Abstract
Background:
In 2017, Zimbabwe adopted a modified version of the World Health Organization 2016 recommendation on HIV birth testing by offering HIV testing at birth only to infants at “high risk” of HIV transmission. There is limited evidence on the effectiveness of this approach. Our study assessed the sensitivity and specificity of birth testing “high risk” infants only.
Methods:
We conducted a cross-sectional study at 10 health facilities from November 2018 to July 2019. A nucleic acid test for HIV was performed on all HIV-exposed infants identified within 48 hours of life, irrespective of risk status. Univariate and bivariate analyses were used to estimate the performance of the risk screening tool.
Results:
HIV nucleic acid test was successfully performed on 1970 infants (95%), of whom 266 (13.5%) were classified as high-risk infants. HIV prevalence for all infants tested was 1.5% (95% CI: 1% to 2%), whereas prevalence among high-risk infants and low-risk infants was 6.8% (95% CI: 3.7% to 9.8%) and 0.6% (95% CI: 0.3% to 1%) respectively. Sensitivity and specificity of the maternal risk screening tool was at 62.1% (95% CI: 44.4% to 79.7%) and 87.2% (95% CI: 85.7% to 88.7%), respectively; positive and negative predictive values were 6.8% (95% CI: 3.7% to 9.8%) and 99.4% (95% CI: 99.0% to 99.7%) respectively.
Conclusions:
Despite high negative predictive value, sensitivity was relatively low, with potential of missing 2 in every 5 HIV infected infants. Given the potential benefits of early ART initiation for all exposed infants, where feasible, universal testing for HIV-exposed infants at birth may be preferred to reduce missing infected infants.
Key Words: risk stratification, birth testing, nucleic acid test
INTRODUCTION
Early infant diagnosis (EID) of HIV is key to infant survival. The 2016 World Health Organization (WHO) Consolidated ARV Guidelines recommended countries with a high burden of vertical transmission of HIV and strong six-week EID testing programs consider adding an HIV nucleic acid test (NAT) at birth to existing EID algorithms to identify in-utero HIV infection among HIV-exposed infants (HEI).1 This guideline is applicable to sub-Saharan Africa, the region with the highest HIV MTCT rates.
For infants infected in-utero or intrapartum, mortality begins to increase at about 3 to 4 weeks old, and is as high as 10% by age 2 months, reaching a peak of 30%–40% between ages 8 and 12 weeks.2,3 Birth testing may be an ideal approach to improve infant survival given that most infants identified through the routine 6-week test are being initiated on antiretroviral therapy (ART) after 12 weeks of age because of delays in result return.4–8 Early identification of HIV-infected infants can accelerate ART initiation which could also result in a reduced HIV reservoir and promote normal immune and brain development for infants.9
Since the WHO released guidance on birth testing in 2016, there has been limited progress in rolling out of birth testing in low- and middle-income countries. The exception is South Africa, which changed its national guidelines in June 2015 to include routine NAT at birth testing for all HEI, with repeat testing at 10 weeks for those with a negative test at birth.10,11 This two-test model allows for earlier detection of in utero HIV transmission while continuing to capture intrapartum transmission although it increases the costs of EID. Most other sub-Saharan countries, including Zimbabwe, are only in a planning stage or piloting HIV birth testing of infants and yet to move to full implementation of routine birth testing.12
In 2017, the Government of Zimbabwe adopted a modified version of the WHO recommendation on HIV birth testing to offer HIV testing at birth only to “high-risk” infants. According to these guidelines, an infant is considered to be “high risk” if they meet any of the following criteria: (1) Mother diagnosed with HIV in labor and delivery stages, (2) mother initiated ART after 32 weeks gestation, (3) mother's viral load above 1000 copies/mL, (4) mother seroconverted during pregnancy, or (5) mother not adhering to ART.1 Previous studies in South Africa, Malawi, and Botswana have demonstrated use in the risk screening approach to increase the yield of birth testing in identifying HIV-infected infants, although the sensitivity of the risk screen for HIV infection in the included neonates ranged from 80% to 100%.13,14
Although the Government of Zimbabwe opted to offer birth testing to high-risk infants, there is paucity of evidence on the effectiveness of this approach to birth testing. It is essential to generate data on the effectiveness of this approach in the early phases of implementing the guidelines to provide evidence for refining their implementation. The Elizabeth Glaser Paediatric AIDS Foundation (EGPAF) implemented the current study, which assessed the sensitivity and specificity of testing “high-risk” infants to inform national birth testing strategies.
