Abstract
Background
Sarcopenia is often conceptualized as a precursor to loss of mobility, but its effect on recovery of mobility after a hip fracture is unknown. We determined the prevalence of low muscle strength (weakness) after hip fracture using putative sarcopenia metrics (absolute grip strength, and grip strength normalized to body mass index, total body fat, arm lean mass, and weight) identified by the Sarcopenia Definitions and Outcomes Consortium (SDOC).
Methods
We examined two well-characterized hip fracture cohorts of community-dwelling older adults from the Baltimore Hip Studies (BHS). The prevalence of muscle weakness was assessed using the SDOC cut points compared to published definitions at 2 and 6 months postfracture. We assessed associations of 2-month weakness with 6-month walking speed <0.6 m/s and calculated the sensitivity and specificity in predicting lack of meaningful change in walking speed (change < 0.1 m/s) at 6 months.
Results
Two hundred and forty-six participants (192 women; 54 men) were included; mean (SD) age of 81 (8) for women and 78 (7) for men. At 2 months, 91% women and 78% men exhibited slow walking speed (< 0.6 m/s). SDOC grip strength standardized by weight (<0.34 kg women, <0.45 kg men) was the most prevalent measure of weakness in men (74%) and women (79%) and provided high sensitivity in men (86%) and women (84%) predicting lack of meaningful change in walking speed at 6 months, although specificity was poor to moderate.
Conclusions
SDOC cut points for grip strength standardized to weight provided consistent indication of poor walking speed performance post-hip fracture.
Keywords: Grip strength, Weakness, Sarcopenia, Gait speed
In 2010, over 250,000 older adults (≥65 years of age) were hospitalized for a hip fracture (1), and the number of hip fractures is expected to increase almost 12% in the United States between 2010 and 2030 (2). Hip fracture patients have significant increases in disability, mortality, and nursing home admission over 12 months postfracture, compared to age-matched controls (3).
The amount and time of maximal recovery without a special postfracture intervention varies by domain of function (4). Most functional gains are achieved by approximately 6 months but additional small gains in some domains continue for as long as 14 months (4). Notably, 30%–75% of patients fail to return to their prefracture levels by 1 year after fracture (5–8).
Due to the acute nature of the hip fracture event, a sudden mobility disability occurs after the fracture with associated changes in body composition which may contribute to poor functional recovery. Loss of lean muscle mass and increases in fat mass have been shown in women postfracture (9). Lean mass has not been shown to influence functional performance in women postfracture (10,11), but it did predict functional outcomes in men (12,13).
Sarcopenia has been operationalized with various clinical characteristics, by the Foundation of the National Institutes of Health Sarcopenia project (FNIH) (14), the International Working Group on Sarcopenia (IWGS) (15), and the European Working Group on Sarcopenia in Older Persons (EWGSOP) (16,17), each of which has a different definition for clinical thresholds based on lean mass and muscle strength. The thresholds from the FNIH predict mobility disability in community-dwelling older adults. Given that hip fracture patients experience a sudden mobility disablement, the utility of these clinical thresholds for predicting improved walking speed post-hip fracture is unclear.
CART analyses conducted by the Sarcopenia Definitions and Outcomes Consortium (SDOC) in epidemiologic cohorts of community-dwelling older adults identified five indices of muscle strength and DXA-derived lean mass that were predictive of mobility limitation (usual walking speed <0.8 m/s) (18). The purpose of the present study was to assess the putative SDOC cut points for muscle weakness (defined using absolute grip strength, and grip strength normalized to body mass index, total body fat, arm lean mass, and weight) in hip fracture cohorts at 2 months postfracture. Another objective was to evaluate the sensitivity and specificity of the five putative SDOC cut points and those for published definitions of sarcopenia (14,16,19–22) in predicting lack of clinically meaningful improvement in walking speed at 6 months postfracture.
Methods
Participants
We examined two well-characterized study cohorts of community-dwelling older adults who experienced a hip fracture from the Baltimore Hip Studies (BHS) research program. Study measures were obtained at baseline (within 15 days of the hip fracture) and at 2 and 6 months postfracture. The two BHS studies (BHS-4 and BHS-7) included in the analyses had the following measures: (i) Body composition measures by dual-energy x-ray absorptiometry (DXA); (ii) Muscle strength by handgrip dynamometry; and (iii) Usual walking speed measures attempted on a 4 m course. Written informed consent was obtained from participants or their proxies and both studies were approved by the institutional review boards at participating institutions. The present study also was reviewed and approved by the Institutional Review Board at the University of Maryland School of Medicine. For this analysis, low muscle strength from the 2-month post-hip fracture visit was used, since that is when walking speed was first collected. Follow-up data were obtained at 6 months post-hip fracture. For the purpose of the longitudinal comparison, only participants with walking speed data at both 2 and 6 months postfracture were included.
