Abstract
Rats and mice have been demonstrated to show episodic-like memory, a prototype of episodic memory, as defined by an integrated memory of the experience of an object or event, in a particular place and time. Such memory can be assessed via the use of spontaneous object exploration paradigms, variably designed to measure memory for object, place, temporal order and object-location inter-relationships. We review the methodological properties of these tests, the neurobiology about time and discuss the evidence for the involvement of the medial prefrontal cortex (mPFC), entorhinal cortex (EC) and hippocampus, with respect to their anatomy, neurotransmitter systems and functional circuits. The systematic analysis suggests that a specific circuit between the mPFC, lateral EC and hippocampus encodes the information for event, place and time of occurrence into the complex episodic-like memory, as a top-down regulation from the mPFC onto the hippocampus. This circuit can be distinguished from the neuronal component memory systems for processing the individual information of object, time and place.
Keywords: entorhinal cortex, episodic memory, CA1, CA3, prefrontal cortex, object recognition
1. Introduction
Episodic memory is conceptualized as recollection of an unique event together with the time and place of its occurrence. Endel Tulving proposed episodic memory as a hypothetical memory system for specific personal experiences, that are “consciously” remembered, encompassing what happened where and when, thus, entailing a kind of “mental time travel” (Tulving, 1983; Tulving, 2002). Since the emergence of this concept of episodic memory, there has been an active debate as to whether nonhuman animals (referred as animals herein) possess this type of memory, particularly because of anthropocentric concepts such as “consciousness” and “mental travel” that have been used to define it. The past two decades studies have revealed new insight on this question with evidence that animals can show a memory akin to human episodic memory, with arguments based on evolution, neuroanatomy and neurobiology (Allen and Fortin, 2013; Fortin et al., 2004; Manns and Eichenbaum, 2006; Templer and Hampton, 2013). These perspectives propose that similar neuroanatomical and neurobiological substrates of memory systems are shared by different species with humans, including a prototype of “episodic memory” which can be studied in animal models to decipher its neurobiological mechanisms.
The pioneering work by Clayton and Dickinson demonstrated the retrieval of specific experiences in animals. Scrub jays were trained to find different food based on their distinct locations and the time when they were confronted. The jays were able to learn that two kinds of foods (what) were placed separately in two different locations (where) and that, dependent on a short or a long delay (when), one of the foods became non-palatable. With this combination of what-where-when components, the authors operationalized the components of episodic memory into observable behavioral terms in an animal model and termed it “episodic-like memory” (Clayton and Dickinson, 1998). Such episodic-like memory (sometimes termed “what-where-when” memory) has been accepted to be a prototype of episodic memory and has been studied in many species (Allen and Fortin, 2013; Binder et al., 2015; Crystal, 2010; Dere et al., 2006; Ergorul and Eichenbaum, 2004; Fugazza et al., 2016; Hamilton et al., 2016; Templer and Hampton, 2013). Cognitive psychologists and neuroscientists also study the nature of episodic-like memory and compare it with episodic memory in humans (Holland and Smulders, 2011; Zlomuzica et al., 2016). Episodic-like memory paradigms have also been adapted to investigate episodic memory in young children, given that they cannot clearly express their experiences with words (Clayton and Russell, 2009; Russell et al., 2011).
There are principally two methodological approaches in the studies of episodic-like memory in animals, namely training-based and training-free models. The training-based models are directly related to the study of Clayton and Dickinson (1998). With this approach, animals are gradually guided to learn certain “what-where-when” rules with the help of positive and/or negative reinforcement. For instance, Babb and Crystal (2006) designed a paradigm to test whether rats remember a specific experience. Regular rat chow and flavored pellets, e.g. grape and raspberry, were placed separately in the ends of four of eight arms of a radial-arm maze, with the other four arms being blocked. Then, a short or a long delay decided what kinds of pellets were available and in which arms they were placed. After a short delay, all arms were accessible and regular chow pellets were placed in the previously blocked arms (no flavored pellets were involved). If a long delay was applied, all arms were accessible, with regular chow pellets placed in the previously blocked arms and the flavored pellets now available in the previously baited arms. Rats revisited the flavored-baited arms more often after a long delay than after a short delay, indicating that they acquired a specific “what-where-when” memory (Babb and Crystal, 2006). Some paradigms have used an odor-span task (Dudchenko et al., 2000) by presenting different odor stimuli placed at distinct contexts or locations across several time delays (Branch et al., 2014; Ergorul and Eichenbaum, 2004). Fear conditioning has also been used to assess episodic-like memory in rats by Li and colleagues (2011), who exposed animals to two distinct contexts at different times of the day - to one context in the morning and the other in the afternoon. They were then given light electrical shock in a different context, either in the morning or in the afternoon. One day after this contextual conditioning, specifically at noon, they were placed back into one of the contexts that had not been paired with electrical shock. Thus, the animals learned to avoid cues for the time point and context of the punishing stimulation. They found that rats exhibited more freezing behavior (indicating fear) in the context that was paired congruent to the time of shock delivery than in the context that was presented incongruent with time of shock application (Li et al., 2011). Similar results were found in an earlier study (O’Brien and Sutherland, 2007). Using classical conditioned licking behavior, Veyrac and colleagues (2015) designed a complicated task demanding rats to learn two odor-drink associations (what) in different locations (where) within two different multisensory enriched environments (in which context/occasion it happened). They found that rats were able to recollect accurately such episodic-like memory for at least 24 hours (Veyrac et al., 2015). In other experiments rats were trained to remember a specific olfactory memory in context and demonstrated their ability to form and recall episodic-like memory (Panoz-Brown et al., 2016; Panoz-Brown et al., 2018). Although training-based models can be interpreted in terms of the expression of “what-where-when” memory, such training procedures can be argued to involve learning about facts (semantic memory), rather than about specific experiences (episodic memory). To overcome this critique, the Crystal laboratory developed a brilliant task to train rats to answer an unexpected question, which mimics a common characteristic of episodic memory in daily life for incidental encoding and unexpected retrieval. Rats were demanded to forage foods in a non-match to sample task (i.e., foods were located in previously non-visited places) and in a response-based T-maze task (i.e., if a sample of food was given in the start arm, turn left; otherwise, turn right), with both tasks being conducted within the same radial maze. After learning the rules of both tasks, rats were asked to retrieve memory of an earlier event that was encoded incidentally: A sample of food was given, or not, in the learning trial of the non-match to sample task, followed by the T-maze test trial. Their findings indicate that rats can answer such a question and that this performance is hippocampus-dependent (Zhou et al., 2012). One of the features of training-based models is that it is time-consuming to train animals and that “emotional” factors (e.g. anxiety/fear reactions to aversive stimulation, incentive/reward-based motivational responses) are likely involved in order to motivate animals to acquire the necessary level of learning. Therefore, in such training-based models the neurobiological system of episodic memory is likely to converge with the amygdala and/or nucleus accumbens for coping with anxiety/fear and/or incentive motivation, respectively.
In contrast, training-free models endeavor to eliminate the application of training altogether, and are designed to decrease or avoid the involvement of emotional and motivational variables (e.g. food restriction or punishing stimulation) and to employ measures that are related to the innate nature of animals to behave in a “what-where-when” setting. For example, Fellini and Morellini (2013) designed a test, which involves different social conspecifics (a female C57BL/6J mouse and a male CD-1 mouse) that are presented at different locations and time points. They found that male C57BL/6J mice approached and avoided the locations that were previously associated with the female C57BL/6J mouse or the male CD-1 mouse, respectively, according to the temporal cues related to social conspecifics (Fellini and Morellini, 2013). Likely, the presence of social stimuli engages brain circuits for the processing of social approach and avoidance behaviors, such as the amygdala.
Alternatively, spontaneous object exploration in rodent studies of recognition memory is a well-established training-free animal model. This test is based on the natural tendency of many species to explore novel stimuli. For example, the length of time spent on exploring a novel object is compared with the time engaged in exploring a familiar object. The difference between the duration of exploration of a novel versus familiar object can be taken as an indication for memory, by the argument that animals tend to explore novel objects more because they remember the familiar ones (Ennaceur and Delacour, 1988). Caution must be exercised to rule out confounding interpretations, such as the absence of preference for a given object, fear of novelty, hyper- or hypo-locomotor activity, sensorimotor malfunctions and others. These factors can be monitored before and during the tests. Spontaneous object exploration can be used to assess memory for “what” (novel object preference; NOP) (Ennaceur and Delacour, 1988), for “where” (object place preference; OPP) (Ennaceur et al., 1997), and for “when” (memory for temporal order or recency; TOM) (Mitchell and Laiacona, 1998). A direct way to measure episodic-like memory is to combine the three “what”, “where” and “when” object exploration tests into one integrated paradigm (this test was first developed for rats (Kart-Teke et al., 2006) and then adapted for mice (Dere et al., 2005b). In this test, two sets of four objects (what) are placed at different locations (where), whereby the temporal appearance of each set of objects is also different (when). Based on the experimental model, episodic-like memory has been found to be not simply a combination of “what”, “where” and “when” memories, but a meta-system which integrates these three components into a complex and distinct compound system (de Souza Silva et al., 2016).
Following the same principle of combining the NOP, OPP and TOM tests, various episodic-like memory paradigms were developed (Davis et al., 2013a; Davis et al., 2013b; Good et al., 2007a; Good et al., 2007b). For example, two distinct objects were presented, followed by another two distinct objects at different locations, and then all the four objects were placed, while the object-locations of one of the two objects from each temporal set was interchanged. As a result, the characteristic of each object applied is determined by what type of object, when it was shown and where it appeared, similar to the Kart-Teke et al paradigm (2006). Recently, Barker and colleagues exploited this variant of episodic-like memory test and found important neurobiological correlates to episodic-like memory (Barker et al., 2017) (see section 4.2).
Here we will detail the properties of spontaneous object exploration paradigms, the NOP (what), OPP (where) and TOM (when) tests, and the integrative episodic-like memory paradigms. We will focus on the evidence primarily found via training-free models in rodents and propose a working hypothesis that identifies the medial prefrontal cortex (mPFC), lateral entorhinal cortex (LEC) and hippocampus as key brain substrates of a neuronal network that subserves episodic memory. The neurotransmitter systems within the mPFC and hippocampus are crucial for the processing of memory and will also be discussed. We will primarily refer to the articles published after 2007 since the findings of NOP, OPP and TOM tests have been summarized (Dere et al., 2007). “Context”-manipulated object exploration tests, e.g., the What-Where-Which test (Davis et al., 2013b; Eacott and Norman, 2004), will not be reviewed. We consider that this topic is worthy to be discussed in a separate article, since it has been actively studied and debated in concept and experimental definition of context, particularly in the context (source) of episodic memory.
2. Basic concepts and methodological factors for measuring object-, place-, time- and episodic-memory based on object exploration
2.1. Novel object preference (NOP)
The term “novel object preference (NOP)” applies to the situation where an animal spends more time exploring a novel object than a familiar one. The NOP test has become one of the most frequently used behavioral paradigms in the fields of neurobiology, psychopharmacology and behavioral neuroscience in the past two decades (Ennaceur and de Souza Silva, 2018).
The NOP test are said to utilize no obvious positive or negative reinforcer and to be dependent on the natural tendency of rodents to explore novel objects more than old ones (Berlyne, 1950; Ennaceur and Delacour, 1988). However, all behaviors are “motivated” and guided by positive and negative outcomes (reinforcers). One can argue that the exploration of novelty is likely motivated by the possibility of a potential source of reward, i.e., positive reinforcement, but also by negative reinforcement, such as escape from boredom and reduction of anxiety/fear, in the case it turns out to be that the novel object is not dangerous or threatening, i.e., has a benign outcome. Novel object exploration might also be influenced by motivation for social interaction (with the object) or by novelty seeking; i.e., novelty per se being a reinforcer. Conversely, animals may fear a novel object, and a neophobic animal might explore less, without necessarily meaning that it does not remember. A change in NOP resulting from a brain lesion, pharmacological challenge or genetic manipulation could, therefore, be a consequence of action on any of these potential variables that can influence object exploration. These variables have not been earnestly considered in the literature using the NOP test.
In rodents, the classical design of the NOP test involves a sample/encoding/learning trial and a test/retrieval/recall trial, separated by a time delay/retention interval. During the sample trial, two identical objects are presented in a testing arena (usually an open field to which animals are previously habituated). In the test trial, one of these is replaced by a novel unknown, but comparably preferred, object (Fig.1A, top). Since two distinct objects are presented in the test trial, their locations should be counter-balanced between subjects to minimize the possible bias of object-location. A preference for exploring the novel object over the familiar one indicates memory of the familiar object that was previously explored in the sample trial. The time differences between the exploration of familiar and novel objects are subtracted and used for the binary judgement of the object memory. Details about how to measure, calculate and perform statistics for the exploration differences can be found in these articles (Akkerman et al., 2012a; Akkerman et al., 2012b). The popular nomenclature of this paradigm, “novel object recognition”, somehow misrepresents the concept of this test, since, instead of recognizing the novel object, the one recognized is the familiar one (Ennaceur, 2010). A term such as “novel object preference” or simply “object recognition”, for exploring the novel object better reflects that the underlying memory is for the previously explored object.
Figure 1.
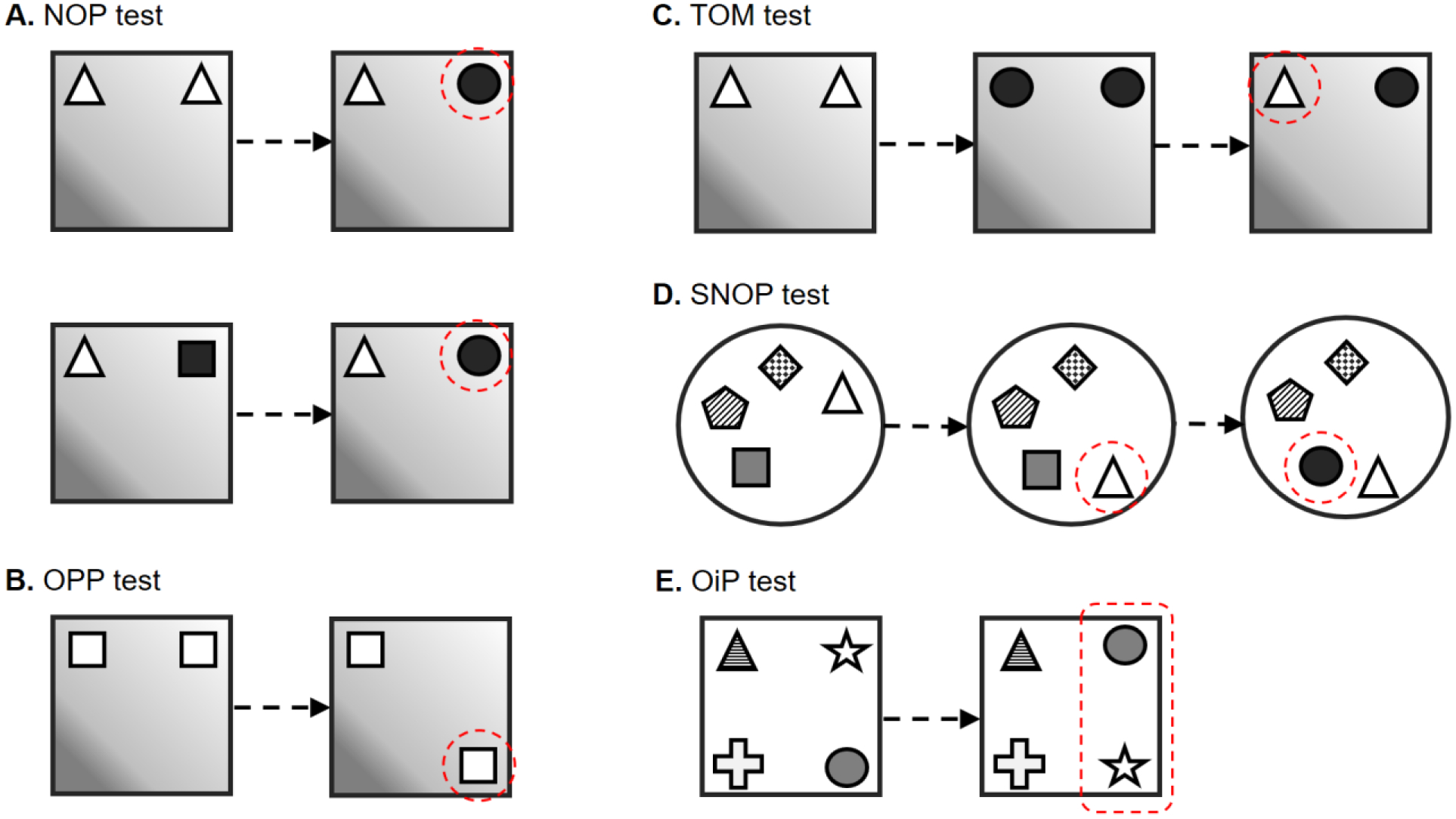
Schematic diagrams of spontaneous object exploration paradigms. (A) Novel object preference (NOP) test. (B) Object place preference (OPP) test. (C) Temporal-order preference memory (TOM) test. (D) Spatial and non-spatial object preference (SNOP) tests. (E) Object-in-place preference memory (OiP) test. Dashed circles indicate the exploratory preference of animals in nature, compared to the other object(s) in that specific trial.
Notwithstanding, some researchers apply two distinct objects in the sample trial of the NOP paradigm (Fig.1A, bottom). Compared to the application of identical objects, that of distinct objects is likely to involve not only novel object identity, but also inter-object relationship (Arain et al., 2012), which presumably activates brain regions for the processing of object-object and/or object-context association more than that for object identity. Not many studies have tried to differentiate the underlying mechanism of the NOP test with applying identical objects in the sample trial from that with distinct objects. When two distinct objects were placed in one context, followed by presenting two copies of one of the familiar objects in another new context, rats showed object preference according to the local cues, but not the spatial ones (Poulter et al., 2013), implying that animals were able to remember the relationship between distinct objects. Animals could recognize them as changes of local features, which has been shown to impact object recognition in animals with lesions on the hippocampus (Piterkin et al., 2008) and LEC (Kuruvilla and Ainge, 2017). In the object-location recognition test in which two distinct objects were presented, followed by two identical objects chosen from either the previously used ones, the LEC lesioned rats showed deficient performance, but not in the NOP test (Wilson et al., 2013c). Although the object-location recognition test measures whether animals remember the location of the changed object, it could potentially also be involved in the memory for the change of inter-object relationship (recognition on the alteration of local features). In a study of disconnecting mPFC and LEC, the memory tested by the NOP test with identical objects was intact, but not when distinct objects were applied during the learning (Chao et al., 2016a). Similarly, deficits were found in LEC lesioned rats when four distinct objects, but not when four identical objects, were presented to be learned (Rodo et al., 2017).This suggests that the recognition memory for identical objects is not as the same as the memory for distinct objects.
Rodents not only use the eyes, nose and forelimbs but also their mechanosensitive whiskers during object exploration (Sofroniew and Svoboda, 2015), and thus, the memory for objects is constructed by a convergence of multiple sensory systems. To study how different sensations contribute to object recognition memory, researchers have tried to dissect the visual (by presenting objects behind transparent barriers, which prevents physical contact), tactile (by presenting objects under red light, which masks the animals’ vision) and visual-tactile interaction (tactile condition for learning, followed by visual condition for testing, or vice versa, called cross-modal object recognition; CMOR) effects (Winters and Reid, 2010). This and related issues (Hu et al., 2018) have been studied by a series of systematic experiments based on the CMOR test (Gaynor et al., 2018; Jacklin et al., 2016; Jacklin et al., 2012; Jacklin et al., 2015; Paylor et al., 2018; Reid et al., 2012, 2014). The interaction between sensations and object recognition is an important topic, but will not be addressed here as it is beyond the scope of this review.
The NOP paradigm has sometimes been called and used to assess “episodic-like memory”, partly due to the conceptual similarity between the memory systems of recognition memory and of episodic memory. Although NOP processing could be involved in episodic memory, at least in the property of “what”, NOP memory does not necessarily demonstrate the properties of “where” and “when”. Thus, the NOP test, per se, is not an appropriate measurement of “episodic-like memory”.
2.1.1. Factors influencing performance
Here we only discuss the methodological factors of NOP. Factors that directly influence the biological states of experimental animals, such as species (Stranahan, 2011), strains, colony conditions, gender (van Goethem et al., 2012), stress (Eagle et al., 2013; Li et al., 2012; Nava-Mesa et al., 2013), early experience (McLean et al., 2010; Plescia et al., 2014), diet (Beilharz et al., 2014; Sarfert et al., 2017) etc., are not in the scope of this review.
Similar to many other behavioral paradigms, any factor involved in either one of the NOP procedural steps can influence the outcome. One factor is the habituation to the testing arena which is used to reduce anxiety and arousal levels of animals (Okuda et al., 2004; Roozendaal et al., 2006). Animals which were not habituated to a testing environment had a twofold higher plasma corticosterone level (Okuda et al., 2004) and displayed more anxiety-related behavior in the open-field test (Maroun and Akirav, 2008) compared to habituated ones. NOP memory tested 24 hours later was found to be disrupted in the non-habituated, but not in the habituated animals (Maroun and Akirav, 2008). Interestingly, exposure to a stressor (30 min on an elevated platform with lights) immediately after the sample trial of NOP, reversed the effects on the habituated and non-habituated animals (Maroun and Akirav, 2008). This suggests that the initial level of arousal (related to habituation versus non-habituation) interacts with the stressor following the learning and influences NOP consolidation. Habituation to the testing environment, which reduces novelty-induced arousal or anxiety, directly contributes to memory for objects. Thus, a habituation procedure is important for NOP testing (Yi et al., 2016) and should be conducted unless experiments are designed to test stress-related issues. In the same vein, any treatment which causes deficiency in habituation learning, such as lesion, pharmacological administration, gene knockout, opto- and chemogenetic manipulations etc., should be considered as a confounding factor when NOP performance is impaired. The procedure of environmental habituation varies among different laboratories, such as absence or presence of objects inside the testing arena (Besheer and Bevins, 2000). For pharmacological experiments, vehicle injections during habituation are suggested, as studies have shown that the first-time injection increases the duration of exploratory activity (Akkerman et al., 2012a).
The degree of habituation learning can be examined by behavioral changes in either within or between sessions (Schildein et al., 2002; Thiel et al., 1998) and used to determine whether a manipulated experimental factor influences the process of habituation.
The second factor is the duration of exploration of objects. In the classic NOP test in which two identical objects were used, studies have shown that a minimum duration of object exploration (reach 10–20 seconds in rats and mice) is essential for testing NOP, both in the sample and test trials, in order to decrease large variation in the test trial (Akkerman et al., 2012a). Once the minimum amount of exploration is reached, the level of exploration in the sample trial is not correlated with the performance in the test trial (Akkerman et al., 2012a; Gaskin et al., 2010). The duration of object exploration is associated with the nature of the objects. The qualities of the objects, e.g. weight, shape, size and texture, should be well-controlled and odors from objects themselves or from animals between trials should be avoided. Objects should be heavy enough to prevent from being moved by the subjects. Any object that incurs significantly higher, lower or no exploration should not be used. Objects made of glass or porcelain are recommended since they can be easily and well cleaned. Objects that can or cannot be climbed by animals have similar but subtle effects in male C57BL/6J mice, which explored mountable objects longer than non-mountable ones and showed better discrimination between them (Heyser and Chemero, 2012). Climbing on an object can be considered as object exploration only when the head of the animal is directed towards the object, as a sign of paying attention on it. Neophobia towards objects, especially when encountering them for the first time, could be another potential factor affecting exploration of objects. Ennaceur and colleagues examined this possibility and found that rats did not exhibit neophobia towards objects (Ennaceur et al., 2009), while this issue is dependent upon the genetic backgrounds of rodents (Binder et al., 2015). A sufficient amount of object exploration time is also required to ensure that animals are not avoiding them. The biological rhythm may contribute to the level of exploration since rodents are nocturnal animals, and many studies have tested them during their “sleeping” time. Some studies on circadian phase have reported that when the NOP test was administered during rodents “sleeping time”, there was no influence on locomotor activity and object recognition (Beeler et al., 2006; Takahashi et al., 2013), but that OPP memory was affected, which has been shown to be better at night (Takahashi et al., 2013). Recent findings indicate that C57BL/6J mice kept under normal light/dark cycles perform better NOP memory at midday than midnight (Tam et al., 2017). Whereas sleep is important for memory consolidation (Binder et al., 2014; Born et al., 2006; Born and Wilhelm, 2012; Chen et al., 2014; Ishikawa et al., 2014; Prince et al., 2014), long-term NOP and OPP performance were impaired when sleep was interrupted after the learning trial (Sawangjit et al., 2018). Significant differences in memory processing may be expected in the comparison of normal versus reversed light-dark cycle. The light intensity applied during the test should not be over 350 lux, as evidenced by an impairment of NOP memory (Tam et al., 2016). To increase exploratory activity, rodents can be isolated in an empty cage for some minutes (Vanmierlo et al., 2016) or food-restricted for one week before the test (Wang et al., 2017a), although food restriction itself has been shown to have promnestic effects in adult and aged mice (Talhati et al., 2014). The definition of object exploration is variable between different laboratories. Some define object exploration as physical contact with objects, while others use exploration within a distance range, e.g. when animals approach an object within 2 cm. Compared to exploration without contact, the former criterion might underestimate the amount of exploration. The criterion for terminating the sample or test trial is also variable between studies and, thus, can influence the amount of exploration. Some apply a fixed time window of exploration, e.g. 5 min per trial, while others use a fixed amount of object exploration, e.g. when animals explore objects for 20 seconds. The amount of time for exploring objects is usually taken as a measure of attention and/or motivation for object exploration. This measure can be obtained in data collected from experiments with a fixed duration (because there is variability in the amount of object exploration), but not from a fixed amount of exploration (because the amount of object exploration is equal for all subjects). Alternatively, animals could be excluded if the duration of object exploration is below a certain level, e.g. less than 10 seconds within 5 minutes. Overall, the level of exploration of objects is essential in NOP testing and the related factors need to be controlled.
If multiple sample trials are applied, the intervals between them influence object memory. For instance, application of five sample trials within one day resulted in weaker object memory than trials spaced over five consecutive days (Bello-Medina et al., 2013), which is consistent with previous studies (Anderson et al., 2008). This suggests that time plays an active role in regards to memory consolidation and this issue should be considered when several sample trials were applied.
NOP performance is variably dependent upon the time interval between the learning and test trials (Ennaceur and Delacour, 1988). Memory weakens over time unless it is strongly consolidated. Thus, the longer the retention interval, the more likely that retrieval/memory is disrupted. The time delay between trials of the NOP is a widely applied experimental factor for studying the extent of memory. Shorter versus longer retention intervals are taken as measures of short-term versus long-term memory (Kesner and Hunsaker, 2010). When a 0 second interval is applied in the NOP test, the performance could be taken as the measurement of “object-based attention” (Alkam et al., 2011; Alkam et al., 2013), and/or of ultra-short/working memory (Chao et al., 2018; Wang et al., 2017a). Early studies have indicated that rodents have memory for objects for up to 24 hours (Ennaceur and Delacour, 1988), and even up to 48 hours (Liu et al., 2016). With the help of sleep for the facilitation of NOP consolidation, the memory can be preserved for up to 3 weeks (Sawangjit et al., 2018). It is noted that the amount of object exploration in the learning trial is positively correlated with the degree of retention in regards to object exploration performance (at least within an amount of object exploration time). The longer the amount of object exploration, the more an animal remembers after a longer retention (Akkerman et al., 2012a; Federman et al., 2013; Ozawa et al., 2011). Therefore, the combination of different durations of object exploration and retention intervals can be used to create different strengths of memory. Discrepancies between studies can be due to the reason that one may apply a shorter object exploration during the sample trial to cause deficient memory after a shorter retention interval, while another might apply a longer object exploration time to establish intact memory after a longer delay. This factor is important in psychopharmacological studies, as researchers manipulate the extent of object memory to examine amnestic or promnestic effects according to the expected effect of the testing substance (Akkerman et al., 2014).
2.2. Object place preference (OPP)
A test similar to the NOP test has been adapted for assessing memory for an object situated in a particular place (Ennaceur et al., 1997). The same procedure as for the NOP test is applied, except that one of the familiar objects is placed in a new location in the test trial to generate a setting for testing memory of place (Fig.1B). This design measures memory for where an object was located by testing for preference for a known object placed in a novel location. More exploration of the object at a new location than at its original location implies memory for the localization of the object in a previous location. OPP memory can be preserved longer than 1 week with the help of sleep (Sawangjit et al., 2018). In addition, it has been found that the half of the tested mice was able to retain OPP information for up to 6 months, but not after 1 year (Atucha et al., 2019).
The methodological factors which impact NOP also influence the OPP test. Spatial features around the testing arena are usually provided to serve as allocentric environmental cues. Thus, possible bias toward object-location could emerge due to spatial bias within the testing arena. This factor should be eliminated or controlled by adjusting the environment and/or counter-balancing the location of objects. In some earlier studies (Escorihuela et al., 1995; Howlett et al., 2004; Hryniewicz et al., 2007), one single object was presented, followed by the copy of the explored object and a novel object. In this case, the novelty pertains not only to the location of object, but also the identity of object (since the new object was novel in both place and identity). To control this factor, the new object can be placed at the previously old location, while the explored object is placed at a novel location (trying to counter-balance the “novelty”). As this design involves complex object and place interaction, the results should be carefully explained, and may likely not reflect only OPP processing. A variant of the OPP test used two distinct objects as the sample, while in the test trial two identical objects selected from either the explored type were placed at the familiar locations (Wilson et al., 2013c). This test is intended to measure the memory for the location of the changed object. However, since two distinct objects are used in the sample trial, it could simply reflect the mnemonic changes of local features (see 2.1.). Another OPP-like paradigm applies a single object during the learning trial, followed by two copies of the explored object, with one of them placed at the old location (Van Cauter et al., 2013). Since the identity of objects remains unchanged, the underlying processing should preferentially be engaged in dealing with place. The placing of animals into the testing arena from the same or different starting locations can potentially impact an OPP-like test, as it was shown that hippocampus-leisoned rats showed a memory impairment when starting to explore from different, but not the same, locations (see 3.3.2; (Langston and Wood, 2010).
2.3. Temporal-order preference
Spontaneous object exploration can be used to assess memory for time or for temporal-order memory (TOM). This test employs two sample trials and one test trial, separated by intervals (Mitchell and Laiacona, 1998). Sets of objects are presented in the two sample trials at distinct time points, and in the test trial one object from each set is placed together. A preference to explore more the object which was presented earlier than the one presented later, suggests a memory for differentiating how recently which object was encountered (Fig.1C).
The methodological factors in the NOP test are suggested to apply to the TOM test as well. Since there are two sample trials in the TOM test, the duration of exploration of objects on both sample trials should be controlled. Otherwise, a bias toward one of the presented object sets could confound the outcome. Another notable factor is the time delay between the two sample trials, which presumably affects the memory strength between the two sets of objects. The TOM test is designed for animals to remember both sets of objects, while forming differential memory decay for each one. One could argue that the earlier presented object is explored more than the later one because of the forgetting of the earlier object. To exclude this argument, a separate NOP test can be used to investigate whether animals have memory for the object encountered in the first time point by applying an inter-interval interval corresponding to the summation of the two intervals applied in the TOM test. Studies have reported that rodents can retain object information for at least 24 hours after the exposure (Ennaceur and Delacour, 1988). Related information can be found in (Hatakeyama et al., 2018), who examined in detail the number of objects used in the sample trial, the exposure time for encoding and the length of the inter-trial interval using the TOM test.
An interesting question is whether animals can sence intervals of hours. Studies in training animals with classical and operant conditioning have shown that animals can behaviorally respond at certain intervals (seconds to minutes) according to the programmed stimulus (Kirsch et al., 2004; Kyd et al., 2008), which implies that animals can program intervals of seconds to minutes dependent on cognitive- and/or motivational-driven mechanisms. Long intervals of days can be timed with internal biological systems of circadian rhythms in animals (Buhusi and Meck, 2005). Timing intervals of hours in animals, however, is controversial. Rats are able to anticipate food with 24-, but not 18-hour intervals (Petersen et al., 2014), while 7–13 hour intervals were later found to be anticipated (Crystal, 2015). It was also reported that object information in the TOM test with interval of 3 (Barker et al., 2007), but not after 24 (Mitchell and Laiacona, 1998), hours could be “recognized” by rats.
2.4. Sense of time
In the sense of the common definition, “when” can be indicative of a specific time point, as well as a period of time (a time interval). Humans recollect an episode with both strategies. What about animals, do they retrieve an experience based only on how long ago? Episodic-like memory in animals might be qualitatively different compared to ours. Or does the essence of episodic-like memory in animals resemble human episodic memory in the ability to apply both strategies?
