Both angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) have repeatedly, but not consistently, been documented to slow progression of pulmonary complications in vulnerable patients. A previously published meta-analysis pointed toward a putative protective role of ACEIs, and to lesser extent of ARBs, in risk of community acquired pneumonia. ACEIs in 7 studies were associated with decreased pneumonia mortality compared with control treatment (odds ratio [OR] 0.66 [95% CI, 0.55–0.80]). ARBs in 1 randomized controlled trial only showed a reduction of borderline significance (OR 0.63 [95% CI, 0.40– 1.00]).1 These seemingly beneficial findings of renin angiotensin system (RAS) blockade on outcomes in pneumonia resurfaced in the recent literature in relation to severe acute respiratory syndrome coronavirus 2 (SARS–CoV-2), also known as coronavirus disease 2019 (COVID-19), infection. Several potential therapeutic/preventive approaches to address angiotensin-converting enzyme 2 (ACE2)-mediated COVID-19 have been described, including the suggestion that ARBs could be administered in the form of a nasal spray to treat COVID-19. Because pneumonia is a common potentially fatal complication in COVID-19 infection, we wondered whether RAS blockade could exert a favorable effect on pneumonia-related outcomes.
Evidence of ACEI/ARB Therapy and Viral Infections of Respiratory System
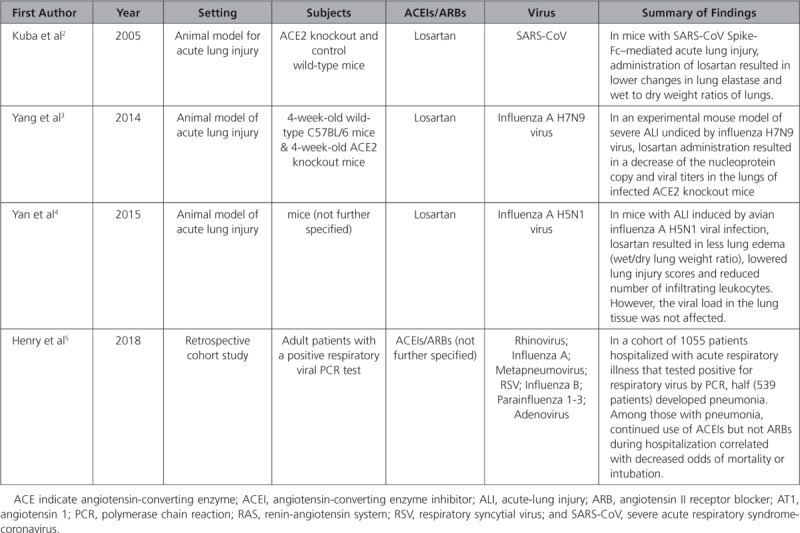
We systematically reviewed the literature for evidence from animal models and clinical data related to the role of ACEIs/ARBs and viral infections. We searched PubMed and EMBASE for original research on animals or humans investigating the impact of ACEIs/ARBs on viral infections. The following search terms were used: viral, virus, infect, pneumonia, acute respiratory distress syndrome, acute lung injury, and angiotensin. Overall, we identified 4 studies2–5 evaluating the role of ACEIs/ARBs in viral infections of respiratory system. In 3 studies of animal models,2–4 virus-induced lung injury and the role of losartan was tested; all reported a key role of decreased RAS activation through losartan (Table). In 1 retrospective study,5 lower rates of death and intubation were noted among patients who continued to be on ACEIs during hospitalization (OR 0.25 [95% CI, 0.09–0.64]). We did not find evidence supporting the continuation, withdrawal, or de novo initiation of ACEIs/ARBs in patients with SARS-CoV-2-infection.
Table.
Studies on Animal Models and Humans Evaluating the Role of ACEIs/ARBs in Viral Infections of Respiratory System
COVID-19 and Hypertension
Epidemiological data suggest that patients with cardiovascular disease and hypertension are more susceptible to SARS–CoV-2-infection and that the course of pneumonia is more severe in hypertensive relative to normotensive subjects. COVID-19–associated pneumonia death cases were marked by more comorbidities, such as cardiovascular disease (22.7%) and hypertension (39.7%). Considerably higher is the prevalence of these comorbidities in black patients. However, susceptibility for infection and complications may be explained by advanced age and multiple comorbidities in many patients. Two large independent studies of influenza type A H1N1 have documented a higher prevalence of hypertension and diabetes. Thus, confounding is likely to be extensive.
Possible Mechanisms
SARS–CoV-2, the recently identified strain responsible for the current COVID-19 epidemic, relies on ACE2 protein as a cellular entry receptor by binding with its spike protein, similar to SARS-CoV. ACE2 participates in a pathway that is counter-regulatory to the effects of angiotensin II, and the cardiorenal protective effect of ARBs may be attributable in part to increased metabolism of angiotensin II by ACE2. Importantly, ACE2 expression protects from lung injury, an action which is downregulated by binding of SARS-CoV via its spike protein.