MATERIAL AND METHODS
Study Design and Setting
We conducted a cross-sectional study to assess the effectiveness of using maternal HIV transmission risk stratification to identify HEI for birth testing in 10 of 51 health facilities that had implemented point-of-care (POC) HIV EID platforms supported by EGPAF. We purposively selected the study sites based on volume of deliveries by HIV-positive women.
Participants
The study participants were HEI and their HIV-positive mothers identified at the study facilities from 1 November 2018 to 31 July 2019. For the purpose of this study, we defined birth testing as testing of infants born to HIV-positive women within 48 hours of birth. All women of HEI who presented to these 10 health facilities during the study period were approached for informed consent, offered NAT for their HEI (regardless of participant risk) and assessed for their HIV transmission risk.
Data Collection and Measurements
We measured maternal HIV transmission risk using a screening tool with the following questions:
Was the mother diagnosed with HIV in labor and delivery?
Did the mother start ART after 32 weeks' gestation?
Was the maternal viral load above 1000 copies/mL in the third trimester?
Did the mother seroconvert during pregnancy?
Was the mother not adhering to ART during pregnancy?
We classified high-risk infants as any infant with a “yes” response to any of the 5 questions. We developed the risk-screening tool based on the Ministry of Health and Child Care (MOHCC) definition of high-risk infants in the birth testing guidelines. Study staff collected the risk-screening data from Antenatal Care (ANC) records, ART registers, delivery registers, and opportunistic infections and ART booklets. We determined whether the mother was diagnosed in labor using the date when the mother was confirmed positive together with date of onset of labor. We used the date when the mother was initiated on ART and the infant's date of birth to determine whether the mother had less than 8 weeks on ART at delivery. We presumed mothers with less than 8 weeks duration on ART to have started ART after the 32-week gestation period. We used the Opportunistic infection and ART register to document viral load test results for mothers who were tested for viral load during the third trimester or 48 hours from delivery. Mothers who had a confirmed negative HIV test at first ANC visit and a positive test during subsequent ANC visits were considered to have seroconverted during pregnancy. Any mother reported to have taken less than 95% of expected doses (based on documentation in ART records of pill count) or reported to have temporarily stopped ART at any visit during ANC period was classified as not adhering to ART while pregnant. Participants with missing information were excluded in the analysis for the missing variable.
Data on HIV testing, including date of test, results, and date of ART initiation, were captured on MoHCC-approved POC EID testing forms, used in all facilities implementing routine POC EID testing. Field staff entered the risk screening and HIV testing data into a cloud database with in-built data validation rules that controlled for missing and inconsistent data, which the investigators accessed and verified in real time.
Laboratory Methods
MoHCC staff trained and certified by Abbott, the manufacturers and distributer of the POC EID devices in use in this study, collected at least 25 mL of capillary blood samples from all the infants screened, using an appropriate lancet from a heel prick. They processed the samples on the m-PIMA HIV-1/2 Detect POC EID devices.
The POC EID device had built-in internal quality controls that could detect, among other things, if the sample was insufficient, machine software needed updating, or cartridges used had expired. If a test resulted in an internal quality control failure, the HEI's mother was approached for repeat sample collection. Post-test counselling was offered to all mothers; those testing HIV-negative were asked to come back for another HIV test at 6 weeks according to national guidelines. A confirmatory test was done for infants who tested HIV-positive, by testing a new sample on the same POC platform. Infants with a positive confirmatory test result were considered HIV-infected and were referred for HIV care and ART services. ART services were available in the same sites where testing took place.
Analysis
Using the Pearson χ2 test, we calculated and compared the proportion of infants who tested HIV-positive by their maternal HIV transmission risk status. We used STATA version 15.0 to perform the calculations.
Ethical Considerations
The study was reviewed and approved by the Research Council of Zimbabwe (RCZ), the Medical Research Council of Zimbabwe (MRCZ/A/2343), and Advarra (formerly Chesapeake) IRB based in United States. We obtained written informed consent from all mothers of HEI for birth testing and collection of data. If the caregiver declined to provide consent, birth testing was still offered according to national guidelines. This study is registered in ClinicalTrials.gov (NCT04206241).
