Abstract
Neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) have been demonstrated for their potential as a neuroregenerative treatment of Alzheimer’s disease (AD). Unfortunately, most proteins cannot be effectively delivered into the brain from the blood stream due to the presence of the blood-brain barrier (BBB). In this study, we delivered BDNF using ADTC5 as BBB modulator (BBBM) into the brains of transgenic APP/PS1 mice, a mouse model for AD. As controls, two groups of APP/PS1 mice were treated with BDNF alone and vehicle, respectively. All three groups were subjected to behavioral/cognitive assessments in Y-maze and novel object recognition (NOR) tests as well as evaluation of the brain markers activated by BDNF. The results showed that BDNF + ADTC5 group performed significantly better in both the Y-maze and NOR assessments compared to mice that received BDNF alone or vehicle. In addition, significant upregulations of NG2 receptors as well as EGR1 and ARC mRNA transcripts were observed in the brain cortex of mice treated with BDNF + ADTC5, further indicating the efficacy of delivered BDNF in the brain. There were high plaque loads in all groups of mice, suggesting no influence of BDNF on the plaque formation. In summary, ADTC5 can deliver BDNF into the brains of APP/PS1 mice and the activity of BDNF in improving cognitive function was likely due to improvement in synaptic plasticity via NG2 glia cells and not by reducing the plaque load.
Keywords: BDNF, Blood-Brain Barrier, Alzheimer’s Diseas, APP/PS1 mice, BBB modulation, Brain delivery, neuroregeneration
1. Introduction and Epidemiology
To date, effective treatments for Alzheimer’s disease (AD) still remain elusive. Currently, treatment of AD focuses on managing symptoms by regulating neurotransmitter levels in the CNS and peripheral nervous system. The primary choice of treatment for moderate to severe AD is acetylcholinesterase inhibitors, including donepezil (Aricept®),1 rivastigmine (Eexelon®),2 and galantamine (Razadyne®).3 Furthermore, N-methyl-d-aspartate (NMDA) receptor regulators (e.g., memantine or Namenda®)4 as well a combination of acetylcholinesterase inhibition and NMDA regulators (i.e., Namzaric®) have been used to treat AD.5, 6 While these treatments may provide temporary relief of symptoms (i.e., moderate confusion, sleeplessness, agitation, anxiety, and aggression), their benefits are temporary and do not halt or reverse the disease; thus, ultimately these drugs are not cost effective.1, 7 Thus, there is an urgent need to find early diagnostic and therapeutic solutions to halt or reverse progress of the disease.
Within the past two decades, there have been extensive attempts to develop “diseasemodifying drugs” rather than “symptomsuppressing drugs.” To block the progression of AD, the drugs must interfere with the pathogenic steps responsible for the manifestation of clinical symptoms, including the early build-up of extracellular amyloid-beta (Aβ) plaques, the intercellular build-up of neurofibrillary tangle Tau formations, neuroinflammation, oxidative damage, metal ion deregulation, and cholesterol metabolism.8 There have been efforts to develop therapeutics that prevent the formation of or removing the Aβ plaques in the brain such as enzyme inhibitors, anti-Aβ monoclonal antibodies (mAbs), and immunotherapies; however, these therapeutics have not been very successful in treating AD. As an alternative, active vaccine therapies, AN1792 and CAD 106, were developed but these vaccines had low efficacy and unforeseen side effects. Some patients treated with AN1792 vaccine developed T cell-mediated meningoencephalitis.9 CAD 106 had advanced to phase-3 trials for prevention of AD; unfortunately, the trial results indicated that such vaccine treatments may be too little too late to provide a significant effect.10 Because active vaccination could generate adverse immune response, passive immunization using monoclonal antibodies (mAbs) has risen as an attractive alternative for treatment of AD.
The use of mAbs as therapeutics could ensure consistent and controlled titers; therefore, the potential adverse side effects can be controlled. The primary drawback of mAh treatments is high costs and the need for repeated administrations.10, 11 Several mAbs have undergone clinical trials for the treatment of AD; to date, none have gained FDA approval for the treatment of AD or other CNS diseases regardless of the extensive target validation, in vitro success, and excellent PK and safety profiles. Bapineuzumab (AAB-001) was the first antibody drug to reach phase-2 clinical trials for AD; unfortunately, the trial was discontinued due to no significant observed improvement on dementia scores in disability assessment. Since the bapineuzumab clinical trial, at least seven other mAbs were evaluated for AD treatment without successful outcomes.10, 12,13 Most recently, aducanumab has been reevaluated following an additional analysis of clinical trials data with pending FDA approval. Alternatively, neurotrophic agents such as brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and insulin-like growth factor (IGF) have been investigated for the treatment of AD.14–18 Similar to mAbs, these neurotrophic proteins have met with challenges for their use in the treatments of AD.
One potential hypothesis for the failure of mAbs and other proteins as therapeutics for the treatment of AD is their inefficiency in crossing the blood-brain barrier (BBB) to have a sufficient dose to exude their efficacies. Several efforts to improve delivery of mAbs and other proteins into the brain such as osmotic BBB disruption (BBBD),19–21 “Trojan Horse” delivery method,22 and ultrasound with microbubbles23, 24 have exhibited various levels of success. Our approach is to use cadherin peptides as BBB modulators (BBBM) to improve the delivery of molecules into the brain. Linear and cyclic cadherin peptides as BBBM (e.g., HAV6, Ac-SHAVSS-NH2; ADTC5, Cyclo(1,7)Ac-CDTPPVC-NH2) have been shown to improve brain depositions of various sizes of proteins (e.g., 15 kDa lysozyme, 65 kDa albumin, 150 kDa IgG mAb) in C57BL/6 mice.25–27 A combination HAV6 peptide and anticancer drug adenanthin has been shown to effectively suppress brain tumor growth and enhance animal survival in the mouse model of medulloblastoma brain tumor28 Recently, multiple treatments of experimental autoimmune encephalomyelitis (EAE) mice (an animal model of multiple sclerosis (MS)) with a combination of ADTC5 and BDNF significantly suppressed disease relapse compared to those treated with BDNF alone, ADTC5 alone, and PBS.29 The results indicate that a combination of BDNF and ADTC5 peptide can be used to treat other brain neurodegenerative disease such as AD.
In the present study, we evaluated the effects of non-invasive systemic delivery of BDNF into the brain using ADTC5 peptide compared to BDNF alone or vehicle in APP/PS1 transgenic mice, an animal model for AD. The efficacy of the treatment was evaluated using cognitive tests, including Y-maze and novel object recognition (NOR). The effects of BDNF brain delivery were also determined by evaluating the activated downstream cellular processes known to be associated with neuroregeneration such as upregulation of NG2 receptors as well as the increased in mRNAs expression of early growth response 1 (EGR1),30, 31 activity-related cytoskeleton-associated protein (ARC),30, 32, 33 and mitogen-activated protein kinase 1 (MAPK1).24, 34–36
2. Materials and Methods
2.1. Animals
All animal studies were carried out under the approved animal protocol (AUS-74-11) granted by Institutional Animal Care and Use Committee (IACUC) at The University of Kansas. Animal Care Unit (ACU) personnel and veterinarians were involved in the care of the animals used in this study. Female transgenic APP/PS1 (MMRRC stock # 34832-Jax) were obtained from Jackson Laboratory (Bar Harbor, ME) and housed until at least 6 months of age. Mice received intravenous (i.v.) injections of either BDNF (5.7 nmol/kg) + ADTC5 (10 μmol/kg; n = 7), BDNF alone (5.7 nmol/kg; n = 6), or vehicle (n = 6) every 4 days, for a total of 8 injections. At the end of the study, the mice were euthanized via CO2 inhalation and perfused with PBS immediately followed by 4% formalin fixative solution. The brains were extracted and post-fixed overnight in the perfusion-fixation solution then transferred to 70% ethanol PBS solution for paraffin embedding.
2.2. Cadherin peptide synthesis and purification
ADTC5 peptide was synthesized using a solid-phase peptide synthesizer (Gyros Protein Technologies, Tucson, AZ) as described previously.26 Briefly, crude peptide was cleaved from the resin with TFA containing scavengers followed by precipitation in cold diethyl ether. The disulfide bond in ADTC5 was formed by stirring the linear peptide precursor in 0.1 M ammonium bicarbonate buffer solution at pH 9.0 in high dilution while bubbling air through the solution. The cyclic ADTC5 was purified using a semi-preparative HPLC X-bridge C18 column (Waters, Milford, MA) and the product was analyzed by analytical HPLC to be > 95 % pure. The exact mass of cyclic ADTC5 was determined by mass spectrometry.
