Abstract
Background
Resuscitation with crystalloid fluid is a cornerstone of pediatric septic shock treatment. However, the optimal type of crystalloid fluid is unknown. We aimed to determine the feasibility of conducting a pragmatic randomized trial to compare balanced (lactated Ringer’s [LR]) with 0.9% normal saline (NS) fluid resuscitation in children with suspected septic shock.
Methods
Open-label pragmatic randomized controlled trial at a single academic children’s hospital from January to August 2018. Eligible patients were >6 months to <18 years old who were treated in the emergency department for suspected septic shock, operationalized as blood culture, parenteral antibiotics, and fluid resuscitation for abnormal perfusion. Screening, enrollment, and randomization were carried out by the clinical team as part of routine care. Patients were randomized to receive either LR or NS for up to 48 hours following randomization. Other than fluid type, all treatment decisions were at the clinical team’s discretion. Feasibility outcomes included proportion of eligible patients enrolled, acceptability of enrollment via the U.S. federal exception from informed consent (EFIC) regulations, and adherence to randomized study fluid administration.
Results
Of 59 eligible patients, 50 (85%) were enrolled and randomized. Twenty-four were randomized to LR and 26 to NS. Only one (2%) of 44 patients enrolled using EFIC withdrew before study completion. Total median (interquartile range [IQR]) crystalloid fluid volume received during the intervention window was 107 (60 to 155) mL/kg and 98 (63 to 128) mL/kg in the LR and NS arms, respectively (p = 0.50). Patients randomized to LR received a median (IQR) of only 20% (13 to 32) of all study fluid as NS compared to 99% (64% to 100%) of study fluid as NS in the NS arm (absolute difference = 79%, 95% CI = 48% to 85%).`
Conclusions
A pragmatic study design proved feasible to study comparative effectiveness of LR versus NS fluid resuscitation for pediatric septic shock.
Crystalloid fluid resuscitation is a key component of treatment for septic shock, but uncertainty remains about the optimal type of fluid. Crystalloid fluids are categorized as unbalanced/unbuffered (i.e., 0.9% “normal” saline [NS]) or balanced/buffered (i.e., lactated Ringer’s [LR], Hartmann’s solution, or PlasmaLyte).1,2 Currently, NS is the most common choice for initial fluid resuscitation worldwide.3,4 However, NS contains a supraphysiologic concentration of chloride and a low strong ion difference that can lead to hyperchloremia, metabolic acidosis, and decreased renal blood flow.5,6 In both adults and children, hyperchloremia, often associated with NS administration, has been associated with increased acute kidney injury, multiple organ dysfunction syndrome, vascular permeability, coagulopathy, fluid overload, and death compared to balanced fluids in surgery, trauma, and sepsis.7,8 In contrast, other studies have suggested either no benefit9 or even harm from balanced fluid resusciation.10
Two recent large pragmatic trials provide the most compelling clinical evidence of benefit for balanced fluid resuscitation in both critically ill and non–critically ill adults, with a small, but meaningful, reduction in major adverse kidney events and hospital mortality.11,12 The largest benefit was observed in adults with sepsis. However, no trial has compared the effectiveness of different crystalloid fluid types for resuscitation in children with sepsis, and the two largest observational pediatric studies reported conflicting results.13,14 Consequently, there is insufficient evidence to strongly recommend a specific crystalloid fluid type for resuscitation of children with septic shock.15,16
A large, prospective, randomized trial is needed to determine the comparative effectiveness of different crystalloid fluid types for the initial resuscitation and ongoing hydration of children with septic shock. Such a trial would require enrollment near onset of resuscitation, consistent adherence to a single fluid type, and a sample size large enough to detect small, but clinically meaningful, differences in risks and benefits. A pragmatic study design could enable efficient and affordable enrollment of a large number of children across multiple sites, as such designs are often used to compare two efficacious therapies in a heterogeneous population as part of routine clinical care.17 The established efficacy, strong safety profile, near-universal availability, familiarity of use, and comparable cost of LR and NS lend well to a pragmatic pediatric trial. Given the complexities of implementing such a trial, we first conducted a singlecenter feasibility study to 1) test whether emergency department (ED) clinicians could screen, randomize, and administer study fluids to children with suspected septic shock during the course of routine clinical care; 2) ensure acceptability to patients and providers of enrollment through the U.S. federal exception from informed consent (EFIC) regulations; and 3) determine whether clinicians would adhere to a randomized fluid type with minimal study team oversight.
METHODS
Study Design
We conducted a pragmatic, two-arm, open-label, randomized controlled trial of LR versus NS for fluid resuscitation in children presenting to the ED with suspected septic shock at a single academic children’s hospital from January 25, 2018, to August 31, 2018. The trial was approved by the institutional review board (IRB) at the Children’s Hospital of Philadelphia with provisions for EFIC under the U.S. Food and Drug Administration (FDA) IND #13698 and was registered at ClinicalTrials.gov/NCT03340805. The trial was monitored by an independent data and safety monitoring board. We have included both the CONSORT extension for reporting pragmatic trials (adapted from Zwarenstein et al.18) and the PRECIS2 tool17 for assessing the pragmatic score of the trial in Data Supplement S1, Appendixes S1 and S2 (available as supporting information in the online version of this paper, which is available at http://onlinelibrary.wiley.com/doi/10.1111/acem.13815/full).
