Abstract
The field of motoneuron and motor unit physiology in mammals has deeply evolved the last decade thanks to the parallel development of mouse genetics and transcriptomic analysis and of in vivo mouse preparations that allow intracellular electrophysiological recordings of motoneurons. We review the efforts made to investigate the electrophysiological properties of the different functional subtypes of mouse motoneurons, to decipher the mosaic of molecular markers specifically expressed in each subtype, and to elucidate which of those factors drive the identity of motoneurons.
Keywords: Spinal motor system, Functional diversity of spinal motoneurons, In vitro and in vivo spinal cord electrophysiology, Molecular markers
Introduction
Although the study of motor unit physiology dates back from the ‘60s and ‘70s, the field has experienced a considerable renewal in the last decade with numerous efforts relying on progress in genetic tools in mice. We will review recent progress in identifying the various types of motoneurons and motor units in mice, based on their electrophysiological properties and the expression of molecular markers. These advances offer unprecedented tools for physiological studies of the spinal motor system under normal and disease conditions.
Electrophysiological properties of motor units
There are several types of motoneurons (MNs) based on the muscle fibers they contact [reviewed in 1]. α-motoneurons (αMNs) innervate extrafusal muscle fibers and are the central components of motor units (MUs), the elementary unit of movements. Depending on the biomechanical properties of the muscle fibers, one can distinguish three types of MUs: slow-contracting, fatigue-resistant MUs (S), fast-contracting, fatigue resistant MUs (FR), and fast-contracting but fatigable units (FF). On the other hand, ɣ-motoneurons (ɣMNs) innervate intrafusal muscle fibers of spindles (an intramuscular proprioceptive organ), not extrafusal muscle fibers, and they do not generate any force at the muscle tendon.
Seminal studies in cats, and later in rats, had shown that there exist correlations between the electrical properties of motoneurons and the contractile properties of their MU [2]. However, how much of these correlations held true in mice, and how to apply them to identify mouse MN types was unknown. The task was made even more arduous by the fact that, for the longest time, mouse spinal MNs could only be recorded in vitro. In these conditions, MNs are disconnected from their muscle fiber, which makes identification of their type difficult. Furthermore, for technical reasons, these recordings were restricted to animals younger than two weeks, i.e. during the postnatal development.
Electrophysiological properties of S- and F-type motoneurons in neonatal mice
Neonatal mouse MNs were shown to display different discharge patterns in response to long current pulses at intensities close to the rheobase [3–5]. In these conditions, they either start to discharge immediately at the onset of the current pulse (immediate firing pattern, 33% of the MN population) or discharge with a delay of a few seconds (delayed firing pattern, 67% of the population [4]). We have recently demonstrated that delayed firing MNs have larger input conductances, higher rheobases, more depolarized voltage thresholds for spiking, narrower action potentials and shorter AHPs, longer dendrites and more dendritic branches than immediate firing MNs (Figure 1). Together with the expression of molecular markers (see below, and Figure 1C), these differential features identify delayed- and immediate-firing MNs as F-type and S-type, respectively [4]. The delayed firing pattern was shown to be caused by a combination of two potassium currents: an A-like current that acts at a short time scale (less than 100 ms) and a slowly-inactivating current that acts at a longer time scale (several seconds) [6]. The channel responsible for the slowly inactivating current has recently been identified as Kv1.2 [7]. This current probably plays a key role in dynamically setting the recruitment threshold of the F-type MNs: the memory effect induced by its long lasting action alters the MN recruitment threshold depending upon its firing history [6,7]. Both patterns of discharge are still present in adults as shown using in vivo intracellular MN recordings [7], but whether they continue to be segregated by type remains to be determined.
