Abstract
Background
Current consensus is to combine a functional measure with muscle quantity to assess/confirm sarcopenia. However, the proper body size adjustment for muscle quantity is debated and sarcopenia in obesity is not well described. Further, functional measures are not muscle-specific or sensitive to etiology, and can be confounded by, for example, fitness/pain. For effective detection/treatment/follow-up, muscle-specific biomarkers linked to function are needed.
Methods
Nine thousand six hundred and fifteen participants were included and current sarcopenia thresholds (EWGSOP2: DXA, hand grip strength) applied to investigate prevalence. Fat-tissue free muscle volume (FFMV) and muscle fat infiltration (MFI) were quantified through magnetic resonance imaging (MRI) and sex-and-body mass index (BMI)-matched virtual control groups (VCGs) were used to extract each participant’s FFMV/height2 z-score (FFMVVCG). The value of combining FFMVVCG and MFI was investigated through hospital nights, hand grip strength, stair climbing, walking pace, and falls.
Results
Current thresholds showed decreased sarcopenia prevalence with increased BMI (underweight 8.5%/normal weight 4.3%/overweight 1.1%/obesity 0.1%). Contrary, the prevalence of low function increased with increasing BMI. Previously proposed body size adjustments (division by height2/weight/BMI) introduced body size correlations of larger/similar magnitude than before. VCG adjustment achieved normalization and strengthened associations with hospitalization/function. Hospital nights, low hand grip strength, slow walking pace, and no stair climbing were positively associated with MFI (p < .05) and negatively associated with FFMVVCG (p < .01). Only MFI was associated with falls (p < .01). FFMVVCG and MFI combined resulted in highest diagnostic performance detecting low function.
Conclusions
VCG-adjusted FFMV enables proper sarcopenia assessment across BMI classes and strengthened the link to function. MFI and FFMV combined provides a more complete, muscle-specific description linked to function enabling objective sarcopenia detection.
Keywords: Sarcopenic obesity, Muscle fat infiltration, Magnetic resonance imaging, Imaging biomarkers, Dual-energy x-ray absorptiometry
Sarcopenia, a condition characterized by progressive loss of muscle mass and function in the aging population, is associated to adverse outcomes in several different disease areas (1,2). The current consensus is to use a combination of a functional measure and muscle quantity/quality to assess/confirm the presence sarcopenia (3,4). A function–mass combination is needed to increase the specificity in diagnosis: Low muscle quantity might not imply sarcopenia-related problems but can simply be attributed to small body size. Likewise, low muscle or physical function might not imply sarcopenia-related problems but could, for example, be attributed to a stroke, neurological disorders, or arthritis.
To identify low muscle mass, dual-energy x-ray absorptiometry (DXA) is most commonly used for the estimation of appendicular lean mass (ALM) (3). Challenges with DXA are, for example, that different DXA brands do not give consistent results, hindering accurate comparison between different patient groups and populations; and that muscle mass measures can be affected by body thickness and hydration status, thus lowering both accuracy and reproducibility and complicating longitudinal assessment (5,6).
To identify low muscle or physical function, several measures, including hand grip strength, chair stand, gait speed, 400-m walk test, timed up and go test, and short physical performance battery are commonly applied (3). Challenges with these measurements, in addition to not being muscle-specific (not directly measured in the muscle) or sensitive to etiology, are that confounding factors such as fitness level, depression, or pain complicates longitudinal assessment by making potential muscle-related functional decline difficult to single out. Further, patients in later stages of disease might eventually be unable to perform these tests, completely hindering continued tracking of their functional decay.
Further challenges in detecting and understanding sarcopenia and its consequences relates to the wide variation in normal physiology within the general population. Low muscle quantity can be found in individuals with small body size without signs of low function or functional decline and the growing obesity epidemic complicates early diagnosis. For patients with obesity, loss of muscle, and fat mass caused by wasting can be misinterpreted as successful weight loss in those striving to lose weight. In addition, the fundamental correlation between body size and muscle quantity further complicates detection of sarcopenia in patients with overweight/obesity. The correlation between muscle quantity and body size has led to suggestions of body size adjustments for ALM, namely through division by height2, weight, or body mass index (BMI) (4,7). However, the recently updated European consensus report (EWGSOP2) and the FNIH sarcopenia project points to different body size adjustments providing thresholds for ALM, ALM/height2, and ALM/BMI without consensus of the proper body size adjustment (3,4).
Simultaneous assessment of muscle quantity and fat infiltration provides a more complete, muscle-specific, description of the muscle that could open up the possibility for objective sarcopenia assessment. Muscle quantity has been extensively used within the field of sarcopenia and muscle fat is a well-established biomarker within muscular dystrophies (8) that has previously been used as a quantitative measure of muscle quality (9,10). Magnetic resonance imaging (MRI) is considered as a reference method for noninvasive assessment of muscle and fat quantity (5). Today, a standardized 6-minute MRI examination can enable simultaneous assessment of muscle volumes and muscle fat infiltration (MFI) as well as traditional body composition (total lean tissue and total adipose tissue), and a detailed characterization of body fat distribution and ectopic fat accumulation (visceral and subcutaneous adipose tissue and liver fat) (11).
For effective detection, treatment and follow-up of sarcopenia-related problems, muscle-specific biomarkers linked to functional performance are needed. The aim of this study was to utilize the MRI-acquired data from the first 10,019 participants in the UK Biobank imaging study to provide basis for individualized, BMI invariant, sarcopenia thresholds identifying unexpectedly low muscle volumes, and investigate the additional value of MFI for sarcopenia assessment.
