Abstract
Oxygen delivery to tissue mitochondria relies on simple diffusion in the target cells and tissues. As such, intracellular availability of O2 in tissue depends on its solubility and diffusivity in complex and heterogeneous macromolecular environments. The path of oxygen diffusion is key to its rate of transfer, especially where pathways of differing favorability are present. Most commonly, aqueous media, such as interstitial fluid and cytoplasm, are assumed to provide the dominant diffusion path. Here, the ‘hydrophobic channeling’ hypothesis is revisited, and several lines of evidence pointing toward lipid-accelerated oxygen diffusion pathways are discussed. The implications of hydrophobic channeling are considered in light of extended membrane networks in cells and tissues.
1. Oxygen Diffusion and Availability
The availability of oxygen (O2) within cells and tissues has significant biomedical implications. Indeed, cellular oxygenation is a critical parameter in tumor therapy [1, 2], anesthesia [3], wound healing [4], reperfusion injury [5], adipose tissue dysfunction [6], as well as brain function [7] and possibly neuronal hypometabolism [8]. Tissue-level hypoxia may select tumor cells resistant to apoptosis [9], and hypoxic cycling may favor tumor aggression and resistance to therapy [10]. Moreover, hypoxia is a major barrier to progress in tissue engineering, as 3D-printed cells toward the center of engineered constructs tend to languish and die because of poor diffusional oxygen delivery [11].
While it is widely known that O2 plays a crucial role in cellular energy metabolism, the mechanism of its delivery to tissue mitochondria is only partially understood. Existing physiological models provide reasonable estimates of the amount of O2 leaving the lungs in the form of oxygenated hemoglobin within red blood cells, and hemoglobin O2 release mechanisms are well studied. Physiological control of microvessel diameter in response to local oxygen demand is also fairly well understood. Yet, preceding uptake by red blood cells and following release in capillaries, O2 transit occurs through simple diffusion [3].
With respect to oxygen diffusion rate, the inhomogeneity of cells and tissues has been largely neglected by the medical and scientific communities, although its importance was previously noted [12]. Tissues vary widely in macromolecular composition and physical properties. Such properties are well known to vary for diseased and aging tissues. For example, variations in lipid composition occur in breast tissues affected by cancer [13]. Moreover, tumor tissue commonly has a greater fraction of interstitium, compared with normal tissue of the same type [14]. Aging eye lens fiber cells have increased membrane cholesterol content [15], which is associated with increased rigidity and reduced oxygen permeability [16–18]. In patients with hypercholesterolemia, membrane cholesterol levels are elevated in red blood cells and possibly also in other cells [19], such as those forming the capillary endothelium. Consequent reduction in the rate of red blood cell O2 unloading may decrease the amount of oxygen supplied to heart tissue under stress conditions [19]. These examples illustrate that tissues may be expected to have different levels of oxygen metabolic availability, depending on physical characteristics of the tissue and of the cells involved in oxygen diffusional delivery.
Metabolic availability is a dynamic parameter, reflecting a balance between metabolic demand and oxygen supply. Differences in tissue function and associated metabolic requirements give a wide range of ‘normal’ tissue oxygen levels [7]. Still, the supply to a particular tissue or portion of tissue depends not only on the amount of oxygen released in the capillaries (or culture media) but also on the rate of oxygen diffusion within the tissue, itself. As such, even tissue-specific O2 measurements provide an incomplete picture of the amount of oxygen metabolically available to mitochondria. Accurate estimation of the kinetics of oxygen transit from air to red blood cells and from capillaries or media to tissue mitochondria will be key to understanding, predicting, and modulating tissue oxygenation.
Here, cellular- and tissue-level oxygen diffusional efficiency is addressed, with a focus on diffusion path and its impact on timely metabolic availability. Widely accepted theoretical aspects of oxygen diffusional flux are first discussed, followed by revisiting the ‘hydrophobic channeling’ hypothesis of lipid-mediated oxygen diffusion. Evidence in support of hydrophobic channeling is considered, especially in the context of interconnected membrane networks prevalent in cells and tissues.
