Abstract
This study experimentally evaluates the performance of different sorbent tubes for sampling acetone vapor in workplace air. A dynamic atmosphere system produced an acetone alone and a mixture with other analytes containing ~73, 483, and 1898 μg acetone mass loading at 25, 50, and 75% relative humidity (RH) at 25°C. Sorbent samples were analyzed in accordance with OSHA Method 69 (Carbosieve S-III) and NMAM 1501, modified to use Anasorb 747 sorbent. Both methods were modified to include the additional analytes. Additional extraction procedures with and without 1% dimethylformamide and anhydrous magnesium sulfate were included in the modified NMAM 1501 using Anasorb 747. Silica gel sorbent tubes analyzed according to NMAM 2027 were included. There were significant reductions in the recovery of acetone from both Anasorb 747 and Carbosieve S-III collected from air at 75% RH, relative to collection at 25 or 50% RH at very low loading compared with that of samples collected at mid to high loading. Silica gel provided a consistent recovery of acetone at all RHs and in the presence of other chemical interferences at 75% RH. The likely cause of mass dependence may arise from the humidity effect on acetone adsorption onto both beaded active carbon and carbon molecular sieve either in sampling or in analysis. The present study confirms not only previous observations but also adds to the literature showing carbonaceous sorbents are not well suited for sampling ketones at high humidity and low concentration.
Keywords: acetone, beaded active carbon, carbon molecular sieve, silica gel, sorbent tube sampling
Introduction
A convenient and popular approach to sampling volatile organic compounds (VOCs) in breathing zones is to draw air through a tube containing a bed of sorbent, most often a porous adsorbent (Harper et al., 2000). The tube and inner sorbent typically weigh only a few grams and are unobtrusive when clipped to workers’ clothes. At the end of the sampling period the adsorbed VOCs are separated from the solid adsorbent by one of two methods, either through solvent extraction or through thermal desorption. Solvent desorption is the most frequently employed method of sample recovery. Thermal desorption has become popular in some countries, but this technique can present problems for the sampling and analysis of highly volatile, reactive or unstable compounds, such as acetone.
Coconut shell charcoal was initially recommended by the National Institute for Occupational Safety and Health (NIOSH) Manual of Analytical Methods (NMAM) 1300 for the measurement of acetone, but this was based on preexisting methods where no account had been taken of relative humidity in the sampled air (NIOSH, 1994). As it became obvious that relative humidity had an important effect on the sampling of polar compounds on coconut charcoal, alternatives were sought (Harper et al., 2000). Carbon molecular sieves (Anasorb CMS and Carbosieve S-III) were evaluated in NMAM 2555, and in the Occupational Safety and Health Administration (OSHA) Analytical Method 69, respectively for the determination of acetone using extraction by carbon disulfide (CS2) alone or with a cosolvent. Presently, however, Anasorb CMS (carbon molecular sieve) is no longer commercially available. Carbosieve S-III has a fine mesh-size, limiting the range of flow-rates that can be used (OSHA, 1988; NIOSH, 2003).
The effect of the adsorption of water vapor on all aspects of the sampling and analysis of polar molecules is of such importance as to require further investigation. The cocollection of water vapor by the sorbent is important because the concentration of water molecules can outnumber the sampled organic vapor molecules by thousands-to-one at high humidity. This can cause three problems with the sampling and analysis: (i) the water molecules may displace collected organic vapor molecules, potentially leading to premature breakthrough and loss of sample, (ii) the water molecules may be displaced by the desorbing solvent into an immiscible aqueous phase into which polar molecules can partition and be lost to the analysis, and (iii) the water molecules can be involved in reactions with organic molecules on the sorbent surface leading to storage losses. Harper et al. evaluated the effect of humidity on the breakthrough of acetone and 2-butanone collected by three different sorbents, including Anasorb 747 (beaded carbon), Anasorb CMS, and active charcoal (Lot 120), and they reported that no displacement of VOCs by water vapor was observed with the Anasorb CMS. They also noted that losses by reaction on storage, as well as migration of adsorbed molecules between the front and rear sections of the tube, can be slowed through refrigeration of the samples (Harper et al., 1993). Per OSHA Method 69, adding a cosolvent (1% dimethylformamide) to the carbon disulfide (CS2), and a drying agent (anhydrous magnesium sulfate) are recommended for desorbing acetone samples to improve recovery. However, the additional extraction procedure is a considerable burden on the analyst and inevitably increases the cost of the analysis. In fact, it seemed possible that Anasorb 747 could be used without the cosolvent and drying agent required in the OSHA Method. This work led to the selection of Anasorb 747 for the sampling of 2-butanone according to NMAM 2500 (NIOSH, 1996). Recently, silica gel was adopted in NMAM 2027 to be used for sampling ketones, with recovery by a ternary solvent mixture of methylene chloride/methanol/water (65:33:2) (NIOSH, 2016).
