Background.
The novel coronavirus severe acute respiratory syndrome coronavirus 2 [coronavirus disease 2019 (COVID-19)] poses unique challenges for immunosuppressed patients. Solid organ transplant (SOT) recipients comprise a large proportion of this group, yet there is limited knowledge about the presentation, clinical course, and immunosuppression management of this novel infection among heart, lung, liver, pancreas, and kidney transplant recipients.
Methods.
We present 21 SOT recipients diagnosed with COVID-19 between January 1, 2020 and April 22, 2020 at a US high-volume transplant center. Diagnostic workup, clinical course, immunosuppression/antiviral management, and immediate outcomes are described.
Results.
Twenty-one (15.9%) of 132 symptomatic patients tested were positive. Mean age at diagnosis was 54.8 ± 10.9 y. Median time from transplant was 5.58 y (interquartile range 2.25, 7.33). Median follow-up was 18 d (interquartile range 13, 30). Fourteen patients required inpatient management, with 7 (50%) placed in the intensive care unit (ICU). All transplant types were represented. Nearly 43% exhibited GI symptoms. Over half (56.2%) presented with elevated serum creatinine suggestive of acute kidney injury. The majority of patients (5/7) with concomitant infections at baseline required the ICU. Eighty percent received hydroxychloroquine ± azithromycin. Ten received toclizumab and/or ribavirin; 1 received remdesivir. Antimetabolites ± calcineurin inhibitors were held or reduced. Over half of hospitalized patients (8/14) were discharged home. Only 1 mortality (4.8%) to date, in a critically ill heart/kidney patient who had been in the ICU before diagnosis.
Conclusions.
COVID-19 positive SOT at our institution had favorable short-term outcomes. Those with concomitant infections had more severe illness. More data will be available to evaluate long-term outcomes and disease impact on graft function.
INTRODUCTION
The novel coronavirus SARS-CoV-2 [coronavirus disease 2019 (COVID-19)] is a highly contagious and devastating virus that has currently infected over 2.5 million people worldwide and resulted in 177 641 deaths as of April 2020.1 Although most people diagnosed with COVID-19 exhibit mild-to-moderate symptoms, early reports from China described vulnerable patient populations, such as the elderly and those with chronic underlying medical conditions including the immunosuppressed, having more severe COVID-19-related illness compared to the general population.2,3 Solid organ transplant (SOT) recipients are one of the largest cohorts of immunosuppressed patients, yet little is known about their risk of contracting the virus, postinfection outcomes, and effect of immunosuppression on the clinical course of the disease. Unique challenges, such as immunosuppression management and interpretation of laboratory data, also exist. Current treatment strategies borrow upon prior experience from other pandemics, such as severe acute respiratory syndrome (SARS) and influenza A virus subtype.4
SARS-CoV-2 primarily affects the respiratory tract, progressing from pneumonia to acute respiratory distress syndrome in severe cases.5 In these cases, there is a recognized cytokine release syndrome (CRS) that when occurs results in multiorgan dysfunction and failure.6 The role of immunosuppression in mounting such inflammatory response is unclear. Inflammatory markers, such as C-reactive protein (CRP), lactate dehydrogenase (LDH), and D-dimer, may reflect disease progression and/or severity.7 Lymphopenia is reported as a common presentation among COVID-19 positive patients.8,9 Treatment options are limited. Antivirals such as hydroxychloroquine (HCQ) with or without azithromycin are widely used empiric options. Remdesivir, an RNA polymerase inhibitor, has shown in vitro activity against SARS-CoV-2 and is currently under phase 3 trial.10 Investigational agents to combat the cytokine response, such as tocilizumab, an interleukin 6 (IL-6) receptor inhibitor, are being studied. Although the exact role of immunosuppression on the progression of COVID-19 is unknown, early case reports of kidney transplant recipients suggest minimizing immunosuppression while continuing steroidal therapy.11
Evaluation of clinical symptoms, utility of biomarkers, and progression of disease are important to understand for optimizing the management in COVID-19 positive SOT recipients. The effect of COVID-19 on the heart, lung, liver, pancreas, and kidney transplant organ systems is not well described. Herein, we present our experience with 21 consecutive SOT recipients diagnosed with COVID-19 at the Houston Methodist J.C. Walter Transplant Center followed to April 22, 2020.