2. Path of Least Resistance
The speed of a molecule’s transit through complex surroundings depends on its path of travel. Where multiple paths are available, the molecule will move primarily along the path of least resistance. In energetic terms, the most favorable path will be that with the lowest free energy. Metabolite molecules such as O2 are understood to diffuse via Brownian motion, exhibiting ‘random walk’ behavior. The overall direction of metabolite flux will be determined by the location of metabolic demand and the resulting concentration gradient.
The rate of metabolite transfer is affected both by the magnitude of the concentration gradient and by the permeability of the medium. Namely, the diffusional flux, J, is directly proportional to the permeability, P, and to the metabolite’s concentration gradient, ΔC, as described by the equation J = −PΔC, derived from Fick’s first law of diffusion [20]. Consistent with intuition, this equation predicts that higher-permeability environments will give rise to greater fluxes. The equation also implies that the impact of permeability on flux will be magnified under conditions of greater metabolic demand, where the magnitude of ΔC is greater.
The solubility–diffusion model relates the permeability of a given environment to the solubility and diffusivity (diffusion coefficient) of a molecule traveling by Brownian motion. Most often used to characterize molecular diffusion across a cell membrane, the solubility–diffusion model can be expressed as P = (KPD)/h [20]. This relation is also known as Overton’s rule. In it, KP is the membrane/water partition coefficient, D is the diffusivity, and h is the thickness of the membrane. The partition coefficient describes the relative solubility of the molecule in the membrane, compared to adjacent bulk water.
Although quantitative application of Overton’s rule may not be meaningful outside the context of membranes, the relation is conceptually valuable for predicting the preferred path of oxygen diffusion in the heterogeneous milieu of tissue. Where two regions of differing permeability are found next to one another, we can expect that oxygen will ‘choose’ a diffusion path through the higher permeability region because its relative solubility and/or diffusivity is greater there. That is, oxygen will diffuse preferentially within the portions of cells and tissues where it is most abundant and able to diffuse most readily. Further, because diffusional flux depends on permeability, we can expect the rate of oxygen transfer to be greatest along such preferential diffusion pathways.
3. Hydrophobic Channeling via Networked Lipids
Given the high water content of tissue, oxygen has been assumed to diffuse primarily via aqueous pathways. To the author’s knowledge, this assumption is not supported by specific evidence but rather by the intuition that aqueous compartments would provide the most direct diffusional paths. It is especially easy to accept this view if one perceives cells as having a primarily aqueous interior and interstitial fluid as ‘mostly water’. Yet, current knowledge of the complex and crowded macromolecular environment of cytoplasm [21] and interstitial fluid [22] poses a challenge. Several researchers have suggested that lipids may provide more favorable pathways and, thus, facilitate rapid oxygen delivery. The related hypothesis has sometimes been called ‘hydrophobic channeling’ [16, 23].
Given its nonpolar nature, O2 is widely known to have substantially greater solubility in lipids versus water, by a factor of at least 3 or 4 [24, 25]. Further, the diffusivity of O2 in lipids is comparable to that in water, although this finding was initially unexpected, given the much higher viscosity of lipids versus water [26, 27]. Taken together with Overton’s rule, the higher solubility and comparable diffusivity in lipids point to enhanced O2 permeability for lipids compared with water. The enhanced permeability would correspond with diffusional flux enhancement, encouraging rapid oxygen transfer within lipids and suggesting that cellular lipids may offer preferential diffusion pathways.
Consistent with these fundamental observations, Longmuir suggested in 1981 that oxygen transit from blood to mitochondria occurs via “channels of high solubility” comprised of lipids [28]. Skulachev later suggested that “extended membranous systems”, especially mitochondrial membrane networks, may function as passageways for oxygen [27]. He proposed “lateral transfer” of oxygen by diffusion within the core of membranes, parallel to the membrane plane. He implied that lateral transfer may occur both within cells via mitochondrial networks and at longer range, within the muscle cell mitochondrial reticulum and through series of cells interconnected by gap junctions.
