Abstract
Background
The combination of sarcopenia and obesity has been associated with physical impairment in older people. However, previous research has relied on assessments of lean mass as a surrogate for muscle mass. We postulate that inaccurate measures of muscle mass may have obscured the role of obesity in sarcopenia and related outcomes. Our aim was to clarify the interactions of muscle and fat with physical performance and adverse outcomes using an accurate measure of muscle mass.
Methods
In a longitudinal study of >1,300 older men (mean age 84 years), we compared a direct measurement of muscle mass (D3 creatine dilution; D3Cr) with an approximation of muscle mass (appendicular lean mass [ALM] by dual-energy x-ray absorptiometry [DXA]) and their associations with measures of physical performance (gait speed, chair stand time) and adverse outcomes (incident injurious falls and mobility problems). We measured percent fat mass by DXA.
Results
Low D3Cr muscle mass was strongly associated with decreased performance and increased risk of adverse outcomes. Increased fat mass had little association after accounting for D3Cr muscle mass. In contrast, DXA ALM was minimally associated with performance or adverse outcomes, and fatness remained associated with both outcomes after accounting for DXA ALM.
Conclusions
When an accurate assessment of muscle mass (rather than lean mass) is used, reduced muscle mass is highly associated with important outcomes and the negative effects of adiposity are minimal, suggesting that obesity has little relevance for the understanding of important adverse health outcomes of sarcopenia in older men.
Keywords: Sarcopenia, Aging, Disability
The loss of muscle mass (sarcopenia) and the loss of muscle function (dynapenia) result in a major public health problem in the geriatric population (1,2). In fact, muscle mass and strength progressively decline with age while fat mass increases. Each of these trends appears to have adverse health consequences, but the combination (sarcopenic obesity) has been proposed as a particularly strong risk factor for declines in physical performance, increased fall risk, disability and mortality (3), and has been postulated to be a major health problem (4). However, while fat mass can be accurately and precisely measured with dual-energy x-ray absorptiometry (DXA), the measurement of muscle mass has been more challenging. Some methods (total body water, bioelectric impedance, DXA) assess lean mass, which includes not only muscle mass but also soft tissues such as vascular, fibrotic, and connective tissue, as well as organ weight and water (5). DXA appendicular lean mass (ALM), or the lean mass in the arms and legs, has been thought to better approximate muscle mass than total body lean mass, and is often standardized to height (eg, DXA ALM/ht2). In studies of lean and fat mass, Baumgartner (6) used DXA ALM/ht2 measurements to propose a definition of sarcopenic obesity; specifically, when DXA ALM/ht2 is lower than −2 SD of that obtained in a healthy population, and the percent of fat mass (also measured by DXA) is greater than the median of a sex-matched population. That approach has been adapted in many subsequent studies of sarcopenic obesity (4). Because studies measuring lean mass do not accurately quantify muscle (7), it has not been possible to adequately evaluate the independent roles of muscle and fat in sarcopenic obesity as risk factors for adverse health conditions in older people.
Muscle mass can now be directly measured using the D3-creatine dilution method (8,9) which is conceptually rigorous and well validated in both animal and human studies (7). For instance, in humans, muscle mass determined by the D3-creatine dilution method is highly correlated to muscle mass measures by MRI (10). To account for body size, total muscle mass is standardized to body weight (D3Cr muscle mass/weight [wgt]). We have recently confirmed the association of low D3Cr muscle mass/wgt prospectively with reduced strength, impaired physical performance, increased functional limitations, and increased risk of incident falls and mobility limitation (11). These associations were much stronger than those with DXA lean mass. This work raised the possibility that DXA-based evaluations of the relationships between obesity, sarcopenia, and important clinical outcomes are misleading.
We previously described strong associations between D3Cr muscle mass measures with physical performance, mobility limitations, and injurious falls in a large, prospective cohort of older men (MrOS) (12). Here, we build on those results to re-examine the issue of sarcopenic obesity. By using an accurate measure of muscle mass (D3Cr dilution), we aimed to better disentangle the independent and joint associations of muscle mass and fat on physical performance, mobility limitations, and injurious falls, and furthermore to contrast these associations when muscle mass is assessed by D3Cr dilution versus DXA-derived measures of lean mass.
Methods
MrOS Cohort and Study Sample
In 2000–2002, 5,994 ambulatory community-dwelling men aged ≥65 and older without bilateral hip replacements were enrolled in MrOS, a multicenter cohort study (13,14). The study was approved by the Institutional Review Board at each center and all participants provided informed consent. In 2014–2016, 2,786 survivors were contacted; 1,841 participated in a clinic visit; and 1,641 agreed to the D3Cr dilution protocol. Of these, 187 were excluded for incorrect completion of the protocol. Six samples were lost and 23 men were excluded because of outlying values for D3Cr muscle mass/wgt. Further, 49 were missing either outcome or DXA variables. Thus, the main analysis sample was 1,376 men; for the incident mobility problem outcome, the sample was limited to those without prevalent mobility problems (N = 1,065).
D3Cr Dilution Method to Estimate Muscle Mass
As described previously (15), the method involves ingesting a 30-mg dose of stable isotope labeled creatine (D3Cr), and providing a fasting, morning urine sample 3–6 days later in which D3-creatinine, unlabeled creatinine and creatine are measured using HPLC and MS/MS; these measures are then included in an algorithm to determine total body creatine pool size and thus skeletal muscle mass.
Dual-Energy X-Ray Absorptiometry
Total body lean mass, ALM, body fat, and bone mineral content were assessed by whole-body DXA scans (Hologic 4500 scanners, Waltham, MA) (16).