As knowledge of the toxicity of chemicals improves, lower occupational exposure limits are often set and this requires sorbent sampling under conditions of low levels of interferences and improved analyte recovery. All three currently available adsorbents suggested for sampling acetone were selected in this study to confirm the method recovery from test atmospheres at various relative humidities (RHs) and concentration levels (mass loadings). In addition, modifications were made to the analytical procedures with the carbon sorbents to determine whether the cosolvent and drying agent were necessary requirements of the methods. Additional benefits resulting from this study include the incorporation of a single acetone method into multianalyte methods and updating of information on the performance of carbonaceous sorbents for sampling ketones at high humidity and low concentration.
Experimental
Sampling of test atmosphere
Three different sorbent tubes, Anasorb 747 (Cat. No. 226–83, SKC, Inc., Eighty Four, PA, USA), ORBO91 Carbosieve S-III (Cat. No. 20360, Supelco Sigma– Aldrich, Inc., Bellefonte, PA, USA), and silica gel (Cat. No. 226–10-03, SKC, Inc.) were used to sample from a glass test chamber (~0.004 m3), which was placed in a 22-m3 walk-in environmental chamber (Nor-Lake Enviroline; Nor-Lake Scientific, Hudson, WI, USA) similar to that previously described (Coffey et al., 2012; LeBouf et al., 2013; Soo et al., 2018). Three levels of relative humidity (25, 50, and 75% RH) at 25°C were maintained through a Miller-Nelson flow-temperature-humidity control system (Model HCS-501, Assay Technology, Inc., Livermore, CA, USA). Two exposure scenarios were selected. First, the dynamically controlled test atmospheres containing ~10, 60, and 260 ppm acetone concentration (73, 438, and 1898 μg mass loading), which are anticipated to be found in the workplace air, were generated at various humidities, by using a certified specialty gas mixture of acetone with nitrogen as balance (Part No. BL1810125, Ideal Speciality Gases and Analytical Services, Houston, TX, USA) controlled by mass flow controller (Aalborg Instruments, Inc., Orangeburg, NY, USA), to evaluate the performance of sorbent tubes with and without drying agent/cosolvent during the solvent desorption procedure. Second, a known concentration mixture of seven analytes of interest (including acetone) was selected to evaluate the effect of chemical interference by using a certified specialty gas mixture with nitrogen as balance (Part No. BL1607134, Ideal Speciality Gases and Analytical Services) controlled by mass flow controller. The other six analytes (ethylbenzene, methyl isobutyl ketone [MIBK], toluene, m-xylene, p-xylene, and o-xylene) are commonly present in paint manufacturing industries. The proportions of the seven selected analytes in the undiluted test gas mixture reflected the relative levels of OSHA’s permissible exposure limit (PEL) for the individual compounds; initial concentrations of each compound were 1000 ppm ± 2% for acetone, 200 ppm ± 2% for toluene, and 100 ppm ± 5% for the other compounds. The final concentration was ~100 times lower (10 ppm acetone), giving a theoretical loading of 73 μg acetone on each tube in each experiment with a 3-l sample. The atmosphere flow rate was ~20 l min−1 within a glass test chamber. Before conducting each experimental trial, a portable Fourier transform infrared spectrometer (FTIR, DX-4040, Gasmet Technologies, Inc., Finland) and a handheld photoionization detector (MiniRAE 2000, RAE Systems, USA) were used to ensure that the test atmosphere was properly mixed with conditioned environmental air. Preliminary sorbent tube analysis results showed all seven analytes were uniformly delivered across all sampling ports (Soo et al., 2018). Samples from the challenge atmospheres were pulled through all three sorbent tube types at a flow rate of 50 ml min−1 for 60 min (3 l). A DryCal® DC-Lite device was used to ensure that the difference between pre- and postsampling flow rates was within ±5%. A factorial experimental design was chosen and performed in the present study. Each experimental trial at each test condition involved three sorbent tube samples and at least 60 consecutive measurements by one portable FTIR. At least three replicate trials of each condition were performed. Note that the silica gel samples were challenged with acetone alone through a test atmosphere generation system at various humidity conditions, whereas in the chemical interference study samples were only taken with silica gel tubes from the mixture of the seven analytes of interest at 75% RH at a temperature of 25°C as a worst case, because silica gel is not normally used to sample these chemicals. (The tube described in NMAM 2027, and which was used in the evaluation of the method, is not identical to SKC 22610–03. However, sorbent tubes with a similar mass of silica gel in the front section of the tube should provide similar results.)
