserves further study because some immune complexes to some extent related to the antigen herein are believed to be proatherogenic 26. There could be some individuals in whom such immune complexes play a negative role. Still, the totality of measured anti‐PCs herein supports mainly a protective role at least of IgG1 anti‐PCs (and IgM anti‐PCs), as in previous publications.
The difference between IgG1 and IgG2 anti‐PCs is also quite interesting. In our earlier studies, anti‐PCs were divided into two populations, group I (IgM and IgG1) and group II (IgG2), depending on their affinity for PC and p‐nitrophenyl phosphorylcholine (NPPC) haptens, respectively. Although group I anti‐PCs recognize both antigens, group II antibodies recognizes only NPPC by recognizing the phenyl ring attached to them. We also demonstrated that IgG2 anti‐PCs only belongs to group II, and our interpretation of the findings was that IgG2 anti‐PCs are directed against capsulated bacteria 15, which is in line with a previous observation of the bactericidal properties of IgG2 anti‐PCs encountering carbohydrate antigens 27, 28. It is also interesting to note that patients with periodontitis have an increased risk of CVD with increased levels of IgG anti‐PCs, in which IgG2, but not IgG1, levels were raised 29, 30. Supporting this finding, vaccinating humans with pneumococcus does not induce IgM anti‐PCs targeted toward OxLDL 31. We thus hypothesize that IgG2 anti‐PCs are related to infections and IgM and IgG1 anti‐PCs are related to CVD and atherosclerosis.
There are many publications that support the notion that IgM anti‐PCs could function as protection markers in different diseases, especially those that are atherosclerosis related 1. An important question is whether these clinical associations also represent causation in relation to protection. Indications of this include increased clearance of dead cells by macrophages, both in mouse 32 and human systems 33, inhibition of uptake of OxLDL by human macrophages and thus inhibition of foam‐cell generation 34, 35, an anti‐inflammatory effect in which human anti‐PCs inhibit phospholipid‐induced inflammatory effects 6, inhibition of cell death by lysophosphatidylcholine (a major phospholipid component of plaque) 15, and promotion of T regulatory cells 36.
Further evidence from experimental mouse models indicates that passive transfer of IgM anti‐PCs ameliorates atherosclerosis 37 and that active immunization with PC also has beneficial effects 38, which is of special interest for the present study because IgG anti‐PCs could also be involved. In another interesting study, immunization with pneumococcus by using a strong adjuvant generated an array of antibodies, including anti‐PCs, and this procedure induced a relatively modest 30% decrease in atherosclerosis, which may to some extent be attributed to anti‐PCs 39. In mouse models, the anti‐PC repertoire is known to be limited, with a dominant clone, TI5. In addition, anti‐PCs are generally described as being T cell independent 21. However, in humans, anti‐PCs showed T cell dependency and are predominantly produced by affinity‐matured B cells and do not exhibit clonal dominance 21, 33. Instead, all healthy donors tested had mounted somatically mutated anti‐PC response using a broad variety of immunoglobulin genes.
To understand the role of IgG1 anti‐PCs, in this study, we used our in‐house–generated, fully human mAbs, which showed variability in binding to PC, some with higher affinity than TI5 21. The antibodies were different clonally and regarding the number of nonsilent mutation. Three different IgG1 mAbs with varying affinity for PC were tested in the present study for functional properties that could shed light on underlying mechanisms by which IgG1 anti‐PCs may protect against atherosclerosis and SLE. Our monoclonal anti‐PCs bind differentially to OSEs. D05 and E01, being the strongest binders (as determined by Biacore), bind efficiently to PC‐BSA, compared with A01. The mAbs had weaker binding to OxLDL and absolutely no binding to native LDL compared with PC, which may be related to the amount of PC exposed upon oxidation (data not shown). Interestingly, these antibodies showed a different profile when binding to the C‐PS of Streptococcus pneumoniae. This might be due to the difference in the recognition of the PC moiety depending on where they are bound (polysaccharide, protein, or even oxidized phospholipids). It is well known that PC is exposed on the teichoic acid of the pneumococcal polysaccharide 40, 41. Also, a competition assay showed that clone E01 induced an almost 100% inhibition (Figure 2B), indicating that these antibodies could play a role in bacterial infections such as S. pneumoniae complications 42.
