Abstract
Background
We examined cross-sectional associations between dietary patterns, macronutrient intake, and measures of muscle mass and lean mass in older men.
Methods
Participants in the Osteoporotic Fractures in Men (MrOS) cohort (n = 903; mean ± SD age 84.2 ± 4 years) completed brief Block food frequency questionnaires (May 2014–May 2016); factor analysis was used to derive dietary patterns. The D3-creatine (D3Cr) dilution method was used to measure muscle mass; dual-energy x-ray absorptiometry (DXA) was used to measure appendicular lean mass (ALM). Generalized linear models were used to report adjusted means of outcomes by dietary pattern. Multiple linear regression models were used to determine associations between macronutrients and D3Cr muscle mass and DXA ALM. Multivariable models were adjusted for age, race, clinic site, education, depression, total energy intake, height, and percent body fat.
Results
Greater adherence to a Western dietary pattern (high factor loadings for red meat, fried foods, and high-fat dairy) was associated with higher D3Cr muscle mass (p-trend = .026). Adherence to the Healthy dietary pattern (high factor loadings for fruit, vegetables, whole grains, and lean meats) was not associated with D3Cr muscle mass or DXA ALM. Total protein (β = 0.09, 95% CI = 0.03, 0.14) and nondairy animal protein (β = 0.16, 95% CI = 0.10, 0.21) were positively associated with D3Cr muscle mass. Nondairy animal protein (β = 0.06, 95% CI = 0.002, 0.11) was positively associated with DXA ALM. Associations with other macronutrients were inconsistent.
Conclusions
Nondairy animal protein intake (within a Western dietary pattern and alone) was positively associated with D3Cr muscle mass in older men.
Keywords: Nutrition, Sarcopenia, Epidemiology
Sarcopenia, which refers to age-related loss of muscle tissue and the accompanying decline in strength and physical performance, may lead to disability, loss of independence, and increased risk of falls and fractures (1,2). Dual-energy x-ray absorptiometry (DXA) has been historically used to estimate lean mass, but it does not measure muscle mass directly (3). The novel deuterated creatine (D3Cr) dilution method allows for assessment of total muscle mass by spot urine sample after subjects have received an oral dose of D3Cr, and this method has been validated as a marker of total muscle mass in humans (3,4). It, therefore, holds potential for use in the diagnosis of sarcopenia, though currently there is a paucity of data regarding associations between D3Cr and clinical outcomes (4).
Dietary patterns, which account for the synergistic and cumulative effects of individual foods, are increasingly recognized as important components of health and disease (5,6). A Healthy (“prudent” or “nutrient-dense”) dietary pattern (typically rich in fruits, vegetables, whole grains, poultry, fish, nuts, legumes, low-fat dairy) and a Western (“energy-dense”) dietary pattern (typically rich in sugar-sweetened beverages, processed foods, red meat, refined grains, sweets/desserts) are the most reproducible dietary patterns across studies that use data-driven techniques (7,8). A few studies have suggested that dietary patterns rich in fruit and vegetables are associated with lower prevalence of sarcopenia (9–11), but to the best of our knowledge, no studies have compared dietary patterns to a direct measure of total muscle mass (4).
Some, but not all, studies have suggested that consumption of the recommended dietary allowance for protein (0.8 g/kg/d) by healthy older people results in negative nitrogen balance and a decrease in muscle cross-sectional area, which implies that dietary protein needs may increase with advancing age (12,13). Adequate-to-high dietary intakes of high-quality protein may promote muscle function (14), protect against the loss of lean body mass (15) and support the maintenance of physical function with age (16).
This cross-sectional study aimed to examine associations between empirically derived dietary patterns, as well as individual macronutrients specified a priori (carbohydrate, fat, total protein, nondairy animal protein, dairy protein, and plant protein), and skeletal muscle mass assessed by the D3Cr dilution method (D3Cr muscle mass) in a population of older community-dwelling men enrolled in the Osteoporotic Fractures in Men (MrOS) study. To account for the possibility that body weight might mediate these associations, we also examined D3Cr muscle mass after adjustment for body weight (D3Cr muscle mass/weight). Additionally, we examined associations between the dietary variables and DXA appendicular lean mass (ALM). We hypothesized that adherence to the Healthy dietary pattern and total dietary protein intake would each be positively associated with D3Cr muscle mass and DXA ALM. Examination of protein subgroups was exploratory.
Method
Study Participants
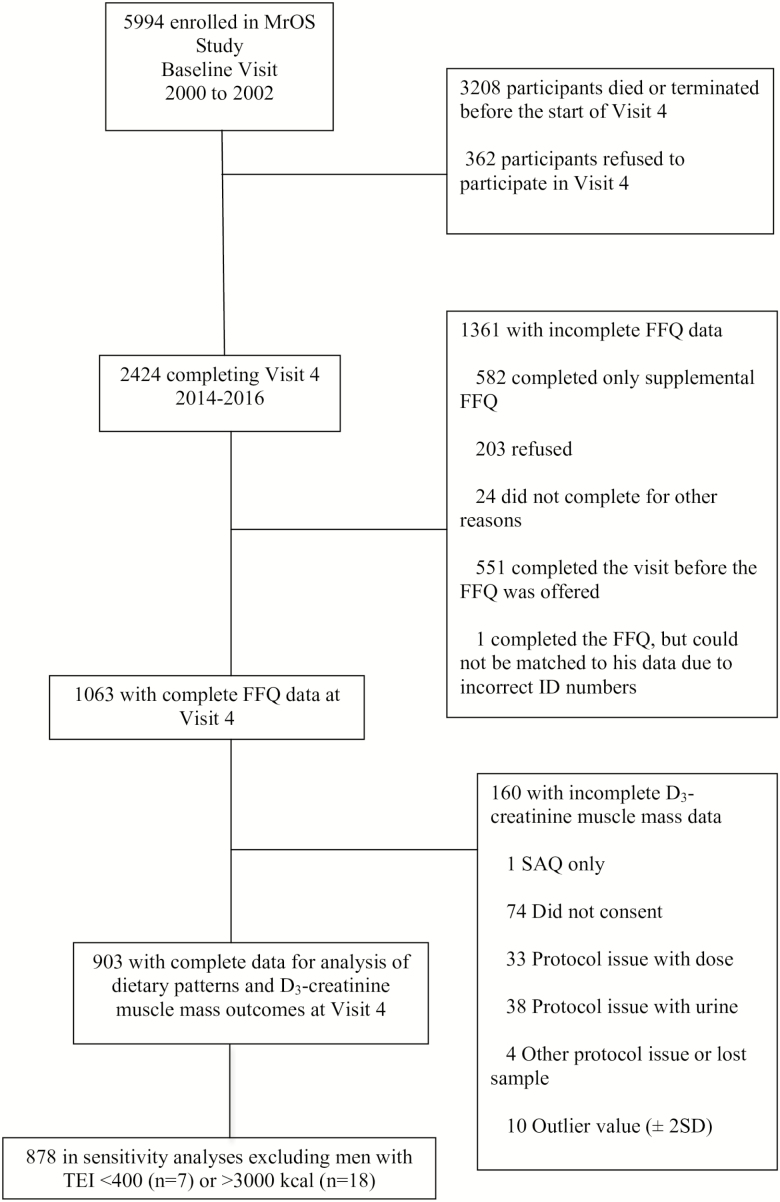
The MrOS prospective cohort study (https://mrosdata.sfcc-cpmc.net/) follows ambulatory men ≥ 65 years at baseline (n = 5,994) at six U.S. clinics (Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California). Men who were unable to walk without the assistance of another person and men who had a history of bilateral hip replacement were excluded from initial enrollment. Men were enrolled from March 2000 through April 2002 (17,18). The fourth follow-up questionnaire and visit, MrOS Visit 4, occurred between May 2014 and May 2016, and 2,424 men completed some part of Visit 4. For the present cross-sectional analysis, we included data from the men who completed the D3Cr dilution protocol and the food frequency questionnaire (FFQ) at Visit 4 (N = 903) (Figure 1).
