Abstract
Background
Most studies have not demonstrated improved survival after prenatal diagnosis of critical congenital heart disease, including hypoplastic left heart syndrome (HLHS). However, the effect of delivery near a cardiac surgical center (CSC), the recommended action after prenatal diagnosis, on HLHS mortality has been poorly investigated.
Methods and Results
Using Texas Birth Defects Registry data, 1999-2007, which monitored >3.4 million births, we investigated the association between distance (calculated driving time) from birth center to CSC and neonatal mortality in 463 infants with HLHS. Infants with extracardiac birth defects or genetic disorders were excluded. The associations between prenatal diagnosis, CSC HLHS volume, and mortality were also examined. Neonatal mortality in infants born <10 minutes from a CSC was 21.0%, 10-90 minutes 25.2%, and >90 minutes 39.6% (p for trend <0.001). Prenatal diagnosis alone was not associated with improved survival (p=0.14). In multivariable analysis, birth >90 minutes from a CSC remained associated with increased mortality, OR 2.03 (95%CI 1.19-3.45), compared to <10 minutes. In subanalysis, birth >90 minutes from a CSC was associated with higher pre-transport mortality (OR 6.69, 95%CI 2.52-17.74) and birth 10-90 minutes with higher pre-surgical mortality (OR 4.45, 95%CI 1.17-17.00). Higher surgical mortality was associated with lower CSC HLHS volume (OR per 10 patients 0.88, 95%CI 0.84-0.91).
Conclusions
Infants with HLHS born far from a CSC have increased neonatal mortality, and most of this mortality is pre-surgical. Efforts to improve prenatal diagnosis of HLHS and subsequent delivery near a large volume CSC may significantly improve neonatal HLHS survival.
Journal Subject Codes: Etiology:[8] Epidemiology, Diagnostic testing:[31] Echocardiography, Cardiovascular (CV) surgery:[41] Pediatric and congenital heart disease, including cardiovascular surgery, Ethics and policy:[100] Health policy and outcome research, Treatment:[25] CPR and emergency cardiac care
Keywords: hypoplastic left heart syndrome, prenatal diagnosis, heart defects, congenital, mortality, population studies, fetal echocardiography, hospital performance, access to care, Stage I palliation
Introduction
Hypoplastic left heart syndrome (HLHS) and other congenital heart diseases are leading causes of infant mortality in the United States (U.S.). 1-3 Despite improved survival due to operative palliation and advances in postoperative care in recent decades, mortality in infants with HLHS remains high.4.
Potentially, prenatal diagnosis and planned delivery near a cardiac surgical center (CSC) should allow prompt stabilization measures and preclude neonatal deterioration.5 Nonetheless, most studies indicate that prenatal diagnosis of HLHS does not improve survival.4,6-13 Many of these studies, however, did not include infants who died before transfer or before surgery and were limited to large volume hospitals. None examined the actual importance of delivery location after prenatal diagnosis. Thus, a child diagnosed prenatally but delivered far from a CSC may be at greater risk, due to the complexity of transfer, than a child diagnosed postnatally but delivered near a CSC, where prompt, effective care is available. We hypothesized that infants with HLHS delivered close to a CSC would have lower neonatal mortality than those delivered at greater distances.
We used the Texas Department of State Health Services’ Texas Birth Defects Registry (TBDR) to investigate the association between distance to a CSC at birth and neonatal mortality in a large group of socioeconomically and racially diverse infants with HLHS. We also investigated the associations among prenatal diagnosis, CSC HLHS volume, and neonatal mortality.
Methods
Patient Population and Study Design
We conducted a retrospective cohort study to evaluate if increased travel time between the birth location and a CSC was associated with increased neonatal mortality among infants with HLHS. This study used data from the TBDR, which has conducted active, statewide surveillance since 1999. Registry staff review records of all delivery units and pediatric hospitals throughout Texas. They abstract detailed demographic and diagnostic information for infants with a registry-monitored birth defect diagnosed within 1 year of delivery, to Texas-resident mothers. TBDR data are routinely linked to Texas vital records.
The TBDR classifies congenital anomalies using 6-digit birth defect codes that are based on the British Pediatric Association (BPA) Classification of Diseases (1979) and the International Classification of Diseases, ninth revision, clinical modification (1979). We included live births during January 1, 1999 -December 31, 2007 with the codes for HLHS (746.700), aortic atresia (746.480), or hypoplastic left ventricle (746.881). To ensure accurate diagnosis, a pediatric cardiologist (S.A.M.) reviewed available TBDR clinical data. A diagnosis of HLHS required at least one of the following:
Severe mitral and aortic obstruction, hypoplasia, or atresia and a hypoplastic left ventricle
“Hypoplastic left heart syndrome” diagnosed on echocardiography, cardiac catheterization, cardiac surgical, or pathologic report
Left ventricular hypoplasia with evidence of Stage 1 palliation and no more appropriate diagnosis
We excluded infants with alternative primary cardiac diagnoses, including atrioventricular septal defect and double outlet or double inlet ventricle. We also excluded twins and infants with gestational age <23 weeks, birthweight <400 grams, or genetic disorders. Patients with either major extracardiac birth defects or more than 3 minor extracardiac birth defects were also excluded. Extracardiac birth defects are carefully catalogued by the TBDR. A major extracardiac birth defects was defined by the National Birth Defects Prevention Network as a congenital abnormality that requires medical or surgical treatment, has a serious adverse effect on health and development, or has significant cosmetic impact.14
The study was approved by the Internal Review Boards of Baylor College of Medicine, the Texas Department of State Health Services, and the University of South Florida.
