Abstract
Background:
Research on the relationship between long-term exposure to particulate matter with aerodynamic diameter () and poor cognitive function is lacking in developing countries, especially in highly polluted areas.
Objectives:
We evaluated associations of long-term exposure to with poor cognitive function in a diverse, national sample of older adults in China.
Methods:
This analysis included data on 13,324 older adults (5,879 who were 65–79 years of age, 3,052 who were 80–89 years of age, 2,634 who were 90–99 years of age, and 1,759 who were of age) with normal cognitive function at baseline from March 2002 to September 2014, with 64,648 person-years of follow-up. We used a geographic information system analysis to estimate the annual average satellite-derived concentration for the geocoded location of the participants’ baseline residences. Poor cognitive function was defined as a score of less than 18 on the Chinese version of the Mini-Mental State Examination (MMSE). Competing risk models were performed to explore the association of with poor cognitive function.
Results:
Each increase in was associated with a 5.1% increased risk of poor cognitive function [adjusted hazard ratio (HR): 1.051; 95% confidence interval (CI): 1.023, 1.079]. Compared to the lowest quartile of (), adjusted values were 1.20 (95% CI: 1.09, 1.33), 1.27 (95% CI: 1.15, 1.41), and 1.21 (95% CI: 1.09, 1.34) for the second (), third (), and fourth () quartiles of , respectively (p for trend ). Subgroup analyses suggested stronger associations between and poor cognitive impairment in men than women. The association was positive in the 65- to 79- and age group but not significant and positive in the other two age groups with similar results.
Conclusion:
was identified as a risk factor for poor cognitive function in Chinese older adults. Improving air quality may reduce the future population burden of poor cognitive function, especially in areas with high air pollution. https://doi.org/10.1289/EHP5304
Introduction
The aging population is projected to continuously increase in developed and developing parts of the world (Gerland et al. 2014). With the decline in fertility and mortality rates, China has become a rapidly aging society. China’s population of 60 years of age and above was 249 million in 2018, accounting for 17.90% of the country’s total population; this group, in turn, accounts for about one-fifth of the world’s total aging population and half of Asia’s aging population (National Bureau of Statistics 2019). The physical health of older adults progressively declines with age. Cognitive decline is the single most feared aspect of growing old (Martin 2004). A systematic review of studies conducted in China between 2001–2016 estimated that the prevalence of mild cognitive impairment (MCI) was 14.7% (Xue et al. 2018). The burden of poor cognitive function is expected to increase with the rapid increase in the elderly population. Therefore, an improved understanding of risk factors for poor cognitive function is needed to inform future disease control and prevention efforts.
Older adults may be especially vulnerable to hazards in their immediate environment, including environmental pollutants (Gouveia and Fletcher 2000; Katsouyanni et al. 2001) and fine particulate matter air pollution specifically (Naghavi et al. 2015). There is emerging evidence for the association between exposure to air pollution and poorer brain health (clinical dementia, neuroimaging correlates, or cognitive impairment) (Russ et al. 2019). Most previous studies of air pollution and cognitive function in the elderly have conducted in developed countries with relatively low air pollution exposures, including cross-sectional studies (Ailshire and Crimmins 2014; Ailshire and Clarke 2015; Gatto et al. 2014; Ranft et al. 2009; Shin et al. 2019; Tzivian et al. 2016) and prospective cohort studies (Power et al. 2011; Tonne et al. 2014; Wellenius et al. 2012; Weuve et al. 2012; Loop et al. 2013). Previous studies of air pollution and poor cognitive function among elderly Chinese participants have been limited to a cross-sectional study (Sun and Gu 2008; Zeng et al. 2014) and a repeated measures analysis (Zhang et al. 2018) that used the air pollution index (API), a simplified measure of air quality that reflects the concentrations of several air pollutants (sulfur dioxide, nitrogen dioxide, and inhalable particulates) to estimate exposure at the city or county level.
In this study, we analyzed data from a large, prospective, nationwide representative cohort of Chinese adults ranging from 65–114 years of age at baseline. To test the hypothesis that long-term exposure to particulate matter with aerodynamic diameter () is associated with poor cognitive function, competing risk models were performed, accounting for possible bias from selective attrition.
Methods
Study Population
This study is based on the Chinese Longitudinal Healthy Longevity Study (CLHLS), conducted in 866 highly diverse counties/cities in 23 provinces of China. Younger elderly (65–79 years of age) were first included in the CLHLS in 2002; in the present study, we included participants enrolled from 2002 to 2014. The CLHLS randomly selected half of the counties and cities in 23 of China’s 31 provinces; it was the first national longitudinal survey on determinants of healthy aging and includes the largest sample of adults of age in China. Details of the study have been provided elsewhere (Lv et al. 2018). The study was approved by the Biomedical Ethics Committee of Peking University (IRB00001052-13074). All participants or their legal representatives signed written consent forms to participate in the baseline and follow-up surveys.
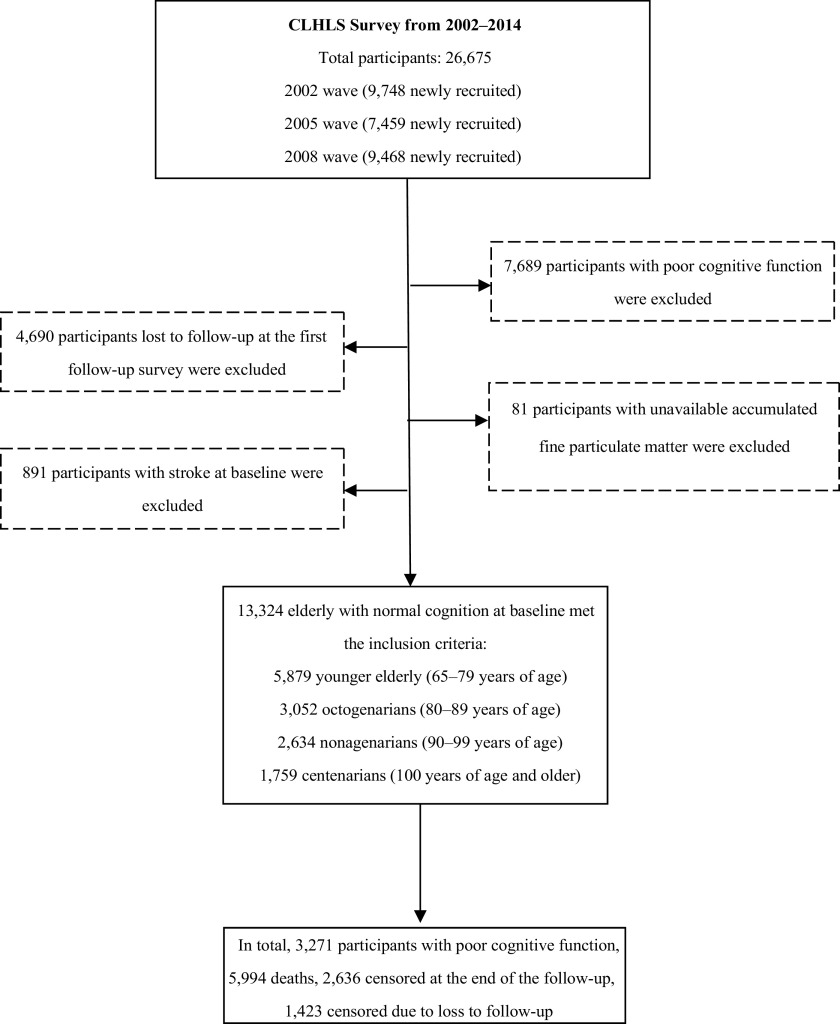
A total of 26,675 older adults were evaluated (standardized questionnaires and physical measurements performed) in the CLHLS from 2002 to 2014. A total of 13,324 adults of age with normal cognition at enrollment were included in the present analysis, including 5,879 who were 65–79 years of age, 3,052 who were 80–89 years of age, 2,634 who were 90–99 years of age, and 1,759 who were of age. Participants were excluded due to poor cognitive function at baseline (score on the Chinese version of Mini-Mental State Examination (MMSE); ), loss to follow-up at the first follow-up survey (), missing information on accumulated (), and a history of stroke at baseline () (Figure 1).