Skulachev additionally noted the presence of fat droplets in very close proximity to mitochondria, seeming to suggest that lipid droplets may also aid in oxygen diffusion [27]. This view was later supported by Sidell and colleagues, who found that fish adapted to cold water temperature (5°C) develop high lipid droplet content in their skeletal muscle [29, 30]. The high lipid content is associated with enhanced oxygen solubility, predicting high oxygen permeability relative to aqueous cytoplasm, which is expected to be highly viscous at low temperature [30]. Skulachev’s view of mitochondrial membranes as passageways for oxygen diffusion is supported by the finding by Sidell and colleagues that mitochondria, themselves, also proliferate in cold-adapted fish [30].
Dutta and Popel further developed the idea of hydrophobic channeling and reviewed a good deal of supportive experimental evidence [12]. Subczynski and colleagues also found support for such a model in their electron spin resonance (ESR) oximetry work, using spin-label probes placed at various depths within model lipid bilayers. The ESR measurements produce profiles of the ‘oxygen transport parameter,’ which reflects the product of O2 solubility and diffusivity. This parameter varies according to the bilayer depth of the spin-label probes, and the resulting profiles indicate that O2 solubility–diffusion is greater in the nonpolar center of the membrane versus the polar headgroup regions. Oxygen concentration and diffusivity curves derived from nuclear magnetic resonance (NMR) experiments [31, 32] and molecular simulation studies [17, 31, 33, 34] support this observation. Simulation data additionally suggest that low molecular packing density promotes O2 solubility and diffusion in the interleaflet region [17, 31].
Both ESR and simulations indicate that the membrane lipid composition substantially influences O2 diffusion patterns [16–18, 35]. In particular, inclusion of membrane cholesterol modestly reduces oxygen permeability across a lipid bilayer but also enhances oxygen channeling toward the bilayer center [17]. Fig. 1 provides a schematic illustration of the channeling effect, based on molecular dynamics simulation data for a bilayer incorporating cholesterol.
Fig 1.
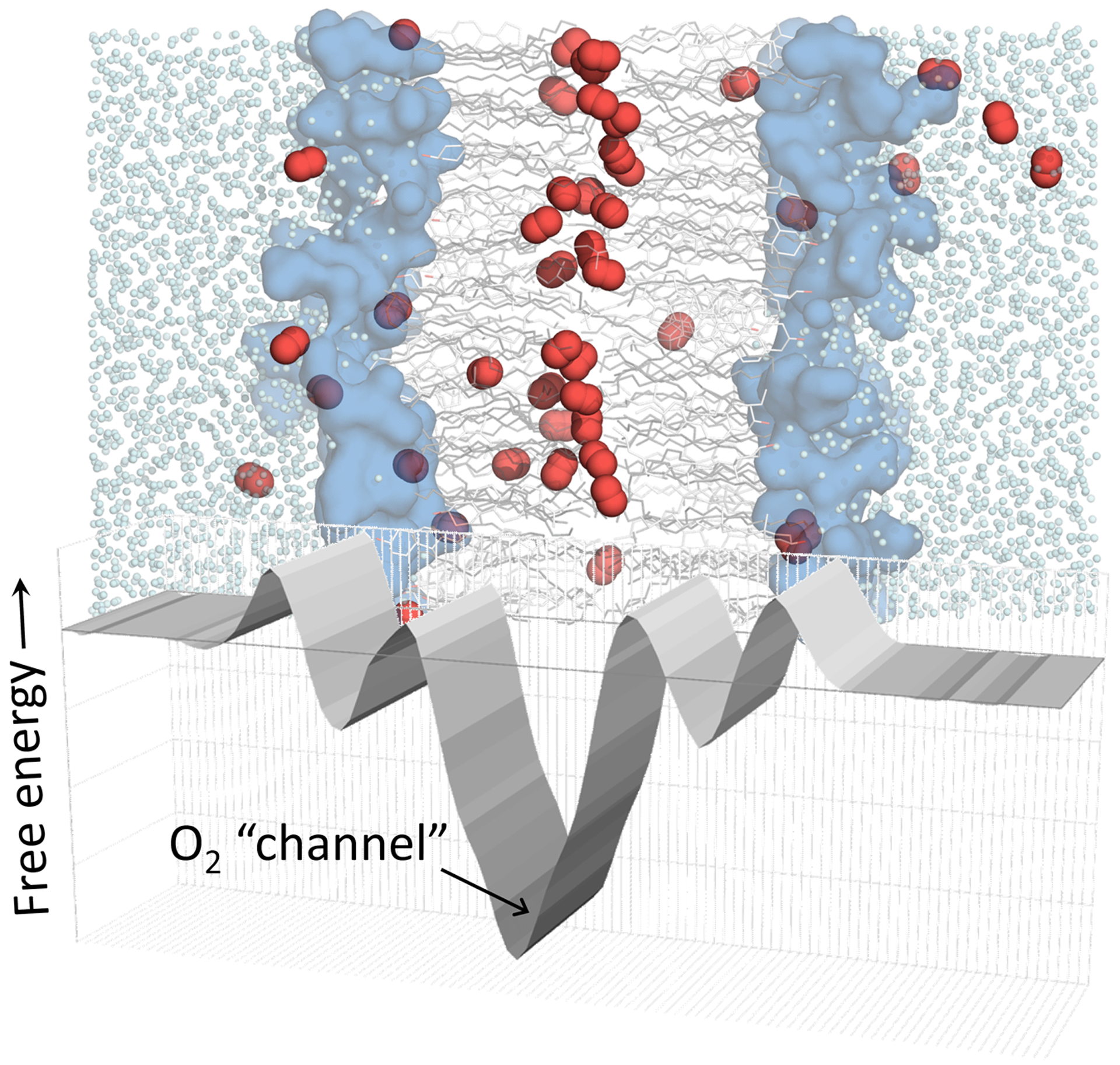
Hydrophobic channeling is facilitated by high O2 solubility and low free energy in the interleaflet region. Schematic representation showing a free energy curve juxtaposed with a molecular dynamics simulation image of a mixed phospholipid/cholesterol bilayer (1:1 ratio). O2 shown as red spheres, phospholipids as gray lines and blue headgroup surface, cholesterol as white lines, and water molecules as cyan dots. H atoms omitted for clarity.