Physical Performance and Incident Falls and Mobility Problems
Gait speed at usual pace was measured over a 6-m course using the average of two trials (m/s) and time to complete five repeated chair stands was assessed (17). During follow-up, MrOS participants answered tri-annual questionnaires about falls and difficulty walking 2–3 blocks or climbing 10 stairs in the preceding 4 months. We used the three questionnaires that followed the participant’s Year 10.5 clinic date to define incident falls and mobility problems (11). Injurious falls were classified as a fall injury for which the participant reported visiting a doctor or other health care provider (versus no falls or a fall which did result in medical attention). We defined mobility problems as any new self-reported difficulty walking 2–3 blocks or climbing 10 steps in the year after the visit.
Statistical Approach
We compared characteristics of participants across quartiles of D3Cr muscle mass/wgt and ALM/Ht2, using analysis of variance (ANOVA), Wilcoxon tests and chi-square tests as appropriate. Beta coefficients from linear regression models describe the relationship between D3Cr muscle mass/wgt or ALM/ht2 (as independent variables in separate models) and physical performance measures (gait speed and chair stands). Models are presented as unadjusted, and adjusted for % fat (as a continuous variable). We also ran unadjusted models that were stratified by quartile of % fat (high: Q4: ≥ 31.86%, low Q1–3: < 31.86%). Interaction p-values are presented from separate models that use these variables continuously. Finally, we report the association of percent fat with the performance outcomes (per SD increment in percent fat) in unadjusted models; models adjusted for D3Cr muscle mass/wgt or DXA ALM/ht2; and unadjusted models stratified by D3Cr muscle mass/wgt or DXA ALM/ht2.
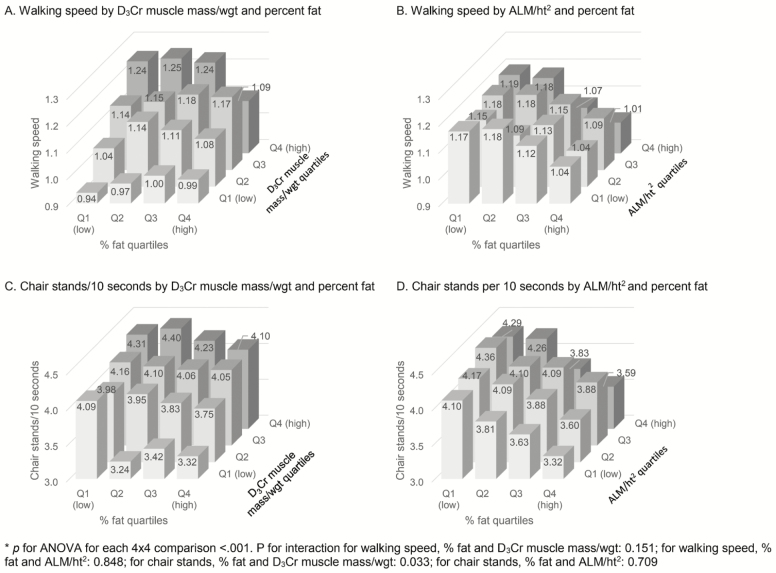
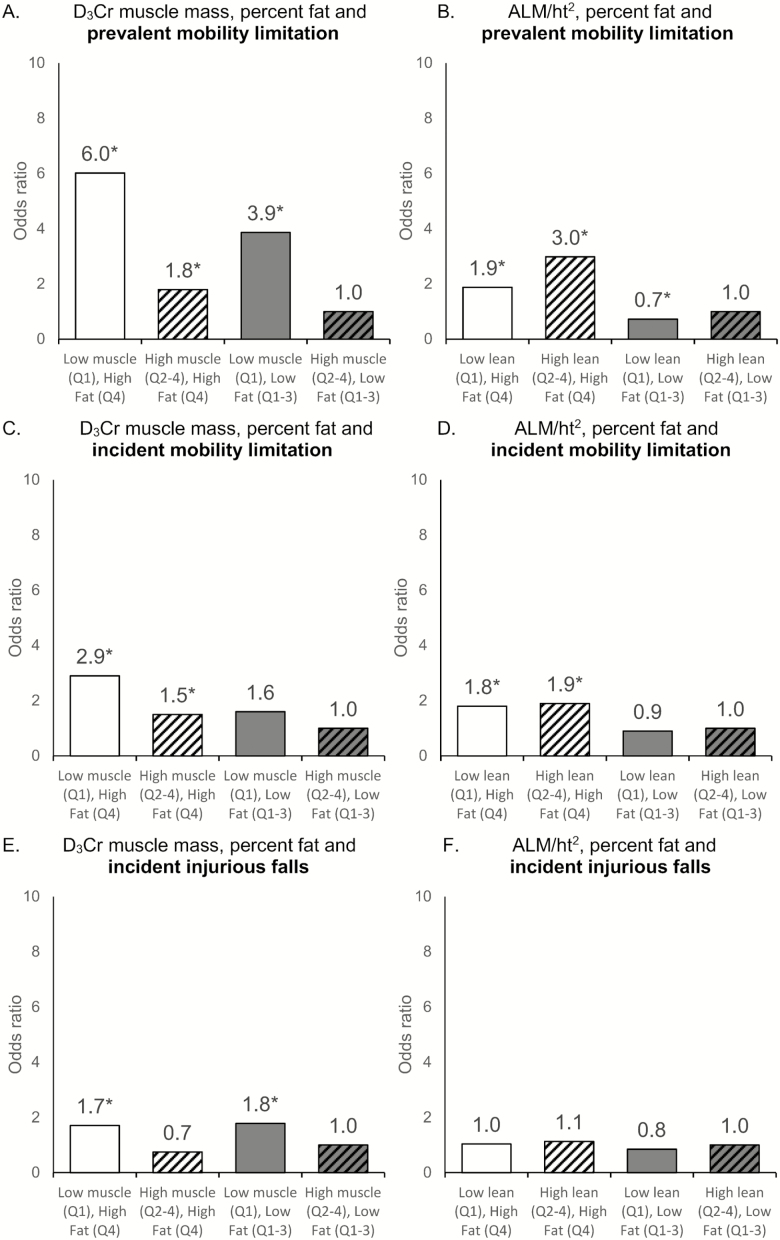
Logistic regression was used to estimate the likelihood of incident falls and mobility problems. D3Cr muscle mass/wgt and ALM/ht2 by DXA were analyzed as quartiles using the highest quartile as the referent group. We adjusted for age, and then age and % fat. Figure 2 presents a bar chart of mean values in physical performance by quartiles. We used a two-way ANOVA to compare physical performance measures across quartiles of % fat and either D3Cr muscle mass/wgt or ALM/ht2. Figure 3 presents odds ratios of mobility limitation and Injurious falls by sarcopenic groups defined using four mutually exclusive combinations of D3Cr muscle mass/wgt and ALM/ht2 with percent fat (eg, high fat/low muscle, high fat/high muscle, etc.).