2.3. Y-maze assessment
Twenty-four hours following the 8th injection of the treatment, the mice were subjected to Y-maze behavioral assessment.37, 38 First, the mice were habituated to the maze for 8 min with one arm of the maze closed off. Three hours following habituation, the mice were reintroduced to the maze for 5 min with all three arms open. All mice were initially placed in the center of the maze oriented toward the same arm; the maze was thoroughly cleaned with 70% ethanol and Virkon between each trial to remove scent cues. Time in Novel Arm was defined as the percent of total time (5 min) spent in the third arm of the maze (previously closed-off arm). An entry into an arm was defined as the head of the mouse entering.
2.4. Novel object recognition assessment
Twenty-four hours following the Y-maze assessment, the mice were subjected to Novel Object Recognition (NOR) assessment.38 First, mice were individually habituated in an empty open field for 5 min. Twenty-four hours after habituation, 2 identical objects were placed in the open field, 5 cm away from the wall; there were two different sets of identical objects that were randomly selected for each mouse. Mice were individually placed in the field facing away from the objects and were allowed to familiarize themselves with the objects for 10 min. Twenty-four hours after familiarization phase, mice were re-subjected to the open field, but one of the objects was replaced with a novel object. The position of the novel object (right or left side) was randomized for each mouse. The mice were allowed to explore the objects for 10 min and the total amount of time each mouse spent interacting with each object was measured. For all steps, the open field and object were cleaned with 70% ethanol and Virkon.
2.5. Histology and immunohistochemistry
Coronal brain sections (10 μm thickness) were generated and mounted onto gelatin-coated slides (Superfrost Plus, Thermo Fisher Scientific, Waltham, MA). For both Aβ histology and NG2 receptor immunohistochemistry, sections were deparaffinized using xylene and serially hydrated from 95% ethanol to distilled water. The positive and negative controls were carried out according to Hewitt et al. (2014).39 For Aβ, slides were stained with Congo Red Solution (Abeam, Cambridge, UK) for 20 min, then dipped in twice in 100% ethanol, cleared with xylene, mounted using synthetic Permount (Fisher Scientific, Hampton, NH) and covered using 2.5 coverslips. Aβ plaque levels were quantified by counting the number of plaques from the hippocampus at lOx magnification from 5 random sections per group (n = 5).
For anti-NG2 mAb staining, slides were first blocked in a 3% hydrogen peroxide blocking agent then subsequently rinsed using distilled water. Next, heat-induced isotope retrieval (HER) was erformed using a 10 nM sodium citrate buffer at pH 6.0.Briefly, the HER buffer was brought to a boil and slides were submerged for 15 min in HER followed by immediate rinsing with PBS containing 0.05% Tween-20 (PBS-T) buffer for 3 min. Slides were then blocked using 10% normal bovine serum albumin (BSA) for 6 min and subsequently rinsed with water. The NG2 primary antibody (Abeam, Cambridge, UK) was then applied to the slides in a dilution of 1:1,000 in PBS-T followed incubation overnight at 4 °C in a moisturizer chamber. The following steps were performed using the Polink-2 HRP plus rabbit DAB detection system for immunohistochemistry (Golden Bridge International Labs, Bothell, WA). Briefly, rabbit antibody enhancer (Reagent 1) was applied to the slides and incubated at room temperature for 30 min. The slides were then rinsed with PBS-T and Polymer-HRP for rabbit (Reagent 2) was applied followed by incubation at room temperature for 30 min. The slides were then rinsed with PBS-T and the chromogen was applied. To prepare the chromogen, 2 drops of DAB Chromogen (Reagent 3B) were added to DAB Reagent buffer (Reagent 3A). The slides were incubated with the DAB mixture for 10 min and then were rinsed with water. Lastly, the slides were dipped into 100% ethanol twice, dried, mounted using Permount and 1.5 coverslips. Anti-NG2-stained slides were imaged using a Leica DM750 Compound Bright-Field Upright Microscope and imaged at 40x (0.65 NA; HI PLAN ∞) magnification under identical exposure times. Anti-NG2 mAb levels were quantified via densitometry analysis at 40x magnification from 5 random sections per group (n = 5).
2.6. Fluorescence in situ hybridization
Coronal brain sections (10 μm thickness) were washed three times in PBS before mounting on gelatin-coated glass slides (Superfrost Plus, Thermo Fisher Scientific). Tissues were allowed to dry at RT and were then stored at −20 °C until use. Fluorescence in situ hybridization (FISH) was performed using Multiplex Reagent Kit V2 from RNAscope® Technology 2.0 (Advanced Cell Diagnostics (ACD), Hayward, CA).40–42 Mounted tissue sections were deparaffinized using xylene and serially dehydrated in 50%, 70%, 95%, and 100% ethanol for 5 min each. Between all pretreatment steps, tissue sections were briefly washed with distilled water. Pretreatment solution 1 (hydrogen peroxide reagent) was applied for 10 min at RT, and then the tissue sections were boiled in pretreatment solution 2 (target retrieval reagent) for 15 min. Mounted slices were pretreated with solution 3 (protease reagent) for 30 min at 40 °C in the HybEz™ hybridization system (ACD). Following tissue pretreatment, the following transcript probes were applied to all sections: Mm-Mapkl-Cl (Cat. # 458161), Mm-Arc-C2 (Cat. # 316911-C2), and Mm-Egrl-C3 (Cat. # 423371-C3), which correspond to MAPK1, ARC, and EGR1, respectively. Probes were hybridized into the brain sections for 2 h at 40 °C and subsequently washed for 2 min at room temperature. Following hybridization, hybridize AMP 1 was applied to each slide, which was then incubated for 30 min at 40 °C. The same process was repeated for hybridize AMP 2 and 3. For HRP-C1 signal development (MAPK1), HRP-C1 was applied to the slides, and they were incubated for 15 min at 40 °C and then washed. For Cl, Opal® 650 (Akoya Biosciences, Menlo Park, CA) was applied and incubated for 30 min at 40 °C and then washed. Following the wash, HRP blocker was applied to each slide, incubated for 15 min at 40 °C followed by washing. This process was repeated for C2 (ARC) and C3 (EGR1) using Opal® 620 and 520, respectively. The resulting transcript - fluorophore labeling is as follows: MAPK-650, ARC-620, EGR1–520. All sections were counterstained by incubating DAPI (4′,6-diamidino-2-phenylindole), fluorescent DNA stain for 30 sec at room temperature following by rinsing. Slides were then covered using ProLong Gold Antifade Mounting Media and 1.5 coverslips. Slides were allowed to dry in the dark overnight at 4 °C. All sections were imaged within 2 weeks.
Fluorescent images were taken using an Olympus IX-81 inverted epifluorescence microscope XI81 (Olympus Life Solutions, Waltham, MA) running SlideBook Version 6.0 (3i, Ringsby, CT) equipped with a digital CMOS camera (2000x2000), automatic XYZ stage position, ZDC autofocus, and a xenon lamp excitation source. Images were taken under identical exposure times (100 msec) using a 40x objective (0.95 NA; UPlanSApo ∞) and appropriate filter sets for each stain or Opal® fluorophore (i.e., DAPI–DAPI, FITC–Opal® 520, Texas red–Opal® 620, and Cy 5.5–Opal® 650). To determine the degree of mRNA transcript expression, 4 images of the CA1 region of the hippocampus regions were randomly selected from mouse samples of each group, and the total fluorescence signal intensity for each channel was quantified. For display purposes, images were pseudo-colored and brightness-adjusted using ImageJ; green was assigned to Opal® 520 (EGR1), red to Opal® 620 (ARC), cyan to Opal® 650 (MAPK1), and grey to DAPI.
2.7. Statistics and data analysis
All statistics and data analyses were performed using GraphPad Prism (San Diego, CA). Analysis of variance (ANOVA) and Student’s T-test were performed with a p-value of less than 0.05 used as the criterion for statistical significance unless otherwise stated.
