Supplemental Digital Content is Available in the Text.
Key Words: point of care, early infant diagnosis, pediatric HIV, stepped-wedge trial, RCT, Kenya, Zimbabwe
Abstract
Background:
Although the World Health Organization recommends HIV-exposed infants receive a 6-week diagnostic test, few receive results by 12 weeks. Point-of-care (POC) early infant diagnosis (EID) may improve timely diagnosis and treatment. This study assesses the impact of routine POC versus laboratory-based EID on return of results by 12 weeks of age.
Methods:
This was a cluster-randomized stepped-wedge trial in Kenya and Zimbabwe. In each country, 18 health facilities were randomly selected for inclusion and randomized to timing of POC implementation.
Findings:
Nine thousand five hundred thirty-nine infants received tests: 5115 laboratory-based and 4424 POC. In Kenya and Zimbabwe, respectively, caregivers were 1.29 times [95% confidence interval (CI): 1.27 to 1.30, P < 0.001] and 4.56 times (95% CI: 4.50 to 4.60, P < 0.001) more likely to receive EID results by 12 weeks of age with POC versus laboratory-based EID. POC significantly reduced the time between sample collection and return of results to caregiver by an average of 23.03 days (95% CI: 4.85 to 21.21, P < 0.001) in Kenya and 62.37 days (95% CI: 58.94 to 65.80, P < 0.001) in Zimbabwe. For HIV-infected infants, POC significantly increased the percentage initiated on treatment, from 43.2% to 79.6% in Zimbabwe, and resulted in a nonsignificant increase in Kenya from 91.7% to 100%. The introduction of POC EID also significantly reduced the time to antiretroviral therapy initiation by an average of 17.01 days (95% CI: 9.38 to 24.64, P < 0.001) in Kenya and 56.00 days (95% CI: 25.13 to 153.76, P < 0.001) in Zimbabwe.
Conclusions:
POC confers significant advantage on the proportion of caregivers receiving timely EID results, and improves time to results receipt and treatment initiation for infected infants. Where laboratory-based EID systems are unable to deliver results to caregivers rapidly, POC should be implemented as part of an integrated testing system.
INTRODUCTION
Despite significant reductions in vertical transmission of HIV due to strong prevention of mother-to-child transmission programs and improved antiretroviral therapy (ART) coverage of pregnant and breastfeeding women, there were still an estimated 160,000 children 0–14 years old who were infected with HIV in 2018, the vast majority of whom were infected through mother-to-child transmission.1
HIV-infected infants tend to have more rapid progression of disease and higher case fatality rates than uninfected infants, with a peak of mortality within 2–3 months of life for those infected in utero.2 Up to 50% of in utero infected infants will die by age 2 if they are not started on ART.3 Early identification of HIV-infected infants is essential for improving timely treatment initiation, and reducing mortality and morbidity.4 Owing to the presence of maternal antibodies in infants, serologic-based HIV diagnostics cannot be used in this population and early infant diagnosis (EID) at 4–6 weeks of age is strongly recommended by the World Health Organization (WHO) for all HIV-exposed infants (HEI).5 Given the clinical urgency of diagnosis and ART in infants, the WHO recommends that results are returned to the caregiver as soon as possible, no more than a month after specimen collection, and positive results are “fast-tracked.”6
Historically, EID was performed at centralized laboratories on high-throughput nucleic acid testing platforms by specialized laboratory staff. Centralized testing has resulted in long turnaround time (TAT) for EID test results, poor return of results to caregivers, and very low rates of ART initiation in infants found to be infected.7,8 The poor performance of centralized laboratory testing may be due to a number of factors including weak sample transport systems, long distances from requesting facilities to centralized laboratories, unpredictable and delayed result return to caregivers, and batching of samples at the laboratory.9
Point-of-care (POC) platforms, which have comparable sensitivity and specificity to laboratory-based EID, allow on-site EID by nonspecialized health workers and produce results within 1.5 hours, are now approved by stringent regulatory agencies, quality-assured by the WHO prequalification of diagnostics program, and available on the market.10–14 POC for EID has shown promise in reducing time to receipt of results to caregivers and initiation on ART in programmatic settings and in previous studies, including one trial and one observational study, and has also been demonstrated to be cost effective.15–18 Additional, larger, comparative studies of POC versus laboratory-based EID can strengthen evidence on the impact of POC to diagnose and treat HIV-infected infants. The Elizabeth Glaser Pediatric AIDS Foundation (EGPAF) worked alongside Ministries of Health (MOH) in 9 countries in Africa to introduce and scale-up POC platforms for EID of HIV as part of routine care for HEI. In Kenya and Zimbabwe, EGPAF conducted a cluster-randomized stepped-wedge trial comparing POC versus laboratory-based EID for HEI tested at the 6-week testing timepoint to assess the impact of POC on return of results to caregivers within 12 weeks of infant age, and timely ART initiation.