Solvent extraction during the desorption procedure
As given in Supplementary Table S1 (available at Annals of Work Exposures and Health online), the combinations of the two carbonaceous sorbent tubes (Anasorb 747 and Carbosieve S-III) and two analytical methods recommended by OSHA or NIOSH, which included different extraction conditions, were adopted in the present study to evaluate whether dimethylformamide (DMF) and magnesium sulfate are required for analysis of acetone. The front and back sorbent sections were put into separate 4 ml vials, with or without 100 mg of magnesium sulfate. The same tubes were further chemically desorbed with use of 1 or 2 ml of carbon disulfide (CS2) with or without 1% DMF. For silica gel tube samples, the front and back sorbent sections were put into separate 10 ml vials. The samples were chemically desorbed with use of 5 ml of methylene chloride (65%), methanol (33%), and deionized water (2%) (NIOSH, 2016). Note that the front glass wool was included for analysis with the front section media.
Sample analysis
All samples were analyzed by a NIOSH contract laboratory (Maxxam Analytics, USA). The samples were analyzed using a GC Trace 1310 (Thermo Fisher Scientific, Inc., Waltham, MA, USA) with Flame Ionization Detector. A Zebron ZB Wax column (60 m × 0.32 mm × 0.5 μm) was selected for Anasorb 747 and Carbosieve SIII. A Zebron ZB-1 column (60 m × 0.32 mm × 1.0 μm) was selected for silica gel. The GC-FID conditions used were as follows: flow rates of column injection were 2.8–3.0 ml min−1 (varied by sorbent type), flow rates of outlet split were 20–21 ml min−1 (varied by sorbent type), a continuous purge flow was 5 ml min−1, the initial oven temperature was 50°C (held for 1 min for silica gel; held for 3 min for Anasorb 747 and Carbosieve SIII), temperature ramp was 10°C min−1 (to 150°C for silica gel; to 230°C for Anasorb 747 and Carbosieve SIII). Samples were provided to the laboratory in three sets, and laboratory control spikes were prepared for each set. Overall, average recovery (%) of laboratory control spikes of acetone with Anasorb 747 (dry, unsampled tubes) varied from 78.0 to 112%, whereas Carbosieve S-III provided ~77.8 to 93.5% average recovery. The average recovery of acetone collected by silica gel varied from 97.5 to 113%. Note that all sorbent tube sample results were corrected for recovery percentage of the laboratory control spikes when recovery was <100%. All blank samples showed results lower than the limit of detection (LOD). The LOD was 0.4 μg for acetone on Anasorb 747 and Carbosieve S-III, whereas the LOD was 3.0 μg for acetone collected by silica gel. The limit of quantitation on Anasorb 747 and Carbosieve S-III ranged from 1.3 to 1.6 μg for all seven analytes. The limit of quantitation for acetone on silica gel was 10 μg.
Statistical analysis
All data were analyzed with JMP software version 13.2 (SAS Institute, Cary, NC, USA). First, descriptive statistics on concentration of acetone for all sorbent samples were calculated. Replicate measures that were collected for each sorbent tube result and variable combination were averaged before the analysis. Measured concentration values from each sorbent tubes were compared with theoretical values (applied values), and mean ratios were calculated. Second, a three-way full factorial analysis of variance was performed to determine whether there were any effects of extraction procedure or humidity on quantification by sorbent sampling methods. All analyses were checked to ensure that the assumptions of the analysis were being met, and all differences were considered significant if probability <0.05. The assumptions of homogeneous variance and normally distributed residuals was examined and found to be satisfied.