Baltimore Hip Study—4 (BHS-4)
BHS-4 was a randomized controlled trial of exercise versus usual care post-hip fracture designed to test the feasibility and efficacy of a home-based Exercise Plus Program, administered by an exercise trainer over the year after hip fracture (23). A total of 180 female hip fracture patients, 65 years and older, were enrolled within 15 days of the fracture from three area hospitals. Eligible patients with surgical repair of a nonpathological hip fracture were community-dwelling and walked without human assistance prior the fracture, were cognitively intact, had no hardware in contralateral hip, and were deemed safe to exercise. There were no differences by treatment group in main hip fracture outcomes, including measures of physical function (23); therefore, we pooled the data for this analysis and included treatment group as a covariate. Nonmissing data from BHS-4 cohort were available for 121 women.
Baltimore Hip Study—7 (BHS-7)
BHS-7 was a prospective observational study that frequency matched (1:1) men and women on calendar time of hip fracture and hospital. The sample comprised 339 hip fracture patients (171 women and 168 men), 65 years and older, who were community-dwelling with surgical repair of a non-pathological hip fracture at one of the eight study hospitals in the Baltimore area (24). Patients were excluded if they were bedbound for 6 months prior to the fracture, weighed greater than 300 pounds, or had hardware in the contralateral hip. Participants were enrolled within 15 days post-hospital admission. Nonmissing data from the BHS-7 cohort were available for 125 participants (54 men and 71 women).
Measures
Body composition
BHS-4 and BHS-7 utilized local DXA facilities near the recruitment hospitals and each participant was scanned on the same machine type (Hologic or Lunar) for all study visits. Total body fat mass and total soft tissue lean mass were acquired using standardized protocols for whole-body DXA scans on Hologic machines (Waltham, MA) in BHS-4 and three DXA facilities in BHS-7, and on Lunar Prodigy machines (Madison, WI) in four DXA facilities in BHS-7 (25,26). Appendicular lean mass (ALM) was the sum of lean mass from both arms and legs.
Grip strength
Grip strength was measured using a JAMAR handheld dynamometer (Sammons Preston Rolyan, Bolingbrook, IL) (27). Both hands were assessed and the maximal value across all trials was used.
Usual walking speed
Walking speed was determined on a 4-m course and defined as the length of the walking course divided by the time required for the participants to complete the course at their usual walking pace. Slow walking speed (slowness) in hip fracture patients was defined as walking speed < 0.6 m/s (28), the primary outcome at 6 months. We chose this cut point for walking speed because very few patients achieved the >0.8 m/s cut point and 0.6 m/s was viewed as a reasonable speed to represent improvement since almost all patients were below this level at 2 months. We also assessed for lack of clinically meaningful improvement in usual walking speed at 6 months, where a small clinically meaningful improvement was defined as an increase of at least 0.1 m/s from Month 2 (29).
Statistical Analysis
Statistical analyses were performed using R version 3.1.1. Analyses were stratified by sex and BHS cohort. Muscle strength cut points were derived from the SDOC CART analyses and ROC curves that were discriminative of slowness (walking speed <0.8 m/s) (18). We applied these cut points in hip fracture cohorts to determine prevalence of muscle weakness, and its association with slow walking speed at Month 6. We used logistic regression models to estimate odds ratios (OR) and accompanying 95% confidence intervals (CI) for lack of meaningful improvement in mobility (slowness defined as improvement in walking speed <0.1 m/s) between baseline and follow-up. We also assessed the sensitivity and specificity of the putative SDOC cut points in predicting lack of clinically meaningful improvement in walking speed (ie, persistent slowness) at 6 months. We then compared the sensitivity and specificity of these new SDOC cut points to those previously proposed by the FNIH Sarcopenia Project (14,19–22) and the European Working Group on Sarcopenia Performance (16).
Results
Of the 519 participants considered for inclusion, 246 had gait speed data at 2 and 6 months postfracture. Baseline (2 months post-hip fracture) characteristics of the two cohorts of hip fracture patients are shown in Table 1. At 2 months, the mean (SD) age of men in BHS-7 was 78 (7) years; the mean (SD) age of women in the two cohorts was 81 (8) years. Fifty percent of men were overweight or obese while 37% of women in BHS-4% and 51% in BHS-7 were overweight or obese. Approximately 90% of women in the two cohorts and 78% of men had 2-month walking speeds slower than 0.6 m/s with mean walking speeds slower in women (0.38 m/s) compared to men (0.45 m/s).
Table 1.