MATERIALS AND METHODS
This is a retrospective review of COVID-19 positive SOT at the Houston Methodist J.C. Walter Jr. Transplant Center in Houston, TX from January 1, 2020 to April 22, 2020. The hospital has an active transplant program with 520 SOTs completed in 2019. The SOT program started in the 1960s, and has completed over 6000 transplants including the heart, lung, liver, kidney, pancreas, islet cell, and all types of multiorgan transplants. Data were initially obtained prospectively and reviewed for the purposes of quality improvement within the transplant center; it was later analyzed retrospectively by the study personnel after obtaining IRB approval (IRB0507-0053). COVID-19 positive cases were identified by the transplant center quality committee and followed clinically by their respective transplant teams and infectious disease specialists. Patient demographics (age, gender, and race), body mass index (BMI), type of organ transplant, time from transplant, comorbidities, angiotensin-converting enzyme inhibitor status (ACEI/ARB), concomitant infections, diagnostic modality, clinical presentation, immunosuppression regimen and subsequent adjustment, diagnostic findings [complete blood count (CBC), liver function tests (LFTs), serum creatinine (SCr), IL-6, CRP, D-dimer, LDH and lactate, and imaging results], clinical course, and treatment modalities were collected.
Descriptive data are reported as median with interquartile range (IQR) or mean ± SD for continuous variables and as frequency and proportion for categorical variables. Statistical analyses were performed using Stata MP v.16.0 (StataCorp LLC, College Station, TX).
Clinical Protocol
SOT recipients were tested for COVID-19 if they exhibited symptoms of fever, cough, and/or shortness of breath (SOB). Diagnostic testing was performed via reverse transcriptase polymerase chain reaction at an institutional laboratory. Patients with concerning symptoms were admitted, and monitored in a COVID-19 unit or ICU until their test returned. If the test was positive, admitted patients remained hospitalized until resolution of symptoms and/or had 2 negative COVID-19 tests. Admission laboratories included CBC, BMP, LFTs, disseminated intravascular coagulation panel, D-dimer, LDH, CRP, IL-6, fibrinogen, and serum triglycerides. Initial chest x-ray (CXR) and/or computed tomography (CT) imaging to evaluate for pneumonia was performed in most patients. Immunosuppression was reduced by holding antimetabolite [mycophenolate mofetil (MMF) or azathioprine] with or without adjustment of calcineurin inhibitors such as tacrolimus (FK) or cyclosporine. FK was adjusted to maintain a trough of 3–7 ng/mL per institutional protocol. Steroids were either kept at the maintenance dose or converted to IV for stress dosing. Administration of HCQ ± azithromycin, ribavirin, toclizumab, remdesivir, nebulized interferon α-2b, anakinra, and convalescent plasma were based on the active institutional algorithm for the treatment of nontransplant COVID-19 positive patients and various investigational study protocols (Figure 1).
FIGURE 1.
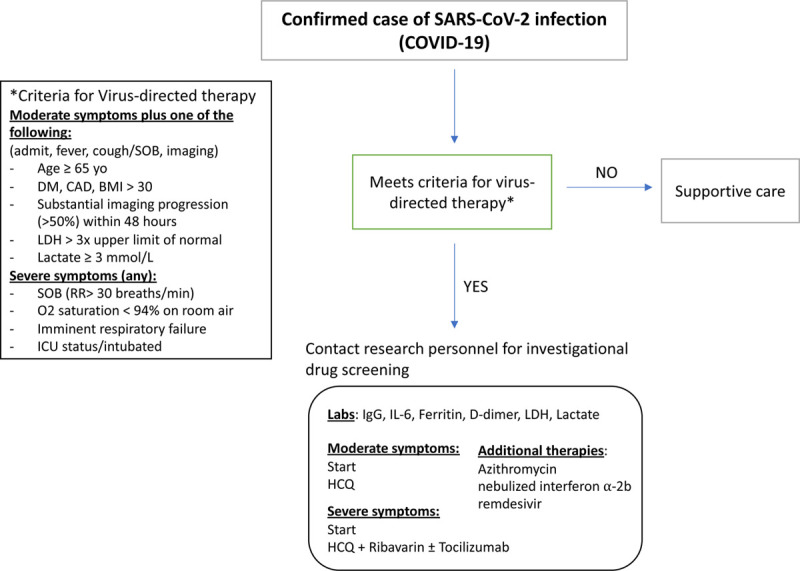
Institutional algorithm for the treatment of COVID-19 positive patients. BMI, body mass index; COVID-19, coronavirus disease 2019; DM, diabetes mellitus; HCQ, hydroxychloroquine; ICU, intensive care unit; IL-6, interleukin 6; LDH, lactate dyhydrogenase; SARS-CoV, severe acute respiratory syndrome coronavirus; SOB, shortness of breath.