Results
Influence of relative humidity
Figure 1 shows the box plots of ratio values of acetone concentration to theoretical values (applied values), grouped according to relative humidity. The differences in the ratios of acetone analyzed according to different methods are not statistically significant (P > 0.05) at the low to mid RHs. There were significant losses in the recovery of acetone when the 75% RH samples were analyzed per modified NMAM 1501 and OSHA method 69, relative to the samples at 25 or 50% RH. However, silica gel sorbent tubes analyzed according to NMAM 2027 provided consistent recovery of acetone samples at all RHs. There are statistically significant differences between silica gel with NMAM 2027 and other two sorbents with their corresponding sampling and analytical methods at 75% RH (P < 0.05). Note that the median (or mean) value of each boxplot is taken over measurements from the dynamically controlled test atmospheres containing ~73, 438, and 1898 μg loading of acetone, anticipated to be found in the workplace. Overall, the mean and median value of ratios (i.e. recovery) were above the threshold (0.75) for acceptability recommended by NIOSH or OSHA but they are not above the preferred criterion (90%) recommended by NIOSH and OSHA. Similar results obtained from mixed atmospheres were found at a case of 73 μg loading of acetone (the lowest loading) with other chemical interferences (Supplementary Figure S1, available at Annals of Work Exposures and Health online).
Figure 1.
Comparison of ratio of measured acetone concentration to applied concentration under various relative humidities. The horizontal lines in the box plot from bottom to top indicate 10th, 25th, 50th (median), 75th, and 90th percentiles. The circles indicates the 5th (lower circle) and 95th (upper circle) percentiles. Dotted line (red) indicates mean value. The median (or mean) value of each boxplot is taken over measurements from the dynamically controlled test atmospheres containing ~10, 60, 260 ppm acetone concentrations (=73, 438, and 1898 μg loading of acetone anticipated to be found in the workplace). The ratio at 0.75 is the threshold for acceptability recommended by NIOSH or OSHA method. The applied concentration is calculated from its concentration in the standard and the dilution factor. *Statistically significant difference (P <0.05).
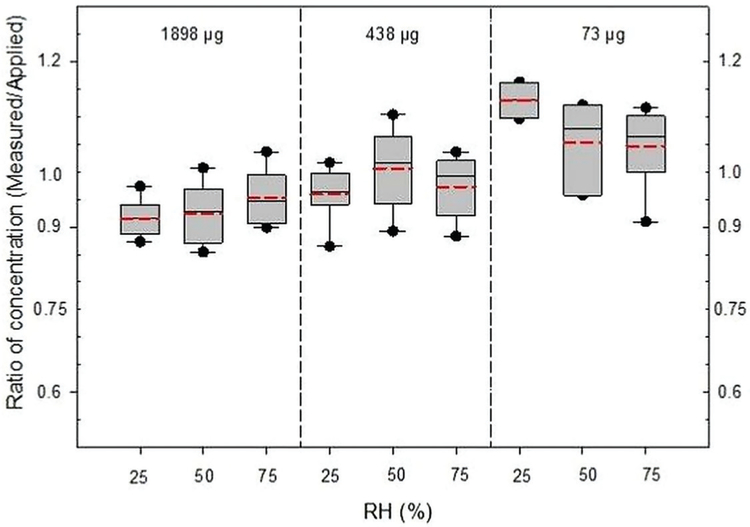
Influence of mass loading
The ratio results grouped according to mass loadings are summarized in Fig. 2. Method comparisons across the different loadings produced results similar to those shown in Fig. 1. There are no significant differences across different observational loading groups through ANOVA analysis (Table 1). But there is no noticeable reduction in recovery of acetone at the very low loading when samples collected by silica gel were analyzed, relative to collection by Carbosieve SIII or Anasorb 747. Overall, both the mean and median values for each method conformed to this 75% acceptability limit while the mean ratio results of modified NMAM 1501 and OSHA method 69 fail to achieve the 90% preferred limit.
Figure 2.
Comparison of ratio of measured acetone concentration to applied concentration under various concentration levels (or loadings). The horizontal lines in the box plot from bottom to top indicate 10th, 25th, 50th (median), 75th, and 90th percentiles. The circles indicates the 5th (lower circle) and 95th (upper circle) percentiles. Dotted line (red) indicates mean value. The median (or mean) value of each boxplot is taken over measurements from the dynamically controlled test atmospheres containing 25, 50, 75% RHs (=5.77, 11.5, and 17.3 mg of water per liter of air anticipated to be found in the workplace) with 1-h sample at 50 ml min−1 (3 l). The ratio at 0.75 is the threshold for acceptability recommended by NIOSH or OSHA method. The applied concentration is calculated from its concentration in the standard and the dilution factor. *Statistically significant difference (P < 0.05).
