Supplemental Digital Content is Available in the Text.
Symptomatic vitreomacular adhesion is associated with reduced visual acuity, which can significantly affect quality of life. This study was a prespecified analysis of the 2-year, sham-controlled OASIS trial. Clinically meaningful (≥5-point) changes in patient-reported outcomes were assessed. Ocriplasmin resulted in clinically meaningful improvements in visual function.
Key words: OASIS, ocriplasmin, patient-reported outcomes, symptomatic vitreomacular adhesion, VFQ-25, vitreomacular traction
Abstract
Purpose:
To evaluate patient-reported visual function after ocriplasmin through the 25-item National Eye Institute Visual Function Questionnaire (VFQ-25) in patients with symptomatic vitreomacular adhesion/vitreomacular traction including macular hole.
Methods:
This was a prespecified analysis of a secondary endpoint from the OASIS trial. Patients received a single intravitreal injection of ocriplasmin (0.125 mg) or sham and completed the VFQ-25 questionnaire at baseline and at Months 6, 12, and 24. Clinically meaningful (≥5-point) changes from baseline were assessed.
Results:
Of the 220 patients enrolled, 146 received ocriplasmin and 74 received sham. At Month 24, the percentage of patients with a ≥5-point improvement from baseline in VFQ-25 composite scores was higher with ocriplasmin versus sham (51.4% vs. 30.1%, 95% confidence interval, 8.1–34.5, P = 0.003). The percentage of patients with ≥5-point worsening at Month 24 was lower with ocriplasmin versus sham (9.5% vs. 15.6%, 95% confidence interval: −15.6 to 3.5, P = 0.191). A larger percentage of patients treated with ocriplasmin versus sham experienced a ≥5-point improvement in VFQ-25 composite and subscale scores at Month 24 regardless of baseline full-thickness macular hole status.
Conclusion:
A larger percentage of patients with symptomatic vitreomacular adhesion/vitreomacular traction reported clinically meaningful improvements in self-assessed visual function with ocriplasmin than sham.
Symptomatic vitreomacular adhesion (VMA) or vitreomacular traction can cause anatomical disturbances of the macula and formation of macular hole.1–3 Reduced visual acuity and visual distortion, such as metamorphopsia, can significantly affect quality of life and is associated with symptomatic VMA.4 The current treatment options for symptomatic VMA, which depend on the disease stage and progression, include watchful waiting, pharmacologic vitreolysis, and pars plana vitrectomy.2,5 Although symptomatic VMA resolves spontaneously in some patients (10%–35% of cases), if left untreated, symptomatic VMA can progress and may lead to vision loss.3,6,7
Ocriplasmin is a recombinant truncated form of human plasmin indicated for treatment of symptomatic VMA,8,9 which acts by hydrolyzing the protein matrix involved in the tractional forces at the macular region.8 Regulatory approvals were based on data from the pivotal Phase 3 Microplasmin for Intravitreous Injection-Traction Release without Surgical Treatment (MIVI-TRUST) ocriplasmin trials (NCT00781859 and NCT00798317) in patients with symptomatic VMA.3,8 The subsequent Phase 3b Ocriplasmin for Treatment for Symptomatic Vitreomacular Adhesion Including Macular Hole (OASIS, NCT01429441) study confirmed the efficacy and safety results of the 6-month MIVI-TRUST trials over 24 months in patients with symptomatic VMA.10
Outcomes from patient self-assessment are needed to understand if the improved clinical outcomes are translating into improved quality of life. The patient's functional ability is as important as the clinical measure of VMA resolution. The validated 25-item National Eye Institute Visual Function Questionnaire (VFQ-25) measures patients' perceptions of vision-related function and of the ways in which treatment affects daily activities related to visual function.11–13 Here, we present patient-reported outcomes for visual function from the OASIS study using the VFQ-25 questionnaire to evaluate the therapeutic and functional benefit of ocriplasmin versus sham.