Figure 2.
The associations between physical performance measures and D3Cr muscle mass or ALM. All models are adjusted for age. ALM = appendicular lean mass. (A) Mean walking speed by quartiles of D3Cr muscle mass/wgt and %fat. Interaction p-value derived from a model with continuous fat, D3Cr muscle mass/wgt and an interaction term. Fat quartile cut points: Q1<23.84, Q2 23.84–<27.82, Q3 27.82–<31.86, Q4≥ 31.86. D3Cr muscle mass/wgt Quartile Cut points: Q1<0.27, Q2 0.27–<0.30, Q3 0.30–<0.34, Q4≥0.34. (B) Mean walking speed by quartiles of ALM/ht2 and %fat. Interaction p-value derived from a model with continuous fat, ALM/ht2, and an interaction term. Fat quartile cut points: Q1<23.84, Q2 23.84–<27.82, Q3 27.82–<31.86, Q4≥31.86. ALM/ht2 Quartile cut points: Q1<6.93, Q2 6.93–<7.49, Q3 7.49–<8.11, Q4≥8.11. (C) Mean number of chair stands per 10 s by quartiles of D3Cr muscle mass/wgt and %fat. Interaction p-value derived from a model with continuous fat, D3Cr muscle mass/wgt and an interaction term. Fat quartile cut points: Q1<23.84, Q2 23.84–<27.82, Q3 27.82–<31.86, Q4≥ 31.86. D3Cr muscle mass/wgt quartile cut points: Q1<0.27, Q2 0.27–<0.30, Q3 0.30–<0.34, Q4≥0.34. (D) Mean number of chair stands per 10 seconds by quartiles of ALM/ht2 and %fat. Interaction p-value derived from a model with continuous fat, ALM/ht2 and an interaction term. Fat quartile cut points: Q1<23.84, Q2 23.84–<27.82, Q3 27.82–<31.86, Q4≥31.86. ALM/ht2 quartile cut points: Q1<6.93, Q2 6.93–<7.49, Q3 7.49–<8.11, Q4≥8.11. ALM = appendicular lean mass; wgt = weight.
Figure 3.
The likelihood of mobility limitation and injurious falls by fat categories and D3Cr muscle mass or ALM. Men are stratified into higher and lower percent fat (Quartile 4 vs Quartiles 1–3) and either higher or lower D3Cr muscle mass/wgt or high or low ALM/ht2 (Quartile 1 vs Quartiles 2–4). * Significant at p < .05 against reference group. (A) Likelihood of prevalent mobility limitation by muscle and fat groups using quartiles of D3Cr muscle mass/wgt and %fat. Interaction p-value derived from a model with continuous fat, D3Cr muscle mass/wgt and an interaction term. Fat quartile cut points: High Q4≥ 31.86, Low Q1–3 <31.86. D3Cr muscle mass/wgt quartile cut points: High Q2–4≥0.27, Low Q1<0.27. (B) Likelihood of prevalent mobility limitation by lean and fat groups using quartiles of ALM/ht2 and %fat. Interaction p-value derived from a model with continuous fat, ALM/ht2 and an interaction term. Fat quartile cut points: High Q4≥ 31.86, Low Q1–3 <31.86. ALM/ht2 quartile cut points: High Q2–4≥6.93, Low Q1<6.93. (C) Likelihood of incident mobility limitation by muscle and fat groups using quartiles of D3Cr muscle mass/wgt and %fat. Interaction p-value derived from a model with continuous fat, D3Cr muscle mass/wgt and an interaction term. Fat quartile cut points: High Q4≥31.00, Low Q1–3<31.00. D3Cr muscle mass/wgt quartile cut points: High Q2–4≥0.28, Low Q1<0.28. (D) Likelihood of incident mobility limitation by lean and fat groups using quartiles of ALM/ht2 and %fat. Interaction p-value derived from a model with continuous fat, ALM/ht2 and an interaction term. Fat quartile cut points: High Q4≥31.00, Low Q1–3<31.00. ALM/ht2 quartile cut points: High Q2–4 ≥6.93, Low Q1<6.93. (E) Likelihood of incident injurious falls by muscle and fat groups using quartiles of D3Cr/wgt and %fat. Interaction p-value derived from a model with continuous fat, D3Cr muscle mass/wgt and an interaction term. Fat quartile cut points: High Q4≥ 31.86, Low Q1–3 <31.86. D3Cr muscle mass/wgt quartile cut points: High Q2–4≥0.27, Low Q1<0.27. (F) Likelihood of incident injurious falls by lean and fat groups using quartiles of ALM/ht2 and %fat. Interaction p-value derived from a model with continuous fat, ALM/ht2 and an interaction term. Fat quartile cut points: High Q4≥ 31.86, Low Q1–3 <31.86. ALM/ht2 quartile cut points: High Q2–4≥6.93, Low Q1<6.93. ALM = appendicular lean mass; wgt = weight.