Figure 1.
Flowchart of study inclusion criteria. Note: CLHLS, Chinese Longitudinal Healthy Longevity Study.
Measurement of Exposure to
Nationwide monitoring data were available beginning in 2013. Our exposure estimates were derived from a remote-sensing concentration grid data product with a resolution of degrees provided by the Institute of Atmospheric Physics, University of Dalhousie (van Donkelaar et al. 2016; Boys et al. 2014). This data set is the longest and highest-resolution–exposure data set available in China and widely used for evaluation of air pollution and health outcomes (Yin et al. 2017; Crouse et al. 2015). The data product used aerosol optical depth (AOD) to simulate concentration with a land-use regression (LUR) model. Subsequently, the data set was calibrated based on global ground-based observations using the geographically weighted regression (GWR) method. In addition, van Donkelaar’s team collected 210 global data sets of ground observations from the literature and used them to estimate for global satellite inversion and found important consistency (), with research areas including northern India and eastern China (2001–2010) (van Donkelaar et al. 2015). Missing concentration data may exist in some grids due to the unavailability of remote-sensing data because of the influence of clouds and snow-capped mountains. We therefore excluded the participants without valid data at the stage of selecting the study population () (Figure 1).
concentrations were assigned to the participants based on the following rules: a) the household address of each surveyed person was obtained via questionnaire, including information on province, city, district (or county), and street (or village). Using R software (version 3.3.1; R Development Core Team), we matched each ascertained address to a series of latitude and longitude coordinates; b) we used geographic information systems vector data to generate a basic map of China’s districts and counties to calculate and display concentration. These vector data are publicly available from the National Geographic Information Center (http://www.webmap.cn/main.do?method=index); and c) each residential address was subsequently matched with the grid of the ambient model it fell into to assign concentrations at a spatial resolution of 0.01 degrees.
In order to characterize the historic long-term exposures more accurately, we averaged the predicted concentrations for all residential locations where individual participants lived from recruitment to a diagnosis of poor cognitive function, death, loss to follow-up, or the end of follow-up (September 2014). We also calculated exposures for the 3 y prior to a diagnosis of poor cognitive function, death, loss to follow-up, or the end of follow-up (September 2014) to explore whether different metrics of exposure may alter the findings.
Assessment of Cognitive Function
Cognitive function was assessed by the Chinese version of the MMSE administered by a trained staff of the Center for Disease Control and Prevention of China or by a trained student. The MMSE has been widely applied in epidemiological studies as a screening test for poor cognitive function and to track changes in cognitive function over time (Daniels et al. 2011; van Exel et al. 2003). The MMSE is also used as a screen for dementia, with high specificity (usually above 0.80) and moderate reliability (24-h test–retest by Pearson correlation usually above 0.85) from a meta-analysis conducted in 2013 (Mitchell 2013). We used a version of the MMSE that has been adapted for the cultural and socioeconomic conditions in China (Zeng and Vaupel 2002). For the sample in the CLHLS 2002 wave, the reliability of the MMSE scale is high (Cronbach’s ) (Sun and Gu 2008). The assessment tool addresses the following aspects of cognitive functioning: orientation, registration, attention, memory, language, and visual construction skills. MMSE scores range from 0 to 30; a higher total score indicates better cognitive function. Because more than half of the participants were illiterate, we used a relatively low cutoff score of 18 to define poor cognitive function and used scores to define normal cognitive function (Tombaugh and Mcintyre 1992; Zhang et al. 1990; Cui et al. 2011).
Covariates
Covariates were obtained using a structured face-to-face questionnaire administered by trained interviewers. Potential confounders included age (continuous), sex, urban or rural residence (city vs. town or countryside), current marital status (either married or divorced, widowed, or never married), living pattern (either with family members, living in a nursing home, or alone), education (literate or illiterate based on or of formal education), smoking status (current smoker, former smoker, or never smoker, defined by the questions “Do you currently smoke?” and “Did you previously smoke?”), alcohol drinking status (current drinker, former drinker, or never drinker, based on the questions “Do you currently drink alcohol?” and “Did you previously drink alcohol?”), and regular exercise (“Do you exercise regularly?”, yes or no). In addition, we collected information on medical history, including self-reported diagnoses of diabetes (yes or no), heart disease (yes or no), and respiratory disease (yes or no), hypertension (yes or no based on self-report of a diagnosis by a doctor or physician, or on measured systolic blood pressure or diastolic blood pressure ), and disability in activities of daily living (ADL) (yes or no) (Han et al. 2019; Li et al. 2018; Tervo et al. 2004). Blood pressure was measured using a mercury sphygmomanometer after 5 min of rest, and the average value of the two measurements was used for analysis. Disability in ADL was defined as the inability to independently perform any of the following tasks: bathing, toileting, dressing, eating, continence, cleaning themselves afterward, or indoor movement (Katz et al. 1963). In addition, we used the gross domestic product (GDP) and number of physicians per z persons at the prefecture-level as indicators of socioeconomic status.
