As our understanding of coronavirus disease 2019 (COVID-19) evolves, it has become clear that severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection causes multisystem disease that is distinct from the typical viral pneumonia. SARS-CoV-2 infection leads to hypercoagulability, microthrombosis, and in some patients, large vessel thrombosis manifesting as ischemic stroke, myocardial infarction, or digital ischemia.1,2 In a cohort study of 184 critically ill COVID-19 patients, the cumulative incidence of thrombosis was 31%.3 In a second cohort study of 107 critically ill COVID-19 patients, pulmonary embolism occurred in 21% of patients, despite widespread use of thromboprophylaxis.4 The incidence of pulmonary embolism was 2-fold higher than in critically ill influenza patients.4
The mechanisms that cause hypercoagulability in COVID-19 are not entirely understood, but there is a strong association between the degree of coagulation system activation and mortality in patients with COVID-19.5 SARS-CoV-2 has been shown to infect endothelial cells, causing endothelialitis.6 Infected endothelial cells propagate proinflammatory and hypercoagulable responses, which can cause small and large vessel thrombosis. These changes are likely in part because of increased tissue factor expression on endothelial cells, as well as increased tissue factor release from injured lung parenchyma.7
Common laboratory changes in sepsis-induced coagulopathy (SIC) and disseminated intravascular coagulation (DIC) include increased thrombin-antithrombin complexes, increased factor(F) VIII activity, thrombocytopenia, increased plasminogen activator inhibitor-1 (PAI-1) activity, low antithrombin and protein C activity, and low or normal fibrinogen.8 Although COVID-19 coagulopathy shares features with SIC and DIC, there are important differences. For example, most critically ill COVID-19 patients have a normal platelet count, elevated fibrinogen (600–1000 mg/dL), and severely elevated FVIII activity (300%–400%).9,10 Likewise, COVID-19 patients are predominately hypercoagulable, with only 2%–3% of patients experiencing serious bleeding even with widespread thromboprophylaxis or systemic anticoagulation.10 This bleeding rate is lower than in other patients with SIC who are treated with anticoagulant drugs, where the bleeding rate may be as high as 15%–25%.11
Activated proteinC (APC) counteracts many of the pathologic changes that occur with COVID-19 (Table 1). Protein C has anticoagulant and anti-inflammatory properties (Figure). When thrombin binds to thrombomodulin on endothelial cell surfaces, proteinC is converted to its active form. APC cleaves activated FV and activated FVIII and neutralizes PAI-1.12 Cleavage of activated FV leads to a dramatic decrease in thrombin generation, as prothrombinase activity is decreased 10,000-fold.12 Protein S further enhances cleavage of activated FV and FVIII by APC. APC’s anti-inflammatory actions occur mainly through protease-activated receptor 1 (PAR1). PAR1 is present on endothelial cell surfaces and is unique in that it can propagate both proinflammatory and anti-inflammatory responses. APC interacts with the endothelial proteinC receptor (EPCR), and this complex cleaves PAR1. Anti-inflammatory signaling is initiated through multiple intracellular pathways including sphingosine kinase and β-arrestin 2.13–15 These pathways lead to antiapoptotic, anti-inflammatory, and barrier-enhancing changes in endothelial cells.12
Table 1.
Pathologic Changes in COVID-19 and Activated Protein C Counteractions
| Pathologic Change | Activated Protein C Counteraction |
|---|---|
| Increased thrombin generation | Cleaves activated factorV, which reduces prothrombinase activity |
| Increased factorVIII activity | Cleaves activated factorVIII shutting down propagation of thrombin generation |
| Increased plasminogen activator inhibitor-1 activity, which inhibits fibrinolysis | Reduces thrombin-activated fibrinolysis inhibitor activation by slowing thrombin generation and facilitates fibrinolysis by neutralizing plasminogen activator inhibitor-1 |
| Increased endothelial cell proinflammatory signaling | Propagates anti-inflammatory signaling in endothelial cells through protease-activated receptor 1 |
Abbreviation: COVID-19, coronavirus disease 2019.
Figure.