RESULTS
A total of 2080 HEI were enrolled in the study; the maternal HIV transmission risk screening tool was administered to all of them. Of the 2080 infants identified and risk-screened, 52 (2.5%) were not tested due to refusal or nonavailability of services at that time. HIV EID testing using the POC platform was completed for 2028 (97.5%) infants, of whom 58 (2.8%) had invalid results (testing errors and the infants were not available for a repeat test) (Fig. 1). HIV positivity among all the infants with a valid result from a POC EID test was 1.5%. Infants who were not tested and those with invalid results were excluded from the final analysis. Of the 1970 infants who had a valid POC EID result, 1900 (96%) of the mothers had booked for ANC and 1287 (68%) of them booked with a known HIV-positive status, whereas 570 (30%) were diagnosed with HIV infection at ANC booking, and 43 (2%) were HIV-negative at booking and seroconverted to become HIV-positive later in ANC (Table 1). The median number of ANC visits was 4 [interquartile range (IQR) 3–5)]. Ninety-six percent of women delivered at a health facility delivery. The median birth weight for all infants tested was 3,000 g (IQR 2700–3270).
FIGURE 1.
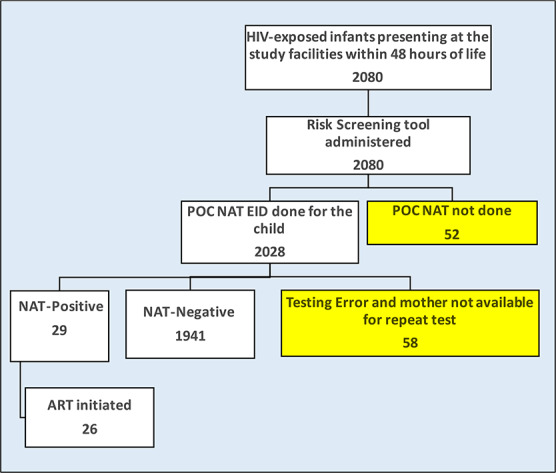
Study participants enrolment procedure.
TABLE 1.
Demographic Characteristics of Mothers and Infants Participating in the Study

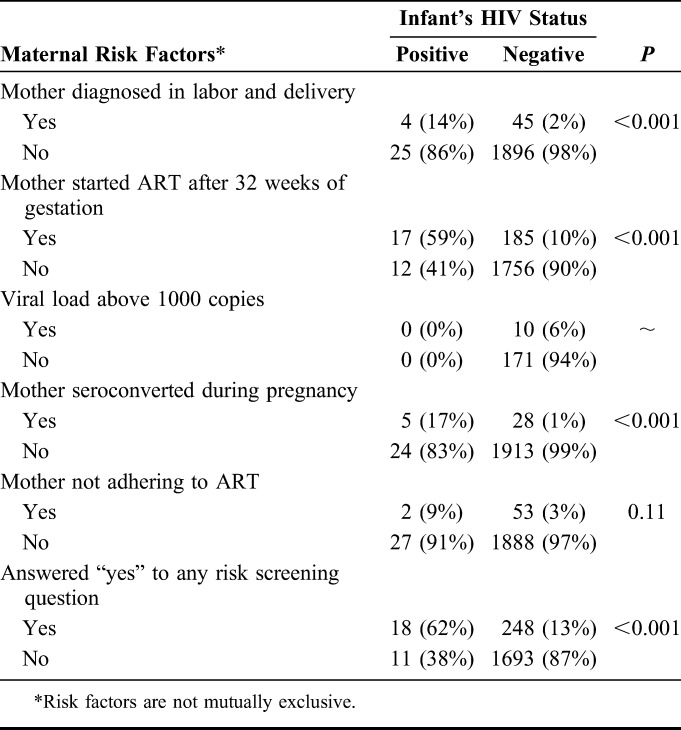
The risk variables of mother being diagnosed in labor and delivery, mother starting ART after 32 weeks of gestation and mother seroconverting during pregnancy were significantly associated with the infant testing HIV-positive at birth (Table 2). The risk variables of mothers having Viral load (VL) >1000 copies/mL or not adhering to ART were not significantly associated with the infant testing HIV-positive (Table 2). It should be noted that viral load results in medical records were available for <10% of women and for none of the women whose infants had positive POC EID test results. HIV prevalence was highest (above 17%) among infants whose mothers seroconverted during pregnancy followed by those whose mothers started ART after 32 weeks of gestation. HIV prevalence increased significantly with increase in the number of risk-assessment questions with an affirmative response, from 0.6% among those scoring zero risk factors to 13.4% among those reporting at least 2 risk factors (P < 0.001).