Statistical Analysis
The differences of categorical variables were tested with the Cochran-Armitage test for trends; the differences of continuous variables were tested with analysis of variance among participants categorized by quartiles of exposure. Multiple imputation (MI) is an attractive approach for missing data problems. To impute missing values of covariates, proc MI in SAS (version 9.4; SAS Institute, Inc.) was performed with the Markov chain Monte Carlo missing pattern. For a continuous variable, a regression method was used; for a classification variable, logistic regression method was used when the classification variable had a categorical response (dichotomous and polytomous variables). An MI procedure (five imputations) replaces each missing value of covariates ( missing for each individual characteristic), so that all 13,324 participants with outcomes of cognitive function were included (Yuan 2011).
Associations between average annual exposures and the incidence of poor cognitive function were estimated using Cox proportional hazard models [PROC PHREG in SAS (version 9.4; SAS Institute, Inc.)], with follow-up time ending at the date of diagnosis or censored at the date of death (since death is a competing risk), loss to follow-up, or the end of follow-up. The date of death was confirmed by the participant’s closest relatives or the village doctor. Covariates included age, sex, urban/rural residence, marital status, education, living pattern, smoking, alcohol drinking, regular exercise, diabetes, heart disease, hypertension, respiratory disease, disability in ADL, and prefecture-level GDP and physicians per 1,000 residents. was categorized by quartiles (, , , and ) or according to Chinese guidelines (, , , ) (MEP and AQSIQ 2012), with tests for linear trend derived by modeling each category of as an ordinal variable. In addition, we modeled as a simple continuous variable [with hazard ratios and 95% confidence intervals (CIs) estimated for increments] and used penalized splines to explore nonlinear associations of with poor cognitive function. Competing risk models were also performed to explore the association of with all-cause mortality with adjustment of the abovementioned covariates.
Subgroup analyses were conducted to assess whether associations with a increase in varied by age group (65–79 y, 80–89 y, 90–99 y, ), sex, education (literate or illiterate), residence (urban or rural), smoking status (current vs. former or never), drinking status (current vs. former or never), regular exercise (yes or no), comorbidity (yes or no), disability in ADL (yes or no), region (east, central, or west China), and prefecture-level GDP by tertiles (, , and ). To explore differences in hazard ratios between/among subgroups, we also calculated -values for interaction. Statistical interaction was performed for building Cox models, including two interaction terms and their product. The maximum likelihood ratios test was used to detect the significance of the interaction effect. For the polytomous variables such as age, region, and GDP, ordinal variables were included to estimate the interaction in the Cox model. In the subgroup analyses, separate stratum-specific models were performed to derive the hazard ratios for each category without interaction terms.
To evaluate the robustness of our estimates, several additional sensitivity analyses were performed. Since loss to follow-up may have been more likely to occur in participants at higher risk of poor cognitive function or death, analyses were conducted excluding the participants lost to follow-up. To assess whether overall results were affected by the inclusion of people who were at high risk of adverse outcomes concurrently with short-term exposure of , models were repeated with exclusion of participants who died in the first year. We also report results from an unadjusted model and a model adjusted for age only.
exposure matching was completed using ArcGIS (version 9.3; Esri) and R (version 3.3.1; R Development Core Team) software. All statistical analyses were conducted using SAS (version 9.4; SAS Institute, Inc.) and R (version 3.3.1; R Development Core Team). Two-sided -values () were considered statistically significant.
Results
The average age of the study sample at baseline was y, ranging from 65 to 114 y; 47.5% were men, 18.8% lived in urban areas, 41.4% were married, and 58.5% were illiterate (Table 1). Men had a higher percentage of smoking and alcohol drinking than women (Table S1).
Table 1.
Descriptive characteristics of 13,324 study participants at baseline by particulate matter with aerodynamic diameter () quartile [ or n (%)].
| Variable | First quartile () | Second quartile () | Third quartile () | Fourth quartile () | -Value | Total |
|---|---|---|---|---|---|---|
| No. of participants | 3,317 (24.9) | 3,366 (25.3) | 3,370 (25.3) | 3,271 (24.5) | — | 13,324 (100) |
| Age (y) | 0.09 | |||||
| Age group | — | — | — | — | — | |
| 65–79 y | 1,374 (41.4) | 1,462 (43.4) | 1,601 (47.5) | 1,442 (44.1) | — | 5,879 (44.1) |
| 80–89 y | 806 (24.3) | 797 (23.7) | 767 (22.8) | 682 (20.9) | — | 3,052 (22.9) |
| 90–99 y | 727 (21.9) | 697 (20.7) | 549 (16.3) | 661 (20.2) | — | 2,634 (19.8) |
| 410 (12.4) | 410 (12.2) | 453 (13.4) | 486 (14.9) | — | 1,759 (13.2) | |
| Sex | — | — | — | — | 0.07 | — |
| Male | 1,640 (49.4) | 1,573 (46.7) | 1,601 (47.5) | 1,520 (46.5) | — | 6,334 (47.5) |
| Female | 1,677 (50.6) | 1,793 (53.3) | 1,769 (52.5) | 1,751 (53.5) | — | 6,990 (52.5) |
| Residence | — | — | — | — | — | |
| Urban | 399 (12.0) | 560 (16.6) | 765 (22.7) | 783 (23.9) | — | 2,507 (18.8) |
| Rural | 2,918 (88.0) | 2,806 (83.4) | 2,605 (77.3) | 2,488 (76.1) | — | 10,817 (81.2) |
| Marital status | — | — | — | — | 0.002 | — |
| Married | 1,348 (40.6) | 1,355 (40.3) | 1,491 (44.2) | 1,328 (40.6) | — | 5,522 (41.4) |