Results
Measurements were available in 1,376 men. The mean age was 84.2 ± 4.1 years, BMI was 26.8 ± 3.6 kg/M2 (17.5% was ≥ 30), and 90% were Caucasian. DXA ALM/ht2 was 7.55 ± 0.9 kg/M2, and D3Cr muscle mass/wgt was 31 ± 5%. Participant characteristics by categories of D3-creatine muscle mass (D3Cr) and adiposity are in Table 1 and by categories of lean mass (DXA) and adiposity are in Supplementary Table 1.
Table 1.
Participant Characteristics by: D3Cr Muscle Mass/wgt and Obesity Status (percent fat)
| Not Low D3Cr Muscle Mass, Not Obese (N = 887) | Low D3Cr Muscle Mass, Not Obese (N = 148) | Not Low D3Cr Muscle Mass, Obese (N = 153) | Low D3Cr Muscle Mass, Obese (N = 188) | Overall (N = 1,376) | p Value | |
|---|---|---|---|---|---|---|
| Age in years, mean (SD) | 83.8 (4.0) | 86.9 (4.2) | 83 (3.1) | 84.5 (3.9) | 84.2 (4.0) | <.001 |
| Age category | <.001 | |||||
| Age <80, n (%) | 84 (9.5) | 3 (2.0) | 18 (11.8) | 13 (6.9) | 118 (8.6) | |
| Age 80–84, n (%) | 475 (53.6) | 45 (30.4) | 91 (59.5) | 90 (47.9) | 701 (50.9) | |
| Age 85–89, n (%) | 240 (27.1) | 59 (39.9) | 39 (25.5) | 64 (34) | 402 (29.2) | |
| Age >90, n (%) | 88 (9.9) | 41 (27.7) | 5 (3.3) | 21 (11.2) | 155 (11.3) | |
| Race | .122 | |||||
| White, n (%) | 787 (88.7) | 137 (92.6) | 138 (90.2) | 177 (94.1) | 1239 (90.0) | |
| African American, n (%) | 23 (2.6) | 1 (0.7) | 6 (3.9) | 3 (1.6) | 33 (2.4) | |
| Asian, n (%) | 39 (4.4) | 5 (3.4) | 2 (1.3) | 1 (0.5) | 47 (3.4) | |
| Hispanic, n (%) | 26 (2.9) | 3 (2.0) | 4 (2.6) | 2 (1.1) | 35 (2.5) | |
| Other, n (%) | 12 (1.4) | 2 (1.4) | 3 (2.0) | 5 (2.7) | 22 (1.6) | |
| BMI, mean (SD) | 25.5 (2.8) | 26.6 (3) | 29.5 (3) | 30.9 (3.7) | 26.8 (3.6) | <.001 |
| BMI ≥30, n (%) | 57 (6.4) | 19 (12.8) | 61 (39.9) | 101 (53.7) | 238 (17.3) | <.001 |
| Walking Speed (m/s) | 1.2 (0.2) | 1 (0.3) | 1.1 (0.2) | 1 (0.3) | 1.1 (0.3) | <.001 |
| Walking Speed ≤0.8 m/s, n (%) | 49 (5.6) | 33 (22.8) | 9 (6.0) | 45 (24.2) | 136 (10.0) | <.001 |
| Incident Fall Injury, N (%) | 80 (9.0) | 26 (17.6) | 10 (6.5) | 28 (14.9) | 144 (10.5) | <.001 |
| ALM (kg), mean (SD) | 22.3 (3.0) | 22.2 (3.1) | 22.4 (3.0) | 23 (3.6) | 22.4 (3.1) | .042 |
| D3Cr muscle mass (kg), mean (SD) | 24.9 (3.8) | 19.8 (2.9) | 26.2 (3.5) | 22.3 (3.7) | 24.1 (4.1) | <.001 |
| D3Cr Muscle Mass/wgt, mean (SD) | 0.33 (0.04) | 0.25 (0.02) | 0.30 (0.02) | 0.24 (0.02) | 0.31 (0.05) | <.001 |
| ALM/ht2 (kg/m2), mean (SD) | 7.5 (0.8) | 7.4 (0.9) | 7.6 (0.9) | 7.7 (1.0) | 7.6 (0.9) | .037 |
Notes: Low muscle mass defined using lowest quartile of D3Cr muscle mass/wgt: Q1 < 0.27. Obesity defined using highest quartile of percent body fat, Q4 ≥ 31.86%. ALM = appendicular lean mass; BMI = appendicular lean mass; wgt = weight.
DXA ALM and D3-Creatine Muscle Mass Are Not Equivalent
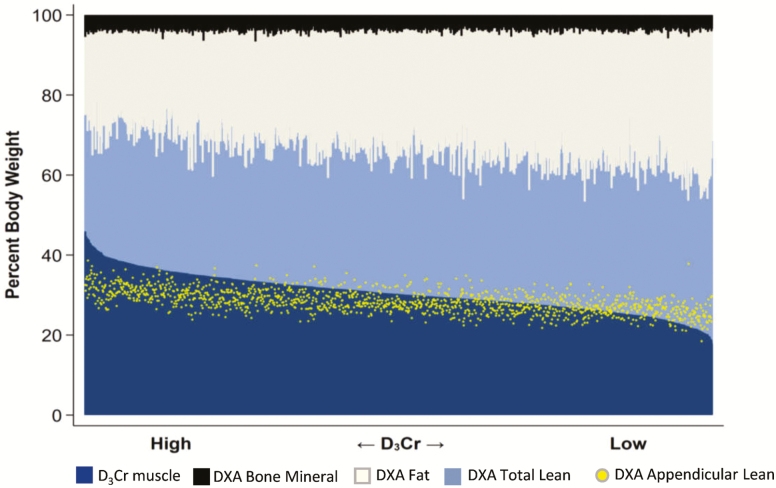
The DXA-based body composition measures and D3Cr muscle mass measurements (as proportions of body weight) are shown graphically in Figure 1. D3Cr muscle mass in the 1,376 men is ranked from highest (left) to lowest (right), with each individual’s corresponding DXA measures of bone mineral mass, fat mass, total lean mass, and ALM. Several patterns are apparent. First, DXA bone mineral mass is a minor component of body composition. Second, the proportion of weight that is DXA total lean mass is, as expected, considerably larger than the proportion that is D3Cr muscle mass. Although the proportions of lean mass and D3Cr muscle mass are correlated, there is considerable variation between them (r2 = .46). Third, ALM is not equivalent to D3Cr muscle mass.