Study Variables
Predictor Variable: Driving Time to a Cardiac Surgical Center
The shortest driving time in minutes between the birth hospital and the infant’s CSC was calculated using geomapping software (CDX technologies and Microsoft MapPoint). Although the actual driving time may be slightly different than calculated, given route changes over years and changes throughout a 24-hour period, average daily driving times were used to minimize this variation. Driving time was used instead of distance in miles to a give a more representative picture of possible delays to a CSC, as distances do not account for differences in speed permitted, urban versus rural travel, or volume of traffic.
A CSC was defined as a hospital that performed >1 Stage 1 palliation (S1P) during the study period. When the CSC was not clearly identified or death occurred before transfer, either the birth hospital’s usual referral CSC or, in the absence of a referral system, the closest CSC was used. Two infants had no birth hospital listed, and their first echocardiograms were performed 4 and 6 days after birth. Thus, their home addresses were used.
Time to CSC was divided into three predetermined groups: <10, 10-90, and >90 minutes. Ten minutes was chosen as a cutoff to separate those delivering close versus not close to a CSC. Ninety minutes was chosen as a cutoff at which transport via air ambulance may be considered.15 The division of travel time into three categories was also performed to minimize the effect of subtle changes in travel time that may have occurred over the course of the study.
Covariates
Maternal socioeconomic status (SES) was estimated from two widely used, validated proxies of SES: education level and percent of population living in poverty in the maternal census tract, according to the 2000 U.S. census.16,17 When a post office box or a non-mappable rural route was the residential address listed, the street or city was used. For categorical analysis of poverty, a cutoff of ≥20% was used in accordance with the U.S. Census Bureau.18.
Preterm birth (PTB) was defined as <37 weeks, low birthweight (LBW) as <2500 grams, and small for gestational age as <10% of expected weight using published criteria19. Birth era was divided into 1999-2002 and 2003-2007. Presence of total anomalous pulmonary venous return and highly restrictive atrial septal defect/intact atrial septum (RAS) also were noted. As RAS information was not available in approximately 25% of infants, it was not included in primary analyses. For secondary analyses, RAS was coded as “present,” “absent,” or “unknown”. The “present” RAS group included those with an atrial septum described as “severely restrictive,” “intact,” or who underwent catheter-based atrial septal intervention. Those with “absent” RAS were those with an atrial septum described as “unrestrictive,” other similar language, or who had no atrial septal descriptors in the echocardiography report but were described throughout the record as clinically stable with no catheter-based atrial septal intervention and Stage 1 palliation (S1P) >3 days of life. All others were included in the “unknown” group, including those with records using only the term “restrictive” atrial septum on the echocardiography report, given wide variation in the use of “restrictive.” CSC HLHS case volume was calculated from the total number of neonates with HLHS presenting to a given CSC, as actual surgical volume was not reliably available in the registry.
Ascertainment of Mortality
Neonatal mortality (death before age 28 days) was chosen as the primary outcome to minimize the competing effects of SES and access to healthcare on post-discharge mortality. Infants were classified as deceased based on the TBDR and Texas vital records. Neonatal death was categorized according to whether it occurred before transfer to a CSC; after arrival at a CSC but before S1P; or after S1P. CSC HLHS volume was used as a variable in the latter two analyses, so only infants with an identifiable CSC were included.
To also investigate the relationship between time to CSC and mortality, three intermediate outcomes were studied: (1) age at diagnosis, using the first abstracted postnatal confirmation of HLHS (typically an echocardiogram); (2) pre-surgical decompensation, determined from review of abstracted data for key phrases (Appendix); and (3) age at S1P.
Statistical Analysis
Univariable comparisons were performed using the Wilcoxon rank sum or Kruskal-Wallis analysis for continuous variables, and Chi-square, Fisher exact, or Chi-square trend analyses for categorical data. For associations with categorical variables with more than two classes, multiple comparisons were adjusted using Bonferroni’s correction. Correlations were calculated using Spearman’s rho.
A multivariable logistic regression model was computed for neonatal mortality outcomes. In the primary analysis, all variables were considered for inclusion if p<0.10 in association with mortality.
For subanalyses evaluating timing of death, the variables noted to be significant in association with overall neonatal mortality were included in the models. For the subanalyses of death prior to surgery (after arrival at a CSC) and death after surgery, which included CSC HLHS volumes in the models, generalized estimating equation models were computed to accommodate correlation between patients at the same center. P values <0.05 were considered statistically significant.
Results
Of 3,401,057 live births during the study period, 558 met criteria for HLHS (birth prevalence, 1.6 per 10,000 live births). Thirteen were from a twin gestation, and 82 had genetic conditions or extracardiac anomalies, leaving 463 cases for analysis. During 1999-2007, nine Texas CSCs performed S1P.
Time to CSC
Time from birthplace to CSC ranged from 0-577 minutes: 210 newborns (45.4%), <10 minutes; 147 (31.7%), 10-90 minutes; and 106 (22.9%), >90 minutes. Mothers delivering farther from a CSC were less educated and more often lived in a poverty area (Table 1). Thirty percent of mothers delivering >90 minutes from a CSC were born in Mexico, compared to 17% of those delivering <90 minutes from a CSC. Very few black mothers delivered >90 minutes from a CSC.
Table 1.