Methods
Study Design and Patient Population
OASIS (NCT01429441) was a Phase 3b, randomized, multicenter, double-masked, sham-controlled, clinical trial of ocriplasmin in patients with symptomatic VMA, including macular hole. Details of the trial design, patient population, and efficacy and safety outcomes were published previously.10 Briefly, eligible patients had symptomatic VMA with a best-corrected visual acuity score of 20/32 or worse on the Early Treatment Diabetic Retinopathy Study chart in the study eye and 20/800 or better in the nonstudy eye. Patients were randomized (2:1) to receive a single intravitreal injection of ocriplasmin (0.125 mg) or sham. The randomization was stratified by the baseline presence of full-thickness macular hole (FTMH).
The study was conducted according to the Declaration of Helsinki and the International Conference for Harmonisation (ICH) Guideline for Good Clinical Practice. Ethics committee and institutional review board approval was obtained. Written informed consent was obtained from each patient before enrollment.
Assessments
Patient-reported visual function was recorded at baseline and at 6, 12, and 24 months after injection using the VFQ-25 questionnaire to measure the vision-targeted health status. The administrator of the VFQ-25 questionnaire was masked to the intervention arm. The VFQ-25 includes a global composite score, a single general health subscale score, and the following 11 vision-related subscale scores: color vision, dependency, distance activities, driving, general vision, mental health, near activities, ocular pain, peripheral vision, role difficulties, and social functioning. Scoring excluded items wherever data were missing and was based on an algorithm for the VFQ-25 questionnaire, in which 0 represents the worst and 100 represents the best possible score.14 Changes (improvement or worsening) of ≥5 points from baseline in VFQ-25 composite and subscale scores were considered clinically meaningful.15–17 VFQ-25 composite and subscale scores were examined by baseline FTMH (yes/no), symptomatic VMA resolution at Day 28 (yes/no), and vitrectomy while on study (yes/no).
Statistical Analysis
VFQ-25 analyses included all randomized patients who received an injection of ocriplasmin in the study eye and had data for at least one follow-up visit. A ≥5-point improvement in VFQ-25 scores at Month 24 was a predetermined secondary endpoint of the OASIS study.10 The threshold for counting a difference between groups in percentages of patients with a ≥5-point change in composite and subscale scores was calculated as 100% (1/N) where N is the number of patients in the smaller subgroup of the comparison. The study was not specifically powered to detect differences between treatment groups in the percentage of patients with a ≥5-point change in VFQ-25 composite score at Month 24, nor any other subscale scores. When a visit did not take place, the scores of the previous visit were carried forward using the last-observation-carried-forward method. The VFQ-25 results were summarized by treatment and visit using descriptive statistics. When calculated across strata (irrespective of FTMH status at baseline), percentages were computed using the inverse of variance formula. The percentages of patients with at least a 5-point change (increase and decrease) in VFQ-25 scores were compared between ocriplasmin and sham groups for the overall population (irrespective of FTMH status at baseline) using the Cochran–Mantel–Haenszel test. Within strata (stratum defined based on the FTMH status at baseline), the Pearson chi-square test was used. Comparisons of changes in VFQ-25 scores (≥5-point) also were made between ocriplasmin and sham groups based on baseline FTMH status (present, absent), the status of nonsurgical resolution of VMA at Day 28 (yes, no), and vitrectomy while on study (yes, no).
Results
Baseline Demographics and Disease Characteristics
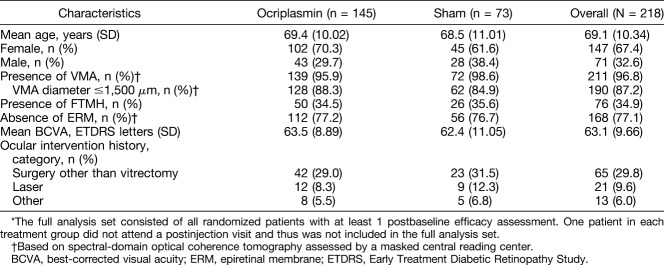
Of the 220 patients enrolled into the OASIS study, 146 received a single intravitreal ocriplasmin injection, and 74 received a sham injection. Two patients, one from each treatment group, did not attend the postinjection visits and were therefore excluded from this analysis. Demographics and baseline ocular characteristics are presented in Table 1.