3. Results
3.1. Effect of BDNF on cognitive performance in Y-maze and NOR assessments
The ability of ADTC5 to deliver BDNF into the brains of mice after i.v. injection was assessed by determining the effects of BDNF on improving cognitive function in APP/PS1 Alzheimer’s disease animal model as determined by Y-maze and NOR assessments. The efficacy of BDNF (5.71 nmol/kg) + ADTC5 (10 μmol/kg; n = 7) was compared to that of BDNF alone (5.71 nmol/kg; n = 6), and vehicle (n = 6). Once mice reached 6 months of age, each treatment was delivered via an i.v. injection every 4 days for a total of 8 injections. Twenty-four hours following the final injection, mice were subjected to Y-maze and NOR assessments.
In the Y-maze, mice that received BDNF + ADTC5 performed significantly better than mice that received BDNF alone or vehicle (Figure 1). The mice that received BDNF + ADTC5 spent a greater percentage of time in the third arm (F(2,15) = 3.99; p < 0.05, Figure 1A) and had a higher number of entries into the third arm of the maze than did the groups that received BDNF alone or vehicle (F(2,15) = 5.63; p < 0.05, Figure 1B).
Figure 1.
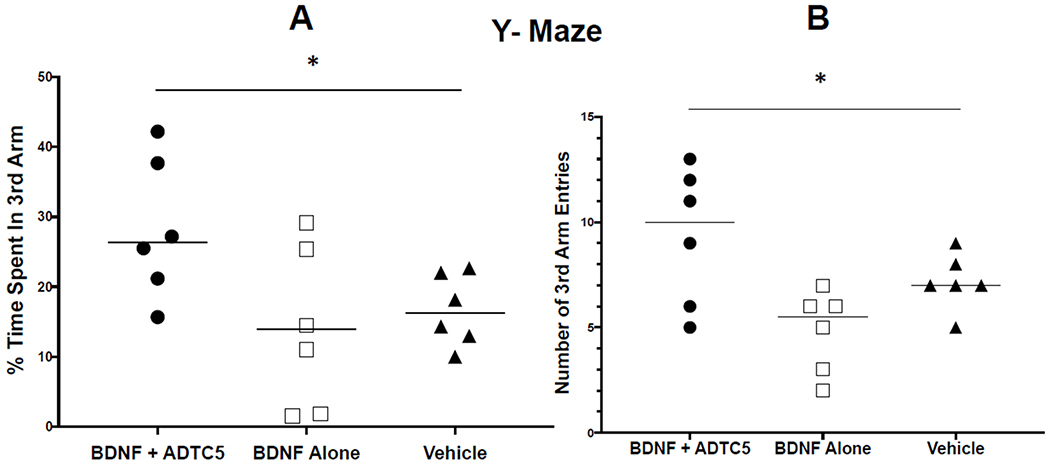
Y-maze cognitive assessment of transgenic APP/PS1 mice, an AD animal model after eight injections of BDNF (5.71 nmol/kg) + ADTC5 (10 μmol/kg), BDNF alone (5.71 nmol/kg), or vehicle. (A) The percent of total time spent in the novel arm or third arm of the Y-maze. (B) The total number of entries made into the third arm of the Y-maze. *p < 0.05; one-way ANOVA (95% confidence, n =5).
For the NOR assessment, the mice that received BDNF + ADTC5 performed significantly better than mice that received BDNF alone or vehicle. The mice that received BDNF + ADTC5 spent a greater percentage of time with the novel object (F(2,16) = 6.55; p < 0.01) than did the mice that received BDNF alone or vehicle (Figure 2A). Lastly, there was no significant difference in total time spent with either of the two objects; in other words, all groups spent similar amounts of time interacting with either object (F(2,16) = 0.682; p = 0.52; Figure 2B).
Figure 2.
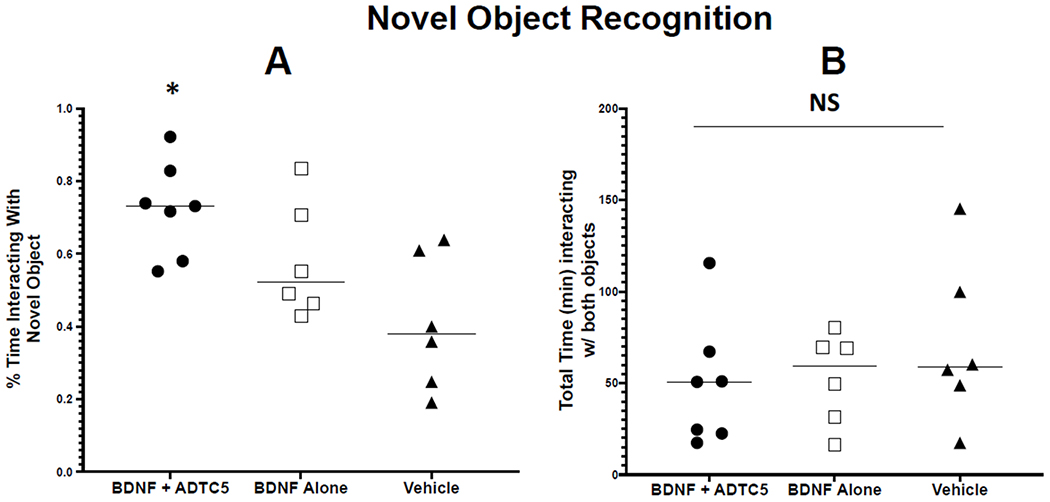
Novel object recognition (NOR) cognitive assessment of transgenic APP/PS1 mice after eight injections with BDNF (5.71 nmol/kg) + ADTC5 (10 μmol/kg), BDNF alone (5.71 nmol/kg), or vehicle on. (A) The percent of total time spent interacting with the novel object. (B) The total amount of time mice spent interacting with either object. *p < 0.05; one-way ANOVA (95% confidence, n =5); NS = No Significant Difference.
3.2. Effect of BDNF delivery on amyloid beta plaques in hippocampus
The effects of BDNF brain delivery on the amounts of Aβ plaques were determined in groups of mice treated with BDNF + ADTC5, BDNF alone, or vehicle. The results indicated that all groups expressed high level and high variability of plaques in the hippocampus regardless of the treatment. There were no significant differences in the amounts of amyloid beta plaques in all three groups (F(2,12) = 0.096; p = 0.91, n = 5; Figure 3).
Figure 3.
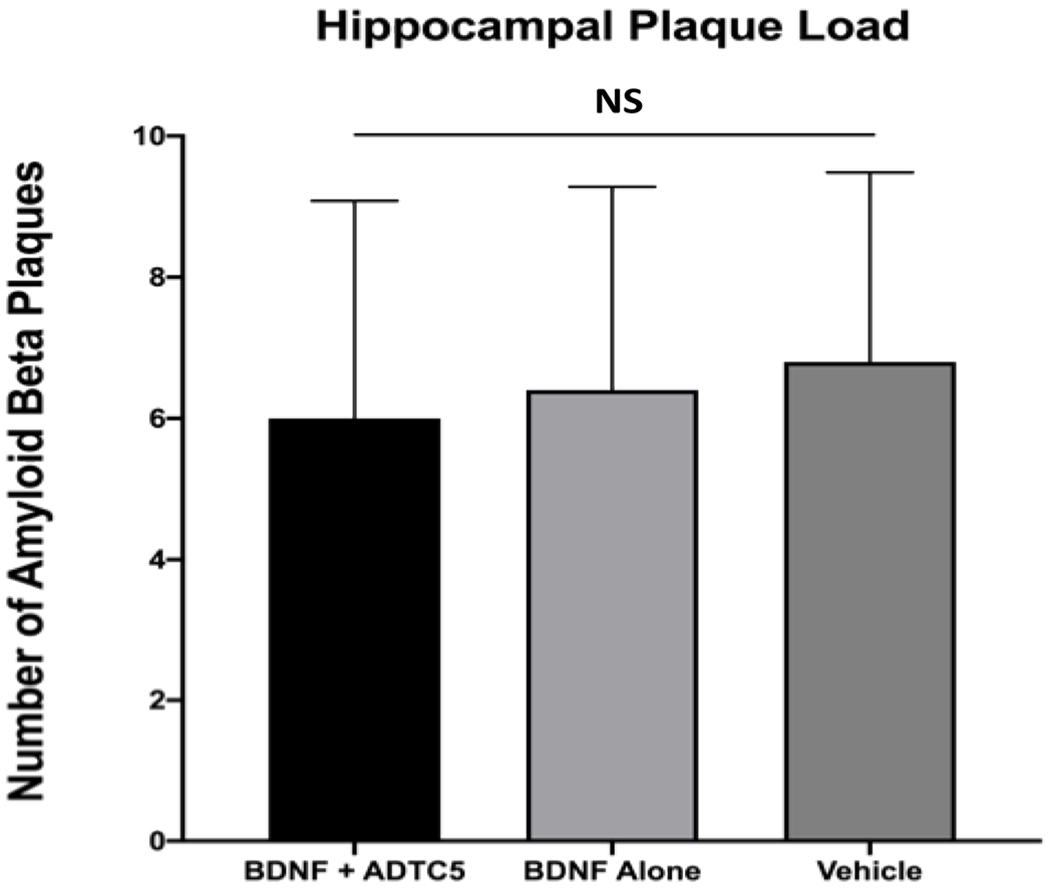
The effect of eight injections of BDNF (5.71 nmol/kg) + ADTC5 (10 μmol/kg), BDNF alone (5.71 nmol/kg), or vehicle in APP/PS1 mice on amyloid plaque loads at the hippocampal region as determined using Congo red staining. The is no significant difference (NS) in all three groups.