Molecular simulation studies by Ghysels and coworkers demonstrated that the radial diffusivity of O2 parallel to the plane of phospholipid bilayers is greater than the transverse diffusivity perpendicular to the bilayer plane [34]. Moreover, O2 molecules travel laterally in the interleaflet region across distances 3 to 10 times the bilayer thickness, prior to exiting into an adjacent aqueous layer and rapidly reentering a bilayer [36]. These findings indicate that oxygen diffuses more readily inside the core of a lipid bilayer, between the leaflets, than it does across a bilayer. Thus, travel within membranes is favored both by the enhanced concentration of oxygen in that region, compared with water, and by the enhanced radial diffusivity of oxygen in the membrane core.
Skulachev pointed out that high density of membrane proteins may attenuate lateral transport within membranous systems [27]. A recent simulation study partially addresses this issue [37]. A major finding of the study is that the apparent diffusivity of oxygen within a lipid bilayer is reduced by the presence of a potassium channel protein. This effect may stem from slow rotational dynamics of the protein side chains, relative to the lipid tail motions. Additionally, the presence of membrane proteins decreases the effective solubility of oxygen in the membrane because the portion of the membrane occupied by protein is not available to solvate O2 [37]. With respect to oxygen permeability, the simulation study agrees with prior experimental work in bilayers incorporating bacteriorhodopsin protein [38]. Both methods indicate that the oxygen permeability is reduced, but the degree of change cannot be predicted solely by the physical presence of protein [17, 38]. Rather, the apparent permeability of the lipid surrounding the protein is also reduced [16, 37, 38], but this effect has not been satisfactorily explained.
It is important to note that the solubility of oxygen in the cytosol is expected to be lower than in pure water [27, 28]. First, the presence of salts can substantially reduce oxygen solubility [39]. Furthermore, water molecules surrounding other cytosolic solutes—proteins, cytoskeletal structures, and membrane polar groups— become tied up in solvent shells and are, thus, less available to solvate O2 [27]. Macromolecular crowding in the cytosol is likely to reduce the effective diffusivity of O2, as well, because collisions with proteins and other macromolecules would tend to obscure the diffusional path.