Figure 1.
Comparison of components of body composition, each as a percent of body weight, in 1,376 older men. In combination, the three-compartment DXA measures of bone mineral, fat and total lean equal 100%. Superimposed are the measures of D3Cr muscle and DXA ALM. The men are ranked with those with highest D3Cr on the left and lowest on the right. ALM = appendicular lean mass; DXA = dual-energy x-ray absorptiometry.
D3Cr Muscle Mass/wgt and DXA ALM/ht2 Have Different Relationships to %Fat.
As reported (11), men with higher fat mass tended to have higher D3Cr muscle mass (r = 0.24, p < .001) and also tended to have higher ALM (r = .40, p < .001). However, the muscle mass proportion by D3Cr (D3Cr muscle mass/wgt) was negatively correlated with % fat (r = −.59, p < .001) while DXA ALM/ht2 and % fat were not related (r = .02, p = .38).
Associations of Physical Performance with Fat Mass and D3Cr Muscle Mass or DXA Lean Mass
There were strong associations between low D3Cr muscle mass and poor physical performance that were essentially independent of adiposity, while DXA ALM/ht2 was weakly if at all associated with physical performance and in those models adiposity retained a significant effect. Figure 2 illustrates these associations when body composition measures are in quartiles. Lower D3Cr muscle mass/wgt was independently associated with lower walking speed and shorter chair stand time, but there was no independent effect of fat (walking speed) or the effect was weak (chair stand time). Lower DXA ALM/ht2 was associated with lower chair stand time but not with walking speed, and percent fat was associated with an independent, deleterious effect on physical performance. The interactions between percent fat and DXA ALM/ht2 were not significant, but the slowest chair stand time was in men with highest percent fat and lowest D3Cr muscle mass/wgt. The relationships were not different when analyses were done using ALM/BMI rather than ALM/ht2.
Adjustment for adiposity, or stratification by % fat (quartiles 1–3 vs quartile 4), in analyses of physical performance revealed the same patterns (Table 2). Each 1 SD decrement in D3Cr muscle mass/wgt was associated with both slower walking speed (−0.11 m/s) and slower chair stands (0.34/10 seconds). Adjustment for percent fat had little impact. Also, when stratified by percent fat, the association between D3Cr muscle mass/wgt and chair stands was minimally but significantly stronger for those in the higher fat group (interaction p = −.033). In contrast, there was no association between ALM/ht2 and walking speed. After adjustment for fat, ALM/ht2 was minimally associated with walking speed. Stratification by percent fat showed no significant association between ALM/ht2 and walking speed in either the high fat or low fat group. Lower ALM/ht2 was associated with worse chair stand time, remained significant after adjustment for fat, and stratification by percent fat did not significantly alter the association. These results were similar when D3Cr muscle mass/ht2 was substituted for D3Cr muscle mass/wgt, when ALM/wgt was substituted for ALM/ht2, or when ALM and D3Cr muscle mass were used without body size adjustment.
Table 2.
Association (beta coefficient) for D3Cr Muscle Mass/wgt, DXA ALM/ht2 and Physical Performance, with Adjustment or Stratification by Percent Fat
| D3Cr muscle mass/wgt | ALM/ht2 | |||
|---|---|---|---|---|
| Walking speed | Chair stands/10 s | Walking speed | Chair stands/10 s | |
| Unadjusted | −0.10 (−0.11, −0.09) | −0.34 (−0.39, −0.28) | −0.001 (−0.015, 0.012) | −0.11 (−0.16, −0.05) |
| Adjusted for % fat | −0.11 (−0.12, −0.09) | −0.32 (−0.39, −0.25) | −0.003 (−0.016, 0.010) | −0.11 (−0.17, −0.06) |
| Stratified models | ||||
| High % fat (quartile 4: ≥ 31.86) | −0.08 (−0.11, −0.06) | −0.30 (−0.44, −0.17) | −0.001 (−0.027, 0.025) | −0.12 (−.26, 0.01) |
| Low % fat (quartiles 1–3: <31.86) | −0.09 (−0.10, −0.07) | −0.28 (−0.34, −0.22) | −0.005 (−0.020, 0.010) | −0.12 (−0.18, −0.06) |
| p-interaction | .151 | .033 | .848 | .709 |
Note: Reported per SD increment: D3Cr muscle mass/wgt: −0.05, percent body fat: 5.9%, ALM/ht2: −0.87. ALM = appendicular lean mass; wgt = weight.
Moreover, adiposity had little effect on measures of physical performance after adjustment for D3Cr muscle mass/wgt but remained important in models using ALM/ht2. Higher percent fat was associated with slower walking speed and chair stands in unadjusted models (Supplementary Table 2) but these associations were no longer significant with adjustment for D3Cr muscle mass/wgt. In contrast, associations between percent fat and slower walking speed and chair stands were essentially unchanged and remained significant after adjustment for ALM/ht2. In stratified models, there was a slight association between percent fat and slower walking speed in those with low D3Cr muscle mass/wgt but the interaction was not significant. The association between percent fat and chair stand time was slightly stronger in those with high (vs low) D3Cr muscle mass (interaction p value = .030). In models stratified by ALM/ht2, however, higher percent fat was associated with slower walking speed and chair stands in both strata of lean mass in a similar direction (p for interaction > .05).