Table 1.
Demographic and Baseline Ocular Characteristics (Full Analysis Set)*
Patient-Reported Visual Function
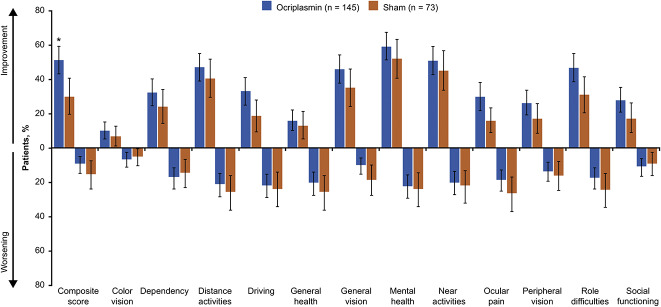
A larger percentage of patients treated with ocriplasmin than with sham achieved a ≥5-point increase in VFQ-25 composite score from baseline at Month 24 (51.4% vs. 30.1%; difference: 95% confidence interval [CI], 8.1–34.5, P = 0.003; Figure 1). A smaller percentage of patients in the ocriplasmin group versus sham experienced a ≥5-point decrease in the VFQ-25 composite score from baseline at Month 24 (9.5% vs. 15.6%; difference: 95% CI, −15.6 to 3.5, P = 0.191; Figure 1). For all subscale scores, a larger percentage of patients treated with ocriplasmin versus sham achieved a ≥5-point improvement in subscale scores from baseline at Month 24 (Figure 1). Except for color vision, dependency, and social functioning, a smaller percentage of ocriplasmin recipients than of sham recipients had a ≥5-point decrease in subscale scores at Month 24 (Figure 1). The Month 6 and 12 results for the VFQ-25 composite and subscale scores followed a similar pattern to the Month 24 data, generally favoring ocriplasmin (see Figure, Supplemental Digital Content 1, http://links.lww.com/IAE/B22, which shows data for patients who had at least a 5-point change in VFQ-25 scores from baseline to Months 6 and 12).
Fig. 1.
Patients with at least a 5-point change in VFQ-25 scores from baseline to Month 24 (LOCF, irrespective of vitrectomy). For driving subscale: ocriplasmin (n = 132) and sham (n = 69). *P < 0.01. Error bars represent 95% CIs. The threshold for counting a difference between groups in percentages of patients with a ≥5-point change in composite and subscale scores was calculated as 100% (1/N) where N is the number of patients in the smaller subgroup of the comparison. LOCF, last observation carried forward; VFQ-25, 25-item National Eye Institute Visual Function Questionnaire.