Recent experimental work with lung surfactant has characterized the lung lipids as a network of interlinked lipid bilayers, thought to facilitate rapid O2 uptake via diffusion from bilayer to bilayer along the network [40]. In that work, protein-mediated connection points among the bilayers are suggested to provide a roughly continuous hydrophobic conduit for oxygen diffusion. Absence of the lung surfactant protein fraction reduced the rate of oxygen movement by about 60% [40], suggesting that the protein contacts facilitate hydrophobic channeling.
In the milieu of cells and tissues, networked membranes are a common occurrence. Protein-mediated junctions such as gap junctions and organelle contact sites [41] place membranes in very close proximity. Relatively rapid exit from the membranes, due to low free energy barriers [17], should enable rapid supply of O2 where needed, as well as diversion of O2 to high-flux pathways affected by large O2 concentration gradients.
4. Conclusion
The common assumption that oxygen diffuses primarily by way of aqueous pathways is challenged by contemporary understanding of cells and tissues as complex and crowded macromolecular environments. Rather, evidence to date supports a model of lipid-accelerated oxygen diffusion within membranes and lipid droplets held in close proximity by protein contacts. Further work is needed to account for the effects of macromolecules in cytoplasm/interstitium as well as in cellular membranes. Work is also needed to evaluate the potential role of lipid droplets as accelerants for oxygen diffusion, under various physiological and pathophysiological conditions. Clarifying the mechanism of oxygen diffusional delivery may reveal strategies for alleviating hypoxia in tumors, adipose tissue, chronic wounds, and engineered tissue constructs.
Acknowledgments
Gary Angles provided simulation data for the figure, and PyMOL [42] was used to generate the image. This research is supported by the National Institute of General Medical Sciences of the National Institutes of Health under grant P20GM103451 and by a gift from the Glendorn Foundation.
References
- 1.Hou H, Dong R, Lariviere JP, et al. (2011) Synergistic combination of hyperoxygenation and radiotherapy by repeated assessments of tumor pO2 with EPR oximetry. J Radiat Res 52:568–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Multhoff G, Radons J, Vaupel P (2014) Critical role of aberrant angiogenesis in the development of tumor hypoxia and associated radioresistance. Cancers (Basel) 6:813–28. doi: 10.3390/cancers6020813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunn JOC, Mythen MG, Grocott MP (2016) Physiology of oxygen transport. BJA Educ 16:341–348. doi: 10.1093/bjaed/mkw012 [DOI] [Google Scholar]
- 4.Castilla DM, Liu Z-J, Velazquez OC (2012) Oxygen: Implications for wound healing. Adv Wound Care 1:225–230. doi: 10.1089/wound.2011.0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granger DN, Kvietys PR (2015) Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol 6:524–551. doi: 10.1016/j.redox.2015.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Netzer N, Gatterer H, Faulhaber M, et al. (2015) Hypoxia, oxidative stress and fat. Biomolecules 5:1143–1150. doi: 10.3390/biom5021143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carreau A, El Hafny-Rahbi B, Matejuk A, et al. (2011) Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med 15:1239–53. doi: 10.1111/j.1582-4934.2011.01258.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rius-Pérez S, Tormos AM, Pérez S, Taléns-Visconti R (2017) Vascular pathology: Cause or effect in Alzheimer disease? Neurol English Ed 33:112–120. doi: 10.1016/j.nrleng.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 9.Graeber TG, Osmanian C, Jacks T, et al. (1996) Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 379:88. [DOI] [PubMed] [Google Scholar]