Associations of Mobility Limitation and Injurious Falls with Fat Mass and D3Cr Muscle Mass or DXA ALM/ht2
In models adjusted for age, men in the lowest quartile of D3Cr muscle mass/wgt were 2.7-fold more likely to experience an incident injurious fall, 9.8-fold more likely to have a prevalent mobility limitation, and 2.7-fold more likely to experience incident mobility limitation (Table 3). Adjustment for percent fat did not substantially change these associations. In contrast, in models adjusted for age, ALM/ht2 was not associated with incident injurious falls or mobility limitation, and further adjustment for percent fat had little effect on this null association. Moreover, men in the lower quartile of ALM/ht2 were significantly less likely to have prevalent mobility limitation, and adjustment for percent fat did not change this association.
Table 3.
Association (odds ratio) for D3Cr Muscle Mass/wgt or DXA ALM/ht2 with Prevalent and Incident Mobility Limitations or Incident Injurious Falls, with Adjustment by Percent Fat
| D3Cr Muscle Mass/wgt | ||||||
|---|---|---|---|---|---|---|
| Incident Injurious fall | Prevalent Mobility Limitation | Incident Mobility Limitation* | ||||
| Age-adjusted | + % fat | Age-adjusted | + % fat | Age-adjusted | + % fat | |
| Quartile 1 D3Cr muscle mass/wgt (low) | 2.7 (1.5, 4.7) | 4.1 (2.1, 7.9) | 9.8 (6.1, 15.7) | 7.8 (4.5, 13.5) | 2.7 (1.8, 4.0) | 2.0 (1.2, 3.1) |
| Quartile 2 D3Cr muscle mass/wgt | 1.4 (0.8, 2.6) | 1.9 (1.0, 3.7) | 3.3 (2.0, 5.4) | 2.9 (1.7, 4.8) | 1.9 (1.3, 2.8) | 1.6 (1.0, 2.4) |
| Quartile 3 D3Cr muscle mass/wgt | 2.0 (1.1, 3.5) | 2.3 (1. 35, 4.1) | 2.0 (1.2, 3.3) | 1.8 (1.0, 3.0) | 1.3 (0.9, 1.9) | 1.1 (0. 85, 1.7) |
| Quartile 4 D3Cr muscle mass/wgt (high) | 1.00 referent | 1.00 referent | 1.00 referent | 1.00 referent | 1.00 referent | 1.00 referent |
| p trend | .003 | <.001 | <.001 | <.001 | <.001 | <.001 |
| ALM/ht2 | ||||||
| Incident Injurious fall | Prevalent Mobility limitation | Incident Mobility Limitation | ||||
| Age-adjusted | + % fat | Age-adjusted | + % fat | Age-adjusted | + % fat | |
| Quartile 1 ALM/ht2 (low) | 1.0 (0.6, 1.8) | 1.0 (0.6, 1.8) | 0.5 (0.4, 0.7) | 0.5 (0.3, 0.7) | 0.8 (0.5, 1.1) | 0.7 (0.5, 1.1) |
| Quartile 2 ALM/ht2 | 1.2 (0.7, 2.0) | 1.2 (0.7, 2.0) | 0.6 (0.4, 0.9) | 0.6 (0.4, 0.8) | 0.8 (0.5, 1.1) | 0.8 (0.5, 1.1) |
| Quartile 3 ALM/ht2 | 1.4 (0.8, 2.2) | 1.4 (0.8, 2.2) | 0.7 (0.5, 1.0) | 0.7 (0.5, 1.0) | 0.9 (0.6, 1.3) | 0.9 (0.6, 1.2) |
| Quartile 4 ALM/ht2 (high) | 1.00 referent | 1.00 referent | 1.00 referent | 1.00 referent | 1.00 referent | 1.00 referent |
| p trend | .926 | .926 | <.001 | <.001 | .125 | .093 |
Note: ALM = appendicular lean mass; wgt = weight.
*Because the number of men with follow-up for incident mobility limitation (N = 1,065) is different than for prevalent mobility limitation (N = 1,376) the quartile limits are slightly different. D3Cr muscle mass/wgt Prevalent Quartile cut points: Q1<0.27, Q2 0.27-<0.30, Q3 0.30-<0.34, Q4 ≥ 0.34. D3Cr muscle mass/wgt Incident Quartile cut points: Q1<0.28, Q2 0.28-<0.31, Q3 0.31-<0.35, Q4 ≥ 0.35 ALM/ht2 Prevalent Quartile cut points: Q1<6.93, Q2 6.93-<7.49, Q3 7.49-<8.11, Q4 ≥ 8.11. ALM/ht2 Incident Quartile cut points: <6.93, Q2 6.93-<7.47, Q3 7.47-<8.04., Q4 ≥ 8.04.
Those with both lower D3Cr muscle mass/wgt and higher percent fat were more likely to have prevalent mobility limitation and to experience mobility limitation during follow-up, but there was not a significant interaction in the effects of muscle mass and adiposity (Figure 3). In contrast, the associations between ALM/ht2 with prevalent or subsequent mobility limitation were small, and higher %fat was highly significant. The likelihood of injurious fall was also higher in men with lower D3Cr muscle mass/wgt while there was little difference by fat category. However, those with higher and lower DXA ALM/ht2 had a comparable likelihood of injurious falls, there was little difference in categories of percent fat. In age-adjusted models, percent fat was not significantly associated with incident injurious falls (Supplementary Table 4). Further adjustment for D3Cr muscle mass/wgt or ALM/ht2 resulted in a lower likelihood of injurious falls for those with higher fat. Men in the highest quartile of percent fat had a higher likelihood of prevalent and incident mobility limitation. After further adjustment for D3Cr muscle mass/wgt, the association between percent fat and prevalent mobility limitation was no longer significant, while the association with incident mobility limitation remained significant but was attenuated. After adjustment for age and ALM/ht2, the associations of high percent fat with increased likelihood of prevalent and incident mobility limitation remained unchanged. These results were similar when ALM/BMI was considered in place of ALM/ht2.