Study Population
All patients >6 months and <18 years of age with suspected septic shock treated in the ED were eligible for enrollment. Suspected septic shock was defined by clinician decision to treat suspected bacterial infection with parenteral antibiotics, collection of a blood culture, and intent to administer at least two fluid boluses for abnormal perfusion (defined as the treating clinician’s determination that either hypotension or flash/delayed capillary refill was present).15 Enrollment was open at all times during the 6-month study period. We leveraged activation of our institution’s sepsis clinical pathway19 to identify eligible patients, although patients with suspected septic shock not identified by this pathway were also eligible. Reliance on clinician judgment of suitability for enrollment best approximates “usual care” and is commonly employed in pragmatic trials.20 Patients were excluded if they had received either >40 mL/kg or an indeterminate amount of resuscitation fluid, the clinician judged it unsafe to administer either LR or NS (including for suspected brain herniation, known serum potassium > 6 mEq/L or total serum calcium > 12 mg/dL, known fulminant hepatic failure, end-stage kidney disease on dialysis, mitochondrial disease, inborn errors of metabolism, or allergy to LR or NS), known to be pregnant or incarcerated, or had opted out of the study (see below for details). Given pragmatic eligibility criteria designed to mimic “real-world” practice, prior enrollment during a previous sepsis episode was not an exclusion criteria.
Consent
Patients were enrolled with prospective informed consent if a legally authorized representative (LAR) was present at the bedside and the patient’s condition allowed sufficient time to conduct an informed consent discussion. When a LAR was not present or if fluid administration was deemed emergent, patients were enrolled under EFIC after having the opportunity to “opt-out” during a brief, focused bedside discussion about the study. Patients enrolled under EFIC were approached as soon as feasible by the study team to inform them of study participation, educate about study details, and offer the opportunity to withdraw from study procedures.
In accordance with requirements for EFIC under 21 CFR 50.24 within the United States, we conducted several community consultation and public disclosure activities prior to beginning study enrollment. Community consultation included surveys and focus groups of patients and families at risk for sepsis who were being treated in the ED or pediatric intensive care unit (PICU) and face-to-face meetings with physicians and staff about the study. Public disclosure of the study intent included dissemination of a study website (https://promptbolus.research.chop.edu), posters and brochures made visible and available within inpatient care and family waiting areas, and direct notification of all attending physicians at the Children’s Hospital of Philadelphia. Individuals who opposed future enrollment into the study under EFIC (should they become eligible) were encouraged to contact the study team through the study website. These individuals were asked to wear a bracelet provided by the study team indicating their desire not to be enrolled. We also maintained an “opt-out” list with the study screening forms. Data and feedback from community consultation and public disclosure activities were summarized and reported to the FDA and IRB and submitted to the FDA public docket prior to commencing enrollment. Following completion of patient enrollment and data analysis, results were publicly disclosed via this publication, via our study website, and at ClinicalTrials.gov.
Intervention
Because NS was the overwhelmingly predominant crystalloid fluid used for resuscitation in the ED prior to this study, NS was considered the control group. Although LR was used in several settings within the hospital (e.g., PICU, operating room), LR fluid resuscitation was rarely used in the ED and thus was considered the intervention group. Because use of other balanced fluids, such as PlasmaLyte, were not available for clinical use, only NS and LR were included in this study. The study was conducted using fluids contained in an existing cart that was utilized in the ED to expedite sepsis care. Prior to the study, the cart contained only 0.9% saline as resuscitative fluid. LR was added to the cart as a second option during the study period.
The randomization sequence was generated prior to the start of the study using permuted blocks and equal allocation into two groups. Patients were enrolled after eligibility was confirmed by a treating attending ED physician or pediatric emergency medicine fellow physician who had received specific training and IRB approval to confirm eligibility. We had a “wash-in” period of research coordinator support for enrolling clinicians, with independence expected after 4 weeks. For the first 2 weeks of enrollment, research coordinators were available in person to assist with enrollment. For the second 2 weeks, they were available via text message. Following this wash-in period, clinicians enrolled independently. In the rare instance that the treating physician had not received training about study eligibility and procedures, a second trained clinician/investigator was required to confirm eligibility and conduct randomization. Treatment allocation was then revealed by a member of the clinical team opening the next serially numbered manila opaque envelope located in the top drawer of a sepsis resuscitation cart at the patient’s bedside. The number on each envelope (and thus the sequence of randomization) was recorded in a log at time of enrollment and tracked on a daily basis by the study team to ensure that all subjects were randomized in appropriate numerical sequence. Fluid administration was by open label, with neither the patient nor the care team blinded to the treatment assignment. Blinding of study fluid was deemed not to be pragmatic based on a prior study demonstrating that electrolyte profiles unintentionally revealed crystalloid fluid allocation in two-thirds of patients.9
After allocation was revealed, the clinical team was instructed to administer all subsequent bolus and maintenance fluids as the assigned crystalloid from the time of randomization until 11:59 PM on the following calendar day. An order set within the computerized ordering system was made available to facilitate correct fluid administration. Only the fluid type was determined by the study protocol. All decisions about timing, volume, and rate of fluid administration were at the discretion of the clinical team. An alternative fluid could be substituted if clinically indicated in the judgment of the treating attending physician. All other therapies were recommended in accordance with the institution’s sepsis clinical pathway, but remained at the discretion of the clinical team. For maintenance fluids, the base NS or LR fluid could be supplemented with dextrose or additional electrolytes as determined clinically by the treating attending physician. Research staff were available for questions and support but did not directly oversee screening, enrollment, randomization, or study fluid administration.