There are two critical studies addressing this issue. Using a similar paradigm as designed by Babb and Crystal (2006), Roberts et al. manipulated temporal cues of “when” (a specific time point during a day), “how long ago”, or both, to test rats’ episodic-like memory. They found that rats are sensitive to the cue of “how long ago”, but not to the cue of “when”, and questioned the similarity between episodic-like memory in animals and human episodic memory (Roberts et al., 2008). Zhou and Crystal (2009) conducted a study which dissociates the cue of “how long ago” with rewards and made rats retrieve an event according to “when”, per se. Their findings illustrated that rats are capable of recollecting episodic information according to the cue of a specific time point, which resembles human episodic memory (Zhou and Crystal, 2009). Another approach to study the when component of episodic memory in animals is to look into the future. Humans recollect a specific experience in the past not only for remembering, but also for planning the future. The concept of “mental time travel” implies that the travel can be retrograde or anterograde. There is evidence that animals are capable of prospective memory defined as an ability to inactivate an action for a current situation but re-activate it again at an appropriate future time point (Wilson and Crystal, 2012; Wilson et al., 2013a).
The remarkable findings of time cells in the hippocampus has implications for the understanding of the neurobiology of time. The first clear evidence came from the hippocampal CA1 neuronal ensembles recorded during a task that required the rat to remember the order of a sequence of odor stimuli. Animals were trained to learn a series of odors presented one after another, and asked to recall the earlier odor in the test trial in which two previous odors selected from different time points were located together. The CA1 neuronal ensembles gradually changed across the learning trial, and the memory performance could be predicted by the strength of this neuronal pattern. Thus, the CA1 neurons encode the temporal context information during the mnemonic learning of the odor presentations (Manns et al., 2007). In head-fixed monkeys were asked to learn the order of two objects, presented one after the other with a time delay, and in the test trial required to indicate the correct sequence of the appearance of shown objects. Their hippocampal firing pattern altered along the passage of time during the delays (Naya and Suzuki, 2011). In an associative task rats were required to remember specific object-odor pairings, with an odor being presented 5 to 20 seconds after the presentation of an object. A profound finding was that some CA1 neurons responded specifically during the waiting period. In addition, these temporal context-relevant firing cells were changed when the delay was increased, irrespective of the locations or behaviors of the animals, and, thus, were termed “time cells” (MacDonald et al., 2011). Time cells were also identified in the CA3 region (Salz et al., 2016). The hippocampal neuronal firing patterns reflecting temporal context have also been shown to gradually change from seconds to minutes (Manns et al., 2007), hours to days (Mankin et al., 2012), and weeks (Rangel et al., 2014; Ziv et al., 2013). In contrast, the concept of time could just be a preconceived idea (e.g., influenced by Immanuel Kant) and the brain generates no such representation of time (and space), but instead, variations in the strength of neuronal communication simply guide the direction of neuronal activity flow underlying behaviors (Buzsáki, 2013; Buzsáki and Llinás, 2017). Nevertheless, the existence of “time cells” in the hippocampus provides the fundamental explanation of when in the neurobiology of memory (Eichenbaum, 2014, 2017a).
2.5. Spatial and non-spatial object preference (SNOP)
The spatial and non-spatial object preference (SNOP) test (Fig.1D) is a paradigm which applies four to five distinct objects for the assessment of object and location memories by changing the place and identity of objects sequentially (Lee et al., 2005; Save et al., 1992). The common version of this paradigm utilizes shorter inter-trial intervals, e.g., 3 min, which assesses spatial and object novelty or short-term memory (Hunsaker et al., 2007a; Lee et al., 2005; Vago and Kesner, 2008), while it can be adapted to longer intervals (Van Cauter et al., 2008a, b). For example, after the habituation to the testing arena, four to five distinct objects were presented and an animal was allowed to freely explore them for several sessions (encoding trials). Then, one of the objects was re-located to a novel place (spatial novelty trial), followed by a novel object replacement (object novelty trial; Fig.1D). Rodents showed preference for exploring the displaced object more than the stationary ones, indicating intact spatial recognition. Also, they explored the novel object more than the familiar ones, as an indication for object recognition (Hunsaker et al., 2007a; Lee et al., 2005; Vago and Kesner, 2008).
Although the procedure of this paradigm is similar to the NOP and OPP tests, the differences between the SNOP and NOP/OPP tests are significant. First, the SNOP test applies more objects and more types of objects in the sample trial than the NOP/OPP tests, which involves complicated inter-object relationships (see section2.1. the discussion of applying distinct objects for learning). In this sense, the nature of this test is akin to the object-in-place test (see section 2.5) for measuring the memory for object/location-associative information. Second, the “quantity” of changed and unchanged information is not comparable between the SNOP test and NOP/OPP tests. For example, one out of five stationary objects is spatially changed in the SNOP test (1:4), compared to one of two stationary objects replaced in the OPP test (1:1). Third, the object novelty trial is followed by the spatial novelty trial, which could potentially be influenced by a sequential effect, e.g., the animal might be more alert in the object-novelty trial since it had learned that the object-contextual environment was altered in the spatial-novelty trial. Cautions should be applied in the interpretations of this paradigm.
Since the SNOP paradigm involves changing spatial and object information sequentially, the methodological factors in the NOP, OPP and TOM tests are also relevant for this test.
2.6. Object-in-place preference (OiP)
This paradigm was designed to evaluate recognition memory for the association of objects and their locations (Barker et al., 2007; Barker and Warburton, 2009, 2011b; Bussey et al., 2000; Good et al., 2007a). The OiP test involves one sample and one test trial, separated by a time delay. Four distinct objects, each at a different location, are presented in the sample trial, and two of them are interchanged by location in the test trial (Fig.1E). Rodents explored the two displaced objects more than the two that were stationary. This preference evidences that the animals have memory for the previous places of the objects, i.e., established an association between specific objects and specific locations (for review see (Warburton et al., 2013).
Compared to the NOP and OPP tests, the OiP test presents four distinct objects, instead of two identical objects, as the information to be learned. This test is similar to the SNOP test that applies multiple distinct objects in the sample trial and presumably requires higher memory capacity to learn than the NOP/OPP tests. In addition, since four distinct objects are presented, inter-object relationships are created which would likely engage somewhat different brain regions for memory establishment (see section 2.1.). Theoretically, a failure to form an OiP memory could result from deficits in either the NOP, the OPP, the association of object and location, or their combinations. Thus, the NOP and OPP tests are usually conducted independently along with the OiP test to exclude the factors for impairments in recognition of object or location (Barker et al., 2007; Barker and Warburton, 2011b, 2015; Barker and Warburton, 2018). Whereas the number of objects are fewer in the NOP/OPP tests than in the OiP test (2 versus 4), another confounding possibility is that the OiP test may require higher memory load. If the OiP memory is disrupted, along with intact NOP and OPP performance, the compromised association of object and location might not be the sole reason.
The methodological factors in NOP and OPP tests are also applicable to the OiP test.
2.7. Episodic-like memory preference
The episodic-like memory test developed by Kart-Teke et al. (2006) is meant to gauge the integration of memory for NOP (what), OPP (where) and TOM (when) (Kart-Teke et al., 2006). The combined “what-where-when” memory, however, is not merely a summation of the three mnemonic components, given that “what-where-when” memory could be impaired under the circumstance of intact individual memory for “what”, “where” and “when” (details see section 4.2). Thus, the development of such tests is essential for the understanding of the nature of the prototype of episodic memory. The Kart-Teke’s paradigm is a training-free test in which two different sets of equal objects are used (Fig.2A). Four identical objects, the first set (sample trial 1), are placed at four of eight possible different locations at the periphery of the arena and animals are allowed to explore them for a period of time. The same procedure is applied after a time delay (inter-trial interval), with four objects from the second set (sample trial 2). Two of these four objects are placed at locations which were occupied by the previously presented objects (sample trial 1), while the other two objects are placed at locations which were not occupied before. After another delay, the test trial is applied with two objects from each set placed together: One object of each set is placed at the same location it occupied before, and the other is placed at a novel location. However, all the objects are placed only at locations that have been previously occupied in sample trial 1 or 2. Thus, a specific “what-where-when” setting is created, including one older familiar object at the stationary location (OS), one older familiar object at a displaced location (OD), one recent familiar object at the stationary location (RS) and one recent familiar object at a displaced location (RD) (called ELM2–2 test here: the episodic-like memory test with the application of 2 sets of objects and displacement of 2 objects). In this test, adult rats exhibit a pattern of exploration preferences, where RD > RS (where), OS > RS (when) and OS > OD. The exploration preference patterns, RD > RS (novel location over old one in recent familiar objects) and OS > OD (old location over novel one in older familiar objects), indicates an interaction between object-location and temporal-order, which demonstrates that the animals have episodic-like memory. Based on the duration of exploration of each object in the test trial, three indices are calculated:
Figure 2.
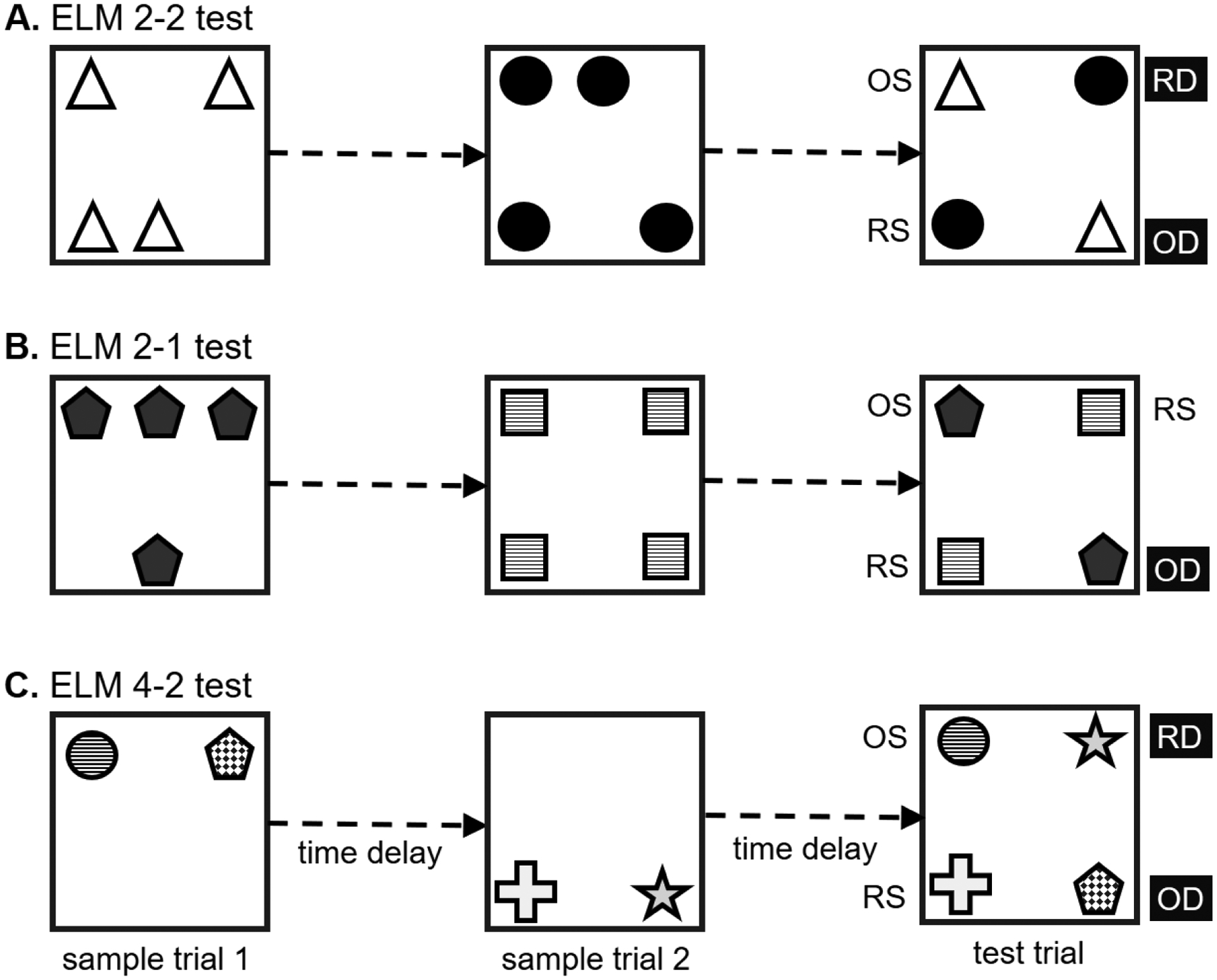
Schematic presentation of the training-free episodic-like memory paradigms with the use of spontaneous object exploration. (A) Test with two sets of objects and displacement of two objects in the test trial (ELM2–2). Two sets of objects, each with four copies, are presented separately at different time points (sample trial 1 and 2). After a delay, two objects from each set are presented together either placed at the same or different location(s). Thus, different spatiotemporal features are attributed to the objects, namely, one older-familiar object at the location that was occupied before (OS), one older-familiar object at a novel location (OD), one recent-familiar object at the location that was occupied before (RS) and one recent-familiar object at a novel location (RD). (B) Test with two sets of objects and displacement of one object in the test trial (ELM2–1). The ELM2–1 paradigm is similar to the ELM2–2 test except that only one object is displaced in the test trial, i.e. the OD. (C) Test with four distinct objects and displacement of two objects in the test trial (ELM4–2). The object arrangement of this test is comparable to the ELM2–2 test but involves four distinct objects.
Positive values of the where and when indices represent the expression of memory for object location and temporal order. A negative value of the Interaction index is shown because of the exploration preference OS > OD exhibited by animals. This counterintuitive result (OS > OD) indicates that the exploration pattern of objects according to their location is influenced by the temporal-order in which they have been experienced in the past (Chao et al., 2016a; Chao et al., 2017; de Souza Silva et al., 2016; Drieskens et al., 2017) and provides a strong argument that the what, where and when information was not inter-communicating independently in this test. The reason for this OS > OD pattern might be due to weaker memory trace for place than for time of the first set of objects, whereby the higher exploration toward OS was proportionally contributed to by the interaction from the second set of objects (temporal order effect). Such an effect would not favor OD. This difference may come from the processing of the second sample trial in which half of the objects were located in previously occupied locations, while the other half was not. Thus, in the second sample trial, those objects at the new locations would form an active memory for place. In the testing trial, OD was placed at one of the locations related to an active place memory; whereas OS had always been located at the location with no such trace memory. The novelty for the displacement of OD could then be “nullified” by the previously active trace for place (reconsolidation effect). Thus, the animals explored OD like another RS. The uniqueness of this model is not only the measurement of where or when memory within a single test trial, but also the integration of distinct what, where and when properties being hypothetically converged into an “episodic-like memory”. Therefore, a neurobiological system which integrates and organizes different sources of information to form such a memory should exist (as discussed below).
In another version of this paradigm the displacement of two objects is simplified to one object (called ELM2–1 test here; the recent familiar object at a displaced location, RD, is not presented; Fig.2B). In this case, object-location and temporal-order memory are tested, while the interaction between these two factors cannot be verified. In this test there is an exploration preference for the novel location over the old location of older-familiar objects (Dere et al., 2005a, b). Three indices can be derived from this paradigm:
Positive values for these indices indicate intact memory for where, when and what.
The third version of episodic-like memory paradigm involves in four distinct objects (called ELM4–2 test here; Fig.2C). The principle concept of the ELM4–2 test (Barker et al., 2017; Davis et al., 2013a; Good et al., 2007a; Good et al., 2007b) is comparable to the Kart-Teke et al. paradigm (2006) by presenting OS, OD, RS and RD objects. However, the differences between ELM4–2 and ELM2–2/2–1 are noted. First, the number of objects used in the sample trials is not identical (4 versus 2). Second, the types of objects are different, as four distinct objects versus two sets of identical objects, are used. This could increase the information complexity, as distinct inter-object relationships are formed in the four distinct objects paradigm. Third, the exploration pattern OD > OS is found, unlike that of the ELM2–2 test. These differences are due to the procedure for the applied number and types of objects in the sample and test trials. The comparison between the ELM4–2 and ELM2–2/ELM2–1 tests is similar to that of the NOP/OPP and SNOP/OiP tests (see sections 2.4 and 2.5). In this ELM4–2 paradigm, two indices can be calculated:
Positive values of Where and When indices demonstrate intact memory for object-location and temporal-order, respectively (Barker et al., 2017).
The original study using the ELM2–2 paradigm reported that the episodic-like memory was preserved for at least 1 hour (Kart-Teke et al., 2006). Studies manipulating the time interval between trials have shown that rats retain the episodic-like memory for over 2 hours (Belblidia et al., 2015), but not after 4–6 hours (Belblidia et al., 2015; Chao et al., 2014). Other studies have shown rats to retain “what-where-when” memory for up to 24 hours in the ELM2–1 test (Barbosa et al., 2010; Barbosa et al., 2013).
The replicability of the ELM2–2 and ELM2–1 tests is verified by independent laboratories (Barbosa et al., 2013; Castilla-Ortega et al., 2012; Castilla-Ortega et al., 2014; Drieskens et al., 2017; Fernandez and Garner, 2008; Inostroza et al., 2013a; Inostroza et al., 2013b; Lanté et al., 2015; Li and Chao, 2008; Lopez-Pigozzi et al., 2016; Wang et al., 2010). In addition, studies in neurodevelopment have taken the concepts and advantages of this model to bypass the expression of language to measure the “prototype of episodic memory” in toddlers and pre-school children (Bauer et al., 2016; Burns et al., 2015; Newcombe et al., 2014; Russell et al., 2011). Also, this model has been adapted for the assessment of episodic-like memory in adult humans for the investigations on age, sleep, emotion and clinical issues (Kinugawa et al., 2013; Mazurek et al., 2015; Pause et al., 2010; Weber et al., 2014; Zlomuzica et al., 2016). Findings and limitations of these tests are also well-reviewed by (Binder et al., 2015).
The episodic-like memory paradigms (Fig.2) are dependent upon the spontaneous object exploration tests of NOP, OPP and TOM, and thus, theoretically are influenced by all the methodological factors involved in these tests. Habituation to the environment, materials and properties of objects, configurations of placement of objects, spatial cues around the environment and time intervals between trials should be carefully controlled.
3. The prefrontal cortex, entorhinal cortex and hippocampus as a memory system
Based on studies of patients with injury to the medial temporal lobe, it is well-accepted that the hippocampus is crucial to the establishment of episodic memory (Burgess et al., 2002; Eichenbaum, 2013; Squire and Zola-Morgan, 1991; Tulving and Markowitsch, 1998). In vivo brain imaging studies during episodic encoding and retrieval have identified several other brain regions that are also critically engaged, including the PFC, retrosplenial cortex, parietal cortex and regions surrounding the hippocampus. The anatomical and functional interactions between these regions are intricate and implicated in the formation and retrieval of episodic memory. Diffusion tensor imaging and functional magnetic resonance imaging (fMRI) studies show strong links between the PFC and medial temporal lobe during episodic encoding (Schott et al., 2011; Schott et al., 2013). Electrophysiological recordings from epileptic patients during correct retrieval of episodic memory suggest the medial temporal lobe to act as a hub to interact with the lateral PFC and parietal cortex to form inter-regional connecting networks (Watrous et al., 2013). We will discuss the roles of the mPFC, EC and hippocampus in determining what, where and when object exploration tests and how their interaction influences the establishment of episodic-like memory.
Studies that do not focus on regional-specific effects, such as systemic or intracerebroventricular pharmacological administrations and global gene manipulations, will not be discussed here.
3.1. The role of the medial prefrontal cortex
Like in primates, the PFC in rodents is considered to participate in functions such as attention, decision making and memory (Chudasama, 2011; Dalley et al., 2004; Preston and Eichenbaum, 2013). The rodent mPFC can be anatomically divided into three subareas, namely the anterior cingulate cortex (ACC), prelimbic cortex (PLC) and infralimbic cortex (ILC), which are located along the dorsal – ventral axis (Dalley et al., 2004; Gabbott et al., 2005), and are reciprocally interconnected (Heidbreder and Groenewegen, 2003). Topographical distribution of projections are mapped from the dorsomedial PFC predominantly to sensorimotor regions and from ventromedial PFC to limbic regions (Hoover and Vertes, 2007). The mPFC could exert control over sensorimotor, emotional and memory systems through its glutamatergic axons (Hoover and Vertes, 2007). Projections of the prefrontal cortical γ-aminobutyric acid (GABA)-ergic neurons to the nucleus accumbens also exist (Lee et al., 2014). The mPFC has been discussed to be involved in planning, temporal processing, attention, behavioral flexibility, goal-directed, social and emotional behaviors (Dalley et al., 2004; Euston et al., 2012; Riga et al., 2014), and its interplay with the hippocampus is implicated in the processing of memory, especially episodic memory (Eichenbaum, 2017b).
The PFC neurons activate for storage of object information (Smith and Jonides, 1999), and ACC neuronal firing is correlated with exploratory behavior in the NOP test (Weible et al., 2009). Furthermore, c-fos (a marker for neuronal activity) expressing in the ACC was found when rats explored a novel object more than a familiar one (Zhu et al., 1995). However, lesions in the mPFC did not affect NOP performance (Ennaceur et al., 1997; Mitchell and Laiacona, 1998), consistent with subsequent studies in rats and mice (Baran et al., 2010; Barker et al., 2007; Barker and Warburton, 2011b; Cross et al., 2012; McAllister et al., 2015; Spanswick and Dyck, 2012). Pharmacological inhibition of the mPFC with muscimol, a GABAA-R agonist, also had no effect in the NOP test when infused before the sample trial (Neugebauer et al., 2018; Pezze et al., 2017). This implies that the mPFC is either not required for object recognition memory, or that other circuits compensate for this function when the mPFC is inactivated. Optogenetic stimulation of the mPFC glutamatergic neurons did not affect memory for NOP and OPP, but facilitated OiP memory, which is involved in object association information by switching inter-object locations (Benn et al., 2016). Whether the mPFC is engaged in memory for NOP, seems to depend on the properties of the object being encoded. When distinct objects were used as samples, object memory consolidation was deficient in animals with inactivated mPFC (Akirav and Maroun, 2006). A possible explanation is that associations between distinct objects (e.g. relative inter-object locations) are processed by the mPFC, while such associative learning is not required when identical objects are applied. This interpretation is in accordance with the perspective of the role of the PFC as a flexible supporter to memory when conditions demand “specificity” (Euston et al., 2012). Consistently, lesion of the mPFC disrupted memory in the OiP test in rats (Barker et al., 2007; Barker and Warburton, 2011b; Cross et al., 2012).
Alternatively, the mPFC has been suggested to play a specific role in memory consolidation and retrieval. A recent study showed that inhibition of the mPFC with Designer Receptors Exclusively Activated by Designer Drug (DREADD) given after the sample trial, impaired both NOP and OPP memories tested 24 and 4 hours later, respectively (Tuscher et al., 2018), suggesting a critical engagement of the mPFC in memory consolidation (Akirav and Maroun, 2006). Pre- (Nagai et al., 2007) or post-sample (Tanimizu et al., 2018) infusions of anisomycin, a protein synthesis inhibitor, into the mPFC disrupted long-term NOP memory in mice. Also, electrical stimulation of the ventromedial PFC facilitated NOP memory and hippocampus proliferation in middle-aged rats (Liu et al., 2015). In addition, pre-test microinfusions of muscimol into the rat ACC impaired the NOP memory tested after 24 hours, but not 20 min, implying the ACC is involved in the retrieval of NOP in a time-dependent manner (Pezze et al., 2017).
The prefrontal cortical neurons were found to respond to spatial goals (Hok et al., 2005) and single-cell recordings in the ACC demonstrated that the ACC is associated with OPP performance (Weible et al., 2009). In a modified NOP test in which only one of the explored objects was presented in the testing trial, some ACC neurons responded to the location of the absent object (Weible et al., 2012). When the PFC was damaged, however, the OPP memory was not influenced (Baran et al., 2010; Barker et al., 2007; Barker and Warburton, 2011b). Conversely, mice with PFC stroke showed impaired OPP, but not NOP, memory, along with reduced structural volume in the dorsal medial nucleus of the thalamus (Zhou et al., 2016). Long-term (24 hours), but not short-term (5 min), OPP memory was deficient when a cAMP response element binding protein (CREB)-binding protein, histone acetyltransferase, was reduced in the mouse PFC (Vieira and Korzus, 2015). Given that the mPFC and hippocampus interact in a complementary way to process spatial information (Chao et al., 2017; Eichenbaum, 2017b; Lee and Kesner, 2003; Maharjan et al., 2018), it is possible that the object-place processing can be taken over by the hippocampus when the PFC is dysfunctional.
The PFC has been considered to account for the processing of time. TOM was impaired in rodents with selective lesions of the mPFC (Barker et al., 2007; Barker and Warburton, 2011b; Cross et al., 2012; Mitchell and Laiacona, 1998) or lidocaine injection (Hannesson et al., 2004). However, TOM deficits were not found in an animal model of ischemic lesion in the mPFC (Deziel et al., 2015). The catecholaminergic systems in the PFC are involved in the processing of TOM, given that catecholamine depletion in the mPFC induced by 6-hydroxydopamine (6-OHDA) disrupted this memory, but neither memory for NOP nor that for OPP (Nelson et al., 2011). However, this is contradicted by a similar study which reported impaired NOP memory after such a lesion (Kadowaki Horita et al., 2013). The findings in rodents are consistent with studies in human patients, with lesions to the ventromedial PFC, showing deficits in remembering past and imagining future events (Bertossi et al., 2016). The macaque ventrolateral PFC was also found to signal specific object-time relationships during a temporal order task (Naya et al., 2017).
The processing of OiP memory requires the participation of the mPFC, as evidenced by studies of lesions or blocking of glutamate-, acetylcholine (ACh)- or dopamine (DA)-R (Barker et al., 2007; Barker and Warburton, 2008, 2009, 2015; Savalli et al., 2015). In addition, medial prefrontal cortical DNA methylation, but not histone deacetylation, is involved in OiP memory (Scott et al., 2017). Thus, the medial prefrontal cortical DNA methylation, glutamate, ACh and DA systems mediate the recognition of specific object-place relationships.
The mPFC is unquestionably important for episodic-like memory. Higher expression of immediate early genes (c-fos and zif-268) was identified in the PFC and ACC after the learning of episodic-like memory employing conditioned-training of licking behavior (Veyrac et al., 2015). Higher zif-268 expression was also found after exposure to the ELM2–2 test of episodic-like memory (Barbosa et al., 2013). Rats with mPFC damage exhibited defective “what-where-when” memory when tested by the contextual conditioning (Li et al., 2011). Mice with lesion of the mPFC showed the impaired where, but not what or when, component assessed in the ELM2–1 test (DeVito and Eichenbaum, 2010).
Neurotransmitter systems have profound impacts on multiple synaptic functions and behaviors. The diversity of neurotransmitters and their receptors (R) mediate a broad spectrum of physiological as well as pathological status.
3.1.1. Glutamate
Glutamate is the major excitatory neurotransmitter in the brain that activate ionotropic glutamate-R (e.g., AMPA-, kainate and NMDA-R) and metabotropic glutamate-R (e.g., mGluR). Intra-infusions of MPEP, a mGlu5-R antagonist, into the rat PLC disrupted spatial memory tested by a cross-maze and NOP memory test (Christoffersen et al., 2008). Infusion of 6-Cyano-7-nitroquinoxaline (CNQX), an AMPA/kainate-R antagonist, into the PLC/ILC region (the dorsal mPFC) had no effect when given before the learning trial of NOP (Barker and Warburton, 2011a). When CNQX was infused into the PLC/ILC, either before the second sample trial or before the test trial of the TOM test, disrupted memory. Alternatively, intra-PLC/ILC infusion of 2-amino-5-phosphonopentanoic acid (AP5), an NMDA-R antagonist, impaired TOM performance when applied before the second sample trial, but not before the test trial (Barker and Warburton, 2011a). In the OiP test, pre-sample microinjections of CNQX or AP5 into the mPFC interfered short-term (5 minutes) memory in rats. The same pre-sample PFC infusions of AP5 also impaired OiP memory tested 1 hour later, but not when given pre-test (Barker and Warburton, 2008).
These findings indicate that the expression of NOP memory is dependent on the mGlu5-R, but not the AMPA/kainate-R, of the mPFC. The encoding/consolidation of TOM is dependent upon the medial prefrontal cortical AMPA/kainate- and NMDA-R, while AMPA/kainate-R, but not NMDA-R, are required for the retrieval of TOM. Both the PFC AMPA/kainate- and NMDA-R are indispensable for the encoding of specific object-location relationships, while NMDA-R engages in the learning and consolidation, but not recall, of OiP memory.
3.1.2. Dopamine
DAergic neurons in the brain largely originate from the substantia nigra pars compacta and ventral tegmental area, forming the nigrostriatal and mesocorticolimbic pathways, respectively. DA binds to a large family of G-protein coupled-R that can be classified into D1-like (D1- and D5-R; activate cyclic AMP production) and D2-like (D2-, D3 and D4-R; inhibit adenylyl cyclase activity). The interaction between the mPFC and midbrain DAergic systems plays a key role in the processing of NOP: A unilateral PFC lesion combined with a midbrain DAergic systems deficiency in the unilateral hemisphere, impaired NOP (90 min interval) if the lesions were in different hemispheres, but not when they were in the same hemisphere. The same disconnected lesions did not influence spatial working memory tested by a T-maze non-matching to place task (Chao et al., 2013). This implies that the midbrain DA interplays with the mPFC in the expression of NOP memory and that the communication between a non-lesioned side of the mPFC and midbrain DA systems is necessary for object memory. Interestingly, the rat with the unilateral DA deficiency also showed deficits in the TOM test, suggestive of a relationship between “time”, DA systems and PFC/hippocampus functions (Chao et al., 2013). Transcranial direct current stimulation onto the PFC also increased DA levels in the hippocampus and striatum, and NOP memory was thereby improved in spontaneous hypertensive rats (Leffa et al., 2016). In addition, DA levels were found to be elevated in the mPFC during the test trial of NOP memory in rats (McLean et al., 2017).
When administrated prior to the sample trial, microinjections into the mPFC of the DA D1/5-R agonist SKF81297 facilitated, and its antagonist SCH23390 disrupted, NOP memory tested 24 hours later (De Bundel et al., 2013; Nagai et al., 2007). Microinjections of SCH23390 into the PFC, but not into the hippocampus, also blocked the facilitating effects of systemic reboxetine, a norepinephrine (NE) reuptake inhibitor, on the performance of long-term NOP (De Bundel et al., 2013). Pre-sample prefrontal microinjections of SCH23390 did not impair the NOP memory tested 1 hour later (Savalli et al., 2015), although contrary results have also been reported (Clausen et al., 2011). Pre-sample infusions of SKF81297 into the PFC impaired short-term (1 hour) NOP (Pezze et al., 2015). In the OiP test, pre-sample infusions of SCH23390 or SKF83566, a DA D1-R antagonist, into the rat mPFC led to an impairment, tested 5 and 1 hour later. Pre-test infusions of SCH23390 into the PFC had no effect in the OiP (1 hour) memory (Savalli et al., 2015). The D1/5-R of the mPFC could bidirectionally regulate the encoding and/or consolidation of NOP memory, probably compatible with their inverted U-shape functions in the processing of working memory (Cools and D’Esposito, 2011). Together, the prefrontal cortical D1-R underlie the learning and/or consolidation, but not retrieval, of object-location relevant information.
Pre-sample microinjections of the DA D2-R antagonist L-741.626 into the PFC dose-dependently disrupted short-term NOP (2 min interval), which is consistent with its effects with acute systemic administration (Watson et al., 2012). Pre-sample infusions of the D3-R antagonist S33084 into the rat PFC dose-dependently improved NOP (4 hours interval), supported by the findings when injected systemically (Watson et al., 2012). Thus, pharmacological blockage of the prefrontal cortical DA D2- and D3-R leads to NOP deficiency and facilitation, respectively.
Microinjections of the D1-R antagonist SCH23390, the D2-R antagonist L-741.626 or the D3-R agonist 7-OH-DPAT into the PFC after the sample trial dose-dependently impaired NOP memory when tested 1 hour later (Papp et al., 2017). Conversely, post-sample microinjections of the D1-R agonist SKF81297, the D2-R agonist quinpirole or the D3-R antagonist SB277,011 into the PFC facilitated NOP memory when tested after 24 hours (Papp et al., 2017). However, a study shows that post-sample PFC infusions of quinpirole did not affect NOP performance (Rossato et al., 2013). Infusions of SCH23390 into the PFC also impaired long-term 24 hours NOP memory when administrated immediately, but not 6 hours, after the sample trial (Rossato et al., 2013). These results suggest that the prefrontal cortical DA D1-, D2- and D3-R critically modulate the NOP consolidation. Pharmacological blockage of DA D1- and D2-R and activation of D3-R disrupt short-term object consolidation, but pharmacological activation of DA D1- and D2-R and blockage of D3-R facilitate long-term object consolidation.