Table 1.
The summary of three-way factorial analysis of variance (adjusted R2 = 0.911; RMSE = 0.039; observations = 111; P value < 0.001).
| Fixed effect | Level | DF | DF Den | F ratio | Prob > F |
|---|---|---|---|---|---|
| RH | 3 | 2 | 56.3 | 54.2 | <0.0001* |
| Loading | 3 | 2 | 55.6 | 2.76 | 0.0722 |
| RH x loading | 9 | 4 | 55.6 | 9.05 | <0.0001* |
| Method | 3 | 2 | 56.1 | 16.4 | <0.0001* |
| RH x method | 9 | 4 | 56.1 | 16.3 | <0.0001* |
| Loading x method | 9 | 4 | 54.6 | 11.8 | <0.0001* |
| RH x loading x method | 27 | 8 | 54.6 | 1.87 | 0.0832 |
| Random effect | Variance ratio | Variance component | Standard error | Wald P-value | Percent of total |
| Sample ID | 1.03 | 0.0015 | 0.0006 | 0.009 | 50.8 |
| Residual | 0.0015 | 0.0003 | 49.2 | ||
| Total | 0.003 | 0.0005 | 100 | ||
Statistical significance.
DF, degree of freedom; DF Den, denominator degrees of freedom.
Comparison by method
Figures 3–5 compare the ratio of concentration (measured/applied) for each individual method across the different RHs and grouped according to the different mass loadings. In general, all three methods worked well at high and medium mass loadings even at the high humidity condition but only the silica gel tube with NMAM 2027 worked well at low mass loadings and low humidity. Nevertheless, except for the interaction of both factors, the sampling media and analytical method can be considered to have no effect on ratios of measured acetone values to theoretical values. This indicates that the Anasorb 747 with the modified NMAM 1501 determined acetone quite well at the low to mid RH’s, thus eliminating the need for DMF and magnesium sulfate, which are necessary when sampling with the Carbosieve SIII and analyzing it by OSHA Method 69 (pairwise correlations = 0.8946). The loss of recovery can be determined as a function of humidity through nonlinear regression as presented in Figs 4(b) and 5(b). The regression coefficients from the nonlinear equations (with cubic term) decreased as RH increased, indicating poor recovery from low loading samples at high humidity. This was observed in both beaded active carbon and carbon molecular sieve adsorbents compared to samples collected by silica gel. The summary of three-way factorial analysis of variance with random effect for illustrating the effects of each parameter and their interaction on method recovery was presented in Table 1. These results suggest that variability among the dependent variables and covariates is dominated while random variation was accommodated by taking each sample ID from repeat measures across different test parameter group. Note that the main effect of loading is not significant in this experiment. However, there are significant interactions between loading and method and loading and RH. With respect to method, the statistical model confirms the silica tubes are unaffected by variation in loading, while the others show a reduced recovery only at low loading. With respect to RH, the silica tubes are again unaffected by changes in RH, while the others show reduced recovery at high humidity. Figures 4 and 5 show that this is primarily occurring at low loading and high RH. The assumptions of homogeneous variance and normally distributed residuals was met, indicating the experimental protocol variation was minimal and does not account for the low recovery from the carbonaceous sorbents at 75% humidity.
Figure 3.
Comparison of ratio of measured acetone concentration to applied concentration under various combinations of concentration levels (or loadings) and relative humidities: Silica gel sorbent tube with NMAM 2027. The horizontal lines in the box plot from bottom to top indicate 10th, 25th, 50th (median), 75th, and 90th percentiles. The circles indicates the 5th (lower circle) and 95th (upper circle) percentiles. Dotted line (red) indicates mean value. The ratio at 0.75 is the threshold for acceptability recommended by NIOSH or OSHA method. The applied concentration is calculated from its concentration in the standard and the dilution factor. *Statistically significant difference (P < 0.05).
Figure 5.