Data Collection
Clinical data were recorded onto a secure, standardized electronic case report form developed with input from all study investigators and the Trial Innovation Center at the University of Utah. A data dictionary defining each variable was developed prior to medical record abstraction. In keeping with the pragmatic design, a parsimonious list of data elements included patient demographics, comorbid conditions, amount and type of study and nonstudy fluid administration, source of infection, and sepsis-related therapies, including time to antibiotics, vasoactive agents, mechanical ventilation, renal replacement therapies, and extracorporeal membrane oxygenation. In addition, specified laboratory values measured as part of routine clinical care were collected (e.g., electrolytes, creatinine). Potential adverse events were collected at two time points—once during the period of study fluid administration and again 5 to 7 days after randomization. Although this pilot and feasibility study was not powered for effectiveness endpoints, we monitored all-cause hospital mortality capped at 90 days, hospital length of stay, and hospital-free days out of 28. In addition, because two large adult trials published after starting enrollment for this trial demonstrated differences in major adverse kidney events at 30 days with balanced fluid treatment,11,12 we added this endpoint post hoc (see Table 1). Safety endpoints included brain herniation, electrolyte abnormalities, and treatment for thromboembolism.
Table 1.
Study Outcomes
| Outcome | Definition |
|---|---|
| Feasibility outcomes | |
| Proportion of eligible patients enrolled | >65% of eligible patients enrolled |
| Acceptability of EFIC | ≤10% of patients withdrawing from study after enrollment under EFIC or |
| >90% of EFIC-eligible patients enrolled with completion of study activities | |
| Adherence to group assignment | Absolute difference in proportion of total isotonic fluids administered as NS between groups of ≥65% or |
| ≥85% of subjects in NS arm received ≥90% of study fluid as NS and ≥80% of subjects in LR arm received ≥75% of study fluid as LRa | |
| Effectiveness outcomes | |
| MAKE30 | Major adverse kidney events at 30 days (defined as at least one of the following): |
| Death | |
| New renal replacement therapy | |
| Persistent kidney dysfunction at hospital discharge or 30 days (serum creatinine ≥2× baseline or median value for age if no baseline available) | |
| All-cause hospital mortality | Censored at 90 days |
| Hospital length of stay | Days from hospital admission until discharge |
| Hospital-free days out of 28 days | Days between enrollment and day 28 in which patient was alive and out of the hospital |
| New inpatient RRT | New continuous renal replacement therapy, hemodialysis, or peritoneal dialysis |
| Safety outcomes | All within 4 calendar days of randomization except thrombosis which was within 7 days |
| Hyperlactatemia | >4 mMol/L |
| Hyperkalemia | >6 mEq/L |
| Hypercalcemia | Ionized calcium > 1.35 mmol/L or total serum calcium > 12 mg/dL |
| Hypernatremia | >155 mEq/L |
| Hyponatremia | <128 mEq/L |
| Hyperchloremia | >110 mEq/L |
| Thrombosis | Therapy for new arterial or venous thrombus with systemic anticoagulant or |
| Clotting of intravenous catheter in subjects receiving ceftriaxone and LR | |
| Cerebral edema | Therapy with hyperosmolar therapy (hypertonic saline and/or mannitol) for radiographic and clinical determination of new impending or present brain herniation |
EFIC = exception from informed consent; LR = lactated Ringer’s; MAKE30 = major adverse kidney events within 30 days; NS = 0.9% normal saline; RRT = renal replacement therapy
Patients were eligible for enrollment after an initial 20 mL/kg fluid bolus, which was most commonly expected to be 0.9% saline. Thus, it was anticipated that patients randomized to lactated Ringer’s would receive at least one 0.9% saline bolus.
Outcomes
We assessed three outcomes related to the feasibility of conducting this pragmatic trial that would inform a subsequent multicenter clinical trial: 1) the proportion of eligible patients randomized, 2) acceptability to patients/families and clinicians of using EFIC for enrollment, and 3) adherence to study group assignment with demonstrable separation between the groups in the type of fluid used. We a priori established criteria to determine feasibility for each outcome measure (Table 1). In addition, we tracked processes for data collection using medical record review and collected data on potential effectiveness and safety endpoints to be used in a future multicenter trial (Table 1).