3.3. Effect of BDNF delivery on NG2-glia
Previously, brain delivery of BDNF using ADTC5 in experimental autoimmune encephalomyelitis (EAE) induced oligodendrocyte maturation which was reflected in the increase in NG2 receptor expression in the brain.29 Furthermore, BDNF+/+ mice have shown a significant upregulation of NG2 glia following the development of cuprizone-induced lesions compared to BDNF+/− and BDNF−/− mice.43, 44 Thus, the effects of BDNF brain delivery using ADTC5 were determined by evaluating the oligodendrocyte progenitor maturation in the APP/PS1 mouse model. In this case, the brain expressions of NG2 receptors were probed in the cortex region using anti-NG2 antibody staining. The brain cortexes of mice treated with BDNF + ADTC5 have higher degree of NG2 staining (darker staining) compared to those treated with BDNF alone or vehicle alone (Figure 4A). Quantification using pixel values of NG2 stain indicated that mice were treated with BDNF + ADTC5 had a higher or darker staining compared to those mice treated with BDNF alone or vehicle (Figure 4B, F(2,12)= 11.16; p < 0.01, n = 5).
Figure 4.
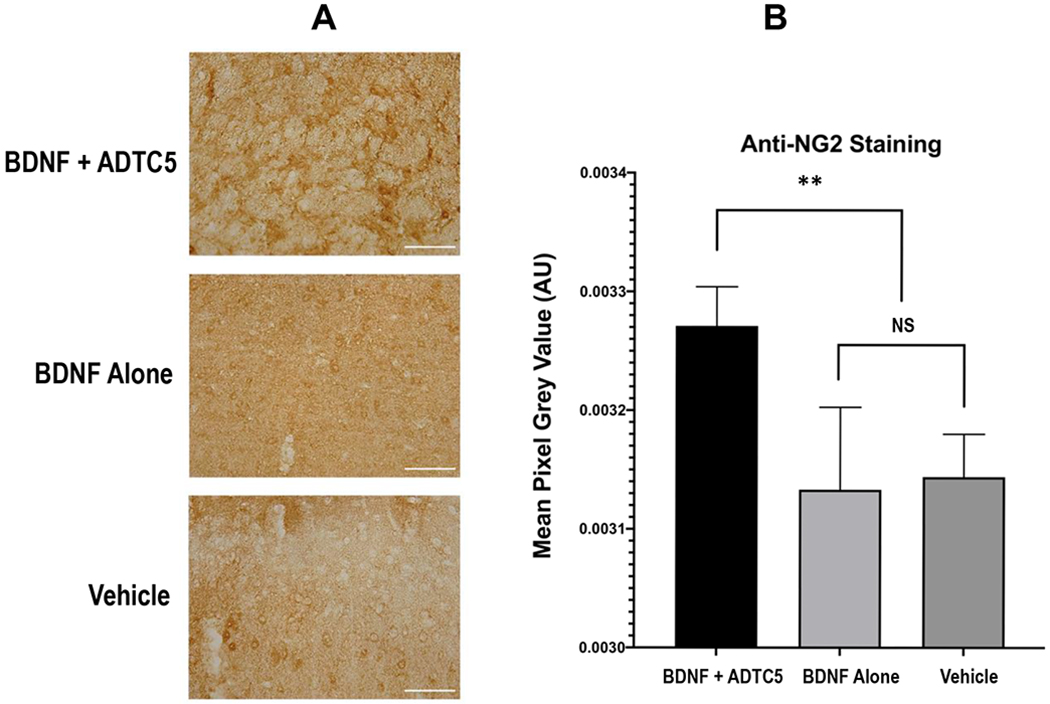
The effect of multiple treatments of APP/PS1 mice with BDNF (5.71 nmol/kg) + ADTC5 (10 μmol/kg), BDNF alone (5.71 nmol/kg), or vehicle on the expression of NG2 receptors in the cortex as stained by DAB. (A) Color photomicrograph of anti-NG2 staining (brown) taken under identical conditions from the cortex of mice treated with BDNF + ADTC5, BDNF alone, and vehicle; slides from BDNF + ADTC5-treated mice showed dense regions of activated NG2-glia. (B) Quantitative NG2 density comparison among the APP/PS1 mice treated with BDNF + ADTC5, BDNF alone, and vehicle; Scale bar = 100 μm; **p ≤ 0.01; NS = No Significant Difference; one-way ANOVA (95% confidence; n = 5).
3.4. Effect of BDNF on EGR1, ARC, and MAPK1 mRNA transcript expression
BDNF is known to stimulate downstream transcription factors such as, tropomyosin receptor kinase B (TrkB), cyclic AMP response element binding protein (CREB), MAPK1, EGR1, and ARC.31, 45, 46 We have previously demonstrated that delivering BDNF using ADTC5 to EAE mice resulted in the increase in EGR1 and ARC mRNA transcript expression. Additionally, others have shown that EGR1 directly targets ARC expression.30, 47 We quantified the levels of EGR1, ARC, and MAPK1 mRNA transcripts via fluorescence in situ hybridization (FISH) method. Qualitatively, the brain sections from the CA1 regions of the hippocampus have higher levels of visual staining from ERG1 and ARC expression in mice treated with BDNF + ADTC5 compared to mice treated with BDNF alone or vehicle (Figure 5A). However, it was difficult to visually differentiate the staining of MAPK1 in all three different groups. Using quantitative method, the number of pixel counts from EGR1 (F(2,9)) = 23.48; p < 0.001, n = 5) and ARC (F(2,9) = 7.33; p < 0.05, n = 5) transcript levels in BDNF + ADTC5 group were significantly higher compared to those mice received BDNF alone or vehicle (Figure 5B). In contrast, very high levels of MAPK1 expression were found in all three groups with no significant differences were observed in all groups (F(2,9) = 0.08; p = 0.92, n = 5; Figure 5B).
Figure 5.