| Not married | 1,969 (59.4) | 2,011 (59.7) | 1,879 (55.8) | 1,943 (59.4) | — | 7,802 (58.6) |
| Educational background | — | — | — | — | — | |
| Illiterate | 1,987 (59.9) | 1,857 (55.2) | 1,943 (57.7) | 2,005 (61.3) | — | 7,792 (58.5) |
| Literate | 1,330 (40.1) | 1,509 (44.8) | 1,427 (42.3) | 1,266 (38.7) | — | 5,532 (41.5) |
| Living pattern | — | — | — | — | — | |
| With family member | 2,682 (80.9) | 2,796 (83.1) | 2,842 (84.3) | 2,823 (86.3) | — | 11,143 (83.6) |
| Alone or in nursing home | 635 (19.1) | 570 (16.9) | 528 (15.7) | 448 (13.7) | — | 2,181 (16.4) |
| Tobacco smoking status | — | — | — | — | — | |
| Never smoker | 2,228 (67.2) | 2,161 (64.2) | 2,125 (63.1) | 2,043 (62.5) | — | 8,557 (64.2) |
| Current smoker | 693 (20.9) | 801 (23.8) | 784 (23.3) | 725 (22.2) | — | 3,003 (22.5) |
| Former smoker | 396 (11.9) | 404 (12.0) | 461 (13.7) | 503 (15.4) | — | 1,764 (13.2) |
| Alcohol drinking status | — | — | — | — | 0.3 | — |
| Never drinker | 2,266 (68.3) | 2,305 (68.5) | 2,276 (67.5) | 2,212 (67.6) | — | 9,059 (68.0) |
| Current drinker | 714 (21.5) | 769 (22.8) | 774 (23.0) | 763 (23.3) | — | 3,020 (22.7) |
| Former drinker | 337 (10.2) | 292 (8.7) | 320 (9.5) | 296 (9.0) | — | 1,245 (9.3) |
| Regular exercise | — | — | — | — | — | |
| No | 2,365 (71.3) | 2,241 (66.6) | 2,273 (67.4) | 2,184 (66.8) | — | 9,063 (68.0) |
| Yes | 952 (28.7) | 1,125 (33.4) | 1,097 (32.6) | 1,087 (33.2) | — | 4,261 (32.0) |
| Hypertension | — | — | — | — | — | |
| No | 1,861 (56.1) | 1,836 (54.5) | 1,624 (48.2) | 1,650 (50.4) | — | 6,971 (52.3) |
| Yes | 1,456 (43.9) | 1,530 (45.5) | 1,746 (51.8) | 1,621 (49.6) | — | 6,353 (47.7) |
| Heart disease | — | — | — | — | — | |
| No | 3,127 (94.3) | 3,144 (93.4) | 3,085 (91.5) | 2,957 (90.4) | — | 12,313 (92.4) |
| Yes | 190 (5.7) | 222 (6.6) | 285 (8.5) | 314 (9.6) | — | 1,011 (7.6) |
| Diabetes | — | — | — | — | 0.005 | — |
| No | 3,258 (98.2) | 3,304 (98.2) | 3,285 (97.5) | 3,177 (97.1) | — | 13,024 (97.7) |
| Yes | 59 (1.8) | 62 (1.8) | 85 (2.5) | 94 (2.9) | — | 300 (2.3) |
| Respiratory disease | — | — | — | — | 0.3 | — |
| No | 2,969 (89.5) | 3,009 (89.4) | 2,980 (88.4) | 2,893 (88.4) | — | 11,851 (88.9) |
| Yes | 348 (10.5) | 357 (10.6) | 390 (11.6) | 378 (11.6) | — | 1,473 (11.1) |
| Disability | — | — | — | — | — | |
| No | 3,012 (90.8) | 3,072 (91.3) | 2,949 (87.5) | 2,771 (84.7) | — | 11,804 (88.6) |
| Yes | 305 (9.2) | 294 (8.7) | 421 (12.5) | 500 (15.3) | — | 1,520 (11.4) |
| Comorbidity | — | — | — | — | — | |
| No | 2,771 (83.5) | 2,784 (82.7) | 2,688 (79.8) | 2,570 (78.6) | — | 10,813 (81.2) |
| Yes | 546 (16.5) | 582 (17.3) | 682 (20.2) | 701 (21.4) | — | 2,511 (18.8) |
Note: Comorbidity represents any positive medical history for diabetes, heart disease, respiratory disease, or hypertension. Urban signifies city residence, and rural signifies town or countryside residence. Illiterate represents not having received education; literate signifies having received formal education of more than 1 y. Disability represents disability in activities of daily living. Missing values include marital status (23), educational background (44), living pattern (31), tobacco smoking status (47), alcohol drinking status (58), regular exercise (38), and hypertension (103).The detailed explanation of the MI procedure is given in the “Methods” section. —, no data available; SD, standard deviation.
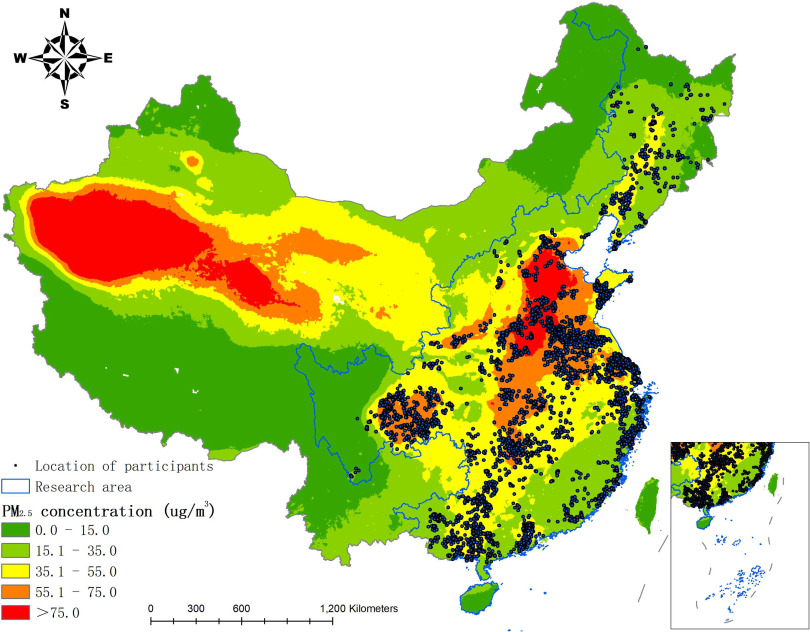
Average annual exposures over the follow-up period ranged from 8.5 to [median: ; interquartile range (IQR): ]. There was substantial variability in the average annual exposures across the study area (Figure 2). Average annual exposures during each year of the study period (2002–2014) were highly correlated (pairwise correlation coefficients: 0.84–0.98) (Table S2). The distributions of exposures among participants followed an approximately normal distribution each year (Figure S1).
Figure 2.
Map of particulate matter with aerodynamic diameter () concentration in China during the study period (2002–2014). The blue lines denote the geographic areas included in this study, covering 23 provinces from the south to the north of China. Black points represent the location of participants. The inset in the lower right corner indicates the South China Sea and the islands. Our large study area included a large sample size and encompassed a broad concentration range of fine particulate matter that included areas of the lowest and highest pollution in China.
A total of 3,271 participants who had normal cognitive function at baseline (MMSE score ) developed poor cognitive function during 64,648 person-years of follow-up. During the follow-up, 7,458 deaths were documented (including 1,464 with poor cognitive function). Based on adjusted competing risk models, a increase in was associated with a 5.1% increase in the risk of poor cognitive function [hazard ratio (HR): 1.051 (95% CI: 1.023, 1.079)] (Figure 3). Corresponding estimates from a crude model and a model adjusted for age only were an HR of 1.044 (95% CI: 1.017, 1.071) and an HR of 1.054 (95% CI: 1.028, 1.081), respectively (Table S3). In contrast, the HR for all-cause mortality with each increase in was 1.007 (95% CI: 0.988, 1.026). Compared to the lowest quartile of (), the adjusted HRs for , , and were 1.20 (95% CI: 1.09, 1.33), 1.27 (95% CI: 1.15, 1.41), and 1.21 (95% CI: 1.09, 1.34), respectively (p for trend ). When categorized by Chinese guidelines, adjusted HRs were 0.50 (95% CI: 0.12, 2.00), 1.20 (95% CI: 1.09, 1.34), and 1.28 (95% CI: 1.04, 1.59) for , , and levels, respectively, compared with (p for trend ) (Figure 3). Compared to the lowest quartile of (), the crude models’ HRs were , , and were 1.12 (95% CI: 1.02, 1.24), 1.23 (95% CI: 1.12, 1.36), and 1.29 (95% CI: 1.17, 1.42), and the models adjusted for age-only HRs were 1.19 (95% CI: 1.08, 1.31), 1.29 (95% CI: 1.17, 1.42), and 1.24 (95% CI: 1.13, 1.37), respectively (Table S3).