Baseline (Month 2) Characteristics of Hip Fracture Patients and Prevalence of Sarcopenia by Different Definitions
| Characteristics | Men | Women | |||
|---|---|---|---|---|---|
| BHS7 (n = 54) | BHS4 (n = 121) | BHS7 (n = 71) | |||
| Age (years), mean (SD) | 78 (7) | 81 (7) | 81 (8) | ||
| Race, white (%) | 48 (89) | 116 (96) | 66 (93) | ||
| Weight (kg), mean (SD) | 80 (13) | 62 (12) | 65 (15) | ||
| Height (m), mean (SD) | 1.78 (0.07) | 1.6 (0.06) | 1.58 (0.09) | ||
| BMI (kg/m2), mean (SD) | 25.6 (4.1) | 24.1 (4.2) | 26.1 (6.2) | ||
| Underweight <25 (%) | 50 (27/54) | 63 (74/118) | 49 (35/71) | ||
| Normal 25–29 (%) | 35 (19/54) | 26 (31/118) | 28 (20/71) | ||
| Overweight ≥30 (%) | 15 (8/54) | 11 (13/118) | 23 (16/71) | ||
| Walking speed (m/s), mean (SD) | 0.45 (0.21) | 0.36 (0.18) | 0.39 (0.18) | ||
| Walking speed <0.6 m/s (%) | 78 (42/54) | 92(111/121) | 90 (64/71) | ||
| Grip (kg), mean (SD) | 32.3 (11) | 16.5 (4.1) | 19.8 (14.4) | ||
| Grip/BMI (m2), mean (SD) | 1.3 (0.52) | 0.69 (0.2) | 0.77 (0.52) | ||
| Grip/TBF, mean (SD) | 1.89 (1.93) | 0.97 (0.57) | 0.98 (0.90) | ||
| ALM (kg), mean (SD) | 20.9 (3.3) | 14.5 (2.2) | 14.6 (3.2) | ||
| Sarcopenia Definition | Cut point | (n = 53) | Cut point | (n = 116) | (n = 71) |
| SDOC Grip (%) | <35.5 | (64) | <20.0 | (82) | (63) |
| SDOC Grip/BMI (%) | <1.05 | (32) | <0.79 | (72) | (58) |
| SDOC Grip/TBF (%) | <1.66 | (60) | <0.65 | (23) | (32) |
| SDOC Grip/Arm lean mass (%) | <6.08 | (69) | <3.26 | (15) | (13) |
| SDOC Grip/Weight (%) | <0.45 | (74) | <0.34 | (85) | (72) |
| FNIH Grip (%) | <26.0 | (25) | <16.0 | (45) | (32) |
| FNIH Grip/BMI (%) | <1.00 | (30) | <0.56 | (26) | (28) |
| EWGSOP* (%) | — | (65) | — | (55) | (51) |
Note: ALM = appendicular lean mass; BMI = body mass index; Grip = grip strength; TBF = total body fat.
*EWGSOP based on low lean mass (ALM/ht2 <7.23 and 5.67 kg/m2 in men and women, respectively) plus low strength (grip strength < 30 and 20 kg in men and women, respectively) and/or performance (walking speed < 0.8 m/s).
The prevalence of low muscle strength (weakness) cut points at 2 months postfracture, by BHS cohort and sex, as determined by five SDOC candidate metrics and FNIH and EWGSOP sarcopenia criteria are also shown in the lower section of Table 1. The number below each threshold varied by definition and exhibited a wide range in both men (25%–74%) and women (13%–85%). Across cohorts and sex, the number below the threshold was greatest for both men and women using grip strength normalized for weight derived by the SDOC (74% men and 79% women). This prevalence was more than double that derived by FNIH definitions. Overall, there tended to be fewer men below the threshold for each cut point than women with the exception of grip strength normalized for arm lean mass and for total body fat (TBF) which had more than four times and two times as many men than women below the cut point, respectively (69% vs 14% and 60% vs 28%).
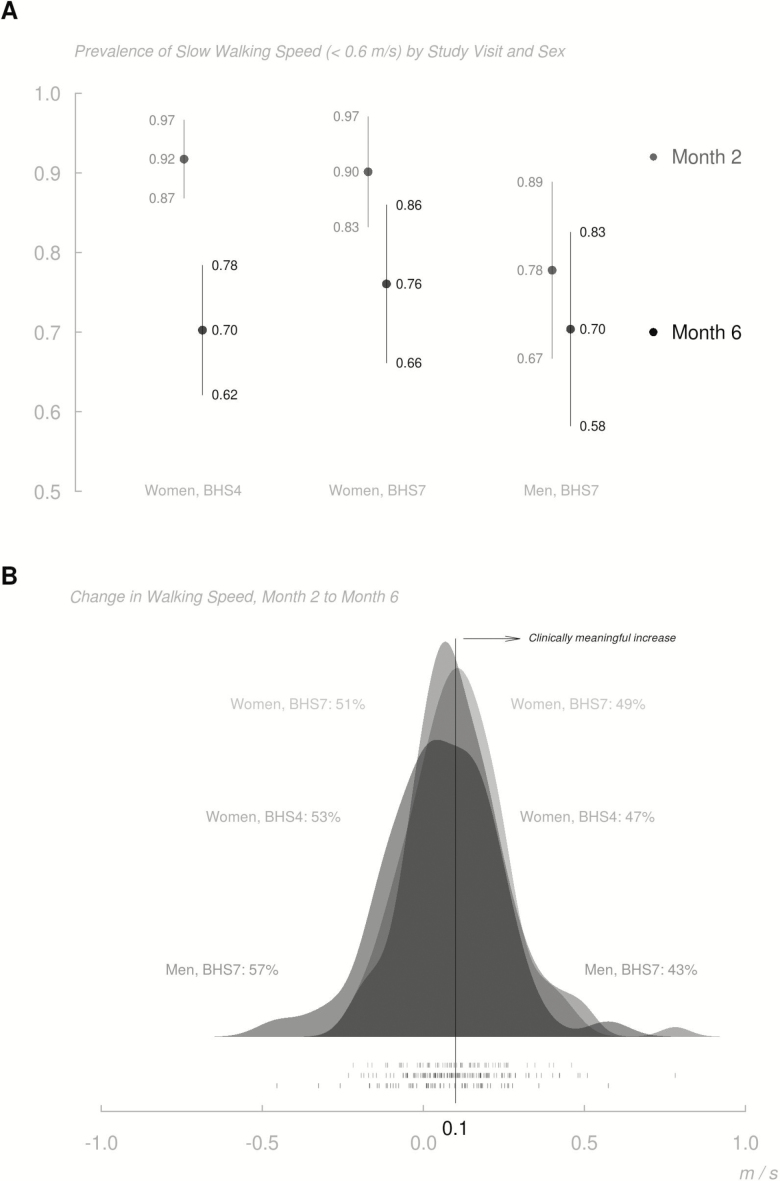