Figure 1.
Selection of Participants. FFQ, food frequency questionnaire; MrOS, osteoporotic fractures in men; SAQ, self-administered questionnaire; TEI, total energy intake.
Assessment of D3Cr Muscle Mass, D3Cr Muscle Mass/Weight, and DXA ALM
D3Cr muscle mass was determined using the protocol described in Cawthon et al. (4). Briefly, each participant took an oral dose of deuterated creatine. Then, 3–6 days later, each participant provided a fasting morning (but not first void) urine sample in which D3-creatine, D3-creatinine, and unlabeled creatine and creatinine were measured. Using the published algorithm, total body creatine pool size was then calculated from the urine measures. Since 98% of the creatine in the body is found in skeletal muscle at a constant concentration, we were able to convert the value of total body creatine pool size to muscle mass after accounting for any dose that was directly excreted into the urine (4,19). DXA (QDR 4500W; Hologic Inc., Bedford, MA) was used to measure ALM (kg), as previously described (20).
Dietary Assessment
MrOS Visit 4 dietary data were derived by FFQ. The Block 98.2 brief FFQ, which assesses nutrient and energy intake and for which validity and reliability are well established (21), was adapted for the MrOS study and analyzed by Block Dietary Systems. There were 69 individual food item questions and 13 questions about food preparation and low-fat foods. A standard portion size graphic was included, and there were four categories of portion size responses, as well as nine categories of frequency responses for foods and beverages. The Block Brief 2000 FFQ nutrient database was used to determine the nutrient composition.
Dietary Patterns
The derivation of dietary patterns in the MrOS cohort using factor analysis has been previously described (22). Briefly, we constructed food groups from the FFQ data using individual food variables based on nutrient similarities, culinary use, and previous studies. We used the PROC FACTOR procedure in SAS to determine the number of dietary factors, based on robustness, interpretability, and eigenvalues on a scree plot. We calculated factor loadings for each food group across the dietary patterns, and we named the dietary patterns based on factor loadings that contributed the most to each pattern. Two major dietary patterns emerged, which we named Western and Healthy. The Western dietary pattern had highest factor loadings for red meat, fried foods, and high-fat dairy, and the Healthy dietary pattern loaded highest in fruit, vegetables, whole grains, and lean meats such as chicken. We use the term “adherence” to describe how closely the men’s usual dietary intakes aligned with the derived dietary patterns (22). Dietary pattern scores are linear combination of foods in each respective dietary pattern and provide a way to compare dietary pattern adherence from one participant’s diet to another. Dietary pattern scores were divided into quartiles, with quartile 1 representing the lowest adherence and quartile 4 representing the highest adherence to the pattern (8,23).
Individual Macronutrients
We examined carbohydrate, fat, and total protein intake (expressed as percentage of total energy intake, %TEI). Because previous work in the MrOS cohort suggested that the source of dietary protein may mediate associations between protein intake and musculoskeletal variables such as fracture (24) and bone strength (25), we performed exploratory analyses to further examine associations between measures of muscle and lean mass and protein intake by source. Dietary protein sources included nondairy animal, dairy, and plant. Plant protein included protein from foods such as vegetables, grains, nuts, and legumes and was calculated as total protein − animal protein (dairy and nondairy) (25).
Clinic Measurements
At MrOS Visit 4, study participants completed a questionnaire about their medical history, which included self-report of diagnoses of comorbid conditions and lifestyle habits. Depressive symptoms were assessed with the Geriatric Depression Scale (26), with the standard cut point of ≥ 6 depressive symptoms used to define depression (26). Current prescription and over-the-counter medications used in the past 30 days were categorized by a computerized medication coding dictionary (27); prescription medications were stored electronically in a medications inventory database (San Francisco Coordinating Center, San Francisco, CA). The Iowa Drug Information Service Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA) was used to match each medication to its ingredient(s). Body weight was measured with a digital scale or with a standard regularly calibrated balance beam scale. A wall-mounted stadiometer was used to measure participants’ height without shoes, using a standard held-expiration technique. Body mass index (BMI; kg/m2) was calculated from height and weight measurements. Physical activity was assessed using the Physical Activity Scale for the Elderly (28). Highest level of education was self-reported. Cognitive function was assessed with the Trails B test (29) and the Modified Mini-Mental State Examination (30).
Statistical Methods
We compared participant characteristics across quartiles of D3Cr muscle mass using analysis of variance (ANOVA) for continuous normally distributed variables, Kruskal–Wallis tests for skewed continuous variables and chi-square tests for categorical variables. Additionally, we calculated mean intakes of energy, carbohydrate, fat, protein, as well as the protein subgroups, based on the FFQ data.
We used generalized linear models to report adjusted means of D3Cr muscle mass, D3Cr muscle mass/weight, and DXA ALM by quartile of dietary pattern adherence. Models were adjusted for age, race, clinic site, education, depression, TEI, height, and percent body fat. Age, race, clinic site, and TEI were selected as covariates per standard practice in previous MrOS publications of dietary data (22,31). Education and depression were selected as covariates since they have been previously associated with dietary patterns (32,33). Height and percent body fat were selected as covariates to account for body size and composition, respectively. These variables were chosen rather than BMI because BMI, by definition, includes muscle mass (the outcome measure), and we wanted to avoid over-adjustment.