METHODS
Country Setting and Platform Selection
Zimbabwe and Kenya are high-HIV burden countries in sub-Saharan Africa and are among the 21 priority countries in the “Global Plan toward the elimination of new HIV infections among children and keeping their mothers alive.”19 Zimbabwe and Kenya have 3 and 8 centralized referral laboratories for EID, respectively, and both have implemented laboratory strengthening activities in recent years. Zimbabwe supports transport of samples from most health facilities to centralized laboratories once weekly. Results are returned through SMS printers; however, approximately 15% of EGPAF-supported facilities included in the POC EID project did not have functioning SMS printers. Significant investment has been made in Kenya's centralized laboratory system, with daily sample transport from requesting facilities to centralized laboratories, and results made available immediately to requesting facilities on an electronic health record system (the EID dashboard).
The project used both WHO prequalified POC assays, Abbott's m-Pima HIV-1/2 Detect and the Cepheid's Xpert HIV-1 Qual. The Ministry of Health and country teams were able to choose whichever mix of platforms best fit the laboratory network and site specifications with no influence of the study team and our analysis does not differentiate between the 2 types of platforms.
Study Design
This was a prospective stepped-wedge cluster-randomized controlled trial.20 Our primary outcome was the percentage of caregivers receiving the 6-week EID results by the time the infant was 12 weeks of age. Secondary outcomes included TAT from sample collection to result return, percentage of HIV-infected infants started on ART within 60 days of sample collection and turnaround time to ART initiation. Project sites were purposively chosen to include a mix of urban and rural sites, as well as high- and lower-level health facilities. To maximize access to POC while containing costs, the project used a hub-and-spoke approach, where POC platforms were placed at health facilities with higher EID demand, which served as “hubs.” Smaller, nearby spoke facilities (within 1-hour transport of the hub) would send samples for EID to the hub. All spoke sites sending samples to the hub were included in calculations. Samples were transported according to existing protocols, and facilities received refreshers on protocols and support from district health officers.
The sample size was calculated for hubs and spokes separately and for each country separately. In both countries, the study was powered to detect at least a 50% increase in the proportion of caregivers receiving results by 12 weeks of infant age after introduction of POC intervention, assuming a design effect of 2.
Of the POC EID project sites that were scheduled to receive a POC platform (45 in Kenya and 50 in Zimbabwe), 18 hub sites in each country were randomly selected to participate in the study. Sites in Kenya were located in 9 counties in Western Kenya and included 251 spoke sites. Sites in Zimbabwe were located in 18 districts and included 82 spoke sites. In both countries, hub sites comprised provincial, district, and subdistrict hospitals, whereas spoke sites were primary health care facilities in both urban and rural areas (see Tables 1 and 2, Supplemental Digital Content, http://links.lww.com/QAI/B466).
Sites were then randomized in groups of 6 and assigned to either the second, third, or fourth “step,” indicating when they would receive the POC platform and begin POC EID testing. All health facilities started with the laboratory-based EID during the first step and transitioned to POC as per randomization schedule. If the selected site also had spokes referring to it, these were included in the study and randomized with the hub site. Steps lasted for 4 months each, with a 1-month transition in between each. This ensured adequate time for POC platforms to be installed and training to take place in each participating facility, so that by the beginning of the next step, all platforms had uninterrupted service (Fig. 1). Transition data were not included in the analysis.
FIGURE 1.
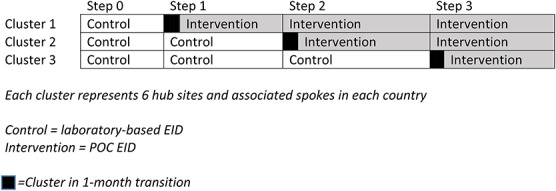
Stepped-wedge design. Each cluster represents 6 hub sites and associated spokes in each country.