Figure 1A displays the prevalence of slow walking speed by study visit and sex, and Figure 1B shows the distributions of change in walking speed from 2 to 6 months by sex and cohort. While there is improvement in walking speed over time, 70% of BHS-4 participants and 74% of BHS-7 participants still had walking speed <0.6 m/s at 6 months after the fracture. Approximately, 53% of participants in both cohorts had a change in walking speed from 2 to 6 months that was <0.10 m/s. While walking speed was somewhat greater among men at 2 months, proportionately fewer men than women exhibited meaningful improvements from 2 to 6 months; at 6 months, mean walking speed was essentially equal among men and women.
Figure 1.
Prevalence of slow walking speed by study visit, and sex (A) and change in walking speed, Month 2 to Month 6 (B). (A) Month 2 in grey and Month 6 in black. 95% confidence intervals are also shown. (B) Kernel density estimates of change in walking speed from Months 2 to 6 are depicted for each cohort. The proportions of each cohort achieving and failing to achieve clinically meaningful increases (0.1 m/s or greater) in walking speed between Months 2 and 6 are noted. One-way “rug” displays at the bottom demarcate measured changes in walking speed at the participant level.
Of the SDOC candidate metrics, grip strength normalized to weight was associated most strongly with slowness (walking speed < 0.6 m/s) at 6 months in men (OR: 9.60; CI 2.50–42.39; Table 2). While this metric was also significant in women (OR: 2.26; CI 1.07–4.74), grip strength normalized to BMI had the strongest association (OR: 3.20; CI 1.64–6.31). OR also showed a high association of grip strength normalized to TBF in both men (OR: 7.71; CI 1.89–36.77) and women (OR: 3.00; CI 1.41–6.88) with slow walking speed at Month 6. Grip strength adjusted for body size (ie, weight, TBF, BMI) always performed better than absolute grip strength.
Table 2.
Associations (OR) of SDOC Sarcopenia Candidate Metrics with Slow Walking Speed (<0.60 m/s) at 6 mo
| Definition | OR (95% CI) | |
|---|---|---|
| Men | Women | |
| Absolute Grip | 4.14 (1.17, 15.57)* | 1.83 (0.91, 3.63) |
| Grip/BMI | 4.22 (1.12, 20.82)* | 3.20 (1.64, 6.31)*** |
| Grip/TBF | 7.71 (1.89, 36.77)** | 3.00 (1.41, 6.88)** |
| Grip/arm lean mass | 4.67 (1.12, 20.64)* | 2.44 (0.94, 7.62) |
| Grip/weight | 9.60 (2.50, 42.39)** | 2.26 (1.07, 4.74)* |
Note: BMI = body mass index; CI = confidence interval; Grip = grip strength; OR = odds ratio; TBF = total body fat. The OR compares those below the cut point with those above the cut point.
*p < .05; **p < .01; ***p < .001.
Table 3 provides the results of the longitudinal sensitivity and specificity analyses that evaluated lack of clinically meaningful change in walking speed (<0.10 m/s) for each metric from 2 to 6 months. Overall, the SDOC metrics performed better in both men and women for predicting those with a lack of clinically meaningful improvement in walking speed compared to the FNIH or EWGSOP definitions. In particular, SDOC grip strength normalized to weight had the best sensitivity in men (86%) and women (84% in the combined cohorts) in predicting who will not exhibit a clinically meaningful walking speed improvement. In men, grip strength standardized by body size also had high sensitivity (arm lean mass [82%] or TBF [79%]) as did absolute grip strength (78%). While SDOC grip strength standardized by BMI had poor sensitivity, it had the highest specificity (80%). The metrics above with the highest sensitivity had specificity ranging from 50% to 67%.
Table 3.