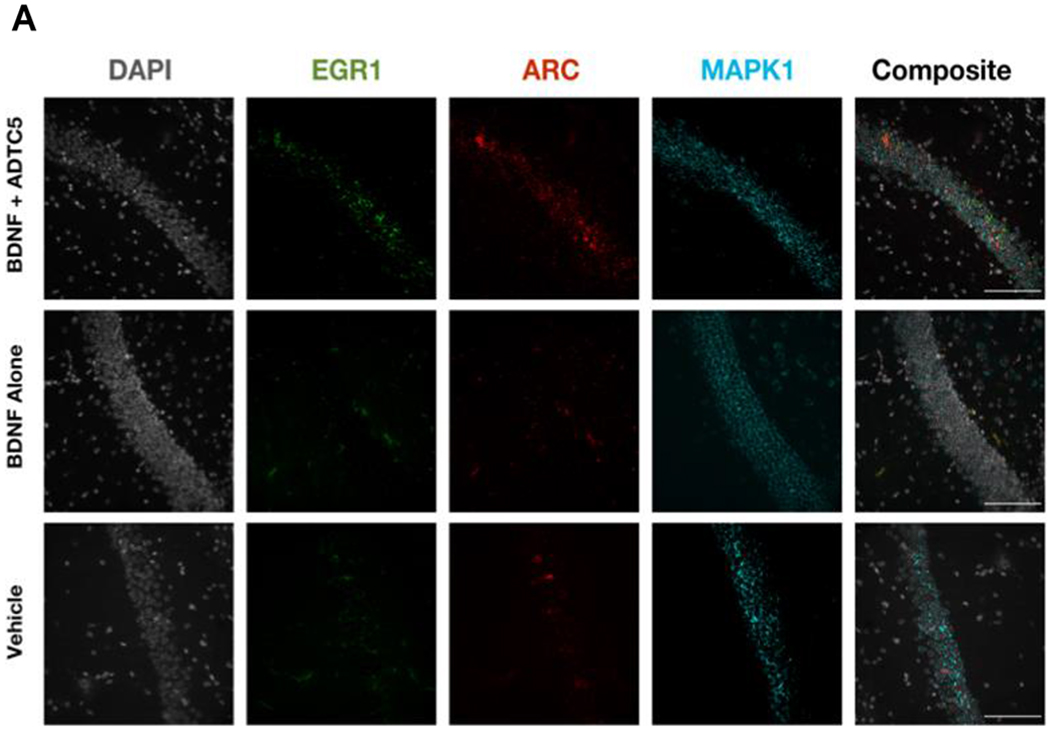
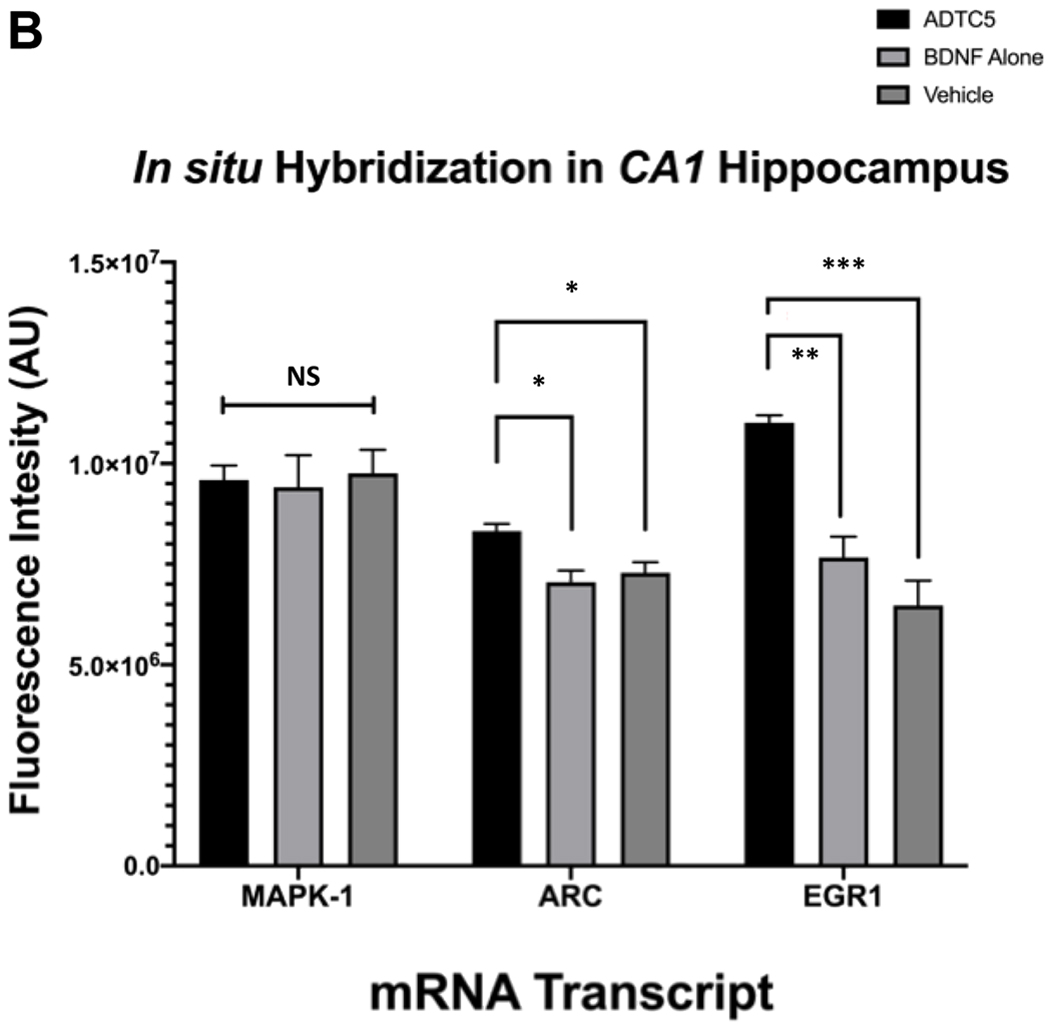
The effects of BDNF (5.71 nmol/kg) + ADTC5 (10 μmol/kg), BDNF alone (5.71 nmol/kg), or vehicle treatments on mRNA expression of MAPK1, EGR1, and ARC in the CA1 region of the brain hippocampus from treated APP/PS1 mice. (A) Photomicrograph of DAPI (grey), EGR1 (green), ARC (red), MAPK (cyan) and composite images taken of the hippocampus of APP/PS1 mice treated with BDNF + ADTC5, BDNF alone, or vehicle. (B) Quantitative comparison using fluorescence intensities of MAPK1 EGR1, and ARC mRNA transcript expressions after multiple treatments with BDNF + ADTC5, BDNF alone, or vehicle. Scale bar = 100 μm; p* ≤ 0.05; ** and ***p ≤ 0.001; one-way ANOVA (99% confidence; n = 4); NS = No significant difference. Contrast and brightness of images were adjusted only for display purposes.
4. Discussion
In this study, we delivered BDNF using ADTC5 into the brains of transgenic APP/PS1 mice, which is one of the animal models for AD. BDNF was selected because it is an endogenous protein that has low potential to induce an adverse side effect in transgenic APP/PS1 mice. Because BDNF alone cannot penetrate the BBB, there is a need to utilize ADTC5 to enhance brain delivery of BDNF. BDNF effects in the brain were determined by monitoring the cognitive improvements in transgenic APP/PS1 mice as well as the induction of neuroregeneration signals.
The results showed that multiple administrations of BDNF + ADTC5 can significantly improve cognitive performance of the animals in Y-maze (Figure 1) and NOR (Figure 2) behavioral assessments compared to those treated with BDNF alone or vehicle. Furthermore, there were significant increases in the expression of NG2 cells (Figure 4) as well as EGR1 and ARC mRNA transcripts (Figure 5) in BDNF + ADTC5-treated mice compared the group of mice treated with BDNF alone or vehicle. Taken together, the results indicate that ADTC5 can enhance the brain delivery of BDNF and its delivery can influence brain physiology and functions of transgenic APP/PS1 mice.
Recently, multiple treatments of EAE mice with BDNF + ADTC5 during disease remission prevented the disease relapse as determined by the disease body scores while multiple treatments of EAE mice with BDNF alone, ADTC5 alone, or vehicle alone did not prevent the disease relapse.29 Similarly, EAE mice treated with BDNF + ADTC5 promoted NG2 glia maturation, increased ARC and EGR1 expressions, and enhanced axon remyelination compared to those treated with BDNF alone or vehicle.29 In the same study, BDNF delivered with ADTC5 was detected in the brain homogenates using Western blots while BDNF delivered alone could not be detected in the brain homogenates. In addition, administration of BDNF + ADTC5 triggered phosphorylation of TrkB (pTrkB) receptors in the brain; in contrast, administration of BDNF alone did not trigger pTrkB formation. Phosphorylation of TrkB has been established to be a marker of BDNF activity in the brain.34, 35 Taken together, the results indicate that ADTC5 deliver BDNF into the brain to improve disease symptoms.
Y-maze and NOR cognitive assessments were used to evaluate the effects of BDNF delivery into the brain and these methods are standard and validated methods to determine cognitive performance of AD animal models in preclinical studies.37, 48–52 They have been used to evaluate the efficacy of potential therapeutic agents for reversing cognitive impairment that could potentially be translated to AD patients. The advantage of these assessment methods is that the animals are not subjected to stressful conditions such as food or water deprivation as well as physical stress. Stressful conditions such as those in the Morris water maze could alter the cognitive performance. In our studies, animals that received multiple treatments of BDNF + ADTC5 had significant behavior improvements in Y-maze and NOR tests compared to the animal groups that received BDNF alone or vehicle. It has been shown that exercise induces upregulation of BDNF, which leads to the enhancement of brain plasticity; in addition, upregulation in BDNF has been associated with improved performance in behavioral tests in AD animal models.53–56 Additionally, Bechara et al. (2014) demonstrated that a single intracerebroventricular (ICV) injection of BDNF can mimic exercise-induced brain plasticity and improve spatial memory in the object displacement task.57 In summary, the positive results from cognitive performance tests in our study were congruent with those of others, suggesting the presence of BDNF in the brain when delivered with ADTC5. Thus, there is a potential utility of non-invasive delivery of BDNF in cognitive performance in AD patients.