We used multiple linear regression models and the multivariate nutrient density method (34) to determine associations between individual nutritional variables and muscle outcomes (D3Cr muscle mass, D3Cr muscle mass/weight, and DXA ALM). Our main macronutrient parameters were expressed as percent TEI and were analyzed both as continuous variables and by quartile. We used statistical methods appropriate for compositional data. Since the percent intake must add up to 100%, an increase in one macronutrient implies a decrease in the referent category (ie, all other macronutrients). Models were run with one model for each macronutrient and one model for protein intake by source, including all sources in order so the referent category was the same as for total protein. Since this is compositional data (percent protein intake) the models depend on the covariate(s) not included. Thus, an increase in percent protein intake is necessarily associated with a decrease in percent non-protein intake. Implicit in the interpretation is that it is an exchange of intake (ie, one cannot directly tell whether it is an increase in protein intake or decrease in non-protein intake that is the causal factor [possibly both]).
Effect size was defined to be the beta-coefficient from a regression model where both exposure and outcome were parameterized to have mean = 0 and standard deviation (SD) = 1. For comparison purposes, models considering protein by source were parameterized to have the same units as for total protein (ie, 1 SD = 3%TEI). Base models were adjusted for age, clinic site, and TEI. Multivariable models were further adjusted for race, education, depression, height, and percent body fat.
To aid our interpretation of the protein subgroup results, we also scaled both exposure variables and outcomes by standard deviation using the following equation:
| (1) |
To account for men with implausible energy intakes, we performed sensitivity analyses excluding men who reported consuming <400 or >3,000 kcal/d.
All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC), and two-sided significance levels were set at p < .05.
Results
The analysis cohort was comprised of 903 primarily Caucasian (89%) men with a mean ± SD age of 84.2 ± 4 years and a mean ± SD BMI of 26.9 ± 3.8 kg/m2.
Table 1 shows participant characteristics stratified by quartile of D3Cr muscle mass. Men in higher quartiles of D3Cr muscle mass were more likely to be younger, have a higher BMI, have greater DXA ALM and be more physically active (higher Physical Activity Scale for the Elderly score) compared to men in lower quartiles. Additionally, men in higher quartiles of D3Cr muscle mass were less likely to report depressive symptoms (lower Geriatric Depression Scale score), more likely to have better cognitive function (shorter time to complete the Trails B test and higher Modified Mini-Mental State Examination score), and less likely to have diabetes or myocardial infarction compared to men in lower quartiles. Men did not differ across quartiles in terms of race, smoking status, education or percent body fat (Table 1), or by history of cancer, heart failure, or stroke (data not shown). Mean intakes of the individual macronutrients are presented in Supplementary Table 1. Of the 903 participants in the present analysis, 377 (41.75%) met the RDA (0.8 g/kg body weight) for total protein.
Table 1.
Characteristics (Mean ± SD) or N (%) of Older Men by Quartile of D3Cr Muscle Mass (n = 903)
| Characteristic | Quartile 1† (lowest) | Quartile 2‡ | Quartile 3§ | Quartile 4|| (highest) | p-value |
|---|---|---|---|---|---|
| Age (y) | 86.8 ± 4.2 | 84.6 ± 3.8 | 83.2 ± 3.3 | 82.1 ± 2.7 | <.001*** |
| White race (n) | 202 (89.8) | 202 (89.4) | 201 (88.9) | 199 (88.1) | .944 |
| nonsmoker (n) | 83 (43.5) | 83 (43) | 96 (48.2) | 92 (44) | .647 |
| Education¶ (n) | 175 (77.8) | 183 (81) | 184 (81.4) | 191 (84.5) | .339 |
| Body fat (%) | 27.7 ± 6.6 | 27.8 ± 6.0 | 28.1 ± 5.1 | 27.8 ± 5.9 | .894 |
| BMI (kg/m2) | 25.4 ± 3.4 | 26.2 ± 3.5 | 27.2 ± 3.3 | 28.9 ± 3.8 | <.001*** |
| ALM (kg/m2) | 20.1 ± 2.1 | 21.5 ± 2.3 | 22.8 ± 2.3 | 25.4 ± 3 | <.001*** |
| PASE score | 103 ± 69.6 | 118.5 ± 63.5 | 125.7 ± 60.4 | 133.9 ± 64.1 | <.001*** |
| GDS (score) | 19 (8.4) | 17 (7.5) | 9 (4) | 7 (3.1) | .036* |
| Trails B (s) | 160.7 ± 70.8 | 143.8 ± 71.3 | 127.1 ± 61.8 | 124.9 ± 58.9 | <.001*** |
| MMSE score | 90.9 ± 7.2 | 91.6 ± 7.2 | 92.9 ± 6.8 | 93.7 ± 5.9 | <.001*** |
| Diabetes (n) | 42 (18.7) | 24 (10.6) | 49 (21.7) | 29 (12.8) | .004** |
| MI (n) | 41 (18.2) | 32 (14.2) | 27 (12) | 18 (8) | .012* |
| Multivitamin use (n) | 128 (57.7) | 117 (52.7) | 128 (57.1) | 118 (52.7) | .570 |
| Health status (good/excellent; n) | 182 (80.9) | 201 (88.9) | 204 (90.3) | 212 (93.8) | <.001*** |
Notes: D3-creatine (D3Cr), body mass index (BMI), appendicular lean mass (ALM) from dual-energy x-ray absorptiometry (DXA), physical activity scale for the elderly (PASE), geriatric depression score (GDS), Modified Mini-Mental State Examination (MMSE), myocardial infarction (MI).
†11.8 to <21 kg, n = 225.
‡21 to <23.7 kg, n = 226.
§23.7 to <26.7 kg, n = 226.
||26.7 to <36.2 kg, n = 226.
¶At least some college.
*p < 0.05, **p < 0.01, ***p < 0.001.
Men in higher quartiles of D3Cr muscle mass consumed significantly less energy from carbohydrate and more energy from fat, total protein, and nondairy animal protein compared to men in lower quartiles. Men did not differ across quartiles of D3Cr muscle mass in terms of dairy protein intake, plant protein intake, or dietary pattern score (Table 2). Final factor loadings for the dietary patterns are presented in Supplementary Table 2.
Table 2.