Maternal/Infant Characteristics and Intermediate Outcomes by Calculated Driving Time to Cardiac Surgical Center among infants with Hypoplastic Left Heart Syndrome, Texas, 1999-2007
| All N= 463 |
<10 Minutes N=210 |
10-90 Minutes N=147 |
>90 minutes N=106 |
P-value | |
|---|---|---|---|---|---|
| Maternal/Child Characteristics | |||||
| Preterm – no. (%) | 59 (13%) | 22 (10%) | 18 (12%) | 19 (18%) | 0.07 |
| Birthweight <2500 g – no. (%) | 50 (11%) | 23 (11%) | 12 (8%) | 15 (14%) | 0.54 |
| SGA – no. (%) | 67 (14%) | 32 (15%) | 18 (12%) | 17 (16%) | 0.99 |
| Male sex – no. (%) | 303 (65%) | 146 (70%) | 90 (61%) | 67 (63%) | 0.18 |
| Maternal race/ethnicity – no. (%) | <0.001* | ||||
| White | 208 (45%) | 89 (42%) | 77 (52%) | 42 (40%) | |
| Black | 46 (10%) | 28 (13%) | 17 (12%) | 1 (1%) | |
| Hispanic | 199 (43%) | 87 (41%) | 50 (34%) | 62 (58%) | |
| Other | 10 (2%) | 6 (3%) | 3 (2%) | 1 d%) | |
| Median maternal age - years (IQR) | 26 (22, 31) | 27 (21, 32) | 26 (22, 30) | 25 (21,29) | 0.28 |
| Maternal education – no. (%) | 0.01 | ||||
| >12th grade | 176 (39%) | 90 (44%) | 53 (36%) | 33 (31%) | |
| 12th grade | 153 (34%) | 68 (33%) | 49 (34%) | 36 (34%) | |
| <12th grade | 127 (28%) | 47 (23%) | 44 (30%) | 36 (34%) | |
| Poverty by census tract ≥20% – no. (%) | 144 (31%) | 55 (26%) | 27 (18%) | 62 (58%) | <0.001 |
| Maternal birth country – no. (%) | 0.005* | ||||
| US | 337 (74%) | 149 (73%) | 115 (78%) | 73 (70%) | |
| Mexico | 91 (20%) | 37 (18%D | 23 (16%) | 31 (30%) | |
| Other | 28 (6%) | 18 (9%) | 9 (6%) | 1 d%) | |
| Median birth year (IQR) | 2003 (2001,2005) |
2004 (2002,2006) |
2003 (2001,2005) |
2002 (2000,2005) |
<0.001 |
| Birth era – no. (%) | <0.001 | ||||
| 1999-2002 | 200(43%) | 72 (34%) | 71 (48%) | 57 (54%) | |
| 2003-2007 | 263 (57%) | 138 (66%) | 76 (52%) | 49 (46%) | |
| Prenatal diagnosis – no. (%) | 180 (39%) | 138 (66%) | 28 (19%) | 14(13%) | <0.001 |
| TAPVR – no. (%) | 15 (3%) | 7 (3%) | 2 (1%) | 6 (6%) | 0.51 |
| Intermediate Outcomes | |||||
| Age at diagnosis – no. (%) | <0.001 | ||||
| <1 day | 260 (58%) | 157 (77%) | 58 (42%) | 45 (43%) | |
| 1-2 days | 134 (30%) | 36 (18%) | 53 (38%) | 45 (43%) | |
| >2 days | 52 (12%) | 10 (5%) | 27 (20%) | 15 (14%) | |
| Pre-surgical decompensation – no. (%) | 35 (8%) | 11 (5%) | 11 (7%) | 13 (12%) | 0.03 |
| Median age at surgery - days (IQR) | 5 (3,8) | 5 (2,8) | 6 (4,8) | 7 (4,10) | 0.02 |
SGA: small for gestational age. IQR: interquartile range (25th percentile, 75th percentile). TAPVR: total anomalous pulmonary venous return.
Significant difference overall noted in multi-category group; for maternal race/ethnicity: <10 vs. 10-90 minutes p=0.32, <10 vs. >90 minutes p<0.001, 10-90 vs. >90 p<0.001. For maternal birth country: <10 vs. 10-90 minutes p=0.52, <10 vs. >90 minutes p=0.002, 10-90 vs. >90 minutes p=0.005.
Intermediate outcomes reported only for cases in which sufficient data were available.
Prenatal Diagnosis
HLHS was prenatally diagnosed in 39% of newborns, with a higher proportion diagnosed prenatally in the later birth era (49.0% versus 25.5%, p<0.001). Prenatally diagnosed infants were delivered significantly closer to a CSC, with median time to CSC 2.6 minutes (interquartile range, IQR 1.2,9.9 minutes) versus 28.8 minutes (IQR 10.0,136.3 minutes, p<0.001).
Neonatal mortality
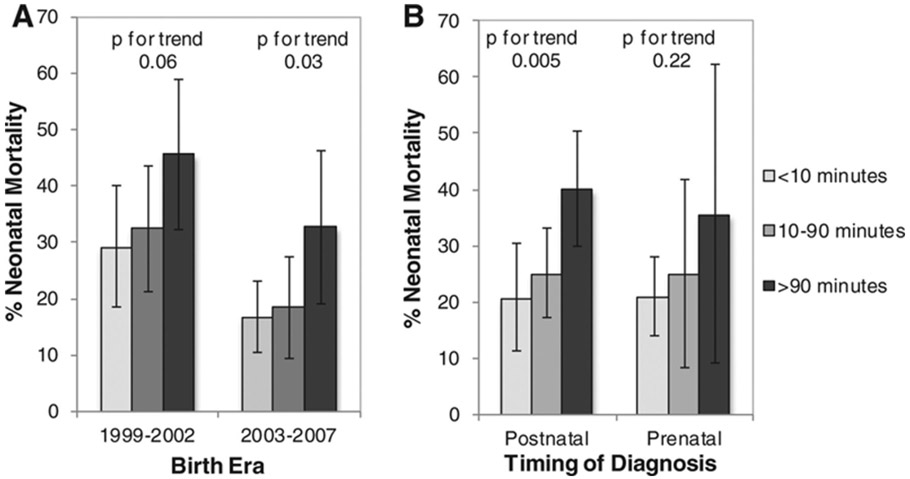
Death before age 28 days occurred in 123 infants (26.6%). Mortality was higher in the earlier birth era (Table 2). In crude analysis, increased time to a CSC was associated with increased mortality: <10 minutes, 21.0%; 10-90 minutes, 25.2%; and >90 minutes, 39.6% (p for trend <0.001, Table 2). This pattern was evident in both birth eras and in both prenatally and postnatally diagnosed infants (Figure 1). In infants diagnosed prenatally, mortality was 22.8% compared to postnatally, 29.0% (p=0.14) (Table 2).