- 10.Michiels C, Tellier C, Feron O (2016) Cycling hypoxia: A key feature of the tumor microenvironment. Biochim Biophys Acta -Rev Cancer 1866:76–86. doi: 10.1016/j.bbcan.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 11.Volkmer E, Drosse I, Otto S, et al. (2008) Hypoxia in static and dynamic 3D culture systems for tissue engineering of bone. Tissue Eng Part A 14:1331–1340. doi: 10.1089/ten.tea.2007.0231 [DOI] [PubMed] [Google Scholar]
- 12.Dutta A, Popel AS (1995) A theoretical analysis of intracellular oxygen diffusion. J Theor Biol 176:433–45. doi: 10.1006/jtbi.1995.0211 [DOI] [PubMed] [Google Scholar]
- 13.Hilvo M, Denkert C, Lehtinen L, et al. (2011) Novel theranostic opportunities offered by characterization of altered membrane lipid metabolism in breast cancer progression. Cancer Res 71:3236–3245. doi: 10.1158/0008-5472.CAN-10-3894 [DOI] [PubMed] [Google Scholar]
- 14.Jain RK (1987) Transport of molecules in the tumor interstitium: A review. Cancer Res 47:3039–3051 [PubMed] [Google Scholar]
- 15.Truscott RJ (2000) Age-related nuclear cataract: A lens transport problem. Ophthalmic Res 32:185–94. doi: 55612 [DOI] [PubMed] [Google Scholar]
- 16.Widomska J, Raguz M, Subczynski WK (2007) Oxygen permeability of the lipid bilayer membrane made of calf lens lipids. Biochim Biophys Acta 1768:2635–2645. doi: S0005–2736(07)00233–7 [pii] 10.1016/j.bbamem.2007.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dotson RJ, Smith CR, Bueche K, et al. (2017) Influence of cholesterol on the oxygen permeability of membranes: Insight from atomistic simulations. Biophys J 112:2336–2347. doi: 10.1016/j.bpj.2017.04.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plesnar E, Szczelina R, Subczynski WK, Pasenkiewicz-Gierula M (2018) Is the cholesterol bilayer domain a barrier to oxygen transport into the eye lens? Biochim Biophys Acta -Biomembr 1860:434–441. doi: 10.1016/j.bbamem.2017.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchwald H, Menchaca HJ, Michalek VN, et al. (2000) Plasma cholesterol: An influencing factor in red blood cell oxygen release and cellular oxygen availability. J Am Coll Surg 191:490–497 [DOI] [PubMed] [Google Scholar]
- 20.Missner A, Pohl P (2009) 110 Years of the Meyer-Overton rule: Predicting membrane permeability of gases and other small compounds. Chem Phys Chem 10:1405–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis RJ (2001) Macromolecular crowding: Obvious but underappreciated. Trends Biochem Sci 26:597–604. doi: 10.1016/S0968-0004(01)01938-7 [DOI] [PubMed] [Google Scholar]
- 22.Wiig H, Gyenge C, Iversen PO, et al. (2008) The role of the extracellular matrix in tissue distribution of macromolecules in normal and pathological tissues: Potential therapeutic consequences. Microcirculation 15:283–296. doi: 10.1080/10739680701671105 [DOI] [PubMed] [Google Scholar]
- 23.Subczynski WK, Wisniewska A, Yin JJ, et al. (1994) Hydrophobic barriers of lipid bilayer membranes formed by reduction of water penetration by alkyl chain unsaturation and cholesterol. Biochemistry 33:7670–7681. doi: 10.1021/bi00190a022 [DOI] [PubMed] [Google Scholar]
- 24.Battino R, Evans FD, Danforth WF (1968) The solubilities of seven gases in olive oil with reference to theories of transport through the cell membrane. J Am Oil Chem Soc 45:830–833 [DOI] [PubMed] [Google Scholar]
- 25.Möller M, Botti H, Batthyany C, et al. (2005) Direct measurement of nitric oxide and oxygen partitioning into liposomes and low density lipoprotein. J Biol Chem 280:8850–4. doi: 10.1074/jbc.M413699200 [DOI] [PubMed] [Google Scholar]