Methods
Data Acquisition
The first 10,019 UK Biobank participants from the imaging study were included. UK Biobank is a long-term biobank study following the health and well-being of 500,000 volunteer participants enrolled at ages from 40 to 69 beginning 2006. The imaging study aims to conduct detailed imaging of over 100,000 by inviting all participants out of those originally participating in the study living within reasonable travelling distance to one of the three imaging assessment centers in Reading, Stockport, and Newcastle (12).
This research was conducted using the UK Biobank resource, project ID 6569. The study was approved by the North West Multicenter Research Ethics Committee, UK. Written informed consent was obtained prior to study entry.
Measurements available through the UK Biobank resource
Height was recorded using a Seca stadiometer (Seca, Hamburg, Germany), weight with a Tanita BC418ma (Tanita Europe, NL), and hand grip strength using a Jamar J00105 hydraulic hand dynamometer (Lafayette Instrument, USA) (protocol is given in Supplementary Material). Handedness, usual walking pace, frequency of stair climbing, and number of falls last year were acquired through touchscreen questionnaires. Number of hospital nights was extracted from the Health Episode Statistics (HES) data obtained through linking to National Health Services (NHS) records (downloaded November 2016, available from 1995 to 2015). Whole-body DXA was performed using a GE-Lunar iDXA (Madison, WI). ALM was calculated by summarizing lean mass for arms and legs (quantified through DXA, UK Biobank Field IDs 23275, 23258).
Outcomes investigated in this study were:
Hand grip strength, comparing participants below and above 16/27 kg (females/males). The hand grip strength was that of the hand reported as dominant. If no information of handedness was present (N = 1) or a participant reported using both hands (N = 167), the mean of the right and left hand was used.
Usual walking pace, comparing participants self-reporting “Slow pace” to those reporting “Steady average pace” or “Brisk pace.”
Frequency of stair climbing, comparing participants self-reporting climbing 0 flights of stairs/day (approximately 10 steps) to those reporting climbing 1 or more flights of stairs/day the last 4 weeks.
Falls, comparing participants self-reporting more than one fall the last year to those reporting only one fall or no falls.
Health care burden, defined as number of hospitalization nights within 10 years prior to scanning, excluding pregnancy-related nights (ICD10-codes under O and P) and truncated at 30 nights.
Supplementary Material presents specifics of data acquisition for outcomes related to functional performance.
Body composition profiling using MRI
MRI-based body composition analysis, resulting in the quantification of fat and muscle volumes by application of a 6-minute dual-echo Dixon Vibe protocol and advanced image analysis (11,13–17), was performed using AMRA Researcher (AMRA Medical AB, Linköping, Sweden). Specifically, fat-tissue free muscle volume (FFMV) in the thighs, MFI in the anterior thighs, and visceral and abdominal subcutaneous adipose tissue were quantified.
FFMV was defined as the sum of all voxels with fat fraction < 50% (what one may call “viable muscle tissue”). This is to be differentiated from lean muscle volume (sometimes called contractile muscle volume), which can be calculated by subtracting the fat volume from the entire muscle volume. FFMV is insensitive to erroneous muscle segmentation in boundaries between muscle tissue and surrounding intermuscular adipose tissue (as intermuscular adipose tissue consists of voxels with high percentage of fat that are effectively removed from FFMV through the 50% threshold).
MFI was measured as the fat fraction in the “viable muscle tissue” (FFMV). This is a 3D-volumetric assessment corresponding to what is measured when fat fraction is assessed in homogenous appearing muscle tissue using single voxel spectroscopy. MFI has the same benefit as FFMV concerning erroneous muscle segmentations.
Details about MRI scanning protocol and image analysis can be found in the Supplementary Material.
Inclusion
Nine thousand six hundred and fifteen out of the 10,019 UK Biobank participants were included. A participant was required to have a non-zero hand grip strength, and data for FFMV with corresponding MFI value of at least one leg. Out of these, 4,586 participants had data available from the DXA assessment. Only the DXA subset was used for the analyses where data from DXA were included.
Statistical Analysis
To illustrate the need of individualized muscle quantity assessment, current sarcopenia thresholds as recommended by EWGSOP2 (hand grip strength: 16/27 kg, DXA-measured ALM/height2: 6.0/7.0 kg/m2 (females/males)) (3), were investigated in different BMI classes: underweight (BMI < 20 kg/m2), normal weight (20 ≤ BMI < 25 kg/m2), overweight (25 ≤ BMI < 30 kg/m2), and obesity (BMI ≥ 30 kg/m2). Further, DXA-based ALM was compared to the suggested MRI-based muscle quantity measurement (FFMV) through receiver operator characteristic (ROC) analysis for the detection of low functional performance.
Individualized muscle volume assessment using MRI
To provide basis for individualized sarcopenia thresholds identifying lower muscle volumes than expected, a virtual control group (VCG) was created for each participant. Each VCG consisted of participants of the same sex and within ±2 kg/m2 of the participants’ BMI. If this group did not include at least 150 virtual controls, the BMI interval was symmetrically and incrementally increased by 0.1 kg/m2 until that number was reached. To measure each individual’s deviation from expected muscle volume while taking body size into account, the FFMV/height2 z-score (number of standard deviations [SDs] from mean FFMV/height2) was calculated from the VCG distributions. This measure will be referred to as FFMVVCG. To put FFMVVCG in the context of other proposed body size adjustments, its BMI correlation was compared to the BMI correlation of the measures obtained through division of ALM and FFMV by height2, weight, and BMI.
Association with aging
The associations of FFMVVCG and MFI with age, were compared to the associations of FFMV and ALM (both adjusted by division with height2, weight, and BMI, respectively) with age through visualization, calculation of the 5-year difference in mean, and calculation of the 5-year effect size. The inferred rates of change for FFMVVCG and MFI (based on cross-sectional data) across the complete age range were investigated by applying a 10-year sliding window (step size 0.5 years) and extracting the slope from the linear regression model (predicting age from muscle measurements) fitted to each 10-year window. The association between age and inferred rate of change was investigated using linear regression.