Table 2.
Maternal/Infant Characteristics and Intermediate Outcomes by Neonatal Mortality among infants with Hypoplastic Left Heart Syndrome, Texas, 1999-2007
| Survivors N= 340 |
Non-Survivors N= 123 |
P-value | |
|---|---|---|---|
| Characteristics | |||
| Median time to CSC - minutes (IQR) | 12 (2, 56) | 22 (3, 142) | 0.001 |
| Time to CSC – no. (%) | <0.001 | ||
| <10 minute | 166 (49%) | 44 (36%) | |
| 10-90 minutes | 110 (32%) | 37 (30%) | |
| >90 minutes | 64 (19%) | 42 (34%) | |
| Preterm – no. (%) | 34 (10%) | 25 (20%) | 0.003 |
| Birthweight <2500g – no. (%) | 28 (8%) | 22 (18%) | 0.003 |
| SGA – no. (%) | 46 (14%) | 21 (17%) | 0.34 |
| Male sex – no. (%) | 230 (68%) | 73 (59%) | 0.10 |
| Maternal race/ethnicity – no. (%) | 0.29 | ||
| White | 158 (46%) | 50 (41%) | |
| Black | 34 (10%) | 12 (10%) | |
| Hispanic | 143 (42%) | 56 (46%) | |
| Other | 5 (1%) | 5 (4%) | |
| Median maternal age - years (IQR) | 26 (22, 31) | 25 (21,30) | 0.30 |
| Maternal education – no. (%) | 0.49 | ||
| >12th grade | 129 (38%) | 47 (40%) | |
| 12th grade | 110 (33%) | 43 (36%) | |
| <12th grade | 98 (29%) | 29 (24%) | |
| Poverty by census tract ≥20% – no. (%) | 102 (30%) | 42 (34%) | 0.40 |
| Maternal birth country – no. (%) | 0.69 | ||
| US | 252 (75%) | 85 (71%) | |
| Mexico | 64 (19%) | 27 (23%) | |
| Other | 21 (6%) | 7 (6%) | |
| Median birth year (IQR) | 2004 (2002,2006) | 2002 (2000,2005) | <0.001 |
| Birth era – no. (%) | <0.001 | ||
| 1999-2002 | 130 (38%) | 70 (57%) | |
| 2003-2007 | 210 (62%) | 53 (43%) | |
| Prenatal diagnosis – no. (%) | 139 (41%) | 41 (33%) | 0.14 |
| TAPVR – no. (%) | 10 (3%) | 5 (4%) | 0.56 |
| Intermediate Outcomes | |||
| Age at Diagnosis – no. (%) | 0.84 | ||
| <1 day | 187 (58%) | 73 (59%) | |
| 1-2 days | 101 (31%) | 33 (27%) | |
| >2 days | 35 (11%) | 17 (14%) | |
| Pre-surgical decompensation – no. (%) | 12 (4%) | 23 (19%) | <0.001 |
| Median age at surgery - days (IQR) | 5 (3,8) | 5 (3,10) | 0.96 |
CSC: cardiac surgical center. SGA: small for gestational age. IQR: interquartile range (25th percentile, 75th percentile). TAPVR: total anomalous pulmonary venous return. Intermediate outcomes reported only for cases in which sufficient data were available.
Figure 1.
Neonatal mortality of infants with hypoplastic left heart syndrome by driving time from birth location to cardiac surgical center, among birth eras (A) and with prenatal versus postnatal diagnosis (B). Comparison of mortality among birth eras, accounting for time to CSC, p=0.002. Comparison of mortality by prenatal diagnosis, accounting for time to CSC, p=0.90. Error bars represent 95% confidence intervals.
In multivariable analysis, factors significantly associated with neonatal mortality were time to CSC, LBW, and earlier birth year (Table 3). PTB was not included in the multivariable model because of its high correlation with LBW.
Table 3.
Multivariable Logistic Regression Models for Overall Neonatal Mortality and Timing-Specific Neonatal Mortality among Infants with Hypoplastic Left Heart Syndrome, Texas, 1999-2007.
| Characteristic | Neonatal Death N=123/463 |
Pre-Transfer Death N=30/463 |
Pre-Stage 1 Death after arrival at CSC N=15/384† |
Stage 1 Death N=52/369 |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Time to CSC | ||||
| <10 minutes | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 10-90 minutes | 1.19 (0.71-1.99) | 0.94 (0.26-3.44) | 4.45 (1.17-17.00) | 0.97 (0.41-2.30) |
| >90 minutes | 2.03 (1.19-3.45) | 6.69 (2.52-17.74) | 2.98 (0.81-10.95) | 1.11 (0.53-2.35) |
| Birthweight <2500g | 2.97 (1.57-5.62) | 4.83 (1.87-12.51) | 2.83 (0.96-8.37) | 1.67 (0.83-3.36 |
| Birth year | 0.83 (0.76-0.91) | 0.84 (0.71-0.99) | 0.97 (0.84-1.13) | 0.82 (0.69-0.96) |
| CSC HLHS volume, per 10 patients |
0.96 (0.90-1.02)* | 0.88 (0.84-0.91)* | ||
OR: odds ratio. CI: confidence interval. CSC: cardiac surgical center. HLHS: Hypoplastic left heart syndrome.