Discussion
Our major finding is that muscle mass measured by D3Cr dilution is strongly and independently associated with physical performance and risk of injurious falls or mobility limitation, and that the degree of body fatness has little influence on these outcomes once D3Cr muscle mass is considered. Thus, in older men, the clinical importance of “sarcopenic obesity”—the combined effect of muscle and fat on adverse physical outcomes—is minimal when muscle mass is accurately measured with D3Cr dilution. On the other hand, an approximation of muscle mass, DXA ALM/ht2, had little independent effect on measures of physical function or incident adverse events, and fatness remained an important contributor after accounting for DXA ALM.
We previously reported that muscle mass measured by D3Cr dilution is robustly associated with physical performance and incident mobility limitation and injurious falls. We reasoned that those novel results could be very relevant for the evaluation of the relative contributions of muscle mass and fatness to those outcomes, and hence, for the understanding of sarcopenic obesity. On the basis of other estimates of muscle mass, it has been previously postulated that lower muscle mass and increased fatness both contributed substantially to adverse outcomes in older adults. In fact, we found that when muscle mass is assessed with D3Cr dilution the influence of overall fatness on physical performance and incident outcomes is very small while muscle mass retains strong associations. These results provide a new framework for considering sarcopenic obesity in which reduced muscle mass plays a dominant role in determining the degree of physical impairment and the risk of related adverse outcomes such as injurious falls and mobility limitation. The contribution of obesity to other health consequences that have been attributed to sarcopenic obesity, such as cardiometabolic syndromes (18), may be greater, and the influence of specific fat compartments, such as muscle fat infiltration (19), might be important in addition to the mass of muscle itself.
Our findings (Figure 1) demonstrate that measurement of muscle mass with D3Cr dilution and its relationship to adiposity are fundamentally different from muscle mass approximated by DXA ALM. As we have previously reported (11) total lean mass is only moderately correlated (r = .66, p < .001) with muscle mass by D3Cr. This is not a surprising finding since lean mass includes organ weights, tissues, and water that are not muscle. Similarly, ALM, commonly used in sarcopenia research as a surrogate measure of skeletal muscle, is only modestly correlated with D3Cr muscle mass, there is considerable individual variation in the correlation and the relationship is inconsistent across the range of muscle mass by D3Cr. Moreover, ALM/ht2 is unrelated to D3Cr muscle mass/wgt. Thus, ALM and ALM/ht2 are inadequate substitutes for directly measured muscle mass by D3Cr dilution.
Our findings also indicate that ALM/ht2 and D3Cr muscle mass are quite different in their associations with body fat. While the proportion of body fat was not correlated with ALM/ht2, a consistent finding in previous studies, percent body fat was lower in men with higher D3Cr muscle mass. This discrepancy may be due to fundamental differences in the measurements. In support of the inverse relationship of D3Cr muscle mass and percent body fat, muscle mass is important in energy metabolism and adiposity might be predicted to be inversely related to muscle mass. We previously reported (11) that D3Cr muscle mass was significantly associated with physical activity. Other research suggests that an estimate of muscle mass is closely associated with basal metabolic rate (BMR) (20). Thus, differences in BMR and physical activity, the two major components of total daily energy expenditure, may explain the relationship between D3Cr muscle mass and body fatness.
In view of our findings, the interpretation of the condition sarcopenic obesity is highly dependent on the method used to measure muscle mass. First, the associations between low D3Cr muscle mass/wgt and poor physical performance were strong and independent of adiposity. Also, while higher percent fat was associated with slower walking speed and worse chair stands performance, adjustment for D3Cr muscle mass/wgt largely attenuated these associations. In contrast, the associations between ALM/ht2 and physical performance were weaker or nonsignificant, adjustment for % fat strengthened those relationships, and the negative associations between percent fat and physical performance remained significant after adjustment for ALM/ht2. Moreover, in stratified models of D3Cr muscle mass/wgt and percent fat there was minimal effect of adiposity on physical performance. Although there was a suggestion that adiposity was more associated with chair stand time in the highest quartile of fatness, the effect was very small. But, in similar models using ALM/ht2 there was a prominent negative association of percent fat. These comparisons suggest that fat has little independent association with physical performance when muscle mass is measured by D3Cr dilution, but has important adverse effects when measured by ALM/ht2. Similarly, there were strong associations between low D3Cr muscle mass/wgt and prevalent and incident mobility limitation and incident injurious falls regardless of adjustment for percent fat. Conversely, there was no association between ALM/ht2 and incident injurious falls or mobility limitation, and the likelihood of prevalent mobility limitation was actually lower in men with higher ALM/ht2. These associations were largely unchanged by adjustment for percent fat.
Why higher %fat was more often associated with poor physical performance and adverse outcomes in models with DXA ALM/ht2 is uncertain, but may reflect that DXA ALM/ht2 is a less accurate measurement of muscle and has little or no independent relationship with adverse outcomes. We have shown here that higher fat mass is associated with lower D3Cr muscle mass/wgt, and thus, in analyses that include DXA ALM/ht2 and %fat, higher fat mass may be a reflection of proportionately lower muscle mass.