Associations with functional outcomes and health care burden
To assess the potential value of combined muscle assessment for sarcopenia (including both FFMV and MFI) the associations with hand grip strength, walking pace, stair climbing, falls, and health care burden were investigated. Logistic regression modeling was performed using FFMVVCG and MFI as predictors for functional performance in the whole cohort. For health care burden, negative binomial regression modeling was performed. The associations to health care burden were additionally investigated among participants with low ALM/height2 (EWGSOP2 thresholds, N = 797) and among participants with low hand grip strength (EWGSOP2 thresholds, N = 612). All models were adjusted for sex and age, and fat distribution/abdominal adiposity (by including visceral and subcutaneous adipose tissue volumes separately in each model). Visceral and subcutaneous adipose tissue volumes are approximate of the fat mass carried by the thigh muscles and highly correlated with weight (excluding the thigh muscles themselves). Further, distribution between visceral and subcutaneous fat is associated with metabolic disorders common in the obesity population such as type 2 diabetes and coronary heart disease (11,18–20). For reference, analyses were repeated using FFMV instead of FFMVVCG.
Diagnostic performance
Logistic regression models were used for prediction of the four functional performance outcomes (low hand grip strength, slow walking pace, no stair climbing, and more than one fall last year) using (i) FFMV, (ii) FFMVVCG, (iii) MFI, and (iv) FFMVVCGand MFI as covariates. The predicted values extracted from these models were used as predictors in ROC analysis, and the area under the ROC curves with corresponding confidence intervals were calculated for comparison of diagnostic performance.
To enable development of sarcopenia thresholds based on combined muscle assessment, the fraction of participants with low functional performance for different FFMVVCG and MFI thresholds were calculated.
Associations were considered significant for α < 0.05. Computations were performed using R version 3.4.4 (The R Foundation, Vienna, Austria).
Results
Table 1 summarizes the characteristics of the full cohort, females and males separately. Supplementary Table S1 shows corresponding characteristics in the DXA subset. No significant differences in characteristics were found.
Table 1.
Characteristics Summarization of the Full Cohort, Females and Males Separately
| All | Females | Males | |
|---|---|---|---|
| N participants | 9,615 | 5,046 | 4,569 |
| Age, years | 62.60 (7.50) | 61.87 (7.34) | 63.41 (7.59) |
| Weight, kg | 75.55 (14.77) | 68.68 (12.91) | 83.14 (12.84) |
| Height, m | 1.69 (0.09) | 1.63 (0.06) | 1.76 (0.06) |
| Body mass index, kg/m2 | 26.64 (4.37) | 26.24 (4.76) | 27.08 (3.85) |
| Waist circumference, cm | 87.29 (12.04) | 81.91 (11.32) | 93.24 (9.82) |
| Appendicular lean mass/height2, kg/m2 | 7.34 (1.23) | 6.55 (0.85) | 8.24 (0.94) |
| Hand grip strength, kg | 31.25 (10.51) | 24.02 (5.96) | 39.24 (8.46) |
| Fat-tissue free muscle volume, L | 10.34 (2.57) | 8.36 (1.18) | 12.54 (1.77) |
| Fat-tissue free muscle volume/height2, L/m2 | 3.57 (0.62) | 3.14 (0.37) | 4.05 (0.48) |
| Muscle fat infiltration, % | 7.41 (1.86) | 7.93 (1.85) | 6.83 (1.69) |
| Visceral adipose tissue volume, L | 3.68 (2.21) | 2.63 (1.51) | 4.84 (2.28) |
| Abdominal subcutaneous adipose tissue volume, L | 7.01 (3.19) | 8.05 (3.41) | 5.85 (2.46) |
| N hand grip strength < 16/27 kg (females/males) | 612 (6.37%) | 338 (6.70%) | 274 (6.00%) |
| N flights of stairs climbed = None | 758 (7.88%) | 385 (7.63%) | 373 (8.16%) |
| N usual walking pace = Slow | 420 (4.37%) | 241 (4.78%) | 179 (3.92%) |
| N falls last year > 1 | 457 (4.75%) | 291 (5.77%) | 166 (3.63%) |
Note: For continuous variables, mean and standard deviation is show. Appendicular lean mass assessed by dual-energy x-ray absorptiometry (DXA) (available for N = 4,586 participants), fat-tissue free muscle volume, muscle fat infiltration, visceral adipose tissue volume, and subcutaneous adipose tissue volume assessed by magnetic resonance imaging (MRI).