CSC HLHS Volume was included for latter two timing-specific mortality analyses using mixed generalized estimating equations models.
Denominator for the group of patients with pre-Stage 1 death is lower than all arriving at a CSC because only patients who’s CSC was clearly documented were included.
In sub-analyses that adjusted for RAS, the results for time to CSC were similar: 10-90 minutes odds ratio (OR) 1.23 (95% confidence interval: CI 0.73-2.08); >90 minutes, OR 1.97 (95% CI 1.14-3.38). The OR for RAS “present” versus “absent” was 4.19 (95% CI 1.92-9.12).
To account for the possible effect of parental intention not to treat, we excluded six infants who were diagnosed prenatally, born ≥10 minutes from a CSC, and never transferred. The results were similar: 10-90 minutes, OR 1.16 (95%CI 0.69-1.95); >90 minutes, OR 1.85 (95% CI 1.07-3.20).
Timing of Death
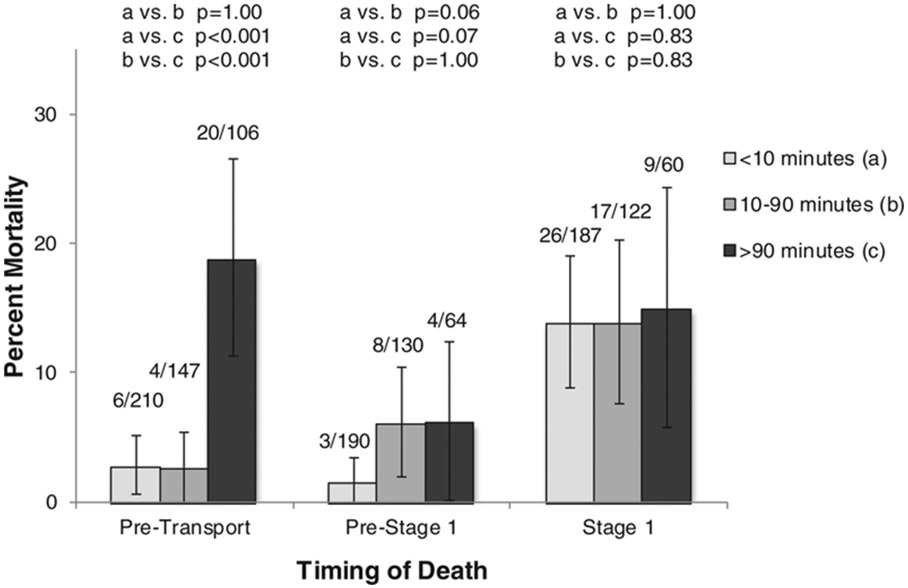
Thirty patients (6.5%) died before arriving at a CSC. Pre-transport mortality in infants delivered >90 minutes from a CSC was 18.9% compared to those born 10-90 minutes (2.7%, p<0.001) and <10 minutes (2.9%, p<0.001, Figure 2). In those prenatally diagnosed, delivery >90 minutes from a CSC was associated with significant pre-transport mortality (28.6%), compared to 7.1% in those born 10-90 minutes (p=0.06), and 2.2% in those born <10 minutes from a CSC (p<0.001).
Figure 2.
Pre-transport, Pre-Stage 1, and Stage 1 neonatal mortality in infants with hypoplastic left heart syndrome by driving time from birth location to cardiac surgical center. Error bars represent 95% confidence intervals.
In multivariable analysis, time to CSC, LBW, and birth year remained associated with pre-transport neonatal death (Table 3). When RAS was included, the results remained similar: 10-90 minutes, OR 0.94 (95% CI 0.26-3.47); >90 minutes, OR 6.26 (95% CI 2.35-16.70).
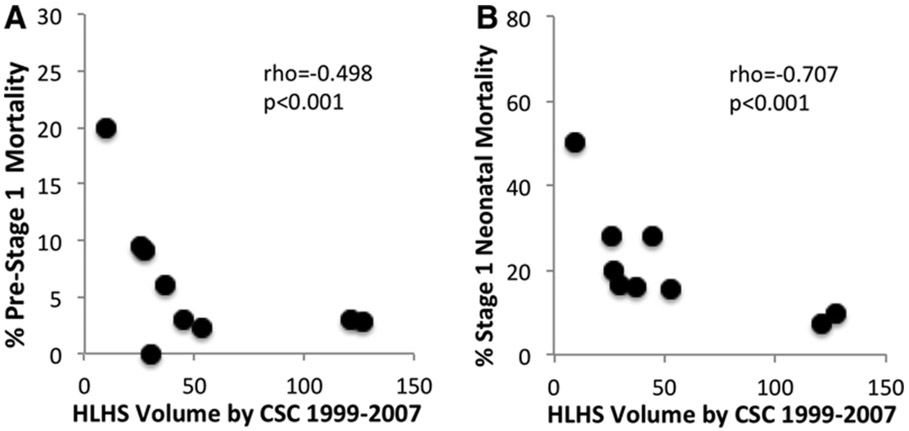
Of 433 newborns arriving at a CSC, 94 (21.7%) died, 15 prior to undergoing S1P, and 52 after S1P; 27 had insufficiently detailed hospital procedure information abstracted. For pre-surgical mortality analysis, 384 infants had sufficient information for classification. In those born <10 minutes from a CSC, 3/190 (1.6%) patients died before surgery, compared to 8/130 (6.2%) in those born 10-90 minutes, and 4/64 (6.3%) in those born >90 minutes (Figure 2). CSC HLHS volume was inversely correlated with CSC pre-Stage 1 mortality (Spearman rho=−0.498, p<0.001, Figure 3A). In the final multivariable model, time to CSC 10-90 minutes was significantly associated with increased pre-Stage I mortality, while there was a trend towards increased mortality in patients delivered >90 minutes from a CSC and those cared for in lower volume CSCs (Table 3). When RAS was included, the results were similar: 10-90 minutes, OR 3.43 (95% CI 0.96-12.33); >90 minutes, OR 4.44 (95% CI 1.12-17.57).