Collectively, DA plays a significant role in the regulation of the prefrontal cortical functions in the encoding and consolidation of NOP and OiP processing, irrespective of short- or long-term memory.
3.1.3. Serotonin
The primary source of serotonin (5-HT) derives from the raphe nuclei that project to the central nervous systems. 5-HT, by executing its action through the binding to more than 14 types of 5-HT-R, have received emphasis in the modulation of PFC-related functions and memory processing (Meneses, 2015). For instance, the selective 5-HT reuptake inhibitor escitaplopram, but not citaplopram, facilitated the NOP memory, increased the neuronal firing rate of ventral tegmental area in vivo, and potentiated the mPFC NMDA-R mediated currents in vitro (Schilstrom et al., 2011).
Pre-test microinjections of MDL 11,939, a 5-HT2A-R antagonist, into the rat mPFC impaired TOM, but not NOP and OPP, all with 3 hours intervals (Bekinschtein et al., 2013). In the object-in-context (OIC) test, different sets of objects are presented at distinct contexts and animals are later asked to recognize which object is incongruent to the testing context. Pre-test prefrontal infusions of MDL 11,939 or 8-OH DPAT, a 5HT1A-R agonist, but not of SB 242084, a 5HT2C-R antagonist, also impaired OIC memory tested 3 hours later (Bekinschtein et al., 2013). These findings suggest that 5HT2A-R in the mPFC are critical for memory retrieval in short-term TOM, but not for NOP and OPP. The prefrontal 5HT1A-R and 2A-R, but not 2C-R, are also important in the retrieval of short-term OIC memory.
3.1.4. Acetylcholine
ACh, binding to the two main nicotinic and muscarinic ACh-R, is profoundly involved in cognition and memory processing (Hasselmo and Sarter, 2011). The medial septum and vertical diagonal band of Broca (MSvDB) send projections to the hippocampus to form the septo-hippocampal cholinergic pathways. The baso-cortical cholinergic pathways from the nucleus basalis magnocellularis (NBM) project to the entire cortex (Mesulam et al., 1983).
Infusion of the muscarinic ACh-R antagonist scopolamine into the PLC/ILC before the second sample trial, but not before the test trial, of the TOM test disrupted memory (Barker and Warburton, 2011a). Similarly, pre-sample, but not pre-test, microinjections of scopolamine into the mPFC caused deficient OiP memory tested 5 minutes and 1 hour later (Barker and Warburton, 2009). When OiP memory was tested 24 hours later, pre-sample, but not pre-test, PFC infusions of the α7 nicotinic ACh-R antagonist, methyllycaconitine citrate, or the α-nicotinic ACh-R blocker, α-bungarotoxin, impaired memory. Conversely, microinjections of the α4β2 nicotinic ACh-R antagonist, dihydro-β-erythroidine hydrobromide, into the PFC led to deficient OiP memory when given pre-test, but not pre-sample. Post-sample PFC infusions of neither methyllycaconitine citrate nor dihydro-β-erythroidine hydrobromide influenced OiP memory. In addition, infusions into the mPFC of methyllycaconitine citrate, before learning, or dihydro-β-erythroidine hydrobromide, before the test trial, did not influence OPP (24 hours) memory (Sabec et al., 2018).
The muscarinic ACh-R of the PLC/ILC are essential for the encoding/consolidation, but not retrieval, of TOM and OiP. The medial prefrontal cortical α7 nicotinic ACh-R underly the learning, but not consolidation and recall, of associative object-location memory, while the α4β2 nicotinic ACh-R are responsible for the retrieval, but not learning and consolidation, of this memory. Neither α7- nor α4β2-nicotinic ACh-R seem to be significantly involved in OPP memory. These findings suggest that the muscarinic and nicotinic ACh-R in the mPFC have different roles in processing the information of time and associative object-location.
3.1.5. Short summary
Lesion and inactivation studies have provided substantial evidence that TOM, OiP and episodic-like, but not NOP and OPP, memories are dependent on the mPFC. However, the mPFC may mediate memory consolidation, regardless of types of object exploration tests. Furthermore, pharmacological studies have found that NOP memory tested even minutes later was impaired by pre-sample infusions of substances into the PFC (Christoffersen et al., 2008; Pezze et al., 2015; Watson et al., 2012). Details of findings of substances injected locally into the mPFC are listed in Table 1. The results suggest that it may not be the mPFC is irrelevant to NOP processing, but rather other neural systems take over when the mPFC is dysfunctional.
Table 1.
Pharmacological studies investigating the role of neurotransmitter systems of medial prefrontal cortex in object exploration tests. Substances are infused into the medial prefrontal cortex.
| Species and strain | Treatment | Test | Time of treatment | Sample trial | Retention | Findings | Reference |
|---|---|---|---|---|---|---|---|
| Pigmented | CNQX, 2.5μg | NOP | Pre-sample | 4min | 3 h | No effect | Barker&Warburton, 2011a |
| male rats | (AMPA/kainate-R | ||||||
| antagonist) | |||||||
| Wistar | AP5, 2.5μg | NOP | Post-sample | 5min | 3h | No effect | Akirav&Maroun, 2006 |
| male rats | (NMDA-R antagonist) | NOP | Post-sample | 5min | 24h | Impaired | |
| Rats | MPEP, 1–10μg | NOP | Pre-sample | 5min | 5min | Impaired | Christoffersen et al., 2008 |
| (mGlu5-R antagonist) | 5 and 10μg | ||||||
| ICR male mice | SCH23390, 1.0μg | NOP | Pre-sample | 10min | 1h | No effect | Nagai et al., 2007 |
| (DA D1/5-R antagonist) | NOP | Pre-sample | 10min | 24h | Impaired | ||
| Sprague-Dawley | SCH23390 | NOP | Pre-sample | 5min | 5min | Impaired | Clausen et al., 2011 |
| male rats | (DA D1/5-R antagonist) | ||||||
| Sprague-Dawley | SCH23390, 1.0μg | NOP | Pre-sample | 15min | 24h | Impaired | De Bundel et al., 2013 |
| male rats | (DA D1-R antagonist) | ||||||
| Dark Agouti | SCH23390, 5mM/1.0μl | NOP | Pre-sample | 4min | 1h | No effect | Savalli et al., 2015 |
| male rats | (DA D1/5-R antagonist) | ||||||
| Wistar male rats | SCH23390, 1.5μg | NOP | Post-sample | 5min | 24h | Impaired | Rossato et al., 2013 |
| (DA D1/5-R antagonist) | NOP | 6 h post-sample | 5min | 24h | No effect | ||
| Wistar | SCH23390, 0.5–3.0μg | NOP | Post-sample | reach 20s | 1h | Impaired | Papp et al., 2017 |
| male rats | (DA D1/5-R antagonist) | 3.0 μg | |||||
| Sprague-Dawley | SKF81297, 0.03–3.0μg | NOP | Pre-sample | 2min | 24h | Facilitated | De Bundel et al., 2013 |
| male rats | (DA D1/5-R agonist) | 0.03μg | |||||
| Wistar | SKF81297, 0.025–0.05μg | NOP | Pre-sample | 5min | 10min | Impaired | Pezze et al., 2015 |
| male rats | (DA D1/5-R agonist) | 0.05μg | |||||
| Wistar | SKF81297, 0.05–0.75μg | NOP | Post-sample | reach 20s | 24h | Facilitated | Papp et al., 2017 |
| male rats | (DA D1-R agonist) | 0.2μg | |||||
| ICR male mice | Raclopride, 5.0μg | NOP | Pre-sample | 10min | 1h | No effect | Nagai et al., 2007 |
| (DA D2-R antagonist) | NOP | Pre-sample | 10min | 24h | No effect | ||
| Lister Hooded | L741,626, 0.63–5.0μg | NOP | Pre-sample | 3min | 2min | Impaired | Watson et al., 2012 |
| male rats | (DA D2-R antagonist) | ||||||
| Wistar | L741.626, 0.5–1.0μg | NOP | Post-sample | reach 20s | 1h | Impaired | Papp et al., 2017 |
| male rats | (DA D2-R antagonist) | 1.0 μg | |||||
| Wistar | Quinpirole, 3.0μg | NOP | Post-sample | 5min | 24h | No effect | Rossato et al., 2013 |
| male rats | (DA D2-R agonist) | ||||||
| Wistar | Quinpirole, 0.1–5.0μg | NOP | Post-sample | reach 20s | 24h | Facilitated | Papp et al., 2017 |
| male rats | (DA D2-R agonist) | 1.0μg | |||||
| Lister Hooded | S33084, 0.63–2.5μg | NOP | Pre-sample | 3min | 4h | Facilitated | Watson et al., 2012 |
| male rats | (DA D3-R antagonist) | ||||||
| Wistar | SB-277,011, 0.1–1.0μg | NOP | Post-sample | reach 20s | 24h | Facilitated | Papp et al., 2017 |
| male rats | (DA D3-R antagonist) | 0.5μg | |||||
| Wistar | 7-OH-DPAT, 0.1–10μg | NOP | Post-sample | reach 20s | 1h | Impaired | Papp et al., 2017 |
| male rats | (DA D3-R agonist) | 10 μg | |||||
| Wistar | MDL11,939, 0.3μg | NOP | Pre-test | 5min | 3h | No effect | Bekinschtein et al., 2013 |
| male rats | (5-HT2A-R antagonist) | ||||||
| Wistar | MDL11,939, 0.3μg | OPP | Pre-test | 5min | 3h | No effect | Bekinschtein et al., 2013 |
| male rats | (5-HT2A-R antagonist) | ||||||
| Lister Hooded | MLA, 100nM | OPP | Pre-sample | 3min | 24h | No effect | Sabec et al., 2018 |
| male rats | (α7nACh-R antagonist) | ||||||
| Lister Hooded | DHβE, 1μM | OPP | Pre-test | 3min | 24h | No effect | Sabec et al., 2018 |
| male rats | (α4β2nACh-R antagonist) | ||||||
| Pigmented | CNQX, 2.5μg | TOM | Pre-sample2 | 4min | 3h | Imapired | Barker&Warburton, 2011a |
| male rats | (AMPA/kainate-R | TOM | Pre-test | 4min | 3h | Impaired | |
| antagonist) | |||||||
| Pigmented | AP5, 5.0μg | TOM | Pre-sample2 | 4min | 3h | Imapired | Barker&Warburton, 2011a |
| male rats | (NMDA-R antagonist) | TOM | Pre-test | 4min | 3h | No effect | |
| Wistar | MDL11,939, 0.3μg | TOM | Pre-test | 5min | 3h | Impaired | Bekinschtein et al., 2013 |
| male rats | (5-HT2A-R antagonist) | ||||||
| Pigmented | Scopolamine, 10μg | TOM | Pre-sample2 | 4min | 3h | Imapired | Barker&Warburton, 2011a |
| male rats | (mAChR antagonist) | TOM | Pre-test | 4min | 3h | No effect | |
| Pigmented | CNQX, 2.5μg | OiP | Pre-sample | 5min | 5min | Impaired | Barker&Warburton, 2008 |
| male rats | (AMPA/kainate-R | ||||||
| antagonist) | |||||||
| Pigmented | AP5, 5.0μg | OiP | Pre-sample | 5min | 5min | Impaired | Barker&Warburton, 2008 |
| male rats | (NMDA-R antagonist) | OiP | Pre-sample | 5min | 1h | Impaired | |
| OiP | Pre-test | 5min | 1h | No effect | |||
| Dark Agouti | SCH23390, 5mM/1.0μl | OiP | Pre-sample | 5min | 5min | Impaired | Savalli et al., 2015 |
| male rats | (DA D1/5-R antagonist) | OiP | Pre-sample | 5min | 1h | Impaired | |
| OiP | Pre-test | 5min | 1h | No effect | |||
| Dark Agouti | SKF83566, 0.2mM/1.0μl | OiP | Pre-sample | 5min | 5min | Impaired | Savalli et al., 2015 |
| male rats | (DA D1-R antagonist) | OiP | Pre-sample | 5min | 1h | Impaired | |
| Dark Agouti | Scopolamine, 10μg | OiP | Pre-sample | 5min | 5min | Impaired | Barker&Warburton, 2009 |
| male rats | (mACh-R antagonist) | OiP | Pre-sample | 5min | 1h | Impaired | |
| OiP | Pre-test | 5min | 1h | No effect | |||
| Lister Hooded | MLA, 100nM | OiP | Pre-sample | 5min | 24h | Impaired | Sabec et al., 2018 |
| male rats | (α7nACh-R antagonist) | OiP | Post-sample | 5min | 24h | No effect | |
| OiP | Pre-test | 5min | 24h | No effect | |||
| Lister Hooded | α-BGT, 1μM | OiP | Pre-sample | 5min | 24h | Impaired | Sabec et al., 2018 |
| male rats | (α-nACh-R blocker) | OiP | Pre-test | 5min | 24h | No effect | |
| Lister Hooded | DHβE, 1μM | OiP | Pre-sample | 5min | 24h | No effect | Sabec et al., 2018 |
| male rats | (α4β2nACh-R antagonist) | OiP | Post-sample | 5min | 24h | No effect | |
| OiP | Pre-test | 5min | 24h | Impaired |
Abbreviations: AP5, 2-amino-5-phosphonopentanoic acid; MPEP, 2-methyl-6-(phenylethynyl)-pyridine; CNQX, 6-Cyano-7-nitroquinoxaline; MLA, methyllycaconitine citrate; α-BGT, α-bungarotoxin; DhβE, Dihydro-β-erythroidine hydrobromide; NOP, novel object preference; OPP, object place preference; TOM, temporal order memory; OiP, object-in-place; s, seconds; min, minutes; h, hours.
3.2. The role of the entorhinal cortex
Evidence has shown that the EC is involved in spatial recognition and long-term memory. EC-lesioned animals showed deficient spatial navigation in the Morris water maze (Nyakas et al., 2009; Parron et al., 2004), but not when spatial cues were provided by proximal objects located in the pool (Parron et al., 2004). The EC-lesioned rats have also been reported to show deficits in the NOP test with an 2-hour interval (Nyakas et al., 2009). Post-sample intra-EC infusions of the protein synthesis inhibitors, anisomycin, emetine or cycloheximide, disrupted long-term (24 hours), but not short-term (3 hours), NOP memory. This disruption was time-dependent as the effect was found when infusions were made immediately, but not 3 or 6 hours, after the sample (Lima et al., 2009). Thus, the EC participates in long-term memory consolidation of object memory. In the SNOP test, impaired spatial recognition and less object exploration were found in EC-lesioned animals (Parron and Save, 2004). The EC lesioned animals also exhibited deficits in habituation to the environment when the time delay was 10, but not 4 min. In addition, impaired spatial recognition, but not object recognition, was found, irrespective of the delays (Van Cauter et al., 2008a). This indicates that the EC is important for maintaining spatial information across time in memory storage. Furthermore, electrical stimulation of EC facilitated NOP memory and water maze spatial navigation in rats infused with the amyloid peptides 1–42 into the hippocampus, as a model of Alzheimer’s disease (Zhang et al., 2015), suggestive of close connections between the EC and hippocampus-dependent functions.
The EC, located in the medial temporal lobe, can be anatomically subdivided into lateral and medial compartments. The LEC preferentially receives projections from the perirhinal cortex, insular cortex, PLC and ILC, while the medial entorhinal cortex (MEC) mainly connects with the postrhinal, occipital and parietal cortical regions in rats (Burwell and Amaral, 1998a, b; Witter et al., 2000). Projections from the perirhinal cortex to LEC and those from the postrhinal cortex to MEC are functionally different, with the former primarily sending inhibitory signals (Apergis-Schoute et al., 2007; Pinto et al., 2006; Willems et al., 2018) and the latter primarily sending excitatory ones (Koganezawa et al., 2015). The dominant projections of the perirhinal-LEC and postrhinal-MEC pathways have inspired scientists to propose a neuroanatomy system of episodic memory that integrates spatial representation from the postrhinal-MEC pathway and non-spatial representation from the perirhinal-LEC pathway into the hippocampus (Davachi, 2006; Deshmukh and Knierim, 2011; Eichenbaum et al., 2007; Hargreaves et al., 2005; Knierim et al., 2006; Schultz et al., 2012). Yet, recent behavioral and electrophysiological evidence indicates that both LEC and MEC process spatial information, with local and global spatial frameworks being represented within LEC and MEC, respectively (Knierim et al., 2014; Neunuebel et al., 2013).
In non-spatial (what) object exploration tests, lesions of the LEC of rats spared memory in the classic NOP test (Kuruvilla and Ainge, 2017; Van Cauter et al., 2013; Wilson et al., 2013b; Wilson et al., 2013c), but impaired object memory when 3 or 4 distinct objects were used during the learning (Hunsaker et al., 2013; Kuruvilla and Ainge, 2017). This finding is in agreement with others, indicating the LEC is critical for the memory of associations between different objects, even contexts (Hunsaker et al., 2013; Kuruvilla and Ainge, 2017; Wilson et al., 2013b; Wilson et al., 2013c). Evidence from the SNOP test has supported this perspective, as object recognition was compromised when four distinct, but not identical, objects were required to be remembered by LEC lesioned rats (Rodo et al., 2017; Van Cauter et al., 2013). When reducing the diversity of objects to three distinct objects, the LEC-lesioned animals were able to show intact object recognition in the SNOP test (Rodo et al., 2017). These results indicate that the LEC processes complicated, but not simple, inter-relationships between objects. Compatible with this view are the imaging findings that the LEC was more recruited in the processing of the number of items (5 versus 10 odors) during the recall of a non-matching to sample task (Ku et al., 2017). In contrast, MEC lesions affected neither the NOP test (Hales et al., 2014; Hales et al., 2018; Hunsaker et al., 2013; Kuruvilla and Ainge, 2017; Van Cauter et al., 2013) nor object novelty as assessed by the SNOP test (Rodo et al., 2017; Van Cauter et al., 2013). Pre-test microinjections of scopolamine into the MEC also had no effect in the NOP test, but decreased the amount of object exploration (Rashid and Ahmed, 2019). Thus, the LEC, but not MEC, critically processes object-object inter-relationships when environmental complexity (object associative information) increases.
In an OPP-like paradigm to test spatial (where) object exploration, in which a single object was presented in the sample trial and another identical object was placed together with the familiar one in the test trial, memory was disrupted in animals with lesion of the MEC, but not LEC (Van Cauter et al., 2013). However, another study showed that lesions of the MEC did not disrupt memory in the OPP test, but impaired spatial memory tested by the water maze escape task and that combined lesions of the MEC and hippocampus led to a stronger impairment (Hales et al., 2014). In the SNOP test, impaired spatial recognition was found after lesion of the MEC (Rodo et al., 2017; Van Cauter et al., 2013). The MEC-lesioned animals exhibited deficits in spatial recognition when three or four distinct, but not identical, objects were used in the sample trial of the SNOP test (Rodo et al., 2017). These data illustrate the engagement of the MEC in the processing of “general” information about space and its interaction with the hippocampus (Hales et al., 2014; Hales et al., 2018), particularly in situation with increased complexity of objects. It should be noted that lesions of the MEC may mask its specific role in the processing of spatial information. For example, inactivation of the superficial layers of MEC stellate cells impaired memory for OPP, but not NOP, in mice (Tennant et al., 2018). Lesions of the LEC, on the other hand, did not affect OPP memory (Van Cauter et al., 2013; Wilson et al., 2013c), but impaired object-location recognition in a test in which two distinct objects were replaced by two identical ones, chosen from either of the explored type (Wilson et al., 2013c). Impaired spatial recognition was also reported in the LEC-lesioned rats tested by the SNOP paradigm (Van Cauter et al., 2013), but see (Rodo et al., 2017). In the same vein, the LEC-lesioned rats showed deficits in searching for foods in an arena in which spatial cues were provided by two distinct objects located within the environment (Kuruvilla and Ainge, 2017). Space-relevant object recognition can be mediated by both LEC and MEC, depending on the diversity of objects explored.
A summary of the roles of LEC and MEC in the object exploration tests is shown in Table 2 and Figure 3. Please note that less object exploration and habituation to the testing environment were sometimes reported in the mentioned studies (Parron and Save, 2004; Van Cauter et al., 2013; Van Cauter et al., 2008a), which could potentially confound the observed performance. Secondly, the majority of lesion studies has applied less than 10-minutes retention intervals (consequences are not clear after longer delays). The whole EC is likely to engage in long-term object memory, given that EC-lesioned or inactivated animals exhibited deficits in the NOP test when tested 24 hours later (Lima et al., 2009; Nyakas et al., 2009). How the LEC and MEC contribute to long-term memory in regards to spatial and non-spatial processing remains unclear.
Table 2.
Lesions and inactivation studies on the entorhinal cortex in object exploration tests.
| Species and strain | Treatment | Test | Time of treatment | Sample trial | Retention | Findings | Reference |
|---|---|---|---|---|---|---|---|
| Harlan Wistar | NMDA lesions, | NOP | Pre-sample | 5min | 2h | Impaired | Nyakas et al., 2009 |
| male rats | whole EC | 2 copies | |||||
| Wistar | Anisomycin, 160μg | NOP | Post-sample | 5min | 3h | No effect | Lima et al., 2009 |
| male rats | (protein synthesis | 2 distinct | Post-sample | 5min | 24h | Impaired | |
| inhibitor), EC | 3h post-sample | 5min | 24h | No effect | |||
| 6h post-sample | 5min | 24h | No effect | ||||
| Wistar | Emetine, 50μg | NOP | Post-sample | 5min | 3h | No effect | Lima et al., 2009 |
| male rats | (protein synthesis | 2 distinct | Post-sample | 5min | 24h | Impaired | |
| inhibitor), EC | 3h post-sample | 5min | 24h | No effect | |||
| 6h post-sample | 5min | 24h | No effect | ||||
| Wistar | Cycloheximide, 20μg | NOP | Post-sample | 5min | 3h | No effect | Lima et al., 2009 |
| male rats | (protein synthesis | 2 distinct | Post-sample | 5min | 24h | Impaired | |
| inhibitor), EC | 3h post-sample | 5min | 24h | No effect | |||
| 6h post-sample | 5min | 24h | No effect | ||||
| Long Evans | Radio-frequency | SNOP | Pre-sample | 4minx6 | 4min | Impaired SD | Parron&Save 2004 |
| male rats | lesions, whole EC | 5 distinct | m-Impaired NO | ||||
| Long Evans | Radio-frequency | SNOP | Pre-sample | 4minx6 | 30s | Impaired SD | van Cauter et al., 2008a |
| male rats | lesions, whole EC | 4 distinct | 4&10min | Impaired SD | |||
| 4&10min | No effect in NO | ||||||
| Long Evans | Radio-frequency | NOP | Pre-sample | reach 15s | 2min | No effect | van Cauter et al., 2013 |
| male rats | lesions, LEC | 2 copies | |||||
| Lister Hooded | Ibotenic acid lesions, | NOP | Pre-sample | 3min or | 2min | No effect | Wilson et al., 2013b |
| male rats | LEC | 2 copies | reach 15s | ||||
| Lister Hooded | Ibotenic acid lesions, | NOP | Pre-sample | 3min or | 2min | No effect | Wilson et al., 2013c |
| male rats | LEC | 2 copies | reach 15s | ||||
| Lister Hooded | Ibotenic acid lesions, | NOP | Pre-sample | 3min or | 1min | No effect | Kuruvilla&Ainge, 2017 |
| male rats | LEC | 2 copies | reach 15s | ||||
| Long Evans | Ibotenic acid lesions, | NOP | Pre-sample | 5minx3 | 3min | Impaired | Hunsaker et al., 2013 |
| male rats | LEC | 3 distinct | |||||
| Lister Hooded | Ibotenic acid lesions, | NOP | Pre-sample | 4minx6 | 1min | Impaired | Kuruvilla&Ainge, 2017 |
| male rats | LEC | 4 distinct | |||||
| Lister Hooded | Ibotenic acid lesions, | OPP | Pre-sample | 3min or | 2min | No effect | Wilson et al., 2013c |
| male rats | LEC | 2 copies | reach 15s | ||||
| Long Evans | Radio-frequency | OPP* | Pre-sample | reach 15s | 2min | No effect | van Cauter et al., 2013 |
| male rats | lesions, LEC | 1 copy | |||||
| Lister Hooded | Ibotenic acid lesions, | OLP** | Pre-sample | 3min or | 2min | Impaired | Wilson et al., 2013c |
| male rats | LEC | 2 distinct | reach 15s | ||||
| Long Evans | LEC lesions | SNOP | Pre-sample | 4minx6 | 4min | No effect in SD | Rodo et al., 2017 |
| male rats | 3 distinct | No effect in NO | |||||
| Long Evans | Radio-frequency | SNOP | Pre-sample | 4minx6 | 4min | Impaired SD | van Cauter et al., 2013 |
| male rats | lesions, LEC | 4 distinct | Imapired NO | ||||
| Long Evans | LEC lesions | SNOP | Pre-sample | 4minx6 | 4min | No effect in SD | Rodo et al., 2017 |
| male rats | 4 distinct | Impaired NO | |||||
| Long Evans | LEC lesions | SNOP | Pre-sample | 4minx6 | 4min | No effect in SD | Rodo et al., 2017 |
| male rats | 4 copies | No effect in NO | |||||
| Long Evans | Radio-frequency | NOP | Pre-sample | reach 15s | 2min | No effect | van Cauter et al., 2013 |
| male rats | lesions, MEC | 2 copies | |||||
| Long Evans | NMDA lesions, | NOP | Pre-sample | 15min | 3h | No effect | Hales et al., 2014 |
| male rats | MEC | 2 copies | |||||
| Lister Hooded | Ibotenic acid lesions, | NOP | Pre-sample | 3min or | 1min | No effect | Kuruvilla&Ainge, 2017 |
| male rats | MEC | 2 copies | reach 15s | ||||
| Long Evans | NMDA lesions, | NOP | Post-sample | 15minx4 | 2w | No effect | Hales et al., 2018 |
| male rats | MEC | 2 copies | |||||
| Sim1 cre | AAV-Flex-TeLC | NOP | Pre-sample | 5min | 3min | No effect | Tennant et al., 2018 |
| mice | MEC | 2 copies | |||||
| Long Evans | Ibotenic acid lesions, | NOP | Pre-sample | 5minx3 | 3min | No effect | Hunsaker et al., 2013 |
| male rats | MEC | 3 distinct | |||||
| Lister Hooded | Ibotenic acid lesions, | NOP | Pre-sample | 4minx6 | 1min | No effect | Kuruvilla&Ainge, 2017 |
| male rats | MEC | 4 distinct | |||||
| Long Evans | NMDA lesions, | OPP | Pre-sample | 15min | 3h | No effect | Hales et al., 2014 |
| male rats | MEC | 2 copies | |||||
| Sim1 cre | AAV-Flex-TeLC | OPP | Pre-sample | 5min | 3min | Impaired | Tennant et al., 2018 |
| mice | MEC | 2 copies | |||||
| Long Evans | Radio-frequency | OPP* | Pre-sample | reach 15s | 2min | Imapired | van Cauter et al., 2013 |
| male rats | lesions, MEC | 1 copy | |||||
| Long Evans | MEC lesions | SNOP | Pre-sample | 4minx6 | 4min | Impaired SD | Rodo et al., 2017 |
| male rats | 3 distinct | No effect in NO | |||||
| Long Evans | Radio-frequency | SNOP | Pre-sample | 4minx6 | 4min | Impaired SD | van Cauter et al., 2013 |
| male rats | lesions, MEC | 4 distinct | No effect in NO | ||||
| Long Evans | MEC lesions | SNOP | Pre-sample | 4minx6 | 4min | Impaired SD | Rodo et al., 2017 |
| male rats | 4 distinct | No effect in NO | |||||
| Long Evans | MEC lesions | SNOP | Pre-sample | 4minx6 | 4min | No effect in SD | Rodo et al., 2017 |
| male rats | 4 copies | No effect in NO |
Abbreviations: EC: entorhinal cortex; LEC: lateral entorhinal cortex; MEC: medial entorhinal cortex; TeLC, tetanus toxin light chain; SD, spatial displaced object; NO, novel object; m-Impaired, moderately impaired; s, seconds; min, minutes; h, hours; w, weeks.
one object was located, followed by two identical objects of the explored type.
two distinct objects were located, followed by two identical objects chosen form either the explored type.
Figure 3.
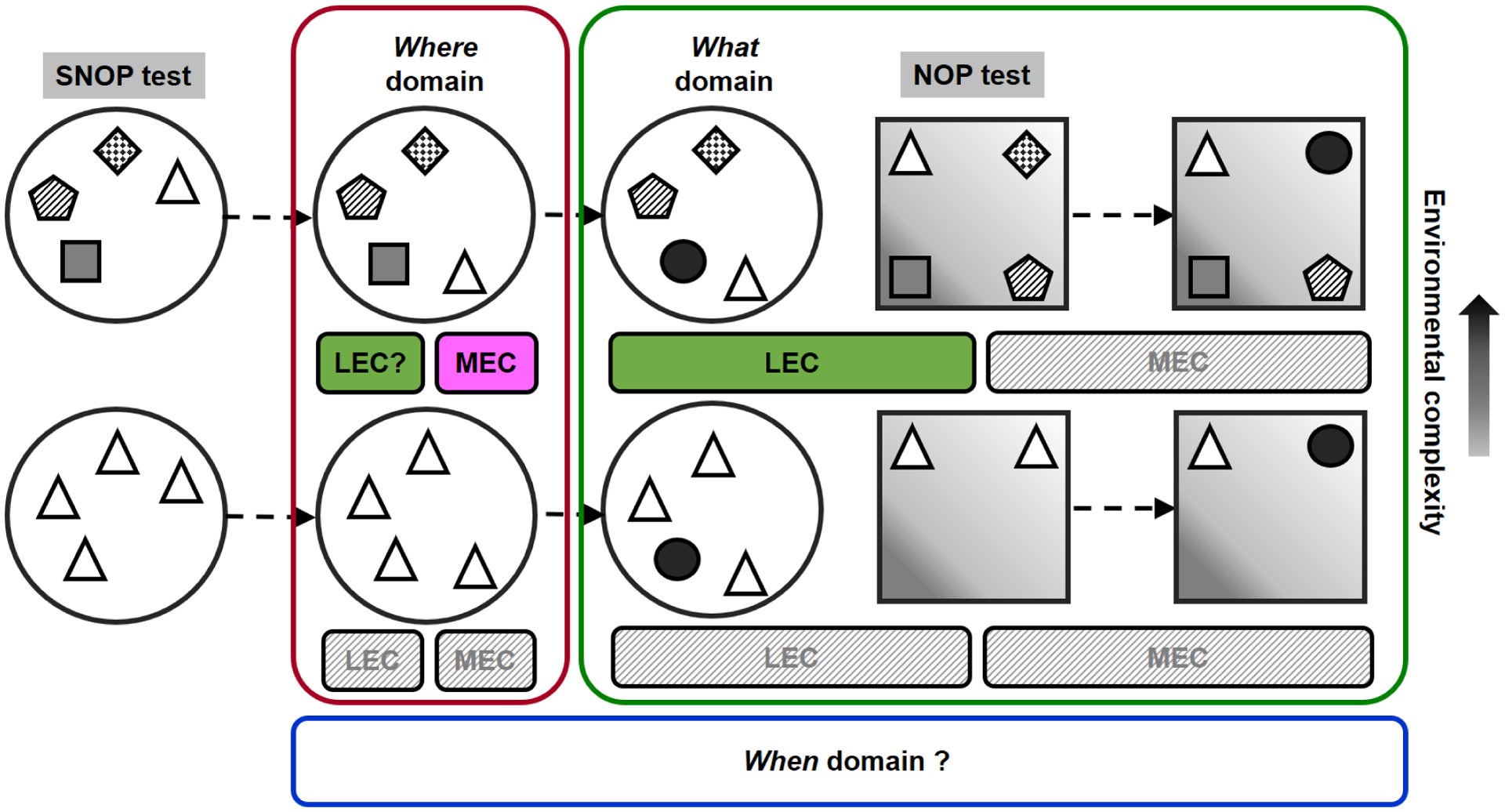
The roles of lateral entorhinal cortex (LEC) and medial entorhinal cortex (MEC) in the spatial and non-spatial object preference (SNOP) and novel object preference (NOP) tests. Both regions are not required in simple situation (identical objects). In complex situation (4 distinct objects), the LEC, but not MEC, is essential for the processing of object recognition (what), while the MEC is essential for spatial recognition (where). Lesions of the LEC are reported to disrupt spatial recognition (van Cauter et al., 2013), but see (Rodo et al., 2017). How the LEC and MEC interact with time (when) remains underexplored since the majority of the studies applies short retentions only (less than 10 minutes; unclear for longer delays).
