Abstract
Background
Acute hospitalization may lead to posthospital syndrome, but no studies have investigated how this syndrome manifests and geriatric syndromes are often used as synonym. However, studies on longitudinal associations between syndromes and adverse outcomes are scarce. We aimed to analyze longitudinal associations between geriatric syndromes and functional decline (FD), readmission, and mortality.
Methods
Prospective cohort study, including 401 acutely hospitalized patients (aged ≥ 70). We performed: (i) logistic regression analyses to assess associations between patterns of geriatric syndromes as they develop over time (between admission and 1 month postdischarge), and FD and readmission; (ii) generalized estimating equations to assess longitudinal associations between geriatric syndromes over five time points (admission, discharge, 1, 2, and 3 months postdischarge) and FD, mortality, and readmission at 3 months postdischarge.
Results
After syndrome absent, syndrome present at both admission and 1 month postdischarge was most prevalent. Persistent patterns of apathy (odds ratio [OR] = 4.35, 95% confidence interval [CI] = 1.54–12.30), pain (OR = 3.26, 95% CI = 1.21–8.8), malnutrition (OR = 3.4, 95% CI = 1.35–8.56), mobility impairment (OR = 6.65, 95% CI = 1.98–22.38), and fear of falling (OR = 3.17, 95% CI = 1.25–8.02) were associated with FD. Developing cognitive impairment (OR = 6.40, 95% CI = 1.52–26.84), fatigue (OR = 4.71, 95% CI = 1.03–21.60), and fall risk (OR = 4.30, 95% CI = 1.21–16.57) postdischarge, was associated with readmission; however, only 4%–6% developed these syndromes. Over the course of five time points, mobility impairment, apathy, and incontinence were longitudinally associated with FD; apathy, malnutrition, fatigue, and fall risk with mortality; malnutrition with readmission.
Conclusion
Most geriatric syndromes are present at admission and patients are likely to retain them postdischarge. Several geriatric syndromes are longitudinally associated with mortality and, particularly, persistently present syndromes place persons are at risk of FD. Although few persons develop syndromes postdischarge, those developing cognitive impairment, fatigue, and fall risk were at increased readmission risk.
Keywords: Geriatric syndromes, Acute hospitalization, Postdischarge, Adverse outcomes
After hospitalization for an acute medical illness, older persons are at great risk of several adverse health outcomes. For over 30% of patients aged 65 and older, hospitalization leads to functional decline (FD) (1–3), even when these individuals tend to have a good baseline functioning level (4). Particularly, the first month postdischarge has been marked as a crucial period for recovery, after which functional impairments are likely to remain persistent (3). Additionally, over 30% of patients require another unplanned hospitalization in the first 90 days postdischarge (5), and postdischarge mortality rates within this same timeframe are as high as 16% (6). Extensive research has been conducted to identify risk factors at admission for adverse postdischarge outcomes and found that, for example, old age, the severity of the acute medical illness and comorbidity are important risk factors for FD (7), readmission (5), and postdischarge mortality (8).
A few years ago, it was proposed to use the term posthospital syndrome to indicate the vulnerable period after acute hospitalization and a failure to recover to baseline functioning (9). It is thought that patients do not only try to recover from the acute medical illness but experience an acquired state of risk for readmission, due to the adverse circumstance of hospitalization, including bed rest and malnutrition. While some studies support the hypothesis that in-hospital factors may increase the risk of poor outcomes postdischarge (2,7), no previous studies have investigated the real construct of a posthospital syndrome, that is, how it manifests itself or how it is defined in terms of actual symptoms that might be present during the first months postdischarge.
Currently, when referring to a posthospital syndrome, geriatric syndromes such as pain, depressive symptoms, and cognitive impairment are often used as a synonym. Previous research shows that older patients present with an average of six geriatric syndromes at time of admission (1) and the great majority of older patients experiences at least one syndrome in the prehospital period (10). Given that patients are likely to retain geriatric syndromes in the postdischarge period (11), and strong associations have been observed between the presence of geriatric syndromes during hospitalization and FD (1,12,13), it is plausible that postdischarge geriatric syndromes may place patients further at risk for adverse health outcomes and, subsequently, form a hindrance to optimal recovery. Currently missing, however, are studies that provide insight into the longitudinal relationship between geriatric syndromes and adverse outcomes over the course from admission until the first three critical months posthospitalization.
In the present study, the Hospital-Associated Disability and Impact on Daily Life (Hospital-ADL) study, we aimed to explore how single geriatric syndromes may contribute to adverse postdischarge outcomes in acutely hospitalized patients aged 70 and older. Specifically, we aimed to describe patterns of single syndromes as they develop over time (between admission and 1 month postdischarge) and to analyze the association between these patterns of syndromes and FD and readmission at 3 months postdischarge. Further, we aimed to assess longitudinal associations between the presence of geriatric syndromes over the course of five time points (admission, discharge, 1, 2, and 3 months postdischarge) and FD, mortality, and readmission at 3 months postdischarge.
Methods
Study Design and Setting
The Hospital-ADL prospective cohort study was conducted between October 2015 and June 2017. Details of the study have been described elsewhere (14). Participants were recruited from Internal Medicine, Cardiology, or Geriatric wards of one University Medical Center and five regional teaching hospitals in the Netherlands. The study was approved by the Institutional Review board of the Amsterdam University Medical Centers, location AMC, in The Netherlands (Protocol ID: AMC2015_150) Local approval was provided by the participating hospitals. We adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting of observational studies (15).