Figure 1. S- and F-type MNs can be distinguished based on their firing pattern in neonatal mice.
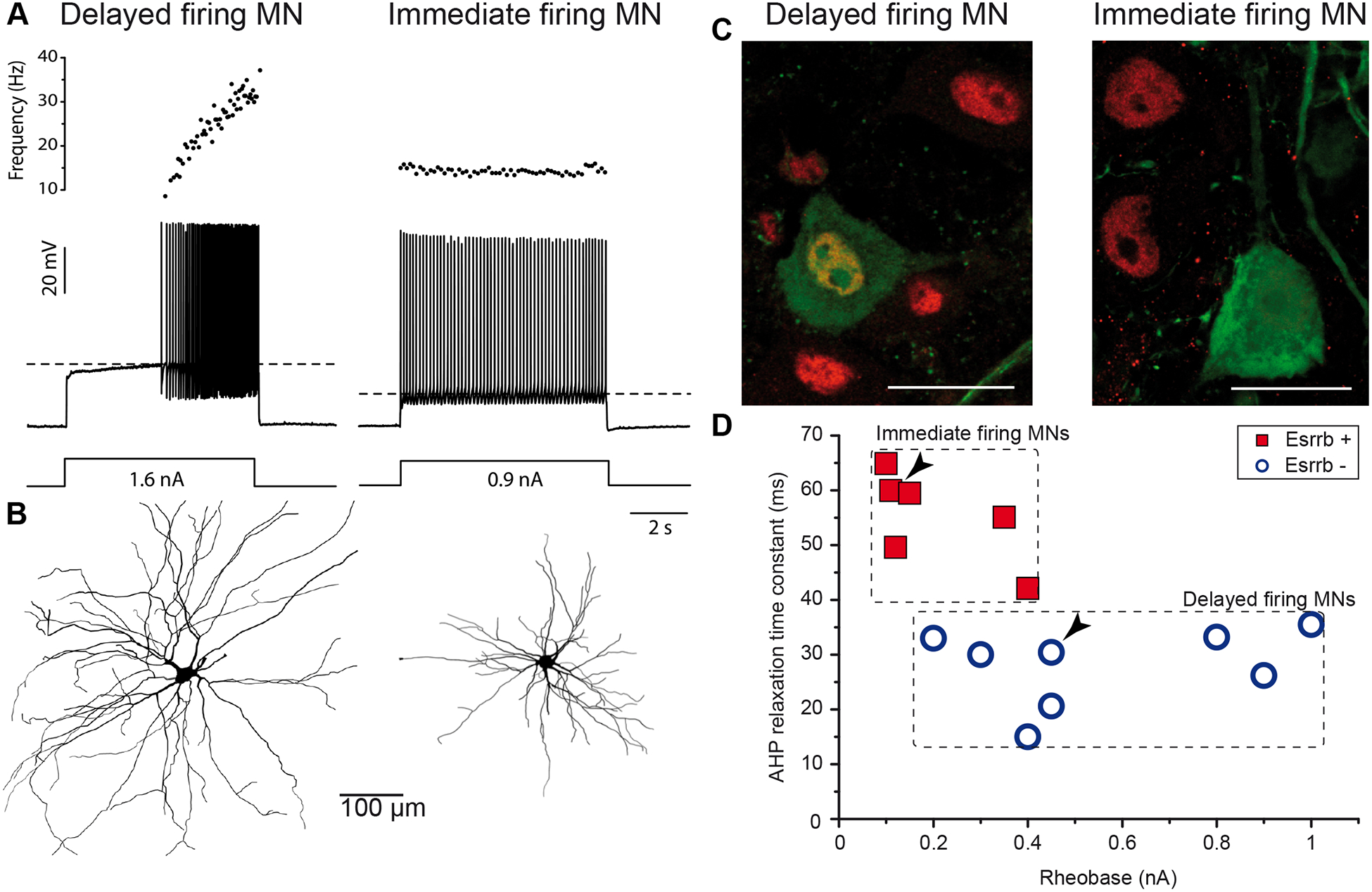
A. Response of a delayed-firing (left) and an immediate-firing (right) motoneuron to a 5 s pulse. The current intensity was the minimal intensity necessary to elicit firing in our searching protocol (rheobase). Bottom: injected-current (square pulses), middle: voltage-response and top: instantaneous firing frequency. The horizontal dashed line shows the voltage threshold for spiking. B. Reconstructed dendritic trees of delayed- (left) and immediate-firing (right) motoneurons. The axon was not reconstructed in either case. C. Examples of Esrrb staining (red) in neurobiotin-filled (green) motoneurons. Scale bar: 30 μm. Both delayed and immediate firing MNs do express NeuN and receive proprioceptive VGluT1-positive synapses (not shown) indicating that they are both α-MNs. However, only immediate firing MNs express Esrrb. In addition, none of the immediate firing MN express Chodl or MMP9 (not shown), whereas half of the delayed firing MN (mainly those with the highest input conductances and rheobases) do. D. Plot of the AHP relaxation time constants against the rheobases for labelled motoneurons. Arrowheads point to the motoneurons illustrated in C. Adapted from [4], licensed under CC-BY.
In vivo intracellular recordings allow functional identification of motor unit type
Over the last decade, technical breakthroughs have made it possible to record spinal MNs in vitro in older animals [8–12], but the problem of identifying MN type without MU output remains. In parallel, preparations allowing intracellular recordings of motoneurons in vivo in adult mice were developed. These preparations allow recording fully mature MNs, which has been difficult to achieve in vitro, as well as to investigate the different compartments of motor units (inputs on motoneurons from specific pathways, intrinsic membrane properties of motoneurons and force output). A number of technical issues had to be solved to reach that point, in particular the maintenance, for several hours, of good physiological conditions of the mouse despite the invasive surgery necessary to get access to spinal cord, nerves and muscles. Actually, the first paper with in vivo motoneuron intracellular recordings was published in 1975 [13], but we had to wait almost 30 years for the publication of a second paper [14], illustrating how difficult such a work is. Since that time, several groups put a lot of efforts in developing in vivo preparations allowing to investigate the intrinsic properties of motoneurons in anaesthetized mice [15,16], the force output of individual motor units [17,18] and the motoneuron response during fictive locomotion in decerebrated mice [19–21].
These technical breakthroughs allowed us to identify the physiological type of the MU and to correlate, for the first time in mice, the electrical properties of MNs to the physiological type of the MU [22]. MUs were classified in S, FR and FF based on twitch amplitude, twitch contraction time, and fatigability (Figure 2). The electrophysiological properties of the different types of MNs followed a similar pattern as previously described: S MNs have a small input conductance and are the most excitable; FR MNs have input conductance generally higher than S, and are therefore generally less excitable; FF MNs are the least excitable, with high input conductances and large recruitment current [22]. However, the distributions of electrophysiological properties overlap widely, and further work is needed to define good criteria, usable in mice, allowing identification of MN type based solely on their electrophysiological properties.
Figure 2. Functional characterization of mouse motor units in vivo.