Dietary Variables (Mean ± SD) of Older Men by Quartile of D3Cr Muscle Mass (n = 903)
| Characteristic | Quartile 1† (lowest) | Quartile 2‡ | Quartile 3§ | Quartile 4|| (highest) | p-value |
|---|---|---|---|---|---|
| Energy (kcal) | 1535.3 ± 653.7 | 1512.8 ± 587.8 | 1486.4 ± 585.6 | 1580.1 ± 649.2 | .651 |
| Carbohydrate (%TEI) | 47 ± 7 | 47 ± 7 | 44 ± 7 | 45 ± 7 | <.001** |
| Fat (%TEI) | 40 ± 7 | 40 ± 7 | 42 ± 7 | 42 ± 7 | .002* |
| Total protein (%TEI) | 16 ± 3 | 16 ± 3 | 17 ± 3 | 17 ± 3 | .005* |
| Nondairy animal protein (%TEI) | 6 ± 3 | 6 ± 3 | 7 ± 3 | 7 ± 3 | <.001** |
| Dairy protein (%TEI) | 4 ± 2 | 4 ± 2 | 3 ± 2 | 3 ± 2 | .098 |
| Plant protein (%TEI) | 7 ± 2 | 6 ± 2 | 6 ± 2 | 6 ± 2 | .112 |
| Western pattern (score) | −0.09 ± 0.98 | −0.09 ± 0.97 | −0.02 ± 0.94 | 0.11 ± 1.07 | .100 |
| Healthy pattern (score) | 0.05 ± 0.97 | −0.05 ± 0.96 | 0.02 ± 1 | 0.03 ± 1.02 | .719 |
Notes: total energy intake (TEI).
†11.8 to <21 kg, n = 225.
‡21 to <23.7 kg, n = 226.
§23.7 to <26.7 kg, n = 226.
||26.7 to <36.2 kg, n = 226.
*p < 0.05, **p < 0.01, ***p < 0.001.
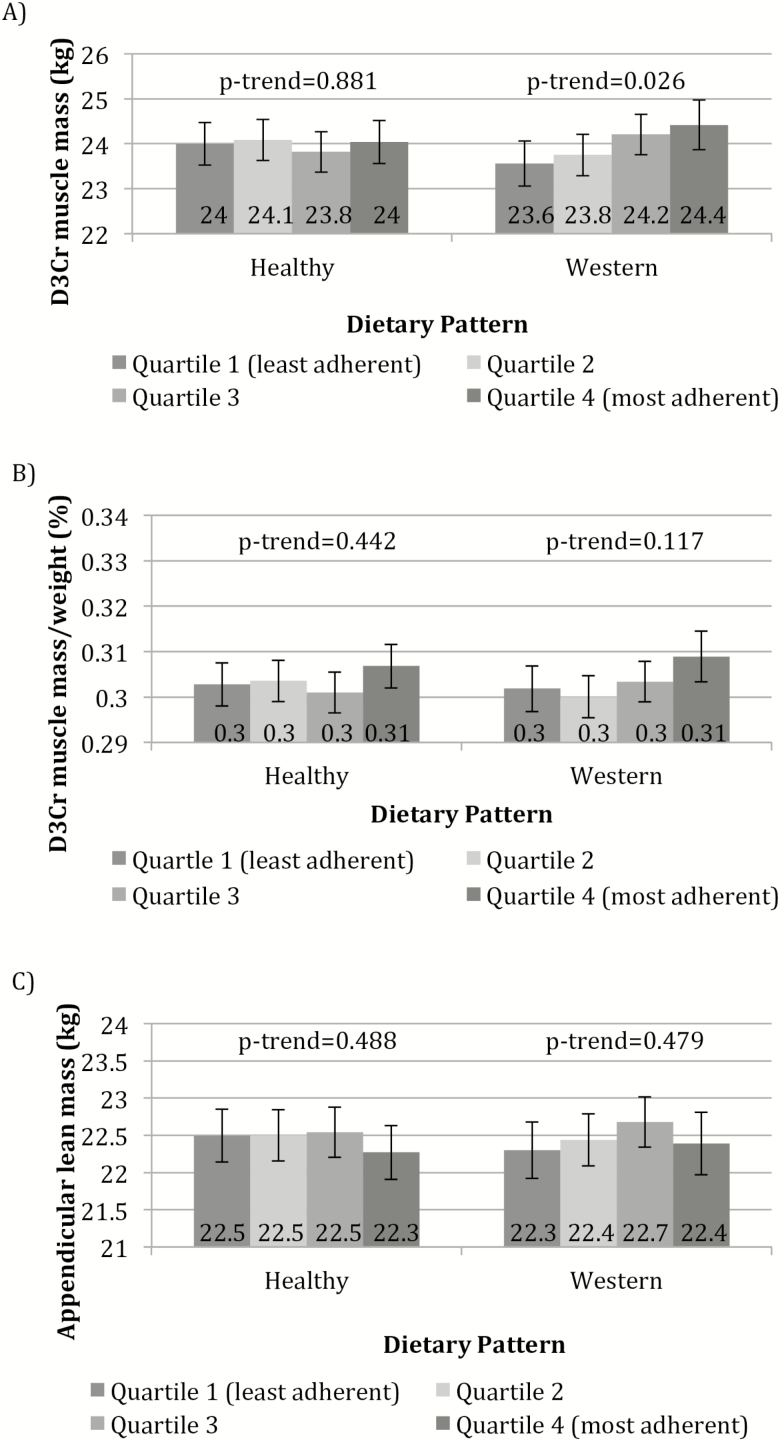
Adjusted means of D3Cr muscle mass, D3Cr muscle mass/weight, and DXA ALM by category of adherence to the dietary patterns are presented in Figure 2. There was a significant positive association between adherence to the Western dietary pattern and D3Cr muscle mass (p-trend = .026); mean D3Cr muscle mass for men in quartile 4 of the Western dietary pattern was 24.4 kg compared to 23.6 kg for men in quartile 1. There were no associations between the Western dietary pattern and D3Cr muscle mass/weight or DXA ALM, or between the Healthy dietary pattern and D3Cr muscle mass, D3Cr muscle mass/weight, or DXA ALM.
Figure 2.
(A) Adjusted means (±95% CI) of D3Cr muscle mass by category of dietary pattern. (B) Adjusted means (±95% CI) of D3Cr muscle mass/weight by category of dietary pattern. (C) Adjusted means (±95% CI) of appendicular lean mass (ALM) by category of dietary pattern. Models were adjusted for age, clinic site, total energy intake (TEI), race, education, depression, height, and percent body fat. For the Healthy dietary pattern, quartile 1 (least adherent) is considered least optimal nutritionally.