Here, we also report that IgG1 monoclonal anti‐PCs increase efferocytosis efficiency by macrophages of apoptotic cells. This is in line with results from mouse models, in which immature dendritic cell uptake of apoptotic cells was increased by IgM anti‐PCs 43, and with our study in human systems using extracted polyclonal IgM anti‐PCs. Phagocytosis of apoptotic cells by macrophages and other phagocytes is an important physiological process that is required to maintain homeostasis by recognizing “eat‐me” signals, a danger‐associated molecular pattern (DAMP) on cells undergoing apoptosis; PC‐ and malondialdehyde‐modified proteins are major examples of such DAMPs. Defective clearance of dead cells could be a common denominator in both atherosclerosis and SLE, which could also partly explain the clinical relevance between these two pathological conditions. Efficient phagocytosis of apoptotic cells even promotes active anti‐inflammatory properties in human macrophages, promoting amelioration of inflammation 44. Defective clearance of dead cells could thus promote a chronic inflammatory state, which could develop into symptom‐giving autoimmune disease 45. Clearance of dead cells is known to be deviant and may be one of the primary causes in initiating SLE and other autoimmune conditions, in which pathogenic autoantibodies, for example, anti–double‐stranded DNA antibodies, are directed toward nucleic acids.
Impaired apoptotic cell clearance is also implicated in atherosclerosis because accumulation of dead cells in a necrotic core and ensuing inflammation can cause progression and increased inflammation in plaque 1. Not only IgM anti‐PCs but also IgG1 anti‐PCs could thus be protective in CVD, atherosclerosis, and SLE, with increased clearance of dead cells as one potential mechanism. Of the three mAbs, D05 has maximum efficiency in aiding apoptotic cell clearance compared with A01 or E01. This is also supported by the evidence that D05 and A01 bind to the apoptotic cells significantly in higher proportion compared with E01. It is also quite intriguing that although D05 and E01 bind with higher efficiency to PC, only D05 improves phagocytosis efficiency, whereas E01 does not. Although E01 is effective in recognizing PC attached to a polysaccharide, it does not recognize PC found on the cell wall of the eukaryotic cells, which could also be attributed to the difference in the CDR3 sequence.
Unlike IgM anti‐PCs, these antibodies did not recruit C1q or C3b complement components 43. One plausible mechanism for the effector function could be direct involvement of Fc‐γ receptors. The role of Fc‐γ receptors in facilitating apoptotic cell clearance is well documented 46. In addition, these antibodies did not improve CD36 expression upon addition at different concentrations (data not shown), which also confirms that these antibodies do not require a complement protein or CD36 to improve phagocytosis efficiency. We reported that anti‐PCs have anti‐inflammatory properties, inhibiting the effects of inflammatory phospholipids 6. In line with this is the finding herein that IgG1 anti‐PC clones, to varying extent, inhibit the effects of LPS. Also, such anti‐inflammatory effects could thus contribute to the antibodies’ protective properties.
Taken together, IgG1, but not IgG2, anti‐PCs could protect against atherosclerosis, CVD, and SLE, in which the mechanism may include clearance of dead cells as well as an anti‐inflammatory effect. We recently reported that both IgG and IgM anti‐PC levels are significantly higher among people from Kitava, Papua New Guinea, living a traditional lives as hunters, gatherers, and horticulturalists. Of note, antibodies in higher titers in SLE (considered pathogenic) were raised in these group of people, but rheumatic diseases and CVD were not prevalent there, which is not likely to be attributed only to shorter life span. Also, other lifestyle and risk factors where more beneficial there, so anti‐PCs may be only one of several factors. We suggested that low exposure to PC‐exposing microorganisms (such as nematodes, parasites, and bacteria) could be one cause, and we identified Treponema as one such organism 47, 48, 49.
Vaccination with PC to prevent and/or treat atherosclerosis, CVD, and SLE could thus be one possibility that deserves further study, and our findings herein suggest that such a vaccine should be constructed to optimize an IgG1 and IgM response to PC bound to noncarbohydrate antigens.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Johan Frostegård.
Acquisition of data
Thiagarajan, Rahman, Lundström.
Analysis and interpretation of data
All authors.
Supporting information
ACKNOWLEDGMENTS
We thank Tomas Jogestrand and Thomas Gustafsson for ultrasound measures and analyses.
Supported by the Swedish Rheumatism Association, the Swedish Heart‐Lung Foundation, King Gustav V's 80‐Year Fund, Vinnova, and the Sixth Framework Programme of the European Union (grant LSHM‐CT‐2006‐037227; CVDImmune; with Dr. Frostegård as coordinator).