COVID-19-specific treatment algorithms were created by a hospital-based multidisciplinary committee designed to standardize treatment protocols and prioritize potential research studies for COVID-19 positive patients at our institution. This group met twice weekly to review hospital data, COVID-19-related patient outcomes, evolving literature, and availability of treatments to adjust protocols as necessary. Based on the committee review, protocol changes were implemented via the hospital electronic medical records, and all medical staff were notified of the changes in the weekly hospital-wide updates. The algorithm divided patients based on the severity of patient symptoms. Moderate symptoms were defined by the presence of fever, cough/SOB and 1 of the following: age ≥ 65 y old, presence of diabetes mellitus (DM), coronary artery disease, obesity (BMI > 30), LDH > 3 times normal, and lactate ≥ 3 mmol/L. Severe symptoms were defined as having 1 of the following: tachypnea (respiratory rate > 30 breaths/min), hypoxia (SpO2 < 94% on room air), respiratory failure, and/or need for ICU admission due to intubation status. Patients with moderate symptoms received HCQ 400 mg twice daily for 2 doses followed by daily for 4 d. Patients with severe symptoms received HCQ at the same dose and ribavirin tapered from 400 mg 3 times daily to 200 mg daily for 10 d. Azithromycin was given depending on the QTc interval, and dosed at 500 mg × 1 dose and 250 mg for 4 d. A CRS grading system initially used at our institution for monitoring chimeric antigen receptor T-cell therapy was adapted for use in COVID-19 patients utilizing the presence of fever, hypotension, and hypoxia as a guide for initiating tocilizumab. In addition, for transplant patients, immunosuppression medical management was adjusted per daily inpatient multidisciplinary review specific to each organ type and in collaboration with infectious disease consultants. ICU patients were managed by the same transplant teams, infectious disease consultants, and intensivist.
Transplant patients who were not admitted were managed by their transplant physicians and infectious disease consultants and instructed to self-isolate, monitor temperature daily, and scheduled for weekly electronic follow-up. Outpatient medications were adjusted by reducing antimetabolite and maintaining an FK trough of 3–7 ng/mL if applicable.
RESULTS
Of the 4100 SOTs actively followed by the Houston Methodist J.C. Walter Jr. Transplant Center, 132 patients were tested for COVID-19 as of April 22, 2020, 35.6% (47/132) of tests were performed in kidney transplant recipients, with fewer performed in the liver (22%, 29/132), lung (15.2%, 20/132), heart (7.6%, 10/132), heart multiorgan (6.8%, 9/132), kidney/pancreas (6.8%, 9/132), and liver multiorgan with kidney (6%, 8/132). Ninety-two percent (121/132) were inpatient tests compared to 8% (11/132) outpatient. Nearly 16% (21/132) of transplant patients tested positive, with 57% (12/21) from kidney transplant recipients. Other COVID-19 positive patients include the liver (3/21), lung (2/21), heart/lung (1/21), liver/kidney (1/21), heart/kidney (1/21), and kidney/pancreas (1/21). These data are summarized in Table 1.
TABLE 1.
Patient demographics
| Characteristics | All patients, n = 21 |
|---|---|
| Age, y | 54.8 ± 10.9 |
| Male, n (%) | 13/21 (62) |
| Race, n (%) | |
| Caucasian | 13/21 (62) |
| African American | 5/21 (24) |
| Hispanic | 3/21 (14) |
| BMI (kg/m2) | 28.1 ± 5.3 |
| Comorbidities, n (%) | 19/21 (90) |
| Diabetes mellitus | 12/19 (63) |
| Hypertension | 16/19 (84) |
| Coronary artery disease | 6/19 (32) |
| Cerebrovascular accident | 1/19 (5) |
| Transplant type, n (%) | |
| Kidney | 12/21 (57) |
| Liver | 3/21 (14) |
| Lung | 2/21 (10) |
| Heart/lung | 1/21 (5) |
| Liver/kidney | 1/21 (5) |
| Heart/kidney | 1/21 (5) |
| Kidney/pancreas (SPK) | 1/21 (5) |
| Time posttransplant (y) | 5.58 (IQR 2.25, 7.33; range 0.42–12.5) |
| Immunosuppression at presentation, n (%) | |
| Tacrolimus (FK) | 20/21 (95) |
| Cyclosporine | 1/21 (5) |
| Mycophenolate mofetil | 17/21 (81) |
| Azathioprine | 1/21 (5) |
| Prednisone | 17/21 (81) |
| Active concomitant infections at presentation, n (%) | 7/21 (33) |
| Inpatient | 6/7 (86) |
| Outpatient | 1/7 (14) |
Data presented as n (%) or median (IQR, range).