Study Population
Patients admitted consecutively, aged 70 and older, acutely admitted for at least 48 hours were approached. The following inclusion criteria were applied: (i) adequate Dutch language proficiency to complete questionnaires; and (ii) Mini-Mental State Examination (MMSE) score ≥15 (16). Although delirium is a common geriatric syndrome (17), we were not able to include delirious patients because an MMSE could not be performed or because patients scored below 15. Further, patients were excluded if they: (i) had a life expectancy of ≤3 months (according to the attending physician); (ii) were disabled in all Katz-ADLs (six items) (18). The attending medical doctor was asked for approval and confirmation that patients could be approached and were, for example, not delirious or terminally ill.
Data Collection
Two researchers (R.S. and L.R.) visited the participating wards on Mondays, Wednesdays, and Fridays. After informed consent was obtained, patients were enrolled. The researchers were trained to administer the study protocol to reduce observer variability. A comprehensive geriatric assessment (CGA) was completed to assess the presence geriatric syndromes at baseline (≤48 hours after admission), discharge, 1 month (home visit), 2 months (by telephone), and 3 months postdischarge (home visit). The CGA evaluated 12 health problems on the psychological, somatic, and functional domain, which are covered by the generally used definition of geriatric syndromes (17,19). Data on the prevalence and course of geriatric syndromes from admission until 3 months postdischarge has been published elsewhere (11).
In the psychological domain, cognitive impairment and depressive symptoms were assessed, by performing, respectively, the MMSE (cutoff point ≤23) (16) and Geriatric Depression Scale-15 (GDS, cutoff point ≥6) (20). Since apathy and depression are considered as separate entities and apathy has been reported to be present postdischarge in qualitative research, we decided to assess apathy separately (using the GDS-3, cutoff point ≥2) (21,22). Although some researchers distinguished apathy and depressive symptoms into two nonoverlapping subscales (GDS-12 and GDS-3), this is particularly done when apathy is the main topic of interest (23,24). When assessing depressive symptoms the GDS-15 (cutoff point ≥6) has been validated and is extensively used to assess depressive symptoms in an older patient population (25,26), which was therefore used in our study, despite the statistical dependence between the GDS-15 and GDS-3.
In the somatic domain, we assessed pain, malnutrition, incontinence (17,19), using a Numerating Rating Scale (NRS, cutoff score ≥4) (27), the Short Nutritional Assessment Questionnaire (SNAQ) (28), and continence item on the Katz-ADL (18), respectively. We considered dizziness, because it is often the result of a multifactorial interplay between medical and functional aspects (29), by asking the question: “do you feel dizzy at this moment?.” Although less commonly described as geriatric syndrome, it was decided to assess fatigue (NRS cutoff score ≥4) (30), since it is an important feature of frailty (17,31), and in previous qualitative research, it was reported to be present postdischarge (22).
In the functional domain, mobility impairment (use of a walking aid) and participants’ fall risk were taken into account. Participants were asked whether they had a fall in the past 6 months prior to hospitalization, during their hospital stay, and in the first, second, and third month postdischarge. Falls are often accompanied by fear of falling (32), which was considered using a NRS, cutoff score ≥4.
The presence of geriatric syndromes was measured at all five time points with two exceptions: (i) malnutrition was not measured at discharge because the SNAQ includes retrospective questions on the previous months, which overlaps with hospital admission; and (ii) cognitive impairment could not be measured at 2 months postdischarge, because a telephone follow-up was performed at that time point.
Outcome Measures
FD was defined as a loss of independence in at least one of the six basic Katz-ADL items (bathing, dressing, toileting, incontinence, transfer from bed-chair, and eating) (18) at 3 months postdischarge compared to 2 weeks prior to admission. Data on unplanned hospital readmissions within 90 days postdischarge were gathered from patient records and we asked at follow-up whether participants were acutely hospitalized in the last month. Readmissions were considered as dichotomous outcome measure (at least one readmission within 3 months). Data on mortality were verified using patient records or obtained from general practitioners or family members.
Longitudinal Relationship Between Geriatric Syndromes and Outcomes
The longitudinal relationship between geriatric syndromes and the outcome variables was analyzed in two ways. First, logistic regressions were performed to analyze the relationship between patterns of syndromes, as they develop between admission and 1 month postdischarge, and FD and readmission. Based on their presence at admission and 1 month postdischarge, the following four patterns of geriatric syndromes were defined: (i) syndrome absent (absent at both time points), (ii) syndrome persistent (syndrome present at both time points), (iii) syndrome resolved (syndrome present at admission and absent at 1 month), and (iv) syndrome developed (syndrome absent at admission and present at 1 month). Because a readmission could occur within 1 month postdischarge, that is before the last measurement point of the pattern, we decided to assess associations between patterns and readmission in the second or third month. It was not possible to determine the association between patterns and mortality, because a large proportion of participants who died, deceased during hospitalization or within 1 month (70%). Note, incontinence is included in the Katz-ADL scale, which was used to determine FD. Therefore, we conducted a sensitivity analysis by removing incontinence from the Katz-ADL scale when assessing the association between patterns of incontinence and FD.