Statistical Analysis
We report subject characteristics, clinical features, and outcomes using counts and relative frequencies for categorical variables and median with interquartile range (IQR) for numeric variables. We compared outcomes between treatment groups using chi-square tests or Wilcoxon rank-sum tests, with confidence intervals (CIs) for proportions calculated by the Wilson score method. CIs for differences in medians were obtained using the nonparametric bootstrap. The study was powered to detect an absolute difference in the mean use of NS between arms of at least 65% with a lower 95% CI border of >60%. Analyses were conducted using R Language and Environment, version 3.3.2.21
RESULTS
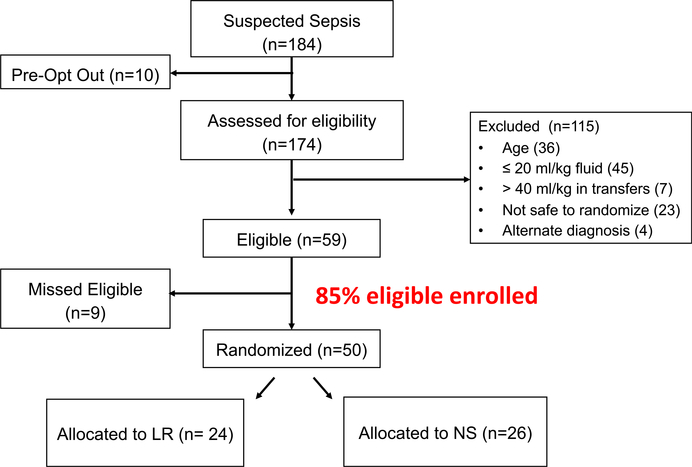
Of 184 patients who presented with suspected septic shock during the study period, 174 (95%) were screened for eligibility. Ten patients had opted out of consideration for study participation and were therefore not considered to be eligible. Of the 59 eligible patients, 50 (85%) unique patients were randomized (Figure 1), with 24 subjects randomized to LR and 26 to NS. Patient characteristics by study group are presented in Table 2. Nineteen patients (38%) had identified bacterial or fungal pathogens, and only two (4%) had final diagnoses other than septic shock. Site of infection is listed in Table 2 and pathogens isolated are listed in Data Supplement S1, Table S1. All patients were admitted to the hospital.
Figure 1.
Diagram of subject enrollment.
Table 2.
Patient Characteristics
| Variable | LR Group (n = 24) | NS Group (n = 26) |
|---|---|---|
| Age (years) | ||
| Median (IQR) | 3.8 (2.0–10.4) | 9.7 (2.6–16.3) |
| 6 months to <1 year | 1 (4) | 2 (8) |
| 1 to <6 years | 14 (58) | 8 (31) |
| 6 to <13 years | 5 (21) | 7 (27) |
| 13 to 17 years | 4 (17) | 9 (35) |
| Sex | ||
| Female | 11 (46) | 12 (46) |
| Race/ethnicity | ||
| Non-Hispanic white | 11 (46) | 11 (42) |
| Non-Hispanic black | 3 (13) | 4 (15) |
| Hispanic | 10 (42) | 9 (35) |
| Asian | 0 (0) | 1 (4) |
| Unknown/not reported | 0 (0) | 1 (4) |
| Comorbid conditions | ||
| Cancer | 4 (17) | 5 (19) |
| Kidney disease (no dialysis) | 2 (8) | 1 (4) |
| Neurologic dysfunction | 7 (29) | 5 (19) |
| Chronic ventilator dependence | 6 (25) | 5 (19) |
| Bone marrow or solid organ transplant | 3 (13) | 2 (8) |
| Sickle cell disease | 0 (0) | 2 (8) |
| Indwelling central venous catheter | 6 (25) | 4 (15) |
| Any comorbid condition | 16 (67) | 16 (62) |
| Site of infection | ||
| Bacteremia | 2 (8) | 4 (15) |
| Pneumonia/lung infection | 5 (21) | 5 (19) |
| Abdominal infection | 1 (4) | 0 (0) |
| Urinary tract infection | 4 (17) | 3 (12) |
| CNS infection | 2 (8) | 3 (12) |
| Skin/soft tissue infection | 2 (8) | 3 (12) |
| Other | 6 (25) | 6 (23) |
| Unknown | 3 (13) | 4 (15) |
| Alternative diagnosis other than infection | 1 (4) | 1 (4) |
| Enrollment method | ||
| EFIC | 22 (92) | 22 (85) |
CNS = central nervous system; EFIC = exception from informed consent; IQR = interquartile range.
Categorical data are presented as n (%); continuous data are presented as median (IQR)
Forty-four (88%) patients were enrolled under EFIC. The LAR of each patient enrolled under EFIC was notified of enrollment by a study team member as soon as feasible and offered the opportunity to withdraw from further study procedures. Contact with the LAR was successful for all but two patients, one of whom had died shortly after PICU admission (unrelated to study fluid administration). One patient (2% of EFIC subjects) withdrew from further study fluid administration but allowed data to be included in the analysis. We are aware of at least two additional patients who were not enrolled because the ED physician was concerned about enrollment via EFIC despite otherwise meeting eligibility criteria. Thus, of the 46 total patients who we can confirm met the specified conditions for EFIC, this method of enrollment was acceptable to 43 (93%).
Total median (IQR) crystalloid fluid volume administered during the intervention window was 107 (60 to 155) mL/kg and 98 (63 to 128) mL/kg in the LR and NS arms, respectively (p = 0.50, Table 3). Approximately one-half of the total crystalloid fluid administered during the intervention window was as bolus fluids and one-half as maintenance fluids in both study groups. Thirty-eight percent of subjects in the LR group and 42% in the NS group received ≥ 60 mL/kg of bolus crystalloid fluid (p = 0.73).
Table 3.