Patient-Reported Visual Function by Baseline Full-Thickness Macular Hole Status
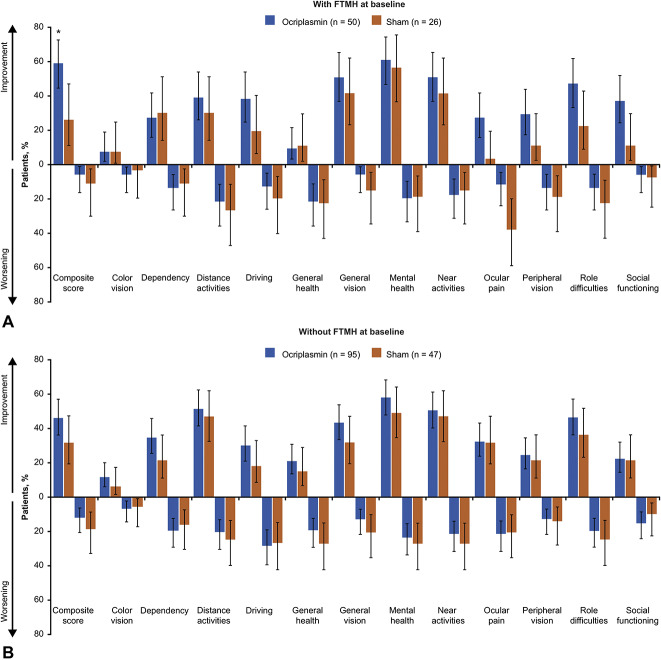
At baseline, 34.5% (50/145) of ocriplasmin recipients and 35.6% (26/73) of sham recipients had FTMH. A larger percentage of patients in the ocriplasmin group than sham achieved a ≥5-point improvement from baseline in the VFQ-25 composite score at Month 24 regardless of baseline FTMH status (with FTMH: 60% vs. 26.9% [95% CI, 11.3–54.9], P = 0.006; without FTMH: 46.3% vs. 31.9% [95% CI, −2.3 to 31.1], P = 0.101; Figure 2). Results for worsening of patient-reported visual function by FTMH status were similar. A smaller proportion of patients in the ocriplasmin group than sham had a ≥5-point decrease in the VFQ-25 composite score at Month 24 regardless of baseline FTMH status (with FTMH: 6.0% vs. 11.5% [95% CI, −19.5 to 8.4], P = 0.396; without FTMH: 12.6% vs. 19.1% [95% CI, −19.6 to 6.6], P = 0.303; Figure 2). As with the composite scores, the proportions of patients with ≥5-point changes in subscale scores generally favored ocriplasmin over sham. For patients with baseline FTMH, a larger percentage of patients in the ocriplasmin group than sham experienced a ≥5-point improvement in 9 of 12 subscale scores at Month 24. For patients without baseline FTMH, a larger percentage of patients achieved a ≥5-point improvement for 10 of 12 subscale scores with ocriplasmin than sham. The ≥5-point worsening in subscale scores occurred in a smaller proportion of patients with ocriplasmin than sham for most subscale scores regardless of baseline FTMH status. The Month 6 and 12 findings were similar to the results at Month 24 for the FTMH subgroups (see Figure, Supplemental Digital Content 2, http://links.lww.com/IAE/B23, which shows data for patients with a ≥5-point change in VFQ-25 scores from baseline to Months 6 and 12 by FTMH at baseline).
Fig. 2.
Patients with at least a 5-point change in VFQ-25 scores from baseline to Month 24 by FTMH at baseline (LOCF, irrespective of vitrectomy). Panel A shows patients with FTMH at baseline, panel B shows patients without FTMH at baseline. For driving subscale: ocriplasmin (n = 46) and sham (n = 25) in subgroup with FTMH at baseline. For driving subscale: ocriplasmin (n = 86) and sham (n = 44) in subgroup without FTMH at baseline. *P < 0.01. Error bars represent 95% CIs. The threshold for counting a difference between groups in percentages of patients with a ≥5-point change in composite and subscale scores was calculated as 100% (1/N) where N is the number of patients in the smaller subgroup of the comparison. FTMH, full-thickness macular hole; LOCF, last observation carried forward; VFQ-25, 25-item National Eye Institute Visual Function Questionnaire.
Patient-Reported Visual Function by Vitreomacular Adhesion Resolution at Day 28
Nonsurgical symptomatic VMA resolution was achieved at Day 28 in 62/145 (41.7%) patients treated with ocriplasmin and 5/73 (6.2%) patients receiving sham (P < 0.001). Regardless of symptomatic VMA resolution at Day 28, a larger percentage of patients in the ocriplasmin group than sham experienced a ≥5-point improvement in VFQ-25 composite score at Month 24 (with VMA resolution: 57.1% vs. 39.8% [95% CI, −27.0 to 61.6] P = 0.483; without VMA resolution: 48.7% vs. 29.5% [95% CI, 4.0–34.5] P = 0.019). A smaller percentage of patients without VMA resolution at Day 28 experienced a ≥5-point worsening in the VFQ-25 composite score at Month 24 with ocriplasmin than sham (13.3% vs. 17.1% [95% CI, −15.2 to 7.7] P = 0.552). The trend was reversed in those with VMA resolution, in whom a larger percentage of ocriplasmin recipients experienced a ≥5-point worsening in the composite score (4.7% vs. 0.0% [95% CI, −0.6 to 9.9] P = 0.632). However, it should be noted that the subgroup of patients in the sham group with symptomatic VMA resolution was small (n = 5), and results should be interpreted with caution.