Second, longitudinal associations between the presence of geriatric syndromes over the course of all five time points (including admission, discharge, 1, 2, and 3 months postdischarge, except for malnutrition and cognitive impairment, which were not measured at discharge and 2 months postdischarge, respectively) and FD, unplanned readmission, and mortality at 3 months postdischarge, were assessed using generalized estimating equations (GEE) analyses with an independent correlation structure. This is also known as a “dynamic logistic regression analysis,” which provides the same regression coefficients as a standard logistic regression analysis, but with increased standard errors. This is necessary to take into account the correlation between the repeated observations within the patient (33). The obtained odds ratios (ORs) represent the averaged odds of FD, readmission or mortality in a subject with a certain syndrome compared to a subject without that syndrome across all five time points for each individual patient. Given that the included covariates are not time-dependent in this relatively short timeframe, the time variable itself was not part of the analysis (34). Note, also with regard to the longitudinal association between incontinence and FD, a sensitivity analysis was conducted in which we removed incontinence from the Katz-ADL scale to measure FD. We present adjusted models where we adjusted for demographics (age, sex, educational level, marital status, living situation) and comorbidity index and length of hospital stay. Sensitivity analyses were performed: All longitudinal analyses were repeated using only complete cases. Furthermore, we corrected for multiple comparisons, using Holm’s sequential rejective Bonferroni procedure (35).All statistical analyses were performed using SPSS Statistics (version 24.0).
Results
Patient Characteristics
A total of 1,024 consecutive patients were eligible for participation, of whom 505 did not meet the inclusion criteria, were too ill to participate or could not be approached within 48 hours as they were not present at the ward during time of inclusion. Of the remaining 519, 401 consented to participate (Table 1). A total of 16.5% participants experienced FD, 10.0% deceased during hospitalization or within 3 months postdischarge, and 33.9% were readmitted within 3 months. At 3 months postdischarge, besides the 40 participants who died, 88 (21.9%) participants were lost to follow up Compared to participants who did not leave the study, those who were lost to follow up or died had a significant higher mean age (SD): 81.0 (6.6), were more often single or divorced (59.4%) instead of married, had a higher mean CCI-index (SD): 2.45 (2.13), had a longer median (interquartile range [IQR]) length of hospital stay: 7.02 (4.6–12.0), and were less often discharged home (76.2%).
Table 1.
Baseline Characteristics of the Study Population
| Patient Characteristics | |
|---|---|
| Age in years, mean (SD)a (N = 401) | 79.7 (6.7) |
| Male, N (%) (N = 401) | 206 (51.4) |
| Living arrangements before admission, N (%) (N = 401) | |
| Independent | 337 (84.0) |
| Nursing home | 9 (2.2) |
| Senior residence/Assisted living | 55 (13.7) |
| Marital status, N (%) (N = 401) | |
| Married or living together | 209 (52.1) |
| Single or divorced | 64 (16.0) |
| Widow/widower | 128 (31.9) |
| Born in the Netherlands, N (%) (N = 401) | 359 (89.5) |
| Education, N (%) (N = 401) | |
| Primary school | 101 (25.2) |
| Elementary technical/domestic science school | 89 (22.2) |
| Secondary vocational education | 120 (29.9) |
| Higher level high school/third-level education | 91 (22.7) |
| Charlson Comorbidity Index b (mean, SD) (N = 401) | 2.14 (1.95) |
| Polypharmacy, N(%)c (N = 401) | 260 (64.8) |
| Hearing impairment, N (%) (N = 401) | 52 (13.0) |
| Vision impairment, N (%) (N = 401) | 41 (10.2) |
| Hospitalization in past 6 mo, N (%) (N = 401) | 133 (33.2) |
| Primary admission diagnosis, N (%) (N = 401) | |
| Infection | 58 (14.5) |
| Gastrointestinal | 45 (11.2) |
| Cardiac | 122 (30.4) |
| Respiratory | 75 (18.7) |
| Cancer (including hematology) | 13 (3.2) |
| Electrolyte disturbance | 11 (2.7) |
| Renal | 15 (3.7) |
| Other | 62 (15.5) |
| Length of hospital stay, Median (IQRd) (N = 387) | 5.8 (3.9–8.9) |
| Discharge destination, N (%) (N = 366) | |
| Home | 317 (86.6) |
| Nursing home | 6 (1.6) |
| Rehabilitation center | 20 (5.5) |
| Assisted living | 6 (1.6) |
| Other (eg, other hospital) | 17 (4.6) |
| Received ambulatory physiotherapy or occupational therapy within 3 mo postdischarge, N (%) (N = 281) | 116 (41.3) |
| Unplanned readmission within 3 mo postdischarge, N (%) (N = 271) | 92 (33.9) |
| Unplanned readmission during the second or third month postdischarge, N (%) (N = 268) | 51 (19.0) |
| Functional decline at 3 mo postdischarge, N (%) (N = 273) | 45 (16.5) |
| Missing cases at discharge, N (%) (N = 401) | 24 (6.0) |
| Lost to follow up | 14 (3.5) |
| Deceased | 10 (2.5) |
| Missing cases at 1 mo postdischarge, N (%) | |
| (N = 401) | 92 (22.9) |
| Lost to follow up | 57 (14.2) |
| Declined to participate at this time point | 15 (3.7) |
| Deceased | 20 (5.0) |
| Missing cases at 2 mo postdischarge, N (%) | |
| (N = 401) | 110 (27.4) |
| Lost to follow up | 69 (17.2) |
| Declined to participate at this time point | 12 (3.0) |
| Deceased | 29 (7.2) |
| Missing cases at 3 mo postdischarge, N (%) | |
| (N = 401) | 128 (32.2) |
| Lost to follow up | 88 (21.9) |
| Deceased | 40 (10.0) |
Note: aStandard Deviation. bRange of 0 to 31, with a higher score indicating more or more severe comorbidity (48). cUse of five or more different medications. dInterquartile range.