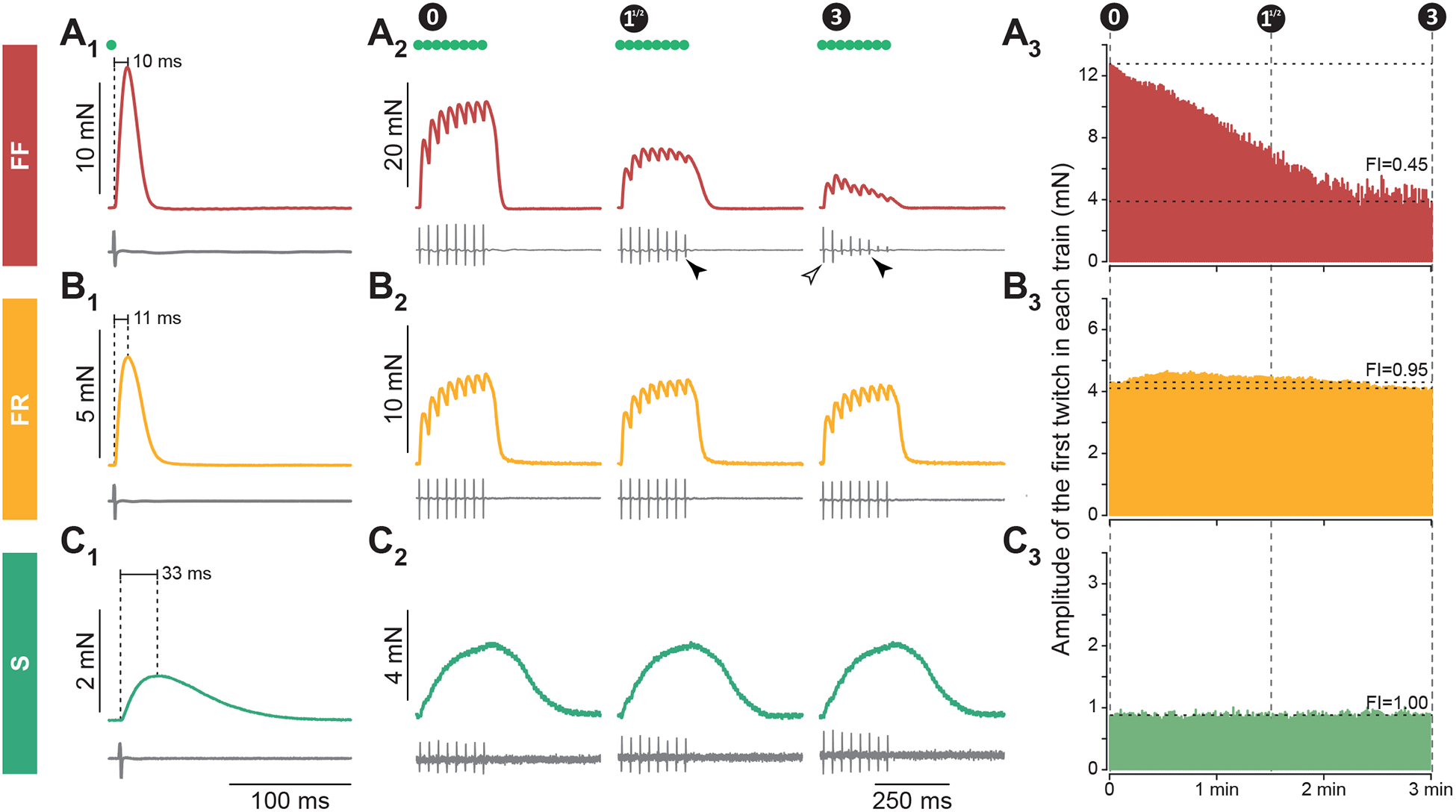
A–C. Procedure for type-identification of mouse MUs in vivo and examples of three MUs: FF (A), FR (B) and S (C). Individual twitch response allow measurement of twitch amplitudes and contraction time (A1, B1, C1). Series of unfused tetani allow to test the fatigability of the muscle fibers (A2–3, B2–3, C2–3). Motor units were classified as S-type if contraction time ≥20 ms; FR if contraction time <20 ms and twitch amplitude <8 mN; FF if contraction time <20 ms and twitch force ≥8 mN. See [22] for details. Figure from [22], licensed under CC-BY.
Molecular markers of motoneuron subtypes
Although electrophysiological experiments are able to provide information about the type of the recorded MNs, these techniques remain challenging, time consuming, and fairly low-throughput. This is the reason why investigators have searched for ways to distinguish the different types of MNs on histological slices.
With the advent of mouse genetics and transcriptomic analysis, it has become possible to identify genes expressed in specific types of MNs (see Figure 3). A first step in this direction was to unequivocally differentiate αMNs from ɣMNs. Early work in cats had demonstrated that ɣMNs are smaller than αMNs [23], and they do not receive monosynaptic connection from Ia afferents [24,25], nor C-boutons [26]. These features were key in identifying several specific markers of α and ɣMNs (Figure 3). αMNs specifically express the neuron-specific nuclear protein NeuN [27] and Osteopontin [28]. On the other hand, ɣMNs are characterized by the lack of NeuN expression, and the selective expression of GDNF receptor Gfrα1 [29], and the serotonin receptor 5HT1d [30]. Interestingly, although both populations are dependent upon the expression of the homeobox gene Hb9 [31,32], ɣMNs do not express GFP in Hb9::GFP mice [29].
Figure 3. Summary of putative molecular markers of motoneuron subtypes.