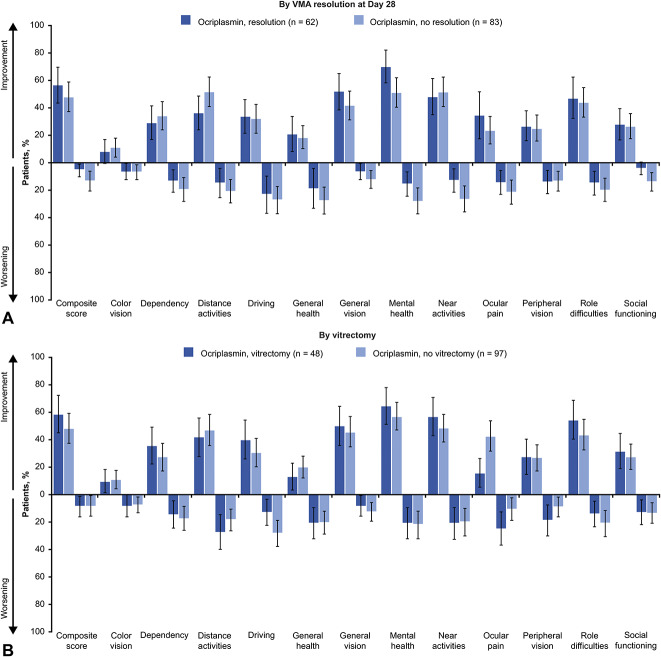
In ocriplasmin recipients, a larger percentage of patients with VMA resolution at Day 28 compared with no VMA resolution achieved a ≥5-point improvement in composite and 6 subscale scores at Month 24 (Figure 3A [top panel]). Except for color vision and peripheral vision subscales, a smaller percentage of patients with VMA resolution in the ocriplasmin group experienced a ≥5-point worsening in composite and subscale scores than patients without VMA resolution. The Month 6 and Month 12 findings were similar to the results at Month 24 for the subgroups defined by symptomatic VMA resolution (see Table, Supplemental Digital Content 3, http://links.lww.com/IAE/B24, which shows a ≥5-point change in VFQ-25 scores from baseline to Month 6 and Month 12 in the ocriplasmin group by VMA resolution at Day 28 and by vitrectomy).
Fig. 3.
Patients with at least a 5-point change in VFQ-25 scores from baseline to Month 24 by VMA resolution at Day 28 (panel A) and vitrectomy (panel B). For driving subscale: ocriplasmin with resolution (n = 58) and ocriplasmin without resolution (n = 74). For driving subscale: ocriplasmin with vitrectomy (n = 45) and ocriplasmin without vitrectomy (n = 87). Error bars represent 95% CIs. The threshold for counting a difference between groups in percentages of patients with a ≥5-point change in composite and subscale scores was calculated as 100% (1/N) where N is the number of patients in the smaller subgroup of the comparison. LOCF, last observation carried forward; VFQ-25, 25-item National Eye Institute Visual Function Questionnaire; VMA, vitreomacular adhesion.