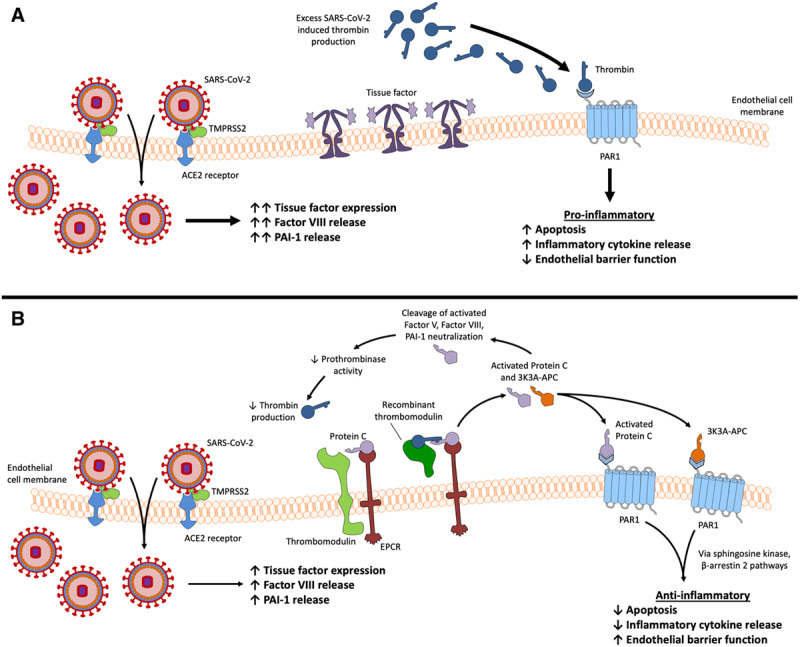
Procoagulant changes that occur with COVID-19 and potential therapeutics. A, SARS-CoV-2 entry leads to increased tissue factor expression on the endothelial cell surface, as well as factor VIII and PAI-1 release. Excessive thrombin generation leads to a proinflammatory state through PAR1 signaling. The net effect is an increase in apoptosis, propagation of proinflammatory signaling, and a decrease in endothelial cell barrier function. B, Recombinant thrombomodulin activates protein C and leads to anti-inflammatory signaling through PAR1, as well as anticoagulant actions including cleavage of activated factor V and factor VIII. Reduction of activated factor V reduces prothrombinase activity and subsequently thrombin production. APC and 3K3A-APC activate PAR1, which leads to anti-inflammatory actions via the sphingosine kinase and β-arrestin 2 pathways. These changes result in a decrease in apoptosis and reduced proinflammatory cytokine release, as well as enhanced endothelial cell barrier function. The Figure created with Motifolio Toolkit (Motifolio Inc, Ellicott City, MD). ACE2 indicates angiotensin-converting enzyme 2; APC, activated protein C; COVID-19, coronavirus disease 2019; PAI-1, plasminogen activator inhibitor-1; PAR1, protease-activated receptor 1; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; TMPRSS2, transmembrane protease serine 2.
APC has a complex history as a therapeutic. In 2001, the results of the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study were published demonstrating that recombinant human APC, drotrecogin alfa, reduced mortality in severe sepsis by 6%.16 After publication of PROWESS, there was great enthusiasm for drotrecogin alfa, but within a decade, the drug was voluntarily withdrawn from the market by its manufacturer due to numerous follow-up studies that either showed no benefit or harm with its use.17 The main concern with APC treatment was that it increased the risk of serious bleeding. In PROWESS, the APC treatment group had a serious bleeding rate of 3.5% compared with 2% in the control group (P = .06).16
Since 2001, new drugs that modulate the proteinC pathway have been studied in clinical trials (Table 2). For example, the APC variant, 3K3A-APC (ZZ Biotech, Houston, TX), was engineered to minimize the bleeding risks observed with drotrecogin alfa. 3K3A-APC is structurally similar to APC, but 3 lysine residues are replaced with alanine, limiting its anticoagulant activity to <10% of APC. 3K3A-APC maintains APC’s anti-inflammatory properties and has been shown to have cytoprotective effects in preclinical studies. In 2019, the results of a phase II clinical trial were published that included 110 patients with ischemic stroke who were revascularized and treated with 3K3A-APC or placebo. In this study, 3K3A-APC reduced the rate of hemorrhagic stroke conversion from 86.5% to 67.4% (P = .04).18 The authors concluded that doses up to 540 μg/kg were well tolerated and that there were barrier enhancing and vascular protective effects with 3K3A-APC. Future plans for the drug are not clear, but it is likely that its manufacturer will be pursuing Food and Drug Administration approval for adjunctive treatment of ischemic stroke. In critically ill patients with COVID-19, 3K3A-APC could be used with or without low dose anticoagulation, depending on whether a particular patient exhibits clinical or laboratory signs of hypercoagulability.