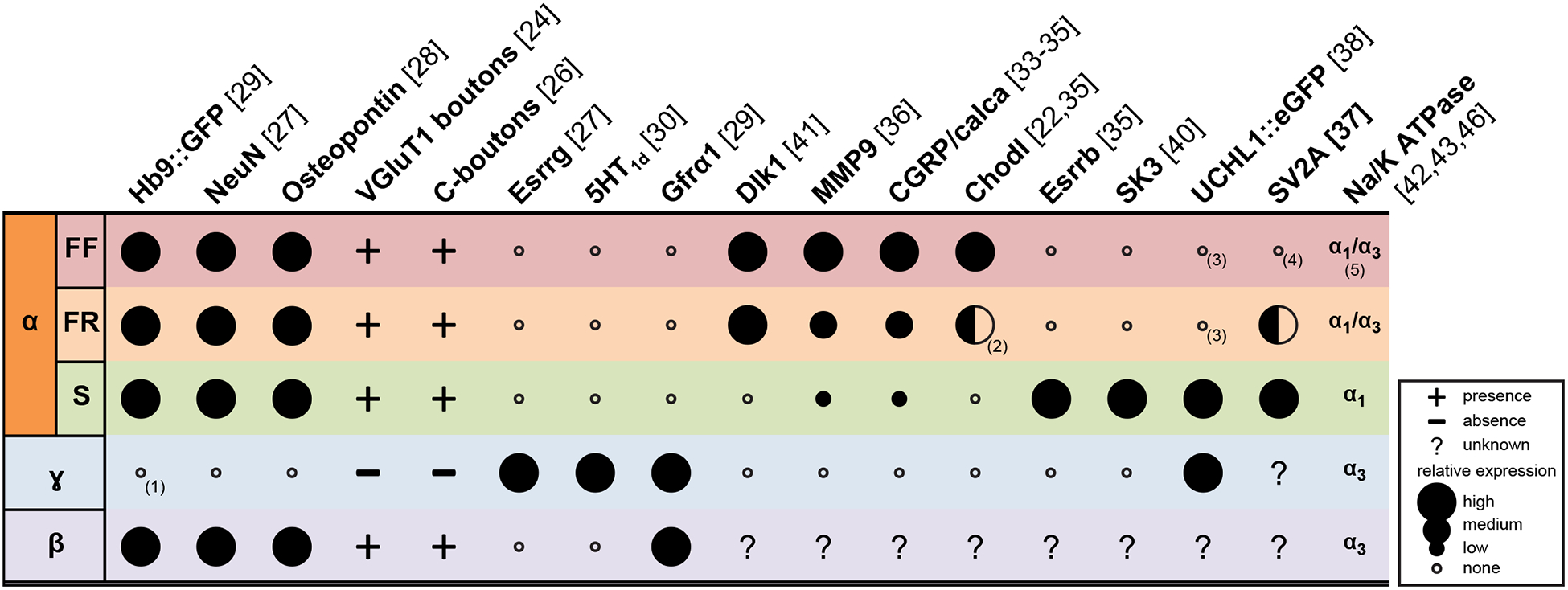
Notes: (1) No expression of GFP in Hb9::GFP mice despite the expression of Hb9 in ɣMNs during development [31]. (2) Expression of Chodl is restricted to the largest FR MUs, see [22] and text for details. (3) True only in rodents. SK3 is expressed in all αMN subtypes in cats. (4) expression is restricted postnatally only: signal is present at P0 but disappears at P14. (5) Several authors seem to disagree on this point, see text for details.
Seminal studies in cats have shown that there are morphological differences between αMNs subtypes [2]. Yet, there are no clear-cut parameters that allow differentiating one type of motoneuron from another. This issue is even more true in neonates where morphological differences are even less pronounced. In order to distinguish S, FR and FF αMNs, investigators have focused on genes that are expressed in some, but not all, αMNs. Doing so, several groups have identified Calcitonin gene-related peptide (CGRP)/calca [33–35], Chondrolectin [35], and Matrix metallopeptidase 9 (MMP-9) [36] as potential markers of F-type MN, and Estrogen-related receptor beta (Esrrb) [35] as a potential maker of S MNs. Since S- and F-type MN innervate different types of muscle fibers, Chakkalakal et al. [37] have studied the distribution of the isoforms of the synaptic vesicle protein SV2 at the neuromuscular junction and have shown that the expression of the SV2A isoform becomes restricted to S-type MNs postnatally. More fortuitously, as a way to study the function of the UCHL1 gene, Yasvoina et al. [38] have generate a mouse expressing eGFP under the UCHL1 promoter. In this mouse, eGFP is expressed by cortico-spinal neurons, as well as a subpopulation of small-size spinal MNs. Observation of their neuromuscular junctions revealed that some of these MNs are ɣMNs, while others are presumably S-type αMNs [38].
Of particular importance for the study of MU physiology are genes that control the differential electrophysiological properties of the MN subtypes. For example, S-type MNs have a longer afterhyperpolarization (AHP) following each spike than F-type MNs [39]. Deardorff et al. [40] have observed that, at least in rodents, the SK3 isoform of the calcium-activated K+ channels responsible for this hyperpolarization is specifically expressed in the smallest, presumably S-type, αMNs. The different electrophysiological properties of each MN type suggest that, at some point during their development, each type expresses transcription factors responsible for the expression of a set of membrane conductances. One such transcription factor, Dlk1 is expressed by the large, presumably F-type MNs, and it is both necessary and sufficient to promote a “fast” electrophysiological signature in αMNs [41].