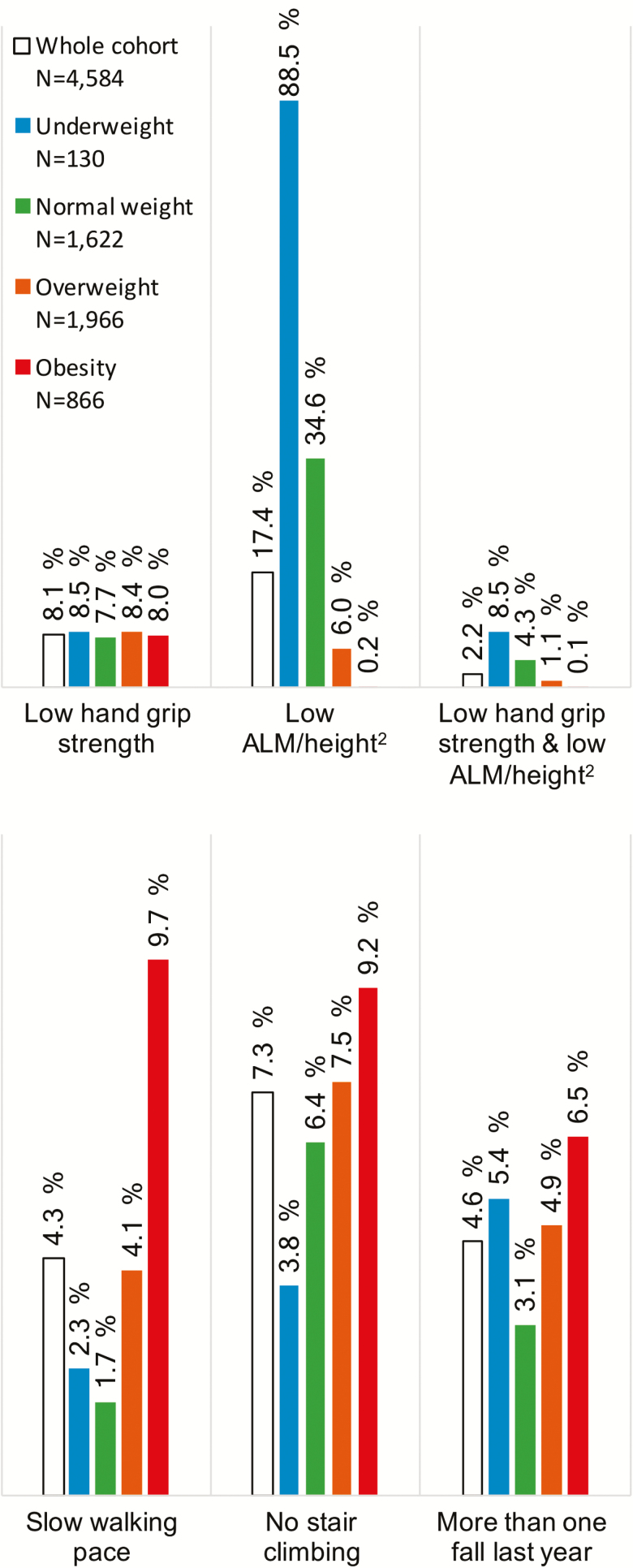
The fractions of participants with low muscle mass (ALM/height2), low hand grip strength, and the combination of the two (EWGSOP2 thresholds) in different BMI classes are shown in Figure 1, together with the fraction of participants reporting low functional performance (slow walking pace, no stair climbing, and more than one fall last year). Application of current thresholds showed decreased sarcopenia prevalence with increased BMI (normal weight 4.3%, overweight 1.1%, obesity 0.1%). Contrary, the prevalence of low functional performance increased with increasing BMI. For example, 1.7% of the normal weight participants reported slow walking pace, compared to 9.7% among participants with obesity. The same pattern was observed for stair climbing and falls. Including only participants older than 65 years showed very similar results.
Figure 1.
Fraction of participants with low appendicular lean mass and/or low hand grip strength for sarcopenia detection (EWGSOP2), and fraction of participants reporting low functional performance across BMI classes. Hand grip strength thresholds: 16/27 kg (females/males), appendicular lean mass/height2 (ALM/height2, assessed by DXA) thresholds: 6.0/7.0 kg/m2 (females/males).
The diagnostic performance for detecting low hand grip strength, slow walking pace, no stair climbing, and more than one fall last year was similar comparing DXA-based ALM and suggested MRI-based muscle quantity measurement FFMV (Supplementary Table S2). The correlation between FFMVVCG and MFI was weak (coefficient of determination [R2] 0.13/0.17 females/males; Supplementary Figure S1).
Individualized Muscle Volume Assessment Using MRI
Supplementary Figure S2 shows the implications of a threshold application to FFMV/height2 compared to FFMVVCG. Threshold application to FFMV/height2 resulted in different percentages of participants stratified depending on BMI class. For example, a FFMV/height2 threshold stratifying 5% of the male participants with obesity at the same time stratifies 100% of those with underweight. The percentage of participants stratified by threshold application to FFMVVCG instead was independent of BMI class: For example, a threshold of −1 SD from mean VCG results in stratification of about 15% in all BMI classes.
Figure 2 shows the association between FFMVVCG and BMI in comparison to other body size adjustments (Supplementary Table S3 holds corresponding R2 values). For females, the correlation between DXA-measured ALM and BMI (R2 = .378) was of 2.4 times higher magnitude than the correlation between MRI-measured FFMV and BMI (R2 = .155). Adjusting ALM through ALM/height2 resulted in a correlation to BMI of larger magnitude (R2 = .640) than what was originally observed. ALM/weight introduced a negative correlation to BMI, also with larger magnitude (R2 = .397) than originally observed, and ALM/BMI introduced a negative correlation of similar magnitude (R2 = .315). In contrast, the VCG adjustment of FFMV (FFMVVCG) effectively normalized the association between muscle volume and BMI (R2 = .002/.006 [females/males]).
Figure 2.
Sex-specific associations between fat-tissue free muscle volumeVCG (FFMVVCG) and BMI in comparison to other body size adjustments. BMIbins with less than 25 participants removed. ALM = Appendicular lean mass (assessed by dual-energy x-ray absorptiometry [DXA]); F = Females; FFMV = Fat-tissue free muscle volume; M = Males; SD = Standard deviation; VCG = Virtual control group.
Associations with Aging
Supplementary Figure S3 shows the associations of FFMVVCG and MFI with age in comparison to ALM and FFMV (both adjusted by division with height2, weight, and BMI, respectively). FFMVVCG showed a negative association to age (average 5-year difference −0.19 SD from mean VCG) between ages 47–77 years and MFI showed a positive association to age (average 5-year difference 0.40 percentage points). Average 5-year difference for ALM/height2 was −0.098 kg/m2 and the 5-year effect size was −0.11. In comparison, the highest 5-year effect size was found for FFMVVCG (−0.21) and MFI (0.24). Age associations were significant for all muscle measurements (both DXA-based and MRI-based). Supplementary Material presents complete table (Supplementary Table S4).