Table 2.
Protein C Variants and Pathway Modulators
| Drug | Mechanism of Action | Phase of Development | Reported BleedingRisk | Rationale for COVID-19 |
|---|---|---|---|---|
| Recombinant thrombomodulin | Binds to thrombin and enhances proteinC activation | Approved in Japan since 2008 | No increased bleeding in 3 randomized controlled trials, which included over 1600 patients | Its anticoagulant actions are thrombin dependent so patients with the most thrombin generation will have the most proteinC activation |
| Has been studied in both phase II and phase III trials in septic patients with disseminated intravascular coagulation | COVID-19 patients have increased factorVIII activity and increased proinflammatory signaling from endothelial cells, which it can counteract | |||
| 3K3A-APC | Induces biased protease-activated receptor 1 anti-inflammatory signaling in endothelial cells | Phase II randomized controlled trial completed in ischemic stroke patients | Less hemorrhagic stroke conversion in treated patients than in placebo control group | Biased anti-inflammatory signaling can be induced in endothelial cells |
| Minimal anticoagulant activity | If anticoagulant actions are desired in patients with hypercoagulability 3K3A-APC can be combined with low molecular weight heparin or unfractionated heparin |
Abbreviation: COVID-19, coronavirus disease 2019.
Recombinant thrombomodulin (Asahi, Tokyo, Japan) has been approved for use in Japan since 2008. In 2019, the results of an international randomized controlled phase III trial of recombinant thrombomodulin treatment in sepsis; Sepsis Coagulopathy Asahi Recombinant LE Thrombomodulin (SCARLET) were published. Although recombinant thrombomodulin did not reduce mortality in bacterial sepsis patients with DIC, COVID-19 has unique features that may be better suited for recombinant thrombomodulin including marked hypercoagulability and increased FVIII activity.19 In SCARLET, there was a trend toward greater survival in patients who had elevated thrombin-antithrombin complexes that received recombinant thrombomodulin.19 There was also no increased risk of bleeding observed with recombinant thrombomodulin. It is possible that COVID-19 patients with severely elevated D-dimer might have a similar benefit if treated with recombinant thrombomodulin.
Recombinant thrombomodulin theoretically has lower bleeding risk than drotrecogin alfa because its anticoagulant effect is thrombin-dependent and hence patients with the most thrombin generation will also have the most APC activation. A recent meta-analysis of recombinant thrombomodulin use for SIC that included over 1600 patients from 3 randomized controlled studies demonstrated that it decreased mortality in patients with SIC (relative risk=0.80, 95% confidence interval [CI], 0.65–0.98).20 We suggest that recombinant thrombomodulin could be used to treat COVID-19 patients with hypercoagulability under a food and drug administration expanded access protocol or a small phase II clinical trial could be performed with an investigational new drug application.
In summary, SARS-CoV-2 causes endothelialitis, marked hypercoagulability, and both micro and macrovascular thrombosis. Given these features, novel modulators of the proteinC pathway may have an adjunctive role in treating critically ill patients with COVID-19. Although drotrecogin alfa failed in septic patients with DIC, both 3K3A-APC and recombinant thrombomodulin are likely to have lower bleeding risk, while maintaining APCs anti-inflammatory properties. Neither drug is approved for use in COVID-19 patients and unanticipated risks or lack of clinical benefit are possible. Nevertheless, given the unique hypercoagulability and endothelial cell dysfunction observed in COVID-19, we believe exploring the efficacy of these drugs is worthwhile.
DISCLOSURES
Name: Michael Mazzeffi, MD, MPH, MSc.
Contribution: This author conceived the manuscript, wrote and approved the final manuscript.
Name: Jonathan H. Chow, MD.
Contribution: This author conceived the manuscript, wrote and approved the final manuscript.
Name: Anthony Amoroso, MD.
Contribution: This author conceived the manuscript, wrote and approved the final manuscript.
Name: Kenichi Tanaka, MD, MSc.