BMI, body mass index; FK, tacrolimus; IQR, interquartile range; SPK, simultaneous pancreas and kidney transplant.
Demographics
COVID-19 positive patients had an average age of 54.8 ± 10.9 y old at the time of diagnosis. Five patients were over the age of 65, with the oldest at 73 y old. About 62% (13/21) of the COVID-19 positive recipients were male. The majority of cases occurred in Caucasian patients (62%, 13/21). The average BMI was 28.1 ± 5.3 kg/m2. The median time from transplant was 5.58 y (IQR 2.25, 7.33). A heart/kidney patient was the most recent from transplant at 0.42 y, and was diagnosed during the index transplant hospitalization. The majority of the SARS-CoV-2 patients (90%, 19/21) had at least 1 comorbidity such as hypertension, DM, obesity, chronic lung disease, and cardiovascular disease. Only 2 patients (2/21) were on ACE inhibitors. Eighty-one percent (17/21) of patients were on triple maintenance immunosuppression involving a calcineurin inhibitor (FK or cyclosporine), an antimetabolite (MMF or azathioprine), and prednisone. At the time of diagnosis, 7 patients (7/21) had concomitant infections.
Clinical Presentation
Of the 21 patients who tested positive, 95.2% (20/21) presented with fever, cough, and/or SOB. This group included 9 patients (42.9%) who also presented with GI symptoms such as diarrhea, vomiting, and abdominal pain. Other secondary symptoms included encephalopathy (1), hallucinations (1), dysosmia and dysgeusia (1), and hypercoagulability with ischemic limb (1). The majority (66.7%) of patients tested positive on the first test, and of the remaining 33%, only 1 patient was positive on a third attempt. Diagnostic imaging (CXR and/or CT scan) were obtained in 16 patients. One patient, who had a prior kidney transplant, presented with fever and GI symptomatology and was tested for COVID-19 after a chest infiltrate suspicious for COVID-19 pneumonia was identified on CXR and abdominal CT. Imaging results from 6 patients (6/16) did not reveal any acute findings suggestive of COVID-19 pneumonia. The remaining 10 patients had imaging demonstrating ground-glass opacities and/or infiltrates with multilobar involvement. These data are summarized in Table 2.
TABLE 2.
Clinical presentation
| Symptoms on presentation, n (%) | 20/21 (95) |
| Fever | 18/21 (86) |
| Respiratory | 11/21 (52) |
| Cough | 8/11 (73) |
| Shortness of breath | 7/11 (64) |
| Dyspnea on exertion | 2/11 (18) |
| Fatigue | 5/21 (24) |
| GI symptoms | 9/21 (43) |
| Diarrhea | 6/9 (67) |
| Abdominal pain | 3/9 (33) |
| Emesis | 2/9 (22) |
| CNS | 5/21 (24) |
| Headache | 3/5 (60) |
| Encephalopathy/hallucinations | 2/5 (40) |
| Dysosmia/dysgeusia | 1/21 (5) |
| DVT | 1/21 (5) |
| Asymptomatic | 1/21 (5) |
| Timing of positive test, n (%) | |
| First attempt | 14/21 (67) |
| Second attempt | 6/21 (29) |
| Third attempt | 1/21 (5) |
| Radiographic imaging, n (%) | 16/21 (76) |
| Multifocal/bilateral patchy opacities or infiltrates | 10/16 (63) |
| No acute findings | 6/16 (37) |
Data presented as n (%) or median (IQR, range).
CNS,; DVT,; GI,; IQR, interquartile range.