Behavioral, imaging and electrophysiological recording data suggest that LEC preferentially processes object-associated information, e.g., the past and current locations of objects (Beer et al., 2013; Deshmukh and Knierim, 2011; Tsao et al., 2013; Van Cauter et al., 2013), and the context of the objects (Wilson et al., 2013b; Wilson et al., 2013c). Intriguingly, populations of LEC neurons also encode temporal information across different contexts (Tsao et al., 2018). This matches the anatomical features of LEC, as being a functional hub for integrating multisensory information (Bota et al., 2015), including both spatial and non-spatial characteristics, probably even time (Tsao et al., 2018). In contrast, the MEC neurons fire for location in the environment and across different contexts, forming “grid-like” activities (Fyhn et al., 2004; Hafting et al., 2005). Strikingly, the MEC neurons respond at fixed distances and directions from objects, irrespective of location, shape and size of objects, and have been called object-vector cells (Hoydal et al., 2019). Anatomical, electrophysiological and behavioral evidence suggests that the LEC and MEC have some convergent functions: a). The perirhinal cortex and postrhinal cortex project to the MEC and LEC, respectively, although in lesser extent than the perirhinal-LEC and postrhinal-MEC connections (van Strien et al., 2009), and there are reciprocal interconnections between the LEC and MEC (Burwell, 2000; Dolorfo and Amaral, 1998), which provide an anatomical basis for the involvement of the mixture processing of “spatial” and “non-spatial” information in both entorhinal compartments, b), the LEC and MEC neurons signal both information about objects and position (Keene et al., 2016), and c), the MEC lesioned rats had deficits in the recollection of an odor-based, non-spatial, recognition task, suggesting a broader role of MEC in memory function (Sauvage et al., 2010). Save and Sargolini thus proposed that the roles of LEC and MEC in non-spatial and spatial processing are environmental complexity-dependent (Rodo et al., 2017), whereby they integrate what and where information into the hippocampus in a tightly cooperative and flexible manner (Save and Sargolini, 2017).
3.3. The role of the hippocampus
3.3.1. Novel object preference
The role of the hippocampus in the processing of NOP is contentious. The hippocampus is not required for short-term NOP memory (less than 10 minutes) shown by lesion and inactivation studies (organized in Table 3). Inconsistent findings were reported in the literature when the retention delay was over 10 minutes in the NOP test. Some studies showed that rats with hippocampal lesions had impaired NOP (across delays from 10 minutes to 24 hours) memory (Ainge et al., 2006; Broadbent et al., 2010; Broadbent et al., 2004; Clark et al., 2000), whereas the majority of the studies employing hippocampal lesions in rats failed to report an influence in the NOP test (Albasser et al., 2012; Albasser et al., 2010; Barker and Warburton, 2011b; Forwood et al., 2005; Gaskin et al., 2003; Good et al., 2007a; Langston and Wood, 2010; Mumby et al., 2005; Piterkin et al., 2008; Tam et al., 2014; Winters et al., 2004). Alternatively, pre-sample intra-hippocampus infusions of lidocaine (Hammond et al., 2004) or muscimol (Cohen et al., 2013) was reported to disrupt NOP memory tested 24 hours later in mice. Similar effects were also found in a circular track in which animals explored newly and repeatedly presented objects in a clockwise order (Bass et al., 2014).
Table 3.
Findings of the novel object preference test based on lesions and inactivation (lidocaine & GABAa receptors ago- and antagonists) studies in the hippocampus (unless specified, the target region is dorsal hippocampus).
| Species and strain | Treatment | Apparatus | Time of treatment | Sample trial | Retention | Findings | Reference |
|---|---|---|---|---|---|---|---|
| Long Evans | Radio-frequency | OF | Pre-sample | reach 30s | 10s or 1min | No effect | Clark et al., 2000 |
| male rats | lesions, whole HPC | Pre-sample | reach 30s | 10min or 1h | Impaired | ||
| Pre-sample | reach 30s | 24h | No effect | ||||
| Long Evans | Ibotenic acid lesions, | OF | Pre-sample | reach 30s | 10s or 1min | No effect | Clark et al., 2000 |
| male rats | whole HPC | Pre-sample | reach 30s | 10min or 1h | Impaired | ||
| Pre-sample | reach 30s | 24h | m-Impaired | ||||
| Long Evans | NMDA lesions, | OF | Pre-sample | 5min | 15min | No effect | Gaskin et al., 2003 |
| male rats | whole HPC | Pre-sample | 5min | 24h | No effect | ||
| Long Evans | Ibotenic acid lesions, | OF | Broadbent et al., 2004 | ||||
| male rats | ~50–75% HPC | Pre-sample | 15min | 3h | No effect | ||
| ~ventral 50% HPC | Pre-sample | 15min | 3h | No effect | |||
| whole HPC | Pre-sample | 15min | 3h | Impaired | |||
| Lister Hooded | Ibotenic acid lesions, | Y-maze | Pre-sample | reach 25s | <30s | No effect | Winters et al., 2004 |
| male rats | whole HPC | Pre-sample | or 5 min | 15min | No effect | ||
| Pre-sample | 1h | No effect | |||||
| Pre-sample | 24h | No effect | |||||
| Lister Hooded | Ibotenic acid lesions, | Y-maze | Pre-sample | reach 25s | 15min, 1h, | No effect | Forwood et al., 2005 |
| male rats | whole HPC | or 3min | 24h & 48h | ||||
| Long Evans | NMDA lesions, | OF | Pre-sample | 5minx5 | 24h | No effect | Mumby et al., 2005 |
| male rats | whole HPC | Pre-sample | 5minx5 | 1w | No effect | ||
| Pre-sample | 5minx5 | 3w | No effect | ||||
| Lister Hooded | ibotenic acid lesions, | OF | Pre-sample | reach 30s | 10s or 1min | No effect | Ainge et al., 2006 |
| male rats | ~60% HPC | Pre-sample | reach 30s | 10min or 1h | Impaired | ||
| Pre-sample | reach 30s | 24h | Impaired | ||||
| Lister Hooded | ibotenic acid lesions, | OF | Pre-sample | reach 30s | 10s or 1min | No effect | Ainge et al., 2006 |
| male rats | whole HPC | Pre-sample | reach 30s | 10min or 1h | No effect | ||
| Pre-sample | reach 30s | 24h | Impaired | ||||
| Lister Hooded | Ibotenic acid lesions, | OF | Pre-sample | 5 min | 2min | No effect | Good et al., 2007a |
| male rats | whole HPC | ||||||
| Long Evans | NMDA lesions, | CT maze | Pre-sample | 1 min | 60min | No effect | Piterkin et al., 2008 |
| male rats | whole HPC | ||||||
| Lister Hooded | Ibotenic acid lesions, | BT maze | Pre-sample | 1 min | <1min | No effect | Albasser et al., 2010 |
| male rats | whole HPC | 2 distinct | |||||
| Long Evans | Ibotenate acid lesions, | OF | Pre-sample | 15min | 3h | m-Impaired | Broadbent et al., 2010 |
| male rats | whole HPC | ||||||
| Long Evans | NMDA lesion, | OF | Pre-sample | 5min | 35s | Impaired | Gaskin et al., 2010 |
| male rats | dorsal HPC | over days | m-Impaired | ||||
| Pre-sample | 5min | 2h | No effect | ||||
| over days | m-Impaired | ||||||
| Lister Hooded | Ibotenic acid lesions, | OF | Pre-sample | reach 15s | 2min | No effect | Langston&Wood, 2010 |
| male rats | whole HPC | ||||||
| Pigmented | NMDA lesions, | OF | Pre-sample | reach 40s | 5 min | No effect | Barker&Warburton, 2011 |
| male rats | whole HPC | Pre-sample | or 4 min | 3 h | No effect | ||
| Pre-sample | 24h | No effect | |||||
| Lister Hooded | Ibotenic acid lesions, | BT maze | Pre-sample | 1 min | 1min-2h | No effect | Albasser et al., 2012 |
| male rats | whole HPC | 2 distinct | |||||
| Wistar | NMDA lesions, | OF | Pre-sample | 5min | 5min | No effect | Haijima&Ichitani, 2012 |
| male rats | dorsal HPC | Pre-sample | 5min | 30min | No effect | ||
| Pre-sample | 5minx4 | 1w | Impaired | ||||
| Pre-sample | 5minx4 | 4w | m-Impaired | ||||
| Lister Hooded | Ibotenic acid lesions, | OF | Pre-sample | 5 min | 5min | No effect | Tam et al., 2014 |
| male rats | dorsal HPC | Pre-sample | 5 min | 2h | No effect | ||
| ICR | Ibotenic acid lesions, | OF | Pre-sample | 5min | 24h | Impaired | Yi et al., 2016 |
| male mice | whole HPC | Pre-sample# | 5min | 24h | No effect | ||
| Long Evans | NMDA lesions, | OF | Post-sample 1w | 5minx5 | 1+2–3w | Impaired | Gaskin et al., 2003 |
| male rats | whole HPC | Post-sample 5w | 5minx5 | 5+2–3w | Impaired | ||
| Long Evans | Ibotenate acid lesions, | OF | Post-sample 1d | 5minx12 | 2w | Impaired | Broadbent et al., 2010 |
| male rats | whole HPC | Post-sample 4w | 5minx12 | 4+2w | Impaired | ||
| Post-sample 8w | 5minx12 | 8+2w | No effect | ||||
| Wistar | NMDA lesions, | OF | Post-sample 1d | 5minx4 | 1w | Impaired | Haijima&Ichitani, 2012 |
| male rats | dorsal HPC | Post-sample 4w | 5minx4 | 4+1w | Impaired | ||
| C57BL/6J | lidocaine, 4%/0.5μl | OF | Pre-sample | reach 38s | 5min | No effect | Hammond et al., 2004 |
| male mice | (Na+ channel blocker) | 24h | Imapired | ||||
| C57BL/6J | Muscimol, 1μg | OF | Pre-sample | reach 30–38s | 24h | Impaired | Cohen et al., 2013 |
| male mice | (GABAa-R agonist) | ||||||
| Long Evans | Muscimol, 0.5μg | CT maze | Pre-sample | < 1h | Impaired | Bass et al., 2014 | |
| male rats | (GABAa-R agonist) | distinct** | |||||
| C57BL/6J | Muscimol, 0.5μg | OF | Post-sample | 15min | 24h | No effect | Oliveira et al., 2010 |
| male mice | (GABAa-R agonist) | Post-sample# | 15min | 24h | Facilitated | ||
| C57BL/6J | Muscimol, 0.5μg | OF | Post-sample | 10min | 24h | Impaired | Haettig et al., 2011 |
| male mice | (GABAa-R agonist) | 2 distinct | |||||
| C57BL/6J | Muscimol, 1μg | OF | Post-sample | reach 30–38s | 24h | Impaired | Cohen et al., 2013 |
| male mice | (GABAa-R agonist) | ||||||
| Sprague Dawley | Bicuculline, 0.5μg | OF | Post-sample | 5min | 24h | No effect | Kim et al., 2014 |
| male rats | (GABAa-R antagonist) | Post-sample# | 5min | 24h | Impaired | ||
| C57BL/6J | Muscimol, 0.5μg | OF | Pre-test | 10min | 24h | No effect | Haettig et al., 2011 |
| male mice | (GABAa-R agonist) | 2 distinct | |||||
| C57BL/6J | Muscimol, 1μg | OF | Pre-test | reach 30–38s | 24h | Impaired | Cohen et al., 2013 |
| male mice | (GABAa-R agonist) | ||||||
| C57BL/6J | Muscimol, 0.35μg | OF | Pre-test | 10minx3 | 24h | Impaired | Stackman et al., 2016 |
| male mice | (GABAa-R agonist) | ||||||
| C57BL/6J | Muscimol, 1μg | OF | Pre-, post-sample | reach 30–38s | 24h | Impaired | Cohen et al., 2013 |
| male mice | (GABAa-R agonist) | &pre-test | |||||
| C57BL/6J | AAV-hSyn-hM3Dq | OF | Pre-sample | 3min | 24h | No effect | Lopez et al., 2016 |
| male mice | AAV-CaMKIIa-hM3Dq | Pre-sample | 3min | 24h | No effect | ||
| AAV-hSyn-hM4Di | Pre-sample | 10min | 24h | No effect | |||
| AAV-CaMKIIa-hM4Di | Pre-sample | 10min | 24h | No effect | |||
| PV cre | Lenti-EF1a-DIO-hM3Dq | OF | Pre-sample | 10min | 1h | No effect | Zou et al., 2016 |
| male mice | dorsal dentate gyrus | Pre-sample | 10min | 24h | No effect | ||
| C57BL/6J | AAV-CaMKIIa-hM4Di | OF | Pre-sample | reach 30s | 24h | No effect | Tuscher et al., 2018 |
| female OCT | AAV-CaMKIIa-hM4Di | Post-sample | reach 30s | 24h | No effect | ||
| mice | AAV-CaMKIIa-KORD | Post-sample | reach 30s | 24h | Impaired | ||
| GAD-65 cre | AAV-CAG-DIO-hM3Dq | OF | Post-sample | 10min | 24h | No effect | Yu et al., 2018 |
| male mice | |||||||
| C57BL/6N | VGAT-cre + | OF | Post-sample | 10min | 24h | No effect | Yu et al., 2018 |
| male mice | AAV-EF1a-DIO-hM4Di |
Abbreviations: OF: open field; CT maze, circular track maze; BT maze, bow-tie shaped maze; OCT: ovariectomized; m-Impaired: moderately impaired; s, seconds; min, minutes; h, hours; w, weeks.
objects were presented one by one;
in a less-habituated context.
AAV and lentiviral vectors carrying excitatory or inhibited receptors are transferred into the dorsal hippocampal neurons and can be activated by clozapine-N-oxide (hM3Di & hM4Dq) and salvinorin B (KORD) at the selected time.
On the other hand, the hippocampus is likely involved in NOP consolidation and/or retrieval. Hippocampal CA1 firing rates were enhanced during NOP testing (Cohen et al., 2013) and NOP consolidation is related to an increase of the hippocampal CA3-CA1 synaptic efficacy (Clarke et al., 2010). Both CA1 and CA3 areas (more CA1 than CA3) were recruited in the NOP test (Beer et al., 2013). In studies investigating retrograde memory, animals with hippocampus lesion, after being submitted to repeated exposure of the sample trial in the NOP test, exhibited impaired recognition memory when tested within 5, but not 8, weeks after the lesions (Broadbent et al., 2010; Gaskin et al., 2003; Haijima and Ichitani, 2012). Thus, an intact learning is not sufficient to support the memory if the hippocampus was damaged afterward. Post-sample intra-hippocampus infusion of muscimol impaired long-term NOP (24 hours) memory (Cohen et al., 2013; Haettig et al., 2011), but see (Oliveira et al., 2010). Interestingly, post-sample infusions of muscimol and the GABAa-R antagonist bicuculline into the dorsal hippocampus respectively facilitated and disrupted long-term NOP memory in a less-habituated environment, but not after sufficient habituation (Kim et al., 2014; Oliveira et al., 2010). This suggests that the dorsal hippocampal GABAa-R dually modulate NOP consolidation when contextual information is relatively novel. The ventral hippocampus is also involved in NOP memory, as muscimol infusions led to NOP deficits (Neugebauer et al., 2018), albeit this was not supported by a lesion study (Broadbent et al., 2004). Post-sample infusions of anisomycin into the hippocampal CA1 area impaired long-term (24 hours), but not short-term (3 hours), NOP memory when it was infused within 3, but not 6, hours after the sample trial (Rossato et al., 2007), whereas no effect was reported (Balderas et al., 2008). Similar results were found with intra-CA1 infusion of anisomycin, which impaired NOP consolidation and reconsolidation, and the reconsolidation effects of which were reversed by the ubiquitin-proteasome system inhibitor β-Lactacystin (Furini et al., 2015). Post-sample intra-CA1 infusions of lactacystin also disrupted long-term NOP memory, but in a time-dependent manner (Figueiredo et al., 2015). A recent study showed that chemogenetic inhibition (KORD), but not hM4Di, of the dorsal hippocampus impaired NOP consolidation tested 24 hours later (Tuscher et al., 2018). Pre-test muscimol microinjections into the dorsal hippocampus was reported to impair (Cohen et al., 2013; Stackman et al., 2016) or not affect (Haettig et al., 2011) NOP memory in mice.
It has also been shown that the hippocampal neurogenesis is important for the existed object information (Suarez-Pereira and Carrion, 2015). The hippocampal dentate gyrus is involved in NOP processing as evidenced by destruction of high K+ infusions into the region leading to NOP deficits, either treated before or after the sample trial (Suzuki et al., 2015). NOP memory also elevated the AMPA/NMDA current ratio in the dentate gyrus (Yang et al., 2017). However, lesions in the dorsal dentate gyrus did not induce NOP/OPP deficits in rats (Beselia et al., 2010), and DREADD activation of GABAergic parvalbumin neurons in the dentate gyrus also showed no effect in the NOP test, but influenced anxiety-like behaviors, social novelty and contextual fear extinction in mice (Zou et al., 2016). Whether the dente gyrus engaging in object memory depends on situations when configurations of object-feature or -context were altered (Dees and Kesner, 2013; Kesner et al., 2016; Kesner et al., 2015).
The hippocampus actively processes contextual cues. Rats with hippocampus lesions exhibited intact NOP tested in the same environment, but not in different one. However, opposite results were found if only local cues proximal to the applied objects were altered: The control animals had deficient NOP memory in the changed context, but intact memory in the same one, while the lesioned animals remembered the objects in both cases (Piterkin et al., 2008). This is consistent with a later study in which pre-test microinjection of muscimol into the dorsal hippocampus impaired NOP memory when encoded and recalled in different environments (Cohen et al., 2013). Thus, the hippocampus processes the information about located objects within a specific context.
Taken together, the hippocampus is not essential for NOP memory when the retention is less than 10 minutes. The hippocampus engagement in the processing of NOP memory over 10 minutes depends on many factors. Lesion extent of the hippocampus has been discussed, in that severe, but not partial, lesions influenced NOP memory (Broadbent et al., 2004). Yet, the effects of severe hippocampal lesions could be confounded by less object exploration during the NOP encoding (Ainge et al., 2006). Lesions of the hippocampus also influenced memory load, i.e., how many types and numbers of objects can be remembered. Studies have shown that the dorsal hippocampus-lesioned mice were impaired in remembering six distinct objects (Sannino et al., 2012), although this finding seems hard to explain the mentioned discrepancies because two identical objects are usually used in the NOP test (Table 3). Method of lesion (e.g., permanent versus transient lesions) can be a factor; in the majority of studies the non-effect of hippocampus lesions is likely to reflect a functional compensation, such as the reorganization of neuronal circuits in hippocampus-associated regions (Cohen and Stackman, 2015). However, a non-effect of pharmacological inactivation on the dorsal hippocampus was reported in the NOP test if animals were well-habituated to the testing environment (Kim et al., 2014; Oliveira et al., 2010). The majority of chemogenetic studies, either activated before or after the sample trial, showed no influence in the 24 hours-delayed NOP test by overall excitation or inhibition on the dorsal hippocampus, or selectively on the excitatory glutamatergic neurons or inhibitory interneurons in the region (Lopez et al., 2016; Tuscher et al., 2018; Yu et al., 2018; Zou et al., 2016). Nevertheless, long-term NOP impairment was consistently found when the hippocampus was damaged after the learning session (Broadbent et al., 2010; Gaskin et al., 2003; Haijima and Ichitani, 2012). Thus, the hippocampus is undoubtedly important for NOP memory when the retention is over 10 minutes (Cohen and Stackman, 2015), while its involvement likely depends on contextual novelty (Kim et al., 2014; Oliveira et al., 2010; Yi et al., 2016), features of context (Piterkin et al., 2008), and probably allocentric signals (Langston and Wood, 2010) (see below).
3.3.2. Object place preference
The OPP test has been shown to be hippocampus-dependent in rodents (Barker and Warburton, 2011b; Mumby et al., 2002). This is compatible with the classical consensus that the hippocampus is a critical brain region for processing spatial information. Electrophysiological recordings of the hippocampal CA1 cells have shown that when the location of two objects was rotated, the CA1 firing pattern remapped, suggesting that the CA1 coordinated the altered spatial information (Lenck-Santini et al., 2005). CA1 firing rate was also increased during the OPP test, especially when a novel object was placed at a new location (Larkin et al., 2014). CA1 and CA3 pyramidal neurons were reported to code object information in location when the objects were spatially displaced (Deshmukh and Knierim, 2013; Manns and Eichenbaum, 2009). CA1 area was required in both object and location conditions, while CA3 was more recruited for OPP than NOP (Beer et al., 2013). Likewise, the CA1 neuronal firing pattern was reorganized during the learning of searching for a new goal in a spatial memory task (Dupret et al., 2010). Higher c-fos expression induced by the OPP, but not NOP, test was found in the hippocampal CA1 and CA3 subregions (Mendez et al., 2015), while Arc mRNA imaging data showed higher involvement of CA3 in OPP than NOP (Beer et al., 2013). OPP memory also increased the expression of other immediate early genes, such as Arc, Zif268 and Narp, and enhanced the protein levels of Arc, postsynaptic density protein 95 (PSD-95) and αCaMKII in the hippocampal dentate gyrus (Soule et al., 2008). In addition, the newborn cells of dentate gyrus are critical for OPP memory (Jessberger et al., 2009). A study has reported that hippocampal lesioned rats showed intact memory when tested by a paradigm similar to OPP with a 2 min interval. Interestingly, in this OPP-like test in which two distinct objects were presented and only one of their kind was presented later, the rats with hippocampus lesions exhibited disrupted memory when placed into the testing arena from different starting locations, but not from the same location (Langston and Wood, 2010). This suggests that the hippocampus encodes allocentric information of located objects, which is compatible with the findings of the landmark-vector properties of CA1 pyramidal neurons (Deshmukh and Knierim, 2013). The dorsal, but not ventral, hippocampus has been shown to be critically involved in OPP memory (Gaskin et al., 2009a), leading to disrupted OPP memory tested 2 hours, but not 5 min, later (Tam et al., 2014). The functional separation of dorsal versus ventral hippocampus in the processing of memory could be associated with the dominant role of the dorsal hippocampus in both, encoding and retrieval processes (Nakamura and Sauvage, 2016).
Pre-sample lidocaine infusion into the CA1 subarea of the dorsal hippocampus disrupted OPP memory in mice (Assini et al., 2009). Post-sample trial muscimol or anisomycin microinjection into the hippocampus also impaired long-term OPP memory (Oliveira et al., 2010; Ozawa et al., 2017). Furthermore, when injected 3 hours, but not 5 days, after the sample trial, intra-hippocampal infusions of muscimol injected twice daily for 4 consecutive days, impaired long-term OPP memory which was tested 2–4 days after the last infusion of muscimol (Gaskin et al., 2011). This finding is consistent with the previous lesion study in which OPP memory was spared in hippocampus-damaged animals, if the lesion was made 3 weeks, but not 1–3 days, after the learning trial (Gaskin et al., 2009b). Pre-sample, but not pre-test (Ozawa et al., 2017), infusions of anisomycin into the dorsal hippocampus impaired long-term OPP memory in the rat (Moncada, 2017; Ozawa et al., 2014, 2017). Similar effects were found with pre-sample infusions of the protein synthesis inhibitor emetine and the mRNA synthesis inhibitor 5,6-dichlorobenzimidazole 1-β-D-ribofuranoside into the dorsal hippocampus, while these infusions did not influence short-term (5 min) OPP memory (Ozawa et al., 2017). This suggests that learning and/or consolidation of long-term OPP is dependent on protein and mRNA synthesis in the hippocampus. Pre-test intra-hippocampus infusions of muscimol also disrupted OPP memory tested 24 hours later in rats (Gaskin et al., 2011), supporting a critical role of the hippocampus in the retrieval of long-term OPP memory.
Mice with specific inactivation of the hippocampal excitatory or inhibitory neurons exhibited long-term OPP deficits, suggesting that both excitatory and inhibitory neurons are cardinal for this memory (Haettig et al., 2013). In line with these findings, application of DREADDs by transferring excitatory- (M3Dq)- or inhibitory- (M4Di)-R into the dorsal hippocampus, led to improved long-term memory for OPP, but not NOP, in the M3Dq-activated mice, while the M4Di-activated animals showed the opposite (Lopez et al., 2016). Deficits in OPP memory (4-hour inter-trial interval) were also found when the dorsal hippocampus was, either pre- or post-sample, chemogenetic inhibited (M4Di or KORD) in the ovariectomized female mice (Tuscher et al., 2018). Post-sample activation of M3Dq selectively in the CA1 interneurons also impaired long-term OPP, but not NOP, memory in mice (Yu et al., 2018). In a simultaneous assessment of mnemonic information processing for NOP versus OPP, rats with lesioned hippocampus showed the preference for novel object over novel location (Chao et al., 2016b). This result implies that information about object, but not location, can be functionally compensated when the hippocampus is damaged. Overall, it is clear that the hippocampus plays an important role in OPP memory, which can be mediated by the hippocampal excitatory and inhibitory neurons.
Distinct hippocampal subregions are activated by different object stimuli that combine “what” and “where” components. For instance, in a setting of exploring three small “positionally” distinct objects, the distal CA1 and proximal CA3, which preferentially receive “what” information (Mishkin et al., 1983), were recruited during the recognition of a novel object (increased mRNA of the immediate early genes Arc and Homer1a), whereas the proximal CA3 and lower blade of dentate gyrus, but not CA1, were involved in novel object recognition during exploring three large “directionally” distinct objects (Hoang et al., 2018). The neuronal plasticity of hippocampal long-term potentiation (LTP) and long-term depression (LTD) underlie object exploration processes. When encountering a novel environment, LTP was enhanced in the CA1, dentate gyrus and CA3 (Hagena and Manahan-Vaughan, 2011; Kemp and Manahan-Vaughan, 2008), suggesting that the cellular mechanism is common for such a situation. In the NOP test, LTD was facilitated in the CA1 (Goh and Manahan-Vaughan, 2013c). During “positional” object exploration, LTD was enhanced in the CA1 region, but not dentate gyrus, while the opposite results were found during “directional” object exploration (Kemp and Manahan-Vaughan, 2008). Interestingly, LTD was facilitated at the commissural-associational fibers in CA3 during the “positional” object exploration test, but not at the mossy fibers from the dentate gyrus projecting to the CA3. In the “directional” object exploration setting, the mossy fibers-CA3 LTD was facilitated, but not the commissural-CA3 LTD (Hagena and Manahan-Vaughan, 2011). Furthermore, fast gamma (~60–100 Hz) synchrony between CA1 and CA3 is found to increase during the encoding of novel object-place information (Zheng et al., 2016).
Studies on proteomes of hippocampal areas CA1 and CA3 were found to show a different level of expression for ~ 31% (532 proteins out of 1697 ones) after an open field exploration. In addition, memories for NOP and OPP explicitly altered CA1 and CA3 proteomes, in which the changes of protein expression were largely consistent in CA1 across the two forms of memory, but distinct in CA3. These results suggest that specialized proteomic roles of CA1 and CA3 areas contribute to recognition memory (von Ziegler et al., 2018).
The memory for object-place associations requires the involvement of the hippocampus. Lesions or NMDA-R blockade in the hippocampus disrupt OiP memory (Barker et al., 2007; Barker and Warburton, 2015; Good et al., 2007a). Post-sample infusions of the DNMT inhibitor RG108 or 5-aza-2’-deoxycytidine into the hippocampus impaired memory in the OiP, but not NOP, test, suggesting that hippocampal DNA methylation engages in the processing of object-place information (Scott et al., 2017).
3.3.3. Temporal order memory
Lesion studies have indicated the hippocampus to have a pivotal role in the processing of memory for object recency and/or for temporal order recognition (Albasser et al., 2012; Barker and Warburton, 2011b), but see (Tam et al., 2014). TOM memory seems to be mildly influenced when tested with a short 2 min interval in hippocampus-lesioned rats (Good et al., 2007a). Studies investigating the roles of different hippocampal subregions have shown that region CA1, but not CA3, is critical in the processing of temporal memory for objects (Hoge and Kesner, 2007). When temporal order judgment is involved in spatial alternation, e.g. when an object is moved to different locations at different time points, both, the CA1 and CA3 subregions were shown to be involved (Hunsaker and Kesner, 2008). These data suggest that the CA1 processes temporal information, irrespective of whether spatial or non-spatial stimuli are involved, while the CA3 only participates in this processing if a spatial component is present. Imaging data also show the CA1 to be activated during both spatial and nonspatial processing, while CA3 activation codes primarily information about place (Beer et al., 2013; Beer et al., 2014). Since both the CA1 (MacDonald et al., 2011; Pastalkova et al., 2008) and CA3 (Salz et al., 2016) cells have been identified for the processing of “time”, an open question is how they contribute to episodic memory with respect to the “when” component. Distinct roles of CA1 and CA3 interacting with “when” is expected, as rats with the CA1 lesion showed deficits in a non-spatial temporal-order task if the inter-trial interval was 10, but not 3, seconds, while the CA3 lesioned rats had deficits in both intervals (Farovik et al., 2010). The hippocampus is important for the processing of memory for “when”, which is fundamental for the construction of episodic memory.
3.3.4. Episodic-like memory
The hippocampus is essential for the formation and storage of episodic-like memory. Intra-hippocampus infusion of muscimol disrupted episodic-like memory, measured by licking behavior, and more c-fos and zif-268 positive-cells were found in the hippocampal CA1, CA3 and dentate gyrus after the retrieval (Veyrac et al., 2015). In an episodic-like memory paradigm, in which social stimuli, i.e. female or dominant male animals, instead of objects were employed, mice displayed intact specific memory for “what-where-when” along with increased Arc/Arg3.1 mRNA expression in the hippocampus. In addition, microinjection of anisomycin into the hippocampus disrupted the consolidation of memory for “what-where-when” (Fellini and Morellini, 2013).
In the ELM2–1 test, the c-fos expression of hippocampal CA1 and dentate gyrus required for episodic-like memory was correlated with the when and where components, respectively (Castilla-Ortega et al., 2012; Castilla-Ortega et al., 2014). Furthermore, higher Arc RNA expression was found in the distal CA1 area for the processing of the when component, while the where component activated both CA1 and CA3 areas after the ELM2–1 test (Beer et al., 2018). In a nonmatching to location task, the distal CA3 (close to CA2), but not proximal CA3 (close to the dente gyrus), area was highly required (Flasbeck et al., 2018). These findings indicate that episodic memory is underpinned by segregated networks of spatial and non-spatial information along the proximo-distal axis of the hippocampus. In experiments studying the effects of chronic restraint stress and voluntary exercise on episodic-like memory and hippocampal neurogenesis, restraint disrupted neurogenesis and the when component, whereas exercise improved it and the where index of the ELM2–1 test (Castilla-Ortega et al., 2014). Pharmacological muscimol microinjections into the hippocampal CA1area disrupted the where and when components, while inactivation of the CA3/dentate gyrus spared the when component, but not the where component (Barbosa et al., 2012). Mice with hippocampal lesions were impaired in the what, where and when components in the ELM2–1 paradigm (DeVito and Eichenbaum, 2010). Transgenic mice with vasopressin 1b knockout exhibited deficient sociability and social novelty assessed by the three-chambered social test, and impaired when memory in the ELM2–1 test. Given that vasopressin 1b is highly expressed in the hippocampal CA2 area, the CA2 vasopressin 1b could be associated with social behaviors and episodic memory (DeVito et al., 2009), while its effects are likely independent of NMDAR as transgenic mice with selective NMDAR knockout in vasopressin 1b neurons showed intact social and object memories (Williams Avram et al., 2019).
Increased zif-268 expression was found in the dorsal CA1 after exposure to the ELM2–2 paradigm in which rats showed intact where, but not when component (Barbosa et al., 2013). Rats with temporal lobe epilepsy induced by kainate treatment exhibited impaired episodic-like memory, assessed by the ELM2–2 test, along with disrupted theta coherence across the CA1 and dentate gyrus, but performance on tests of memory for what (NOP), where (OPP) or when (TOM) were not influenced (Inostroza et al., 2013b). In this epilepsy model, decreased theta-gamma (30–60 Hz) coupling in the hippocampal CA1 area was associated with the impaired episodic-like memory (Lopez-Pigozzi et al., 2016). In the ELM2–2 test, pre-sample intra-CA1 muscimol infusions disrupted the interaction as well as the where and when components (Drieskens et al., 2017). Importantly, CA3 lesioned rats exhibited intact NOP, TOM and spatial recognition memories, but were not capable of episodic-like memory in the ELM2–2 test, indicating that the CA3 region is critical for the integration of “what-where-when” components into an episodic-like memory (Li and Chao, 2008). Intra-CA3 infusion of lidocaine also impaired the performance of rats tested by a task of source memory, which is critical for identifying different episodes (Crystal et al., 2013). Distinct, but complementary, roles of differential hippocampal subregions and circuits are expected in the processing of episodic-like memory.