D. Thiagarajan, MSc, PhD, A. Frostegård, MD, PhD, J. Steen, PhD, M. Rahman, PhD, M. Vikström, MSc, S. Lundström, PhD, J. Frostegård, MD, PhD: Karolinska Institutet, Stockholm, Sweden; 2R. Fiskesund, MD, PhD: Karolinska University Hospital, Huddinge, Sweden.
Dr. Frostegård is named inventor on patents relating to antibodies against phosphorylcholine. No other disclosures relevant to this article were reported.
REFERENCES
- 1. Frostegård J. Immunity, atherosclerosis and cardiovascular disease. BMC Med 2013;11:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frostegård J, Huang YH, Rönnelid J, Schäfer‐Elinder L. Platelet‐activating factor and oxidized LDL induce immune activation by a common mechanism. Arterioscler Thromb Vasc Biol 1997;17:963–8. [DOI] [PubMed] [Google Scholar]
- 3. Caidahl K, Hartford M, Karlsson T, Herlitz J, Pettersson K, de Faire U, et al. IgM‐phosphorylcholine autoantibodies and outcome in acute coronary syndromes. Int J Cardiol 2013;167:464–9. [DOI] [PubMed] [Google Scholar]
- 4. Su J, Georgiades A, Wu R, Thulin T, de Faire U, Frostegård J. Antibodies of IgM subclass to phosphorylcholine and oxidized LDL are protective factors for atherosclerosis in patients with hypertension. Atherosclerosis 2006;188:160–6. [DOI] [PubMed] [Google Scholar]
- 5. Anania C, Gustafsson T, Hua X, Su J, Vikström M, de Faire U, et al. Increased prevalence of vulnerable atherosclerotic plaques and low levels of natural IgM antibodies against phosphorylcholine in patients with systemic lupus erythematosus. Arthritis Res Ther 2010;12:R214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Su J, Hua X, Concha H, Svenungsson E, Cederholm A, Frostegård J. Natural antibodies against phosphorylcholine as potential protective factors in SLE [published erratum appears in Rheumatology (Oxford) 2008;47:1593]. Rheumatology (Oxford) 2008;47:1144–50. [DOI] [PubMed] [Google Scholar]
- 7. Vas J, Gronwall C, Marshak‐Rothstein A, Silverman GJ. Natural antibody to apoptotic cell membranes inhibits the proinflammatory properties of lupus autoantibody immune complexes. Arthritis Rheum 2012;64:3388–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sobel M, Moreno KI, Yagi M, Kohler TR, Tang GL, Clowes AW, et al. Low levels of a natural IgM antibody are associated with vein graft stenosis and failure. J Vasc Surg 2013;58:997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gleissner CA, Erbel C, Haeussler J, Akhavanpoor M, Domschke G, Linden F, et al. Low levels of natural IgM antibodies against phosphorylcholine are independently associated with vascular remodeling in patients with coronary artery disease. Clinical Res Cardiol 2015;104:13–22. [DOI] [PubMed] [Google Scholar]
- 10. Wilde B, Slot M, van Paassen P, Theunissen R, Kemna M, Witzke O, et al. Phosphorylcholine antibodies are diminished in ANCA‐associated vasculitis. Eur J Clin Invest 2015;45:686–91. [DOI] [PubMed] [Google Scholar]
- 11. Imhof A, Koenig W, Jaensch A, Mons U, Brenner H, Rothenbacher D. Long‐term prognostic value of IgM antibodies against phosphorylcholine for adverse cardiovascular events in patients with stable coronary heart disease. Atherosclerosis 2015;243:414–20. [DOI] [PubMed] [Google Scholar]
- 12. Nguyen TG, McKelvey KJ, March LM, Hunter DJ, Xue M, Jackson CJ, et al. Aberrant levels of natural IgM antibodies in osteoarthritis and rheumatoid arthritis patients in comparison to healthy controls. Immunol Lett 2016;170:27–36. [DOI] [PubMed] [Google Scholar]
- 13. Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Impaired phagocytosis of apoptotic cell material by monocyte‐derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum 1998;41:1241–50. [DOI] [PubMed] [Google Scholar]
- 14. Panda S, Zhang J, Tan NS, Ho B, Ding JL. Natural IgG antibodies provide innate protection against ficolin‐opsonized bacteria. EMBO J 2013;32:2905–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fiskesund R, Su J, Bulatovic I, Vikström M, de Faire U, Frostegård J. IgM phosphorylcholine antibodies inhibit cell death and constitute a strong protection marker for atherosclerosis development, particularly in combination with other auto‐antibodies against modified LDL. Results Immunol 2012;2:13–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Griffiths B, Mosca M, Gordon C. Assessment of patients with systemic lupus erythematosus and the use of lupus disease activity indices. Best Pract Res Clin Rheumatol 2005;19:685–708. [DOI] [PubMed] [Google Scholar]