Supplementary Figure S4 shows the inferred rate of change (based on cross-sectional data) for FFMVVCG and MFI in females and males across the complete age range. Females showed a decelerated inferred decrease in muscle volume (p < .001), whereas the males showed and accelerated inferred decrease in muscle volume (p < .001). These results were similar for FFMV as compared to FFMVVCG. For MFI, both females and males showed an accelerated inferred increase of MFI (p < .05 and p < .001, respectively).
Associations with Functional Outcomes and Health Care Burden
Table 2 presents the results from the statistical modeling investigating functional outcomes (hand grip strength, stair climbing, walking pace, and falls) and health care burden. Results were overall similar for the models including FFMV (without VCG adjustment) and MFI as compared to when including FFMVVCG and MFI. The trend was that FFMVVCG showed stronger associations to all functional outcomes as compared to FFMV, although confidence intervals were partly overlapping. The associations of FFMVVCG with hand grip strength, stair climbing, and walking pace were significant within the whole cohort and females/males separately. For falls however, the associations with both FFMV and FFMVVCG were nonsignificant within all groups. The associations between MFI and all functional outcomes were significant within all groups with exception of the males, where the associations with stair climbing and falls were nonsignificant.
Table 2.
Multivariable Statistical Modeling of Handgrip Strength: Comparing Participants Above and Below 16/27 kg (females/males), Walking Pace: Comparing Slow Pace to Steady Average Pace and Brisk Pace, Stair Climbing (flights of stairs climbed last 4 weeks comparing none to 1 or more than 1 times a day), Number of Falls: Comparing More Than one Fall Last Year to None or Only one Fall, and Health Care Burden: Number of Hospital Nights
| Odds ratios Functional Outcome | Group | Model 1 | Model 2 | ||
|---|---|---|---|---|---|
| Fat-Tissue Free Muscle Volume | Muscle Fat Infiltration | Fat-Tissue Free Muscle VolumeVCG | Muscle Fat Infiltration | ||
| Hand grip strength† (N = 612) | All | 0.63 (0.58–0.68)*** | 1.10 (1.04–1.16)*** | 0.64 (0.58–0.70)*** | 1.11 (1.06–1.18)*** |
| Females | 0.59 (0.52–0.67)*** | 1.07 (1.00–1.15)*** | 0.64 (0.56–0.73)*** | 1.08 (1.00–1.16)* | |
| Males | 0.65 (0.59–0.71)*** | 1.13 (1.04–1.23)*** | 0.62 (0.53–0.72)*** | 1.15 (1.06–1.25)** | |
| Stair climbing† (N = 758) | All | 0.90 (0.85–0.95)*** | 1.09 (1.03–1.14)*** | 0.83 (0.76–0.91)*** | 1.08 (1.02–1.13)** |
| Females | 0.82 (0.74–0.92)*** | 1.11 (1.04–1.19)*** | 0.77 (0.68–0.87)*** | 1.09 (1.02–1.17)** | |
| Males | 0.92 (0.85–0.98)* | 1.04 (0.97–1.12) ns | 0.87 (0.77–0.99)* | 1.04 (0.97–1.12)ns | |
| Walking pace† (N = 420) | All | 0.75 (0.69–0.82)*** | 1.27 (1.20–1.35)*** | 0.67 (0.60–0.76)*** | 1.26 (1.19–1.34)*** |
| Females | 0.61 (0.53–0.71)*** | 1.22 (1.13–1.32)*** | 0.66 (0.56–0.77)*** | 1.22 (1.13–1.32)*** | |
| Males | 0.84 (0.76–0.93)** | 1.33 (1.21–1.45)*** | 0.68 (0.56–0.82)*** | 1.30 (1.19–1.43)*** | |
| Number of falls† (N = 457) | All | 1.03 (0.95–1.11) ns | 1.13 (1.06–1.19)*** | 1.06 (0.96–1.18)ns | 1.13 (1.07–1.20)*** |
| Females | 1.03 (0.92–1.16) ns | 1.15 (1.07–1.24)*** | 1.05 (0.92–1.20)ns | 1.16 (1.08–1.24)*** | |
| Males | 1.02 (0.92–1.12)ns | 1.07 (0.96–1.18)ns | 1.08 (0.90–1.29)ns | 1.08 (0.96–1.19)ns | |
| Health care burden†† | All | 0.96 (0.91–1.01)ns | 1.10 (1.04–1.16)*** | 0.91 (0.84–0.99)* | 1.09 (1.03–1.15)** |
| Females | 0.88 (0.80–0.96)** | 1.08 (1.02–1.16)* | 0.85 (0.77–0.94) ** | 1.07 (1.00–1.15). | |
| Males | 1.02 (0.95–1.09) ns | 1.11 (1.03–1.20)** | 0.98 (0.87–1.11)ns | 1.11 (1.03–1.19)* | |
| Low ALM/ height2 | 1.09 (0.79–1.49) ns | 1.28 (1.06–1.56)* | 0.79 (0.52–1.21)ns | 1.21 (0.99–1.47) | |
| Low hand grip strength | 0.98 (0.81–1.20) ns | 1.14 (1.00–1.30)* | 0.80 (0.61–1.04). | 1.10 (0.97–1.26)ns |
Note: Values are odds ratios and associated confidence intervals. Models adjusted for (sex), age, visceral adipose tissue volume, and subcutaneous adipose tissue volume. Low ALM/height2 (ALM, appendicular lean mass assessed by dual-energy x-ray absorptiometry (DXA)) defined as below 6.0/7.0 kg/m2 (females/males); Low hand grip strength defined as below 16/27 kg (females/males) [3] †Logistic regression, ††Negative binomial regression. Level of significance:. p < .01, *p < .05, **p < .01, ***p < .001, ns = Nonsignificant; VCG = Virtual control group.