Disease Course
Seven patients (7/21), who had lung/heart, liver, or kidney transplants, had mild disease and were treated as outpatients. The 14 hospitalized patients included 8 kidney transplant recipients (57.1%), 2 lung (14.3%), 1 liver (7.1%), 1 kidney/pancreas (7.1%), 1 liver/kidney (7.1%), and 1 heart/kidney (7.1%). All 14 inpatients and 2 outpatients had transplant protocol laboratory tests at the time of COVID-19 testing. Laboratory data are summarized in Table 3. The median WBC on admission was 6.4 k/µL (IQR 3.8, 8.5, range 1.9–14.6) with a median absolute lymphocyte count of 524.5 cells/mm3 (IQR 335, 845). Median CRP was 11.8 mg/dL (IQR 5.2, 23.2), which was >5 mg/dL in 83% (10/12). Median D-dimer was 1.46 µg/mL (IQR 0.57, 2.98). IL-6 ranged from <1 to 1081 pg/mL, and was abnormal in 75% (9/12) patients. One liver/kidney patient had an IL-6 of 1081 pg/mL, but had concomitant soft tissue infections with mucormycosis and pseudomonas of the extremities before COVID-19 diagnosis. For all patients, LFTs were normal, except in 1 kidney transplant recipient, who presented with acute hepatitis. Lactate levels were elevated in only 2 patients (heart/kidney and liver/kidney), both of whom had clinical evidence of sepsis. Median LDH was 253 (IQR 210, 321). Median SCr at presentation was 1.7 mg/dL (IQR 1.1, 3.3, range 0.8–7.6). Eleven patients (68.8%) had elevated SCr suggestive of acute kidney injury (AKI). This number did not include the heart/kidney patient who was diagnosed during the index transplant admission and was on maintenance dialysis before and after testing for COVID-19.
TABLE 3.
Medical management and hospital course
| Hospitalization, n (%) | 14/21 (67) |
| Kidney | 8/14 (57) |
| Lung | 2/14 (14) |
| Liver | 1/14 (7) |
| Kidney/pancreas | 1/14 (7) |
| Liver/kidney | 1/14 (7) |
| Heart/kidney | 1/14 (7) |
| Admission location | |
| ICU | 7/14 (50) |
| Ventilatory support | 5/7 (71) |
| Inpatient floor | 7/14 (50) |
| Laboratory tests on admission, n (%) | 16/21 (76) |
| WBC, ×1000/µL (n = 16) | 6.4 (IQR 3.8, 8.5; range 1.9–14.6 |
| Absolute lymphocyte count, cells/mm3 (n = 16) | 524.5 (IQR 335, 845; range 237–1922) |
| C-reactive protein, mg/dL (n = 12) | 11.8 (IQR 5.2, 23.2; range 1.56–>30) |
| D-dimer, µg/mL (n = 11) | 1.46 (IQR 0.57, 2.98; range 0.56–8.72) |
| IL-6, pg/mL (n = 12) | 21 (IQR 5,71.5; range <5–1081) |
| Lactate, mmol/L (n = 14) | 1.7 (IQR 1.4, 2.1; range 0.8–3.9) |
| Lactate dehydrogenase, U/L (n = 11) | 253 (IQR 210, 321; range 110–370) |
| Serum creatinine, mg/dL (n = 16) | 1.8 (IQR 1.13, 3.28; range 0.78–7.58) |
| Immunosuppression management | |
| Antimetabolite held | 12/14 (86) |
| Calcineurin inhibitor reduced | 3/14 (14) |
| Increased baseline steroids | 3/14 (5) |
| Antiviral therapy, n (%) | 12/21 (57) |
| Hydroxychloroquine with azithromycin | 7/12 (58) |
| Hydroxychloroquine without azithromycin | 2/12 (17) |
| Azithromycin without hydroxychloroquine | 2/12 (17) |
| Ribavirin | 6/12 (50) |
| Remdesivir | 1/12 (8) |
| Convalescent plasma | 1/12 (8) |
| Immunomodulating therapy, n ($%) | 4/21 (19) |
| Toclizumab | 4/4 (100) |
| Nebulized interferon alpha | 1/4 (25) |
| Anakinra | 1/4 (25) |
| Patient disposition | |
| Deceased | 1/21 (5) |
| Discharged home | 8/14 (57) |
| Remains hospitalized | 6/14 (43) |
| Length of stay if discharged, d | 6 (IQR 4, 11) |
| ICU d | 11 (IQR 7, 15) |
| Follow-up to datea | 18 d (IQR 13, 30) |
aAs of April 22, 2020.
Data presented as n (%) or median (IQR) or median (IQR with range).
ICU, intensive care unit; IL-6, interleukin 6; IQR, interquartile range.