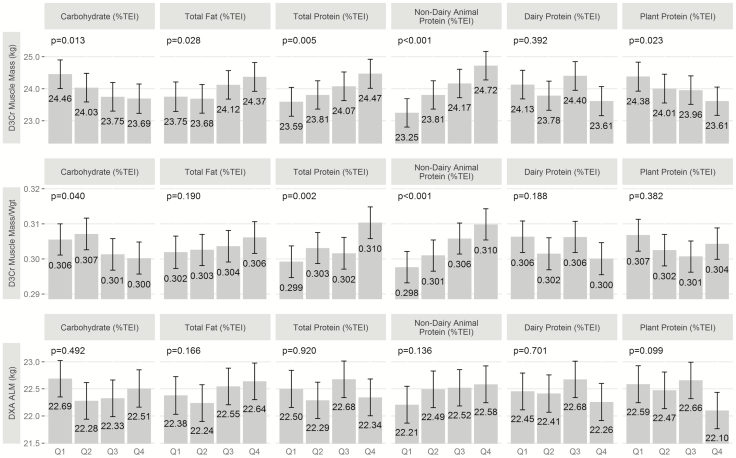
Multiple linear regression models of D3Cr muscle mass, D3Cr muscle mass/weight, and DXA ALM with macronutrients (as continuous variables) are presented in Table 3. Carbohydrate intake was inversely associated with D3Cr muscle mass in the base model and multivariable model and with D3Cr muscle mass/weight in the multivariable model. Dietary fat intake was positively associated with D3Cr muscle mass in both the base and multivariable models, though statistical significance was lost after adjustment for weight (D3Cr muscle mass/weight); dietary fat was positively associated with DXA ALM in the base model but not in the multivariable model. Total protein intake was positively associated with D3Cr muscle mass and D3Cr muscle mass/weight in the base and multivariable models. Nondairy animal protein intake was positively associated with D3Cr muscle mass and D3Cr muscle mass/weight in the base and multivariable models and with DXA ALM in the multivariable model. Plant protein was inversely associated with D3Cr muscle mass, D3Cr muscle mass/weight, and DXA ALM in the base model and with D3Cr muscle mass and DXA ALM in the multivariable model. Multiple linear regression models of D3Cr muscle mass, D3Cr muscle mass/weight, and DXA ALM with macronutrients (as quartiles) are presented in Figure 3 (multivariable models only). Findings were similar to the analysis by continuous variables. However, the association between nondairy animal protein and DXA ALM was nonsignificant. Furthermore, the association between plant protein and DXA ALM was also nonsignificant.
Table 3.
Multiple Linear Regression Models of Muscle Mass and Lean Mass With Nutritional Variables in Older Men (n = 903)
| D3Cr Muscle Mass (kg) | D3Cr Muscle Mass/Weight | DXA Appendicular Lean Mass (kg) | ||||
|---|---|---|---|---|---|---|
| Base | Multivariable | Base | Multivariable | Base | Multivariable | |
| Carbohydrate (%TEI) | −0.11 (−0.17, −0.06)*** | −0.09 (−0.15, −0.04)** | 0.02 (−0.05, 0.08) | −0.06 (−0.11, −0.01)* | −0.06 (−0.13, 0.001) | −0.03 (−0.09, 0.02) |
| Fat (%TEI) | 0.08 (0.02, 0.14)* | 0.06 (0.004, 0.12)* | −0.05 (−0.11, 0.01) | 0.03 (−0.02, 0.08) | 0.07 (0.004, 0.13)* | 0.04 (−0.02, 0.09) |
| Total protein (%TEI) | 0.10 (0.04, 0.16)** | 0.09 (0.03, 0.14)** | 0.10 (0.04, 0.16)** | 0.08 (0.03, 0.13)** | −0.005 (−0.07, 0.06) | −0.003 (−0.06, 0.05) |
| Nondairy Animal protein (%TEI) | 0.17 (0.11, 0.23)*** | 0.16 (0.10, 0.21)*** | 0.08 (0.02, 0.14)* | 0.11 (0.07, 0.16)*** | 0.06 (−0.004, 0.12) | 0.06 (0.002, 0.11)* |
| Dairy protein (%TEI) | −0.04 (−0.10, 0.02) | −0.04 (−0.10, 0.02) | −0.04 (−0.10, 0.03) | −0.03 (−0.08, 0.02) | −0.03 (−0.09, 0.04) | −0.04 (−0.09, 0.02) |
| Plant protein (%TEI) | −0.07 (−0.13, −0.01)* | −0.07 (−0.13, −0.02)* | 0.07 (0.01, 0.13)* | −0.02 (−0.07, 0.03) | −0.07 (−0.14, −0.01)* | −0.06 (−0.11, −0.0005)* |
Notes: Base model adjusted for age, clinic site, TEI (total energy intake). Multivariable model adjusted for age, clinic site, TEI, race, education, depression, height, and percent body fat. Effect size (beta-coefficient with unit = SD) and 95% confidence interval. Dietary protein intake is calculated as a percentage of TEI (%TEI) and scaled to have 1 unit change = 3% TEI = SD (total protein intake). Source-specific protein intakes were included in a single model (ie, each one was adjusted for the other two source-specific intakes). SD’s for outcomes and exposures include the following: D3Cr muscle mass 4.01; D3Cr muscle mass/weight 0.05; DXA Appendicular lean mass 3.12; Carbohydrate (%TEI) 0.07; Fat (%TEI) 0.07; Total protein (%TEI) 0.03; nondairy animal protein (%TEI) 0.03; Dairy protein (%TEI) 0.02; Plant protein (%TEI) 0.02.
*p < .05, **p < .01, ***p < .001.
Figure 3.
Multiple linear regression models of D3Cr muscle mass, D3Cr muscle mass/weight, and DXA ALM with macronutrients (as quartiles). Models were adjusted for age, clinic site, total energy intake (TEI), race, education, depression, height, and percent body fat. Multivariable models shown only.
After controlling for age, race, site, education, depression, TEI, percent body fat, and height (multivariable regression model), each SD increment in percent nondairy animal protein (3%) was associated with 0.64 kg higher D3Cr muscle mass and 0.19 kg higher DXA ALM. Each SD increment in percent plant protein (2%) was associated with 0.28 kg lower D3Cr muscle mass and 0.19 kg lower DXA ALM.
Sensitivity analyses excluding men with very low energy intakes (<400 kcal/d; n = 7) and very high energy intakes (>3,000 kcal/d; n = 18) did not change the main results (data not shown).