3.3.5. Glutamate
Glutamate plays an important role in hippocampal plasticity and object memory. For example, the extracellular glutamate content in the hippocampus was increased in the NOP test (Cohen et al., 2013; Stanley et al., 2012). NOP memory and hippocampal LTP were both impaired even several weeks later by a single systemic administration of MK-801, a NMDA-R blocker, in rats (Wiescholleck and Manahan-Vaughan, 2013). The enhanced hippocampal LTD induced by the learning of novel objects can be blocked by the systemic administrations of the NMDA-R antagonist CPP or mGlu5-R antagonist MPEP (Goh and Manahan-Vaughan, 2013a). In an earlier study, mice with selective NMDA-R subunit 1 knockout in the hippocampal CA1 area exhibited NOP deficits (Rampon et al., 2000).
Pre-sample hippocampal infusions of AP5 disrupted NOP memory tested 3 hours, but not 5 minutes, later in rats (Baker and Kim, 2002). With manipulations of the number of objects used in the sample of the NOP test, pre-sample AP5 infusions into the hippocampus impaired memory when there were four, but not two, sample objects (Sugita et al., 2015). Pre-sample AP5 microinjections into the hippocampus impaired performance on OPP (Cassini et al., 2013), but not NOP (Barker and Warburton, 2015; Yamada et al., 2017). Pre-sample infusion of CNQX or AP5 into the rat postsubiculum also impaired long-term OPP, but not NOP, performance (Bett et al., 2013). Post-sample AP5 infusion into the hippocampus impaired OPP, while pre-test AP5 infusion had no influence, suggesting that the treatment influenced consolidation of information, but not retrieval processes (Yamada et al., 2017).
In a study that the strength of encoding (exposure to the sample from 2 – 5 times) and delay between trials (24 hours - 6 weeks) were manipulated, pre-test infusions of AP5 into the hippocampus disrupted NOP performance only when the memory was weak, while infusion of 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX), a potent AMPA-R antagonist, impaired the memory irrespective of the delays (Iwamura et al., 2016). The above results suggest that the hippocampal AMPA- and NMDA-R are important for OPP memory, while whether they account for NOP performance depends on the extent of memory load.
NMDA-R GluN1 and GluN2A levels were elevated in the hippocampus, but not in the PFC, after habituation to an open field and exploration in the NOP sample trial, while they did not change after the NOP test (Cercato et al., 2017), suggesting their roles in novelty discrimination of an environment. Reduction of NMDA-R surface dynamics in the dorsal hippocampus had no effect in the long-term (24 hours) OPP test (Potier et al., 2016). Maintenance of long-term OPP memory required GluA2/AMPA-R stabilization, as evidenced by intra-hippocampus infusions of peptides that intervene in the binding process of N-ethylmaleimide-sensitive factor to GluA2 (Migues et al., 2014). Likewise, post-sample hippocampal infusions of GluA23Y or G2CT that interfered with the GluA2/AMPA-R removal, reinstated the forgotten OPP long-term memory and did not influence new OPP learning (Migues et al., 2016). In the ELM2–1 test, selective knockout of the NMDA-R subunit NR1 in the hippocampal CA1 region impaired where, but not when, memory in mice. In contrast, mice with the same knockout, but in the CA3 region, exhibited intact where and when memory (Place et al., 2012). This suggests that the CA1 NMDA signaling is essential for the processing of spatial, but not temporal, information in an episodic-like context.
3.3.6. Dopamine
Simultaneous release of DA and NE was observed in the hippocampus during the OPP test, and catecholamine depletion in the dorsal hippocampus induced by 6-OHDA disrupted memory for OPP, but not NOP (Moreno-Castilla et al., 2017), indicating that hippocampal catecholaminergic neurotransmission has an active role in coding place of object.
In the SNOP test, infusions of the DA D1/2-R agonist apomorphine into the hippocampal CA1, but not CA3, disrupted short-term 3-min recognition of object location. The same infusions of apomorphine into the CA1 or CA3 region did not affect the object novelty (Vago and Kesner, 2008). Likewise, pre-sample microinjections of SCH23390 or SKF38393 into the hippocampus did not influence NOP memory tested 90 min or 24 hours later (Balderas et al., 2013). Thus, the hippocampal DA D1/2-R are key for the performance of location novelty, but not object novelty.
Pre-sample hippocampal infusion of the DA D1/5-R agonist SKF81297 improved long-term (24 hours interval) NOP memory (De Bundel et al., 2013), while that of SCH23390, a D1/5-R antagonist, impaired it (De Bundel et al., 2013). Pre-sample microinjections of SCH23390 into hippocampus did not affect OPP memory when tested 1 hour later (Savalli et al., 2015), but impaired long-term (24 hours) OPP memory (Moncada, 2017). The hippocampal DA D1/5-R mediate long-term NOP memory, while their roles in OPP memory are dependent on the extent of memory strength.
Post-sample trial microinjections of the D1-R antagonist SCH23390 and D3-R agonist 7-OH-DPAT, but not D2-R antagonist L-741.626, into the hippocampus impaired NOP when tested 1 hour later, whereas post-sample hippocampal infusions of SCH23390 had no effect on long-term NOP memory (Rossato et al., 2015; Rossato et al., 2013), although in another study an impairment by SCH23390 was reported (Furini et al., 2014). Post-sample microinjections of the D1-R agonist SKF81297, the D2-R agonist quinpirole and the D3-R antagonist SB277,011 into the dorsal hippocampus facilitated NOP when tested after 24 hours (Papp et al., 2017). Thus, the hippocampal DA D1-, D2- and D3-R are involved in NOP consolidation in a bidirectional manner, while D1-R showed variable effects.
In experiments on memory reconsolidation, in which the second sample trial can be considered as the test trial for NOP, pre-test infusions of SCH23390 into the dorsal hippocampal CA1 area showed no effects on long-term NOP memory (Rossato et al., 2015).
In a series of experiments studying the DAergic projections to the dorsal hippocampus, which originate primarily from the locus coeruleus, rather than ventral tegmental area (Kempadoo et al., 2016; Takeuchi et al., 2016), hippocampal opto-stimulation of the DA axon terminals from the locus coeruleus during learning facilitated long-term OPP memory. This facilitation effect was blocked by pre-sample trial infusions of SCH23390, but not of the β-adrenergic-R antagonist propranolol, into the dorsal hippocampus (Kempadoo et al., 2016). Although the ventral tegmental area-hippocampal DA projections are sparse, they likely stabilize memory of searching for a spatial goal (McNamara et al., 2014) and regulate the hippocampal theta rhythm (Orzel-Gryglewska et al., 2015). Memory consolidation induced by contextual novelty was modulated by the DA release from the locus coeruleus, rather than ventral tegmental area, which is dependent on the hippocampal DA D1/5-R, but not adrenergic-R (Takeuchi et al., 2016).
3.3.7. Serotonin
Hippocampal plasticity has been shown to be mediated by 5-HT. For instance, hippocampal CA1 pyramidal neurons exhibited reduced spontaneous inhibitory postsynaptic currents (IPSC) after the application of SLV, a 5-HT6-R antagonist (de Bruin et al., 2016). Systemic post-sample administration of fluoxetine recovered long-term OPP memory, activated Akt/GSK-3β signaling in the hippocampus and increased hippocampal LTP in mice (Yi et al., 2018).
Post-sample, but not pre-sample or pre-test, infusions of TCB-2, a 5-HT2A-R agonist, into the dorsal hippocampal CA1 improved NOP when tested 24 hours later in mice. TCB-2 also enhanced the hippocampal glutamate levels and the mean firing rate of CA1 neurons (Zhang et al., 2016). The NOP-facilitating effect of the 5-HT1B-R agonist CP94253 in 5-HT1B-R adapter protein p11 knockout mice was prevented by overexpression of p11 selectively in the hippocampus (Eriksson et al., 2013). Post-sample infusions of RS67333, a 5-HT4-R agonist, or RS23597, a 5-HT4-R antagonist, into the dorsal CA1 area impaired spatial recognition tested by the SNOP paradigm (Nasehi et al., 2017).
Different subtypes of hippocampal 5-HT-R play distinct roles in object recognition, whereby 5-HT1A-, 5-HT1B- and 5-HT2A-R are involved in facilitation of consolidation, while 5-HT4-R seems to impair the processing of object consolidation and/or retrieval.
3.3.8. Acetylcholine
In the SNOP test including five different objects (Vago and Kesner, 2008), infusions of scopolamine and physostigmine, an acetylcholinesterase inhibitor, into the hippocampal CA3 area impaired and enhanced spatial and object recognition, respectively (Hunsaker et al., 2007b). Selective transection of dorsal CA3 projections in the fimbria, which constitutively blocks the MSvDB signals toward CA3, resulted in deficient spatial and object recognition in the SNOP test (Hunsaker et al., 2007b). Increased hippocampal ACh efflux was also found, regardless of whether a familiar or novel object was presented (Stanley et al., 2012).
Pre-sample infusions of nicotine into the dorsal hippocampus facilitated long-term NOP and OPP memories when tested 72 hours later in rats (Melichercik et al., 2012). Post-sample microinjections of scopolamine into the hippocampus disrupted NOP memory after 90 min, but not 24 hours; long-term NOP memory was also not influenced when the injection was made 160 min after the sample trial or 90 min before the test trial (Balderas et al., 2012). Pre-test infusions of scopolamine into the dorsal CA1 area did not affect NOP memory, but reduced the overall object exploration time (Rashid and Ahmed, 2019). The hippocampal ACh-R play key roles in memory consolidation, with nicotinic and muscarinic ACh-R being related to facilitation and disruption of mnemonic storage, respectively.
The interaction between cholinergic and NMDA systems contributes to memory. In cultured hippocampal neurons, for example, co-application of choline and NMDA/glycine caused larger currents than the application of NMDA/glycine alone; choline enhanced NMDA-R-mediated LTP of miniature excitatory postsynaptic currents (EPSC). Intervention with the interaction between the nicotinic α7 ACh-R and NMDA-R blocked these effects in vitro, and disrupted NOP memory in mice (Li et al., 2013). Application of the nicotinic α7 ACh-R agonist FRM-17874 facilitated NOP in rats and dose-dependently enhanced the theta oscillation induced by electrical stimulation in the rodent hippocampus (Stoiljkovic et al., 2015).
3.3.9. Histamine
The tuberomammillary nucleus of the posterior hypothalamus, the origin of histaminergic neurons, projects axons to wide areas of the brain; e.g. histamine H1-, H2- and H3-R are found in the cortex, hippocampus and amygdala (Esbenshade et al., 2008; Panula et al., 1984; Ryu et al., 1995; Watanabe et al., 1984). In histamine H1-R and H2-R knockout mice, LTP in the hippocampus CA1 area was decreased together with impairment of NOP memory (Dai et al., 2007).
The hippocampal histamine-R play a critical role in object memory consolidation: Post-sample infusions of the histamine H1-R antagonist pyrilamine, the H2-R antagonist ranitidine or the H3-R agonist imetit into the hippocampal CA1 area impaired long-term NOP memory (24 hours) when infused 30 min or 2 hours later, but not when applied immediately or 6 hours later (da Silveira et al., 2013). Conversely, the histamine H1-R agonist pyridylethylamine, the H2-R agonist dimaprit and the H3-R antagonist thioperamide had no effect on NOP consolidation when infused into the CA1 (da Silveira et al., 2013).
3.3.10. Norepinephrine
The locus coeruleus located in the brainstem is a cluster of adrenergic neurons that release NE across the central nervous system. It is well-known that NE modulates multiple functions, including arousal, attention, stress, and learning and memory (Benarroch, 2009; Schwarz and Luo, 2015). Systemic administration of propranolol, a β-adrenergic antagonist, blocked the learning-induced hippocampal LTD and spatial object recognition in freely moving mice (Goh and Manahan-Vaughan, 2013b).
The rat hippocampal NE levels were found to increase after object exploration (Mello-Carpes et al., 2016). Post-sample infusions of the β-adrenergic antagonist timolol into the hippocampal CA1 area disrupted long-term NOP memory in rats (Furini et al., 2010; Mello-Carpes et al., 2016; Mello-Carpes and Izquierdo, 2013). Interestingly, intra-CA1 infusions of NE reversed the long-term NOP memory impairment induced by infusions of muscimol into the locus coeruleus (Mello-Carpes and Izquierdo, 2013). Post-sample infusions of NE into the rat CA1 region also promoted NOP memory tested 21 days later (Mello-Carpes et al., 2016). Thus, NE systems in the hippocampal CA1 control long-term NOP consolidation.
3.3.11. Cannabinoid
The endocannabinoid system critically mediates development, synaptic plasticity, cognition and neuropsychiatric disorders through cannabinoid-R, endocannabinoids and the enzymes regulating the synthesis and degradation of the endocannabinoids. The G-protein-coupled cannabinoid 1-R is enriched in axonal terminals in the brain, while cannabinoid 2-R is mainly, but not exclusively, expressed in the periphery system (Lu and Mackie, 2016; Lutz et al., 2015). Application of JWH-018, a synthetic cannabinoid 1/2-R agonist, decreased potassium-evoked glutamate and GABA release and LTP in hippocampus slices (Barbieri et al., 2016; Basavarajappa and Subbanna, 2014). Mice with selective knockout of cannabinoid 1-R in the GABAergic neurons, exhibited disruption of NOP (30 min) memory, and facilitation of spontaneous IPSC in the CA1 pyramidal neurons in vitro (Albayram et al., 2016). Furthermore, knockdown of the cannabinoid 1-R-interacting protein 1 in the mouse hippocampus increased cell proliferation and neuroblast differentiation in the dentate gyrus and improved NOP memory (Jung et al., 2017).
Intra-hippocampus infusions of WIN 55,212–2, a non-selective cannabinoid-R agonist, dose-dependently disrupted the performance in the OPP, but not NOP, test in rats (Suenaga and Ichitani, 2008). Post-sample infusions of WIN 55,212–2 or VDM-11, an endocannabinoid membrane transporter inhibitor, into the hippocampal CA1 region impaired long-term, but not short-term, NOP memory in a dose-dependent manner. Similar effects were found by the CA1 infusion of the cannabinoid 1-R agonist ACEA, but not the cannabinoid 2-R agonist JWH-015 or palmitoylethanolamide, an endogenous fatty acid amide with affinity to cannabinoid-R GPR55 and GPR119, suggesting a role of hippocampal cannabinoid 1-R in NOP consolidation (Clarke et al., 2008). Microinjections of the cannabinoid 1-R antagonist AM251 into the CA1 region facilitated object novelty tested by the SNOP paradigm (Nasehi et al., 2017).
The cannabinoid 1-R in the hippocampus play distinct roles in the modulation of NOP and OPP memory, as evidenced by memory being impaired and improved through pharmacological activation and blockage of the R, respectively.
3.3.12. Signaling pathway
Many molecular pathways within the hippocampus are involved in object memory. For instance, inhibition of d-amino acid oxidase through d-serine modulation, augmented NMDA-R-mediated long-term potentiation (LTP) in the hippocampus ex vivo and improved long-term NOP memory in vivo (Hopkins et al., 2013). Intra-hippocampal infusions of rapamycin, an inhibitor of mTOR signaling, either pre- or post-sample, impaired NOP memory tested 24 hours later, suggesting that mTOR mechanisms in the hippocampus are underlying long-term object memory (Jobim et al., 2012), compatible with previous findings (Myskiw et al., 2008). Intra-hippocampal infusions of PD98059, a selective MEK inhibitor, disrupted long-term NOP memory in mice (Nagai et al., 2007). The RGS14 gene knockout mouse exhibited improved NOP memory and facilitation of LTP in the CA2, but not CA1, area. This effect is dependent upon the ERK/MAPK pathways, given that facilitated LTP in the CA2 was blocked by U0126, a MEK inhibitor (Lee et al., 2010). Expression of the nuclear factor kappa B (NF-kB) and the immediate-early gene zif268 were increased after object exploration, and post-trial intra-hippocampal inhibition of NF-kB or zif268 impaired NOP memory (Zalcman et al., 2015). Object exploration also increased the expression of the immediate-early genes Nr4a1 and Nr4a2, while intra-hippocampal knockdown of either one impaired long-term OPP (24 hours) memory (McNulty et al., 2012). Post-sample infusions of zeta inhibitory peptide into the rat hippocampus impaired NOP memory (Hales et al., 2015). Intra-hippocampus injection of the AAV vector carrying either a negative or active form of Rac1, facilitated and impaired NOP memory, respectively, suggesting that Rac1 activity in the hippocampus dually controls NOP memory (Liu et al., 2016). Inactivating the atypical protein kinase C isoform M zeta (PKMζ) in the dorsal hippocampus abolished memory for OPP, but not NOP (Hardt et al., 2010); these effects were regulated by glutamate subunit 2-dependent AMPA-R (Migues et al., 2010).
Object exploration elevated the rat hippocampal BDNF levels (Furini et al., 2010; Mello-Carpes et al., 2016), and selective BDNF deletion in the mouse hippocampus disrupted NOP memory (Heldt et al., 2007). Post-sample intra-hippocampus BDNF infusions were found to compensate OPP deficits induced by anisomycin, and hippocampal BDNF infusions facilitated long-term OPP memory (Ozawa et al., 2014), suggesting hippocampal BDNF is involved in protein synthesis for spatial memory consolidation. Increased expression of BDNF in the hippocampal CA1 with the transduction of AAV9-BDNF, elevated the phosphorylation level of CaMKII, CREB, tropomyosin receptor kinase (TrkB) and p38 MAPK, and facilitated long-term OPP, but not NOP, memory (Wang et al., 2017b). Thus, BDNF signaling pathway in the hippocampus mediates NOP and OPP memory.
Post-sample hippocampal infusions of histone acetylase inhibitors decreased histone acetylation and impaired NOP memory (Federman et al., 2013; Zhao et al., 2012), while the infusion of a HDAC inhibitor had the opposite effects (Federman et al., 2013). Longer learning of the sample trial of the NOP test increased hippocampal histone H3 acetylation and persistence of NOP memory, which could be prevented by NF-kB inhibition (Federman et al., 2013). OPP memory was enhanced by post-sample microinjections of the HDAC inhibitor sodium butyrate into the dorsal hippocampus (Roozendaal et al., 2010). HDAC3-flox mice injected with AAV-Cre recombinase into the hippocampal CA1 region to selectively deplete HDAC3 in CA1, showed enhanced long-term OPP memory. Similar results were found in mice with the intra-CA1 infusions of RGFP136, a selective HDAC3 inhibitor (McQuown et al., 2011). The deacetylase domain of HDAC3 in the hippocampus was also found to be critical for long-term OPP memory in mice (Alaghband et al., 2017). Disrupted long-term OPP, but not NOP, memory, along with impaired hippocampal LTP and reduced histone H2B, H3 and H4 acetylation, were found in the CREB-binding protein-flox mice microinjected with AAV-Cre into the dorsal hippocampus to produce hippocampal CREB-binding protein depletion (Barrett et al., 2011).
3.3.13. Short summary
Although the involvement of hippocampus in the NOP test is controversial (see 3.3.1), the hippocampus is essential for the processing of long-term NOP consolidation and retrieval. In addition, the hippocampus cardinally regulates the processing of OPP, OiP and episodic-like memories. Distinct neurotransmitter systems in the hippocampus have complementary and interactive roles in the regulation of different object exploration tests. The hippocampal DA, ACh and NE bi-directionally modulate NOP and OPP memories, while glutamate, 5-HT, histamine and cannabinoids are also engaged. The functions of hippocampal neurotransmitter systems are governed by different subtypes of receptors. Details of findings of substances injected locally into the hippocampus (points 3.3.5–11) are listed in Table 4.
Table 4.
Pharmacological studies investigating the role of neurotransmitter systems in the hippocampus in object exploration tests. Substances are infused into the dorsal hippocampus, primarily the CA1 area.
| Species and strain | Treatment | Test | Time of treatment | Sample trial | Retention | Findings | Reference |
|---|---|---|---|---|---|---|---|
| Rats | NBQX | NOP | Pre-test | 5minx5 | 24h | Impaired | Iwamura et al., 2016 |
| (AMPA-R antagonist) | NOP | Pre-test | 5minx5 | 1w | Impaired | ||
| NOP | Pre-test | 5minx5 | 3w | Impaired | |||
| Long-Evans | AP5, 3.75μg | NOP | Pre-sample | 1min | 5min | No effect | Baker&Kim, 2002 |
| male rats | (NMDA-R antagonist) | NOP | Pre-sample | 1min | 3h | Impaired | |
| Pigmented | AP5, 25mM/0.5μl | NOP | Pre-sample | 4min | 1h | No effect | Barker&Warburton, 2015 |
| male rats | (NMDA-R antagonist) | ||||||
| Long Evans | AP5, 40mM/1.0μl | NOP | Pre-sample | 5min | 5min | No effect | Sugita et al., 2015 |
| male rats | (NMDA-R antagonist) | NOP | Pre-sample | 5min | 5min | Impaired/4obj | |
| Wistar | AP5, 20–40mM | NOP | Pre-sample | 3min | 20min | No effect | Yamada et al., 2017 |
| male rats | (NMDA-R antagonist) | ||||||
| Rats | AP5 | NOP | Pre-test | 5minx2 | 24h | No effect | Iwamura et al., 2016 |
| (NMDA-R antagonist) | NOP | Pre-test | 5minx2 | 1w | Impaired | ||
| Rats | AP5 | NOP | Pre-test | 5minx5 | 1w | No effect | Iwamura et al., 2016 |
| (NMDA-R antagonist) | NOP | Pre-test | 5minx5 | 3w | Impaired | ||
| Wistar | SCH23390, 2.0μg | NOP | Pre-sample | 5min | 90min | No effect | Balderas et al., 2013 |
| male rats | (DA D1/5-R antagonist) | NOP | Pre-sample | 5min | 24h | No effect | |
| Sprague-Dawley | SCH23390, 1.0μg | NOP | Pre-sample | 15min | 24h | Impaired | De Bundel et al., 2013 |
| male rats | (DA D1/5-R antagonist) | ||||||
| Wistar | SCH23390, 1.5μg | NOP | Post-sample | 5min | 24h | No effect | Rossato et al., 2013 |
| male rats | (DA D1/5-R antagonist) | NOP | 6 h post-sample | 5min | 24h | No effect | |
| Wistar | SCH23390 | NOP | Post-sample | 5min | 24h | Impaired | Furini et al., 2014 |
| male rats | (DA D1/5-R antagonist) | NOP | 1h post-sample | 5min | 24h | Impaired | |
| NOP | 3h post-sample | 5min | 24h | No effect | |||
| Wistar | SCH23390, 1.5μg | NOP | Post-sample | 5 min | 24h | No effect | Rossato et al., 2015 |
| male rats | (DA D1/5-R antagonist) | NOP | 6 h post-sample | 5 min | 24h | No effect | |
| NOP | Pre-test | 5 min | 24h | No effect | |||
| Wistar | SCH23390, 0.5–3.0μg | NOP | Post-sample | reach 20s | 1h | Impaired | Papp et al., 2017 |
| male rats | (DA D1/5-R antagonist) | 3.0 μg | |||||
| Wistar | SKF38393, 12.5μg | NOP | Pre-sample | 3min | 90min | No effect | Balderas et al., 2013 |
| male rats | (DA D1-R agonist) | NOP | Pre-sample | 3min | 24h | No effect | |
| Sprague-Dawley | SKF81297, 0.03–3.0μg | NOP | Pre-sample | 2min | 24h | Facilitated | De Bundel et al., 2013 |
| male rats | (DA D1/5-R agonist) | ||||||
| Wistar | SKF81297, 0.05–0.5μg | NOP | Post-sample | reach 20s | 24h | Facilitated | Papp et al., 2017 |
| male rats | (DA D1/5-R agonist) | 0.5μg | |||||
| Wistar | L741.626, 0.5–2.5μg | NOP | Post-sample | reach 20s | 1h | No effect | Papp et al., 2017 |
| male rats | (DA D2-R antagonist) | ||||||
| Wistar | Quinpirole, 3.0μg | NOP | Post-sample | 5min | 24h | No effect | Rossato et al., 2013 |
| male rats | (DA D2-R agonist) | ||||||
| Wistar | Quinpirole, 0.1–5.0μg | NOP | Post-sample | reach 20s | 24h | Facilitated | Papp et al., 2017 |
| male rats | (DA D2-R agonist) | 5.0μg | |||||
| Wistar | SB-277,011, 0.1–1.0μg | NOP | Post-sample | reach 20s | 24h | Facilitated | Papp et al., 2017 |
| male rats | (DA D3-R antagonist) | 1.0μg | |||||
| Wistar | 7-OH-DPAT, 0.1–10μg | NOP | Post-sample | reach 20s | 1h | Impaired | Papp et al., 2017 |
| male rats | (DA D3-R agonist) | 0.1–1.0μg | |||||
| C57BL/6J | TCB-2, 1μg | NOP | Post-sample | 10min | 24h | Facilitated | Zhang et al., 2016 |
| male mice | (5-HT2A-R agonist) | ||||||
| Wistar | Scopolamine, 30μg | NOP | Post-sample | 10min | 90min | Impaired | Balderas et al., 2012 |
| male rats | (mACh-R antagonist) | NOP | Post-sample | 10min | 24h | No effect | |
| NOP | 160min post-sample | 10min | 24h | No effect | |||
| NOP | 90min pre-test | 10min | 24h | No effect | |||
| Long-Evans | Nicotine, 0.5–2μg | NOP | Pre-sample | 3 min or | 72h | Facilitated | Melichercik et al., 2012 |
| male rats | (nACh-R agonist) | reach 25s | 0.5–1.0μg | ||||
| Wistar | Pyrilamine, 50mM/1.0μl | NOP | Post-sample | 5min | 24h | No effect | de Silveira et al., 2013 |
| male rats | (H1-R antagonist) | NOP | 30min post-sample | 5min | 24h | Impaired | |
| NOP | 2h post-sample | 5min | 24h | Impaired | |||
| NOP | 6 h post-sample | 5min | 24h | No effect | |||
| Wistar | Pyridylethylamine, | NOP | Post-sample | 5min | 24h | No effect | de Silveira et al., 2013 |
| male rats | 10mM/1.0μl | NOP | 30min post-sample | 5min | 24h | No effect | |
| (H1-R agonist) | NOP | 2h post-sample | 5min | 24h | No effect | ||
| NOP | 6 h post-sample | 5min | 24h | No effect | |||
| Wistar | Ranitidine, 50mM/1.0μl | NOP | Post-sample | 5min | 24h | No effect | de Silveira et al., 2013 |
| male rats | (H2-R antagonist) | NOP | 30min post-sample | 5min | 24h | Impaired | |
| NOP | 2h post-sample | 5min | 24h | Impaired | |||
| NOP | 6 h post-sample | 5min | 24h | No effect | |||
| Wistar | Dimaprit, 10mM/1.0μl | NOP | Post-sample | 5min | 24h | No effect | de Silveira et al., 2013 |
| male rats | (H2-R agonist) | NOP | 30min post-sample | 5min | 24h | No effect | |
| NOP | 2h post-sample | 5min | 24h | No effect | |||
| NOP | 6 h post-sample | 5min | 24h | No effect | |||
| Wistar | Thioperamide, | NOP | Post-sample | 5min | 24h | No effect | de Silveira et al., 2013 |
| male rats | 50mM/1.0μl | NOP | 30min post-sample | 5min | 24h | No effect | |
| (H3-R antagonist) | NOP | 2h post-sample | 5min | 24h | No effect | ||
| NOP | 6 h post-sample | 5min | 24h | No effect | |||
| Wistar | Imetit, 10mM/1.0μl | NOP | Post-sample | 5min | 24h | No effect | de Silveira et al., 2013 |
| male rats | (H3-R agonist) | NOP | 30min post-sample | 5min | 24h | Impaired | |
| NOP | 2h post-sample | 5min | 24h | Impaired | |||
| NOP | 6 h post-sample | 5min | 24h | No effect | |||
| Wistar | Timolol, 1μg | NOP | Post-sample | 5min | 24h | Impaired | Furini et al., 2010 |
| male rats | (β-adrenergic antagonist) | ||||||
| Wistar | Timolol, 1μg | NOP | Post-sample | 5min | 24h | Impaired | Mello-Carpes&Izquierdo, |
| male rats | (β-adrenergic antagonist) | 2013 | |||||
| Wistar | Timolol, 1μg | NOP | Post-sample | 5min | 24h | Impaired | Mello-Carpes et al., 2016 |
| male rats | (β-adrenergic antagonist) | ||||||
| Wistar | Norepinephrine, 1μg | NOP | Post-sample | 5min | 24h | No effect | Mello-Carpes&Izquierdo, |
| male rats | 2013 | ||||||
| Wistar | Norepinephrine, 1μg | NOP | Post-sample | 5min | 24h | No effect | Mello-Carpes et al., 2016 |
| male rats | NOP | Post-sample | 5min | 3w | Facilitated | ||
| Wistar | WIN55,212–2, 1–2.0μg | NOP | Pre-sample | 3min | 20min | No effect | Suenaga&Ichitani, 2008 |
| male rats | (CB-R agonist) | ||||||
| Wistar | WIN55,212–2, 10nM/0.8μl | NOP | Post-sample | 5min | 3h | No effect | Clarke et al., 2008 |
| male rats | (CB-R agonist) | NOP | Post-sample | 5min | 24h | Impaired | |
| NOP | Pre-test | 5min | 24h | No effect | |||
| Wistar | AM-251, 1–100pM/0.8μl | NOP | Post-sample | 5min | 24h | No effect | Clarke et al., 2008 |
| male rats | (CB 1-R antagonist) | ||||||
| Wistar | ACEA, 0.001–0.01fM/0.8μl | NOP | Post-sample | 5min | 24h | Impaired | Clarke et al., 2008 |
| male rats | (CB 1-R agonist) | ||||||
| Wistar | JWH-015, 10–300fM/0.8μl | NOP | Post-sample | 5min | 24h | No effect | Clarke et al., 2008 |
| male rats | (CB 2-R agonist) | ||||||
| Wistar | PLL, 1–100pM/0.8μl | NOP | Post-sample | 5min | 24h | No effect | Clarke et al., 2008 |
| male rats | (CB 2-R agonist) | ||||||
| Wistar | VDM-11, 100pM/0.8μl | NOP | Post-sample | 5min | 3h | No effect | Clarke et al., 2008 |
| male rats | (ECB transporter inhibitor) | NOP | Post-sample | 5min | 24h | Impaired | |
| Wistar male rats | AP5, 5μg | OPP | Pre-sample | 4min | 1h | Impaired | Cassini et al., 2013 |
| (NMDA-R antagonist) | OPP | Pre-sample | 4min | 24h | No effect | ||
| Pigmented | AP5, 25mM/0.5μl | OPP | Pre-sample | 3min | 1h | Impaired | Barker&Warburton, 2015 |
| male rats | (NMDA-R antagonist) | ||||||
| Wistar male rats | AP5, 20–40mM | OPP | Pre-sample | 3min | 5min | Impaired | Yamada et al., 2017 |
| (NMDA-R antagonist) | |||||||
| Wistar male rats | AP5, 20–40mM | OPP | Pre-sample | 10min | 2h | Impaired | Yamada et al., 2017 |
| (NMDA-R antagonist) | OPP | Post-sample | 10min | 2h | Impaired | ||
| OPP | Pre-test | 10min | 2h | No effect | |||
| Dark Agouti | SCH23390, 5mM/1.0μl | OPP | Pre-sample | 5min | 1h | No effect | Savalli et al., 2015 |
| male rats | (DA D1/5-R antagonist) | ||||||
| Wistar male rats | SCH23390, 2μg | OPP | Pre-sample | 8min | 24h | Impaired | Moncada, 2017 |
| (DA D1/5-R antagonist) | |||||||
| Long-Evans | Nicotine, 0.5–2μg | OPP | Pre-sample | 3 min or | 72h | Facilitated | Melichercik et al., 2012 |
| male rats | (nACh-R agonist) | reach 25s | 0.5–2.0μg | ||||
| Wistar | WIN55,212–2, 1–2.0μg | OPP | Pre-sample | 3minx2 | 5min | Impaired | Suenaga&Ichitani, 2008 |
| male rats | (cannabinoid-R agonist) | ||||||
| Long-Evans | Apomorphine, 10–15μg | SNOP | Post-sample* | 6min | 3min | Impaired | Vago&Kesner, 2008 |
| male rats | (DA D1/2-R agonist) | SD | |||||
| NMRI | RS23597, 0.016μg | SNOP | Post-sample* | 6minx3 | 24h | Impaired | Nasehi et al., 2017 |
| male mice | (5-HT4-R antagonist) | SD | |||||
| NMRI | RS67333, 0.016μg | SNOP | Post-sample* | 6minx3 | 24h | Impaired | Nasehi et al., 2017 |
| male mice | (5-HT4-R agonist) | SD | |||||
| Long-Evans | Scopolamine, 30μM/0.4μl | SNOP | Post-sample* | 6min | 3min | Impaired | Hunsaker et al., 2007b |
| male rats | (mACh-R antagonist) | CA3 | SD and NO | ||||
| Long-Evans | Physostigmine, 30μM/0.4μl | SNOP | Post-sample* | 6min | 3min | Facilitated | Hunsaker et al., 2007b |
| male rats | (ACh-esterase inhibitor) | CA3 | SD and NO | ||||
| NMRI | AM251, 0.2μg | SNOP | Post-sample* | 6minx3 | 24h | Faciliated | Nasehi et al., 2017 |
| male mice | (CB1-R antagonist) | SD | |||||
| Pigmented | AP5, 25mM/0.5μl | OiP | Pre-sample | 5min | 5min | Impaired | Barker&Warburton, 2015 |
| male rats | (NMDA-R antagonist) | OiP | Pre-sample | 5min | 1h | Impaired | |
| OiP | Pre-test | 5min | 1h | No effect | |||
| Dark Agouti | SCH23390, 5mM/1.0μl | OiP | Pre-sample | 5min | 5min | No effect | Savalli et al., 2015 |
| male rats | (DA D1/5-R antagonist) | OiP | Pre-sample | 5min | 1h | No effect | |
Abbreviations: AP5, 2-amino-5-phosphonopentanoic acid; NBQX, 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide; PLL, palmitoylethanolamide; H-R: histamine receptors; CB-R: cannabinoid receptors; ECB, endocannabinoid; NOP, novel object preference; OPP, object place preference; SNOP, spatial and non-spatial object preference; OiP, object-in-place preference; obj, objects; SD, spatial displaced object; NO, novel object; s, seconds; min, minutes; h, hours; w, weeks.