Figure 3.
A. Percent pre-Stage 1 mortality in infants with hypoplastic left heart syndrome (HLHS) after arrival at a cardiac surgical center (CSC), by CSC, with HLHS volume (cases) on the x-axis, and mortality on the y-axis. B. Percent Stage 1 neonatal mortality by CSC, with HLHS volume on the x-axis, and mortality on the y-axis.
Three infants never underwent S1P but were alive at 28 days and ultimately died, one underwent S1P in another state, two were referred out of state for a primary cardiac transplant, and one had no detailed hospital information abstracted and died at 40 days of life.
Evidence of S1P at an identifiable CSC existed for 369 infants. CSC HLHS volume was inversely correlated with S1P mortality (Spearman rho=−0.707, p<0.001, Figure 3B). Only low HLHS volume and earlier birth year remained associated with higher S1P mortality in multivariable regression (Table 3), even when RAS was included.
Mechanism of Effect of Increased Time to CSC and Mortality
We assessed intermediate outcomes including age at diagnosis (in most cases, time to first echocardiogram), presurgical decompensation, and age at surgery. Age at diagnosis was available for 96% of infants. Although infants born further from a CSC were older at diagnosis (Table 1), older age at diagnosis was not associated with increased mortality (Table 2), even after restricting the analysis to postnatally diagnosed infants (p=0.50) or to no RAS (p=0.74).
Thirty-five infants had evidence of pre-surgical decompensation (Appendix). Infants born further away from a CSC had greater frequency of decompensation (Table 1), which was associated with increased mortality (Table 2) even after restricting to no RAS (p=0.03). Of note, almost two-thirds (n=22) of infants with decompensation were diagnosed at ≤2 days of life. Of these 22 patients, 2 of 8 (25%) born <10 minutes from a CSC died, while 5 of 5 (100%) patients born 10-90 minutes from a CSC died, and 8 of 9 (89%) patients born >90 minutes from a CSC died.
Age at surgery was available for 302 of 384 infants undergoing S1P. Infants born further from a CSC were older at surgery (Table 1), but this factor was not associated with mortality (Table 2), even after restricting to no RAS (p=0.84).
Discussion:
This population-based study is the first to examine the interactions among prenatal diagnosis, birth location, and CSC volume in determining neonatal survival in infants with HLHS. Our findings suggest that prenatal diagnosis may reduce mortality if mothers living far from a CSC deliver close to a CSC. Mortality was high in newborns diagnosed prenatally and born far from a CSC, whereas mortality in those diagnosed postnatally but delivered near a CSC was comparable to those prenatally identified born near a CSC. Specifically, we demonstrate that birth near a CSC results in lower pre-transport and pre-surgical mortality. This finding is paramount, as more than 36% of deaths in our cohort occurred prior to S1P.
Although longer driving time to a CSC was strongly associated with increased mortality in our cohort, we recognize that longer time to CSC may be associated with a more complex risk factor of limited access to care, including lack of prenatal diagnosis and delivery at institutions less experienced with HLHS.
Our study also confirmed the well-known association between CSC volume and S1P mortality.20-22 However, our results suggest that volume may also be inversely related to pre-surgical mortality. Although in multivariable analysis this association was not statistically significant, the unadjusted data were compelling and the analysis was likely underpowered given the small number of pre-surgical deaths after arrival at a CSC (n=15). If this association is present, it suggests that not only surgical experience but experience of the multidisciplinary cardiac team accounts for improved survival in these children. This association should be investigated further.
To date, there have been few modifiable risk factors shown to significantly affect mortality in HLHS. However, the findings in this study suggest that improved fetal detection of HLHS, planned delivery near a CSC, and referral for care at a high-volume institution have the potential to dramatically improve outcomes in HLHS. As an example, in the 2003-2007 cohort, for those with a prenatal diagnosis, born <10 minutes from a CSC, and cared for at a large volume CSC, neonatal mortality was 6.3%. For those born in the same era without a prenatal diagnosis, >10 miles from a CSC, and cared for at a low volume CSC, 28-day mortality was 28.9%.
Age at diagnosis (typically the first echocardiogram) was not associated with neonatal mortality. Although this finding is not initially intuitive, it emphasizes the importance of delivery at or near a CSC with a dedicated cardiac team that can provide immediate, effective resuscitation and treatment. Increased frequency of decompensation at increased distance from a CSC was the likely cause of increased mortality in our study, regardless of the age at diagnosis. Hence, improved prenatal diagnosis of HLHS with delivery close to a CSC may decrease neonatal mortality. Cost effectiveness analysis should be done to explore if universal transfer of mothers living far from a CSC to a nearby birth hospital is reasonable policy, especially in states with a large rural population.