Comparison of ratio of measured acetone concentration to applied concentration under various combinations of concentration level (loading) and relative humidity: Anasorb 747® sorbent tube (active carbon with low ash content adsorbent) with NMAM 1501. (a) The horizontal lines in the box plot from bottom to top indicate 10th, 25th, 50th (median), 75th, and 90th percentiles. The circles indicates the 5th (lower circle) and 95th (upper circle) percentiles. Dotted line (red) indicates mean value. The ratio at 0.75 is the threshold for acceptability recommended by NIOSH or OSHA method. The applied concentration is calculated from its concentration in the standard and the dilution factor. *Statistically significant difference (P < 0.05). (b) The ratio of concentration (measured/applied) as a function of RH at different loading regions, measured and predicted values as indicated in the trend line (or blue shield region) on the forecast plots.
Figure 4.
Comparison of ratio of measured acetone concentration to applied concentration under various combinations of concentration level (loading) and relative humidity: ORBO 91® sorbent tube (Carbosieve SIII adsorbent) with OSHA Method 69. (a) The horizontal lines in the box plot from bottom to top indicate 10th, 25th, 50th (median), 75th, and 90th percentiles. The circles indicates the 5th (lower circle) and 95th (upper circle) percentiles. Dotted line (red) indicates mean value. The ratio at 0.75 is the threshold for acceptability recommended by NIOSH or OSHA method. The applied concentration is calculated from its concentration in the standard and the dilution factor. *Statistically significant difference (P < 0.05). (b) The ratio of concentration (measured/ applied) as a function of RH at different loading regions, measured and predicted values as indicated in the trend line (or blue shield region) on the forecast plots.
Discussion
The effect of humidity on the performance of carbon based solid sorbent tubes used to sample VOCs in air has been an issue observed in several studies because the water molecules may occupy the sites in the pores competitively (Dubinin, 1980, 1981; Vermisoglou et al., 2007; Lee et al., 2010; Bradley et al., 2011; Zhang et al., 2017). Helmig and Vierlig (1995) showed that the carbon molecular sieve had a significant water uptake, in particular at >50% RH. Gawlowaski et al. (1999) also reported that either microporous active carbon or carbon molecular sieve sorbent tubes adsorb substantial amounts of water used to sample VOCs form the atmosphere. As observed in this study, Maceira et al. (2017) described humidity problems with carbon-based sorbent tubes. The present study confirms not only previous observations but also adds to the literature showing carbonaceous sorbents are not well suited for sampling ketones at high humidity and low concentrations because the water molecules may occupy the sites in the pores competitively as mentioned previously (Dubinin, 1980, 1981).
The present study confirms that NMAM 2027 has a recovery >95% of acetone vapor in humid air at 80% RH with 20°C. In contrast, in neither Harper et al., nor in NMAM 2555, nor in OSHA Method 69 was there a failure to recover acetone at high humidity proportional to the magnitudes seen here. It should be noted that neither in Harper et al. (1993) nor in OSHA method 69 was the method recovery (or % recovery of storage sample from test atmosphere) at very low loadings of acetone measured. For example the lowest loading in Harper et al. (1993) was 530 μg. In OSHA method, it was 3548 μg. The research presented here supports the position that the humidity issue is concentration (or loading) dependent, so that the previous studies are not wholly invalidated.
Conclusions
Our study showed that neither anhydrous magnesium sulfate, nor 1% DMF, alone or together, improved the recovery of acetone from either Carbosieve S-III or Anasorb 747 at low or mid RH as previously noted by Harper et al., but recovery from both sorbents was poor (<75%) with low loading at high (75%) humidity. The present study confirms previous observations that carbonaceous sorbents are not well suited for sampling ketones at high humidity. Our study further showed all methods can be used at high loadings, even at high RH, but only the silica gel tube method works well for low concentrations at high humidity. The method using silica gel for sampling acetone has better recovery of acetone in the situation of low concentrations and high humidity, resulting in a lower expanded uncertainty for the method in this range of conditions.
Supplementary Material
Acknowledgments
The authors are sincerely thankful to Dr Evanly Vo (National Personal Protective Technology Laboratory/NIOSH) for reviewing the article before journal submission.
Funding
This project was funded internally by the National Institute for Occupational Safety and Health (Project CAN number: 93909PX).
Footnotes
Disclaimer
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention or the Occupational Safety and Health Administration. Mention of any company or product does not constitute endorsement by NIOSH/CDC.
Supplementary Material
Supplementary data are available at Annals of Work Exposures and Health online.