This study has major strengths. It is a unique comparison of a direct measurement of muscle mass using D3Cr with assessment of ALM in the evaluation of the sarcopenic obesity. We examined a large cohort of community dwelling, older men, a group at risk of impaired physical performance and adverse health outcomes, in a longitudinal, observational study design. The cohort included a wide range of muscle and fat mass, allowing adequately testing of the hypotheses proposed. Study sites were experienced and assessment methods were standardized. The major limitation of our work is that that the participants were older men who were primarily Caucasian; our findings may not pertain to other groups. We purposefully did not address the complex issue of defining sarcopenia in general, but rather concentrated on the more focused issue of sarcopenic obesity.
In sum, these results suggest that the independent and joint associations of adiposity and muscle mass vary substantially by the method used to approximate muscle mass. In contrast to previous studies that were based on measures of DXA ALM, our results using the D3Cr dilution method suggest that muscle mass is a primary determinate of physical performance and adverse outcomes, and that the effects of higher body fatness are less important. While there may be a small adverse effect of adiposity combined with low muscle mass in older men, the importance of sarcopenic obesity appears to be minor when muscle mass is measured accurately, and the term “sarcopenic obesity” has few implications for physical function, injurious falls and mobility limitation. Overall, these findings have implications for further examination of the interactions of muscle and fat, and how these contribute to adverse health outcomes.
Funding
This work is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. Funding for the D3Cr muscle mass measure was provided by NIAMS (grant number R01 AR065268). GlaxoSmithKline provided in-kind support by providing the D3-creatine dose and analysis of urine samples.
Author Contributions
Study concept and design: E.S.O. and P.M.C. Acquisition of subjects and/or data: E.S.O. and P.M.C. Analysis and interpretation of data: All the authors. Preparation of initial manuscript: E.S.O., P.M.C., and K.E.P.
Conflict of Interest
None of the authors have relationships or activities that could appear to have influenced the submitted work. W.J.E. and M.H. are listed as coinventors on the granted patents for the D3-Cr dilution method. However, they do not derive any income or royalties or own the intellectual property for the method.
Supplementary Material
References
- 1. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x [DOI] [PubMed] [Google Scholar]
- 2. Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63:829–834. doi: 10.1093/gerona/63.8.829 [DOI] [PubMed] [Google Scholar]
- 3. Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. 2008;18:388–395. doi: 10.1016/j.numecd.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 4. Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14:513–537. doi: 10.1038/s41574-018-0062-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Evans WJ, Hellerstein M, Orwoll E, Cummings S, Cawthon PM. D3-creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2019;10(1):14–21. doi: 10.1002/jcsm.12390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x [DOI] [PubMed] [Google Scholar]
- 7. Evans WJ, Hellerstein M, Orwoll E, Cummings S, Cawthon PM. D3 -Creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2019;10:14–21. doi: 10.1002/jcsm.12390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stimpson SA, Turner SM, Clifton LG, et al. Total-body creatine pool size and skeletal muscle mass determination by creatine-(methyl-D3) dilution in rats. J Appl Physiol (1985). 2012;112:1940–1948. doi: 10.1152/japplphysiol.00122.2012 [DOI] [PubMed] [Google Scholar]
- 9.Stimpson SA, Leonard MS, Clifton LG, et al. Longitudinal changes in total body creatine pool size and skeletal muscle mass using the D3-creatine dilution method. J Cachexia Sarcopenia Muscle. 2013;4(3):217–223. doi: 10.1007/s13539-013-0110-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clark RV, Walker AC, O’Connor-Semmes RL, et al. Total body skeletal muscle mass: estimation by creatine (methyl-d3) dilution in humans. J Appl Physiol (1985). 2014;116:1605–1613. doi: 10.1152/japplphysiol.00045.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cawthon PM, Orwoll ES, Peters KE, et al. Strong relation between muscle mass determined by d3-creatine dilution, physical performance and incidence of falls and mobility limitations in a prospective cohort of older men. J Gerontol A Biol Sci Med Sci. 2018;74(6):844–852. doi: 10.1093/gerona/gly129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cawthon PM, Orwoll ES, Peters KE, et al. ; Osteoporotic Fractures in Men (MrOS) Study Research Group Strong relation between muscle mass determined by D3-creatine dilution, physical performance, and incidence of falls and mobility limitations in a prospective cohort of older men. J Gerontol A Biol Sci Med Sci. 2019;74:844–852. doi: 10.1093/gerona/gly129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 14. Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 15. Shankaran M, Czerwieniec G, Fessler C, et al. Dilution of oral D3 -Creatine to measure creatine pool size and estimate skeletal muscle mass: development of a correction algorithm. J Cachexia Sarcopenia Muscle. 2018;9:540–546. doi: 10.1002/jcsm.12278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee CG, Boyko EJ, Nielson CM, et al. ; Osteoporotic Fractures in Men Study Group Mortality risk in older men associated with changes in weight, lean mass, and fat mass. J Am Geriatr Soc. 2011;59:233–240. doi: 10.1111/j.1532-5415.2010.03245.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cawthon PM, Fullman RL, Marshall L, et al. ; Osteoporotic Fractures in Men (MrOS) Research Group Physical performance and risk of hip fractures in older men. J Bone Miner Res. 2008;23:1037–1044. doi: 10.1359/jbmr.080227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dominguez LJ, Barbagallo M. The cardiometabolic syndrome and sarcopenic obesity in older persons. J Cardiometab Syndr. 2007;2:183–189. doi: 10.1111/j.1559-4564.2007.06673.x [DOI] [PubMed] [Google Scholar]
- 19. Linge J, Heymsfield SB, Dahlqvist Leinhard O. On the definition of sarcopenia in the presence of aging and obesity-initial results from UK Biobank. J Gerontol A Biol Sci Med Sci, doi: 10.1093/gerona/glz229 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tzankoff SP, Norris AH. Longitudinal changes in basal metabolism in man. J Appl Physiol Respir Environ Exerc Physiol. 1978;45:536–539. doi: 10.1152/jappl.1978.45.4.536 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.