TABLE 2.
Maternal Risk Factors Stratified by HIV Status
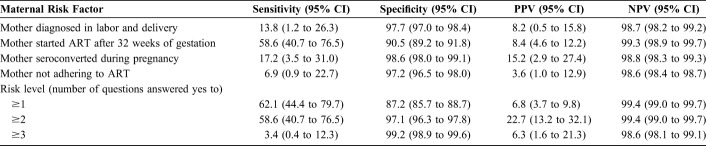
Sensitivity and specificity in detecting HIV status varied for different screening questions. Starting ART after 32 weeks' gestation had the highest sensitivity in predicting HIV infection, 58.6%, (95% CI: 40.7 to 76.5), and mother not adhering to ART had the lowest sensitivity, 7.1% (95% CI: −2.4% to 16.7%) (Table 3). Specificity was highest among infants whose mothers seroconverted during pregnancy, 98.6% (95% CI: 98.0% to 99.1%), and mother starting ART after the 32-week gestation period was lowest, 90.5% (95% CI: 89.2% to 91.8%). Having at least one maternal risk factor had the highest sensitivity 62.1% (95% CI: 44.4% to 79.7%) and sensitivity decreased with increase in the number of maternal risk factors that an infant was exposed from 62.1% for score ≥one risk factor to 3.4% for score ≥ 3 risk factors, whereas specificity increased from 87.2% to 99.2% for the same categories (Table 3). The highest positive predictive value (PPV) was recorded among infants with at least 2 maternal risk factors, 22.7% (95% CI: 13.2% to 32.1%), whereas the negative predictive value (NPV) was high regardless of number of risk factors, from 99.4% with ≥1 (99.0–99.7) to 98.6% (98.1–99.1) with ≥3 risk factors (Table 3).
TABLE 3.
Sensitivity, Specificity, and Positive and Negative Predictive Values for the Maternal HIV Transmission Risk-Screening Tool
DISCUSSION
This study assessed the use of maternal HIV transmission risk stratification in identifying HEI who may benefit from additional NAT for HIV at birth in Zimbabwe. In general, such screening assessments should have a high sensitivity to reduce missed diagnoses. A sensitivity of 62% represents a potential missed opportunity of 2 in every 5 infants with in utero HIV infection. Other studies that assessed the use of maternal transmission risk screening tools to identify infants for birth testing observed a higher sensitivity of around 80%, which represents potential missed opportunity of one in every 5 HIV-positive infants.13–15
Our study observed a relatively low sensitivity and positive predictive value of the risk screening tool, but high NPV. This is because of the overall low HIV prevalence among HEI tested at birth (1.5%). Maternal and infant-related issues and risks not assessed in the study might have contributed to the low performance of the screening tool. Additional factors identified by other studies included presence of maternal sexually transmitted infection, substance use, poor access to ANC, having a symptomatic infant, and/or having a low birth weight infant as risk predictors for HIV infection in infants at birth.15,16 Modification of the risk screening tool to include these additional factors may increase its sensitivity.
Despite the risk screen's low sensitivity, the risk screen may still be considered for use because it did predict positivity and thus, the HIV positivity of infants at high risk was higher than that of infants not identified at high risk. Factors such as resource availability and MTCT rates must be taken into account in deciding whether such a risk screen should be used. Both the costs of NAT and the costs and human resource implications for training and administering the risk screen itself must be considered when weighing costs and benefits. In addition, the risk screen may be used not for targeting birth testing, but instead for decisions on the provision of enhanced ARV prophylaxis (eg, three drugs for prophylaxis) if birth testing were not available or if the infant initially tests negative.
This study reports high HIV prevalence among infants whose mothers started ART after the 32-week gestation period. This is consistent with Townsend et al who found that vertical transmission probability declined with increase in number of weeks the mother was on ART.4,17 This finding also resonates with the Zimbabwe MOHCC policy, which encourages early booking in ANC and the “HIV test and treat” policy, because these will reduce maternal transmission rates and enhance infant survival.