Patient-Reported Visual Function by Vitrectomy Status
Overall 48/145 (33.1%) patients in the ocriplasmin group and 32/73 (43.8%) patients in the sham group underwent vitrectomy. A larger percentage of patients in the ocriplasmin group than sham achieved a ≥5-point improvement in the VFQ-25 composite score at Month 24 regardless of vitrectomy status (with vitrectomy: 58.5% vs. 39.9% [95% CI, −2.7 to 39.9] P = 0.121; without vitrectomy: 48.0% vs. 16.1% [95% CI, 17.6–46.2] P = 0.006). Results for worsening of patient-reported visual function by vitrectomy status were similar. A smaller percentage of patients in the ocriplasmin group than sham experienced a ≥5-point worsening in the VFQ-25 composite score at Month 24, regardless of vitrectomy status (with vitrectomy: 8.6% vs. 16.5% [95% CI, −22.8 to 7.0] P = 0.304; without vitrectomy: 8.3% vs. 6.9% [95% CI, −7.6 to 10.4] P = 0.523).
In the ocriplasmin group, a larger percentage of patients with vitrectomy than no vitrectomy achieved a ≥5-point improvement in composite and 7 subscale scores at Month 24 (Figure 3B). Similar percentages of patients in the ocriplasmin group experienced a ≥5-point worsening in the VFQ-25 composite score at Month 24 regardless of vitrectomy status. A larger percentage of patients with vitrectomy than without vitrectomy in the ocriplasmin group experienced a ≥5-point worsening in distance activities, ocular pain, and peripheral vision subscale scores. The Month 6 and Month 12 findings for the subgroups defined by vitrectomy are presented in Supplemental Digital Content 3 (see Table, http://links.lww.com/IAE/B24). In the sham group, a larger percentage of patients with vitrectomy than without experienced a ≥5-point improvement in composite and 10 of 12 subscale scores at Month 24. A greater percentage of patients in the sham group with vitrectomy than without experienced a ≥5-point worsening in composite and 6 subscale scores.
Discussion
The Phase 3b OASIS study patient-reported visual function outcomes demonstrate that treatment with a single intravitreal injection of ocriplasmin in patients with symptomatic VMA improved self-reported visual function during 2 years of follow-up. Ocriplasmin recipients also were less likely to report worsening visual functioning compared with patients treated with sham injection. The OASIS patient-reported visual function outcomes are consistent with those reported in the MIVI-TRUST trials, in which the percentage of patients with at least a 5-point improvement in the VFQ-25 composite score at Month 6 was comparable (39.4% OASIS and 36.0% MIVI-TRUST).18 The lower percentages of patients with a ≥5-point worsening in the VFQ-25 composite score at Month 6 also were comparable (11.8% OASIS and 15.0% MIVI-TRUST).18 These findings complement clinical endpoints and can aid in clinical decision-making. Indeed, patient-reported outcomes are powerful tools for validating the effects of a treatment on patient health and daily-life activities, both in terms of benefits and potential adverse effects.
Clinically meaningful improvements in VFQ-25 composite scores favored ocriplasmin over sham regardless of baseline FTMH status or symptomatic VMA resolution at Day 28. Results for ≥5-point improvements at Month 24 in the VFQ-25 subscale scores also generally favored ocriplasmin over sham across the subgroups analyzed. Within the ocriplasmin group, patients with FTMH at baseline had better patient-reported outcomes than those without FTMH at baseline.
As might be expected, larger improvements in patient-reported visual function correlated with VMA resolution at Day 28. In addition, even in those patients with persistent symptomatic VMA, the improvements in the VFQ-25 composite score were larger with ocriplasmin treatment than sham. A similar finding was observed in the MIVI-TRUST trial, in which larger improvements in patient-reported visual function correlated with partial or complete symptomatic VMA resolution, suggesting that partial symptomatic VMA release may be sufficient to reduce traction and improve visual function.18 In addition, recent case studies suggest improved clinical symptoms and visual acuity after ocriplasmin treatment despite achieving only partial symptomatic VMA release at Day 28.19 However, the analysis by symptomatic VMA resolution should be taken with caution because the number of patients in the sham group with symptomatic VMA resolution at Day 28 was small (n = 5).