After the infusions, one more sample trial was given before the test trials of SNOP paradigm.
4. The functional hippocampal-cortical network of episodic memory
As the mPFC, LEC and hippocampus have major functions in the processes governing memory for object, place, time and their integration, we thus dissect their anatomical, electrophysiological and behavioral roles in the context of episodic memory.
4.1. The pathways between the prefrontal cortex, entorhinal cortex and hippocampus
There are strong multi-synaptic (indirect pathway) connections between the mPFC and hippocampus, but monosynaptic projections (direct pathway) also exist (Rajasethupathy et al., 2015). A direct hippocampus-PFC pathway originates from the ventral hippocampal CA1 area and subiculum onto the mPFC, while sparse projections from the intermediate third of the hippocampus also exist (Cenquizca and Swanson, 2007; Jay and Witter, 1991). This hippocampus-mPFC pathway makes synaptic contact both with glutamatergic pyramidal neurons and GABAergic interneurons within the mPFC (Gabbott et al., 2002; Jay et al., 1992; Tierney et al., 2004). The majority studies have focused on the dorsal hippocampus, and thus, the understanding of functions of the hippocampal ventral compartment is not as solid as that of its dorsal part. The dorsal and ventral hippocampus exhibit functional heterogeneity (Fanselow and Dong, 2010). For example, the ventral CA1 cells required stronger afferent stimulation to elicit action potentials, showed lower neuronal excitability and attenuated LTP induced by theta burst stimulation than the dorsal CA1 cells. The levels of NMDA GluN1-, GluN2-, mGlu1-, mGlu2/3- and DA D1-R were higher, but GABAA-R was lower, in the ventral than dorsal hippocampus, while mGlu5-, GABAB- and DA D2-R were unchanged across the hippocampus (Dubovyk and Manahan-Vaughan, 2018). The functional topography along the hippocampus dorsal-ventral axis likely affects the interaction with the mPFC, which receives strong projections from the ventral hippocampus.
A direct monosynaptic projection from the mPFC back to the hippocampus could not be reveal until recently, when a projection from the prefrontal cortical ACC to the dorsal hippocampal CA1 and CA3 regions in mice was found through viral vector tracing. This monosynaptic ACC-hippocampus projection was further examined by transferring channelrhodopsin-2 (ChR2) into the ACC, and by patch-clamp recording in vitro dorsal CA1 and CA3 neurons. It has found that light-stimulation of the axonal terminals of ACC was sufficient to incur EPSC and evoked action potentials in the CA1 and CA3 neurons, but not the dentate gyrus. This monosynaptic projection is also functionally essential for memory retrieval, as assessed by contextual fear conditioning (Rajasethupathy et al., 2015). Thus, direct and mutual information processing between the PFC and hippocampus exists in rodents. The intermediate and ventral hippocampus send projections to the mPFC, which presumably transfers hippocampus-relevant information to the PFC. The prefrontal cortical ACC projects back to the hippocampus and delivers PFC-relevant information onto the hippocampus (Fig. 4).
Figure 4.
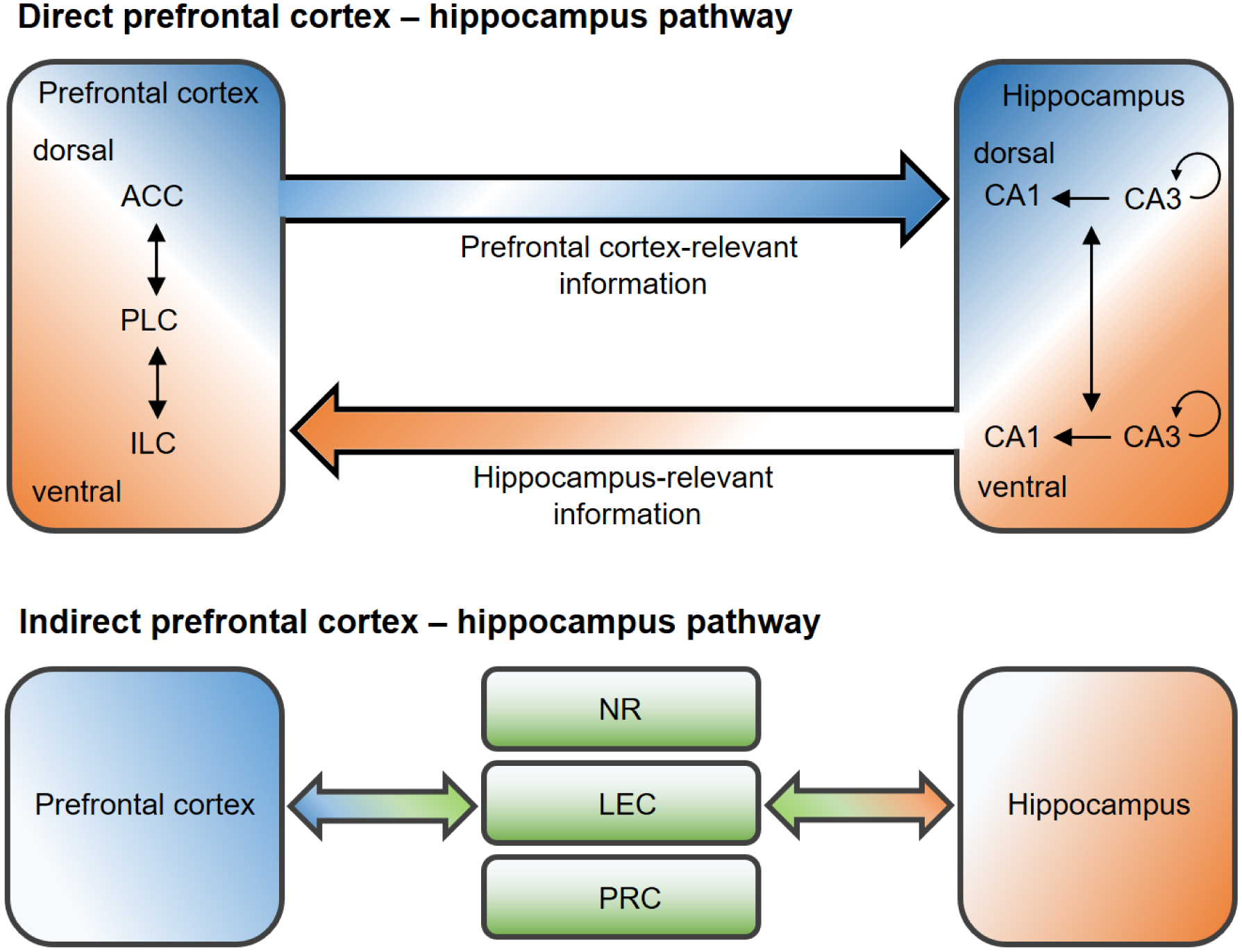
Diagram of direct (top) and indirect (bottom) neuroanatomical pathways between the prefrontal cortex and hippocampus. ACC: anterior cingulate cortex; PLC: prelimbic cortex; ILC: infralimbic cortex; NR: nucleus reuniens; LEC: lateral entorhinal cortex; PRC: perirhinal cortex.
The indirect communication between the PFC and hippocampus was via other substrates to form many multi-synaptic routes. The nucleus reuniens (NR) in the medial thalamus, the perirhinal cortex and LEC are mutually connected with the PFC and hippocampus (Apergis-Schoute et al., 2006; Burwell and Amaral, 1998a; Hoover and Vertes, 2007; Varela et al., 2014). Anatomical interconnections are also found among the NR, perirhinal cortex and LEC (Burwell and Amaral, 1998a, b; Varela et al., 2014). Besides these regions, other neural substrates that project to both mPFC and hippocampus include the amygdala, ventral tegmental area, MSDB, and hypothalamus (Hoover and Vertes, 2007; Varela et al., 2014). DA and ACh neurotransmission play critical roles in the regulation of memory via the ventral tegmental area and MSDB projections to the PFC-hippocampus-associated circuits (Hasselmo, 2006; Lisman and Grace, 2005). Importantly, a subpopulation of single NR and LEC neurons sends axonal collaterals to both mPFC and hippocampus, implicating that the NR and LEC can directly and simultaneously modulate the activities of mPFC and hippocampus (Hoover and Vertes, 2012; Varela et al., 2014). The inhibitory projections from the LEC, but not MEC (since the projections are sparse (Melzer et al., 2012), to the hippocampal CA1 area form a disinhibition mechanism which blunts NOP processing (Basu et al., 2016). Thus, the indirect PFC-hippocampus pathway could engage in the functions required for the interaction between PFC and hippocampus. For example, the PFC-NR circuit is proposed to modulate memory generalization versus specification, at least in hippocampus-dependent contextual fear memory (Xu and Sudhof, 2013). The interplay between the mPFC and medial thalamus is also critical for TOM and OiP, but not NOP, tests (Cross et al., 2012). Electrical stimulation of the EC has been found to reduce PFC pyramidal cellular activity (Valenti and Grace, 2009), and to enhance neurogenesis in the dentate gyrus of the hippocampus (Stone et al., 2011). Brain activities, measured by fMRI, were increased in the rat mPFC and hippocampus by 5–20 Hz electrical stimulation of the LEC (Krautwald et al., 2019). Imaging studies have also indicated that the LEC-CA1 pathway is recruited for the processing of exploration of familiar objects and of object-recency, while the pathway of LEC-dentate gyrus-CA3 is activated for the processing of recognition of novel objects and for object-recency (Aggleton et al., 2012; Kinnavane et al., 2014; Olarte-Sanchez et al., 2014). Evidence also shows increased functional connectivity of hippocampal CA1, CA3, insular cortex, perirhinal cortex and mPFC formed by the NOP test (Tanimizu et al., 2018). The interaction between the mPFC and LEC is crucially involved in episodic-like memory in rats (Chao et al., 2016a). While the direct PFC-hippocampal and indirect PFC-NR/perirhinal/LEC-hippocampal pathways form anatomical loops for memory, the functional distinctions between the direct and indirect pathways are elusive, particularly in the context of episodic memory.
4.2. Functional connectivity between the prefrontal cortex and hippocampus
4.2.1. Electrophysiological studies
Electrophysiological and behavioral evidence has implicated the PFC-hippocampus circuits in the processing of memory. For instance, a portion of the PFC neurons were found to be coupled to the theta rhythm (4–10 Hz) of the hippocampus and to best match with a delay of approximately 50 ms. This illustrates that the PFC-hippocampal projections generate an oscillatory synchronization and supports the concept of a directional pathway of hippocampus towards the PFC as focal for information processing (Siapas et al., 2005). In a reward-based Y-maze learning paradigm, the theta coherence between the PFC and hippocampus was increased, especially after rats had learned how to reliably procure food (Benchenane et al., 2010). Lesions in the ventral hippocampus disrupted the PFC activity for anticipating a spatial goal (Burton et al., 2009), indicating that the hippocampus processes and guides the PFC with respect to spatial information. In a complex experimental design, rats were trained to discriminate object-reward pairings based on different contexts: Object A, but not object B, was paired with food in context 1, with the opposite condition in context 2. Local field potentials of the PFC and hippocampus were recorded during the onset of exploring the contexts and objects. The strength of theta synchronization between the PFC and hippocampus was shifted based on different onsets. Theta oscillations in the hippocampus precede those in the PFC during the onset of contextual exploration, while the prefrontal cortical theta precedes that in the hippocampus during the onset of object exploration (Place et al., 2016). In addition, the PFC-hippocampus theta oscillations were enhanced after an error made in a paired associative learning task, and were primarily directed by the PFC to hippocampus information flow. By contrast, the opposite information flow was found after correct answers together and with stronger alpha/beta oscillations (Brincat and Miller, 2015). In humans, the PFC-hippocampus theta coherence was also increased in an inferential task which required participants to integrate associations that shared common features (Backus et al., 2016). Alternatively, an oscillation varying between 2–5 Hz could also be involved in the PFC-hippocampus coupling (Fujisawa and Buzsaki, 2011), which can be modulated by the NR. Microinjection of lidocaine into the NR decreased the coherence of 2–5 Hz, but not theta, between the PFC and hippocampus in rats (Roy et al., 2017). These findings suggest that the direct and indirect pathways of PFC-hippocampus could be distinctly associated with the theta and 2–5 Hz PFC-hippocampus synchronization, respectively. The hippocampus apparently generates a spatiotemporal event and contacts the PFC for information, while the role of PFC is that of an executor that guides the hippocampus in correctly retrieving that event. Such reasoning receives support from brain imaging studies indicating strong links between the PFC and medial temporal lobe during episodic encoding (Schott et al., 2011; Schott et al., 2013), and both regions are involved in the integration of temporal-context memory (Jenkins and Ranganath, 2010; Naya et al., 2017; Tubridy and Davachi, 2011).
4.2.2. Lesion and inactivation studies
The application of disconnection procedures together with behavioral tests has proven useful in exploring the causality of the mPFC-hippocampus interaction in the control of episodic memory. The basic proposition of the disconnection approach is that, if two brain areas interact for a specific function, disrupting one of the areas in one hemisphere combined with a simultaneous disruption of the other area in the opposite hemisphere (i.e. disconnecting the circuit at two different levels) should lead to a functional deficit. Disconnecting the PFC-hippocampus pathway did not influence performance in NOP and OPP tests, either by lesions (Barker and Warburton, 2011b) or infusions of NBQX or AP5 before the sample trial (Barker and Warburton, 2015). However, disconnection of the PFC and hippocampus disrupted TOM and OiP (remembrance for altering relative object-location associations) memories (Barker and Warburton, 2011b). Disconnected inactivation of the mPFC and hippocampus with pre-sample NBQX or AP5 infusions impaired OiP performance, independent of time delay. Retrieval of OiP memory was dependent on the PFC-hippocampus AMPA-R, but not NMDA-R (Barker and Warburton, 2015). Furthermore, pre-test infusions of NBQX into the unilateral PFC and hippocampus with the disconnection approach impaired the where and when components of the ELM4–2 test (Barker et al., 2017). These findings suggest that the interplay between the PFC and hippocampus is critical for “when” and “what-where-when” memories.
A meta-system of episodic-like memory which integrates the “what”, “where” and “when” component memories:
The question that follows is whether the PFC-hippocampus pathway directly contributes to episodic memory per se or merely to the individual memory systems for what, where or when memory, given that, by logic, an impairment of one of the components should cause a failure of episodic memory. To investigate this issue, we conducted an episodic-like memory test along with separate tests for the components “what”, “where” and “when” in the same animals. The mPFC and dorsal hippocampus CA1 or CA3 regions were disconnected by employing the ELM2–2 test (For details see (Chao et al., 2017; de Souza Silva et al., 2016). When the PFC-CA1 circuit was interrupted, the ability to integrate distinct components into an episodic-like memory was impaired. Furthermore, performance in the OPP test (where) was disrupted, whereas memories for NOP (what) and TOM (when), were intact. The disruption of episodic-like memory could not have resulted from the impairment of object location memory since the PFC-CA1 disconnected animals showed intact spatial recognition in the ELM2–2 test (positive where index). This argues that they were not incapable of recognizing different locations. These findings indicate that the PFC-CA1 circuit is critically involved in the processing of episodic-like memory itself (Chao et al., 2017). By contrast, disconnecting the PFC-CA3 circuit also impaired performance in the ELM2–2 test, but did not interfere with performance in the individual NOP, OPP and TOM tests. Pharmacological inactivation of the PFC in one hemisphere with CNQX, but not with AP-5, combined with lesions of CA3 in the opposite hemisphere (disconnecting the CA3 and PFC gultamatergic circuit) disrupted episodic-like memory (de Souza Silva et al., 2016).
These results lead to the conclusions regarding the neurobiological bases of episodic-like memory: (a). Since the disconnected PFC-CA3 circuit resulted in intact individual memory for what, where and when, but disrupted episodic-like memory, it follows that episodic-like memory is not simply a combination of “what”, “where” and “when” memories, but a meta-system that integrates these components into episodic-like memory. (b). The PFC AMPA/kainate-R, but not NMDA-R, are involved in the processing of episodic-like memory. These results are compatible with the finding that the AMPA-R, but not NMDA-R, in the PFC are essential for the learning and memory of object-place associations (Barker and Warburton, 2008, 2015; Tse et al., 2011), probably due to the fast synaptic transmission provided by AMPA-R. (c). Disconnecting the PFC-CA1 and PFC-CA3 pathways revealed differential effects on episodic-like memory, indicating distinct functional roles of the PFC-CA1 versus -CA3 pathways.
The indirect PFC-hippocampus pathway also plays an important role in the processing of episodic-like memory. For instance, the NR mutually connects with the PFC and hippocampus and has been proposed to have an active role in the processing of memory. The NR-lesioned rats exhibited intact NOP, OPP and short-term OiP memories, but long-term OiP memory was impaired. Furthermore, the muscarinic and nicotine ACh-R, but not NMDA-R, of the NR can modulate the encoding of OiP memory (Barker and Warburton, 2018). Alternatively, the LEC, which is essential for object-associations (Wilson et al., 2013c), projects mutually to the CA1, CA3 and PFC. In order to test whether the indirect pathway is causally involved in episodic-like memory, the PFC-LEC circuit was disconnected (Chao et al., 2016a). As expected, memory tested by the ELM2–2 test was deficient, suggesting the PFC-LEC circuit is indispensable for episodic-like memory. This pathway is also engaged in the processing of object-associated identity, location and context information, but not in NOP, OPP and TOM (Chao et al., 2016a).
4.2.3. Functional circuits
Attempts to dissect functions of differential neuronal circuits are progressing with the development of advanced biological tools, such as chemo- and opto-genetics. Barker et al. (2017) selectively inactivated the circuits from the posterior-dorsal or intermediate CA1 projecting to the mPFC in rats. In the ELM4–2 test, inactivation of the posterior-dorsal CA1 to PFC pathway impaired the when, but not where, component of episodic-like memory, while inactivation of the intermediate CA1-PFC pathway disrupted the where, but not when, component. Furthermore, the posterior-dorsal CA1 to PFC pathway was crucial for the memory for temporal-order, but not for NOP, OPP and OiP. In contrast, the intermediate CA1 to PFC pathway was important for OiP memory, but not for NOP, OPP and temporal-order. The findings pinpoint that the CA1 projection to PFC pathway is functionally divergent, with the posterior-dorsal and intermediate circuits accounting for the processing of temporal and inter-item locational information, accordingly (Barker et al., 2017).
Different extents of episodic-like memory deficits were observed, depending on the region disconnected in the studies of disconnecting the mPFC either from the CA1, CA3 or LEC (Chao et al., 2016a; Chao et al., 2017; de Souza Silva et al., 2016) (Table 5). The results indicate that there is a bias for the episodic-like information to be processed in the direct pathways than indirect PFC-hippocampal ones. Using the ELM2–2 test, it was found that the where component was still functional, although weakened after disconnecting the PFC-LEC pathway, which spared the direct CA3-CA1-PFC circuit. Disconnection of the PFC-CA1 pathway, while preserving the indirect CA3-LEC-PFC circuit, impaired the interaction index. When the PFC-CA3 pathway was disconnected, the interaction, as well as the where and when indexes were disrupted. These results reveal that, although the CA1-PFC pathway was preserved, episodic-like and associated spatial memories were still impaired, which highlights the role of the interplay between the PFC and CA3 in the processing of episodic information. In sum, these findings imply that (a). Processing of episodic information is more actively involved in the direct PFC-hippocampal pathway than in the indirect pathway. (b). Both the CA1 and CA3 regions are cardinal in the integration of “what-where-when” components into episodic-like memory. (c). The direct pathway from the CA1 to the PFC alone may not to be sufficient for coding episodic-like memory unless it receives additional inputs from the CA3 region. However, the direction of information flow in these studies is hard to ascertain, since the connections are ablated. Experiments with precise circuity control, e.g. optogenetics, are necessary to establish the distinct involvement of the links between CA1 and CA3 to the PFC and of the direct versus indirect hippocampal-PFC connections in the control of episodic-like memory. Recent human imaging studies shed light on the distinct but complementary characteristics of CA1 and CA3 in episodic memory (Copara et al., 2014; Dimsdale-Zucker et al., 2018). Nevertheless, the disconnection studies have contributed new insights into the neural networks that determine episodic-like memory and its component memory systems of object recognition in space and time, with emphasis on the cirtical involvement of the direct and indirect PFC-hippocampal pathways (Fig.5).
Table 5.
Lesion or inactivation studies performed with various episodic-like memory tests. ELM2–2 test: assesses episodic-like memory with 2 types of object, each type presented at different time points, and in the test trial one of the objects from each type is displaced to a new location (2 types of objects and 2 displaced ones). ELM2–1 test: assesses episodic-like memory with 2 types of object, each type presented at different time points, and in the test trial one of the recent familiar objects is displaced (2 types of objects and 1 displaced one). ELM4–2 test: assesses episodic-like memory with 4 distinct objects, 2 as a group presented at different time points, and in the test trial 2 out of 4 objects are displaced (4 different objects and 2 displaced ones).
| Findings in ELM 2–2 test | Additional tests: | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal | Sex | Treatment | Region | Where | When | Interaction | Delay | NOP | OPP | TOM | Ref. |
| Wistar | Male | Electrolytic lesions | CA3 | P | P | Impair | 1 h | P | P* | P | Li&Chao 2008 |
| Wistar | Male | Infusion of muscimol | CA1 | Impair | Impair | Impair | 1 h | ( | not tested | ) | Drieskens, 2017 |
| before sample | |||||||||||
| Wistar | Male | NMDA lesions | PFC+CA3 | Impair | Impair | Impair | 1 h | P | P | P | de Souza Silva |
| disconnection | et al., 2016 | ||||||||||
| Wistar | Male | NMDA lesions | PFC+CA1 | P | P | Impair | 1 h | P | Impair | P | Chao et al., 2017 |
| disconnection | |||||||||||
| Wistar | Male | NMDA lesions | PFC+LEC | P** | P | Impair | 1 h | P | P | P | Chao et al., 2016 |
| disconnection | |||||||||||
| Findings in ELM 2–1 test | Additional tests: | ||||||||||
| Animal | Sex | Treatment | Region | Where | When | What | Delay | NOP | OPP | TOM | Ref. |
| Wistar | Male | Infusion of Muscimol | DG/CA3 | Impair | P | (not tested) | 24 h | ( | not tested | ) | Barbosa 2012 |
| before sample | |||||||||||
| Wistar | Male | Infusion of Muscimol | CA1 | Impair | Impair | (not tested) | 24 h | ( | not tested | ) | Barbosa 2012 |
| before sample | |||||||||||
| C57BL | Male | NMDA lesions | PFC | Impair | P | P | 50 min | ( | not tested | ) | DeVito 2010 |
| C57BL | Male | NMDA lesions | HPC | Impair | Impair | Impair | 50 min | ( | not tested | ) | DeVito 2010 |
| Findings in ELM 4–2 test | Additional tests: | ||||||||||
| Animal | Sex | Treatment | Region | Where | When | Delay | OiP | NOP | OPP | TOM | Ref. |
| Lister | Male | Infusion of NBQX | PFC-HPC | Impair | Impair | 1 h | ( | not | testing | ) | Barker et al 2017 |
| Hooded | before test | disconnection | |||||||||
| Lister | Male | Infusion of Daun02 | pdCA1->PFC | P | Impair | 1 h | P | P | P | Impair | Barker et al 2017 |
| Hooded | inactivation | ||||||||||
| Lister | Male | Infusion of Daun02 | iCA1->PFC | Impair | P | 1 h | Impair | P | P | P | Barker et al 2017 |
| Hooded | inactivation | ||||||||||
NOP: novel object preference test; OPP: object place preference test; TOM: temporal order memory test; OiP: object-in-place test. P: presence of the test.
spatial recognition was tested by the preference of time spent on explored location versus unexplored one in a radial-arm maze.
the disconnected PFC-LEC group showed positive, but reduced, values of the where index.
Figure 5.
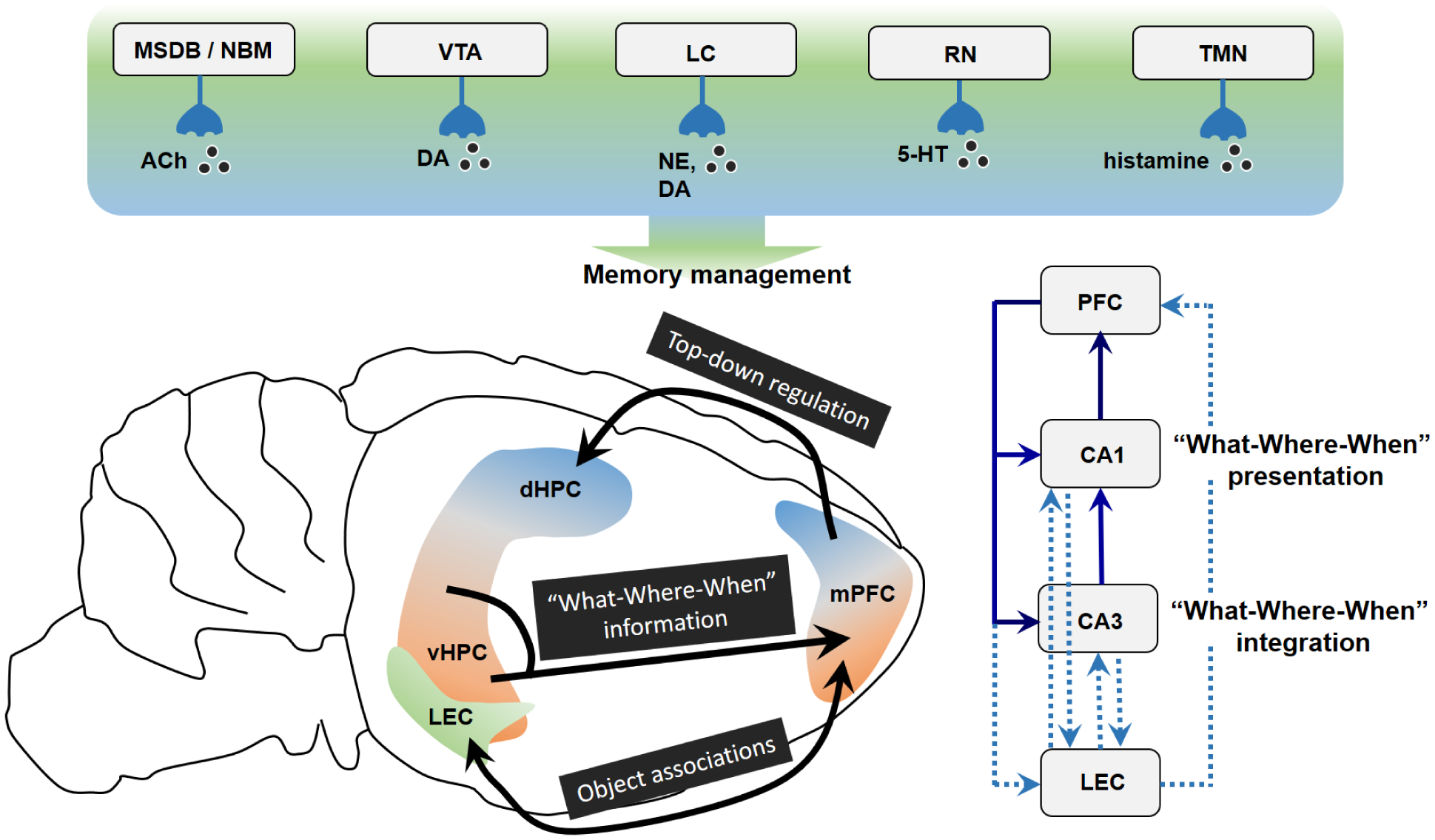
A hypothetical system of episodic-like memory. The interaction between PFC and HPC underlies episodic-like memory, whereby the HPC conveys specific “What-Where-When” information unto the PFC that constantly regulates information selection. The indirect PFC-HPC pathway via LEC provides object-related, including contextual and temporal, details. Contrasting roles of CA1 and CA3 process the presentation or integration of “What-Where-When” representation, respectively. Different neurotransmitters, including ACh, DA, NE, 5-HT and histamine, modulate memories in a distinct but complementary manner. Left panel: Locations of mPFC, HPC and LEC in the mouse brain. Right-bottom panel: Black and dashed arrows demonstrate direct and indirect PFC-HPC pathways, respectively. mPFC: medial prefrontal cortex; dHPC: dorsal hippocampus; vHPC: ventral hippocampus; LEC: lateral entorhinal cortex; MSDB: medial septum and diagonal band of Broca; NBM: nucleus basalis magnocellularis; RN: raphe nucleus; VTA: ventral tegmental area; TMN: tuberomammillary nucleus; LC: locus coeruleus; ACh: acetylcholine; 5-HT: serotonin; DA: dopamine; NE: norepinephrine.
5. Conclusion
Episodic-like memory has been investigated and characterized as a prototype of episodic memory in the fields of behavioral neuroscience, psychopharmacology and cognitive psychology. The training-free episodic-like memory tests exploit the spontaneous object exploration which is shown by many species as behavioral measures, and thus, avoid using strong positive and negative reinforcements and extensive training procedures. These paradigms combine the properties of the NOP, OPP and TOM tests in the attempt to decipher the neurobiological mechanisms that underlie episodic-like memory. The direct and indirect pathways between the mPFC and hippocampus employ distinct, but complementary, functions to structure the “what”, “where” and “when” components that subserve episodic memory. Moreover, different neurotransmitter systems within the mPFC and hippocampus behave synergistically during the learning, storage and retrieval processes inherent in the NOP, OPP and TOM tests. The anatomical and functional connections between the mPFC, LEC and hippocampus inclusively, but not exclusively, underpin the formation of episodic-like memory. A meta-system of episodic-like memory which integrates the “what”, “where” and “when” component memories likely comprises the hippocampal CA1/CA3-prefrontal cortical circuitry.
Acknowledgements
We acknowledge funding by a Heisenberg Fellowship and grant from the DFG (SO 1032/5-2 and SO 1032/2-5) to MAdSS, grant from the DFG (HU 306/27-3) to JPH, start-up funding from University of Minnesota Medical School and University of Minnesota Foundation to YMY, and the National Institute of Neurological Disorders And Stroke (NINDS) of the National Institutes of Health (NIH) grant R15NS112964 to YMY.