- 17. Lemne C, Jogestrand T, de Faire U. Carotid intima‐media thickness and plaque in borderline hypertension. Stroke 1995;26:34–9. [DOI] [PubMed] [Google Scholar]
- 18. Nowak J, Nilsson T, Sylven C, Jogestrand T. Potential of carotid ultrasonography in the diagnosis of coronary artery disease: a comparison with exercise test and variance ECG. Stroke 1998;29:439–46. [DOI] [PubMed] [Google Scholar]
- 19. Wendelhag I, Liang Q, Gustavsson T, Wikstrand J. A new automated computerized analyzing system simplifies readings and reduces the variability in ultrasound measurement of intima‐media thickness. Stroke 1997;28:2195–200. [DOI] [PubMed] [Google Scholar]
- 20. Gray‐Weale AC, Graham JC, Burnett JR, Byrne K, Lusby RJ. Carotid artery atheroma: comparison of preoperative B‐mode ultrasound appearance with carotid endarterectomy specimen pathology. J Cardiovasc Surg (Torino) 1988;29:676–81. [PubMed] [Google Scholar]
- 21. Fiskesund R, Steen J, Amara K, Murray F, Szwajda A, Liu A, et al. Naturally occurring human phosphorylcholine antibodies are predominantly products of affinity‐matured B cells in the adult. J Immunol 2014;192:4551–9. [DOI] [PubMed] [Google Scholar]
- 22. Horkko S, Bird DA, Miller E, Itabe H, Leitinger N, Subbanagounder G, et al. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid‐protein adducts inhibit macrophage uptake of oxidized low‐density lipoproteins. J Clin Invest 1999;103:117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kabat EA, Wu TT. Identical V region amino acid sequences and segments of sequences in antibodies of different specificities: relative contributions of VH and VL genes, minigenes, and complementarity‐determining regions to binding of antibody‐combining sites. J Immunol 1991;147:1709–19. [PubMed] [Google Scholar]
- 24. Lundstrom SL, Fernandes‐Cerqueira C, Ytterberg AJ, Ossipova E, Hensvold AH, Jakobsson PJ, et al. IgG antibodies to cyclic citrullinated peptides exhibit profiles specific in terms of IgG subclasses, Fc‐glycans and a fab‐peptide sequence. PLoS One 2014;9:e113924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lundstrom SL, Zhang B, Rutishauser D, Aarsland D, Zubarev RA. SpotLight Proteomics: uncovering the hidden blood proteome improves diagnostic power of proteomics. Sci Rep 2017;7:41929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lopes‐Virella MF, Virella G. Pathogenic role of modified LDL antibodies and immune complexes in atherosclerosis. J Atheroscler Thromb 2013;20:743–54. [DOI] [PubMed] [Google Scholar]
- 27. Scott MG, Shackelford PG, Briles DE, Nahm MH. Human IgG subclasses and their relation to carbohydrate antigen immunocompetence. Diagn Clin Immunol 1988;5:241–8. [PubMed] [Google Scholar]
- 28. Goldenberg HB, McCool TL, Weiser JN. Cross‐reactivity of human immunoglobulin G2 recognizing phosphorylcholine and evidence for protection against major bacterial pathogens of the human respiratory tract. J Infect Dis 2004;190:1254–1263. [DOI] [PubMed] [Google Scholar]
- 29. Blaizot A, Vergnes JN, Nuwwareh S, Amar J, Sixou M. Periodontal diseases and cardiovascular events: meta‐analysis of observational studies. Int Dent J 2009;59:197–209. [PubMed] [Google Scholar]
- 30. Schenkein HA, Gunsolley JC, Best AM, Harrison MT, Hahn CL, Wu JH, et al. Antiphosphorylcholine antibody levels are elevated in humans with periodontal diseases. Infect Immun 1999;67:4814–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Damoiseaux J, Rijkers G, Tervaert JW. Pneumococcal vaccination does not increase circulating levels of IgM antibodies to oxidized LDL in humans and therefore precludes an anti‐atherogenic effect. Atherosclerosis 2007;190:10–1. [DOI] [PubMed] [Google Scholar]