Few studies have investigated the role of birth location in critical congenital heart disease (CHD). In 2000, Simpson, et al. compared neonatal physiology scores in infants with CHD born at their tertiary care facility with those born at community hospitals, and noted no statistically significant difference23. However, a wide variety of lesions, including low mortality lesions such as ventricular septal defect and atrioventricular septal defect, were included in the study. Bennet, et al. investigated the role of birth in a specialty vs. non-specialty hospital on mortality in children with duct-dependent lesions, and demonstrated no statistically significant difference24. However, only patients who underwent surgery were included. Our study is consistent with Bennett’s study in showing no statistically significant association between birth far from a CSC and mortality when the sample was limited to only those undergoing surgery.
Our study helps to explain why numerous studies have shown no effect of prenatal diagnosis. First, benefit accrues when prenatal awareness of the diagnosis results in delivery close to a CSC. Those who happened to deliver close may have attenuated the beneficial effect in prior studies. Second, the benefit of prenatal diagnosis is primarily pre-surgical. Survival bias (the sickest die before arriving and undergoing surgery) is likely present in the majority of prior studies.
Limitations
This study was limited by the use of abstracted registry data. However, this registry source is exceptional for its active surveillance for HLHS in all Texas delivery hospitals and pediatric hospitals and extensive data collection. A study of similar content and size cannot be prospectively accomplished.
The use of registry data did not allow us to distinguish families who chose nonintervention, which would result in death. If prenatally-diagnosed mothers chose more often to deliver >10 minutes from a CSC, those with longer times to CSCs would have increased mortality, as our data demonstrate. However, only 42 mothers with a prenatal diagnosis delivered >10 minutes from a CSC. Thirty-six of these infants were transferred to a CSC, suggesting that the families desired care. When excluding the other six children, the results were unchanged, confirming that this factor was not responsible for our results. Although families of infants diagnosed postnatally and born far from a CSC may have chosen non-intervention at a higher rate, that factor would not negate our findings, as differential counseling could serve as an additional mechanism of increased mortality with increased distance from a CSC.
Data on termination of pregnancy (TOP) was not included in this study. The TBDR does collect information on TOP in the facilities in which data are collected for births. Although this does not likely include all TOPs, only 0.71% of all 1999-2007 cases of HLHS in the TBDR, as defined by birth defect code 746.700, were induced terminations.25
Whereas the primary study variables, time to CSC and neonatal mortality, were universally available, some variables of interest were not, the most important of which was the presence of RAS. Hence, RAS was incorporated into the analyses secondarily and did not significantly alter the effect estimates of time to CSC. Medication use was not available in the birth defects registry, so time to prostaglandin (PGE) initiation was not evaluated in this study.
The findings of this study may not be applicable in other states or countries with less variation in geography or access to healthcare. However, most states in the United States have even a greater proportion of the population considered rural than Texas. Per the 2010 US Census, 35 states ranked above Texas in proportion of households living rurally.26. Therefore, we believe a significant proportion of infants with HLHS would benefit from prenatal diagnosis and relocating close to a high volume CSC.
Conclusions
Birth far from a CSC is associated with increased neonatal mortality in HLHS, as is care in a low-HLHS volume CSC. Efforts to improve prenatal diagnosis of HLHS and subsequent delivery near a large volume CSC should significantly improve neonatal HLHS survival. Given that the effect of distance on mortality appears to be at least partially due to differential ability to deal with the decompensating infant with HLHS, education of medical teams far from a CSC and advanced telemedicine also have the potential to improve neonatal outcomes.
Supplementary Material
Acknowledgments:
The authors thank the TBDR staff for excellent registry maintenance, Drs. Hugh D. Allen and B. Lee Ligon for manuscript editing, and the Texas Children’s Hospital Cardiovascular Clinical Research Core for overall research support.
Footnotes
Conflict of Interest Disclosures: None.
References:
- 1.Members Writing Group, Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee, On behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics--2012 Update: A Report From the American Heart Association. Circulation. 2012;125:e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boneva RS, Botto LD, Moore CA, Yang Q, Correa A, Erickson JD. Mortality Associated With Congenital Heart Defects in the United States : Trends and Racial Disparities, 1979–1997. Circulation. 2001;103:2376–2381. [DOI] [PubMed] [Google Scholar]
- 3.Kuehl KS, Loffredo CA, Ferencz C. Failure to diagnose congenital heart disease in infancy. Pediatrics. 1999;103:743–747. [DOI] [PubMed] [Google Scholar]