In contrast to some other studies, our study did not find an association between either reported maternal ART adherence or VL >1000 copies/mL in the third trimester and neonatal HIV infection.18–20 The limited availability of maternal VL results in this study limits our ability to interpret this finding. However, a study by Garcia et al21 concluded that although these risk factors can predict transmission, they cannot predict timing of transmission. Finally, the number of women with nonadherence to ART was relatively small, which may limit the ability to detect a small association between nonadherence and HIV infection.
Viral load monitoring is a central tool to evaluate ART effectiveness and make clinical decisions for all people on ART, including pregnant women.22–24 The nonavailability of such important information for 99% of women not only significantly limits the interpretation of maternal VL as risk factor for infant HIV infection in this study, but also the ability of health care workers to use maternal VL as part of a risk stratification tool. As such, risk stratification in this regard can only be possible when appropriate VL scale up is guaranteed.
One limitation of this study was low numbers of HIV-positive infants identified, which resulted in wider confidence intervals around sensitivity and PPVs. However, the study was sufficiently powered to see a difference in positivity yields between targeted vs. universal testing of HEI. Other strengths of the study were active recruitment of study participants and the study facilities were a good representation of various levels of care. This study did not assess the cost of implementing targeted vs. universal testing of HEI, and further research may be required to compare the cost and cost-effectiveness of various screening tools. The study did not also assess health care worker feasibility and acceptability of universal birth testing versus targeted testing using the risk screening tool.
CONCLUSIONS
Although there was an association between maternal HIV transmission risk and HIV infection among infants coupled with a high NPV, the sensitivity was relatively low, and 2 of every 5 HIV-infected infants would be missed if birth testing were based solely on a positive risk screen. Although the use of the risk screening tool may be cost-efficient in the face of widespread resource constraints, there is need to balance this with the potential benefits of early diagnosis and early treatment of infants living with HIV. Late diagnosis will delay ART initiation, and this could potentially undermine the long-term impact of HIV care scale-up on reducing mortality and controlling the HIV epidemic.25–27 Further, if risk-based targeted birth testing is used, it will be equally important to strengthen universal HIV virologic testing at 6 weeks in to identify children who were missed at birth because they were not identified as high risk or were too early in infection to be detected on HIV virologic testing. Although there are significant logistical and resource challenges to identifying and treating HIV-infected neonates, universal testing of all HEI at birth may be preferred to risk-based testing, to avoid missing as many as 38% of neonates that are HIV-infected.
ACKNOWLEDGMENTS
The authors are grateful to the mothers and infants who participated in this study and to the Hospital Management and staff from the 10 Health facilities that participated in this study who gave their time and provided support. The authors thankfully acknowledge the data collection team, for their commitment during the data collection process.
Footnotes
Funded and supported by, Unitaid, Geneva, Switzerland.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.WHO Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: WHO; Available at: http://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed September 9, 2019. [Google Scholar]
- 2.Bourne DE, Thompson M, Brody LL, et al. Emergence of a peak in early infant mortality due to HIV/AIDS in South Africa. AIDS Lond Engl. 2009;23:101–106. [DOI] [PubMed] [Google Scholar]
- 3.Newell ML, Coovadia H, Cortina-Borja M, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. [DOI] [PubMed] [Google Scholar]
- 4.Townsend CL, Byrne L, Cortina-Borja M, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000-2011. AIDS Lond Engl. 2014;28:1049–1057. [DOI] [PubMed] [Google Scholar]