The effects of BDNF on Aβ plaques were evaluated by staining the brain slices with Congo red. The results showed that there was no significant reduction in the amounts of Aβ plaques in BDNF + ADTC5-treated group compared to the groups treated with BDNF alone or vehicle (Figure 3). Our results were consistent with the finding by Nagahara et al. (2013) where brain delivery of BDNF using a Lenti-BDNF vector did not show any reduction of Aβ plaques while it improved hippocampal-dependent learning in APP transgenic mice.58 In addition, the study also demonstrated that BDNF treatment ameliorated cell loss and improved synaptophysin immunoreactivity in the hippocampus.58 Similarly, our studies showed that treatments with BDNF + ADTC5 increased in mRNA transcripts of EGR1 and ARC (Figure 5) compared to BDNF-treated or control group. EGR1 is a downstream transcription factor that is activated by BDNF, in addition to the activation of cAMP response element binding protein (CREB) and c-Fos.31, 46 Subsequently, it has been shown that EGR1 also triggered the activation of ARC upon upregulation by BDNF.30, 33 EGR1-deficient mice have lower expression of ARC protein in some neurons compared to normal mice, suggesting that there is a connection between EGR1 and ARC. Therefore, it is proposed that BDNF is ameliorating APP/PS1 symptoms by modulating hippocampal plasticity and not by reducing amyloid-beta plaque loads.
Another possible pathway to improve cognitive behavior is by upregulating oligodendrocyte progenitor cells (OPC) or NG2 glia cells that have an integral role in modulation of synaptic response, remyelination, and integration of neurons into synaptic networks.59 Other researchers have found that the presence of high Aβ plaques and exacerbated APP/PS1 symptoms could increase the levels of NG2 glia cells as a counter response to the increased of neuroinflimation.60, 61 In BDNF + ADTC5 group, a significant upregulation of NG2 receptors compared to controls indicates the role of delivered BDNF on neuroregeneration and cognitive improvement in APP/PS1 mice (Figure 4). A significant upregulation of NG2 glia cells in the corpus callosum was also observed when EAE mice were treated with BDNF + ADTC5.29 Similarly, Nakajima et al. (2010) showed that targeted retrograde gene delivery of BDNF in rats after spinal cord injury suppressed apoptosis of neurons and oligodendroglia, which resulted in a significant promotion of NG2 expression.62 Furthermore, McTigue et al. (1998) demonstrated that transplanting fibroblasts producing BDNF into contused adult rat spinal cords resulted in a significant increase in expansion of oligodendrocyte lineage cells.63 These combined findings provide strong evidence that BDNF delivery can ameliorate CNS damage via promotion of NG2 oligodendrocytes and they provide a good motivation to utilize BDNF for treatment of neurodegenerative diseases.
Other than delivering BDNF, ADTC5 has been shown to improve delivery of 15 kDa lysozyme, 65 kDa albumin, and 150 kDa IgG mAh into the brains of C57BL/6 mice; however, ADTC5 could not deliver 220 kDa fibronectin into the brain.21, 22 The results suggest that there is a size limit of molecule that can be delivered into the brain by ADTC5. Using a quantitative method, it was found that the amount of brain deposition of 150 kDa IgG mAh was lower than that of 65 kDa albumin when delivered using the same conditions.26 This suggests that there is a potential correlation between the size of delivered molecule to its effectiveness to cross the BBB. As predicted, the kidney glomerular filtration can influence the brain delivery of proteins. We found that brain delivery of 15 kDa lysozyme by ADTC5 was less effective than those of 65 kDa albumin or 150 kDa IgG mAh using the same conditions. It was found that a high fraction of lysozyme was found in the kidney.26 This indicates that the low brain deposition of lysozyme was due to the rapid clearance by the kidney.
One of the potential pitfalls of our BBBM method is that it may allow the delivery of unwanted molecules into the brain from the blood stream. To counter this potential problem, one could control the size and timing of pore size opening in the paracellular pathway of the BBB to limit or exclude unwanted molecules to enter the brain. As indicated previously, ADTC5 cannot deliver 220 kDa fibronectin, which provides a size limit for delivering protein to the brain.26 Another important factor of BBB modulation is the duration of pore opening created by BBBM and this factor reflects the reversibility of BBB modulation by the modulator. Therefore, time-dependent opening of the BBB was evaluated using a pretreatment experiment. In this case, ADTC5 was administered first, and after a certain period of time, different sizes of molecules were delivered and detected in the brain. The result showed that ADTC5 allowed the BBB permeation of a small molecule such as gadopentetic acid (Gd-DTPA; MW = 547.6 Da) in a time window of 0 to 2 h; however, no molecules could pass through the BBB after 4-h.64 This indicates that the pores created by ADTC5 to allow gadopentetic acid to cross the BBB were closed between 2 to 4 h. In contrast, ADTC5 allowed only 65 kDa galbumin to enter the brain after 10-min delay, but no albumin could cross the BBB after 40-min delay.27 Thus, for a large molecule such as galbumin, the pores closed between 10 to 40 min. Finally, a 20-min delay between administrations of ADTC5 and IgG mAb did not allow the penetration of IgG mAb across the BBB. These results suggest that there is a time-dependent collapse of the pore openings created by ADTC5 in the paracellular pathway of the BBB. We propose that the ADTC5 modulates the cadherin-cadherin interactions in the BBB intercellular junctions to create large, medium, and small pores. The large pores disintegrate to become medium and small pores in time-dependent manner, followed by disintegration of medium pores to small pores until the paracellular pathway reseals into the normal condition. The reversibility of the BBB to the normal condition after modulation with ADTC5 was also evaluated using transmission electron microscopy (TEM).64 The BBB endothelial cells in mice 2 h after treatment with ADTC5 have similar morphology compared to those found in vehicle treated mice.64 Two hours after pretreatment with ADTC5, the vehicular activity in the BBB endothelial cells was similar to that of vehicle treated mice. Taken together, the BBB modulation by ADTC5 is time- and molecular size-dependent and the modulation is reversible.
To test the general applicability of BBBM, another cadherin peptide, HAV6 peptide (Ac-SHAVSS-NH2), was used to deliver adenanthin, an anticancer drug, into the brains of mice with medulloblastoma brain tumors.28 With its hydrophobic and soluble properties, adenanthin is expected to favorably penetrate the BBB; however, because it is a substrate for an efflux pump, P-glycoprotein (Pgp), it cannot effectively cross the BBB. In healthy mice, coadministration of adenanthin with HAV6 peptide significantly enhanced its brain deposition up to brain concentration of 22 μM compared to undetectable amount or below the detection limit (BQL: 0.1 mg/mL) when it was administered alone.28 Three cycles of treatment with adenanthin + HAV6 increased the survival median of the brain tumor mice to 30 days compared to 19 or 20 days, when treated with adenanthin alone or placebo, respectively. Furthermore, additional two cycles of treatment (a total of five cycles) with adenanthin + HAV6 for the surviving mice extended survivability the end of the study on day 45 and these mice have no sign of brain tumors.28 Interestingly, multiple treatments of mice with HAV6 alone didn’t generate astrogliosis or microglia activation, suggesting the BBB modulation with HAV6 peptide did not induce neuroinflammation.28 It was determined previously using magnetic resonance imaging (MRI) studies that the enhancement of molecule brain delivery was not due to the change in the blood flow in the brain caused by the HAV6 peptide.65 This study provides additional support for the applicability of BBBM in delivering therapeutic molecules for potential treatments of brain diseases.
5. Conclusion
This study showed that the non-invasive brain delivery of BDNF using ADTC5 improved the cognitive behavior in Y-maze and NOR tests in transgenic APP/PS1 mice, an animal model for AD. In addition, BDNF + ADTC5 injected every 4 days for a total of eight injections increased brain expressions of NG2 receptors and mRNAs of EGR1 and ARC. These markers are related to BDNF activity in the brain. These results were similar to that of brain delivery of BDNF using ADTC5 in the EAE mice.29 The results from this study provide initial data for future evaluation of the effects of brain delivery of protein drugs such as mAbs, enzymes, neurotrophic factors, and hormones in other animal models of brain diseases.