Sensitivity/Specificity of Various Definitions in Predicting Lack of Clinically Meaningful Improvement in Walking Speed at 6 mo (change in walking speed <0.10 m/s)
| BHS4 | BHS7 | ||||
|---|---|---|---|---|---|
| Definition | Cut point | Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) |
| Men | |||||
| SDOC Grip | <35.5 | — | — | 78 (29/37) | 53 (8/15) |
| SDOC Grip/BMI | <1.05 | — | — | 51 (19/37) | 80 (12/15) |
| SDOC Grip/TBF | <1.66 | — | — | 79 (27/34) | 67 (8/12) |
| SDOC Grip/arm lean mass | <6.08 | — | — | 82 (28/34) | 50 (6/12) |
| SDOC Grip/weight | <0.45 | — | — | 86 (32/37) | 60 (9/15) |
| FNIH Grip | <26.0 | — | — | 35 (13/37) | 80 (12/15) |
| FNIH Grip/BMI | <1.00 | — | — | 41 (15/37) | 80 (12/15) |
| EWGSOP* | — | — | — | 63 (22/35) | 46 (6/13) |
| Women | |||||
| SDOC Grip | <20.0 | 82 (68/83) | 36 (13/36) | 69 (37/54) | 35 (6/17) |
| SDOC Grip/BMI | <0.79 | 79 (63/80) | 47 (17/36) | 74 (40/54) | 53 (9/17) |
| SDOC Grip/TBF | <0.65 | 35 (25/72) | 74 (26/35) | 58 (25/43) | 93 (13/14) |
| SDOC Grip/arm lean mass | <3.26 | 22 (16/72) | 86 (30/35) | 21 (9/43) | 100 (14/14) |
| SDOC Grip/weight | <0.34 | 86 (71/83) | 25 (9/36) | 81 (44/54) | 41 (7/17) |
| FNIH Grip | <16.0 | 52 (43/83) | 67 (24/36) | 48 (26/54) | 71 (12/17) |
| FNIH Grip/BMI | <0.056 | 31 (25/80) | 81 (29/36) | 39 (21/54) | 88 (15/17) |
| EWGSOP* | — | 58 (41/71) | 51 (18/35) | 30 (13/43) | 14 (2/14) |
Note: BMI = body mass index; Grip = grip strength; TBF = total body fat.
*EWGSOP based on low lean mass (ALM/ht2 <7.23 and 5.67 kg/m2 in men and women, respectively) plus low strength (grip strength < 30 and <20 kg in men and women, respectively) and/or performance (walking speed < 0.8 m/s).
Among women in combined cohorts, SDOC grip strength adjusted for weight had the highest sensitivity (84%). Grip strength adjusted for BMI (77%) or absolute grip strength (76%) also had good sensitivity and were better predictors than the FNIH or EWGSOP definitions. None of these cut points had specificity values above 53%. In contrast, specificity was highest for SDOC grip strength adjusted for arm lean mass (93%) or TBF (84%), as well as for FNIH grip strength adjusted for BMI (85%).
Discussion
In this analysis of hip fracture cohorts with high prevalence of slow walking speed, we found that SDOC grip strength relative to weight was the most consistent metric across the analyses in men and women after hip fracture for muscle weakness and for predicting mobility disability. It provided the highest prevalence of muscle weakness at 2 months post-hip fracture and it was a significant predictor of poor gait speed performance at 6 months, either sustained slow walking speed (<0.6 m/s) or lack of clinically meaningful improvement in walking speed (change < 0.10 m/s). Overall, SDOC muscle weakness definitions based on low grip strength, absolute or adjusted to body size, were better predictors of poor gait speed at 6 months than the cut points used by FNIH and EWGSOP.
There were some sex differences in the sensitivity and specificity of the various weakness and low lean mass metrics in hip fracture patients. Among the SDOC metrics, grip strength measures consistently had high sensitivity in men with adjustment for body weight, arm lean mass or total body fat, improving on absolute grip strength slightly. In contrast, the specificity was higher when SDOC grip strength was adjusted for BMI or TBF, or with the FNIH absolute grip strength or adjusted for BMI metric. Similarly, in women, the sensitivity of SDOC grip strength measures (adjusted for weight or BMI or absolute grip strength) consistently performed better than the FNIH or EWGSOP metrics. The only SDOC measures with relatively high specificity were grip strength adjusted for arm lean mass or TBF. The FNIH grip strength (absolute or adjusted for BMI) also had high specificity. The variable performance of the metrics overall and by sex is likely due to the fact that the cut points were derived from community-dwelling older adults, rather than from male and female hip fracture patients. We know, for example, that men with hip fracture have higher grip strength, and have proportionately more lean mass, higher weight and BMI, and are less likely to be underweight than are women with hip fracture. The impaired status of hip fracture patients raises the question as to whether hip fracture-specific metrics are needed. On the other hand, cut points that define nearly everyone with hip fracture as “slow” or “weak” may be appropriate given the impaired function of these individuals.