There exists another type of motoneuron, the so-called β-motoneurons that are innervating both extrafusal and intrafusal muscle fibers [1]. Studying this type of MN has been hindered by the extreme difficulty in identifying them. Initially, the α3 isoform of the Na/K ATPase was suggested as a selective marker for ɣMNs [42]. However, others have observed the expression of that protein in αMNs [43–45]. In particular, Ruegsegger et al. have shown, using retrograde labeling from either a muscle subcompartment containing only FF fibers or a muscle containing both S and FR muscle fibers, that FF MNs express α3, while S MNs only express the α1 subunit [43]. This contradiction may be resolved by the observation, in sections of ventral roots, that α3 is present in the plasma membrane of small-diameter myelinated fibers (i.e. ɣ), as well as some (but not all) substantially larger fibers, suggesting that this isoform is expressed by both ɣ- and larger MNs, which could be βMNs [46]. Whatever the case, more work is required to identify unequivocally βMNs.
Despite great strides in the identification of potential markers of MN type, we believe it is necessary to remain cautious. These putative markers rely, particularly for the different types of αMNs, on size differences between MNs; but, as mentioned above, size distributions overlap widely. In vivo recordings open the possibility to validate the selectivity of putative molecular markers in MNs that have been functionally identified in adult animals. Chodl was the first marker to be investigated this way (Figure 4). We found that Chodl is specifically expressed in FF as well as the largest FR MUs but not in the smaller FR, nor in the S-type MUs [22]. In other words, contrary to the initial study presenting Chodl as a marker of F-type MNs [35], we show that Chodl is a marker of size, rather than type: even though all Chodl+ MNs are fast, not all fast MNs are Chodl+, and Chodl− MNs can be either slow or fast. Interestingly, Chodl has been implicated in motor axon growth during development [47,48], which could explain why it is expressed by large MUs, whose axon displays extensive branching within the muscle.
Figure 4. Chondrolectin is a marker of size rather than type.
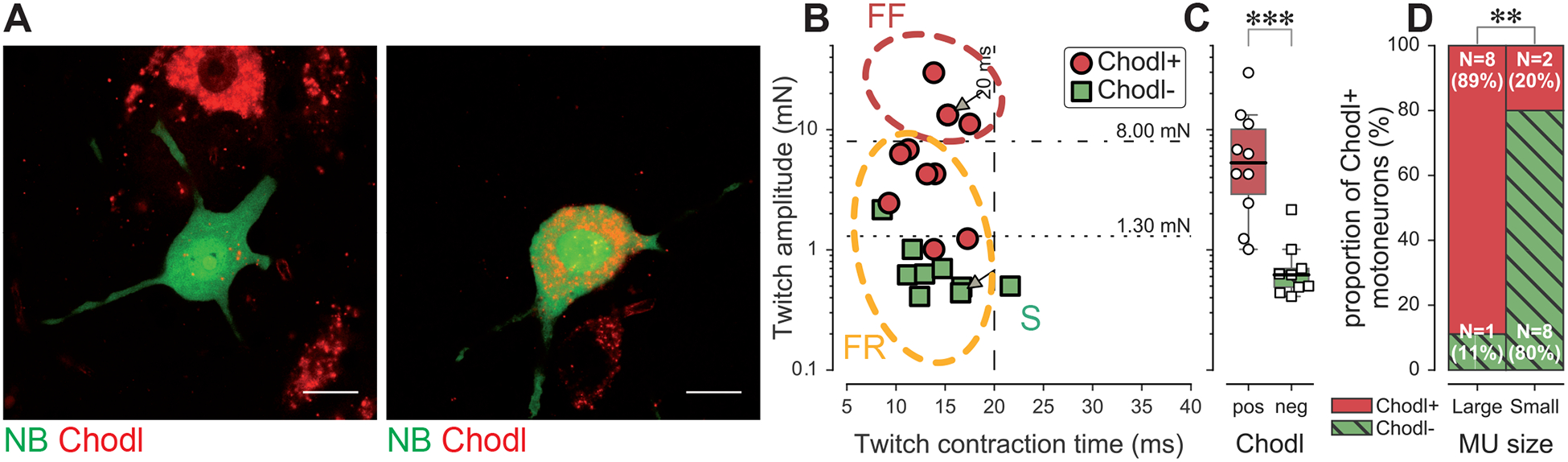
A. Two examples of intracellular-labeled (neurobiotin, NB, green) motoneurons, coupled with ISH revelation of Chodl RNA (Chodl, red). In each experiment, a single MN was type-identified, labelled with an intracellular dye (NB), in order to recognize it among all the other MNs; and in situ hybridization against Chodl mARN was performed after fixation and slicing of the spinal cord. Left panel: Chodl− small FR motoneuron; Right panel: Chodl+ FF motoneuron. Scale bars: 15 μm. B. Contractile properties of the motor units tested for Chodl expression. The motoneurons indicated with arrows correspond to the two cells in D. Red circles are the motoneurons that expressed Chodl, while green squares are those that did not. The dashed lines at 8 mN and 20 ms separate the different types of MUs, and the dash-dotted line at 1.3 mN separates large from small MUs. C. Comparison of the average twitch amplitude of motor units split according to their expression of Chodl. D. Comparison of the proportion of cells expressing Chodl in the population of tested cells, split in two categories, large and small. Adapted from [22], licensed under CC-BY.