All infants who had a sample collected for the 6-week testing timepoint (defined as an HEI presenting for EID at 4–8 weeks of age) at sites included in the study during one of the steps were included in the study. HEI with tests conducted for any other indication, including confirmatory tests after an initial positive test, or testing above 8 weeks of age, were excluded.
EID Procedures
All centralized laboratory testing sites were provided with a brief on-site EID refresher training, including EID record keeping, at the beginning of the study, which was performed by EGPAF staff. In addition, all sites had quarterly EID monitoring and mentorship visits using a standard EID monitoring checklist. If issues with the quality or process of EID were identified, additional mentorship and/or on-site trainings were provided.
When infants presented for EID to study sites during the centralized laboratory steps, the caregiver was counseled on EID according to national guidelines. A lancet was used to collect dried blood spot samples, usually from a heel prick. The dried blood spot cards were then packaged and sent to centralized laboratories according to standard national procedures.
Sites were enrolled into POC testing according to the randomization schedule. The POC system manufacturer installed all POC platforms and provided the initial end-user training to staff identified by the site to perform the POC testing. In addition, site staff involved in EID testing or who would see infants eligible for EID testing received on-site training on the availability and indications for POC EID at the time of system installation. After a site began POC testing, a monitoring and mentorship visit was conducted at weeks 2 and 6 and quarterly thereafter. These visits used a standardized POC EID monitoring checklist, similar in length content to that used during the centralized testing periods. If issues in EID were identified, additional mentorship and/or on-site refresher trainings were implemented.
In Zimbabwe, once a result was received by the requesting facility, caregivers were sent SMS alerts that results have returned. In Kenya, when a result was made available to the requesting facility, the caregiver was contacted through phone and SMS. In addition, the centralized laboratory or testing site called the requesting facilities to alert them of HIV-positive results. Finally, in Kenya, which had an extensive sample transport network, several EID strengthening campaigns were underway during the period of the study that involved line-listing of HEI to ensure that samples for EID were collected and results returned.
Data Collection
Data were collected from routine health facility registers and test request forms. Test request forms include demographic and clinical information about the mother (including ART and breastfeeding status), clinical information about the infant (including sex, age, prophylactic medications, previous HIV tests, and testing indication), details about specimen collection and testing (time, date, and type), and test result information (including result and when communicated to the facility and the caregiver). For infants identified as positive, the form also includes information about ART initiation date, regimen, and health center if referred for additional care.
Copies of paper forms with redacted personal information were transported to study offices for data entry. Data were quality checked on-site, at study offices, and by off-site study team members. Data were entered into server-based Excel forms, and exported into STATA 16.0 (StataCorp, College Station, TX). A real-time aggregated data dashboard was also created for monitoring throughout the study.
Statistical Analysis
Baseline demographic and clinical characteristics of children in each country were summarized using means for continuous variables and proportions for categorical variables. Exploratory analysis of study outcomes included summarizing binary variables as proportions and means for TAT by step and testing mode (POC versus laboratory testing). The effect of POC on proportion of results returned to caregiver within 12 weeks was estimated using generalized linear mixed models with the random effects accounting for clustering. The model included a time variable to account for trends in the outcome and the POC indicator variable to estimate the effect. Similarly, the effect of POC on TAT was estimated using linear mixed-effects models. Only nonconfirmatory routine 6-week tests (defined as EID at 4–8 weeks of age) are included. Outcomes were analyzed both by country and together, using the statistical analysis described above. Data were analyzed using STATA 16.0 and R statistical software.
Ethical Approvals
This study was approved by Advarra (formerly Chesapeake) IRB in Maryland, USA (Pro00021150 & Pro00023239), the Kenyatta National Hospital IRB in Nairobi, Kenya (P191/04/2017), the Medical Research Council of Zimbabwe (MRCZ/A/2176), and the Research Council of Zimbabwe. All IRBs granted waivers of individual consent for the primary outcome measures. This study was registered in ClinicalTrials.gov (NCT03824067).