Fluid Administration During Intervention Window
| Variable | LR Group | NS Group | Difference (95% CI) |
|---|---|---|---|
| Total crystalloid volumes administered | |||
| Crystalloid fluid volume (mL/kg) | |||
| Total fluid | 107 (60 to 155) | 98 (63 to 128) | −9 (−57 to 35) |
| Bolus fluid | |||
| Prerandomization | 20 (19 to 23) | 20 (17 to 27) | 0 (−1 to 4) |
| Postrandomization | 38 (20 to 60) | 33 (20 to 40) | −5 (−28 to 18) |
| Maintenance fluid | |||
| Total | 49 (18 to 88) | 35 (24 to 78) | −14 (−50 to 18) |
| NS | 0 (0 to 0) | 21 (2 to 41) | 21 (4 to 39) |
| LR | 40 (9 to 78) | 0 (0 to 0) | −40 (−68 to −11) |
| Other | 0 (0 to 0) | 0 (0 to 21) | 0 (0 to 17) |
| Proportion of patients receiving ≥60 mL/kg as bolus fluid | 9 (38) | 11 (42) | |
| Adherence to study arm | |||
| Fluid compliance among isotonic fluidsa,b | |||
| Pre- and postrandomization fluidc | 15 (63) | 23 (88) | |
| Postrandomization fluid | 20 (83) | 24 (92) | |
| Proportion of isotonic fluid as NS | |||
| Pre- and postrandomizationc | 20 (13 to 35) | 100 (100 to 100) | 80 (69 to 85) |
| Postrandomization | 0 (0 to 0) | 100 (100 to 100) | 100 (100 to 100) |
| Proportion of isotonic fluid as LR | |||
| Pre- and postrandomization | 80 (65 to 87) | 0 (0 to 0) | −80 (−85 to −69) |
| Postrandomization | 100 (100 to 100) | 0 (0 to 0) | −100 (−100 to −100) |
Data are reported as n (%) or median (IQR).
IQR = interquartile range; LR = lactated Ringer’s; NS = 0.9% normal saline.
Fluid compliance was defined as receipt of ≥75% of study fluid as LR in LR group and ≥90% of study fluid as NS in NS group.
Isotonic fluids included LR, NS, D5 LR, or D5 NS with additional electrolytes permitted as per clinical team.
Elements that were determined a priori as feasibility metrics.
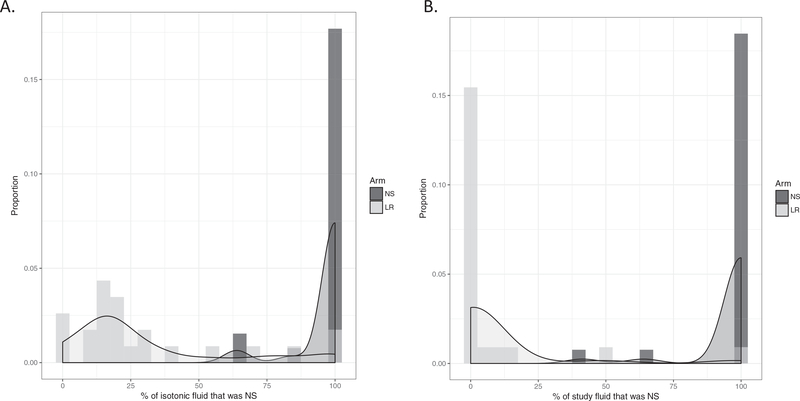
Overall, there was strong adherence to study arm assignment (Table 3) with appropriate separation of fluid type administered between study groups (Figure 2). Patients randomized to LR received a median (IQR) of only 20% (13% to 35%) of all (including preand postrandomization) isotonic fluids as NS compared to 100% (100% to 100%) of fluid as NS in the NS arm (difference = 80%, 95% CI = 65% to 89%), which met our prespecified feasibility goal of an absolute difference in the proportion of total isotonic fluids administered as NS between groups of ≥65%. When only postrandomization fluids were considered, patients randomized to the LR arm received a median (IQR) of 0% (0% to 0%) of isotonic fluids as NS and 100% (100% to 100%) as LR, while patients in the NS arm received a median (IQR) of 100% (100% to 100%) of isotonic fluids as NS and 0% (0% to 0%) as LR. Sixty-three percent of patients in the LR arm received ≥75% of their isotonic fluid as LR, which fell slightly short of our second a priori established feasibility goal. Eighty-eight percent of patients in the NS arm received ≥90% of their isotonic fluid as NS, which met our feasibility goal. Of note, there were two patients in the LR arm who were randomized based on intent to give ≥40 mL/kg resuscitative fluids who ultimately did not receive any study fluids. These patients are included in the analysis based on intention-to-treat principles. Additional details regarding fluid administration can be found in Data Supplement S1, Table S2.
Figure 2.
Histograms depicting distribution of fluid type including (A) both pre- and postrandomization and (B) postrandomization only.
Median (IQR) time per patient for data extraction from the medical record was 105 (90 to 165) minutes. Data were available for all specified variables from each enrolled patient. Potential effectiveness and safety endpoints to be used for a multicenter trial are shown in Table 4. All endpoints were able to be ascertained from routine clinical data collection. Rates laboratory testing sent are shown in Data Supplement S1, Table S3. Adverse events by group are also listed in Table 4. There were no unexpected serious adverse events related to study fluid administration.
Table 4.