Clinically meaningful improvements in VFQ-25 composite scores also favored ocriplasmin over sham regardless of vitrectomy. Composite scores were 18.6% higher with vitrectomy and 31.9% higher without vitrectomy in the ocriplasmin group than in the sham group. These improvements were observed despite the fact that patients with vitrectomy had better patient-reported outcomes than those without vitrectomy within the ocriplasmin and sham groups.
The analyses of patient-reported visual function outcomes described here have some limitations. First, the subgroup analyses were descriptive in nature only. Moreover, the symptomatic VMA resolution and vitrectomy subgroups were based on treatment-dependent variables as opposed to a random assignment. Another limitation is that 28% of patients discontinued the study before the conclusion of the 2-year follow-up period, which led to missing values.10 Furthermore, the 2-year time frame of the OASIS study allowed additional confounding factors, such as cataract surgeries and vitrectomies during the study follow-up period. However, the 2-year follow-up period and the randomized, double-masked design of the study suggest that the differences observed are unbiased and causal.
In conclusion, treatment with a single ocriplasmin injection led to clinically meaningful improvements in patient-reported visual function measured by the VFQ-25 questionnaire over 24 months in patients with symptomatic VMA.
Acknowledgments
The authors acknowledge the principal investigators and study site staff who participated in the OASIS clinical study as well as all patients enrolled in the study. Statistical support was provided by Esmeralda Meunier from Oxurion (formerly ThromboGenics), Gaston Geenslaan 1, 3001 Leuven, Belgium. Medical writing assistance was provided by Usha Gutti and Duprane Pedaci Young from Fishawack Communications Inc and was funded by Oxurion (formerly ThromboGenics).
Footnotes
This study was funded by Oxurion (formerly ThromboGenics), Iselin, New Jersey. The sponsor participated in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Presented in part at the American Academy of Ophthalmology (AAO) annual meeting, New Orleans, LA, November 11–14, 2017, and the American Society of Retina Specialists (ASRS) annual meeting, Vancouver, BC, Canada, July 20–25, 2018.
C. Mein receives research grants from Allergan, Alcon, Iconic, Ora, Acucella, and the DRCR.net. P. U. Dugel is a consultant to Bausch + Lomb, Genentech, Alcon, NeoVista, MacuSight, ArticDx, ORA, Novartis, Allergan, Santen, Inc., Opthotech, Lux Bioscience, DigiSight, Roche, TopCon, Acucela, Pentavision, ORA, Stealth BioTherapeutics, Annidis, Clearside Biomedical, Optovue, Pentavision, Neurotech, Lutronic, Alimera Sciences, DOSE Medical, Aerpio, Omeros, Shire Human Genetics, Opthea, Graybug Vision, Irenix, ByeOnics, Clearside Biomedical, PanOptica, Chengdu Kanghong Biotechnology, and SciFluor Life Sciences. He is an equity owner to ArticDx, Opthotech, DigiSight, Annidis, Clearside Biomedical, Alimera Sciences, Aerpio, and PanOptica. L. Feiner is a consultant to Roche and received lecture fees from Roche, Regeneron, Oxurion (formerly ThromboGenics), and Bayer. K. Drenser is/has previously served as a consultant to Spark Therapeutics, Allergan, and Genentech/Roche and as a member of the Spark Therapeutics data safety monitoring board. She also is serving/has served on the speaker bureau for Allergan and is an equity owner of Interview Medical Systems (Focus ROP), Phoenix, and Retinal Solutions. D. Miller receives research support from Genentech, Alcon, Novartis, and Ophthotech. He is an equity owner of Vortex Surgical. M. Benz has no financial disclosures. E. Meunier is employed as a consultant to Oxurion (formerly ThromboGenics). L. Moro is an employee of Oxurion (formerly ThromboGenics). M. S. Fineman is a consultant to PRN, Oxurion (formerly ThromboGenics), and Allergan.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.retinajournal.com).