Conclusion and perspectives
A lot of work has been done in the last decade to identify the mosaic of genes/protein that confer to each motoneuron subtype its identity. Even if electrophysiological validation remains to be done, the studies presented here offer new exciting tools for the study of the spinal motor system. Case in point, being able to recognize MN-type using molecular markers has already allowed demonstrating that vestibular and proprioceptive systems project differently on F- and S-type MNs [49]. Furthermore, we believe that these tools will be especially important for the study of Amyotrophic Lateral Sclerosis (ALS), the most prominent neurodegenerative disease of MNs, where the order of MN degeneration depends on their type: FF MUs degenerate first, followed by FR units, whereas S-type MUs are the most resistant [50,51].
Coupled with the cre/lox or crispr/cas9 technologies, highly selective molecular markers would have potentially limitless applications. For instance, optogenetic tools could be expressed in specific subpopulations of MNs to study their role in various behavioral tasks. The future is bright for the young generation of spinal cord physiologists, whose imagination is the sole limit in applying these new technologies to answering questions that no one has yet dared to ask.
Footnotes
Declarations of interest: none
References
Papers of particular interest have been highlighted as:
● of special interest
●● of outstanding interest
- 1.Manuel M, Zytnicki D: Alpha, beta and gamma motoneurons: functional diversity in the motor system’s final pathway. Journal of integrative neuroscience 2011, 10:243–276. [DOI] [PubMed] [Google Scholar]
- 2.Burke R: Motor units: anatomy, physiology, and functional organization BT - Handbook of Physiology. The Nervous System. Motor Control In Handbook of Physiology. The Nervous System. Motor Control. Edited by: American Physiological Society; 1981:345–421. [Google Scholar]
- 3.Pambo-Pambo A, Durand J, Gueritaud J-P: Early excitability changes in lumbar motoneurons of transgenic SOD1G85R and SOD1G(93A-Low) mice. Journal of Neurophysiology 2009, 102:3627–3642. [DOI] [PubMed] [Google Scholar]
- 4.●.Leroy F, Lamotte d’Incamps B, Imhoff-Manuel RD, Zytnicki D: Early intrinsic hyperexcitability does not contribute to motoneuron degeneration in amyotrophic lateral sclerosis. eLife 2014, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that S- and F- type motoneurons have a different firing patterns in neonatal spinal slices. In this work, MN identity was established on the basis of their electrical properties, their anatomy and the expression of molecular markers.
- 5.Durand J, Filipchuk A, Pambo-Pambo A, Amendola J, Borisovna Kulagina I, Gueritaud JP: Developing electrical properties of postnatal mouse lumbar motoneurons. Front Cell Neurosci 2015, 9:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leroy F, Lamotte d’Incamps B, Zytnicki D: Potassium currents dynamically set the recruitment and firing properties of F-type motoneurons in neonatal mice. Journal of Neurophysiology 2015, 114:1963–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.●●.Bos R, Harris-Warrick RM, Brocard C, Demianenko LE, Manuel M, Zytnicki D, Korogod SM, Brocard F: Kv1.2 Channels Promote Nonlinear Spiking Motoneurons for Powering Up Locomotion. Cell Reports 2018, 22:3315–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work identified the channel responsible for the slow depolarization observed in delayed-firing motoneurons. It further shows that this channel can act as a gain control mechanism and helps generate a smooth transition from quiescence to steady-state locomotion.
- 8.Mitra P, Brownstone RM: An In Vitro Spinal Cord Slice Preparation for Recording from Lumbar Motoneurons of the Adult Mouse. Journal of Neurophysiology 2011. [DOI] [PubMed] [Google Scholar]
- 9.Husch A, Cramer N, Harris-Warrick RM: Long duration perforated patch recordings from spinal interneurons of adult mice. Journal of Neurophysiology 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang MC, Heckman CJ: In vitro sacral cord preparation and motoneuron recording from adult mice. The Journal of neuroscience : the official journal of the Society for Neuroscience 2006, 156:31–36. [DOI] [PubMed] [Google Scholar]
- 11.Carp JS, Tennissen AM, Mongeluzi DL, Dudek CJ, Chen XY, Wolpaw JR: An in vitro protocol for recording from spinal motoneurons of adult rats. Journal of Neurophysiology 2008, 100:474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadzipasic M, Tahvildari B, Nagy M, Bian M, Horwich AL, McCormick Da: Selective degeneration of a physiological subtype of spinal motor neuron in mice with SOD1-linked ALS. Proceedings of the National Academy of Sciences of the United States of America 2014, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huizar P, Kuno M, Miyata Y: Electrophysiological properties of spinal motoneurones of normal and dystrophic mice. The Journal of Physiology 1975, 248:231–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alstermark B, Ogawa J: In vivo recordings of bulbospinal excitation in adult mouse forelimb motoneurons. Journal of Neurophysiology 2004, 92:1958–1962. [DOI] [PubMed] [Google Scholar]
- 15.Manuel M, Iglesias C, Donnet M, Leroy F, Heckman CJ, Zytnicki D: Fast kinetics, high-frequency oscillations, and subprimary firing range in adult mouse spinal motoneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 2009, 29:11246–11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meehan CF, Sukiasyan N, Zhang M, Nielsen JB, Hultborn H: Intrinsic properties of mouse lumbar motoneurons revealed by intracellular recording in vivo. Journal of Neurophysiology 2010, 103:2599–2610. [DOI] [PubMed] [Google Scholar]
- 17.Manuel M, Heckman CJ: Adult mouse motor units develop almost all of their force in the subprimary range: a new all-or-none strategy for force recruitment? The Journal of neuroscience : the official journal of the Society for Neuroscience 2011, 31:15188–15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manuel M, Heckman CJ: Simultaneous intracellular recording of a lumbar motoneuron and the force produced by its motor unit in the adult mouse in vivo. Journal of visualized experiments : JoVE 2012:e4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakanishi ST, Whelan PJ: A decerebrate adult mouse model for examining the sensorimotor control of locomotion. Journal of Neurophysiology 2012, 107:500–515. [DOI] [PubMed] [Google Scholar]
- 20.Meehan CF, Grondahl L, Nielsen JB, Hultborn H: Fictive locomotion in the adult decerebrate and spinal mousein vivo. Journal of Physiology 2012, 590:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meehan CF, Mayr KA, Manuel M, Nakanishi ST, Whelan PJ: Decerebrate mouse model for studies of the spinal cord circuits. Nature protocols 2017, 12:732–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.●.Martínez-Silva MdL, Imhoff-Manuel RD, Sharma A, Heckman CJ, Shneider NA, Roselli F, Zytnicki D, Manuel M: Hypoexcitability precedes denervation in the large fast-contracting motor units in two unrelated mouse models of ALS. eLife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study is the first to identify functionally MN subtypes, in mice, based on the force developed by their MUs.