The results for health care burden showed differences between females and males. For females, muscle volume was more strongly associated with health care burden. With this exception, the associations with health care burden were nonsignificant when including FFMV and strengthened by the VCG adjustment. When including FFMV, a higher MFI was positively associated to health care burden within all groups (whole cohort, females, males, and for participants with low ALM/height2, and participants with low hand grip strength). Applying VCG adjustment resulted in similar associations between health care burden and MFI.
Diagnostic Performance
Table 3 presents results from the ROC analysis investigating diagnostic performance for detection of low functional performance. Between FFMV and FFMVVCG, diagnostic performance was comparable for hand grip strength and number of falls but significantly higher for FFMVVCG for stair climbing and walking pace. The diagnostic performance using MFI in comparison to FFMVVCG was generally higher for walking pace and falls and comparable for hand grip strength and stair climbing. Combining FFMVVCG and MFI generally resulted in the highest diagnostic performance for all functional outcomes.
Table 3.
Diagnostic Performance of Muscle Measurements for Detecting Low Functional Performance (hand grip strength: comparing participants above and below 16/27 kg (females/males), Walking Pace: Comparing Slow Pace to Steady Average Pace and Brisk Pace, Stair Climbing (flights of stairs climbed last 4 weeks comparing none to 1 or more than 1 times a day), and Number of Falls: Comparing More than One Fall Last Year to None or Only One Fall)
| ROC Functional Outcome |
Group | Fat-Tissue Free Muscle Volume (FFMV) | FFMVVCG | Muscle Fat Infiltration (MFI) | FFMVVCG + MFI |
|---|---|---|---|---|---|
| Hand grip strength (N = 612) |
All | 0.62 (0.59–0.64) | 0.65 (0.63–0.68) | 0.62 (0.60–0.65) | 0.67 (0.65–0.69) |
| Females | 0.65 (0.62–0.68) | 0.65 (0.62–0.68) | 0.63 (0.60–0.66) | 0.67 (0.64–0.69) | |
| Males | 0.71 (0.68–0.74) | 0.66 (0.63–0.69) | 0.62 (0.59–0.66) | 0.67 (0.64–0.70) | |
| Stair climbing (N = 758) |
All | 0.48 (0.46–0.50) | 0.59 (0.57–0.61) | 0.59 (0.57–0.61) | 0.60 (0.58–0.62) |
| Females | 0.57 (0.54–0.60) | 0.61 (0.58–0.64) | 0.63 (0.60–0.65) | 0.64 (0.61–0.67) | |
| Males | 0.55 (0.52–0.58) | 0.57 (0.54–0.60) | 0.57 (0.54–0.60) | 0.58 (0.55–0.61) | |
| Walking pace (N = 420) |
All | 0.55 (0.53–0.58) | 0.66 (0.64–0.69) | 0.75 (0.72–0.77) | 0.76 (0.74–0.78) |
| Females | 0.55 (0.51–0.59) | 0.65 (0.61–0.69) | 0.76 (0.73–0.78) | 0.76 (0.73–0.79) | |
| Males | 0.56 (0.51–0.61) | 0.68 (0.64–0.72) | 0.74 (0.70–0.78) | 0.76 (0.72–0.80) | |
| Number of falls (N = 457) |
All | 0.55 (0.53–0.58) | 0.53 (0.50–0.55) | 0.61 (0.58–0.64) | 0.61 (0.58–0.64) |
| Females | 0.51 (0.48–0.55) | 0.53 (0.49–0.56) | 0.62 (0.58–0.65) | 0.62 (0.58–0.65) | |
| Males | 0.51 (0.46–0.55) | 0.53 (0.48–0.57) | 0.57 (0.52–0.61) | 0.57 (0.53–0.61) |
Values are area under the receiver operator characteristic (ROC) curves with associated confidence intervals. Predictors were the predicted values extracted from four logistic regressions predicting the functional outcomes using (i) fat–free muscle volume/height2 (FFMV/height2), (ii) FFMVVCG, (iii) muscle fat infiltration (MFI), and (iv) FFMVVCG and MFI. VCG, virtual control group.
In Supplementary Material, sex-specific threshold tables for FFMVVCG and MFI with descriptions of functional performance associated to each threshold (Supplementary Tables S5–S8) can be found. Supplementary Table S9 shows FFMV/height2 values for each BMI and FFMVVCG threshold value presented in Supplementary Tables S5 and S7.
Discussion
This study provided basis for BMI-invariant muscle quantity assessment and investigated the value of combined muscle assessment (FFMV and MFI) for sarcopenia assessment. There were three key findings. First, the application of virtual control groups, resulting in FFMVVCG, effectively normalized the association between muscle volume and BMI (R2 = .002/.006 [females/males]) and strengthened the link with functional outcomes. Second, MFI and FFMVVCG were weakly correlated (R2 = 0.13/017 [females/males], and both were significantly associated with hospitalization, muscle function [hand grip strength], and mobility function [stair climbing, walking pace, and falls]). Lastly, MFI was consistently more strongly associated with outcomes and the addition of MFI to FFMVVCG strengthened the link to functional outcomes resulting in the highest diagnostic performance for detecting low functional performance.