Figure 3.
The association of particulate matter with aerodynamic diameter () and poor cognitive function [Mini-Mental State Examination (MMSE) ] in competing risk models among Chinese older adults 65 years of age and older. Adjusted covariates include age (continuous), sex, residence, current marital status, living pattern, education (literacy status), smoking status, alcohol drinking status, regular exercise, diabetes, heart disease, hypertension, respiratory disease, disability in activities of daily living, gross domestic product (GDP), physicians per z persons at prefecture level. Note: CI, confidence interval; HR, hazard ratio.
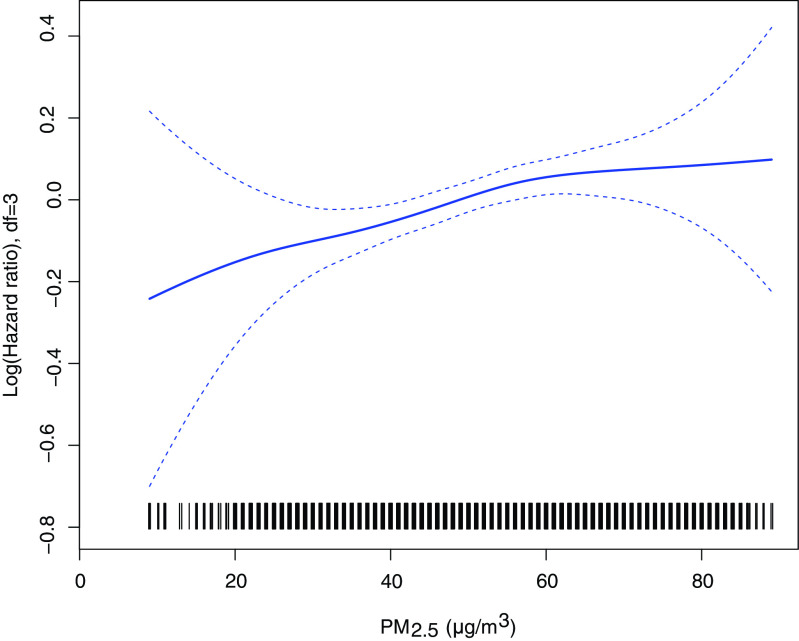
The association between poor cognitive function and during the 3 y prior to the end of each individual’s follow-up was positive but weaker than associations with average exposure over the entire follow-up period; the adjusted HR for a increase in was 1.033 (95% CI: 1.007, 1.058) (Figure 3). When was modeled using penalized splines, the association with the ln(HR) for poor cognitive function was approximately linear; with the increment of , the risk of poor cognitive function significantly increased (Figure 4).
Figure 4.
The association of fine particulate matter with poor cognitive function among Chinese older adults in Cox models with penalized splines. Adjusted covariates include age (continuous), sex, residence, current marital status, living pattern, education (literacy status), smoking status, alcohol drinking status, regular exercise, diabetes, heart disease, hypertension, respiratory disease, disability in activities of daily living, gross domestic product (GDP), physicians per z persons at the prefecture level. Note: df, degrees of freedom; HR, hazard ratio.
Adjusted associations were consistent with the primary model estimates after excluding 1,423 participants lost to follow-up (HR: 1.062; 95% CI: 1.034, 1.089 for a increase in ) and after excluding 865 participants who died during the first year of follow-up (HR: 1.056; 95% CI: 1.029, 1.084) (Table S4).
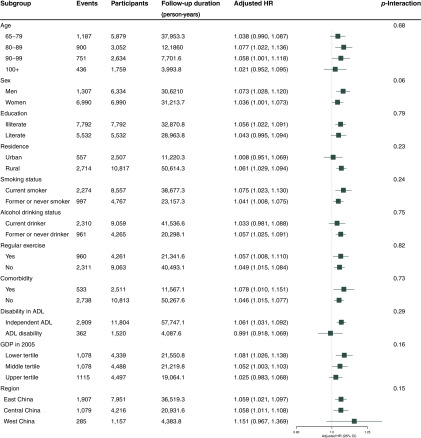
In subgroup analyses, the association between a increase in and poor cognitive function differed by sex, with a stronger association among men (HR: 1.073; 95% CI: 1.028, 1.120) than women (HR: 1.036; 95% CI: 1.001, 1.073) (). When evaluated by region, associations were very similar for eastern and central China (HR: 1.059; 95% CI: 1.021, 1.097 and HR: 1.058; 95% CI: 1.011, 1.108, respectively), while the association was stronger for western China (HR: 1.151; 95% CI: 0.967, 1.369) but much less precise, consistent with the smaller numbers of events and participants in this region ().The association between poor cognitive function and a increase in increased as prefecture-level GDP decreased, with HRs of 1.025 (95% CI: 0.983, 1.068), 1.052 (95% CI: 1.003, 1.103), and 1.081 (95% CI: 1.026, 1.138) for the upper, middle, and lower tertiles, respectively (), with a slight inverse association for the relatively small number of participants classified as disabled (HR: 0.991; 95% CI: 0.918, 1.069) and a positive association for nondisabled participants (HR: 1.061; 95% CI: 1.031, 1.092, ). The HR for those with comorbidities is stronger than for those without (HR: 1.078; 95% CI: 0.010, 1.151 and HR: 1.046; 95% CI: 1.105, 1.077, respectively), but the difference is not significant (). For smoking, the association was stronger for current smokers than for former or never smokers, though the difference was not significant (). For age, the association was weakest for the group and similar for the three younger age groups. The association was essentially null for urban residents (HR: 1.008; 95% CI: 0.951, 1.069) but positive for rural residents (HR: 1.061; 95% CI: 1.029, 1.094), though the differences were not significant, in part due to the small numbers of participants in urban areas () (Figure 5).
Figure 5.