RESULTS
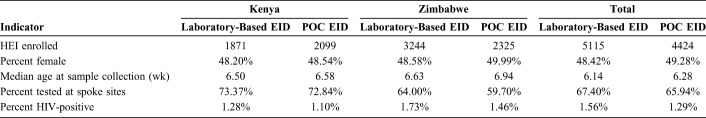
Study enrollment was conducted between December 2017 and March 2019. One hundred forty-four facility-months of data contributed to each arm (POC and laboratory-based testing). Overall, 9539 HEI had a sample collected for the 6-week EID indication during the study period: 5115 using laboratory-based testing and 4424 with POC. Overall, median infant age at sample collection under laboratory-based testing was 6.14 months and under POC EID was 6.28 months. Forty-eight percent of infants in the laboratory-based arm and 49.2% in the POC EID arm were female (Table 1). In Kenya, 3970 infants were tested: 1871 with laboratory-based and 2099 with POC EID. In Zimbabwe, 5569 infants received tests during the study period: 3244 with laboratory-based and 2325 with POC EID. Overall, HIV positivity was 1.4%, with 137 infants diagnosed with HIV. Forty-seven infants with HIV were identified in Kenya: 24 with laboratory-based and 23 with POC testing. Ninety infants with HIV were identified in Zimbabwe: 56 with laboratory-based and 34 with POC EID (Table 1). Across both countries, the majority of infants (66.7%) were tested at spoke sites. In Kenya, 2900 infants had a specimen collected for testing at spoke sites (1371 with laboratory-based and 1529 with POC EID) and 1070 infants were tested at hub sites (500 with laboratory-based and 570 with POC EID). In Zimbabwe, 3464 were tested at spoke sites (2076 with laboratory-based and 1388 with POC EID) and 2105 infants were tested at hub sites (1168 with laboratory-based and 937 with POC EID). As per design, no infants in the study received a POC test during the first step or a laboratory-based test during the last step.
TABLE 1.
Study Population Demographic Data
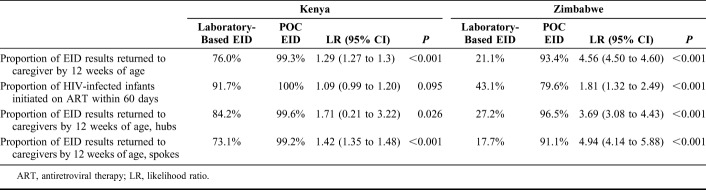
In Kenya, the proportion of test results returned to the caregiver by age 12 weeks was 76.0% for laboratory-based and 99.3% with POC EID. Caregivers were 1.29 [95% confidence interval (CI): 1.27 to 1.3, P < 0.001] times more likely to receive EID results by age 12 weeks with POC than with laboratory-based EID. In Zimbabwe, the proportion of test results returned to caregiver by age 12 weeks was 21.1% with laboratory-based and 93.4% with POC EID. In Zimbabwe, caregivers were 4.56 (95% CI: 4.50 to 4.6, P < 0.001) times more likely to receive infant EID results by age 12 weeks with POC than with laboratory-based EID (Table 2).
TABLE 2.
Proportions and Likelihood Ratios of Key Steps Along the EID Care Cascade
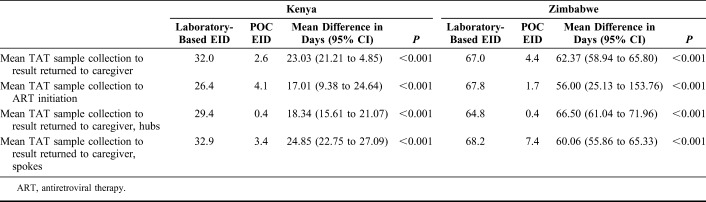
The mean TAT from sample collection to caregiver receipt of results was 32.0 days with laboratory-based and 2.6 days with POC testing in Kenya. In Zimbabwe, the mean TAT from sample collection to caregiver receipt of results was 67.0 with laboratory-based and 4.4 days with POC testing. POC EID significantly reduced the time between sample collection and return of infant HIV test results to caregiver by an average of 23.0 days (95% CI: 21.2 to 24.9, P < 0.001) in Kenya and 62.4 days (95% CI: 58.9 to 65.8, P < 0.001) in Zimbabwe (Table 3). For both the proportion of tests results returned to caregiver by 12 weeks of infant age and the TAT from sample collection to caregiver receipt of results, there was some heterogeneity among clusters and between countries when using laboratory-based EID testing, but very little heterogeneity among clusters and between countries under POC EID (see Figures 1 and 2, Supplemental Digital Content, http://links.lww.com/QAI/B466).