6. Acknowledgements
This work was supported by grants from KU Alzheimer’s Disease Center–National Institute of Aging (P30-AG035982) and National Institute of Neurological Disorders and Stroke (R01-NS075374). BMK was supported by NIH T32 Predoctoral Training Program on Pharmaceutical Aspects of Biotechnology (T32-GM008359). We would like to thank Dr. Noraida Martinez-Rivera for her optical microscopy and immunohistochemical training and assistance. The Leica DM750 Compound Bright-Field Upright Microscope and Olympus IX-81 inverted microscope imaging experiments were conducted at the KU Microscopy and Analytical Imaging Research Resource Core Laboratory, supported by KU Office of Research, NIH Clinical and Translational Science Award (UL1TR002366), and Institutional Development Award (IDeA) from NIH NIGMS (P20 GM103418). We also thank Nancy Harmony for proofreading this manuscript.
7. References:
- 1.Courtney C, Farrell D, Gray R, Hills R, Lynch L, Sellwood E, Edwards S, Hardyman W, Raftery J, Crome P, Lendon C, Shaw H, Bentham P, Group ADC, Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet 2004;363:2105–15. [DOI] [PubMed] [Google Scholar]
- 2.Rosler M, Anand R, Cicin-Sain A, Gauthier S, Agid Y, Dal-Bianco P, Stahelin HB, Hartman R, Gharabawi M, Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ 1999;318:633–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loy C, Schneider L, Galantamine for Alzheimer’s disease. John Wiley & Sons, Ltd: 1996; Vol. 16. [Google Scholar]
- 4.Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ, Memantine Study G., Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 2003;348:1333–41. [DOI] [PubMed] [Google Scholar]
- 5.Prasher VP, Review of donepezil, rivastigmine, galantamine and memantine for the treatment of dementia in Alzheimer’s disease in adults with Down syndrome:implications for the intellectual disability population. Int J Geriatr Psychiatry 2004;19:509–15. [DOI] [PubMed] [Google Scholar]
- 6.Scarpini E, Scheltens P, Feldman H, Treatment of Alzheimer’s disease:current status and new perspectives.Lancet Neurol 2003;2:539–47. [DOI] [PubMed] [Google Scholar]
- 7.Loveman E, Green C, Kirby J, Takeda A, Picot J, Payne E, Clegg A, The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s disease. Health Technol Assess 2006;10:iii–iv, ix,–xi, 1–160. [DOI] [PubMed] [Google Scholar]
- 8.Yiannopoulou KG, Papageorgiou SG, Current and future treatments for Alzheimer’s disease. Ther Adv Neurol Disord 2013;6:19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, Orgogozo JM, Team ANS, Clinical effects of Abetaimmunization (AN1792) in patients with AD in an interrupted trial.Neurology 2005;64:1553–62. [DOI] [PubMed] [Google Scholar]
- 10.van Dyck CH, Anti-Amyloid-beta Monoclonal Antibodies for Alzheimer’s Disease: Pitfalls and Promise. Biol Psychiatry 2018;83:311–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemere CA, Immunotherapy for Alzheimer’s disease: hoops and hurdles. Mol Neurodegener 2013;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, Reichert M, Ketter N, Nejadnik B, Guenzler V, Miloslavsky M, Wang D, Lu Y, Lull J, Tudor IC, Liu E, Grundman M, Yuen E, Black R, Brashear HR, Bapineuzumab,Clinical Trial, I., Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med 2014;370:322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummings JL, Cohen S, van Dyck CH, Brody M, Curtis C, Cho W, Ward M, Friesenhahn M, Rabe C, Brunstein F, Quartino A, Honigberg LA, Fuji RN, Clayton D, Mortensen D, Ho C, Paul R, ABBY: A phase 2 randomized trial of crenezumab in mild to moderate Alzheimer disease. Neurology 2018;90:e1889–e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagahara AH, Tuszynski MH, Potential therapeutic uses of BDNF in neurological and psychiatric disorders.Nat Rev Drug Discov 2011;10:209–19. [DOI] [PubMed] [Google Scholar]
- 15.Tuszynski MH, Thal L, Pay M, Salmon DP, U HS, Bakay R, Patel P, Blesch A, Vahlsing HL, Ho G, Tong G, Potkin SG, Fallon J, Hansen L, Mufson EJ, Kordower JH, Gall C, Conner J, A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med 2005;11:551–5. [DOI] [PubMed] [Google Scholar]
- 16.Mitra S, Behbahani H, Eriksdotter M, Innovative Therapy for Alzheimer’s Disease-With Focus on Biodelivery of NGF. Front Neurosci 2019;13:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGregor CE, English AW, The Role of BDNF in Peripheral Nerve Regeneration: Activity-Dependent Treatments and Val66Met. Front Cell Neurosci 2018;12:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonthius DJ, Karacay B, Dai D, Pantazis NJ, FGF-2, NGF and IGF-1, but not BDNF, utilize a nitricoxide pathway to signal neurotrophic and neuroprotective effects against alcohol toxicity in cerebellar granule cell cultures. Brain Res Dev Brain Res 2003;140:15–28. [DOI] [PubMed] [Google Scholar]
- 19.Chakraborty S, Filippi CG, Wong T, Ray A, Fralin S, Tsiouris AJ, Praminick B, Demopoulos A, McCrea HJ, Bodhinayake I, Ortiz R, Langer DJ, Boockvar JA,Superselective intraarterial cerebralinfusion of cetuximab after osmoticblood/brain barrier disruption for recurrent malignant glioma: phase I study. J Neurooncol 2016;128:405–15. [DOI] [PubMed] [Google Scholar]
- 20.Riina HA, Fraser JF, Fralin S, Knopman J, Scheff RJ, Boockvar JA, Superselective intra arterial cerebral infusion of bevacizumab: arevival of interventional neuro-oncology for malignant glioma. J Exp Ther Oncol 2009;8:145–50. [PubMed] [Google Scholar]
- 21.Williams PC, Henner WD, Roman-Goldstein S, Dahlborg SA, Brummett RE, Tableman M, Dana BW, Neuwelt EA, Toxicity and efficacy of carboplatin and etoposidein conjunction with disruption of the blood-brain tumor barrier in the treatment of intracranial neoplasms.Neurosurgery 1995;37:17–27;discussion 27-8. [DOI] [PubMed] [Google Scholar]
- 22.Pardridge WM, Delivery of Biologics Across the Blood-Brain Barrier with Molecular Trojan Horse Technology. BioDrugs 2017;31:503–19. [DOI] [PubMed] [Google Scholar]
- 23.Downs ME, Buch A, Sierra C, Karakatsani ME, Teichert T, Chen S, Konofagou EE, Ferrera VP,Long-Term Safety of Repeated Blood-Brain Barrier Opening via Focused Ultrasound with Microbubbles in Non-Human Primates Performing a Cognitive Task. PLoS One 2015;10:e0125911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baseri B, Choi JJ, Deffieux T, Samiotaki G, Tung YS, Olumolade O, Small SA, Morrison B, Konofagou EE, Activation of signaling pathways following localized delivery of systemically administered neurotrophic factors across the blood-brain barrier using focused ultrasoundand microbubbles. Phys Med Biol 2012;57:N65–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulapane KR, Kopec BM, Siahaan TJ, Improving In Vivo Brain Delivery of Monoclonal Antibody Using Novel Cyclic Peptides.Pharmaceutics 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ulapane KR, Kopec BM, Siahaan TJ, In Vivo Brain Delivery and Brain Deposition of Proteins with Various Sizes. Mol Pharm 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ulapane KR, On N, Kiptoo P, Williams TD, Miller DW, Siahaan TJ, Improving Brain Delivery of Biomolecules via BBB Modulation in Mouse and Rat: Detection using MRI, NIRF, and Mass Spectrometry. Nanotheranostics 2017;1:217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sajesh BV, On NH, Omar R, Alrushaid S, Kopec BM, Wang W-G, Sun H-D, Lillico R, Lakowski TM, Siahaan TJ, Davies NM, Puno P-T, Vanan MI, Miller DW, Validation of Cadherin HAV6 Peptide in the Transient Modulation of the Blood-Brain Barrier for the Treatment of Brain Tumors. Pharmaceutics 2019;11: 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kopec BM, Kiptoo P, Zhao L, Rosa-Molinar E, Siahaan TJ,Noninvasive Brain Delivery and Efficacy of BDNF to Stimulate Neuroregeneration and Suppression of Disease Relapse in EAE Mice. Mol Pharm 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L, Carter J, Gao X, Whitehead J, Tourtellotte WG, The neuroplasticity-associated arc gene is a direct transcriptional target of early growth response (Egr) transcription factors. Mol Cell Biol 2005;25:10286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alder J, Thakker-Varia S, Bangasser DA, Kuroiwa M, Plummer MR, Shors TJ, Black IB, Brain-derived neurotrophic factor-induced gene expression reveals novel actions of VGF in hippocampal synaptic plasticity. J Neurosci 2003;23:10800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR, Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci 2002;22:1532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin Y, Edelman GM, Vanderklish PW, The brain-derived neurotrophic factor enhances synthesis of Arc in synaptoneurosomes. Proc Natl Acad Sci U S A 2002;99:2368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshii A, Constantine-Paton M, Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol 2010;70:304–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, Kunugi H, BDNF function and intracellular signaling in neurons. Histol Histopathol 2010;25:237–58. [DOI] [PubMed] [Google Scholar]