Stratified analyses of association of each increase in particulate matter with aerodynamic diameter () concentration with poor cognitive function (Mini-Mental State Examination (MMSE) ) in competing risk models among Chinese older adults of 65 years of age and older. Adjusted covariates include age (continuous), sex, residence, current marital status, living pattern, education (literacy status), smoking status, alcohol drinking status, regular exercise, diabetes, heart disease, hypertension, respiratory disease, disability in activities of daily living, gross domestic product (GDP), physicians per z persons at the prefecture level. Note: CI, confidence interval; HR, hazard ratio.
Discussion
In this prospective cohort study based on a nationwide sample of older adults living in 866 highly diverse counties and cities of China, including areas exposed to high concentrations of , exposure to () was associated with a higher risk of poor cognitive function after accounting for potential confounders.
Studies of the impact of on cognitive function in elderly populations living in developed countries (the United States and United Kingdom) have also reported evidence of an adverse effect (Weuve et al. 2012; Wellenius et al. 2012; Tonne et al. 2014; Ailshire and Clarke 2015). Loop et al. (2013) did not find clear evidence of an association between and the incidence of cognitive impairment in a geographically diverse, biracial U.S. cohort of men and women (), with an odds ratio (95% CI: 0.97, 1.64) for increased odds of incident impairment with a increase in concentration. The OR was attenuated toward 1 after adding more covariates. The highest quartile of concentration was in Loop et al. (2013)’s study, which would have fallen within the lowest quartile for our study (). Compared to other prospective cohort studies focusing on (Tonne et al. 2014; Weuve et al. 2012), the exposure data reported by Loop et al. (2013) are similar to these two studies. Tzivian et al. (2016) reported positive cross-sectional associations between long-term air pollutant exposures and MCI during 5 y of follow-up in a cohort of German adults (45–75 years of age at baseline), with an OR of 1.16 (95% CI: 1.05, 1.27) for overall MCI in association with an IQR increase in () and similar or slightly weaker associations with IQR increases in long-term PM with aerodynamic diameter , , absorbance, , and concentrations.
The results of our study provide further support the potential influence of long-term exposure to on the development of poor cognitive function in a population with exposure concentrations ranging from relatively low values to concentrations that are much higher than those observed in developed countries. In China, previous studies have explored the role of air pollution in global cognitive performance (Sun and Gu 2008; Zeng et al. 2014; Zhang et al. 2018). Sun and Gu (2008) reported that a 1-point increase in API is associated with cognitive function test score (; 95% CI: , ). Zeng et al. (2014) explored the relationship between API and cognitive dysfunction in the CLHLS study; each unit increase in API was associated with a 9% increased odds of age-related cognitive decline. Zhang et al. (2018) found that long-term exposure to air pollution impedes cognitive performance, with a 1–standard deviation increase in average 3-y API associated with a 1.132-point drop in verbal test scores. The API includes values for various contaminants, making it difficult to attribute their findings to a specific pollutant or set of pollutants. Our findings complement and extend these previous findings by estimating the risk of poor cognitive function in association with ambient exposures in a large population of elderly Chinese. contains a variety of components. The main components are elemental carbon, organic carbon compounds, sulfates, nitrates, ammonium salts, and various metal elements. Although we found an association between and poor cognitive function, we could not determine whether specific components of were primarily responsible. In addition, we cannot rule out confounding by other air pollutants.
As observed in previous studies (Ailshire and Clarke 2015; Shin et al. 2019), subgroup analyses suggested stronger associations between and cognitive impairment in men than women. Men in our study were more likely than women to be current smokers (39% vs. 7.4%), alcohol consumers (36% vs. 11%), and regular exercisers (38% vs. 27%). Differences in gender-related lifestyle factors may result in differential exposure patterns between men and women and may modify the effects of environmental exposures. The association between and poor cognitive function was stronger among residents of rural areas, though the difference was not significant. This could reflect a greater susceptibility among rural residents, differences in ambient air pollutants between rural and urban areas, confounding (e.g., by exposure to indoor air pollution), or other mechanisms.
Epidemiological studies have shown that poor cognitive function in old age is a multifactorial disease (Avila et al. 2010; del Valle 2011). However, the specific relevance or causative nature of these factors and the potential for independent or synergistic effects of air pollution on the causes and pathogenesis of cognitive decline remain unclear. Atmospheric particulate matter inhaled through the respiratory tract activates human macrophages and inflammatory cytokines, causing inflammation and subsequent oxidative stress. Inflammatory compounds spread through the body’s circulatory system and penetrate the blood–brain barrier, thus potentially affecting nervous system function (Genc et al. 2012; Hirano et al. 2003). A second potential mechanism is related to the direct entry of PM into the nervous system through the olfactory bulb following nasal inhalation. Pathological changes in the olfactory bulb were observed in the early stages of Alzheimer’s disease (AD) (Doty 2008). Ultrafine particles (UFP) were observed in human olfactory bulb periglomerular neurons after exposure to air pollution (Calderón-Garcidueñas et al. 2004).
Our research has the following strengths. First, based on the prospective cohort design, we were able to identify individuals with normal cognition at baseline to assess poor cognitive function in relationship to . Second, our study also provided an opportunity to explore the exposure–response relationship between and poor cognitive function over a wide range of concentrations (from 8.5 to ). Third, the large sample size of community-based older adults, especially of age, permitted us to focus not only on the association of with poor cognitive function but also to estimate associations in different subgroups. The association was weakest and not significant for the group, positive in the 65–79 age group but not significant and positive in the other two age groups with similar results. Finally, our cohort contained a wide geographical distribution of residents that covered nearly all densely populated areas in China.
There are some limitations of the present study worth noting. First, the duration of residence at the current address for each study participant was unknown. We averaged exposures over all residences during the follow-up period, and because we were unable to weigh exposures according to the duration of residence in each location, some exposure misclassification would have occurred. According to the home address obtained in each follow-up visit, we have roughly counted that 356 of the 13,324 respondents changed their addresses and moved 1 times. Older adults (chosen for this study) tend to maintain geographic stability. Second, only outdoor exposures to were estimated based on residential location; indoor air pollution and secondhand smoking, which may play a potential role in confounding the association of ambient with poor cognitive function, were not measured. Third, only was measured; other ambient pollutants were not assessed due to a lack of these data for China. There is a possibility that the effects observed in the present study are not due to but to another pollutant or combination of pollutants whose concentrations strongly correlate with that of . Fourth, the simulation of the exposure data did not separately calibrate the model based on the values of the ground monitoring stations in different regions, but cross-validation was performed at a later stage. Fifth, the covariates or confounding factors we have collected may not be comprehensive or accurate; for example, in the definition of hypertension, we cannot completely exclude the possibility of individual blood pressure fluctuations, although we used reliable measurement methods. In addition, we did not have access to data about the number of cigarettes consumed, only whether the participant was a current, former, or never smoker. The smoking and drinking information in this study were self-reported, so they are subject to recall bias. Finally, the large portion of participants who died or were lost to follow-up may have led to selection bias. However, the competitive risk models and sensitivity analyses helped address this issue and provide evidence in support of an association of and poor cognitive function.