- 26.Fischkoff S, Vanderkooi JM (1975) Oxygen diffusion in biological and artificial membranes determined by the fluorochrome pyrene. J Gen Physiol 65:663–676. doi: 10.1085/jgp.65.5.663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skulachev VP (1990) Power transmission along biological membranes. J Membr Biol 114:97–112 [DOI] [PubMed] [Google Scholar]
- 28.Longmuir IS (1981) Channels of oxygen transport from blood to mitochondria. Adv Physiol Sci 25:19–22. doi: 10.1016/B978-0-08-027346-4.50007-3 [DOI] [Google Scholar]
- 29.Desaulniers N, Moerland TS, Sidell BD (1996) High lipid content enhances the rate of oxygen diffusion through fish skeletal muscle. Am J Physiol 271:R42–7 [DOI] [PubMed] [Google Scholar]
- 30.Sidell BD (1998) Intracellular oxygen diffusion: The roles of myoglobin and lipid at cold body temperature. J Exp Biol 201:1119–1128 [DOI] [PubMed] [Google Scholar]
- 31.Al-Abdul-Wahid MS, Yu CH, Batruch I, et al. (2006) A combined NMR and molecular dynamics study of the transmembrane solubility and diffusion rate profile of dioxygen in lipid bilayers. Biochemistry 45:10719–10728. doi: 10.1021/bi060270f [DOI] [PubMed] [Google Scholar]
- 32.Al-Abdul-Wahid MS, Evanics F, Prosser RS (2011) Dioxygen transmembrane distributions and partitioning thermodynamics in lipid bilayers and micelles. Biochemistry 50:3975–83. doi: 10.1021/bi200168n [DOI] [PubMed] [Google Scholar]
- 33.Marrink SJ, Berendsen HJC (1996) Permeation process of small molecules across lipid membranes studied by molecular dynamics simulations. J Phys Chem 100:16729–16738. doi: 10.1021/jp952956f [DOI] [Google Scholar]
- 34.Ghysels A, Venable RM, Pastor RW, Hummer G (2017) Position-dependent diffusion tensors in anisotropic media from simulation: Oxygen transport in and through membranes. J Chem Theory Comput 13:2962–2976. doi: 10.1021/acs.jctc.7b00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subczynski WK, Hyde JS, Kusumi A (1991) Effect of alkyl chain unsaturation and cholesterol intercalation on oxygen transport in membranes: A pulse ESR spin labeling study. Biochemistry 30:8578–8590 [DOI] [PubMed] [Google Scholar]
- 36.De Vos O, Venable RM, Van Hecke T, et al. (2018) Membrane permeability: Characteristic times and lengths for oxygen and a simulation-based test of the inhomogeneous solubility-diffusion model. J Chem Theory Comput 14:3811–3824. doi: 10.1021/acs.jctc.8b00115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dotson RJ, Pias SC (2018) Reduced oxygen permeability upon protein incorporation within phospholipid bilayers. Adv Exp Med Biol 1072:405–411. doi: 10.1007/978-3-319-91287-5_65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashikawa I, Yin JJ, Subczynski WK, et al. (1994) Molecular organization and dynamics in bacteriorhodopsin-rich reconstituted membranes: Discrimination of lipid environments by the oxygen transport parameter using a pulse ESR spin-labeling technique. Biochemistry 33:4947–4952 [DOI] [PubMed] [Google Scholar]
- 39.Battino R, Rettich TR, Tominaga T (1983) The solubility of oxygen and ozone in liquids. J Phys Chem Ref Data 12:163–178 [Google Scholar]
- 40.Olmeda B, Villén L, Cruz A, et al. (2010) Pulmonary surfactant layers accelerate O2 diffusion through the air-water interface. Biochim Biophys Acta -Biomembr 1798:1281–1284. doi: 10.1016/j.bbamem.2010.03.008 [DOI] [PubMed] [Google Scholar]
- 41.Helle SCJ, Kanfer G, Kolar K, et al. (2013) Organization and function of membrane contact sites. Biochim Biophys Acta -Mol Cell Res 1833:2526–2541. doi: 10.1016/j.bbamcr.2013.01.028 [DOI] [PubMed] [Google Scholar]
- 42.The PyMOL Molecular Graphics System, Version 1.7.6.5 Schrödinger, LLC