Effectiveness and Safety Outcomes
| Outcomes | LR Group | NS Group |
|---|---|---|
| Effectiveness outcomes | ||
| MAKE30 | 1 (4) | 1 (4) |
| All-cause hospital mortality, censored at 90 days | 0 | 1 (4) |
| Hospital length of stay (days) | 5 (3–8) | 3 (2–9) |
| Hospital-free days out of 28 (days) | 23 (20–25) | 25 (20–26) |
| New inpatient RRT | 0 | 0 |
| Safety outcomes | ||
| Hyperlactatemia | 2 (8) | 4 (15) |
| Hyperkalemia | 1 (4) | 2 (8) |
| Hypercalcemia | 0 | 4 (15) |
| Hypernatremia | 0 | 1 (4) |
| Hyponatremia | 0 | 0 |
| Hyperchloremia | 3 (13) | 7 (27) |
| Thrombosis | 0 | 0 |
| Cerebral edema | 1 (4) | 0 |
| Additional adverse events | ||
| Vasoactive infusion | 3 (13) | 3 (12) |
| Mechanical ventilation | 7 (29) | 6 (23) |
| Seizure | 1 4) | 2 (8) |
| Acute kidney injury | 0 | 1 (4) |
| Hepatic dysfunction | 0 | 1 (4) |
| Rash | 4 (17) | 4 (15) |
| Pressure injury | 1 (4) | 0 |
Categorical variables are listed as n (%); continuous outcomes are listed as median (IQR). Shock was defined as need for vasoactive medication; respiratory failure was defined as need for invasive or noninvasive mechanical ventilation.
LR = lactated Ringer’s; MAKE30 = major adverse kidney events within 30 days; NS = 0.9% normal saline; RRT == renal replacement therapy.
DISCUSSION
We have demonstrated feasibility to use a pragmatic study design to randomize children with suspected septic shock to LR versus NS fluid resuscitation. Specifically, we enrolled 85% of eligible subjects and enrollment under EFIC had 93% acceptability overall among patients and providers, with only one postenrollment withdrawal. We also demonstrated adherence to study group assignment with acceptable separation between the two arms. Finally, we established that collection of a parsimonious set of variables about patient characteristics, fluid administration and other sepsis therapies, and clinical outcomes was efficient to abstract from the medical record with minimal missing data. The results of our pilot study support use of this pragmatic study design in a larger multicenter trial to test the comparative effectiveness of balanced/buffered fluids versus NS resuscitation for pediatric septic shock.
Pragmatic trials are intended to help clinicians choose among efficacious therapies, while explanatory trials test causal research hypotheses.22 In reality, however, the degree of trial “pragmatism” exists along a spectrum.23 Our ultimate aim is to determine the comparative effectiveness of different types of crystalloid fluids for resuscitation of pediatric septic shock with maximal generalizability of trial results across usual care practices. We therefore tested whether our study design was sufficiently pragmatic for busy emergency physicians to expeditiously enroll a large number of children with a heterogeneous, life-threatening condition concurrent with their clinical care. Although conducted at a single center, we included more than 50 pediatric emergency medicine attending and fellow physicians and screened 184 patients. We utilized an existing systematic sepsis recognition program19 to assist screening, but accurate enrollment ultimately relied on clinician judgment of key e9lements that defined septic shock, i.e., suspicion of bacterial infection and abnormal perfusion. With this approach, only 15% of eligible patient were missed and only 4% of enrolled patients did not have septic shock. Notably, we purposely did not utilize the comprehensive research support services available within our ED to simulate future enrollment at other sites where research infrastructure may differ. In addition, the case mix of enrolled children in terms of presence of comorbid conditions was similar to published estimates in U.S. children’s hospitals.24,25 Moreover, we anticipate that the proliferation of sepsis screening and recognition programs will help to facilitate enrollment in a future multicenter trial in a similar manner as in our pilot study.
Although there is debate about the appropriate volume and rate of fluid administration in children with septic shock, crystalloid fluid resuscitation is recommended as a critical, early intervention to address hypovolemia.15,16 Most fluid resuscitation of children with septic shock occurs within the initial hours after sepsis recognition. Delaying enrollment until after admission to an intensive care unit or even after several hours of ED resuscitation may ensure septic shock as the correct diagnosis and concentrate enrollment of patients most likely to have the study outcome, but risks substantial volume of prestudy fluid resuscitation that may dilute the main effect of the fluid intervention. Such contamination with prestudy fluid resuscitation has been noted in previous trials in adults, in which receipt of fluid volumes of ≥2000 mL prior to study enrollment was common.26–28
To enroll patients as early as possible after eligibility, we conducted the study under the U.S. exception from informed consent (EFIC) regulations for emergency research in the United States. EFIC studies are justified under the following strict conditions: 1) patients are in a life-threatening situation, available treatments are unproven or unsatisfactory, and the collection of valid scientific evidence is necessary to determine the safety and effectiveness of a therapy; 2) obtaining prospective informed consent is not feasible; 3) patients may directly benefit from the research; and 4) research could not be practicably be carried out without using EFIC. Additional safeguards include pretrial community consultation among potential subjects and their families and pre- and posttrial public disclosure to promote awareness and transparency. These criteria set an appropriately high bar and, thus, EFIC studies have been uncommon to date in children. However, several EFIC-based studies have been successfully carried out in the United States29,30 and, using a related ethical design, in Europe, Canada, and Australia.31–35 For this study of two efficacious resuscitation fluids, enrollment with EFIC was approved by our IRB, endorsed by the FDA through our IND application, and proved acceptable to patients/families and clinicians.