Today’s sarcopenia definitions are highly BMI-dependent, complicating diagnosis across BMI classes and hindering detection within obesity. This study showed that none of the previously proposed body size adjustments (division by height2, weight, or BMI) normalized the correlation between muscle quantity (ALM or FFMV) and body size. In fact, ALM/height2 introduced a BMI correlation of larger magnitude than before and division by weight or BMI introduced negative BMI correlations, with larger/similar magnitudes than before. In contrast, VCG adjustment (FFMVVCG) showed an effective normalization of body size enabling BMI-invariant assessment for sarcopenia detection, and opening up the possibility to properly address sarcopenia across all BMI classes, also within overweight/obesity. Few, if any, studies on sarcopenia have controlled whether the application of a body size adjustment actually achieved normalization as intended, which might have caused misinterpretation of results. The rationale behind adjusting muscle volume for body size is the fundamental correlation between the two caused by the natural response of the body to increase muscle volume as a reaction to increased body weight. In this study, MFI was not adjusted for body size because higher MFI is, in contrast to higher muscle volume, weakly correlated with body size and is associated with lower functional performance and poor outcome (8,21,22).
The VCG adjustment was preferred over, for example, adjusting using residuals (23) as the VCG adjustment is nonparametric and restricts the data used for prediction to individuals similar to each specific patient, hindering extrapolation of associations and lowering the effect of approximation in intervals where data is sparse (20). The reason for the effectiveness of the VCG adjustment is that it takes the specific distribution of FFMV/height2, for each BMI value, into account. In addition to the effective normalization, the VCG adjustment further strengthened the link between muscle volume and both functional outcomes and hospital nights. This indicates a higher clinical relevance of assessing the deviation from an individual’s expected muscle volume instead of direct muscle volume.
The older population presented with lower muscle volumes (FFMV and FFMVVCG) and higher MFI. At the same time, FFMVVCG and MFI were weakly correlated indicating that the inferred decrease in muscle volume was not due to an inferred increase in MFI and that both FFMV and MFI could hold unique information that may help explain age-related wasting. There were clear differences between females and males when investigating inferred rate of change for muscle volume and MFI. Although conclusions cannot be drawn based on these results (as the data is cross-sectional), they indicate that the age-related wasting in general is more accelerated in males as compared to females. These results need to be replicated in a longitudinal study.
Comparison between DXA-measured ALM and MRI-measured FFMV showed similar diagnostic performance for detecting low functional performance. However, FFMVVCG and MFI were both more strongly associated with outcomes with the best diagnostic performance consistently observed for MFI. Further, FFMVVCG and MFI both were associated with hospitalization and low functional performance, indicating they can be used as complimentary information providing clinically meaningful descriptions of muscle composition and that there is additional value in measuring MFI to describe muscle composition in greater detail. This was further strengthened by the results on diagnostic performance for detecting low functional performance, where the combination of FFMVVCG and MFI resulted in the highest diagnostic performance for all outcomes. The most notable difference between FFMVVCG and MFI was the association with falls, where the association with MFI was positively significant but with FFMVVCG nonsignificant. This association with MFI may reflect a neurological link connected to balance and/or motor function—muscle fat has been shown to increase with the progression of neuromuscular disorders (24).
The only exceptions where FFMV or FFMVVCG proved a better predictor as compared to MFI were for the health care burden associations within the female population and for stair climbing within the male population. For health care burden, the differences between females and males might be due biological differences or related to differences in causes for hospitalization between sexes (although all pregnancy-related ICD-10 codes were removed). For stair climbing, the differences might also occur due to differences in self-reporting between sexes. Differences in self-reporting in general was probably largest for walking pace, as the perception of what a “slow,” “steady/average,” and “brisk” pace is can be very different. Confounders for self-reported stair climbing (flights of stairs climbed) and number of falls last year should instead mainly be restricted to memory and misreporting.
The coupled description of decrease in muscle volume and increase in MFI in the aging population offers a more complete, muscle-specific, description of functional decline. Combined muscle assessment for sarcopenia (MFI and FFMVVCG) can be performed using a 6-minute MRI scan coupled with automated image analysis (13–17), including quantification of visceral fat, subcutaneous fat, and liver fat, allowing complete wasting assessment. Today, this is a solution available also outside the image processing research community. Such assessment results in quantifiable, muscle-specific, imaging biomarkers with direct links to functional outcomes that might allow objective sarcopenia assessment. The standardization and high accuracy and precision (13–17) enable close tracking of longitudinal changes and comparison over, and between, large cohorts. Today, MRI is not readily available to detect sarcopenia at population scale and is not suitable as first sarcopenia assessment due to limitations in cost and access. Sources of cost are, for example, the scanning time and laborious manual segmentation of anatomical regions. However, the rapid development of fast and standardized MRI protocols and automated segmentation techniques might be the start of changing this. In the future, screening could be used to decide what patients might benefit from an MRI examination for detection and tracking of progression.
We make no recommendation of suitable thresholds for FFMVVCG and MFI. Instead, a basis for defining thresholds to identify individuals with a low muscle function due to abnormally low muscle volumes (FFMVVCG) and poor muscle quality (MFI) was provided. Since FFMVVCG are body size-adjusted FFMV values, a lookup table showing what each FFMVVCG threshold value corresponds to in FFMV (per BMI value) was provided. UK Biobank consists mainly of Caucasians and has low prevalence of underweight. Hence, the results might not be applicable to other ethnicities and/or need adjustment in reference values. Another limitation was the self-reported information for the functional outcomes. Although the study benefitted from using hand grip strength acquired through a gold standard method, results should be replicated using other commonly used functional measurements or mobility information gathered through motion sensors.
Conclusion
Adjusting muscle volume for body size using virtual control groups strengthens the link between muscle volume and functional outcomes and enables BMI-invariant sarcopenia assessment, also within overweight and obesity. Further, the addition of MFI for combined muscle assessment provides a more complete, muscle-specific description more strongly associated with muscle- and mobility function enabling objective sarcopenia detection using quantifiable imaging biomarkers.
Conflict of Interest
J.L. and O.D.L. are employees of AMRA Medical AB. O.D.L. is stockholder in AMRA Medical AB.