Conclusions
In this community-based prospective cohort study among older adults in China, long-term exposure to was associated with an increased risk of poor cognitive function. Overall, our findings suggest that reducing air pollution exposure may delay or prevent poor cognitive function in the population as a whole. This is of great significance to the economic and social development of China’s aging society. Future studies with longer periods of follow-up are needed to focus more comprehensively on the multiple components of air pollution and specific areas of cognition to better explore causality.
Supplementary Material
Acknowledgments
The CLHLS, which provided the data analyzed in this paper, is jointly supported by the National Science and Technology Planning Project (2018YFC2000300), the National Natural Sciences Foundation of China (81573247, 71233001, 71490732, 81273160, and 91543111), the U.S. National Institute of Aging (NIA) (2P01AG031719), the United Nations Fund for Population Activities, and a Claude D. Pepper Older Americans Independence Centers grant (5P30 AG028716 from NIA).
References
- Ailshire JA, Clarke P. 2015. Fine particulate matter air pollution and cognitive function among U.S. older adults. J Gerontol B Psychol Sci Soc Sci 70(2):322–328, PMID: 24906394, 10.1093/geronb/gbu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ailshire JA, Crimmins EM. 2014. Fine particulate matter air pollution and cognitive function among older US adults. Am J Epidemiol 180(4):359–366, PMID: 24966214, 10.1093/aje/kwu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J, Perry G, Martínez-Martín P. 2010. Prospects on the origin of Alzheimer’s disease. J Alzheimers Dis 20(2):669–672, PMID: 20505238, 10.3233/JAD-2010-1421. [DOI] [PubMed] [Google Scholar]
- Boys BL, Martin RV, van Donkelaar A, MacDonell RJ, Hsu NC, Cooper MJ, et al. 2014. Fifteen-year global time series of satellite-derived fine particulate matter. Environ Sci Technol 48(19):11109–11118, PMID: 25184953, 10.1021/es502113p. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Reed W, Maronpot RR, Henríquez-Roldán C, Delgado-Chavez R, Calderón-Garcidueñas A, et al. 2004. Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol 32(6):650–658, PMID: 15513908, 10.1080/01926230490520232. [DOI] [PubMed] [Google Scholar]
- Crouse DL, Peters PA, Hystad P, Brook JR, van Donkelaar A, Martin RV, et al. 2015. Ambient PM2.5, O3, and NO2 exposures and associations with mortality over 16 years of follow-up in the Canadian Census Health and Environment Cohort (CanCHEC). Environ Health Perspect 123(11):1180–1186, PMID: 26528712, 10.1289/ehp.1409276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G-H, Yao Y-H, Xu R-F, Tang H-D, Jiang G-X, Wang Y, et al. 2011. Cognitive impairment using education-based cutoff points for CMMSE scores in elderly Chinese people of agricultural and rural Shanghai China. Acta Neurol Scand 124(6):361–367, PMID: 21303351, 10.1111/j.1600-0404.2010.01484.x. [DOI] [PubMed] [Google Scholar]
- Daniels LB, Laughlin GA, Kritz-Silverstein D, Clopton P, Chen W-C, Maisel AS, et al. 2011. Elevated natriuretic peptide levels and cognitive function in community-dwelling older adults. Am J Med 124(7):670.e1–e8, PMID: 21683832, 10.1016/j.amjmed.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Valle L. 2011. Oxidative stress in aging: theoretical outcomes and clinical evidences in humans. Biomed Aging Pathol 1(1):1–7, 10.1016/j.biomag.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Doty RL. 2008. The olfactory vector hypothesis of neurodegenerative disease: is it viable? Ann Neurol 63(1):7–15, 10.1002/ana.21327. [DOI] [PubMed] [Google Scholar]
- Gatto NM, Henderson VW, Hodis HN, St John JA, Lurmann F, Chen J-C, et al. 2014. Components of air pollution and cognitive function in middle-aged and older adults in Los Angeles. Neurotoxicology 40:1–7, PMID: 24148924, 10.1016/j.neuro.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc S, Zadeoglulari Z, Fuss SH, Genc K. 2012. The adverse effects of air pollution on the nervous system. J Toxicol 2012(4):782462, PMID: 22523490, 10.1155/2012/782462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerland P, Raftery AE, Sevčíková H, Li N, Gu D, Spoorenberg T, et al. 2014. World population stabilization unlikely this century. Science 346(6206):234–237, PMID: 25301627, 10.1126/science.1257469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia N, Fletcher T. 2000. Time series analysis of air pollution and mortality: effects by cause, age and socioeconomic status. J Epidemiol Community Health 54(10):750–755, PMID: 10990478, 10.1136/jech.54.10.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han R, Tang Z, Ma L. 2019. Related factors of cognitive impairment in community-dwelling older adults in Beijing Longitudinal Study of Aging. Aging Clin Exp Res 31(1):95–100, PMID: 29633170, 10.1007/s40520-018-0943-8. [DOI] [PubMed] [Google Scholar]
- Hirano S, Furuyama A, Koike E, Kobayashi T. 2003. Oxidative-stress potency of organic extracts of diesel exhaust and urban fine particles in rat heart microvessel endothelial cells. Toxicology 187(2–3):161–170, 10.1016/S0300-483X(03)00053-2. [DOI] [PubMed] [Google Scholar]
- Katsouyanni K, Touloumi G, Samoli E, Gryparis A, Le Tertre A, Monopolis Y, et al. 2001. Confounding and effect modification in the short-term effects of ambient particles on total mortality: results from 29 European cities within the APHEA2 project. Epidemiology 12(5):521–531, PMID: 11505171, 10.1097/00001648-200109000-00011. [DOI] [PubMed] [Google Scholar]
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. 1963. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA 185:914–919, PMID: 14044222, 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- Li N, Chen G, Zeng P, Pang J, Gong H, Han Y, et al. 2018. Prevalence and factors associated with mild cognitive impairment among Chinese older adults with depression. Geriatr Gerontol Int 18 (2):263–268, PMID: 28880438, 10.1111/ggi.13171. [DOI] [PubMed] [Google Scholar]
- Loop MS, Kent ST, Al-Hamdan MZ, Crosson WL, Estes SM, Estes MG Jr, et al. 2013. Fine particulate matter and incident poor cognitive function in the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort. PLoS One 8(9):e75001, PMID: 24086422, 10.1371/journal.pone.0075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Y-B, Gao X, Yin Z-X, Chen H-S, Luo J-S, Brasher MS, et al. 2018. Revisiting the association of blood pressure with mortality in oldest old people in China: community based, longitudinal prospective study. BMJ 361:k2158, PMID: 29871897, 10.1136/bmj.k2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GM. 2004. Defeating dementia. Nature 431(7006):247–248, 10.1038/431247b. [DOI] [Google Scholar]
- MEP (Ministry of Environmental Protection of the People’s Republic of China), AQSIQ (General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. 2012. National Ambient Air Quality Standard, GB, 3095-2012. http://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/dqhjbh/dqhjzlbz/201203/t20120302_224165.htm [accessed 1 January 2016].