Relationship Between Patterns of Geriatric Syndromes and FD and Readmission
Syndrome absent at both admission and 1 month postdischarge, was the most frequently observed pattern for all geriatric syndromes. Persistent patterns (syndrome present at both admission and 1 month postdischarge) were the second most prevalent for depressive symptoms, apathy, malnutrition, incontinence, fatigue, mobility impairment, functional impairment, and fear of falling. Syndrome resolved (present at admission, absent at 1 month postdischarge) was the second most often observed for cognitive impairment, pain, dizziness, and fall risk. Cognitive impairment, fall risk, fatigue, depressive symptoms, dizziness, fear of falling, mobility impairment, incontinence were developed in less than 9% of patients postdischarge. Slightly more participants developed pain, malnutrition, and apathy postdischarge (Table 2).
Table 2.
Patterns of Geriatric Syndromes from Admission Until 1 Month Postdischarge
| Geriatric Syndrome (total number of cases) | Syndrome Absent Absent at admission and postdischarge | Syndrome Persistent Present at admission and postdischarge | Syndrome Resolved Present at admission, recovery postdischarge | Syndrome Developed Absent at admission, present postdischarge |
|---|---|---|---|---|
| Cognitive impairment (268) | 79.1 (212) | 7.5 (20) | 9.7 (26) | 3.7 (10) |
| Depressive symptoms (297) | 76.4 (227) | 9.8 (29) | 7.1 (21) | 6.7 (20) |
| Apathy (297) | 30.3 (90) | 31.0 (92) | 22.6 (67) | 16.2 (48) |
| Pain (305) | 51.1 (156) | 17.0 (52) | 17.7 (54) | 14.1 (43) |
| Malnutrition (304) | 51.3 (156) | 20.4 (62) | 14.5 (44) | 13.8 (42) |
| Incontinence (310) | 53.5 (166) | 27.4 (85) | 10.3 (32) | 8.7 (27) |
| Dizziness (306) | 68.3 (209) | 9.2 (28) | 14.7 (45) | 7.8 (24) |
| Fatigue (306) | 16.0 (49) | 51.6 (158) | 26.5 (81) | 5.9 (18) |
| Mobility impairment (310) | 39.4 (122) | 48.7 (151) | 3.5 (11) | 8.4 (26) |
| Fall risk (309) | 56.0 (173) | 9.4 (29) | 29.4 (91) | 5.2 (16) |
| Fear of Falling (305) | 52.8 (161) | 23.3 (71) | 15.7 (48) | 8.2 (25) |
Note: In most participants, syndromes were absent both at admission and 1 month postdischarge. Syndrome persistent indicates that the syndrome is present both at admission and at 1 month postdischarge. For example, in 23.3% of participants, fear of falling was both present at admission 1 month postdischarge; in 26.5% of participants, fatigue was present at admission but resolved at 1 month postdischarge. 13.8% of participants were not malnourished at admission but was malnourished at 1 month postdischarge.
Compared to patients in which these syndromes were absent at both time points, patients with persistent apathy (OR = 4.35, 95% confidence interval [CI] = 1.54–12.30), pain (OR = 3.26, 95% CI = 1.21–8.8), malnutrition (OR = 3.4, 95% CI = 1.35–8.56), mobility impairment (OR = 6.65, 95% CI = 1.98–22.38), and fear of falling (OR = 3.17, 95% CI = 1.25–8.02) were associated with FD (Supplementary Table 1). When depressive symptoms, incontinence, and mobility impairment were absent at admission but present postdischarge, participants had higher odds on FD compared to patients who had no syndromes at both time points. Note, when the incontinence item was removed from the Katz-ADL scale, no significant association was observed between developing incontinence and FD (OR = 1.56, 95% CI = 0.53–4.53, adjusted for age, sex, educational level, marital status, living situation, comorbidity index, and length of hospital stay). Syndrome resolved patterns of cognitive impairment, mobility impairment, and fear of falling were associated with FD. Malnutrition persistently present at admission and 1 month postdischarge, was significantly associated with readmission. Participants who developed cognitive impairment (OR = 6.40, 95% CI = 1.52–26.84), fatigue (OR = 4.71, 95% CI = 1.03–21.60), and fall risk (OR = 4.30, 95% CI = 1.21–16.57) were at increased readmission risk.