- 5.Phiri NA, Lee HY, Chilenga L, et al. Early infant diagnosis and outcomes in HIV-exposed infants at a central and a district hospital, Northern Malawi. Public Health Action. 2017;21:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finocchario-Kessler S, Gautney BJ, Khamadi S, et al. If you text them, they will come: using the HIV infant tracking system to improve early infant diagnosis quality and retention in Kenya. AIDS Lond Engl. 2014;28(suppl 3):S313–S321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatterjee A, Tripathi S, Gass R, et al. Implementing services for Early Infant Diagnosis (EID) of HIV: a comparative descriptive analysis of national programs in four countries. BMC Public Health. 2011;11:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kayumba K, Nsanzimana S, Binagwaho A, et al. TRACnet internet and short message service technology improves time to antiretroviral therapy initiation among HIV-infected infants in Rwanda. Pediatr Infect Dis J. 2016;35:767–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell W. Neurological and developmental effects of HIV and AIDS in children and adolescents. Ment Retard Dev Disabil Res Rev. 2001;7:211–216. [DOI] [PubMed] [Google Scholar]
- 10.Lilian RR, Kalk E, Technau KG, et al. Birth diagnosis of HIV infection in infants to reduce infant mortality and monitor for elimination of mother-to-child transmission. Pediatr Infect Dis J. 2013;32:1080–1085. [DOI] [PubMed] [Google Scholar]
- 11.Chapter2.pdf. Available at: https://www.who.int/hiv/pub/arv/chapter2.pdf. Accessed September 9, 2019. [Google Scholar]
- 12.Sandbulte MR, Gautney BJ, Maloba M, et al. Infant HIV testing at birth using point-of-care and conventional HIV DNA PCR: an implementation feasibility pilot study in Kenya. Pilot Feasibility Stud. 2019;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim M, Maswabi K, Ajibola G, et al. Targeted HIV testing at birth supported by low and predictable mother-to-child transmission risk in Botswana. J Int AIDS Soc. 2018;21:e25111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moucheraud C, Chasweka D, Nyirenda M, et al. Simple screening tool to help identify high-risk children for targeted HIV testing in Malawian inpatient wards. J Acquir Immune Defic Syndr. 2018;79:352–357. [DOI] [PubMed] [Google Scholar]
- 15.Plessis NMD, Muller CJB, Avenant T, et al. An early infant HIV risk score for targeted HIV testing at birth. Pediatrics. 2019;143:e20183834. [DOI] [PubMed] [Google Scholar]
- 16.Nesheim SR, Rose C, Pan Y, et al. A clinical score to support antiretroviral management of HIV-exposed infants on the day of birth. Pediatr Infect Dis J. 2019;38:939–943. [DOI] [PubMed] [Google Scholar]
- 17.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potty RS, Sinha A, Sethumadhavan R, et al. Incidence, prevalence and associated factors of mother-to-child transmission of HIV, among children exposed to maternal HIV, in Belgaum district, Karnataka, India. BMC Public Health. 2019;19:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chagomerana MB, Miller WC, Tang JH, et al. Optimizing prevention of HIV mother to child transmission: duration of antiretroviral therapy and viral suppression at delivery among pregnant Malawian women. PLoS One. 2018;13:e0195033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prendergast AJ, Goga AE, Waitt C, et al. Transmission of CMV, HTLV-1, and HIV through breastmilk. Lancet Child Adolesc Health. 2019;3:264–273. [DOI] [PubMed] [Google Scholar]
- 21.Garcia PM, Kalish LA, Pitt J, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999;341:394–402. [DOI] [PubMed] [Google Scholar]
- 22.Calmy A, Ford N, Hirschel B, et al. HIV viral load monitoring in resource-limited regions: optional or necessary? Clin Infect Dis. 2007;44:128–134. [DOI] [PubMed] [Google Scholar]
- 23.Bachmann N, von Braun A, Labhardt ND, et al. Importance of routine viral load monitoring: higher levels of resistance at ART failure in Uganda and Lesotho compared with Switzerland. J Antimicrob Chemother. 2019;74:468–472. [DOI] [PubMed] [Google Scholar]
- 24.Myer L, Essajee S, Broyles LN, et al. Pregnant and breastfeeding women: a priority population for HIV viral load monitoring. PLoS Med. 2017;14:e1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Innes S, Lazarus E, Otwombe K, et al. Early severe HIV disease precedes early antiretroviral therapy in infants: are we too late? J Int AIDS Soc. 2014;17:18914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lahuerta M, Ue F, Hoffman S, et al. The problem of late ART initiation in sub-saharan Africa: a transient aspect of scale-up or a long-term phenomenon? J Health Care Poor Underserved. 2013;24:359–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marston M, Becquet R, Zaba B, et al. Net survival of perinatally and postnatally HIV-infected children: a pooled analysis of individual data from sub-Saharan Africa. Int J Epidemiol. 2011;40:385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]