References
- 1.Ocriplasmin for vitreomacular traction. Aust Prescr 2016;39:224–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mec-Slomska AE, Adamiec-Mroczek J, Kuzmicz E, Misiuk-Hojlo M. Intravitreal ocriplasmin: a breakthrough in the treatment of vitreomacular traction? Adv Clin Exp Med 2017;26:527–531. [DOI] [PubMed] [Google Scholar]
- 3.Stalmans P, Benz MS, Gandorfer A, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med 2012;367:606–615. [DOI] [PubMed] [Google Scholar]
- 4.Steel DH, Lotery AJ. Idiopathic vitreomacular traction and macular hole: a comprehensive review of pathophysiology, diagnosis, and treatment. Eye (Lond) 2013;27:S1–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amoaku W, Cackett P, Tyagi A, et al. Redesigning services for the management of vitreomacular traction and macular hole. Eye (Lond) 2014;28:S1–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almeida DCE, Folk JC, Rahim K, Russell SR. Predictive factors for the spontaneous resolution of vitreomacular traction. Invest Ophthalmol Vis Sci 2014;55:327. [Google Scholar]
- 7.Stalmans P. A retrospective cohort study in patients with tractional diseases of the vitreomacular interface (ReCoVit). Graefes Arch Clin Exp Ophthalmol 2016;254:617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.JETREA (ocriplasmin) Intravitreal Injection, 2.5 mg/mL. Prescribing information. 2012. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/125422s000lbl.pdf. Accessed June 12, 2018.
- 9.JETREA (ocriplasmin) summary of product characteristics. 2018. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002381/WC500142158.pdf. Accessed June 12, 2018.
- 10.Dugel PU, Tolentino M, Feiner L, et al. Results of the 2-year ocriplasmin for treatment for symptomatic vitreomacular adhesion including macular hole (OASIS) randomized trial. Ophthalmology 2016;123:2232–2247. [DOI] [PubMed] [Google Scholar]
- 11.Anker SD, Agewall S, Borggrefe M, et al. The importance of patient-reported outcomes: a call for their comprehensive integration in cardiovascular clinical trials. Eur Heart J 2014;35:2001–2009. [DOI] [PubMed] [Google Scholar]
- 12.Weldring T, Smith SM. Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Serv Insights 2013;6:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 2001;119:1050–1058. [DOI] [PubMed] [Google Scholar]
- 14.Mangione CM. The National Eye Institute 25-Item Visual Function Questionnaire. 2000. Available at: https://www.nei.nih.gov/sites/default/files/nei-pdfs/manual_cm2000.pdf. Accessed November 6, 2018.
- 15.Globe DR, Wu J, Azen SP, Varma R. The impact of visual impairment on self-reported visual functioning in Latinos: the Los Angeles Latino Eye Study. Ophthalmology 2004;111:1141–1149. [DOI] [PubMed] [Google Scholar]
- 16.Miskala PH, Hawkins BS, Mangione CM, et al. Responsiveness of the National Eye Institute Visual Function Questionnaire to changes in visual acuity: findings in patients with subfoveal choroidal neovascularization—SST Report No. 1. Arch Ophthalmol 2003;121:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Submacular Surgery Trials Research Group. Evaluation of minimum clinically meaningful changes in scores on the National Eye Institute Visual Function Questionnaire (NEI-VFQ) SST report number 19. Ophthalmic Epidemiol 2007;14:205–215. [DOI] [PubMed] [Google Scholar]
- 18.Varma R, Haller JA, Kaiser PK. Improvement in patient-reported visual function after ocriplasmin for vitreomacular adhesion: results of the Microplasmin for intravitreous injection-traction release without surgical treatment (MIVI-TRUST) trials. JAMA Ophthalmol 2015;133:997–1004. [DOI] [PubMed] [Google Scholar]
- 19.Jeng KW, Baumal CR, Witkin AJ, et al. Incomplete release of vitreomacular attachments after intravitreal ocriplasmin. Ophthalmic Surg Lasers Imaging Retina 2015;46:271–274. [DOI] [PubMed] [Google Scholar]