TABLE 3.
Mean Turnaround Times in Days at Key Steps Along the EID Care Cascade
Outcomes were also analyzed separately for hubs and spokes in each country. In Kenya, infants tested with POC were 3.16 (95% CI: 2.46 to 3.87, P < 0.001) and 1.71 (95% CI: 0.21 to 3.22, P = 0.026) times more likely to receive results by age 12 weeks than those tested with laboratory-based methods, in spoke and hub sites, respectively (Table 2). The TAT from sample collection to result receipt by caregiver was 24.9 (95% CI: 22.8 to 27.1, P < 0.001) and 18.3 (95% CI: 15.6 to 21.1, P < 0.001) days shorter with POC compared with laboratory-based testing in spokes and hubs, respectively. In Zimbabwe, infants tested with POC were 4.5 (95% CI: 4.1 to 4.9, P < 0.001) and 5.1 (95% CI: 4.5 to 5.8, P < 0.001) times more likely to receive results by age 12 weeks than those tested with laboratory-based methods, in spoke and hub sites, respectively. The TAT to caregiver was 60.1 (95% CI: 55.9 to 65.3, P < 0.001) and 66.5 (95% CI: 61.0 to 72.0, P < 0.001) days shorter with POC compared with laboratory-based testing in spokes and hubs, respectively (Table 3).
Overall, HIV-infected infants from both Kenya and Zimbabwe were 2.4 (95% CI: 0.80 to 4.1, P = 0.004) times more likely to be initiated on ART within 60 days of sample collection with POC as compared to laboratory-based methods. However, when analyzing the results by country, only the difference in Zimbabwe was significant (Table 2). In Kenya, the mean (SD) TAT from sample collection to infant ART initiation was 26.6 days (SD = 14.1) with laboratory-based and 4.2 days (SD = 5.6) with POC. In Zimbabwe, the mean TAT from sample collection to infant ART initiation was 67.9 days (SD = 51.8) with laboratory-based and 1.4 days (SD = 2.1) with POC. For HIV-infected infants, POC EID significantly reduced the time between sample collection and infant ART initiation by an average of 17.0 days (95% CI: 9.4 to 24.6, P < 0.001) in Kenya and 56.0 days (95% CI: 25.1 to 153.8, P < 0.001) in Zimbabwe (Table 3).
DISCUSSION
This large, 2-country study comparing the use of POC and laboratory-based EID provides compelling evidence that POC significantly improves timely return of EID results and ART initiation. Caregivers were 1.29 times as likely to receive infant EID results in Kenya and 4.56 times more likely to receive infant EID results in Zimbabwe by 12 weeks of age using POC compared with centralized laboratory testing. In particular, the evidence that POC leads to improved ART initiation for HIV-infected infants is likely to lead to reduced morbidity and mortality, especially for infants who are infected in utero or peripartum. The impact is further evident where the baseline percent of infected infants initiating on treatment quickly is low. Previous modeling work supports this hypothesis and has demonstrated that POC versus laboratory-based testing improves life expectancy by 2.8 years for HIV-infected infants.15
This evidence is consistent with previous studies such as those by Jani et al in Mozambique.13,21 The current study adds important information and generalizability as it was performed in 2 countries with mature EID systems and, especially in the case of Kenya, strong centralized laboratory systems. Kenya's stronger laboratory system and greater number of spoke sites per hub likely contributed to the lower turnaround time both using conventional and POC EID, as compared to Zimbabwe. Despite the strength and investment in the centralized laboratory systems, POC still significantly improved key EID outcomes, such as TAT to ART initiation. In addition, this study confirms previous programmatic findings that POC EID is feasible in larger-scale routine use.12,22
Our study also confirmed programmatic data supporting the use of hub-and-spoke models for POC EID. Use of a hub-and spoke model increases access to POC EID and, in most cases, should be able to contain costs associated with POC platform procurement and maintain operator proficiency by keeping POC platforms only at higher-volume sites. Despite POC platforms not being directly on-site at spoke sites, this study demonstrates that the significant improvements produced by POC EID in the percent of results returned by 12 weeks of life and TAT from sample collection to caregiver receipt of results are maintained at both hub and spoke sites.