Of the 14 patients admitted, 7 (50%) were admitted to the ICU and 5 of the ICU patients (71.4%) required ventilatory support (Table 3). One of these patients was the critically ill heart/kidney recipient, who had a tracheostomy and was on the ventilator before being diagnosed with COVID-19. Those requiring ICU monitoring consisted of kidney (3/7), heart/kidney (1/7), kidney/pancreas (1/7), liver/kidney (1/7), and lung transplant (1/7) recipients. Immunosuppression was adjusted by reducing or holding MMF or azathioprine (12/14) and maintaining baseline steroid dose. For the inpatients, FK was kept at a trough level between 3 and 7 ng/mL, and only 3 patients required dose reduction to reach this goal. One patient (liver/kidney) was administered high-dose steroids. Azithromycin ± HCQ were administered in 11 of the 14 hospitalized patients and in 1 outpatient. Other than the heart/kidney patient, there were no deaths in this group. Tocilizumab was given to 4 patients (3 kidney and 1 kidney/pancreas), 3 of whom were in the ICU and 1 was on the inpatient floor. There were no deaths in the tocilizumab group. Of the 4 patients who received tocilizumab, 1 ICU patient and the 1 inpatient did not require ventilatory support. That floor patient was discharged home after 10 d in the hospital. Remdesivir was given to a liver transplant patient. This patient did not require ICU admission and was discharged home after 5 d. Six patients received ribavirin. One patient, who had a prior kidney transplant, received nebulized interferon α-2b. This patient remains intubated in the ICU. Another patient, who had a kidney/pancreas transplant, received anakinra; but, as the patient’s condition worsened, the patient received convalescent plasma, under emergency use authorization/emergency investigational new drug. This patient is currently extubated but continues to be monitored in the ICU.
To date, only 1 patient has expired. This patient was the heart/kidney transplant recipient who had an atypical and prolonged postoperative transplant course before his COVID-19 diagnosis. Four months into a hospitalization for heart failure, the patient received a combined heart and kidney transplant, but required venoarterial extracorporeal membrane oxygenation, followed by intra-aortic balloon pump support, vasopressors, and dialysis for delayed graft function. He required a tracheostomy and prolonged ventilatory support, eventually developing escherichia coli ESBL pneumonia. He was deconditioned because of prolonged immobilization. Two months after his transplant, the patient developed fungal infiltration of his heart allograft and continued to require dialysis. After intermittent fevers and a suspicious sick contact, the patient was tested for COVID-19. At the time of diagnosis, his D-dimer was elevated at 6.45 µg/mL and he had lymphopenia with an absolute lymphocyte count 297 cells/mm3. He received HCQ and ribavirin. The patient expired 7 d after diagnosis.
Of the remaining 6 patients in the ICU, 2 have been discharged home, and 4 continue to be managed in the ICU. The remaining 6 inpatients have been discharged home. The median length of stay for those discharged was 6 d (IQR 4, 11). The median ICU days to date was 5 d (IQR 7,15). All 7 outpatients did not require hospitalization and continued to be monitored as an outpatient. Median follow-up days to date for all patients was 18 d (IQR 13, 30).
DISCUSSION
In this case series, we describe 21 consecutive SOT recipients who were diagnosed with COVID-19. The majority of these patients had relatively favorable short-term outcomes, with a mortality rate of 4.8% and nearly 50% of inpatients discharged home. All nonhospitalized patients were successfully managed in the outpatient setting. This mortality rate is reflective of the US patients in the general population, which is currently estimated to be around 1%–11%.12
Our preliminary outcomes show a lower mortality rate compared to recently published series of kidney transplant recipients alone and SOT from the UK, New York (Montefiore, Columbia, and Cornell), and Madrid, which reported 14%, 13%–28%, and 27.8% rates, respectively.8,13-15 The locations of these 4 centers had significantly higher number of COVID-19 cases to date in the general population compared to Houston (approximately 143 464, 75 795, and 158 000, respectively, versus 5729).16,17 In New York, the epicenter of the COVID-19 outbreak in the United States, a multicenter report of SOT from Columbia and Cornell observed worse outcomes in transplant patients compared to nontransplant patients, with higher rates of severe disease and mortality among those hospitalized.14
There are several differences among our cohort of patients compared to these other published reports.11,13-15 The case series with the highest mortality rates, Madrid and Montefiore, described patients with higher median age of 71 and 60 y, respectively, compared to 54.8 y at our center. Our hospitalization rate was somewhat lower than that seen in Montefiore (66.7% versus 78%),8 suggesting earlier presentation and/or diagnosis in our patients. Although respiratory insufficiency alone was associated with worse outcomes in the Columbia and Cornell reports,14 in our patients, concomitant infections had the highest need for ICU care and worse outcomes.