Experimentally and in humans, RAS blockade has been shown to upregulate ACE2 activity, thereby potentially antagonizing some effects of COVID-19. Injection of SARS-CoV spike protein into mice worsens acute lung failure in vivo that can be attenuated by inhibiting the RAS pathway.2 Conversely, speculation has been put forward that the enhanced ACE2 expression with RAS antagonists might increase the number of binding sites thereby increasing the odds of infection with SARS–CoV-2.
Clinical Implications
Given that ACE2 is a cellular entry receptor for SARS–CoV-2, careful evaluation of efficacy and safety of antihypertensive therapy with RAS blockade is needed. There is experimental and clinical evidence that RAS blockade can mitigate pulmonary outcome in some forms of viral pneumonia. Presently, there are no data regarding a favorable effect of RAS blockade on pulmonary outcome in SARS–CoV-2-infected patients. Whether or not infectivity to viral infection is increased in patients treated with RAS blockers remains unknown. However, recent news media coverage of this issue has provoked concern and unfortunately even motivated some patients to discontinue RAS blockers all together. Of note, the RAS effects among available blockers is markedly different. Direct renin inhibitors have been shown to diminish activity of the RAS and also downregulate ACE2 in animal models. Thus with direct renin inhibitors, RAS blockade and cardiopulmonary protection is maintained, but ACE2 seems to be downregulated
In general, RAS blockers are efficacious and well-tolerated drugs with few adverse side effects. However, once fever and dehydration set in, the RAS will become upregulated to the extent that its blockade can result in profound hypotension, syncope, cardiovascular collapse, renal failure, and shock. More often than not, this sequence of events occurs unexpectedly and is abrupt.
Recommendations
Presently, all guidelines recommend continuing ACEI/ARBs in patients diagnosed with COVID-19 infection. Until the evidence in aggregate becomes firmer (and it will), we recommend the following with regard to COVID-19 and RAS blockade:
In noninfected patients and patients at risk, there is currently no valid reason to discontinue RAS blockade.
In healthy subjects at risk, evidence is not (yet?) sufficient to prophylactically recommend RAS blockade.
If apprehension about increased infectivity persists, patients on ACEIs or ARBs could temporarily be switched to a direct renin inhibitor.
In COVID-19–positive patients on RAS blockers, the drugs should be continued.
In febrile patients with pulmonary symptoms on RAS blockers, close monitoring of blood pressure and renal function is advisable; RAS blockers should be discontinued only as clinically indicated.
Conclusions
Cardiovascular disease in general, and hypertension and diabetes mellitus specifically, are prevalent in SARS–CoV-2–infected patients. RAS blockers are extensively used for cardiovascular disease and hypertension therapy. There is inconsistent evidence to suggest that RAS blockers exert a favorable effect on pulmonary outcome in viral pneumonia, but no data are available specifically for SARS–CoV-2–infected patients. Conversely, patients with cardiovascular disease and hypertension seem to be prone to SARS–CoV-2-infection, although this most likely is attributable to confounding factors. RAS blockade should be continued in infected patients as clinically indicated. Present evidence is insufficient to recommend use of RAS blockade prophylactically in subjects at risk or therapeutically in those infected with SARS–CoV-2.
Disclosures
None.
Footnotes
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
On April 17, 2020, the ahead-of-print version of this article was revised. The authors revised the first paragraph of the article with an update concerning the difference in the risk of pneumonia in patients who were taking ARBs versus those who were not. Also, the reference found in the second paragraph of page 4 was been revised.
References
- 1.Caldeira D, Alarcão J, Vaz-Carneiro A, Costa J. Risk of pneumonia associated with use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers: systematic review and meta-analysis. BMJ. 2012;345:e4260. doi: 10.1136/bmj.e4260. doi: 10.1136/bmj.e4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang P, Gu H, Zhao Z, Wang W, Cao B, Lai C, Yang X, Zhang LY, Duan Y, Zhang S, et al. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci Rep. 2014;4:7027. doi: 10.1038/srep07027. doi: 10.1038/srep07027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan Y, Liu Q, Li N, Du J, Li X, Li C, Jin N, Jiang C. Angiotensin II receptor blocker as a novel therapy in acute lung injury induced by avian influenza A H5N1 virus infection in mouse. Sci China Life Sci. 2015;58:208–211. doi: 10.1007/s11427-015-4814-7. doi: 10.1007/s11427-015-4814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henry C, Zaizafoun M, Stock E, Ghamande S, Arroliga AC, White HD. Impact of angiotensin-converting enzyme inhibitors and statins on viral pneumonia. Proc (Bayl Univ Med Cent) 2018;31:419–423. doi: 10.1080/08998280.2018.1499293. doi: 10.1080/08998280.2018.1499293. [DOI] [PMC free article] [PubMed] [Google Scholar]