Strengths of this study include its multicountry nature, inclusion of different health facility levels (including primary care), and the large sample size of both number of health facilities and HEI enrolled. In addition, this study added data to support a pragmatic model using short-haul hub-and-spoke networks and existing transport networks without compromising impact. A longitudinal follow-up of HIV-infected infants identified during this study is on-going and will add additional evidence about the impact of POC EID on retention on ART treatment.
The study had several limitations. As the study relied on routinely collected data from health care workers, some data quality errors were inevitable. However, there were very few missing data and extensive and frequent data quality checks minimized the potential for errors. There were fewer POC tests in Zimbabwe as compared to laboratory-based tests. Owing to the stepped-wedge nature of the design, most POC tests were performed in the later period of the study. Seasonal variations in testing demand may have contributed to this imbalance in testing. However, the reasons for this decrease in testing demand in the POC arm are not clear. Health workers recorded data in different paper-based records under the centralized laboratory and POC steps; in the centralized laboratory step, a standard EID register or test request form was used, while in the POC step, a specific POC EID test request form was used. Both records included the same general variables, although the format and some wording were different which may have introduced bias if one record was easier to fill out than the other. However, EGPAF provided training and support to improve record keeping in both centralized laboratory and POC steps. In addition, the low numbers of missing data suggest that both types of records were acceptable and well-used by health workers. Because each facility developed individual messaging on when caregivers should return for results, there may have been variation in the turnaround times between facilities, and results may have been available before caregivers returned. Finally, although the overall sample size was large, due to the success of prevention of mother-to-child transmission, the absolute number of HIV-infected infants was relatively small, limiting the power to perform subanalyses such as the percent of HIV-infected infants initiating ART by 60 days from diagnosis in hub versus spoke sites.
CONCLUSIONS
POC EID has clear benefit, even where there have been efforts to strengthen availability of centralized testing labs, sample referral network, and online result transmission such as in Kenya. In the many countries where the laboratory system more closely resembles that of Zimbabwe, the conferred advantage of POC testing is even more dramatic. POC EID offers significantly improved care for HEI and HIV-infected infants, and countries should consider the strategic addition of POC testing to their diagnostic algorithms and health facilities to improve EID.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank EGPAF POC EID Study Teams for their work on the day-to-day implementation of the POC EID project including but not limited to ensuring the collection of complete and high-quality data. The authors thank Cephas Muchuchuti, Jared Onsase, and Stephen Siamba for data management assistance. The authors thank the Ministries of Health in Kenya and Zimbabwe for their patience, collaboration, and support for the project. The authors thank the infants and caregivers involved in this project evaluation. The authors thank Dr. Lynne Mofenson for reviewing a final draft of this article.
Footnotes
Funded and supported by Unitaid, Geneva, Switzerland.
Preliminary results of this study were presented at the Conference on Retroviruses and Opportunistic Infections; March 11, 2020; Boston, MA.
The authors have no conflicts of interest to disclose.
E.S., R.M., and J.C. developed the study design, supervised data collection, and led statistical analysis. J.-F.L. and F.B. designed and oversaw the data monitoring systems. B.O., H.M., A.C., and C.O. oversaw data collection in Kenya and Zimbabwe, with the support of R.M., G.G., A. Mahomva, and A. Mushavi. E.S. and J.C. drafted the manuscript. All authors collaborated on key decisions throughout the course of the writing and review, provided critical feedback on preliminary drafts and interpretation of results, and approved the final manuscript.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
*E.S. and J.C. contributed equally.