Supplementary Material
Acknowledgments
J.L.: Conception and design, analysis and interpretation of data, writing first draft, critical revision of the manuscript, approved final submission. S.B.H.: Critical revision of the manuscript, approved final submission. O.D.L.: Conception and design, interpretation of data, drafting of manuscript, critical revision of the manuscript, approved final submission. Mikael Petersson: Proof reading/statistical guidance.
References
- 1. Bone AE, Hepgul N, Kon S, Maddocks M. Sarcopenia and frailty in chronic respiratory disease. Chron Respir Dis. 2017;14:85–99. doi:10.1177/1479972316679664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Buyser SL, Petrovic M, Taes YE, et al. Validation of the FNIH sarcopenia criteria and SOF frailty index as predictors of long-term mortality in ambulatory older men. Age Ageing. 2016;45:602–608. doi:10.1093/ageing/afw071 [DOI] [PubMed] [Google Scholar]
- 3. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. ; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended G roup for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:601. doi:10.1093/ageing/afz046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi:10.1093/gerona/glu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prado CM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr. 2014;38:940–953. doi:10.1177/0148607114550189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buckinx F, Landi F, Cesari M, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle. 2018;9:269–278. doi:10.1002/jcsm.12268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim KM, Jang HC, Lim S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J Intern Med. 2016;31:643–650. doi:10.3904/kjim.2016.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hollingsworth KG, de Sousa PL, Straub V, Carlier PG. Towards harmonization of protocols for MRI outcome measures in skeletal muscle studies: consensus recommendations from two TREAT-NMD NMR workshops, 2 May 2010, Stockholm, Sweden, 1–2 October 2009, Paris, France. Neuromuscular Disorders. Elsevier B.V; 2012;22(S2):S54–S67. doi:10.1016/j.nmd.2012.06.005 [DOI] [PubMed] [Google Scholar]
- 9. Reinders I, Murphy RA, Brouwer IA, et al. ; Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study. Muscle quality and myosteatosis: novel associations with mortality risk: the age, Gene/Environment Susceptibility (AGES)-Reykjavik Study. Am J Epidemiol. 2016;183:53–60. doi:10.1093/aje/kwv153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heymsfield SB, Gonzalez MC, Lu J, Jia G, Zheng J. Skeletal muscle mass and quality: evolution of modern measurement concepts in the context of sarcopenia. Proc Nutr Soc. 2015;74:355–366. doi:10.1017/S0029665115000129 [DOI] [PubMed] [Google Scholar]
- 11. Linge J, Borga M, West J, et al. Body composition profiling in the UK Biobank Imaging Study. Obesity (Silver Spring). 2018;26:1785–1795. doi:10.1002/oby.22210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sudlow C, Gallacher J, Allen N, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi:10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karlsson A, Rosander J, Romu T, et al. Automatic and quantitative assessment of regional muscle volume by multi‐atlas segmentation using whole‐body water-fat MRI. J Magn Reson Imaging. 2015;41:1558–1569. doi:10.1002/jmri.24726 [DOI] [PubMed] [Google Scholar]
- 14. West J, Romu T, Thorell S, et al. Precision of MRI-based body composition measurements of postmenopausal women. PLoS One. 2018;13:e0192495. doi:10.1371/journal.pone.0192495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. West J, Dahlqvist Leinhard O, Romu T, et al. Feasibility of MR-based body composition analysis in large scale population studies. PLoS One. 2016;11:e0163332. doi:10.1371/journal.pone.0163332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borga M, Thomas EL, Romu T, et al. Validation of a fast method for quantification of intra-abdominal and subcutaneous adipose tissue for large-scale human studies. NMR Biomed. 2015;28:1747–1753. doi:10.1002/nbm.3432 [DOI] [PubMed] [Google Scholar]
- 17. Leinhard OD, Johansson A, Rydell J, et al. Quantitative abdominal fat estimation using MRI. Presented at: Proceedings of the 19th International Conference on Pattern Recognition (ICPR); December 8–11, 2008; Tampa, FL. doi:10.1109/ICPR.2008.4761764
- 18. Neeland IJ, Turer AT, Ayers CR, et al. Body fat distribution and incident cardiovascular disease in obese adults. J Am Coll Cardiol. 2015;65:2150–2151. doi:10.1016/j.jacc.2015.01.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee JJ, Pedley A, Hoffmann U, Massaro JM, Fox CS. Association of changes in abdominal fat quantity and quality with incident cardiovascular disease risk factors. J Am Coll Cardiol. 2016;68:1509–1521. doi:10.1016/j.jacc.2016.06.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Linge J, Whitcher B, Borga M, Dahlqvist Leinhard O. Sub-phenotyping metabolic disorders using body composition: an individualized, nonparametric approach utilizing large data sets. Obesity (Silver Spring). 2019;27:1190–1199. doi:10.1002/oby.22510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol (1985). 2001;90:2157–2165. doi:10.1152/jappl.2001.90.6.2157 [DOI] [PubMed] [Google Scholar]
- 22. Reinders I, Murphy RA, Koster A, et al. Muscle quality and muscle fat infiltration in relation to incident mobility disability and gait speed decline: the Age, Gene/Environment Susceptibility-Reykjavik Study. J Gerontol A Biol Sci Med Sci. 2015;70:1030–1036. doi:10.1093/gerona/glv016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Newman AB, Kupelian V, Visser M, et al. ; Health ABC Study Investigators. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. doi:10.1046/j.1532-5415.2003.51534.x [DOI] [PubMed] [Google Scholar]
- 24. Barnard AM, Willcocks RJ, Finanger EL, et al. Skeletal muscle magnetic resonance biomarkers correlate with function and sentinel events in Duchenne muscular dystrophy. PLoS One. 2018;13:e0194283. doi:10.1371/journal.pone.0194283 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.