The lack of specificity probably reflects that the injury was to a lower extremity and a measure of leg strength might be more sensitive and specific; however, an important consideration for developing clinical tests used to determine the likelihood of a clinically meaningful improvement in walking speed is that it can be easily applied in a busy “real-world” practice setting. These results show that obtaining DXA measures of lean mass do not provide better prediction of gait speed improvement than grip strength adjusted for weight. Also, the SDOC absolute grip strength measure does not perform well in men or women post-hip fracture and adjustment for body size is necessary in this subpopulation. Researchers and clinicians working with hip fracture patients are encouraged to consider SDOC grip strength/weight thresholds in men and women as having good sensitivity for identifying patients whose walking ability is unlikely to improve at 6 months. This is a particularly important point for most hip fracture patients as standard post-hip fracture rehabilitation ends around this time even though significant mobility disability remains.
Although sarcopenia has been shown to be a risk factor for falls and fractures, including hip fracture (30), the prevalence of sarcopenia in the acute post-fracture period varies greatly, from 12% (31) to 95% (13) in men and 18% (31) to 68% (32) in women, depending on the definition used. Previous FNIH cut points were discriminative of mobility disability in community-dwelling older adults; however, those thresholds may be inadequate for older adults with preexisting mobility limitations (33). In this study, the prevalence of muscle weakness below the SDOC cut points was higher at 2 months postfracture than cut points used in the FNIH or EWGSOP sarcopenia definitions, with the grip strength/weight cut point providing the highest prevalence for men and women (74% and 79%, respectively) versus 28% and 33% for the FNIH cut points or EWGSOP
A few studies have examined the relationship of sarcopenia, either using published definitions or components of sarcopenia such as lean mass and/or strength, with short-term post-hip fracture outcomes. Di Monaco and colleagues assessed lean mass and function in hip fracture patients within a rehabilitation hospital setting. Low lean mass by DXA was significantly associated with poorer functional outcomes in men, but not in women (11,12). González-Montalvo and colleagues found that the EWGSOP sarcopenia definition was associated with greater functional loss, in crude models during the acute hospital stay (31). Similarly, the FNIH sarcopenia definition was associated with worse functional status at discharge from an acute rehabilitation hospital and at 3-month follow-up (34). Over longer follow-up periods, grip strength was associated with worse disability outcomes over 12 months post-hip fracture and lean mass was not (10,35).
There is some logic to the use of sarcopenia measures involving grip strength without a measure of body composition after hip fracture. Prior research indicates that grip strength and upper body task performance do not change very much over the year after hip fracture (36) while lower body tasks and aspects of body composition such as lean body mass, TBF, and BMI, are more likely to be affected by the fracture and its sequelae and are changing as a result of the fracture and subsequent recovery over time. Thus, grip strength adjusted for body size using weight is a sensible metric as a static marker at 2 months predicting subsequent walking ability. If the interest is in how changes in body composition affect gait and other lower extremity tasks, different measures that are reflective of these changes may be more appropriate and may prove useful to target mechanisms for interventions.
Because we sought to analyze within-participant change in walking speed, we were limited in including only those who had gait measurements at both 2 and 6 months postfracture. For this reason, a substantial number of participants were not included in the analytic sample (see Methods). Though comparisons of those included to those excluded did not reveal a systematic difference (data not shown), we nevertheless cannot guarantee that these results would have been replicated had all BHS-4 and BHS-7 participants been observed over the entire study period. Due to the small number of fast walkers at 2 months, we were not able to assess the effect modification of participants’ initial walking speed on the trajectory of continued recovery to 6 months. Another limitation was the analytic approach used to derive the SDOC cut points could not develop race/ethnicity-specific cut points, which may ultimately be necessary for defining sarcopenia. Additionally, the cohorts of hip fracture patients were mostly white, but population-based studies of hip fracture have shown a lower incidence of hip fracture in African Americans (37). Finally, there were some study characteristics that varied between the BHS-4 (RCT) and BHS-7 (observational) cohorts, mainly due to the more stringent eligibility criteria for the RCT, although the women are remarkably similar in the two cohorts (Table 1).
In summary, we have evaluated SDOC cut points for low muscle strength in hip fracture patients. Consistent with previous attempts to define clinically relevant thresholds for low muscle strength (14,17), this analysis showed that cut points inclusive of grip strength adjusted for weight in men and women assessed at 2 months postfracture are predictive of slow walking speed performance (walking speed <0.60 m/s), and accurately identify those who do not have a clinically meaningful improvement in walking speed from 2 to 6 months. Analyses also demonstrated that a measure of lean body mass or body composition is not necessary for predicting gait speed performance. SDOC grip strength/weight thresholds are sensitive indicators of poor gait performance over 6 months post-hip fracture.
Funding
The Sarcopenia Definitions and Outcomes Consortium (SDOC) is supported by the National Institute on Aging (NIA, grant number AG51421), the Foundation for the National of Institutes of Health (FNIH, grant numbers CAWT16SARC2 and BHAS16SARC2), and the California Pacific Medical Center Foundation. Additional support was also provided by the National Institute on Aging awards (U01AG05142, R37AG009901, R01AG018668, R01 AG029315, T32AG00262, and P30AG028747). This research was supported in part by the intramural research program at the National Institute on Aging. Dr. Fielding’s contribution was also supported by the U.S. Department of Agriculture (USDA), under agreement No. 58-1950-4-003. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA.