- 32. Chen Y, Khanna S, Goodyear CS, Park YB, Raz E, Thiel S, et al. Regulation of dendritic cells and macrophages by an anti‐apoptotic cell natural antibody that suppresses TLR responses and inhibits inflammatory arthritis. J Immunol 2009;183:1346–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rahman M, Sing S, Golabkesh Z, Fiskesund R, Gustafsson T, Jogestrand T, et al. IgM antibodies against malondialdehyde and phosphorylcholine are together strong protection markers for atherosclerosis in systemic lupus erythematosus: regulation and underlying mechanisms. Clin Immunol 2016;166–167:27‐37. [DOI] [PubMed] [Google Scholar]
- 34. De Faire U, Su J, Hua X, Frostegård A, Halldin M, Hellenius ML, et al. Low levels of IgM antibodies to phosphorylcholine predict cardiovascular disease in 60‐year old men: effects on uptake of oxidized LDL in macrophages as a potential mechanism. J Autoimmun 2010;34:73–9. [DOI] [PubMed] [Google Scholar]
- 35. Shaw PX, Goodyear CS, Chang MK, Witztum JL, Silverman GJ. The autoreactivity of anti‐phosphorylcholine antibodies for atherosclerosis‐associated neo‐antigens and apoptotic cells. J Immunol 2003;170:6151–7. [DOI] [PubMed] [Google Scholar]
- 36. Sun J, Lundstrom SL, Zhang B, Zubarev RA, Steuer J, Gillgren P, Rahman M, et al. IgM antibodies against phosphorylcholine promote polarization of T regulatory cells from patients with atherosclerotic plaques, systemic lupus erythematosus and healthy donors. Atherosclerosis 2018;268:36–48. [DOI] [PubMed] [Google Scholar]
- 37. Faria‐Neto JR, Chyu KY, Li X, Dimayuga PC, Ferreira C, Yano J, et al. Passive immunization with monoclonal IgM antibodies against phosphorylcholine reduces accelerated vein graft atherosclerosis in apolipoprotein E‐null mice. Atherosclerosis 2006;189:83–90. [DOI] [PubMed] [Google Scholar]
- 38. Caligiuri G, Khallou‐Laschet J, Vandaele M, Gaston AT, Delignat S, Mandet C, et al. Phosphorylcholine‐targeting immunization reduces atherosclerosis. J Am Coll Cardiol 2007;50:540–6. [DOI] [PubMed] [Google Scholar]
- 39. Binder CJ, Horkko S, Dewan A, Chang MK, Kieu EP, Goodyear CS, et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med 2003;9:736–43. [DOI] [PubMed] [Google Scholar]
- 40. Vollmer W, Tomasz A. Identification of the teichoic acid phosphorylcholine esterase in Streptococcus pneumoniae. Mol Microbiol 2001;39:1610–22. [DOI] [PubMed] [Google Scholar]
- 41. Fischer W. Phosphocholine of pneumococcal teichoic acids: role in bacterial physiology and pneumococcal infection. Res Microbiol 2000;151:421–7. [DOI] [PubMed] [Google Scholar]
- 42. Briles DE, Forman C, Hudak S, Claflin JL. Anti‐phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med 1982;156:1177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen Y, Park YB, Patel E, Silverman GJ. IgM antibodies to apoptosis‐associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J Immunol 2009;182:6031–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF‐β, PGE2, and PAF. J Clin Invest 1998;101:890–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell 2010;140:619–30. [DOI] [PubMed] [Google Scholar]
- 46. Indik ZK, Park JG, Hunter S, Schreiber AD. The molecular dissection of Fc gamma receptor mediated phagocytosis. Blood 1995;86:4389–99. [PubMed] [Google Scholar]
- 47. Frostegård J, Tao W, Råstam L, Lindblad U, Lindeberg S. Antibodies against phosphorylcholine among New Guineans compared to Swedes: an aspect of the hygiene/missing old friends hypothesis. Immunol Invest 2017;46:59–69. [DOI] [PubMed] [Google Scholar]
- 48. Frostegård J, Tao W, Georgiades A, Rastam L, Lindblad U, Lindeberg S. Atheroprotective natural anti‐phosphorylcholine antibodies of IgM subclass are decreased in Swedish controls as compared to non‐Westernized individuals from New Guinea. Nutr Metab (Lond) 2007;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Agmon‐Levin N, Bat‐sheva PK, Barzilai O, Ram M, Lindeberg S, Frostegård J, et al. Antitreponemal antibodies leading to autoantibody production and protection from atherosclerosis in Kitavans from Papua New Guinea. Ann N Y Acad Sci 2009;1173:675–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