- 23.Moschovakis AK, Burke RE, Fyffe RE: The size and dendritic structure of HRP-labeled gamma motoneurons in the cat spinal cord. The Journal of comparative neurology 1991, 311:531–545. [DOI] [PubMed] [Google Scholar]
- 24.Eccles JC, Eccles RM, IGGO A, Lundberg A: Electrophysiological studies on gamma motoneurones. Acta physiologica Scandinavica 1960, 50:32–40. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez FJ, Villalba RM, Zerda R, Schneider SP: Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. The Journal of comparative neurology 2004, 472:257–280. [DOI] [PubMed] [Google Scholar]
- 26.Arvidsson U, Svedlund J, Lagerbäck PA, Cullheim S: An ultrastructural study of the synaptology of gamma-motoneurones during the postnatal development in the cat. Brain research 1987, 465:303–312. [DOI] [PubMed] [Google Scholar]
- 27.Friese A, Kaltschmidt JA, Ladle DR, Sigrist M, Jessell TM, Arber S: Gamma and alpha motor neurons distinguished by expression of transcription factor Err3. Proceedings of the National Academy of Sciences of the United States of America 2009, 106:13588–13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misawa H, Hara M, Tanabe S, Niikura M, Moriwaki Y, Okuda T: Osteopontin is an alpha motor neuron marker in the mouse spinal cord. Journal of neuroscience research 2012, 90:732–742. [DOI] [PubMed] [Google Scholar]
- 29.Shneider NA, Brown MN, Smith CA, Pickel J, Alvarez FJ: Gamma motor neurons express distinct genetic markers at birth and require muscle spindle-derived GDNF for postnatal survival. Neural development 2009, 4:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enjin A, Leão KE, Mikulovic S, Le Merre P, Tourtellotte WG, Kullander K: Sensorimotor function is modulated by the serotonin receptor 1d, a novel marker for gamma motor neurons. Molecular and cellular neurosciences 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S: Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron 1999, 23:659–674. [DOI] [PubMed] [Google Scholar]
- 32.Dasen JS, Jessell TM: Hox networks and the origins of motor neuron diversity. Current topics in developmental biology 2008, 88:169–200. [DOI] [PubMed] [Google Scholar]
- 33.Piehl F, Arvidsson U, Hökfelt T, Cullheim S: Calcitonin gene-related peptide-like immunoreactivity in motoneuron pools innervating different hind limb muscles in the rat. Experimental brain research 1993, 96:291–303. [DOI] [PubMed] [Google Scholar]
- 34.Ringer C, Weihe E, Schütz B: Calcitonin gene-related peptide expression levels predict motor neuron vulnerability in the superoxide dismutase 1-G93A mouse model of amyotrophic lateral sclerosis. Neurobiology of disease 2012, 45:547–554. [DOI] [PubMed] [Google Scholar]
- 35.Enjin A, Rabe N, Nakanishi ST, Vallstedt A, Gezelius H, Memic F, Lind M, Hjalt T, Tourtellotte WG, Bruder C, et al. : Identification of novel spinal cholinergic genetic subtypes disclose Chodl and Pitx2 as markers for fast motor neurons and partition cells. The Journal of comparative neurology 2010, 518:2284–2304. [DOI] [PubMed] [Google Scholar]
- 36.●●.Kaplan A, Spiller KJK, Towne C, Kanning KKC, Choe GT, Geber A, Akay T, Aebischer P, Henderson CE: Neuronal matrix metalloproteinase-9 is a determinant of selective neurodegeneration. Neuron 2014, 81:333–348. [DOI] [PMC free article] [PubMed] [Google Scholar]; This transcriptomic study attempts to identify ALS resistance and susceptibility genes by comparing resistant and vulnerable motor pools. They identified MMP9 as a susceptibility gene and showed that MMP9 is expressed in the largest, most vulnerable MNs. Furthermore, knocking out MMP9 protects against neuromuscular junction denervation, improve motor performance and lifespan.