Contribution: This author conceived the manuscript, wrote and approved the final manuscript.
This manuscript was handled by: Roman M. Sniecinski, MD.
FOOTNOTES
GLOSSARY
- ACE2
- angiotensin-converting enzyme 2
- APC
- activated protein C
- COVID-19
- coronavirus disease 2019
- CI
- confidence interval
- DIC
- disseminated intravascular coagulation
- EPCR
- endothelial protein C receptor
- F
- factor
- PAI-1
- plasminogen activator inhibitor-1
- PAR1
- protease-activated receptor 1
- PROWESS
- Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus-2
- SCARLET
- Sepsis Coagulopathy Asahi Recombinant LE Thrombomodulin
- SIC
- sepsis-induced coagulopathy
- TMPRSS2
- transmembrane protease serine 2
Funding: None.
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
REFERENCES
- 1.Oxley TJ, Mocco J, Majidi S. Large-vessel strokeas a presenting featureof COVID-19 in the young. N Engl J Med. 2020;382:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bangalore S, Sharma A, Slotwiner A, et al. ST-segment elevationin patientswith COVID-19 - a case series. N Engl J Med. 2020. April 17 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poissy J, Goutay J, Caplan M, et al. Pulmonary embolismin COVID-19 patients: awarenessof an increased prevalence. Circulation. 2020. April 24 [Epub ahead of print]. [Google Scholar]
- 5.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesisin COVID-19. N Engl J Med. 2020. May 21 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visseren FL, Bouwman JJ, Bouter KP, Diepersloot RJ, de Groot PH, Erkelens DW. Procoagulant activity of endothelial cells after infection with respiratory viruses. Thromb Haemost. 2000;84:319–324. [PubMed] [Google Scholar]
- 8.Wada H, Sase T, Matsumoto T. Increased soluble fibrin in plasma of patients with disseminated intravascular coagulation. Clin Appl Thromb Hemost. 2003;9:233–240. [DOI] [PubMed] [Google Scholar]
- 9.Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in intensive care unit. A reportof thromboelastography findingsand other parametersof hemostasis. J Thromb Haemost. 2020. April 17 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020. May 4 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamakawa K, Umemura Y, Hayakawa M. Japan Septic Disseminated Intravascular Coagulation (J-Septic DIC) study group. Benefit profile of anticoagulant therapy in sepsis: a nationwide multicentre registry in Japan. Crit Care. 2016;20:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouwens EA, Stavenuiter F, Mosnier LO. Mechanisms of anticoagulant and cytoprotective actions of the protein C pathway. J Thromb Haemost. 2013;11suppl1242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soh UJ, Trejo J. Activated protein C promotes protease-activated receptor-1 cytoprotective signaling through beta-arrestin and dishevelled-2 scaffolds. Proc Natl Acad Sci U S A. 2011;108:E1372–E1380.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105:3178–3184. [DOI] [PubMed] [Google Scholar]
- 15.Sinha RK, Wang Y, Zhao Z. PAR1 biased signaling is required for activated protein C in vivo benefits in sepsis and stroke. Blood. 2018;131:1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernard GR, Vincent JL, Laterre PF. Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z. The efficacy of activated protein C for the treatment of sepsis: incorporating observational evidence with a Bayesian approach. BMJ Open. 2015;5:e006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyden P, Pryor KE, Coffey CS. NeuroNEXT Clinical Trials Network NN104 Investigators. Final resultsof the RHAPSODY trial: amulti-center, phase 2 trial usinga continual reassessment methodto determinethe safetyand tolerabilityof 3K3A-APC, a recombinant variantof human activated proteinC, in combinationwith tissue plasminogen activator, mechanical thrombectomyor both in moderateto severe acute ischemic stroke. Ann Neurol. 2019;85:125–136.30450637 [Google Scholar]
- 19.Vincent JL, Francois B, Zabolotskikh I. SCARLET Trial Group. Effect of a recombinant human soluble thrombomodulinon mortalityin patients with sepsis-associated coagulopathy: theSCARLET randomized clinical trial. JAMA. 2019;321:1993–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valeriani E, Squizzato A, Gallo A, et al. Efficacy and safety of recombinant human soluble thrombomodulin in patients with sepsis-associated coagulopathy: asystematic review and meta-analysis. J Thromb Haemost. 2020. April 1 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