- Mitchell AJ. 2013. The Mini-Mental State Examination (MMSE): an update on its diagnostic validity for cognitive disorders. In: Cognitive Screening Instruments: A Practical Approach. Larner AJ, ed. London, UK: Springer Verlag. [Google Scholar]
- Naghavi M, Wang H, Lozano R, Davis A, Liang X, Zhou M, et al. 2015. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385(9963):117–171, PMID: 25530442, 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Bureau of Statistics. 2019. Statistical Communique of China’s National Economic and Social Development in 2018. http://www.stats.gov.cn/tjsj/zxfb/201902/t20190228_1651265.html [accessed 28 February 2019].
- Power MC, Weisskopf MG, Alexeeff SE, Coull BA, Spiro A 3rd, Schwartz J. 2011. Traffic-related air pollution and cognitive function in a cohort of older men. Environ Health Perspect 119(5):682–687, PMID: 21172758, 10.1289/ehp.1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranft U, Schikowski T, Sugiri D, Krutmann J, Krämer U. 2009. Long-term exposure to traffic-related particulate matter impairs cognitive function in the elderly. Environ Res 109(8):1004–1011, PMID: 19733348, 10.1016/j.envres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Russ TC, Reis S, von Tongeren M. 2019. Air pollution and brain health: defining the research agenda. Curr Opin Psychiatry 32(2):97–104, PMID: 30543549, 10.1097/YCO.0000000000000480. [DOI] [PubMed] [Google Scholar]
- Shin J, Han S-Choi H J . 2019. Exposure to ambient air pollution and cognitive impairment in community-dwelling older adults: the Korean Frailty and Aging Cohort Study. Int J Environ Res Public Health 16(19):3767, PMID: 31591354, 10.3390/ijerph16193767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R, Gu D. 2008. Air pollution, economic development of communities, and health status among the elderly in urban China. Am J Epidemiol 168(11):1311–1318, PMID: 18936437, 10.1093/aje/kwn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo S, Kivipelto M, Hänninen T, Vanhanen M, Hallikainen M, Mannermaa A, et al. 2004. Incidence and risk factors for mild cognitive impairment: a population-based three-year follow-up study of cognitively healthy elderly subjects. Dement Geriatr Cogn Disord 17(3):196–203, PMID: 14739544, 10.1159/000076356. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, Mcintyre NJ. 1992. The Mini–Mental State Examination: a comprehensive review. J Am Geriatr Soc 40(9):922–935, PMID: 1512391, 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- Tonne C, Elbaz A, Beevers S, Singh-Manoux A. 2014. Traffic-related air pollution in relation to cognitive function in older adults. Epidemiology 25(5):674–681, PMID: 25036434, 10.1097/EDE.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzivian L, Dlugaj M, Winkler A, Weinmayr G, Hennig F, Fuks KB, et al. 2016. Long-term air pollution and traffic noise exposures and mild cognitive impairment in older adults: a cross-sectional analysis of the Heinz Nixdorf Recall Study. Environ Health Perspect 124(9):1361–1368, PMID: 26863687, 10.1289/ehp.1509824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Donkelaar A, Martin RV, Brauer M, Boys BL. 2015. Use of satellite observations for long-term exposure assessment of global concentrations of fine particulate matter. Environ Health Perspect 123(2):135–143, PMID: 25343779, 10.1289/ehp.1408646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Donkelaar A, Martin RV, Brauer M, Hsu NC, Kahn RA, Levy RC, et al. 2016. Global estimates of fine particulate matter using a combined geophysical-statistical method with information from satellites, models, and monitors. Environ Sci Technol 50(7):3762–3772, PMID: 26953851, 10.1021/acs.est.5b05833. [DOI] [PubMed] [Google Scholar]
- van Exel E, de Craen AJM, Remarque EJ, Gussekloo J, Houx P, Bootsma-van der Wiel A, et al. 2003. Interaction of atherosclerosis and inflammation in elderly subjects with poor cognitive function. Neurology 61(12):1695–1701, PMID: 14694032, 10.1212/01.WNL.0000098877.07653.7C. [DOI] [PubMed] [Google Scholar]
- Wellenius GA, Boyle LD, Coull BA, Milberg WP, Gryparis A, Schwartz J, et al. 2012. Residential proximity to nearest major roadway and cognitive function in community-dwelling seniors: results from the MOBILIZE Boston Study. J Am Geriatr Soc 60(11):2075–2080, PMID: 23126566, 10.1111/j.1532-5415.2012.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F. 2012. Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med 172(3):219–227, PMID: 22332151, 10.1001/archinternmed.2011.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Li J, Liang J, Chen S. 2018. The prevalence of mild cognitive impairment in China: a systematic review. Aging Dis 9(4):706–715, PMID: 30090658, 10.14336/AD.2017.0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin P, Brauer M, Cohen A, Burnett RT, Liu J, Liu Y, et al. 2017. Long-term fine particulate matter exposure and nonaccidental and cause-specific mortality in a large national cohort of Chinese men. Environ Health Perspect 125(11):117002, PMID: 29116930, 10.1289/EHP1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y. 2011. Multiple imputation using SAS software. J Stat Softw 45:1–25, 10.18637/jss.v045.i06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Gu D, Purser J, Hoenig H, Christakis N. 2014. Associations of environmental factors with health and mortality among Chinese elderly: a sample survey in 22 provinces in China. Chin J Health Policy 7(6):53–62, 10.3969/j.issn.1674-2982.2014.06.010. [DOI] [Google Scholar]
- Zeng Y, Vaupel JW. 2002. Functional capacity and self-evaluation of health and life of the oldest-old in China. J Social Issues 58(4):733–748, 10.1111/1540-4560.00287. [DOI] [Google Scholar]
- Zhang MY, Katzman R, Salmon D, Jin H, Cai GJ, Wang ZY, et al. 1990. The prevalence of dementia and Alzheimer’s disease in Shanghai, China: impact of age, gender, and education. Ann Neurol 27(4):428–437, PMID: 2353798, 10.1002/ana.410270412. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen X, Zhang X. 2018. The impact of exposure to air pollution on cognitive performance. Proc Natl Acad Sci USA 115(37):9193–9197, PMID: 30150383, 10.1073/pnas.1809474115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.