- 36.Van’t Veer A, Du Y, Fischer TZ, Boetig DR, Wood MR, Dreyfus CF, Brain-derived neurotrophic factor effects on oligodendrocyte progenitors of the basal forebrain are mediated through trkB and the MAP kinase pathway. J Neurosci Res 2009;87:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HY, Kim HV, Yoon JH, Kang BR, Cho SM, Lee S, Kim JY, Kim JW, Cho Y, Woo J, Kim Y, Taurine in drinking water recovers learning and memory in the adult APP/PS1 mouse model of Alzheimer’s disease. Sci Rep 2014;4:7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webster SJ, Bachstetter AD, Nelson PT, Schmitt FA, Van Eldik LJ, Using mice to model Alzheimer’s dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front Genet 2014;5:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hewitt SM, Baskin DG, Frevert CW, Stahl WL, Rosa-Molinar E, Controls for immunohistochemistry: the Histochemical Society’s standards of practice for validation of immunohistochemical assays. J Histochem Cytochem 2014;62:693–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasquez JJ, Hussien R, Aguilar-Rodriguez B, Junger H, Dobi D, Henrich TJ, Thanh C, Gibson E, Hogan LE, McCune J, Hunt PW, Stoddart CA, Laszik ZG, Elucidating the Burden of HIV in Tissues Using Multiplexed Immunofluorescence and In Situ Hybridization: Methods for the Single-Cell Phenotypic Characterization of Cells Harboring HIV In Situ. J Histochem Cytochem 2018;66:427–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gershon TR, Crowther AJ, Liu H, Miller CR, Deshmukh M, Cerebellar granule neuron progenitors are the source of Hk2 in the postnatal cerebellum. Cancer Metab 2013;1:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith PA, Schmid C, Zurbruegg S, Jivkov M, Doelemeyer A, Theil D, Dubost V, Beckmann N, Fingolimod inhibits brain atrophy and promotes brain-derived neurotrophic factor in an animal model of multiple sclerosis. J Neuroimmunol 2018;318:103–13. [DOI] [PubMed] [Google Scholar]
- 43.Vondran MW, Clinton-Luke P, Honeywell JZ, Dreyfus CF,BDNF+/− mice exhibit deficits in oligodendrocyte lineage cells of the basal forebrain. Glia 2010;58:848–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.VonDran MW, Singh H, Honeywell JZ, Dreyfus CF, Levels of BDNF impact oligodendrocyte lineage cells following a cuprizonelesion. J Neurosci 2011;31:14182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li N, Liu GT, The novel squamosamide derivative FLZ enhances BDNF/TrkB/CREB signaling and inhibits neuronal apoptosis in APP/PS1 mice. Acta Pharmacol Sin 2010;31:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu Y, Russek SJ, BDNF and the diseased nervous system: a delicate balance between adaptive and pathological processes of gene regulation. J Neurochem 2008;105:1–17. [DOI] [PubMed] [Google Scholar]
- 47.Edelmann E, Lessmann V, Brigadski T, Pre- and postsynaptictwists in BDNF secretion and action in synaptic plasticity. Neuropharmacology 2014;76 Pt C:610–27. [DOI] [PubMed] [Google Scholar]
- 48.Saito T, Matsuba Y, Mihira N, Takano J, Nilsson P, Itohara S, Iwata N, Saido TC, Single Appknock-in mouse models of Alzheimer’s disease. Nat Neurosci 2014;17:661–3. [DOI] [PubMed] [Google Scholar]
- 49.Conrad CD, A critical review of chronic stress effects on spatial learning and memory. Prog Neuropsychopharmacol Biol Psychiatry 2010;34:742–55. [DOI] [PubMed] [Google Scholar]
- 50.Ohno M, Cole SL, Yasvoina M, Zhao J, Citron M, Berry R, Disterhoft JF, Vassar R, BACE1 gene deletion prevents neuron loss and memory deficits in 5XFAD APP/PS1 transgenic mice. Neurobiol Dis 2007;26:134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McClean PL, Holscher C, Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer’s disease. Neuropharmacology 2014;76 Pt A:57–67. [DOI] [PubMed] [Google Scholar]
- 52.McLean S, Ganong AH, Seeger TF, Bryce DK, Pratt KG, Reynolds LS, Siok CJ, Lowe JA 3rd, Heym J, Activity and distribution of binding sites in brain of a nonpeptide substance P (NK1)receptor antagonist. Science 1991;251:437–9. [DOI] [PubMed] [Google Scholar]
- 53.Bo H, Kang W, Jiang N, Wang X, Zhang Y, Ji LL, Exercise-induced neuroprotection of hippocampus in APP/PS1 transgenic mice viaupregulation of mitochondrial 8-oxoguanine DNA glycosylase. Oxid Med Cell Longev 2014;2014:834502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim SE, Ko IG, Shin MS, Kim CJ, Jin BK, Hong HP, Jee YS, Treadmill exercise and wheel exercise enhance expressions of neutrophic factors in the hippocampus of lipopolysaccharide-injected rats.Neurosci Lett 2013;538:54–9. [DOI] [PubMed] [Google Scholar]
- 55.Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM,Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab 2013;18:649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bechara RG, Kelly AM, Exercise improves object recognition memory and induces BDNF expression and cell proliferation in cognitively enriched rats. Behav Brain Res 2013;245:96–100. [DOI] [PubMed] [Google Scholar]
- 57.Bechara RG, Lyne R, Kelly AM,BDNF-stimulated intracellular signalling mechanisms underlie exercise-induced improvement in spatial memory in the male Wistar rat. Behav Brain Res 2014;275:297–306. [DOI] [PubMed] [Google Scholar]
- 58.Nagahara AH, Mateling M, Kovacs I, Wang L, Eggert S, Rockenstein E, Koo EH, Masliah E, Tuszynski MH, Early BDNF treatment ameliorates cell loss in the entorhinal cortex of APP transgenic mice. J Neurosci 2013;33:15596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dimou L, Gallo V, NG2-glia and their functions in the central nervous system. Glia 2015;63:1429–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nielsen HM, Ek D, Avdic U, Orbjorn C, Hansson O, Netherlands Brain, B., Veerhuis R, Rozemuller AJ, Brun A, Minthon L, Wennstrom M, NG2 cells, a new trail for Alzheimer’s disease mechanisms? Acta Neuropathol Commun 2013;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dong YX, Zhang HY, Li HY, Liu PH, Sui Y, Sun XH,Association between Alzheimer’s disease pathogenesis and early demyelination and oligodendrocyte dysfunction. Neural Regen Res 2018;13:908–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakajima H, Uchida K, Yayama T, Kobayashi S, Guerrero AR, Furukawa S, Baba H, Targeted retrograde gene delivery of brain-derived neurotrophic factor suppressesapoptosis of neurons and oligodendroglia after spinal cord injury in rats. Spine (Phila Pa 1976) 2010;35:497–504. [DOI] [PubMed] [Google Scholar]
- 63.McTigue DM, Horner PJ, Stokes BT, Gage FH, Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci 1998;18:5354–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laksitorini MD, Kiptoo PK, On NH, Thliveris JA, Miller DW, Siahaan TJ, Modulation of intercellular junctions by cyclic-ADT peptides as a method to reversibly increase blood-brain barrier permeability. J Pharm Sci 2015;104:1065–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.On NH, Kiptoo P, Siahaan TJ, Miller DW, Modulation of blood-brain barrier permeability in mice using synthetic E-cadherin peptide. Mol Pharm 2014;11:974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]