Longitudinal Relationship Between Geriatric Syndromes and FD, Mortality, and Readmission
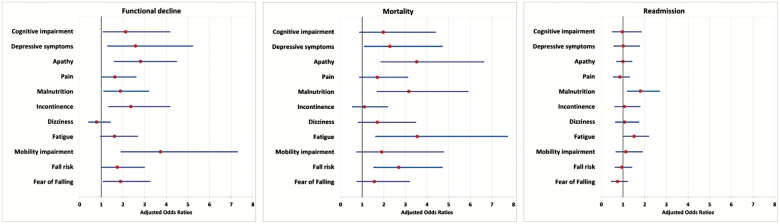
Figure 1 and Supplementary Table 2 show the associations between geriatric syndromes and FD over the course of five time points (admission, discharge, 1, 2, and 3 months postdischarge). Mobility impairment (OR = 3.73, 95% CI = 1.92–7.28, p < .001, α-value = 0.0045), apathy (OR = 2.81, 95% CI = 1.65–4.80, p < .001, α-value = 0.005), and incontinence (OR = 2.37, 95% CI = 1.35–4.17, p = .003, α-value = 0.0056) were significantly associated with FD. Note, the associations between depressive symptoms, malnutrition, fear of falling, cognitive impairment, pain, and fall risk did not remain significant after adjusting for repeated statistical procedures. Sensitivity analyses using only complete cases with data on geriatric syndromes at all time points provided slightly less strong associations with cognitive impairment, depressive symptoms, apathy, pain, fatigue, and fall risk; the associations with malnutrition, incontinence, mobility impairment, and fear of falling became slightly stronger. When removing incontinence from the Katz-ADL, the significant association between incontinence and FD disappeared (OR = 1.73, 95% CI = 0.87–3.37, adjusted for age, sex, educational level, marital status, living situation, comorbidity index, and length of hospital stay). Over all five time points, the presence of apathy (OR = 3.52, 95% CI = 1.87–6.62, p < .001, α-value = 0.0045), fall risk (OR = 2.69, 95% CI = 1.55–4.69, p < .001, α-value = 0.005), malnutrition (OR = 3.16, 95% CI = 1.70–5.89, p < .001, α-value = 0.0056), and fatigue (OR = 3.54, 95% CI = 1.63–7.71, p < .001, α-value = 0.00625) were significantly longitudinally associated with mortality. Depressive symptoms (OR = 2.28, 95% CI = 1.11–4.70, p = .025) did not remain significant after adjusting for repeated statistical procedures (α-value = 0.007). Over all five time points, the presence of malnutrition (OR = 1.80, 95% CI = 1.21–2.68, p = .004, α-value = 0.0045) was longitudinal associated with readmission. Fatigue (OR = 1.51, 95% CI = 1.04–2.18, p = .029) did not remain significant after adjusting for repeated statistical procedures (α-value = 0.005).
Figure 1.
Forest plot of the adjusted odds ratios and their 95% confidence intervals for the longitudinal relationship between geriatric syndromes and functional decline, mortality and readmission over the course of five time points (admission, discharge, 1, 2, and 3 months postdischarge). Generalized estimating equations models adjusted for age, sex, educational level, marital status, living situation, comorbidity index, and length of hospital stay. See Supplementary Table 2 for unadjusted results.
Discussion
This prospective cohort study among acutely hospitalized older patients showed that in particular persistent patterns (present at admission and 1 month postdischarge) of apathy, pain, malnutrition, mobility impairment, and fear of falling seem to place patients at risk of FD. Although patients were unlikely to develop syndromes after admission until 1 month postdischarge, it was found that persons who developed cognitive impairment, fatigue, and fall risk after admission until 1 month postdischarge, had significantly greater odds of readmission in the second or third month postdischarge. Over the course of five time points (admission, discharge, 1, 2, and 3 months postdischarge), the presence of apathy, incontinence, and mobility impairment was longitudinally associated with an increased risk of FD. Over all five time points, apathy, malnutrition, fatigue, and fall risk were longitudinally associated with mortality. Only the presence of malnutrition was longitudinally associated with readmission over the total course from admission until 3 months postdischarge.
Whereas cognitive impairment, pain, and dizziness were relatively often observed to resolve postdischarge, depressive symptoms, apathy, malnutrition, incontinence, fatigue, mobility impairment, and fear of falling, were more often persistently present, indicating that patients’ vulnerability might be persistent. Besides, given that the onset of the acute illness might be 2 weeks prior to admission (7) and geriatric syndromes are often already present in the premorbid period (10), it is likely that this vulnerability is already present before time of admission. In fact, it is conceivable that many of the older patients in our study can be referred to as frail, given that fatigue, malnutrition, and mobility impairment, are common features of frailty (31). In those frail patients, a relatively small acute health problem might have evoked acute hospitalization and, subsequently, caused a health status change resulting in the onset of FD (7,9,31). Strong associations between the presence of geriatric syndromes during hospitalization and FD have already been reported in previous studies (1,12,13), and the current study provides novel information particularly persistently present syndromes from admission until the first month postdischarge seem to place patients at elevated risk of decline in ADL. Our results showed that persons who developed cognitive impairment, fatigue or fall risk at 1 month postdischarge were more likely to be readmitted in the second or third month postdischarge than persons who did not have these syndromes at any time point. Yet, this may account for a very small part of our study population, since only 4, 5, and 6% developed cognitive impairment, fall risk, and fatigue, respectively. Whereas the presence of several syndromes places patients at risk of both death and, to a lesser extent, FD, longitudinal associations with readmission risk was only observed for malnutrition over the course from admission until 3 months postdischarge. This is in line with a previous study in which an association between geriatric syndromes and FD was observed but not with readmission (36). Additionally, previous studies showed that patient factors in general are much better in predicting mortality than readmissions; rather hospitals and health system factors seem to place patients at risk of readmission (37–39).
In line with previous studies (1), it was found that several geriatric syndromes are associated with an increased risk of mortality. The presence of geriatric syndromes might hence also reflect that these older patients are in their last phase of life. Acute hospitalizations occur more often in patient’s last year of life and may, in turn, play a role in further functional deterioration and the progression of frailty in these already vulnerable patients (31). In these patients advance care planning seems warranted to prevent unnecessary hospitalizations and initiate appropriate interventions to minimize the presence, for example, depressive symptoms and fatigue, thereby improving end of life care (40).