Acknowledgments
D.L.O. and T.G.T. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: D.L.O., J.M., R.A.F., P.M.C., S.B., T.M., S.P., and T.G.T. Acquisition of data: D.L.O. and J.M. Analysis and interpretation of data: D.L.O., J.M., R.A.F., H.Z., E.F.B., P.M.C., S.B., and T.G.T. Drafting of the manuscript: D.L.O., J.M., R.A.F., H.Z., E.F.B., P.M.C., and T.G.T. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: T.G.T and H.Z. Obtained funding: D.L.O., J.M., R.A.F., E.F.B., P.M.C., S.B., and T.G.T. The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of Interest
D.L.O., H.Z., R.C.A., T.M., S.P., M.S., and T.G.T. have no conflicts to report. The following coauthors have received support over the past year. J.M. consulted or served on advisory boards for: American Orthopaedic Association; Novartis; UCB; Pluristem; Viking, Inc. None of these entities provided funding for the current project. R.A.F. received personal fees and/or other support from Axcella Health, Inside Tracker, Biophytis, Astellas, Cytokinetics, Amazentis, Nestle, and Glaxo Smith Kline. E.F.B. received grant support from Astellas Pharma USA and consulting fees from Pfizer. P.M.C. served as a consultant for Bioage, and had grant support from Abbott and Nestle. S.B. received grant support from Abbvie, Transition Therapeutics, Abbott, Metro International Biotechnology, LLC, and Alivegen. These grants and contracts are managed by the Brigham and Women’s Hospital. He also reports receiving consulting fees from AbbVie and OPKO and holding equity interest in FPT, LLC.
References
- 1.National Hospital Discharge Survey (NHDS). Number of discharges from short-stay hospitals, by first-listed diagnosis and age: United States; 2010. Available at: https://www.cdc.gov/nchs/data/nhds/3firstlisted/2010first3_numberage.pdf. Accessed June 19, 2019. [Google Scholar]
- 2. Stevens JA, Rudd RA. The impact of decreasing U.S. hip fracture rates on future hip fracture estimates. Osteoporos Int. 2013;24:2725–2728. doi: 10.1007/s00198-013-2375-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leibson CL, Tosteson AN, Gabriel SE, Ransom JE, Melton LJ. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc. 2002;50:1644–1650. doi: 10.1046/j.1532-5415.2002.50455.x [DOI] [PubMed] [Google Scholar]
- 4. Magaziner J, Hawkes W, Hebel JR, et al. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci. 2000;55: M498–M507. doi: 10.1093/gerona/55.9.m498 [DOI] [PubMed] [Google Scholar]
- 5. Jette AM, Harris BA, Cleary PD, Campion EW. Functional recovery after hip fracture. Arch Phys Med Rehabil. 1987;68:735–740. [PubMed] [Google Scholar]
- 6. Zuckerman JD. Hip fracture. N Engl J Med. 1996;334:1519–1525. doi: 10.1056/NEJM199606063342307 [DOI] [PubMed] [Google Scholar]
- 7. Magaziner J, Simonsick EM, Kashner TM, Hebel JR, Kenzora JE. Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study. J Gerontol. 1990;45:M101–M107. doi: 10.1093/geronj/45.3.m101 [DOI] [PubMed] [Google Scholar]
- 8. Dyer SM, Crotty M, Fairhall N, et al. ; Fragility Fracture Network (FFN) Rehabilitation Research Special Interest Group . A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr. 2016;16:158. doi: 10.1186/s12877-016-0332-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fox KM, Magaziner J, Hawkes WG, et al. Loss of bone density and lean body mass after hip fracture. Osteoporos Int. 2000;11:31–5. doi: 10.1007/s001980050003 [DOI] [PubMed] [Google Scholar]
- 10. Wehren LE, Hawkes WG, Hebel JR, Orwig DL, Magaziner J. Bone mineral density, soft tissue body composition, strength, and functioning after hip fracture. J Gerontol A Biol Sci Med Sci. 2005;60:80–84. doi: 10.1093/gerona/60.1.80 [DOI] [PubMed] [Google Scholar]
- 11. Di Monaco M, Vallero F, Di Monaco R, Tappero R, Cavanna A. Muscle mass and functional recovery in women with hip fracture. Am J Phys Med Rehabil. 2006;85:209–215. doi: 10.1097/01.phm.0000200387.01559.c0 [DOI] [PubMed] [Google Scholar]
- 12. Di Monaco M, Vallero F, Di Monaco R, Tappero R, Cavanna A. Muscle mass and functional recovery in men with hip fracture. Am J Phys Med Rehabil. 2007;86:818–825. doi: 10.1097/PHM.0b013e318151fec7 [DOI] [PubMed] [Google Scholar]
- 13. Di Monaco M, Castiglioni C, Vallero F, Di Monaco R, Tappero R. Men recover ability to function less than women do: an observational study of 1094 subjects after hip fracture. Am J Phys Med Rehabil. 2012;91:309–315. doi: 10.1097/PHM.0b013e3182466162 [DOI] [PubMed] [Google Scholar]