Discussion
The results of this study support a modest, positive association between nondairy animal protein intake (alone and within the context of a Western dietary pattern that loaded high in red meat) and D3Cr muscle mass in older American men. When macronutrients were analyzed as continuous variables, nondairy animal protein intake was modestly associated with DXA ALM, and there were weak, inverse associations between plant protein and D3Cr muscle mass and DXA ALM. Although these associations were attenuated in the analysis by macronutrient quartiles (a discrepancy we attribute to loss of power), results remained consistent, with effects in the same directions. Associations between the other macronutrients (fat and carbohydrate) and muscle mass and lean mass outcomes were inconsistent overall. Contrary to our initial hypothesis, adherence to the Healthy dietary pattern (rich in fruit, vegetables, whole grains, and lean meats) was not associated with D3Cr muscle mass or DXA ALM.
Mean intakes of energy and protein (Supplementary Table 1) were lower in the MrOS cohort compared to those of older men in the Framingham Heart Study Offspring cohort (16), the Berlin Aging Study (35), and the Health, Aging and Body Composition Study (36), though men in MrOS were older, on average, compared to the men in the aforementioned cohorts. Mean intakes of both nondairy animal protein and plant protein were substantial and nearly equal, which allowed for the possibility of capturing and comparing associations between these protein subgroups and muscle mass and lean mass outcomes.
To the best of our knowledge, no previous studies have examined associations between these same dietary exposure variables and D3Cr muscle mass, so we are unable to directly compare our findings to other work. Moreover, among related studies in the literature, there is considerable heterogeneity in study design, participant population, dietary assessment methodology, dietary pattern definitions and derivation, and outcome measurements, making comparisons difficult.
Our findings contrast with studies that have reported positive associations between dietary patterns rich in vegetables and fruit and muscle-related outcomes in older individuals. Among Chinese adults 65 years and older (n = 3,957), greater adherence to a dietary pattern rich in vegetables and fruit was associated with lower prevalence of sarcopenia after adjustment for lifestyle and sociodemographic factors; this finding was observed only in men (9). In older men and women, the Mediterranean dietary pattern (rich in fruits, vegetables, olive oil, fish, and nuts) has been associated with lower odds of sarcopenia (defined as a combination of low appendicular muscle mass [<5.45 kg/m2 for women and <7.26 kg/m2 for men] and either low muscle strength or low muscle performance) (10,11). A recent systematic review reported a small body of observational evidence supporting associations of dietary patterns rich in fruits and vegetables, whole grains, fish, lean meat, low-fat dairy, nuts, and olive oil (ie, a “Healthy” dietary pattern) with lean mass and physical performance outcomes in older adults (37). This review focused primarily on ALM, and it is possible that the true effects of dietary intake were masked by the failure to measure changes (or lack thereof) on a direct measure of muscle mass, such as D3Cr muscle mass as in the present study.
There is not a great deal of evidence for beneficial associations between a Western dietary pattern and muscle-related outcomes. Mohseni and colleagues reported that a Western dietary pattern (which was derived by factor analysis and loaded highest in commercial beverages [soft drinks and fruit juice], sugar/dessert, snacks, solid fat, potatoes, high-fat dairy, legumes, organ meat and fast food) was not associated with the odds of having sarcopenia in postmenopausal Iranian women (11). Hashemi and colleagues derived dietary patterns by principal component analysis and reported that neither a “Western” dietary pattern (characterized by high intake of tea, soy, sweets, desserts, sugars, and fast foods) nor a “Mixed” dietary pattern (characterized by high factor loadings in animal proteins, legumes, potatoes, and refined grains) were associated with odds of developing sarcopenia in older adults (10).
We also speculate that the high factor loading of red meat, a rich source of protein and essential amino acids, in the Western dietary pattern of the present study may partially explain the association between this dietary pattern and D3Cr muscle mass, D3Cr muscle mass/weight, and DXA ALM. Protein intake is important for muscle protein synthesis and also acts as an anabolic stimulus (14). There is evidence that animal protein exerts greater anabolic effects than plant protein, possibly due to differences in essential amino acids, which contribute to improved nitrogen balance (14,25). In particular, the amino acid leucine has been identified as a key activator of the mammalian target rapamycin (mTOR) pathway, which is crucial for nutrient and energy-sensing signaling in skeletal muscle (38,39). Plant proteins, most of which are low in leucine, have been attributed to lower anabolic properties compared to animal protein (40). Similarly, in the Health ABC Study, among 2,066 men and women (mean age 74.5 years), energy-adjusted total and animal protein intakes were significantly associated with 3-year changes in DXA ALM. This study found no association between vegetable protein intake and DXA ALM in fully adjusted models (36). More recently, Alexandrov et al. reported that, after adjustment for lifestyle factors, intakes of total protein, animal protein, and fish/meat/egg protein (but not dairy protein or plant protein) were positively associated with 24-hour urinary creatinine excretion (a proxy for muscle mass) among men in the Lifelines Cohort (41).
An important caveat of the present study is that although we observed a positive association between the Western dietary pattern and D3Cr muscle mass, this association lost statistical significance after adjustment for body weight (D3Cr muscle mass/weight), which suggests that body weight may have also mediated this association.
To the best of our knowledge, protein has been the predominant macronutrient of interest in most published studies relating to nutrition and muscle mass or lean mass. However, Kim and Song reported a weak, positive association between carbohydrate intake and total limb lean mass (assessed by DXA) in older Asian men (β = 0.141, p = .046) (42), and Atkins et al. found that older men with a higher carbohydrate intake had reduced odds of low muscle mass (as assessed by mid-arm muscle circumference [odds ratio = 0.73, 95% CI = 0.55–0.96] and fat free mass index [odds ratio = 0.76, 95% CI = 0.58–0.99] (43). These reports contrast with our findings of an inverse association between carbohydrate intake and D3Cr muscle mass; further research is necessary to fully elucidate the associations between carbohydrate intake and muscle mass and lean mass in older men.
The present study has a number of strengths, as well as limitations. To the best of our knowledge, this is the first study to examine associations between dietary variables and total muscle mass as measured by the novel D3Cr dilution method. The MrOS cohort is well-characterized for dietary patterns, body composition and physical function outcomes, and numerous potentially confounding factors such as lifestyle habits and comorbidities. Our statistical approach included factor analysis, which enables examination of dietary patterns that exist in free-living subjects without depending on preconceived ideas of diet-disease relationships; factor analysis also utilizes a continuous variable, which provides increased power to examine diet-disease relationships (7,44). However, factor analysis is exploratory by nature and requires some subjective decisions on handling the data, such as the eigenvalue cut point and data transformation (44). In addition, the factor analysis method has been more difficult to reproduce compared to other methods of dietary pattern analysis such as diet indices and scoring (7).