Our study provides a first exploration of single factors contributing to adverse outcomes and our findings seem to indicate that negative outcomes might be rooted in a person’s vulnerable health status that is persistently present from admission, or even the premorbid period, onwards. Further research is warranted, to assess the longitudinal association between an accumulation of these factors and adverse outcomes, using a comprehensive instrument such a frailty index or clustering approach based on, for example, a comprehensive geriatric assessment (CGA). In fact, CGA has already proven to be effective in the identification of vulnerable older individuals in previous interventional studies and has increasingly become standard practice in the hospital setting (41). Nevertheless, CGA has limited effects on postdischarge outcomes, including functional status (42). This is probably due to a lack of follow-up on recommendations in the CGA treatment plan and a focus on more commonly acknowledged syndromes such as cognitive impairment and incontinence. Integrating CGA in transitional care interventions, including nurse care coordination to ensure a safe transition from hospital to primary care setting seems crucial. Efforts to improve discharge and transitional care have already proven to be effective in reducing readmission rates (43), and mortality (44). To also prevent (further) FD or stimulate recovery, geriatric assessment and management should thus be important elements of such (rehabilitation) interventions (45), including less commonly acknowledged syndromes such as apathy and fear of falling. Although few patients develop new syndromes postdischarge, further research is also warranted to assess which patients are at risk to develop cognitive impairment, fatigue, and fall risk, and subsequently to be readmitted. At the same time, however, care providers should be aware that the presence of geriatric syndromes could reflect that patients are at their end of life. In these patients, advance care planning might be helpful to improve end-of-life care (40).
Limitations
Some limitations of the study need to be addressed. Firstly, although our study provided important information on associations between geriatric syndromes and adverse outcomes, the study design, that is, cohort, precluded determination of causality. Thirdly, since the presence of most geriatric syndromes was determined through self-report, the data may involve reporting bias. Fourthly, an extensive questionnaire was administered and to decrease the burden of participation, it was decided to assess pain, fatigue, and fear of falling with numeric rating scales (NRS) instead of comprehensive scales such as the Falls Efficacy Scale (FES) (46), or Geriatric Pain Measure (GPM) (47). However, using NRS might be a limitation, since NRS and the applied cutoff scores have not been validated in this patient population. Fifthly, we did not collect data on the presence of any home-based or ambulatory rehabilitation interventions during the postdischarge period, which could limit any potential effect on our outcomes. However, such interventions are not part of standard care in The Netherlands and it is plausible that no or a very limited number of participants received such interventions. Lastly, we did not include delirious or severely cognitively impaired patients (MMSE <15), patients who were too ill to participate and patients who had a life expectancy of less than 3 months. This may have led to an underestimation of the observed odds ratios in our study, limiting generalizability of the results to these populations.
Conclusion
In acutely hospitalized older patients, most geriatric syndromes are present at admission and patients are likely to retain them postdischarge. Several geriatric syndromes are longitudinally associated with an increased risk mortality and, particularly, persistently present syndromes place persons are at risk of FD. Although few persons develop geriatric syndromes postdischarge, those who developed cognitive impairment, fatigue, and fall risk were at increased readmission risk, which underscores the relevance of adequate transitional care interventions. Although few persons develop geriatric syndromes postdischarge, those who developed cognitive impairment, fatigue, and fall risk were at increased readmission risk, which underscores the relevance of adequate transitional care interventions. To also prevent FD or stimulate recovery, geriatric syndrome assessment and management during and posthospitalization should be important elements of such interventions.
Supplementary Material
Acknowledgments
Author contributions: R.V.S., L.A., J.A., M.V.D.S., R.E., J.B., and B.B. contributed to the design of the original multicenter cohort study. R.V.S., L.R., and J.A. were responsible for acquisition of data. R.V.S., C.K., J.T., and B.B. contributed to analyses and interpretation of data. All authors contributed substantially to drafting the article or revising it critically and gave final approval of the version to be published. The authors would like to acknowledge the contribution of the Hospital study group. In addition to the authors, the study group consists of the following members: Ingeborg Kuper, Annemarieke de Jonghe, Maike Leguit-Elberse, Ad Kamper, Nynke Posthuma, Nienke Brendel, and Johan Wold. Further, we thank Suzanne Schilder, Daisy Kolk, Angelique Heinen, Robin Kwakman, and Jan Jaap Voigt for assistance with data collection.
On behalf of the Hospital-ADL study group: In addition to the authors, the study group consists of the following members: Ingeborg Kuper, MD, Annemarieke de Jonghe, MD, PhD, Maike Leguit-Elberse, RN, Ad Kamper, MD, PhD, Nynke Posthuma, MD, PhD, Nienke Brendel, MD, Johan Wold, MD.
Funding
This work was supported by The Netherlands Organization for Health Research and Development (NWO-ZonMw), grant number 16156071, awarded to B.B. The funder had no role in the design and conduct of the study, data collection, management, analysis, or interpretation of the data, drafting or reviewing the manuscript, and submission of the manuscript.
Conflict of Interest
None reported.