Pragmatic trials are conducted in typical care settings without intense efforts to standardize interventions beyond the intervention being studied and with the expectation that care will vary between subjects as a matter of chance, clinician preference, and institutional policies.20 Ideally, the intervention and comparator (in this case, LR and NS, respectively) are already widely used, such that clinicians feel comfortable with either treatment arm. In the United States, emergency clinicians caring for children rarely use fluids other than NS, while hospitalists and intensive care clinicians may be more familiar with LR. We therefore provided a brief educational overview to our emergency staff prior to commencing study enrollment, but otherwise relied on established institutional protocols to support safe administration of both fluids. For example, routine protocol is to avoid concurrent administration of LR with ceftriaxone and blood products. The SMART11 and SALT-ED12 trials used a bioinformatics approach to guide fluid administration. Although we considered a similar approach, this was deemed unlikely to be reproducible across multiple centers. Instead, with prestudy education and study team assistance offered remotely as needed, we were able to successfully and safely implement randomized allocation to LR or NS beginning in the ED and continuing on through hospital and/or PICU with adequate separation of study arms. In addition, we made available a single-page screening form, paper envelope randomization, and access to both study fluids within a “sepsis cart” that could be wheeled to the patient’s bedside. We also instructed ED providers to “hand off” study group assignment to inpatient physicians and nurses. We believe that these simple study procedures would be generally reproducible across multiple centers with varying resources. Finally, we confirmed that maintenance fluids comprised a large proportion of fluid administration within the first 24 to 48 hours, supporting the importance of including maintenance fluids in a larger trial, as previously noted.36
LIMITATIONS
There are several limitations to this study. First, despite attempts to minimize site-specific features in screening, enrollment, study interventions, and data collection, it is unlikely that multiple sites would have the exact experience as we found in this pilot study. Second, acceptability of enrollment with EFIC may vary across regions or sites and attention to local culture and values will be important. In addition, it is possible that there were additional patients who were considered not to be safe for administration of study fluids because of unstated concerns about EFIC, thus resulting in an overestimate of acceptability of EFIC. However, withdrawal of only one subject after EFIC is reassuring that families found this mode of enrollment acceptable for this study. Fourth, the pragmatic nature of our inclusion criteria necessitated clinician determination of “suspected shock,” which resulted in enrollment of patients with a spectrum of illness severity. Fifth, the intervention window ranged from 24 to 48 hours to ensure that all subjects were exposed to at least 24 hours of study fluid resuscitation while providing a straightforward and clear stopping point for clinicians. Although such variability could have affected the volume of study fluid between subjects, this variable should be balanced by randomization and previous studies have demonstrated that the majority of fluid was administered within the initial 24 hours.11,37 Sixth, this pilot study was not able to inform sample size or endpoint selection for a multicenter trial. However, we have pursued other efforts for this purpose.38 Finally, our pilot study was conducted for a short time period. For a larger multicenter trial, enrollment will occur over several years and we can expect that adherence may wax and wane over time and vary by site. Efforts to maintain study adherence and ensure enrollment of eligible patients will likely need to be more extensive than in our pilot study.
CONCLUSIONS
In conclusion, we demonstrated feasibility to use a pragmatic study design to study the comparative effectiveness of lactated Ringer’s versus normal saline fluid resuscitation for pediatric septic shock. This pilot trial supports using a similar pragmatic study design for a definitive multicenter comparative effectiveness trial.
Supplementary Material
Acknowledgments
Financial support was provided by 5K12HL109009-04 and U24 TR001597. Dr. Balamuth received support from K23 HD082368. Dr. Weiss was also supported by K23GM110496.
Footnotes
The authors have no relevant financial information or potential conflicts to disclose.
This study was performed at the Children’s Hospital of Philadelphia.
Supervising Editor: Michelle L. Macy, MD, MS.