This study was also limited by the under-representation of minorities in the MrOS cohort and the use of a brief FFQ, which may have underestimated energy and macronutrients (45). Our measure of D3Cr muscle mass depends on the derivation of the total body creatine pool size. Creatine is a component of uncooked animal meat; nearly all creatine in meat is converted to creatinine in the cooking process. While high doses of creatine supplementation increase the total body creatine pool size, dietary creatinine is rapidly excreted after ingestion. Thus, our use of a fasting (but not first-morning void) urine sample should eliminate any influence on the assessment of urine D3-creatinine enrichment. Lastly, the cross-sectional nature of the study precludes establishment of causality.
Further observational studies of dietary factors and D3Cr muscle mass in other populations, including women and minorities, are needed. In particular, the association of the Western dietary pattern and D3Cr muscle mass observed in the present study requires confirmation and clarification. Future work in the MrOS cohort may include examination of associations between specific foods that comprised our Western dietary pattern (eg, red meat, fried foods, high-fat dairy) and D3Cr muscle mass, as well as other muscle-related outcomes.
Conclusion
Adherence to a Western dietary pattern (rich in red meat, fried foods, and high-fat dairy) was associated with higher D3Cr muscle mass in older men (before adjustment for body weight), whereas adherence to a Healthy dietary pattern (rich in vegetables, fruit, whole grains, and lean meats) was not associated with D3Cr muscle mass or DXA ALM. Additionally, results of this analysis support a modest, positive association between intake of nondairy animal protein and D3Cr muscle mass and DXA ALM in older men.
Funding
This work was supported by the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and the National Center for Advancing Translational Sciences (NCATS) (grant numbers U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128). Funding for the D3Cr muscle mass measure was provided by NIAMS (grant number R01 AR065268). GlaxoSmithKline provided in-kind support by providing the d3creatine dose and analysis of urine samples.
Ethical Approval
All procedures performed in the MrOS study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board at each clinic site, and all participants provided written informed consent. For this type of retrospective analysis, additional formal consent was not required.
Conflict of Interest
P.M.C. has institutional research grants from Abbott Nutrition and Nestle. L.L. has institutional research grants from Abbott Nutrition. The other authors declare no conflicts of interest.
Supplementary Material
Acknowledgments
The authors wish to thank the participants of the Osteoporotic Fractures in Men (MrOS) study for their contributions to public health. The authors also thank Danielle Young for preparation of figures. P.M.C., T.R.S., and N.E.L. designed the research. K.E.P. performed statistical analysis. T.R.S. wrote the manuscript. J.M.S., S.J., L.L., A.R.H., and W.J.E. provided subject matter expertise. All authors contributed to interpreting the data and edited, reviewed, and approved the manuscript. T.R.S. and P.M.C. are responsible for the final content of the manuscript.
References
- 1. Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010;21:543–559. doi:10.1007/s00198-009-1059-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fielding RA, Vellas B, Evans WJ, et al. . Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi:10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clark RV, Walker AC, O’Connor-Semmes RL, et al. . Total body skeletal muscle mass: estimation by creatine (methyl-d3) dilution in humans. J Appl Physiol (1985). 2014;116:1605–1613. doi:10.1152/japplphysiol.00045.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cawthon PM, Orwoll ES, Peters KE, et al. . Strong relation between muscle mass determined by d3-creatine dilution, physical performance and incidence of falls and mobility limitations in a prospective cohort of older men. J Gerontol A Biol Sci Med Sci. 2019;74:844–852. doi:10.1093/gerona/gly129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Millen BE, Abrams S, Adams-Campbell L, et al. . The 2015 Dietary guidelines advisory committee scientific report: development and major conclusions. Adv Nutr. 2016;7:438–444. doi:10.3945/an.116.012120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inzitari M, Doets E, Bartali B, et al. ; International Association Of Gerontology And Geriatrics (IAGG) Task Force For Nutrition In The Elderly. Nutrition in the age-related disablement process. J Nutr Health Aging. 2011;15:599–604. doi:10.1007/s12603-011-0053-1 [DOI] [PubMed] [Google Scholar]
- 7. Movassagh EZ, Vatanparast H. Current evidence on the association of dietary patterns and bone health: a scoping review. Adv Nutr. 2017;8:1–16. doi:10.3945/an.116.013326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Judd SE, Letter AJ, Shikany JM, Roth DL, Newby PK. Dietary patterns derived using exploratory and confirmatory factor analysis are stable and generalizable across race, region, and gender subgroups in the REGARDS Study. Front Nutr. 2014;1:29. doi:10.3389/fnut.2014.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan R, Leung J, Woo J. A prospective cohort study to examine the association between dietary patterns and sarcopenia in Chinese community-dwelling older people in Hong Kong. J Am Med Dir Assoc. 2016;17:336–342. doi:10.1016/j.jamda.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 10. Hashemi R, Motlagh AD, Heshmat R, et al. . Diet and its relationship to sarcopenia in community dwelling Iranian elderly: a cross sectional study. Nutrition. 2015;31:97–104. doi:10.1016/j.nut.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 11. Mohseni R, Aliakbar S, Abdollahi A, Yekaninejad MS, Maghbooli Z, Mirzaei K. Relationship between major dietary patterns and sarcopenia among menopausal women. Aging Clin Exp Res. 2017;29:1241–1248. doi:10.1007/s40520-016-0721-4 [DOI] [PubMed] [Google Scholar]
- 12. Campbell WW, Crim MC, Dallal GE, Young VR, Evans WJ. Increased protein requirements in elderly people: new data and retrospective reassessments. Am J Clin Nutr. 1994;60:501–509. doi:10.1093/ajcn/60.4.501 [DOI] [PubMed] [Google Scholar]
- 13. Campbell WW, Trappe TA, Wolfe RR, Evans WJ. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci. 2001;56:M373–M380. doi:10.1093/gerona/56.6.m373 [DOI] [PubMed] [Google Scholar]
- 14. Robinson SM, Reginster JY, Rizzoli R, et al. ; ESCEO working group. Does nutrition play a role in the prevention and management of sarcopenia? Clin Nutr. 2018;37:1121–1132. doi:10.1016/j.clnu.2017.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim JS, Wilson JM, Lee SR. Dietary implications on mechanisms of sarcopenia: roles of protein, amino acids and antioxidants. J Nutr Biochem. 2010;21:1–13. doi:10.1016/j.jnutbio.2009.06.014 [DOI] [PubMed] [Google Scholar]