Similar to the published reports,13-15 measured inflammatory markers were uniformly elevated on presentation in most patients. There did not appear to be a correlation between inflammatory markers and patient outcomes. Lymphopenia, a common finding in nontransplant COVID-19 patients,8,9 was also present in our cohort and in the kidney transplant patients from Montefiore. Despite the prevalence of lymphopenia, its significance in transplant patients outcome or disease progression remains unknown.
Unlike the COVID-19-related risk factors associated with severe illness in the general population18 and observed by the Columbia and Cornell experience,14 both age ≥65 y and the presence of comorbidity did not appear to play a factor in prognosis for our cohort. Of the 5 patients ≥65 y old at our center, 3 were admitted, with 1 patient admitted to the ICU without ventilatory support after transfer from an outside hospital. There were no deaths in these older patients. At-risk comorbidities, such as hypertension, DM, obesity, chronic lung disease, and cardiovascular disease,2,9,18 were present in 90% of our transplant patients, suggesting comorbidities did not drive hospitalization or severity of illness. Additionally, over half (4/7) of the obese patients (BMI ≥ 30 kg/m2) in our cohort were treated in the outpatient setting.
We also observed 73% of patients presented with elevated SCr suggestive of AKI (11/15). Although the majority of our COVID-19 positive patients had kidney transplants (57%), 3 of the 4 patients who did not have elevated SCr were kidney transplant recipients. Our observed trend of elevated SCr is higher than the 15%–29% reported of the general population19 and the 40% and 57% observed in case series of kidney transplant recipients from Columbia13 and the UK.11 Such observation may reflect the potential association between SARS-CoV-2 uptake via ACE2 into the proximal tubular epithelium of the kidney, thus increasing the risk for AKI.11
Despite uncertainty and lack of evidence regarding the optimal management of COVID-19 in transplant patients, our approach to management focused primarily on early diagnosis and treatment, with reduction of immunosuppression. Before the onset of COVID-19 positive cases in our region, we devised a testing protocol for our SOT that would be performed in the outpatient setting at our transplant center or in an isolated area in the hospital after hours. Patients were instructed to wear masks and self-isolate. Similar to the Columbia experience,13 we utilized telemedicine to aid with triage of symptomatic patients and with follow-up during self-isolation. If patients exhibited symptoms suggestive of COVID-19 (ie, fever, cough, and SOB), they were tested and assigned to a designated transplant COVID-19 isolation unit or home depending on the severity of their symptoms. Antiviral therapy was initiated early following a positive test for inpatients. Most hospitalized patients in the early part of our experience received HCQ ± azithromycin, although the use of azithromycin has since been removed from our most current protocol and HCQ is now restricted mostly to clinical trials. Patients with symptom progression were immediately evaluated for enrollment in clinical studies if applicable. Although early case series reported withholding FK across patients20 or in the critically ill,8 we opted to keep FK at low levels, as there is experimental evidence that calcineurin inhibitors may inhibit coronavirus replication.21 We have also continued maintenance steroid dosing, reserving high dose steroids for critically ill and deteriorating patients. Several of our patients, who exhibited severe symptoms on presentation, received immunomodulatory therapy, such as tocilizumab. One patient received remdesivir. The short-term outcomes from these agents have been positive, with no adverse events (such as infection) or deaths and 1 patient from each group discharged home.
The effect of immunosuppression on the progression of COVID-19 is unclear, and may be dependent on the severity of disease. Our institutional algorithm borrows from the theoretical framework by Siddiqi et al22 that the pathologic response to COVID-19 consists of 2 phases: a viral phase and a host inflammatory phase. In the early viral phase, host autoimmunity is important for recovery against viral infection. This is similar to the management of cytomegalovirus or BK virus in SOT, where in reduction of immunosuppression is necessary to combat viral replication. In the host response phase, immunosuppression may be beneficial in reducing the inflammatory sequalae of the cytokine response, which can otherwise lead to multiorgan dysfunction and failure.6 Use of cytokine inhibitors may be potentially beneficial at this later stage. Although there may be a concern that immunosuppression may increase the risk of secondary infection, we did not observe this as none of our inpatients developed subsequent infection. It is intriguing to speculate that some transplant patients may not progress to CRS because of their present immunomodulatory state related to their long-term immune suppression, although this hypothesis needs further investigation to substantiate.