References
- 1.Aggleton JP, Brown MW, Albasser MM, 2012. Contrasting brain activity patterns for item recognition memory and associative recognition memory: insights from immediate-early gene functional imaging. Neuropsychologia 50, 3141–3155. [DOI] [PubMed] [Google Scholar]
- 2.Ainge JA, Heron-Maxwell C, Theofilas P, Wright P, de Hoz L, Wood ER, 2006. The role of the hippocampus in object recognition in rats: examination of the influence of task parameters and lesion size. Behav Brain Res 167, 183–195. [DOI] [PubMed] [Google Scholar]
- 3.Akirav I, Maroun M, 2006. Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cereb Cortex 16, 1759–1765. [DOI] [PubMed] [Google Scholar]
- 4.Akkerman S, Blokland A, Prickaerts J, 2014. Mind the gap: delayed manifestation of long-term object memory improvement by phosphodiesterase inhibitors. Neurobiol Learn Mem 109, 139–143. [DOI] [PubMed] [Google Scholar]
- 5.Akkerman S, Blokland A, Reneerkens O, van Goethem NP, Bollen E, Gijselaers HJ, Lieben CK, Steinbusch HW, Prickaerts J, 2012a. Object recognition testing: methodological considerations on exploration and discrimination measures. Behav Brain Res 232, 335–347. [DOI] [PubMed] [Google Scholar]
- 6.Akkerman S, Prickaerts J, Steinbusch HW, Blokland A, 2012b. Object recognition testing: statistical considerations. Behav Brain Res 232, 317–322. [DOI] [PubMed] [Google Scholar]
- 7.Alaghband Y, Kwapis JL, Lopez AJ, White AO, Aimiuwu OV, Al-Kachak A, Bodinayake KK, Oparaugo NC, Dang R, Astarabadi M, Matheos DP, Wood MA, 2017. Distinct roles for the deacetylase domain of HDAC3 in the hippocampus and medial prefrontal cortex in the formation and extinction of memory. Neurobiol Learn Mem 145, 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albasser MM, Amin E, Lin TC, Iordanova MD, Aggleton JP, 2012. Evidence that the rat hippocampus has contrasting roles in object recognition memory and object recency memory. Behav Neurosci 126, 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albasser MM, Chapman RJ, Amin E, Iordanova MD, Vann SD, Aggleton JP, 2010. New behavioral protocols to extend our knowledge of rodent object recognition memory. Learn Mem 17, 407–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albayram O, Passlick S, Bilkei-Gorzo A, Zimmer A, Steinhauser C, 2016. Physiological impact of CB1 receptor expression by hippocampal GABAergic interneurons. Pflugers Arch 468, 727–737. [DOI] [PubMed] [Google Scholar]
- 11.Alkam T, Hiramatsu M, Mamiya T, Aoyama Y, Nitta A, Yamada K, Kim HC, Nabeshima T, 2011. Evaluation of object-based attention in mice. Behav Brain Res 220, 185–193. [DOI] [PubMed] [Google Scholar]
- 12.Alkam T, Kim HC, Mamiya T, Yamada K, Hiramatsu M, Nabeshima T, 2013. Evaluation of cognitive behaviors in young offspring of C57BL/6J mice after gestational nicotine exposure during different time-windows. Psychopharmacology (Berl) 230, 451–463. [DOI] [PubMed] [Google Scholar]
- 13.Allen TA, Fortin NJ, 2013. The evolution of episodic memory. Proc Natl Acad Sci U S A 110 Suppl 2, 10379–10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson MJ, Jablonski SA, Klimas DB, 2008. Spaced initial stimulus familiarization enhances novelty preference in Long-Evans rats. Behav Processes 78, 481–486. [DOI] [PubMed] [Google Scholar]
- 15.Apergis-Schoute J, Pinto A, Pare D, 2006. Ultrastructural organization of medial prefrontal inputs to the rhinal cortices. Eur J Neurosci 24, 135–144. [DOI] [PubMed] [Google Scholar]
- 16.Apergis-Schoute J, Pinto A, Pare D, 2007. Muscarinic control of long-range GABAergic inhibition within the rhinal cortices. J Neurosci 27, 4061–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arain M, Parameswaran V, Cohen J, 2012. Changing within-trial array location and target object position enhances rats’ (Rattus norvegicus) missing object recognition accuracy. Anim Cogn 15, 771–782. [DOI] [PubMed] [Google Scholar]
- 18.Assini FL, Duzzioni M, Takahashi RN, 2009. Object location memory in mice: pharmacological validation and further evidence of hippocampal CA1 participation. Behav Brain Res 204, 206–211. [DOI] [PubMed] [Google Scholar]
- 19.Atucha E, Fuerst C, Sauvage M, 2019. Functional architecture of memory, Leibniz Inst. for Neurobio., Magdeburg, Germany. Imaging object-in-place memories over half a life time Program No. 164.21.2019 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience, 2019. Online. [Google Scholar]
- 20.Babb SJ, Crystal JD, 2006. Episodic-like memory in the rat. Curr Biol 16, 1317–1321. [DOI] [PubMed] [Google Scholar]
- 21.Backus AR, Schoffelen JM, Szebenyi S, Hanslmayr S, Doeller CF, 2016. Hippocampal-prefrontal theta oscillations support memory integration. Curr Biol 26, 450–457. [DOI] [PubMed] [Google Scholar]
- 22.Baker KB, Kim JJ, 2002. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learn Mem 9, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balderas I, Moreno-Castilla P, Bermudez-Rattoni F, 2013. Dopamine D1 receptor activity modulates object recognition memory consolidation in the perirhinal cortex but not in the hippocampus. Hippocampus 23, 873–878. [DOI] [PubMed] [Google Scholar]
- 24.Balderas I, Morin JP, Rodriguez-Ortiz CJ, Bermudez-Rattoni F, 2012. Muscarinic receptors activity in the perirhinal cortex and hippocampus has differential involvement in the formation of recognition memory. Neurobiol Learn Mem 97, 418–424. [DOI] [PubMed] [Google Scholar]
- 25.Balderas I, Rodriguez-Ortiz CJ, Salgado-Tonda P, Chavez-Hurtado J, McGaugh JL, Bermudez-Rattoni F, 2008. The consolidation of object and context recognition memory involve different regions of the temporal lobe. Learn Mem 15, 618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baran SE, Armstrong CE, Niren DC, Conrad CD, 2010. Prefrontal cortex lesions and sex differences in fear extinction and perseveration. Learn Mem 17, 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbieri M, Ossato A, Canazza I, Trapella C, Borelli AC, Beggiato S, Rimondo C, Serpelloni G, Ferraro L, Marti M, 2016. Synthetic cannabinoid JWH-018 and its halogenated derivatives JWH-018-Cl and JWH-018-Br impair novel object recognition in mice: behavioral, electrophysiological and neurochemical evidence. Neuropharmacology 109, 254–269. [DOI] [PubMed] [Google Scholar]
- 28.Barbosa FF, Pontes IM, Ribeiro AM, Silva RH, 2010. Extending possible applications of an episodic-like memory task in rats. Behav Brain Res 215, 326–331. [DOI] [PubMed] [Google Scholar]
- 29.Barbosa FF, Pontes IM, Ribeiro S, Ribeiro AM, Silva RH, 2012. Differential roles of the dorsal hippocampal regions in the acquisition of spatial and temporal aspects of episodic-like memory. Behav Brain Res 232, 269–277. [DOI] [PubMed] [Google Scholar]
- 30.Barbosa FF, Santos JR, Meurer YS, Macedo PT, Ferreira LM, Pontes IM, Ribeiro AM, Silva RH, 2013. Differential cortical c-Fos and Zif-268 expression after object and spatial memory processing in a standard or episodic-like object recognition task. Front Behav Neurosci 7, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barker GR, Banks PJ, Scott H, Ralph GS, Mitrophanous KA, Wong LF, Bashir ZI, Uney JB, Warburton EC, 2017. Separate elements of episodic memory subserved by distinct hippocampal-prefrontal connections. Nat Neurosci 20, 242–250. [DOI] [PubMed] [Google Scholar]
- 32.Barker GR, Bashir ZI, Warburton EC, 2019. Physiology, Pharmacol. & Neurosci., Univ. of Bristol, Bristol, United Kingdom. There and back again: identification of distinct neural circuits for associative recognition memory encoding and retrieval Program No. 601.05. 2019 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience, 2019. Online. [Google Scholar]
- 33.Barker GR, Bird F, Alexander V, Warburton EC, 2007. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci 27, 2948–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barker GR, Warburton EC, 2008. NMDA receptor plasticity in the perirhinal and prefrontal cortices is crucial for the acquisition of long-term object-in-place associative memory. J Neurosci 28, 2837–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barker GR, Warburton EC, 2009. Critical role of the cholinergic system for object-in-place associative recognition memory. Learn Mem 16, 8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barker GR, Warburton EC, 2011a. Evaluating the neural basis of temporal order memory for visual stimuli in the rat. Eur J Neurosci 33, 705–716. [DOI] [PubMed] [Google Scholar]
- 37.Barker GR, Warburton EC, 2011b. When is the hippocampus involved in recognition memory? J Neurosci 31, 10721–10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barker GR, Warburton EC, 2015. Object-in-place associative recognition memory depends on glutamate receptor neurotransmission within two defined hippocampal-cortical circuits: a critical role for AMPA and NMDA receptors in the hippocampus, perirhinal, and prefrontal cortices. Cereb Cortex 25, 472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barker GRI, Warburton EC, 2018. A critical role for the nucleus reuniens in long-term, but not short-term associative recognition memory formation. J Neurosci 38, 3208–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrett RM, Malvaez M, Kramar E, Matheos DP, Arrizon A, Cabrera SM, Lynch G, Greene RW, Wood MA, 2011. Hippocampal focal knockout of CBP affects specific histone modifications, long-term potentiation, and long-term memory. Neuropsychopharmacol 36, 1545–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basavarajappa BS, Subbanna S, 2014. CB1 receptor-mediated signaling underlies the hippocampal synaptic, learning, and memory deficits following treatment with JWH-081, a new component of spice/K2 preparations. Hippocampus 24, 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bass DI, Nizam ZG, Partain KN, Wang A, Manns JR, 2014. Amygdala-mediated enhancement of memory for specific events depends on the hippocampus. Neurobiol Learn Mem 107, 37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Basu J, Zaremba JD, Cheung SK, Hitti FL, Zemelman BV, Losonczy A, Siegelbaum SA, 2016. Gating of hippocampal activity, plasticity, and memory by entorhinal cortex long-range inhibition. Science 351, aaa5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bauer PJ, Stewart R, White EA, Larkina M, 2016. A place for every event and every event in its place: memory for locations and activities by 4-year-old children. J Cogn Dev 17, 244–263. [Google Scholar]
- 45.Beeler JA, Prendergast B, Zhuang X, 2006. Low amplitude entrainment of mice and the impact of circadian phase on behavior tests. Physiol Behav 87, 870–880. [DOI] [PubMed] [Google Scholar]
- 46.Beer Z, Chwiesko C, Kitsukawa T, Sauvage MM, 2013. Spatial and stimulus-type tuning in the LEC, MEC, POR, PrC, CA1, and CA3 during spontaneous item recognition memory. Hippocampus 23, 1425–1438. [DOI] [PubMed] [Google Scholar]
- 47.Beer Z, Chwiesko C, Sauvage MM, 2014. Processing of spatial and non-spatial information reveals functional homogeneity along the dorso-ventral axis of CA3, but not CA1. Neurobiol Learn Mem 111, 56–64. [DOI] [PubMed] [Google Scholar]
- 48.Beer Z, Vavra P, Atucha E, Rentzing K, Heinze HJ, Sauvage MM, 2018. The memory for time and space differentially engages the proximal and distal parts of the hippocampal subfields CA1 and CA3. PLoS Biol 16, e2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beilharz JE, Maniam J, Morris MJ, 2014. Short exposure to a diet rich in both fat and sugar or sugar alone impairs place, but not object recognition memory in rats. Brain Behav Immun 37, 134–141. [DOI] [PubMed] [Google Scholar]
- 50.Bekinschtein P, Renner MC, Gonzalez MC, Weisstaub N, 2013. Role of medial prefrontal cortex serotonin 2A receptors in the control of retrieval of recognition memory in rats. J Neurosci 33, 15716–15725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belblidia H, Abdelouadoud A, Jozet-Alves C, Dumas H, Freret T, Leger M, Schumann-Bard P, 2015. Time decay of object, place and temporal order memory in a paradigm assessing simultaneously episodic-like memory components in mice. Behav Brain Res 286, 80–84. [DOI] [PubMed] [Google Scholar]
- 52.Bello-Medina PC, Sanchez-Carrasco L, Gonzalez-Ornelas NR, Jeffery KJ, Ramirez-Amaya V, 2013. Differential effects of spaced vs. massed training in long-term object-identity and object-location recognition memory. Behav Brain Res 250, 102–113. [DOI] [PubMed] [Google Scholar]
- 53.Benarroch EE, 2009. The locus ceruleus norepinephrine system: functional organization and potential clinical significance. Neurology 73, 1699–1704. [DOI] [PubMed] [Google Scholar]
- 54.Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI, 2010. Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron 66, 921–936. [DOI] [PubMed] [Google Scholar]
- 55.Benn A, Barker GR, Stuart SA, Roloff EV, Teschemacher AG, Warburton EC, Robinson ES, 2016. Optogenetic stimulation of prefrontal glutamatergic neurons enhances recognition memory. J Neurosci 36, 4930–4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berlyne DE, 1950. Novelty and curiosity as determinants of exploratory behaviour. B J Psychol-Gen Sect 41, 68–80. [Google Scholar]
- 57.Bertossi E, Tesini C, Cappelli A, Ciaramelli E, 2016. Ventromedial prefrontal damage causes a pervasive impairment of episodic memory and future thinking. Neuropsychologia 90, 12–24. [DOI] [PubMed] [Google Scholar]
- 58.Beselia G, Maglakelidze G, Chkhikvishvili N, Burjanadze M, Dashniani M, 2010. Object exploration and reactions to spatial and nonspatial changes in dentate gyrus lesioned rats. Georgian Med News, 61–65. [PubMed] [Google Scholar]
- 59.Besheer J, Bevins RA, 2000. The role of environmental familiarization in novel-object preference. Behav Processes 50, 19–29. [DOI] [PubMed] [Google Scholar]
- 60.Bett D, Stevenson CH, Shires KL, Smith MT, Martin SJ, Dudchenko PA, Wood ER, 2013. The postsubiculum and spatial learning: the role of postsubicular synaptic activity and synaptic plasticity in hippocampal place cell, object, and object-location memory. J Neurosci 33, 6928–6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Binder S, Berg K, Gasca F, Lafon B, Parra LC, Born J, Marshall L, 2014. Transcranial slow oscillation stimulation during sleep enhances memory consolidation in rats. Brain Stimulation 7, 508–515. [DOI] [PubMed] [Google Scholar]
- 62.Binder S, Dere E, Zlomuzica A, 2015. A critical appraisal of the what-where-when episodic-like memory test in rodents: achievements, caveats and future directions. Prog Neurobiol 130, 71–85. [DOI] [PubMed] [Google Scholar]
- 63.Born J, Rasch B, Gais S, 2006. Sleep to remember. Neuroscientist 12, 410–424. [DOI] [PubMed] [Google Scholar]
- 64.Born J, Wilhelm I, 2012. System consolidation of memory during sleep. Psychol Res 76, 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bota M, Sporns O, Swanson LW, 2015. Architecture of the cerebral cortical association connectome underlying cognition. Proc Natl Acad Sci U S A 112, E2093–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Branch CL, Galizio M, Bruce K, 2014. What-Where-When memory in the rodent Odor Span Task. Learn Motiv 47, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brincat SL, Miller EK, 2015. Frequency-specific hippocampal-prefrontal interactions during associative learning. Nat Neurosci 18, 576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Broadbent NJ, Gaskin S, Squire LR, Clark RE, 2010. Object recognition memory and the rodent hippocampus. Learn Mem 17, 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Broadbent NJ, Squire LR, Clark RE, 2004. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A 101, 14515–14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buhusi CV, Meck WH, 2005. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci 6, 755–765. [DOI] [PubMed] [Google Scholar]
- 71.Burgess N, Maguire EA, O’Keefe J, 2002. The human hippocampus and spatial and episodic memory. Neuron 35, 625–641. [DOI] [PubMed] [Google Scholar]
- 72.Burns P, Russell C, Russell J, 2015. Preschool children’s proto-episodic memory assessed by deferred imitation. Memory 23, 1172–1192. [DOI] [PubMed] [Google Scholar]
- 73.Burton BG, Hok V, Save E, Poucet B, 2009. Lesion of the ventral and intermediate hippocampus abolishes anticipatory activity in the medial prefrontal cortex of the rat. Behav Brain Res 199, 222–234. [DOI] [PubMed] [Google Scholar]
- 74.Burwell RD, 2000. The parahippocampal region: corticocortical connectivity. Ann N Y Acad Sci 911, 25–42. [DOI] [PubMed] [Google Scholar]
- 75.Burwell RD, Amaral DG, 1998a. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol 398, 179–205. [DOI] [PubMed] [Google Scholar]
- 76.Burwell RD, Amaral DG, 1998b. Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. J Comp Neurol 391, 293–321. [DOI] [PubMed] [Google Scholar]
- 77.Bussey TJ, Duck J, Muir JL, Aggleton JP, 2000. Distinct patterns of behavioural impairments resulting from fornix transection or neurotoxic lesions of the perirhinal and postrhinal cortices in the rat. Behav Brain Res 111, 187–202. [DOI] [PubMed] [Google Scholar]
- 78.Buzsáki G, 2013. Cognitive neuroscience: time, space and memory. Nature 497, 568–569. [DOI] [PubMed] [Google Scholar]
- 79.Buzsáki G, Llinás R, 2017. Space and time in the brain. Science 358, 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cassini LF, Sierra RO, Haubrich J, Crestani AP, Santana F, de Oliveira Alvares L, Quillfeldt JA, 2013. Memory reconsolidation allows the consolidation of a concomitant weak learning through a synaptic tagging and capture mechanism. Hippocampus 23, 931–941. [DOI] [PubMed] [Google Scholar]
- 81.Castilla-Ortega E, Pedraza C, Chun J, de Fonseca FR, Estivill-Torrus G, Santin LJ, 2012. Hippocampal c-Fos activation in normal and LPA(1)-null mice after two object recognition tasks with different memory demands. Behav Brain Res 232, 400–405. [DOI] [PubMed] [Google Scholar]
- 82.Castilla-Ortega E, Rosell-Valle C, Pedraza C, Rodriguez de Fonseca F, Estivill-Torrus G, Santin LJ, 2014. Voluntary exercise followed by chronic stress strikingly increases mature adult-born hippocampal neurons and prevents stress-induced deficits in ‘what-when-where’ memory. Neurobiol Learn Mem 109, 62–73. [DOI] [PubMed] [Google Scholar]
- 83.Cenquizca LA, Swanson LW, 2007. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev 56, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cercato MC, Vazquez CA, Kornisiuk E, Aguirre AI, Colettis N, Snitcofsky M, Jerusalinsky DA, Baez MV, 2017. GluN1 and GluN2A NMDA receptor subunits increase in the hippocampus during memory consolidation in the Rat. Front Behav Neurosci 10, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chao OY, Huston JP, Li JS, Wang AL, de Souza Silva MA, 2016a. The medial prefrontal cortex-lateral entorhinal cortex circuit is essential for episodic-like memory and associative object-recognition. Hippocampus 26, 633–645. [DOI] [PubMed] [Google Scholar]
- 86.Chao OY, Huston JP, Nikolaus S, de Souza Silva MA, 2016b. Concurrent assessment of memory for object and place: evidence for different preferential importance of perirhinal cortex and hippocampus and for promnestic effect of a neurokinin-3 R agonist. Neurobiol Learn Mem 130, 149–158. [DOI] [PubMed] [Google Scholar]
- 87.Chao OY, Nikolaus S, Huston JP, de Souza Silva MA, 2014. The neurokinin-3 receptor agonist senktide facilitates the integration of memories for object, place and temporal order into episodic memory. Neurobiol Learn Mem 114, 178–185. [DOI] [PubMed] [Google Scholar]
- 88.Chao OY, Nikolaus S, Lira Brandao M, Huston JP, de Souza Silva MA, 2017. Interaction between the medial prefrontal cortex and hippocampal CA1 area is essential for episodic-like memory in rats. Neurobiol Learn Mem 141, 72–77. [DOI] [PubMed] [Google Scholar]
- 89.Chao OY, Pum ME, Huston JP, 2013. The interaction between the dopaminergic forebrain projections and the medial prefrontal cortex is critical for memory of objects: implications for Parkinson’s disease. Exp Neurol 247, 373–382. [DOI] [PubMed] [Google Scholar]
- 90.Chao OY, Yunger R, Yang YM, 2018. Behavioral assessments of BTBR T+Itpr3tf/J mice by tests of object attention and elevated open platform: implications for an animal model of psychiatric comorbidity in autism. Behav Brain Res 347, 140–147. [DOI] [PubMed] [Google Scholar]
- 91.Chen L, Tian S, Ke J, 2014. Rapid eye movement sleep deprivation disrupts consolidation but not reconsolidation of novel object recognition memory in rats. Neurosci Lett 563, 12–16. [DOI] [PubMed] [Google Scholar]
- 92.Christoffersen GR, Simonyi A, Schachtman TR, Clausen B, Clement D, Bjerre VK, Mark LT, Reinholdt M, Schmith-Rasmussen K, Zink LV, 2008. MGlu5 antagonism impairs exploration and memory of spatial and non-spatial stimuli in rats. Behav Brain Res 191, 235–245. [DOI] [PubMed] [Google Scholar]
- 93.Chudasama Y, 2011. Animal models of prefrontal-executive function. Behav Neurosci 125, 327–343. [DOI] [PubMed] [Google Scholar]
- 94.Clark RE, Zola SM, Squire LR, 2000. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci 20, 8853–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Clarke JR, Cammarota M, Gruart A, Izquierdo I, Delgado-Garcia JM, 2010. Plastic modifications induced by object recognition memory processing. Proc Natl Acad Sci U S A 107, 2652–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Clarke JR, Rossato JI, Monteiro S, Bevilaqua LR, Izquierdo I, Cammarota M, 2008. Posttraining activation of CB1 cannabinoid receptors in the CA1 region of the dorsal hippocampus impairs object recognition long-term memory. Neurobiol Learn Mem 90, 374–381. [DOI] [PubMed] [Google Scholar]
- 97.Clausen B, Schachtman TR, Mark LT, Reinholdt M, Christoffersen GR, 2011. Impairments of exploration and memory after systemic or prelimbic D1-receptor antagonism in rats. Behav Brain Res 223, 241–254. [DOI] [PubMed] [Google Scholar]
- 98.Clayton NS, Dickinson A, 1998. Episodic-like memory during cache recovery by scrub jays. Nature 395, 272–274. [DOI] [PubMed] [Google Scholar]
- 99.Clayton NS, Russell J, 2009. Looking for episodic memory in animals and young children: prospects for a new minimalism. Neuropsychologia 47, 2330–2340. [DOI] [PubMed] [Google Scholar]
- 100.Cohen SJ, Munchow AH, Rios LM, Zhang G, Asgeirsdottir HN, Stackman RW Jr., 2013. The rodent hippocampus is essential for nonspatial object memory. Curr Biol 23, 1685–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cohen SJ, Stackman RW Jr., 2015. Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res 285, 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cools R, D’Esposito M, 2011. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry 69, e113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Copara MS, Hassan AS, Kyle CT, Libby LA, Ranganath C, Ekstrom AD, 2014. Complementary roles of human hippocampal subregions during retrieval of spatiotemporal context. J Neurosci 34, 6834–6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cross L, Brown MW, Aggleton JP, Warburton EC, 2012. The medial dorsal thalamic nucleus and the medial prefrontal cortex of the rat function together to support associative recognition and recency but not item recognition. Learn Mem 20, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Crystal JD, 2010. Episodic-like memory in animals. Behav Brain Res 215, 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]; Crystal JD, 2015. Rats time long intervals: evidence from several cases. Int J Comp Psychol 28. [PMC free article] [PubMed] [Google Scholar]
- 106.Crystal JD, Alford WT, Zhou W, Hohmann AG, 2013. Source memory in the rat. Curr Biol 23, 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.da Silveira CK, Furini CR, Benetti F, Monteiro Sda C, Izquierdo I, 2013. The role of histamine receptors in the consolidation of object recognition memory. Neurobiol Learn Mem 103, 64–71. [DOI] [PubMed] [Google Scholar]
- 108.Dai H, Kaneko K, Kato H, Fujii S, Jing Y, Xu A, Sakurai E, Kato M, Okamura N, Kuramasu A, Yanai K, 2007. Selective cognitive dysfunction in mice lacking histamine H1 and H2 receptors. Neurosci Res 57, 306–313. [DOI] [PubMed] [Google Scholar]
- 109.Dalley JW, Cardinal RN, Robbins TW, 2004. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 28, 771–784. [DOI] [PubMed] [Google Scholar]
- 110.Davachi L, 2006. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol 16, 693–700. [DOI] [PubMed] [Google Scholar]
- 111.Davis KE, Eacott MJ, Easton A, Gigg J, 2013a. Episodic-like memory is sensitive to both Alzheimer’s-like pathological accumulation and normal ageing processes in mice. Behav Brain Res 254, 73–82. [DOI] [PubMed] [Google Scholar]
- 112.Davis KE, Easton A, Eacott MJ, Gigg J, 2013b. Episodic-like memory for what-where-which occasion is selectively impaired in the 3xTgAD mouse model of Alzheimer’s disease. J Alzheimers Dis 33, 681–698. [DOI] [PubMed] [Google Scholar]
- 113.de Bruin N, van Loevezijn A, Wicke KM, de Haan M, Venhorst J, Lange JHM, de Groote L, van der Neut MAW, Prickaerts J, Andriambeloson E, Foley AG, van Drimmelen M, van der Wetering M, Kruse CG, 2016. The selective 5-HT6 receptor antagonist SLV has putative cognitive- and social interaction enhancing properties in rodent models of cognitive impairment. Neurobiol Learn Mem 133, 100–117. [DOI] [PubMed] [Google Scholar]
- 114.De Bundel D, Femenia T, DuPont CM, Konradsson-Geuken A, Feltmann K, Schilstrom B, Lindskog M, 2013. Hippocampal and prefrontal dopamine D1/5 receptor involvement in the memory-enhancing effect of reboxetine. Int J Neuropsychopharmacol 16, 2041–2051. [DOI] [PubMed] [Google Scholar]
- 115.de Souza Silva MA, Huston JP, Wang AL, Petri D, Chao OY, 2016. Evidence for a specific integrative mechanism for episodic memory mediated by AMPA/kainate receptors in a circuit involving medial prefrontal cortex and hippocampal CA3 region. Cereb Cortex 26, 3000–3009. [DOI] [PubMed] [Google Scholar]
- 116.Dees RL, Kesner RP, 2013. The role of the dorsal dentate gyrus in object and object-context recognition. Neurobiol Learn Mem 106, 112–117. [DOI] [PubMed] [Google Scholar]
- 117.Dere E, Huston JP, De Souza Silva MA, 2005a. Episodic-like memory in mice: simultaneous assessment of object, place and temporal order memory. Brain Res Brain Res Protoc 16, 10–19. [DOI] [PubMed] [Google Scholar]
- 118.Dere E, Huston JP, De Souza Silva MA, 2005b. Integrated memory for objects, places, and temporal order: evidence for episodic-like memory in mice. Neurobiol Learn Mem 84, 214–221. [DOI] [PubMed] [Google Scholar]
- 119.Dere E, Huston JP, De Souza Silva MA, 2007. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev 31, 673–704. [DOI] [PubMed] [Google Scholar]
- 120.Dere E, Kart-Teke E, Huston JP, De Souza Silva MA, 2006. The case for episodic memory in animals. Neurosci Biobehav Rev 30, 1206–1224. [DOI] [PubMed] [Google Scholar]
- 121.Deshmukh SS, Knierim JJ, 2011. Representation of non-spatial and spatial information in the lateral entorhinal cortex. Front Behav Neurosci 5, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Deshmukh SS, Knierim JJ, 2013. Influence of local objects on hippocampal representations: landmark vectors and memory. Hippocampus 23, 253–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.DeVito LM, Eichenbaum H, 2010. Distinct contributions of the hippocampus and medial prefrontal cortex to the “what-where-when” components of episodic-like memory in mice. Behav Brain Res 215, 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.DeVito LM, Konigsberg R, Lykken C, Sauvage M, Young WS 3rd, Eichenbaum H, 2009. Vasopressin 1b receptor knock-out impairs memory for temporal order. J Neurosci 29, 2676–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Deziel RA, Ryan CL, Tasker RA, 2015. Ischemic lesions localized to the medial prefrontal cortex produce selective deficits in measures of executive function in rats. Behav Brain Res 293, 54–61. [DOI] [PubMed] [Google Scholar]
- 126.Dimsdale-Zucker HR, Ritchey M, Ekstrom AD, Yonelinas AP, Ranganath C, 2018. CA1 and CA3 differentially support spontaneous retrieval of episodic contexts within human hippocampal subfields. Nat Commun 9, 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dolorfo CL, Amaral DG, 1998. Entorhinal cortex of the rat: organization of intrinsic connections. J Comp Neurol 398, 49–82. [DOI] [PubMed] [Google Scholar]
- 128.Drieskens DC, Neves LR, Pugliane KC, de Souza I, Lima ADC, Salvadori M, Ribeiro AM, Silva RH, Barbosa FF, 2017. CA1 inactivation impairs episodic-like memory in rats. Neurobiol Learn Mem 145, 28–33. [DOI] [PubMed] [Google Scholar]
- 129.Dubovyk V, Manahan-Vaughan D, 2018. Less means more: the magnitude of synaptic plasticity along the hippocampal dorso-ventral axis is inversely related to the expression levels of plasticity-related neurotransmitter receptors. Hippocampus 28, 136–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dudchenko PA, Wood ER, Eichenbaum H, 2000. Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory but produce significant impairments on spatial span, recognition, and alternation. J Neurosci 20, 2964–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dupret D, O’Neill J, Pleydell-Bouverie B, Csicsvari J, 2010. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat Neurosci 13, 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Eacott MJ, Norman G, 2004. Integrated memory for object, place, and context in rats: a possible model of episodic-like memory? J Neurosci 24, 1948–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Eagle AL, Fitzpatrick CJ, Perrine SA, 2013. Single prolonged stress impairs social and object novelty recognition in rats. Behav Brain Res 256, 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eichenbaum H, 2013. What H.M. taught us. J Cogn Neurosci 25, 14–21. [DOI] [PubMed] [Google Scholar]
- 135.Eichenbaum H, 2014. Time cells in the hippocampus: a new dimension for mapping memories. Nat Rev Neurosci 15, 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Eichenbaum H, 2017a. On the integration of space, time, and memory. Neuron 95, 1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Eichenbaum H, 2017b. Prefrontal-hippocampal interactions in episodic memory. Nat Rev Neurosci 18, 547–558. [DOI] [PubMed] [Google Scholar]
- 138.Eichenbaum H, Yonelinas AP, Ranganath C, 2007. The medial temporal lobe and recognition memory. Annual Review of Neuroscience 30, 123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ennaceur A, 2010. One-trial object recognition in rats and mice: methodological and theoretical issues. Behav Brain Res 215, 244–254. [DOI] [PubMed] [Google Scholar]
- 140.Ennaceur A, de Souza Silva MA, 2018. Handbook of object novelty recognition. Academic Press, United States. [Google Scholar]
- 141.Ennaceur A, Delacour J, 1988. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res 31, 47–59. [DOI] [PubMed] [Google Scholar]
- 142.Ennaceur A, Michalikova S, Chazot PL, 2009. Do rats really express neophobia towards novel objects? Experimental evidence from exposure to novelty and to an object recognition task in an open space and an enclosed space. Behav Brain Res 197, 417–434. [DOI] [PubMed] [Google Scholar]
- 143.Ennaceur A, Neave N, Aggleton JP, 1997. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res 113, 509–519. [DOI] [PubMed] [Google Scholar]
- 144.Ergorul C, Eichenbaum H, 2004. The hippocampus and memory for “what,” “where,” and “when”. Learn Mem 11, 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Eriksson TM, Alvarsson A, Stan TL, Zhang X, Hascup KN, Hascup ER, Kehr J, Gerhardt GA, Warner-Schmidt J, Arango-Lievano M, Kaplitt MG, Ogren SO, Greengard P, Svenningsson P, 2013. Bidirectional regulation of emotional memory by 5-HT1B receptors involves hippocampal p11. Mol Psychiatry 18, 1096–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Escorihuela RM, Fernandez-Teruel A, Tobena A, Vivas NM, Marmol F, Badia A, Dierssen M, 1995. Early environmental stimulation produces long-lasting changes on beta-adrenoceptor transduction system. Neurobiol Learn Mem 64, 49–57. [DOI] [PubMed] [Google Scholar]