References
- 1. Buurman BM, Hoogerduijn JG, de Haan RJ, et al. . Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLoS One. 2011;6:e26951. doi: 10.1371/journal.pone.0026951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zisberg A, Shadmi E, Gur-Yaish N, Tonkikh O, Sinoff G. Hospital-associated functional decline: the role of hospitalization processes beyond individual risk factors. J Am Geriatr Soc. 2015;63:55–62. doi: 10.1111/jgs.13193 [DOI] [PubMed] [Google Scholar]
- 3. Boyd CM, Landefeld CS, Counsell SR, et al. . Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56:2171–2179. doi: 10.1111/j.1532-5415.2008.02023.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boyd CM, Xue QL, Guralnik JM, Fried LP. Hospitalization and development of dependence in activities of daily living in a cohort of disabled older women: the Women’s Health and Aging Study I. J Gerontol A Biol Sci Med Sci. 2005;60:888–893. doi: 10.1093/gerona/60.7.888 [DOI] [PubMed] [Google Scholar]
- 5. Kansagara D, Englander H, Salanitro A, et al. . Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688–1698. doi: 10.1001/jama.2011.1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clark D, Armstrong M, Allan A, Graham F, Carnon A, Isles C. Imminence of death among hospital inpatients: prevalent cohort study. Palliat Med. 2014;28:474–479. doi: 10.1177/0269216314526443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure”. JAMA. 2011;306:1782–1793. doi: 10.1001/jama.2011.1556 [DOI] [PubMed] [Google Scholar]
- 8. Walter LC, Brand RJ, Counsell SR, et al. . Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285:2987–2994. doi: 10.1001/jama.285.23.2987 [DOI] [PubMed] [Google Scholar]
- 9. Krumholz HM. Post-hospital syndrome–an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100–102. doi: 10.1056/NEJMp1212324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lakhan P, Jones M, Wilson A, Courtney M, Hirdes J, Gray LC. A prospective cohort study of geriatric syndromes among older medical patients admitted to acute care hospitals. J Am Geriatr Soc. 2011;59:2001–2008. doi: 10.1111/j.1532-5415.2011.03663.x [DOI] [PubMed] [Google Scholar]
- 11. van Seben R, Reichardt LA, Aarden JJ, et al. ; Hospital-ADL Study Group The course of geriatric syndromes in acutely hospitalized older adults: the hospital-ADL study. J Am Med Dir Assoc. 2019;20:152–158.e2. doi: 10.1016/j.jamda.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 12. Hoogerduijn JG, Schuurmans MJ, Duijnstee MS, de Rooij SE, Grypdonck MF. A systematic review of predictors and screening instruments to identify older hospitalized patients at risk for functional decline. J Clin Nurs. 2007;16:46–57. doi: 10.1111/j.1365-2702.2006.01579.x [DOI] [PubMed] [Google Scholar]
- 13. Chaudhry SI, McAvay G, Ning Y, Allore HG, Newman AB, Gill TM. Geriatric impairments and disability: the cardiovascular health study. J Am Geriatr Soc. 2010;58:1686–1692. doi: 10.1111/j.1532-5415.2010.03022.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reichardt LA, Aarden JJ, van Seben R, et al. ; Hospital-ADL study group Unravelling the potential mechanisms behind hospitalization-associated disability in older patients; the Hospital-Associated Disability and impact on daily Life (Hospital-ADL) cohort study protocol. BMC Geriatr. 2016;16:59. doi: 10.1186/s12877-016-0232-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296. doi: 10.1371/journal.pmed.0040296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 17. Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–791. doi: 10.1111/j.1532-5415.2007.01156.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. the index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016 [DOI] [PubMed] [Google Scholar]
- 19. Olde Rikkert MG, Rigaud AS, van Hoeyweghen RJ, de Graaf J. Geriatric syndromes: medical misnomer or progress in geriatrics? Neth J Med. 2003;61:83–87. [PubMed] [Google Scholar]
- 20. Yesavage JA, Brink TL, Rose TL, et al. . Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- 21. van der Mast RC, Vinkers DJ, Stek ML, et al. . Vascular disease and apathy in old age. The Leiden 85-Plus Study. Int J Geriatr Psychiatry. 2008;23:266–271. doi: 10.1002/gps.1872 [DOI] [PubMed] [Google Scholar]
- 22. van Seben R, Reichardt LA, Essink DR, van Munster BC, Bosch JA, Buurman BM. “I Feel Worn Out, as if I Neglected Myself”: older patients’ perspectives on post-hospital symptoms after acute hospitalization. Gerontologist. 2018;59:315–326. doi: 10.1093/geront/gnx192 [DOI] [PubMed] [Google Scholar]
- 23. Ayers E, Shapiro M, Holtzer R, Barzilai N, Milman S, Verghese J. Symptoms of apathy independently predict incident frailty and disability in community-dwelling older adults. J Clin Psychiatry. 2017;78:e529–e536. doi: 10.4088/JCP.15m10113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Richard E, Schmand B, Eikelenboom P, et al. ; Alzheimer’s Disease Neuroimaging Initiative Symptoms of apathy are associated with progression from mild cognitive impairment to Alzheimer’s disease in non-depressed subjects. Dement Geriatr Cogn Disord. 2012;33:204–209. doi: 10.1159/000338239 [DOI] [PubMed] [Google Scholar]