Supporting Information
The following supporting information is available in the online version of this paper available at http://onlinelibrary.wiley.com/doi/10.1111/acem.13815/full
References
- 1.Bartels K, Thiele RH, Gan TJ. Rational fluid management in today’s ICU practice. Crit Care 2013;17 Suppl 1:S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yunos NR, Bellomo R, Hegarty FC, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 2012;308:1566–72. [DOI] [PubMed] [Google Scholar]
- 3.Finfer S, Liu B, Taylor C, et al. Resuscitation fluid use in critically ill adults: an international cross-sectional study in 391 intensive care units. Crit Care 2010;14:R185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awad S, Allison SP, Lobo DN. The history of 0.9% saline. Clin Nutr 2008;27:179–88. [DOI] [PubMed] [Google Scholar]
- 5.Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med 2013;369:2462–3. [DOI] [PubMed] [Google Scholar]
- 6.Karakala N, Raghunathan K, Shaw AD. Intravenous fluids in sepsis: what to use and what to avoid. Curr Opin Crit Care 2013;19:537–43. [DOI] [PubMed] [Google Scholar]
- 7.Barhight MF, Lusk J, Brinton J, et al. Hyperchloremia is independently associated with mortality in critically ill children who ultimately require continuous renal replacement therapy. Pediatr Nephrol 2018;33:1079–85. [DOI] [PubMed] [Google Scholar]
- 8.Stenson EK, Cvijanovich NZ, Anas N, et al. Hyperchloremia is associated with complicated course and mortality in pediatric patients with septic shock. Pediatr Crit Care Med 2018;19:155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young P, Bailey M, Beasley R, et al. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA 2015;314:1701–10. [DOI] [PubMed] [Google Scholar]
- 10.Nhan NT, Phuong CX, Kneen R, et al. Acute management of dengue shock syndrome: a randomized doubleblind comparison of 4 intravenous fluid regimens in the first hour. Clin Infect Dis 2001;32:204–13. [DOI] [PubMed] [Google Scholar]
- 11.Semler MW, Self WH, Rice TW. Balanced crystalloids versus saline in critically ill adults. N Engl J Med 2018;378:1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med 2018;378:819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss SL, Keele L, Balamuth F, et al. Crystalloid fluid choice and clinical outcomes in pediatric sepsis: a matched retrospective cohort study. J Pediatr 2017;182 304–10.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emrath ET, Fortenberry JD, Travers C, McCracken CE, Hebbar KB. Resuscitation with balanced fluids is associated with improved survival in pediatric severe sepsis. Crit Care Med 2017;45:1177–83. [DOI] [PubMed] [Google Scholar]
- 15.Davis AL, Carcillo JA, Aneja RK, et al. The American College of Critical Care Medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: executive summary. Pediatr Crit Care Med 2017;18:884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NICE. Sepsis: recognition, diagnosis and early management NICE Guideline [NG51]. London: National Institute for Health and Care Excellence, 2016. [PubMed] [Google Scholar]
- 17.Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009;62:464–75. [DOI] [PubMed] [Google Scholar]
- 18.Zwarenstein M, Treweek S, Gagnier JJ, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337:a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balamuth F, Alpern ER, Abbadessa MK, et al. Improving recognition of pediatric severe sepsis in the emergency department: contributions of a vital sign–based electronic alert and bedside clinician identification. Ann Emerg Med 2017;70:759–68.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zwarentein M, Treweek S, Gagnier JJ, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. J Chinese Integr Med 2009;7:392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing, 2018. [Google Scholar]
- 22.Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Clin Epidemiol 2009;62:499–505. [DOI] [PubMed] [Google Scholar]
- 23.Tosh G, Soares-Weiser K, Adams CE. Explanatory and pragmatic trials: a tool to measure differences. Dialogues Clin Neurosci 2011;13:209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balamuth F, Weiss SL, Neuman MI, et al. Pediatric severe sepsis in U.S. children’s hospitals. Pediatr Crit Care Med 2014;15:798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruth A, McCracken CE, Fortenberry JD, Hall M, Simon HK, Hebbar KB. Pediatric severe sepsis. Pediatr Crit Care Med 2014;15:798–805. [DOI] [PubMed] [Google Scholar]
- 26.Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004;350:2247–56. [DOI] [PubMed] [Google Scholar]
- 27.Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015;372:1301–11. [DOI] [PubMed] [Google Scholar]
- 28.ARISE Investigators; ANZICS Clinical Trials Group, Peake SL, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014;371:1496–506. [DOI] [PubMed] [Google Scholar]
- 29.Chamberlain JM, Okada P, Holsti M, et al. Lorazepam vs diazepam for pediatric status epilepticus: a randomized clinical trial. JAMA 2014;311:1652–60. [DOI] [PubMed] [Google Scholar]
- 30.Cock HR; ESETT Group. Established status epilepticus treatment trial (ESETT). Epilepsia 2011;52 Suppl 8:50–2. [DOI] [PubMed] [Google Scholar]
- 31.Parker MJ, Thabane L, Fox-Robichaud A, Liaw P. Choong K; Canadian Critical Care Trials Group and the Canadian Critical Care Translational Biology Group. A trial to determine whether septic shock-reversal is quicker in pediatric patients randomized to an early goal-directed fluidsparing strategy versus usual care (SQUEEZE): study protocol for a pilot randomized controlled trial. Trials 2016;17:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Hearn K, McNally D, Choong K, et al. Steroids in fluid and/or vasoactive infusion dependent pediatric shock: study protocol for a randomized controlled trial. Trials 2016;17:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macdonald SP, Taylor DM, Keijzers G, et al. Restricted Fluid REsuscitation in Sepsis-associated Hypotension (REFRESH): study protocol for a pilot randomised controlled trial. Trials 2017;18:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inwald DP, Canter R, Woolfall K, et al. Restricted fluid bolus volume in early septic shock: results of the Fluids in Shock pilot trial. Arch Dis Child 2019;104:426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilbert RE, Mok Q, Dwan K, et al. Impregnated central venous catheters for prevention of bloodstream infection in children (the CATCH trial): a randomised controlled trial. Lancet 2016;387:1732–42. [DOI] [PubMed] [Google Scholar]
- 36.Bihari S, Gelbart B, Seppelt I, et al. Maintenance fluid practices in paediatric intensive care units in Australia and New Zealand. Crit Care Resusc 2017;19:310–7. [PubMed] [Google Scholar]
- 37.Self W, Semler M, Rice T. Balanced crystalloids vs saline for noncritically ill adults in the emergency department. Chest 2018;378:819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss SL, Balamuth F, Thurm CW, Downes KJ, Fitzgerald JC, Laskin BL. Major adverse kidney events in pediatric sepsis. Clin J Am Soc Nephrol 2019;14:664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.