REFERENCES
- 1.UNAIDS. Global AIDS Update. 2019. Available at: https://www.unaids.org/sites/default/files/media_asset/2019-global-AIDS-update_en.pdf. Accessed January 1, 2020. [Google Scholar]
- 2.Bourne DE, Thompson M, Brody LL, et al. Emergence of a peak in early infant mortality due to HIV/AIDS in South Africa. AIDS. 2009;23:101–106. [DOI] [PubMed] [Google Scholar]
- 3.Newell ML, Coovadia H, Cortina-Borja M, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. [DOI] [PubMed] [Google Scholar]
- 4.Violary A, Cotton MF, Gibb DM, et al. Early antiviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV infection. Recommendations for a Public Health Approach. 2016. Available at: https://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed January 1, 2020. [PubMed] [Google Scholar]
- 6.World Health Organization. Recommendations on the Diagnosis of HIV Infection in Infants and Children, 2010. Available at: http://www.who.int/hiv/pub/paediatric/diagnosis/en/. Accessed January 1, 2020. [PubMed] [Google Scholar]
- 7.Manumbu S, Smart LR, Mwale A, et al. Shortening turnaround times for newborn HIV testing in rural Tanzania: a report from the field. PLoS Med. 2015;12:e1001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phiri NA, Lee HY, Mtika C, et al. Early infant diagnosis and outcomes in HIV-exposed infants at a central and district hospital, northern Malawi. Pub Health Action. 2017;7:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tiam A, Gill MM, Hoffman HJ, et al. Conventional early infant diagnosis in Lesotho from specimen collection to results usage to manage patients: where are the bottlenecks? PLoS One. 2017;12:e0184769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carmona S, Wedderburn C, Macleod W, et al. Field performance of point-of-care HIV testing for early infant diagnosis: pooled analysis from six countries from the EID Consortium. Abstract #10602. Presented at: International AIDS Conference; 2016; Durban, South Africa.
- 11.Essajee S, Vojnov L, Penazzato M, et al. Reducing mortality in HIV-infected infants and achieving the 90-90-90 target through innovative diagnosis approaches. J Int AIDS Soc. 2015;18(suppl 6):20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunning L, Hsaio N, Myer L. Point-of-care HIV early infant diagnosis: is test sensitivity everything? J Int AIDS Soc. 2015;18:20235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. WHO Prequalification of in Vitro Diagnostics Public Report: Product: m-PIMA HIV-1/2 Detect. 2016. Available at: http://www.who.int/diagnostics_laboratory/evaluations/pq-list/hiv-vrl/160902_amended_public_report_0226_032_00_alere_hiv_detect_v3.pdf. Accessed January 1, 2020. [Google Scholar]
- 14.WHO. WHO Prequalification of in Vitro Diagnostics Public Report: Product: Xpert HIV-1 Qual Assay. 2016. Available at: https://www.who.int/diagnostics_laboratory/evaluations/pq-list/hiv-vrl/160613PQPublicR eport_0259-0700-00_XpertQualHIV_v2.pdf. Accessed January 1, 2020. [Google Scholar]
- 15.Bianchi F, Cohn J, Sacks E, et al. Closing the diagnostic gap in pediatric HIV using routine point-of-care for early infant diagnosis: results from an eight-country intervention evaluation. Lancet HIV. 2019;6:PE373–E381. [DOI] [PubMed] [Google Scholar]
- 16.Jani I, Meggi B, Loquiha O. Effects of point-of-care testing on antiretroviral-therapy initiation rates in infants. Presented at: Conference on Retroviruses and Opportunistic Infections; Februaru 13–16, 2017; Seattle, WA.
- 17.Mwenda R, Fong Y, Magobo T, et al. Significant patient impact observed upon implementation of point-of-care early infant diagnosis technologies in an observational study in Malawi. Clin Inf Dis. 2018;67:701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank S, Cohn J, Dunning L, et al. Clinical impact and cost-effectiveness of incorporating point-of-care (POC) assays into early infant HIV diagnosis (EID) programs at 6 Weeks of age in Zimbabwe: a model-based analysis. Lancet HIV. 2019;6:e182–e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.UNAIDS. The Global Plan Towards the Elimination of New HIV Infections Among Children by 2015 and Keeping Their Mothers Alive. Prepared for the iERG. 2015. Available at: https://www.who.int/woman_child_accountability/ierg/reports/UNAIDS_submission_iERG_2015.pdf. Accessed January 1, 2020. [Google Scholar]
- 20.Hemming K, Haines TP, Chilton JP, et al. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ. 2019;6:e182–e190. [DOI] [PubMed] [Google Scholar]
- 21.Jani I, Meggi B, Loquiha O. Effect of point-of-care early infant diagnosis on antiretroviral therapy initiation and retention of patients. AIDS. 2018;32:1453–1463. [DOI] [PubMed] [Google Scholar]
- 22.Essajee S, Vojnov L, Penazzato M, et al. Reducing mortality in HIV-infected infants and achieving the 90–90–90 target through innovative diagnosis approaches. J Int AIDS Soc. 2015;18:20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.