- 25. Nyunt MS, Fones C, Niti M, Ng TP. Criterion-based validity and reliability of the Geriatric Depression Screening Scale (GDS-15) in a large validation sample of community-living Asian older adults. Aging Ment Health. 2009;13:376–382. doi: 10.1080/13607860902861027 [DOI] [PubMed] [Google Scholar]
- 26. Marc LG, Raue PJ, Bruce ML. Screening performance of the 15-item geriatric depression scale in a diverse elderly home care population. Am J Geriatr Psychiatry. 2008;16:914–921. doi: 10.1097/JGP.0b013e318186bd67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Puntillo KA, Neighbor ML. Two methods of assessing pain intensity in English-speaking and Spanish-speaking emergency department patients. J Emerg Nurs. 1997;23:597–601. doi: 10.1016/s0099-1767(97)90276-2 [DOI] [PubMed] [Google Scholar]
- 28. Kruizenga HM, Seidell JC, de Vet HC, Wierdsma NJ, van Bokhorst-de van der Schueren MA. Development and validation of a hospital screening tool for malnutrition: the short nutritional assessment questionnaire (SNAQ). Clin Nutr. 2005;24:75–82. doi: 10.1016/j.clnu.2004.07.015 [DOI] [PubMed] [Google Scholar]
- 29. Tinetti ME, Williams CS, Gill TM. Dizziness among older adults: a possible geriatric syndrome. Ann Intern Med. 2000;132:337–344. doi: 10.7326/0003-4819-132-5-200003070-00002 [DOI] [PubMed] [Google Scholar]
- 30. Hwang SS, Chang VT, Cogswell J, Kasimis BS. Clinical relevance of fatigue levels in cancer patients at a Veterans Administration Medical Center. Cancer. 2002;94:2481–2489. doi: 10.1002/cncr.10507 [DOI] [PubMed] [Google Scholar]
- 31. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 32. Friedman SM, Munoz B, West SK, Rubin GS, Fried LP. Falls and fear of falling: which comes first? A longitudinal prediction model suggests strategies for primary and secondary prevention. J Am Geriatr Soc. 2002;50:1329–1335. doi: 10.1046/j.1532-5415.2002.50352.x [DOI] [PubMed] [Google Scholar]
- 33. Twisk JW. Longitudinal data analysis. A comparison between generalized estimating equations and random coefficient analysis. Eur J Epidemiol. 2004;19:769–776. doi: 10.1023/b:ejep.0000036572.00663.f2 [DOI] [PubMed] [Google Scholar]
- 34. Twisk JWR. Applied Longitudinal Data Analysis for Epidemiology A Practical Guide. Cambridge: Cambridge University Press; 2013. [Google Scholar]
- 35. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 36. Anpalahan M, Gibson SJ. Geriatric syndromes as predictors of adverse outcomes of hospitalization. Intern Med J. 2008;38:16–23. doi: 10.1111/j.1445-5994.2007.01398.x [DOI] [PubMed] [Google Scholar]
- 37. Amarasingham R, Moore BJ, Tabak YP, et al. . An automated model to identify heart failure patients at risk for 30-day readmission or death using electronic medical record data. Med Care. 2010;48:981–988. doi: 10.1097/MLR.0b013e3181ef60d9 [DOI] [PubMed] [Google Scholar]
- 38. Hammill BG, Curtis LH, Fonarow GC, et al. . Incremental value of clinical data beyond claims data in predicting 30-day outcomes after heart failure hospitalization. Circ Cardiovasc Qual Outcomes. 2011;4:60–67. doi: 10.1161/CIRCOUTCOMES.110.954693 [DOI] [PubMed] [Google Scholar]
- 39. van Walraven C, Dhalla IA, Bell C, et al. . Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ 2010;182:551–557. doi: 10.1503/cmaj.091117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345. doi: 10.1136/bmj.c1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ellis G, Whitehead MA, Robinson D, O’Neill D, Langhorne P. Comprehensive geriatric assessment for older adults admitted to hospital: meta-analysis of randomised controlled trials. BMJ. 2011;343:d6553. doi: 10.1136/bmj.d6553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Deschodt M, Flamaing J, Haentjens P, Boonen S, Milisen K. Impact of geriatric consultation teams on clinical outcome in acute hospitals: a systematic review and meta-analysis. BMC Med. 2013;11:48. doi: 10.1186/1741-7015-11-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Verhaegh KJ, MacNeil-Vroomen JL, Eslami S, Geerlings SE, de Rooij SE, Buurman BM. Transitional care interventions prevent hospital readmissions for adults with chronic illnesses. Health Aff (Millwood). 2014;33:1531–1539. doi: 10.1377/hlthaff.2014.0160 [DOI] [PubMed] [Google Scholar]
- 44. Buurman BM, Parlevliet JL, Allore HG, et al. . Comprehensive geriatric assessment and transitional care in acutely hospitalized patients: the transitional care bridge randomized clinical trial. JAMA Intern Med. 2016;176:302–309. doi: 10.1001/jamainternmed.2015.8042 [DOI] [PubMed] [Google Scholar]
- 45. Verweij L, van de Korput E, Daams JG, et al. . Effects of postacute multidisciplinary rehabilitation including exercise in out-of-hospital settings in the aged: systematic review and meta-analysis. Arch Phys Med Rehabil. 2019;100:530–550. doi: 10.1016/j.apmr.2018.05.010 [DOI] [PubMed] [Google Scholar]
- 46. Kempen GI, Yardley L, van Haastregt JC, et al. . The Short FES-I: a shortened version of the falls efficacy scale-international to assess fear of falling. Age Ageing. 2008;37:45–50. doi: 10.1093/ageing/afm157 [DOI] [PubMed] [Google Scholar]
- 47. Ferrell BA, Stein WM, Beck JC. The Geriatric Pain Measure: validity, reliability and factor analysis. J Am Geriatr Soc. 2000;48:1669–1673. doi: 10.1111/j.1532-5415.2000.tb03881.x [DOI] [PubMed] [Google Scholar]
- 48. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.