- 16. Hruby A, Sahni S, Bolster D, Jacques PF. Protein intake and functional integrity in aging: the Framingham Heart Study Offspring. J Gerontol A Biol Sci Med Sci. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Orwoll E, Blank JB, Barrett-Connor E, et al. . Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi:10.1016/j.cct.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 18. Blank JB, Cawthon PM, Carrion-Petersen ML, et al. . Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials. 2005;26:557–568. doi:10.1016/j.cct.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 19. Shankaran M, Czerwieniec G, Fessler C, et al. . Dilution of oral D3 -Creatine to measure creatine pool size and estimate skeletal muscle mass: development of a correction algorithm. J Cachexia Sarcopenia Muscle. 2018;9:540–546. doi:10.1002/jcsm.12278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee CG, Boyko EJ, Nielson CM, et al. ; Osteoporotic Fractures in Men Study Group. Mortality risk in older men associated with changes in weight, lean mass, and fat mass. J Am Geriatr Soc. 2011;59:233–240. doi:10.1111/j.1532-5415.2010.03245.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boucher B, Cotterchio M, Kreiger N, Nadalin V, Block T, Block G. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr. 2006;9:84–93. doi:10.1079/PHN2005763 [DOI] [PubMed] [Google Scholar]
- 22. Rogers TS, Harrison S, Judd S, et al. ; Osteoporotic Fractures in Men (MrOS) Study Research Group. Dietary patterns and longitudinal change in hip bone mineral density among older men. Osteoporos Int. 2018;29:1135–1145. doi:10.1007/s00198-018-4388-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shikany JM, Safford MM, Newby PK, Durant RW, Brown TM, Judd SE. Southern dietary pattern is associated with hazard of acute coronary heart disease in the reasons for geographic and racial differences in stroke (REGARDS) study. Circulation. 2015;132:804–814. doi:10.1161/CIRCULATIONAHA.114.014421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Langsetmo L, Shikany JM, Cawthon PM, et al. ; Osteoporotic Fractures in Men (MrOS) Research Group. The association between protein intake by source and osteoporotic fracture in older men: a prospective cohort study. J Bone Miner Res. 2017;32:592–600. doi:10.1002/jbmr.3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Langsetmo L, Shikany JM, Burghardt AJ, et al. ; Osteoporotic Fractures in Men (MrOS) Study Research Group. High dairy protein intake is associated with greater bone strength parameters at the distal radius and tibia in older men: a cross-sectional study. Osteoporos Int. 2018;29:69–77. doi:10.1007/s00198-017-4261-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999;14:858–865. doi:10.1002/(SICI)1099-1166(199910)14: 10<858::AID-GPS35>3.0.CO;2-8 [DOI] [PubMed] [Google Scholar]
- 27. Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411. [DOI] [PubMed] [Google Scholar]
- 28. Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi:10.1016/0895-4356(93)90053-4 [DOI] [PubMed] [Google Scholar]
- 29. Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. doi:10.2466/PMS.8.7.271-276 [Google Scholar]
- 30. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 31. Shikany JM, Barrett-Connor E, Ensrud KE, et al. ; Osteoporotic Fractures in Men (MrOS) Research Group. Macronutrients, diet quality, and frailty in older men. J Gerontol A Biol Sci Med Sci. 2014;69:695–701. doi:10.1093/gerona/glt196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Akbaraly TN, Brunner EJ, Ferrie JE, Marmot MG, Kivimaki M, Singh-Manoux A. Dietary pattern and depressive symptoms in middle age. Br J Psychiatry. 2009;195:408–413. doi:10.1192/bjp.bp.108.058925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wall CL, Gearry RB, Pearson J, Parnell W, Skidmore PM. Dietary intake in midlife and associations with standard of living, education and nutrition literacy. N Z Med J. 2014;127:30–40. [PubMed] [Google Scholar]
- 34. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65 (4 Suppl):1220S–1228S; discussion 1229S. doi:10.1093/ajcn/65.4.1220S [DOI] [PubMed] [Google Scholar]
- 35. Nikolov J, Spira D, Aleksandrova K, et al. . Adherence to a mediterranean-style diet and appendicular lean mass in community-dwelling older people: results from the Berlin Aging Study II. J Gerontol A Biol Sci Med Sci. 2016;71:1315–1321. doi:10.1093/gerona/glv218 [DOI] [PubMed] [Google Scholar]
- 36. Houston DK, Nicklas BJ, Ding J, et al. ; Health ABC Study. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87:150–155. doi:10.1093/ajcn/87.1.150 [DOI] [PubMed] [Google Scholar]
- 37. Bloom I, Shand C, Cooper C, Robinson S, Baird J. Diet quality and sarcopenia in older adults: a systematic review. Nutrients. 2018;10:308. doi:10.3390/nu10030308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nowson C, O’Connell S. Protein requirements and recommendations for older people: a review. Nutrients. 2015;7:6874–6899. doi:10.3390/nu7085311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baum JI, Kim IY, Wolfe RR. Protein consumption and the elderly: what is the optimal level of intake? Nutrients. 2016;8:359. doi:10.3390/nu8060359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Vliet S, Burd NA, van Loon LJ. The skeletal muscle anabolic response to plant- versus animal-based protein consumption. J Nutr. 2015;145:1981–1991. doi:10.3945/jn.114.204305 [DOI] [PubMed] [Google Scholar]
- 41. Alexandrov NV, Eelderink C, Singh-Povel CM, Navis GJ, Bakker SJL, Corpeleijn E. Dietary protein sources and muscle mass over the life course: the Lifelines Cohort Study. Nutrients. 2018;10:1471. doi:10.3390/nu10101471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim HN, Song SW. Association between carbohydrate intake and body composition: the Korean National Health and Nutrition Examination Survey. Nutrition. 2019;61:187–193. doi:10.1016/j.nut.2018.11.011 [DOI] [PubMed] [Google Scholar]
- 43. Atkins JL, Whincup PH, Morris RW, Wannamethee SG. Low muscle mass in older men: the role of lifestyle, diet and cardiovascular risk factors. J Nutr Health Aging. 2014;18:26–33. doi:10.1007/s12603-013-0336-9 [DOI] [PubMed] [Google Scholar]
- 44. Newby PK, Muller D, Hallfrisch J, Andres R, Tucker KL. Food patterns measured by factor analysis and anthropometric changes in adults. Am J Clin Nutr. 2004;80:504–513. doi:10.1093/ajcn/80.2.504 [DOI] [PubMed] [Google Scholar]
- 45. Fung TT, Feskanich D. Dietary patterns and risk of hip fractures in postmenopausal women and men over 50 years. Osteoporos Int. 2015;26:1825–1830. doi:10.1007/s00198-015-3081-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.