References
- Bradley RH, Smith MW, Andreu A et al. (2011) Surface studies of novel hydrophobic active carbons. Appl Surf Sci; 257: 2912–19. [Google Scholar]
- Coffey C, LeBouf R, Lee L et al. (2012) Effect of calibration and environmental condition on the performance of direct-reading organic vapor monitors. J Occup Environ Hyg; 9: 670–80. [DOI] [PubMed] [Google Scholar]
- Dubinin MM. (1980) Water vapor adsorption and the microporous structures of carbonaceous adsorbents. Carbon; 18: 355–64. [Google Scholar]
- Dubinin MM, Serpinsky VV. (1981) Isotherm equation for water vapor adsorption by microporous carbonaceous adsorbents. Carbon; 19: 402–3. [Google Scholar]
- Gawlowaki J, Gierczak T, Jezo A et al. (1999) Adsorption of water vapour in the solid sorbents used for the sampling of volatile organic compounds. Analyst; 124: 1553–8. [Google Scholar]
- Harper M (2000) Sorbent trapping of volatile organic compounds from air. J Chromatogr A; 885: 129–51. [DOI] [PubMed] [Google Scholar]
- Harper M, Kimberland ML, Orr RJ et al. (1993) An evaluation of sorbents for sampling ketones in workplace air. Appl Occup Environ Hyg; 8: 293–304. [Google Scholar]
- Helmig D, Vierlig L. (1995) Water adsorption capacity of the solid adsorbents Tenax TA, Tenax GR, carbotrap, carbotrap C, carbosieve SIII, and carboxen 569 and water management techniques for the atmospheric sampling of volatile organic trace gases. Anal Chem; 67: 4380–6. [Google Scholar]
- LeBouf RF, Slaven JE, Coffey CC. (2013) Effect of calibration environment on the performance of direct-reading organic vapor monitors. J Air Waste Manag Assoc; 63: 528–33. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Shiratori N, Lee GH et al. (2010) Activated carbon nanofiber produced from electrospun polyacrylonitrile nanofiber as a highly efficient formaldehyde adsorbent. Carbon; 48: 4248–55. [Google Scholar]
- Maceira A, Vallecillos L, Borrull F et al. (2017) New approach to resolve the humidity problem in VOC determination in outdoor air samples using solid adsorbent tubes followed by TD-GC-MS. Sci Total Environ; 599–600: 1718–27. [DOI] [PubMed] [Google Scholar]
- NIOSH. (1994) Ketones I: Method 1300. NIOSH manual of analytical methods (NMAM), Issue 2. National Institute for Occupational Safety and Health; Available at https://www.cdc.gov/niosh/docs/2003-154/pdfs/1300.pdf Accessed 1 March 2019. [Google Scholar]
- NIOSH. (1996). Methyl ethyl ketone: Method 2500. NIOSH manual of analytical methods (NMAM), Issue 2. National Institute for Occupational Safety and Health; Available at https://www.cdc.gov/niosh/docs/2003-154/pdfs/2500.pdf Accessed 1 March 2019. [Google Scholar]
- NIOSH. (2003). Ketones I: Method 2555. NIOSH manual of analytical methods (NMAM), Issue 1. National Institute for Occupational Safety and Health; Available at https://www.cdc.gov/niosh/docs/2003-154/pdfs/2555.pdf Accessed 1 March 2019. [Google Scholar]
- NIOSH. (2016). Ketones: Method 2027. NIOSH manual of analytical methods (NMAM), Issue 1. National Institute for Occupational Safety and Health; Available at https://www.cdc.gov/niosh/docs/2014-151/pdfs/methods/2027.pdf Accessed 1 March 2019. [Google Scholar]
- OSHA. (1988). Acetone: Method 69. OSHA sampling and analytical method. Occupational Safety and Health Administration; Available at http://www.osha.gov/dts/sltc/methods/index.html Accessed 1 March 2019. [Google Scholar]
- Soo JC, Lee EG, LeBouf RF et al. (2018) Evaluation of a portable gas chromatograph with photoionization detector under variations of VOC concentration, temperature, and relative humidity. J Occup Environ Hyg; 15: 351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermisoglou EC, Georgakilas V, Kouvelos E. et al. (2007) Sorption properties of modified single-walledcarbon nanotubes. Micropor Mesopor Mater; 99: 98–105. [Google Scholar]
- Zhang X, Gao B, Creamer AE et al. (2017) Adsorption of VOCs onto engineered carbon materials: a review. J Hazard Mater; 338: 102–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.