- 37.Chakkalakal JV, Nishimune H, Ruas JL, Spiegelman BM, Sanes JR: Retrograde influence of muscle fibers on their innervation revealed by a novel marker for slow motoneurons. Development (Cambridge, England) 2010, 137:3489–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasvoina MV, Genç B, Jara JH, Sheets PL, Quinlan KA, Milosevic A, Shepherd GMG, Heckman CJ, Ozdinler PH: eGFP Expression under UCHL1 Promoter Genetically Labels Corticospinal Motor Neurons and a Subpopulation of Degeneration-Resistant Spinal Motor Neurons in an ALS Mouse Model. The Journal of neuroscience : the official journal of the Society for Neuroscience 2013, 33:7890–7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gustafsson B, Pinter MJ: An investigation of threshold properties among cat spinal alpha-motoneurones. The Journal of Physiology 1984, 357:453–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.●●.Deardorff AS, Romer SH, Deng Z, Bullinger KL, Nardelli P, Cope TC, Fyffe REW: Expression of postsynaptic Ca2+-activated K+ (SK) channels at C-bouton synapses in mammalian lumbar-motoneurons. The Journal of physiology 2013, 591:875–897. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work shows very high quality immunolabeling pictures showing the restricted expression of the SK3 isoform of the Ca2+-activated K+ (SK) channels in the smallest αMNs. Using correlations with electrophysiological features (AHP), the authors make the convincing argument that this protein is only expressed in S-type αMNs. Remarkably, these channels are exclusively located on the post-synaptic side of the C-boutons.
- 41.●●.Müller D, Cherukuri P, Henningfeld K, Poh CH, Wittler L, Grote P, Schlüter O, Schmidt J, Laborda J, Bauer SR, et al. : Dlk1 promotes a fast motor neuron biophysical signature required for peak force execution. Science (New York, N.Y.) 2014, 343:1264–1266. [DOI] [PubMed] [Google Scholar]; This work demonstrates that the transcription factor Dlk1 is both necessary and sufficient to promote a “fast” signature to MNs. Dlk1 controls an network of genes setting the excitability and firing properties of F-type MNs.
- 42.Edwards IJ, Bruce G, Lawrenson C, Howe L, Clapcote SJ, Deuchars SA, Deuchars J: Na+/K+ ATPase α1 and α3 isoforms are differentially expressed in α- and γ-motoneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 2013, 33:9913–9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.●●.Ruegsegger C, Maharjan N, Goswami A, Filézac de L’Etang A, Weis J, Troost D, Heller M, Gut H, Saxena S: Aberrant association of misfolded SOD1 with Na(+)/K(+)ATPase-α3 impairs its activity and contributes to motor neuron vulnerability in ALS. Acta neuropathologica 2016, 131:427–451. [DOI] [PubMed] [Google Scholar]; This study reveals that misfolded mutSOD1 proteins interacts with the α3 isoform of the Na/K ATPase, reducing its activity. Inhibition of Na/K ATPase α3, which is highly express in FF MNs (the most vulnerable in ALS), accelerates the disease.
- 44.Mata M, Siegel GJ, Hieber V, Beaty MW, Fink DJ: Differential distribution of (Na,K)-ATPase alpha isoform mRNAs in the peripheral nervous system. Brain research 1991, 546:47–54. [DOI] [PubMed] [Google Scholar]
- 45.Dolapchieva SD: Expression of Na+,K(+)-ATPase alpha and beta subunit isoforms in the motor neurons of the rat spinal cord. Membrane & cell biology 1998, 12:355–361. [PubMed] [Google Scholar]
- 46.Dobretsov M, Stimers JR: Neuronal function and alpha3 isoform of the Na/K-ATPase. Front Biosci 2005, 10:2373–2396. [DOI] [PubMed] [Google Scholar]
- 47.Zhong Z, Ohnmacht J, Reimer MM, Bach I, Becker T, Becker CG: Chondrolectin mediates growth cone interactions of motor axons with an intermediate target. The Journal of neuroscience : the official journal of the Society for Neuroscience 2012, 32:4426–4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sleigh JN, Barreiro-Iglesias A, Oliver PL, Biba A, Becker T, Davies KE, Becker CG, Talbot K: Chondrolectin affects cell survival and neuronal outgrowth in in vitro and in vivo models of spinal muscular atrophy. Human molecular genetics 2014, 23:855–869. [DOI] [PubMed] [Google Scholar]
- 49.●●.Basaldella E, Takeoka A, Sigrist M, Arber S: Multisensory Signaling Shapes Vestibulo-Motor Circuit Specificity. Cell 2015, 163:301–312. [DOI] [PubMed] [Google Scholar]; This work used cutting-edge viral technologies and molecular markers to demonstrate that vestibular inputs target preferentially extensor vs. flexor motor pools, and that, within the extensor motor pool, they target preferentially S-type vs. F-type MNs. Interestingly, the density of proprioceptive inputs on F- and S-type MNs is anti-correlated to the distribution of vestibular inputs. They go on to show that the two systems interact, probably through retrograde and homeostatic mechanisms, so that genetically imposed changes to one system are counterbalanced by the other.
- 50.Pun S, Santos AF, Saxena S, Xu L, Caroni P: Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nature neuroscience 2006, 9:408–419. [DOI] [PubMed] [Google Scholar]
- 51.Hegedus J, Putman CT, Tyreman N, Gordon T: Preferential motor unit loss in the SOD1 G93A transgenic mouse model of amyotrophic lateral sclerosis. The Journal of physiology 2008, 586:3337–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]