- 14. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. ; European Working Group on Sarcopenia in Older People . Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2018;48(1):16–31. doi: 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manini TM, Patel SM, Newman AB, et al. Identification of sarcopenia components that discriminate slow walking speed: a pooled data analysis from the Sarcopenia Definitions and Outcomes Consortium. Currently under review. J Am Geriatr Soc. 2020. doi: 10.1111/jgs.16524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cawthon PM, Peters KW, Shardell MD, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci. 2014;69:567–575. doi: 10.1093/gerona/glu023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alley DE, Shardell MD, Peters KW, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69:559–566. doi: 10.1093/gerona/glu011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dam TT, Peters KW, Fragala M, et al. An evidence-based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci. 2014;69:584–590. doi: 10.1093/gerona/glu013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McLean RR, Shardell MD, Alley DE, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci. 2014;69:576–583. doi: 10.1093/gerona/glu012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Orwig DL, Hochberg M, Yu-Yahiro J, et al. Delivery and outcomes of a yearlong home exercise program after hip fracture: a randomized controlled trial. Arch Intern Med. 2011;171:323–331. doi: 10.1001/archinternmed.2011.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Orwig D, Hochberg MC, Gruber-Baldini AL, et al. Examining differences in recovery outcomes between male and female hip fracture patients: design and baseline results of a prospective cohort study from the baltimore hip studies. J Frailty Aging. 2018;7:162–169. doi: 10.14283/jfa.2018.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Visser M, Fuerst T, Lang T, Salamone L, Harris TB. Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. Health, Aging, and Body Composition Study--Dual-Energy X-ray Absorptiometry and Body Composition Working Group. J Appl Physiol (1985). 1999;87(4):1513–20. doi: 10.1152/jappl.1999.87.4.1513 [DOI] [PubMed] [Google Scholar]
- 26. Salamone LM, Fuerst T, Visser M, et al. Measurement of fat mass using DEXA: a validation study in elderly adults. J Appl Physiol (1985). 2000;89(1):345–52. doi: 10.1152/jappl.2000.89.1.345 [DOI] [PubMed] [Google Scholar]
- 27. Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40:423–429. doi: 10.1093/ageing/afr051 [DOI] [PubMed] [Google Scholar]
- 28. Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility–giving mobility clinical visibility: a Mobility Working Group recommendation. JAMA. 2014;311:2061–2062. doi: 10.1001/jama.2014.3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alley DE, Hicks GE, Shardell M, et al. Meaningful improvement in gait speed in hip fracture recovery. J Am Geriatr Soc. 2011;59:1650–1657. doi: 10.1111/j.1532-5415.2011.03560.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoo JI, Ha YC, Kwon HB, Lee YK, Koo KH, Yoo MJ. High prevalence of sarcopenia in Korean patients after hip fracture: a case-control study. J Korean Med Sci. 2016;31:1479–1484. doi: 10.3346/jkms.2016.31.9.1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. González-Montalvo JI, Alarcón T, Gotor P, et al. Prevalence of sarcopenia in acute hip fracture patients and its influence on short-term clinical outcome. Geriatr Gerontol Int. 2016;16:1021–1027. doi: 10.1111/ggi.12590 [DOI] [PubMed] [Google Scholar]
- 32. Ho AW, Lee MM, Chan EW, et al. Prevalence of pre-sarcopenia and sarcopenia in Hong Kong Chinese geriatric patients with hip fracture and its correlation with different factors. Hong Kong Med J. 2016;22:23–29. doi: 10.12809/hkmj154570 [DOI] [PubMed] [Google Scholar]
- 33. Kotlarczyk MP, Perera S, Nace DA, Resnick NM, Greenspan SL. Identifying Sarcopenia in female long-term care residents: a comparison of current guidelines. J Am Geriatr Soc. 2018;66:316–320. doi: 10.1111/jgs.15213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Landi F, Calvani R, Ortolani E, et al. The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in-hospital rehabilitation. Osteoporos Int. 2017;28:1569–1576. doi: 10.1007/s00198-017-3929-z [DOI] [PubMed] [Google Scholar]
- 35. Visser M, Harris TB, Fox KM, et al. Change in muscle mass and muscle strength after a hip fracture: relationship to mobility recovery. J Gerontol A Biol Sci Med Sci. 2000;55A(8):M434–40. doi: 10.1093/gerona/55.8.m434 [DOI] [PubMed] [Google Scholar]
- 36. Magaziner J, Fredman L, Hawkes W, et al. Changes in functional status attributable to hip fracture: a comparison of hip fracture patients to community-dwelling aged. Am J Epidemiol. 2003;157:1023–1031. doi: 10.1093/aje/kwg081 [DOI] [PubMed] [Google Scholar]
- 37. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573–1579. doi: 10.1001/jama.2009.1462 [DOI] [PMC free article] [PubMed] [Google Scholar]