- 4.Feinstein JA, Benson DW, Dubin AM, Cohen MS, Maxey DM, Mahle WT, Pahl E, Villafañe J, Bhatt AB, Peng LF, Johnson BA, Marsden AL, Daniels CJ, Rudd NA, Caldarone CA, Mussatto KA, Morales DL, Ivy DD, Gaynor JW, Tweddell JS, Deal BJ, Furck AK, Rosenthal GL, Ohye RG, Ghanayem NS, Cheatham JP, Tworetzky W, Martin GR. Hypoplastic Left Heart Syndrome. J Am Coll Cardiol.. 2012;59:S1–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verheijen PM, Lisowski LA, Stoutenbeek P, Hitchcock JF, Brenner JI, Copel JA, Kleinman CS, Meijboom EJ, Bennink GBWE. Prenatal diagnosis of congenital heart disease affects preoperative acidosis in the newborn patient. J Thorac Cardiovasc Surg. 2001;121:798–803. [DOI] [PubMed] [Google Scholar]
- 6.Atz AM, Travison TG, Williams IA, Pearson GD, Laussen PC, Mahle WT, Cook AL, Kirsh JA, Sklansky M, Khaikin S, Goldberg C, Frommelt M, Krawczeski C, Puchalski MD, Jacobs JP, Baffa JM, Rychik J, Ohye RG. Prenatal diagnosis and risk factors for preoperative death in neonates with single right ventricle and systemic outflow obstruction: Screening data from the Pediatric Heart Network Single Ventricle Reconstruction Trial. J Thorac Cardiovasc Surg. 2010;140:1245–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Copel JA, Tan AS, Kleinman CS. Does a prenatal diagnosis of congenital heart disease alter short-term outcome? UltrasoundObstet Gynecol. 1997;10:237–241. [DOI] [PubMed] [Google Scholar]
- 8.Kipps AK, Feuille C, Azakie A, Hoffman JIE, Tabbutt S, Brook MM, Moon-Grady AJ. Prenatal Diagnosis of Hypoplastic Left Heart Syndrome in Current Era. Am J Cardiol. 2011;108:421–427. [DOI] [PubMed] [Google Scholar]
- 9.Kumar RK, Newburger JW, Gauvreau K, Kamenir SA, Hornberger LK. Comparison of outcome when hypoplastic left heart syndrome and transposition of the great arteries are diagnosed prenatally versus when diagnosis of these two conditions is made only postnatally. Am J Cardiol. 1999;83:1649–1653. [DOI] [PubMed] [Google Scholar]
- 10.Levey A, Glickstein JS, Kleinman CS, Levasseur SM, Chen J, Gersony WM, Williams IA. The Impact of Prenatal Diagnosis of Complex Congenital Heart Disease on Neonatal Outcomes. Pediatr Cardiol. 2010;31:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahle WT, Clancy RR, McGaurn SP, Goin JE, Clark BJ. Impact of Prenatal Diagnosis on Survival and Early Neurologic Morbidity in Neonates With the Hypoplastic Left Heart Syndrome. Pediatrics. 2001;107:1277–1282. [DOI] [PubMed] [Google Scholar]
- 12.Montana E, Khoury MJ, Cragan JD, Sharma S, Dhar P, Fyfe D. Trends and outcomes after prenatal diagnosis of congenital cardiac malformations by fetal echocardiography in a well defined birth population, Atlanta, Georgia, 1990–1994. JAm Coll Cardiol. 1996;28:1805–1809. [DOI] [PubMed] [Google Scholar]
- 13.Tworetzky W, McElhinney DB, Reddy VM, Brook MM, Hanley FL, Silverman NH. Improved Surgical Outcome After Fetal Diagnosis of Hypoplastic Left Heart Syndrome. Circulation. 2001;103:1269–1273. [DOI] [PubMed] [Google Scholar]
- 14.National Birth Defects Prevention Network (NBDPN). Guidelines for Conducting Birth Defects Surveillance. Sever LE, ed. Atlanta, GA: National Birth Defects Prevention Network, Inc.:June 2004:3–3. [Google Scholar]
- 15.Roslonski D Guidelines for air medical dispatch. American College of Emergency Physicians and National Association of EMS Physicians; 2008. [Google Scholar]
- 16.Pappas G, Queen S, Hadden W, Fisher G. The increasing disparity in mortality between socioeconomic groups in the United States, 1960 and 1986. N Engl J Med. 1993;329:103–109. [DOI] [PubMed] [Google Scholar]
- 17.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health. 2005;95:312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poverty Definitions - U.S Census Bureau [Internet]. Available from http://www.census.gov/hhes/www/poverty/methods/definitions.html.
- 19.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. [DOI] [PubMed] [Google Scholar]
- 20.Tabbutt S, Ghanayem N, Ravishankar C, Sleeper LA, Cooper DS, Frank DU, Lu M, Pizarro C, Frommelt P, Goldberg CS, Graham EM, Krawczeski CD, Lai WW, Lewis A, Kirsh JA, Mahony L, Ohye RG, Simsic J, Lodge AJ, Spurrier E, Stylianou M, Laussen P, Investigators PHN. Risk factors for hospital morbidity and mortality after the Norwood procedure: A report from the Pediatric Heart Network Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirsch JC, Gurney JG, Donohue JE, Gebremariam A, Bove EL, Ohye RG. Hospital Mortality for Norwood and Arterial Switch Operations as a Function of Institutional Volume. Pediatr Cardiol. 2007;29:713–717. [DOI] [PubMed] [Google Scholar]
- 22.Checchia PA, McCollegan J, Daher N, Kolovos N, Levy F, Markovitz B. The effect of surgical case volume on outcome after the Norwood procedure. J Thorac Cardiovasc Surg. 2005;129:754–759. [DOI] [PubMed] [Google Scholar]
- 23.Simpson LL, Harvey-Wilkes K, D’Alton ME. Congenital heart disease: the impact of delivery in a tertiary care center on SNAP scores (scores for neonatal acute physiology). Am. J. Obstet Gynecol 2000;182:184–191. [DOI] [PubMed] [Google Scholar]
- 24.Bennett TD, Klein MB, Sorensen MD, De Roos AJ, Rivara FP. Influence of Birth Hospital on Outcomes of Ductal-Dependent Cardiac Lesions. Pediatrics. 2010;126:1156–1164. [DOI] [PubMed] [Google Scholar]
- 25.Texas Birth Defects Registry. Report of Defects Among 1999-2007 Deliveries. Texas Department of State Health Services; Austin, TX: http://www.dshs.state.tx.us/birthdefects/data/annl99-07.shtm; 2010. [Google Scholar]
- 26.US Census Bureau. Census 2010, Urban and Rural, Summary File 1, Generated by SA Morris; using American Fact Finder http://factfinder2.census.gov. 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.