This paper is a retrospective review of 21 COVID-19 positive SOTs at a US high-volume transplant center. Although we are able to describe our patients and provide short-term outcomes, we are unable to make any definitive conclusions regarding long-term outcomes of our treatment strategies or in this patient population given the limitations of a single-center observational study. Additionally, there was no standardized treatment protocol for COVID-19 positive patients, as our center protocols are constantly adjusted based on new data from the growing number of COVID-19 published reports. These limitations will be improved upon in future publications, as our experience with COVID-19 continues to grow.
Future studies will include evaluation of graft function and rejection risk via monitoring of donor-specific antibodies, long-term impact of antivirals, immunomodulatory therapy with tocilizumab, nebulized interferon alpha or anakinra on inflammatory markers and disease progression, and comparison of COVID-19 outcomes between transplant versus nontransplant patients.
ACKNOWLEDGMENTS
The authors acknowledge The Houston Methodist Hospital COVID-19 clinical protocol committee, Dr Jenny Cheng, MD, for her role in COVID-19 protocol development, and The J.C. Walter Jr. Transplant Center nurses, coordinators and staff who worked tirelessly ensuring the safety and wellbeing of our transplant patients.
Footnotes
The authors declare no funding or conflicts of interest.
S.G.Y., A.W.R., A.S., and M.A. were involved in research design. S.G.Y., A.W.R., M.A., R.F., and S.B. were involved in performance of research and data acquisition. S.G.Y., A.W.R., and A.O.G. were involved in data analysis and interpretation.
S.G.Y. and A.S. were involved in writing of this paper. R.J.K., K.G., H.J.H., A.B., R.M.G., A.O.G., C.M., M.M., M.H., and R.M. were involved in critical review of this paper.
REFERENCES
- 1.World Health Organization. Coronavirus disease 2019 (COVID-19): situation report, 72. 2020; 72 [Google Scholar]
- 2.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020; 395:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu W, Tao ZW, Lei W, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chinese Med J. 2020; 133:1032–1038. doi:10.1097/CM9.0000000000000775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gainer SM, Patel SJ, Seethamraju H, et al. Increased mortality of solid organ transplant recipients with H1N1 infection: a single center experience. Clin Transplant. 2012; 26:229–237. doi:10.1111/j.1399-0012.2011.01443.x [DOI] [PubMed] [Google Scholar]
- 5.Wilder-Smith A, Chiew CJ, Lee VJ. Can we contain the COVID-19 outbreak with the same measures as for SARS? Lancet Infect Dis. 2020; 20:e102–e107. doi:10.1016/S1473-3099(20)30129-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020; 368:473–474. doi:10.1126/science.abb8925 [DOI] [PubMed] [Google Scholar]
- 7.Velavan TP, Meyer CG. Mild versus severe COVID-19: Laboratory markers. Int J Infect Dis. 2020; 95:304–307. doi:10.1016/j.ijid.2020.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 20202374; 382(24):2372 doi:10.1056/NEJMc2010419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323:1061–1069. doi:10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020; 382(24):2327–2336. doi:10.1056/NEJMoa2007016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banerjee D, Popoola J, Shah S, et al. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020; 97:1076–1082. doi:10.1016/j.kint.2020.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020; 69:343–346. doi:10.15585/mmwr.mm6912e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Columbia University Kidney Transplant Program. Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol. 2020; 31:1150–1156. doi:10.1681/asn.2020030375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020; Apr 24. 10.1111. doi:10.1111/ajt.15941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Eng J Med. 2020; 382:2475–2477. doi:10.1056/NEJMc2011117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Coronavirus disease (COVID-2019) situation reports. 2020. Available at https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200423-sitrep-94-covid-19.pdf?sfvrsn=b8304bf0_4. Accessed April 23, 2020
- 17.Harris County Public Health. Harris County/Houston COVID-19 cases. Available at http://publichealth.harriscountytx.gov/Resources/2019-Novel-Coronavirus. Accessed April 27, 2020
- 18.Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 states, March 1-30, 2020. Morb Mortal Wkly Rep. 2020; 69:458–464. doi:10.15585/mmwr.mm6915e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020; 8:475–481. doi:10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu L, Xu X, Ma K, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant. 2020; 20:1859–1863. doi:10.1111/ajt.15869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka Y, Sato Y, Sasaki T. Suppression of coronavirus replication by cyclophilin inhibitors. Viruses. 2013; 5:1250–1260. doi:10.3390/v5051250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020; 39:405–407. doi:10.1016/j.healun.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]