- 147.Euston DR, Gruber AJ, McNaughton BL, 2012. The role of medial prefrontal cortex in memory and decision making. Neuron 76, 1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fanselow MS, Dong HW, 2010. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65, 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Farovik A, Dupont LM, Eichenbaum H, 2010. Distinct roles for dorsal CA3 and CA1 in memory for sequential nonspatial events. Learn Mem 17, 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Federman N, de la Fuente V, Zalcman G, Corbi N, Onori A, Passananti C, Romano A, 2013. Nuclear factor kappaB-dependent histone acetylation is specifically involved in persistent forms of memory. J Neurosci 33, 7603–7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fellini L, Morellini F, 2013. Mice create what-where-when hippocampus-dependent memories of unique experiences. J Neurosci 33, 1038–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fernandez F, Garner CC, 2008. Episodic-like memory in Ts65Dn, a mouse model of Down syndrome. Behav Brain Res 188, 233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Figueiredo LS, Dornelles AS, Petry FS, Falavigna L, Dargel VA, Kobe LM, Aguzzoli C, Roesler R, Schroder N, 2015. Two waves of proteasome-dependent protein degradation in the hippocampus are required for recognition memory consolidation. Neurobiol Learn Mem 120, 1–6. [DOI] [PubMed] [Google Scholar]
- 154.Flasbeck V, Atucha E, Nakamura NH, Yoshida M, Sauvage MM, 2018. Spatial information is preferentially processed by the distal part of CA3: implication for memory retrieval. Behav Brain Res 354, 31–38. [DOI] [PubMed] [Google Scholar]
- 155.Fortin NJ, Wright SP, Eichenbaum H, 2004. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature 431, 188–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Forwood SE, Winters BD, Bussey TJ, 2005. Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus 15, 347–355. [DOI] [PubMed] [Google Scholar]
- 157.Fugazza C, Pogany A, Miklosi A, 2016. Recall of others’ actions after incidental encoding reveals episodic-like memory in dogs. Curr Biol 26, 3209–3213. [DOI] [PubMed] [Google Scholar]
- 158.Fujisawa S, Buzsaki G, 2011. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron 72, 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Furini CR, Myskiw JC, Schmidt BE, Marcondes LA, Izquierdo I, 2014. D1 and D5 dopamine receptors participate on the consolidation of two different memories. Behav Brain Res 271, 212–217. [DOI] [PubMed] [Google Scholar]
- 160.Furini CR, Myskiw Jde C, Schmidt BE, Zinn CG, Peixoto PB, Pereira LD, Izquierdo I, 2015. The relationship between protein synthesis and protein degradation in object recognition memory. Behav Brain Res 294, 17–24. [DOI] [PubMed] [Google Scholar]
- 161.Furini CR, Rossato JI, Bitencourt LL, Medina JH, Izquierdo I, Cammarota M, 2010. beta-adrenergic receptors link NO/sGC/PKG signaling to BDNF expression during the consolidation of object recognition long-term memory. Hippocampus 20, 672–683. [DOI] [PubMed] [Google Scholar]
- 162.Fyhn M, Molden S, Witter MP, Moser EI, Moser MB, 2004. Spatial representation in the entorhinal cortex. Science 305, 1258–1264. [DOI] [PubMed] [Google Scholar]
- 163.Gabbott P, Headlam A, Busby S, 2002. Morphological evidence that CA1 hippocampal afferents monosynaptically innervate PV-containing neurons and NADPH-diaphorase reactive cells in the medial prefrontal cortex (Areas 25/32) of the rat. Brain Res 946, 314–322. [DOI] [PubMed] [Google Scholar]
- 164.Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ, 2005. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 492, 145–177. [DOI] [PubMed] [Google Scholar]
- 165.Gaskin S, Gamliel A, Tardif M, Cole E, Mumby DG, 2009a. Incidental (unreinforced) and reinforced spatial learning in rats with ventral and dorsal lesions of the hippocampus. Behav Brain Res 202, 64–70. [DOI] [PubMed] [Google Scholar]
- 166.Gaskin S, Tardif M, Cole E, Piterkin P, Kayello L, Mumby DG, 2010. Object familiarization and novel-object preference in rats. Behav Processes 83, 61–71. [DOI] [PubMed] [Google Scholar]
- 167.Gaskin S, Tardif M, Mumby DG, 2009b. Patterns of retrograde amnesia for recent and remote incidental spatial learning in rats. Hippocampus 19, 1212–1221. [DOI] [PubMed] [Google Scholar]
- 168.Gaskin S, Tardif M, Mumby DG, 2011. Prolonged inactivation of the hippocampus reveals temporally graded retrograde amnesia for unreinforced spatial learning in rats. Neurobiol Learn Mem 96, 288–296. [DOI] [PubMed] [Google Scholar]
- 169.Gaskin S, Tremblay A, Mumby DG, 2003. Retrograde and anterograde object recognition in rats with hippocampal lesions. Hippocampus 13, 962–969. [DOI] [PubMed] [Google Scholar]
- 170.Gaynor LS, Johnson SA, Mizell JM, Campos KT, Maurer AP, Bauer RM, Burke SN, 2018. Impaired discrimination with intact crossmodal association in aged rats: a dissociation of perirhinal cortical-dependent behaviors. Behav Neurosci 132, 138–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Goh JJ, Manahan-Vaughan D, 2013a. Endogenous hippocampal LTD that is enabled by spatial object recognition requires activation of NMDA receptors and the metabotropic glutamate receptor, mGlu5. Hippocampus 23, 129–138. [DOI] [PubMed] [Google Scholar]
- 172.Goh JJ, Manahan-Vaughan D, 2013b. Hippocampal long-term depression in freely behaving mice requires the activation of beta-adrenergic receptors. Hippocampus 23, 1299–1308. [DOI] [PubMed] [Google Scholar]
- 173.Goh JJ, Manahan-Vaughan D, 2013c. Spatial object recognition enables endogenous LTD that curtails LTP in the mouse hippocampus. Cereb Cortex 23, 1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Good MA, Barnes P, Staal V, McGregor A, Honey RC, 2007a. Context- but not familiarity-dependent forms of object recognition are impaired following excitotoxic hippocampal lesions in rats. Behav Neurosci 121, 218–223. [DOI] [PubMed] [Google Scholar]
- 175.Good MA, Hale G, Staal V, 2007b. Impaired “episodic-like” object memory in adult APPswe transgenic mice. Behav Neurosci 121, 443–448. [DOI] [PubMed] [Google Scholar]
- 176.Haettig J, Stefanko DP, Multani ML, Figueroa DX, McQuown SC, Wood MA, 2011. HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learn Mem 18, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Haettig J, Sun Y, Wood MA, Xu X, 2013. Cell-type specific inactivation of hippocampal CA1 disrupts location-dependent object recognition in the mouse. Learn Mem 20, 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hafting T, Fyhn M, Molden S, Moser MB, Moser EI, 2005. Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806. [DOI] [PubMed] [Google Scholar]
- 179.Hagena H, Manahan-Vaughan D, 2011. Learning-facilitated synaptic plasticity at CA3 mossy fiber and commissural-associational synapses reveals different roles in information processing. Cereb Cortex 21, 2442–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Haijima A, Ichitani Y, 2012. Dissociable anterograde amnesic effects of retrosplenial cortex and hippocampal lesions on spontaneous object recognition memory in rats. Hippocampus 22, 1868–1875. [DOI] [PubMed] [Google Scholar]
- 181.Hales JB, Ocampo AC, Broadbent NJ, Clark RE, 2015. Hippocampal infusion of zeta inhibitory peptide impairs recent, but not remote, recognition memory in rats. Neural Plast 2015, 847136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hales JB, Schlesiger MI, Leutgeb JK, Squire LR, Leutgeb S, Clark RE, 2014. Medial entorhinal cortex lesions only partially disrupt hippocampal place cells and hippocampus-dependent place memory. Cell Rep 9, 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hales JB, Vincze JL, Reitz NT, Ocampo AC, Leutgeb S, Clark RE, 2018. Recent and remote retrograde memory deficit in rats with medial entorhinal cortex lesions. Neurobiol Learn Mem 155, 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hamilton TJ, Myggland A, Duperreault E, May Z, Gallup J, Powell RA, Schalomon M, Digweed SM, 2016. Episodic-like memory in zebrafish. Anim Cogn 19, 1071–1079. [DOI] [PubMed] [Google Scholar]
- 185.Hammond RS, Tull LE, Stackman RW, 2004. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem 82, 26–34. [DOI] [PubMed] [Google Scholar]
- 186.Hannesson DK, Howland JG, Phillips AG, 2004. Interaction between perirhinal and medial prefrontal cortex is required for temporal order but not recognition memory for objects in rats. J Neurosci 24, 4596–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hardt O, Migues PV, Hastings M, Wong J, Nader K, 2010. PKMzeta maintains 1-day- and 6-day-old long-term object location but not object identity memory in dorsal hippocampus. Hippocampus 20, 691–695. [DOI] [PubMed] [Google Scholar]
- 188.Hargreaves EL, Rao G, Lee I, Knierim JJ, 2005. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science 308, 1792–1794. [DOI] [PubMed] [Google Scholar]
- 189.Hasselmo ME, 2006. The role of acetylcholine in learning and memory. Curr Opin Neurobiol 16, 710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hasselmo ME, Sarter M, 2011. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacol 36, 52–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hatakeyama T, Sugita M, Yamada K, Ichitani Y, 2018. Temporal order memory of the rat in spontaneous object recognition: effects of number of items, exposure interval, and retention time. Learn Mem 25, 574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Heidbreder CA, Groenewegen HJ, 2003. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev 27, 555–579. [DOI] [PubMed] [Google Scholar]
- 193.Heldt SA, Stanek L, Chhatwal JP, Ressler KJ, 2007. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry 12, 656–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Heyser CJ, Chemero A, 2012. Novel object exploration in mice: not all objects are created equal. Behav Processes 89, 232–238. [DOI] [PubMed] [Google Scholar]
- 195.Hoang TH, Aliane V, Manahan-Vaughan D, 2018. Novel encoding and updating of positional, or directional, spatial cues are processed by distinct hippocampal subfields: evidence for parallel information processing and the “what” stream. Hippocampus 28, 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hoge J, Kesner RP, 2007. Role of CA3 and CA1 subregions of the dorsal hippocampus on temporal processing of objects. Neurobiol Learn Mem 88, 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hok V, Save E, Lenck-Santini PP, Poucet B, 2005. Coding for spatial goals in the prelimbic/infralimbic area of the rat frontal cortex. Proc Natl Acad Sci U S A 102, 4602–4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Holland SM, Smulders TV, 2011. Do humans use episodic memory to solve a What-Where-When memory task? Anim Cogn 14, 95–102. [DOI] [PubMed] [Google Scholar]
- 199.Hoover WB, Vertes RP, 2007. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 212, 149–179. [DOI] [PubMed] [Google Scholar]
- 200.Hoover WB, Vertes RP, 2012. Collateral projections from nucleus reuniens of thalamus to hippocampus and medial prefrontal cortex in the rat: a single and double retrograde fluorescent labeling study. Brain Struct Funct 217, 191–209. [DOI] [PubMed] [Google Scholar]
- 201.Hopkins SC, Campbell UC, Heffernan ML, Spear KL, Jeggo RD, Spanswick DC, Varney MA, Large TH, 2013. Effects of D-amino acid oxidase inhibition on memory performance and long-term potentiation in vivo. Pharmacol Res Perspect 1, e00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Howlett DR, Richardson JC, Austin A, Parsons AA, Bate ST, Davies DC, Gonzalez MI, 2004. Cognitive correlates of Abeta deposition in male and female mice bearing amyloid precursor protein and presenilin-1 mutant transgenes. Brain Res 1017, 130–136. [DOI] [PubMed] [Google Scholar]
- 203.Hoydal OA, Skytoen ER, Andersson SO, Moser MB, Moser EI, 2019. Object-vector coding in the medial entorhinal cortex. Nature 568, 400–404. [DOI] [PubMed] [Google Scholar]
- 204.Hryniewicz A, Bialuk I, Kaminski KA, Winnicka MM, 2007. Impairment of recognition memory in interleukin-6 knock-out mice. Eur J Pharmacol 577, 219–220. [DOI] [PubMed] [Google Scholar]
- 205.Hu X, Urhie O, Chang K, Hostetler R, Agmon A, 2018. A novel method for training mice in visuo-tactile 3-D object discrimination and recognition. Front Behav Neurosci 12, 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hunsaker MR, Chen V, Tran GT, Kesner RP, 2013. The medial and lateral entorhinal cortex both contribute to contextual and item recognition memory: a test of the binding of items and context model. Hippocampus 23, 380–391. [DOI] [PubMed] [Google Scholar]
- 207.Hunsaker MR, Kesner RP, 2008. Evaluating the differential roles of the dorsal dentate gyrus, dorsal CA3, and dorsal CA1 during a temporal ordering for spatial locations task. Hippocampus 18, 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hunsaker MR, Mooy GG, Swift JS, Kesner RP, 2007a. Dissociations of the medial and lateral perforant path projections into dorsal DG, CA3, and CA1 for spatial and nonspatial (visual object) information processing. Behav Neurosci 121, 742–750. [DOI] [PubMed] [Google Scholar]
- 209.Hunsaker MR, Rogers JL, Kesner RP, 2007b. Behavioral characterization of a transection of dorsal CA3 subcortical efferents: comparison with scopolamine and physostigmine infusions into dorsal CA3. Neurobiol Learn Mem 88, 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Inostroza M, Binder S, Born J, 2013a. Sleep-dependency of episodic-like memory consolidation in rats. Behav Brain Res 237, 15–22. [DOI] [PubMed] [Google Scholar]
- 211.Inostroza M, Brotons-Mas JR, Laurent F, Cid E, de la Prida LM, 2013b. Specific impairment of “what-where-when” episodic-like memory in experimental models of temporal lobe epilepsy. J Neurosci 33, 17749–17762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ishikawa H, Yamada K, Pavlides C, Ichitani Y, 2014. Sleep deprivation impairs spontaneous object-place but not novel-object recognition in rats. Neurosci Lett 580, 114–118. [DOI] [PubMed] [Google Scholar]
- 213.Iwamura E, Yamada K, Ichitani Y, 2016. Involvement of hippocampal NMDA receptors in retrieval of spontaneous object recognition memory in rats. Behav Brain Res 307, 92–99. [DOI] [PubMed] [Google Scholar]
- 214.Jacklin DL, Cloke JM, Potvin A, Garrett I, Winters BD, 2016. The dynamic multisensory engram: neural circuitry underlying crossmodal object recognition in rats changes with the nature of object experience. J Neurosci 36, 1273–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jacklin DL, Goel A, Clementino KJ, Hall AW, Talpos JC, Winters BD, 2012. Severe cross-modal object recognition deficits in rats treated sub-chronically with NMDA receptor antagonists are reversed by systemic nicotine: implications for abnormal multisensory integration in schizophrenia. Neuropsychopharmacol 37, 2322–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Jacklin DL, Kelly P, Bianchi C, MacDonald T, Traquair H, Winters BD, 2015. Evidence for a specific role for muscarinic receptors in crossmodal object recognition in rats. Neurobiol Learn Mem 118, 125–132. [DOI] [PubMed] [Google Scholar]
- 217.Jay TM, Thierry AM, Wiklund L, Glowinski J, 1992. Excitatory amino acid pathway from the hippocampus to the prefrontal cortex. contribution of AMPA receptors in hippocampo-prefrontal cortex transmission. Eur J Neurosci 4, 1285–1295. [DOI] [PubMed] [Google Scholar]
- 218.Jay TM, Witter MP, 1991. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol 313, 574–586. [DOI] [PubMed] [Google Scholar]
- 219.Jenkins LJ, Ranganath C, 2010. Prefrontal and medial temporal lobe activity at encoding predicts temporal context memory. J Neurosci 30, 15558–15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Jessberger S, Clark RE, Broadbent NJ, Clemenson GD Jr., Consiglio A, Lie DC, Squire LR, Gage FH, 2009. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem 16, 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Jobim PF, Pedroso TR, Werenicz A, Christoff RR, Maurmann N, Reolon GK, Schroder N, Roesler R, 2012. Impairment of object recognition memory by rapamycin inhibition of mTOR in the amygdala or hippocampus around the time of learning or reactivation. Behav Brain Res 228, 151–158. [DOI] [PubMed] [Google Scholar]
- 222.Jung HY, Kim DW, Nam SM, Kim JW, Chung JY, Won MH, Seong JK, Yoon YS, Yoo DY, Hwang IK, 2017. Pyridoxine improves hippocampal cognitive function via increases of serotonin turnover and tyrosine hydroxylase, and its association with CB1 cannabinoid receptor-interacting protein and the CB1 cannabinoid receptor pathway. Biochim Biophys Acta Gen Subj 1861, 3142–3153. [DOI] [PubMed] [Google Scholar]
- 223.Kadowaki Horita T, Kobayashi M, Mori A, Jenner P, Kanda T, 2013. Effects of the adenosine A2A antagonist istradefylline on cognitive performance in rats with a 6-OHDA lesion in prefrontal cortex. Psychopharmacology (Berl) 230, 345–352. [DOI] [PubMed] [Google Scholar]
- 224.Kart-Teke E, De Souza Silva MA, Huston JP, Dere E, 2006. Wistar rats show episodic-like memory for unique experiences. Neurobiol Learn Mem 85, 173–182. [DOI] [PubMed] [Google Scholar]
- 225.Keene CS, Bladon J, McKenzie S, Liu CD, O’Keefe J, Eichenbaum H, 2016. Complementary functional organization of neuronal activity patterns in the perirhinal, lateral entorhinal, and medial entorhinal cortices. J Neurosci 36, 3660–3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kemp A, Manahan-Vaughan D, 2008. The hippocampal CA1 region and dentate gyrus differentiate between environmental and spatial feature encoding through long-term depression. Cereb Cortex 18, 968–977. [DOI] [PubMed] [Google Scholar]
- 227.Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER, 2016. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci U S A 113, 14835–14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kesner RP, Hunsaker MR, 2010. The temporal attributes of episodic memory. Behav Brain Res 215, 299–309. [DOI] [PubMed] [Google Scholar]
- 229.Kesner RP, Kirk RA, Yu Z, Polansky C, Musso ND, 2016. Dentate gyrus supports slope recognition memory, shades of grey-context pattern separation and recognition memory, and CA3 supports pattern completion for object memory. Neurobiol Learn Mem 129, 29–37. [DOI] [PubMed] [Google Scholar]
- 230.Kesner RP, Taylor JO, Hoge J, Andy F, 2015. Role of the dentate gyrus in mediating object-spatial configuration recognition. Neurobiol Learn Mem 118, 42–48. [DOI] [PubMed] [Google Scholar]
- 231.Kim JM, Kim DH, Lee Y, Park SJ, Ryu JH, 2014. Distinct roles of the hippocampus and perirhinal cortex in GABAA receptor blockade-induced enhancement of object recognition memory. Brain Res 1552, 17–25. [DOI] [PubMed] [Google Scholar]
- 232.Kinnavane L, Amin E, Horne M, Aggleton JP, 2014. Mapping parahippocampal systems for recognition and recency memory in the absence of the rat hippocampus. Eur J Neurosci 40, 3720–3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kinugawa K, Schumm S, Pollina M, Depre M, Jungbluth C, Doulazmi M, Sebban C, Zlomuzica A, Pietrowsky R, Pause B, Mariani J, Dere E, 2013. Aging-related episodic memory decline: are emotions the key? Front Behav Neurosci 7, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kirsch I, Lynn SJ, Vigorito M, Miller RR, 2004. The role of cognition in classical and operant conditioning. J Clin Psychol 60, 369–392. [DOI] [PubMed] [Google Scholar]
- 235.Knierim JJ, Lee I, Hargreaves EL, 2006. Hippocampal place cells: parallel input streams, subregional processing, and implications for episodic memory. Hippocampus 16, 755–764. [DOI] [PubMed] [Google Scholar]
- 236.Knierim JJ, Neunuebel JP, Deshmukh SS, 2014. Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local-global reference frames. Philos Trans R Soc Lond B Biol Sci 369, 20130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Koganezawa N, Gisetstad R, Husby E, Doan TP, Witter MP, 2015. Excitatory postrhinal projections to principal cells in the medial entorhinal cortex. J Neurosci 35, 15860–15874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Krautwald K, Mahnke L, Angenstein F, 2019. Electrical stimulation of the lateral entorhinal cortex causes a frequency-specific BOLD response pattern in the rat brain. Front Neurosci 13, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ku SP, Nakamura NH, Maingret N, Mahnke L, Yoshida M, Sauvage MM, 2017. Regional specific evidence for memory-load dependent activity in the dorsal subiculum and the lateral entorhinal cortex. Front Syst Neurosci 11, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kuruvilla MV, Ainge JA, 2017. Lateral entorhinal cortex lesions impair local spatial frameworks. Front Syst Neurosci 11, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kyd RJ, Pearce JM, Haselgrove M, Amin E, Aggleton JP, 2008. The effects of hippocampal system lesions on a novel temporal discrimination task for rats. Behav Brain Res 187, 159–171. [DOI] [PubMed] [Google Scholar]
- 242.Langston RF, Wood ER, 2010. Associative recognition and the hippocampus: differential effects of hippocampal lesions on object-place, object-context and object-place-context memory. Hippocampus 20, 1139–1153. [DOI] [PubMed] [Google Scholar]
- 243.Lanté F, Chafai M, Raymond EF, Pereira AR, Mouska X, Kootar S, Barik J, Bethus I, Marie H, 2015. Subchronic glucocorticoid receptor inhibition rescues early episodic memory and synaptic plasticity deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacol 40, 1772–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Larkin MC, Lykken C, Tye LD, Wickelgren JG, Frank LM, 2014. Hippocampal output area CA1 broadcasts a generalized novelty signal during an object-place recognition task. Hippocampus 24, 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lee AT, Vogt D, Rubenstein JL, Sohal VS, 2014. A class of GABAergic neurons in the prefrontal cortex sends long-range projections to the nucleus accumbens and elicits acute avoidance behavior. J Neurosci 34, 11519–11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lee I, Hunsaker MR, Kesner RP, 2005. The role of hippocampal subregions in detecting spatial novelty. Behav Neurosci 119, 145–153. [DOI] [PubMed] [Google Scholar]
- 247.Lee I, Kesner RP, 2003. Time-dependent relationship between the dorsal hippocampus and the prefrontal cortex in spatial memory. J Neurosci 23, 1517–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lee SE, Simons SB, Heldt SA, Zhao M, Schroeder JP, Vellano CP, Cowan DP, Ramineni S, Yates CK, Feng Y, Smith Y, Sweatt JD, Weinshenker D, Ressler KJ, Dudek SM, Hepler JR, 2010. RGS14 is a natural suppressor of both synaptic plasticity in CA2 neurons and hippocampal-based learning and memory. Proc Natl Acad Sci U S A 107, 16994–16998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Leffa DT, de Souza A, Scarabelot VL, Medeiros LF, de Oliveira C, Grevet EH, Caumo W, de Souza DO, Rohde LAP, Torres ILS, 2016. Transcranial direct current stimulation improves short-term memory in an animal model of attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol 26, 368–377. [DOI] [PubMed] [Google Scholar]
- 250.Lenck-Santini PP, Rivard B, Muller RU, Poucet B, 2005. Study of CA1 place cell activity and exploratory behavior following spatial and nonspatial changes in the environment. Hippocampus 15, 356–369. [DOI] [PubMed] [Google Scholar]
- 251.Li JS, Chao YS, 2008. Electrolytic lesions of dorsal CA3 impair episodic-like memory in rats. Neurobiol Learn Mem 89, 192–198. [DOI] [PubMed] [Google Scholar]
- 252.Li JS, Hsiao KY, Chen WM, 2011. Effects of medial prefrontal cortex lesions in rats on the what-where-when memory of a fear conditioning event. Behav Brain Res 218, 94–98. [DOI] [PubMed] [Google Scholar]
- 253.Li S, Fan YX, Wang W, Tang YY, 2012. Effects of acute restraint stress on different components of memory as assessed by object-recognition and object-location tasks in mice. Behav Brain Res 227, 199–207. [DOI] [PubMed] [Google Scholar]
- 254.Li S, Nai Q, Lipina TV, Roder JC, Liu F, 2013. alpha7nAchR/NMDAR coupling affects NMDAR function and object recognition. Mol Brain 6, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Lima RH, Rossato JI, Furini CR, Bevilaqua LR, Izquierdo I, Cammarota M, 2009. Infusion of protein synthesis inhibitors in the entorhinal cortex blocks consolidation but not reconsolidation of object recognition memory. Neurobiol Learn Mem 91, 466–472. [DOI] [PubMed] [Google Scholar]
- 256.Lisman JE, Grace AA, 2005. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron 46, 703–713. [DOI] [PubMed] [Google Scholar]
- 257.Liu A, Jain N, Vyas A, Lim LW, 2015. Ventromedial prefrontal cortex stimulation enhances memory and hippocampal neurogenesis in the middle-aged rats. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Liu Y, Du S, Lv L, Lei B, Shi W, Tang Y, Wang L, Zhong Y, 2016. Hippocampal activation of Rac1 regulates the forgetting of object recognition memory. Curr Biol 26, 2351–2357. [DOI] [PubMed] [Google Scholar]
- 259.Lopez-Pigozzi D, Laurent F, Brotons-Mas JR, Valderrama M, Valero M, Fernandez-Lamo I, Cid E, Gomez-Dominguez D, Gal B, Menendez de la Prida L, 2016. Altered oscillatory dynamics of cA1 parvalbumin basket cells during theta-gamma rhythmopathies of temporal lobe epilepsy. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lopez AJ, Kramar E, Matheos DP, White AO, Kwapis J, Vogel-Ciernia A, Sakata K, Espinoza M, Wood MA, 2016. Promoter-specific effects of DREADD modulation on hippocampal synaptic plasticity and memory formation. J Neurosci 36, 3588–3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Lu HC, Mackie K, 2016. An Introduction to the Endogenous Cannabinoid System. Biol Psychiatry 79, 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Lutz B, Marsicano G, Maldonado R, Hillard CJ, 2015. The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci 16, 705–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H, 2011. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron 71, 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Maharjan DM, Dai YY, Glantz EH, Jadhav SP, 2018. Disruption of dorsal hippocampal - prefrontal interactions using chemogenetic inactivation impairs spatial learning. Neurobiology of Learning and Memory 155, 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mankin EA, Sparks FT, Slayyeh B, Sutherland RJ, Leutgeb S, Leutgeb JK, 2012. Neuronal code for extended time in the hippocampus. Proc Natl Acad Sci U S A 109, 19462–19467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Manns JR, Eichenbaum H, 2006. Evolution of declarative memory. Hippocampus 16, 795–808. [DOI] [PubMed] [Google Scholar]
- 267.Manns JR, Eichenbaum H, 2009. A cognitive map for object memory in the hippocampus. Learn Mem 16, 616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Manns JR, Howard MW, Eichenbaum H, 2007. Gradual changes in hippocampal activity support remembering the order of events. Neuron 56, 530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Maroun M, Akirav I, 2008. Arousal and stress effects on consolidation and reconsolidation of recognition memory. Neuropsychopharmacol 33, 394–405. [DOI] [PubMed] [Google Scholar]
- 270.Mazurek A, Bhoopathy RM, Read JC, Gallagher P, Smulders TV, 2015. Effects of age on a real-world What-Where-When memory task. Front Aging Neurosci 7, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.McAllister KA, Mar AC, Theobald DE, Saksida LM, Bussey TJ, 2015. Comparing the effects of subchronic phencyclidine and medial prefrontal cortex dysfunction on cognitive tests relevant to schizophrenia. Psychopharmacology (Berl) 232, 3883–3897. [DOI] [PubMed] [Google Scholar]
- 272.McLean S, Grayson B, Harris M, Protheroe C, Woolley M, Neill J, 2010. Isolation rearing impairs novel object recognition and attentional set shifting performance in female rats. J Psychopharmacol 24, 57–63. [DOI] [PubMed] [Google Scholar]
- 273.McLean SL, Harte MK, Neill JC, Young AM, 2017. Dopamine dysregulation in the prefrontal cortex relates to cognitive deficits in the sub-chronic PCP-model for schizophrenia: a preliminary investigation. J Psychopharmacol 31, 660–666. [DOI] [PubMed] [Google Scholar]
- 274.McNamara CG, Tejero-Cantero A, Trouche S, Campo-Urriza N, Dupret D, 2014. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat Neurosci 17, 1658–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.McNulty SE, Barrett RM, Vogel-Ciernia A, Malvaez M, Hernandez N, Davatolhagh MF, Matheos DP, Schiffman A, Wood MA, 2012. Differential roles for Nr4a1 and Nr4a2 in object location vs. object recognition long-term memory. Learn Mem 19, 588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, Mullican SE, Jones S, Rusche JR, Lazar MA, Wood MA, 2011. HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci 31, 764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Melichercik AM, Elliott KS, Bianchi C, Ernst SM, Winters BD, 2012. Nicotinic receptor activation in perirhinal cortex and hippocampus enhances object memory in rats. Neuropharmacology 62, 2096–2105. [DOI] [PubMed] [Google Scholar]
- 278.Mello-Carpes PB, da Silva de Vargas L, Gayer MC, Roehrs R, Izquierdo I, 2016. Hippocampal noradrenergic activation is necessary for object recognition memory consolidation and can promote BDNF increase and memory persistence. Neurobiol Learn Mem 127, 84–92. [DOI] [PubMed] [Google Scholar]
- 279.Mello-Carpes PB, Izquierdo I, 2013. The Nucleus of the Solitary Tract --> Nucleus Paragigantocellularis --> Locus Coeruleus --> CA1 region of dorsal hippocampus pathway is important for consolidation of object recognition memory. Neurobiol Learn Mem 100, 56–63. [DOI] [PubMed] [Google Scholar]
- 280.Melzer S, Michael M, Caputi A, Eliava M, Fuchs EC, Whittington MA, Monyer H, 2012. Long-range-projecting GABAergic neurons modulate inhibition in hippocampus and entorhinal cortex. Science 335, 1506–1510. [DOI] [PubMed] [Google Scholar]
- 281.Mendez M, Arias N, Uceda S, Arias JL, 2015. c-Fos expression correlates with performance on novel object and novel place recognition tests. Brain Res Bull 117, 16–23. [DOI] [PubMed] [Google Scholar]
- 282.Meneses A, 2015. Serotonin, neural markers, and memory. Front Pharmacol 6, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mesulam MM, Mufson EJ, Wainer BH, Levey AI, 1983. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience 10, 1185–1201. [DOI] [PubMed] [Google Scholar]
- 283.Migues PV, Hardt O, Finnie P, Wang YW, Nader K, 2014. The maintenance of long-term memory in the hippocampus depends on the interaction between N-ethylmaleimide-sensitive factor and GluA2. Hippocampus 24, 1112–1119. [DOI] [PubMed] [Google Scholar]
- 284.Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K, 2010. PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci 13, 630–634. [DOI] [PubMed] [Google Scholar]
- 285.Migues PV, Liu L, Archbold GE, Einarsson EO, Wong J, Bonasia K, Ko SH, Wang YT, Hardt O, 2016. Blocking synaptic removal of GluA2-containing AMPA receptors prevents the natural forgetting of long-term memories. J Neurosci 36, 3481–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Mishkin M, Ungerleider LG, Macko KA, 1983. Object vision and spatial vision: two cortical pathways. Trends Neurosci 6, 414–417. [Google Scholar]
- 287.Mitchell JB, Laiacona J, 1998. The medial frontal cortex and temporal memory: tests using spontaneous exploratory behaviour in the rat. Behav Brain Res 97, 107–113. [DOI] [PubMed] [Google Scholar]
- 288.Moncada D, 2017. Evidence of VTA and LC control of protein synthesis required for the behavioral tagging process. Neurobiol Learn Mem 138, 226–237. [DOI] [PubMed] [Google Scholar]
- 289.Moreno-Castilla P, Perez-Ortega R, Violante-Soria V, Balderas I, Bermudez-Rattoni F, 2017. Hippocampal release of dopamine and norepinephrine encodes novel contextual information. Hippocampus 27, 547–557. [DOI] [PubMed] [Google Scholar]
- 290.Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H, 2002. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem 9, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Mumby DG, Tremblay A, Lecluse V, Lehmann H, 2005. Hippocampal damage and anterograde object-recognition in rats after long retention intervals. Hippocampus 15, 1050–1056. [DOI] [PubMed] [Google Scholar]
- 292.Myskiw JC, Rossato JI, Bevilaqua LR, Medina JH, Izquierdo I, Cammarota M, 2008. On the participation of mTOR in recognition memory. Neurobiol Learn Mem 89, 338–351. [DOI] [PubMed] [Google Scholar]
- 293.Nagai T, Takuma K, Kamei H, Ito Y, Nakamichi N, Ibi D, Nakanishi Y, Murai M, Mizoguchi H, Nabeshima T, Yamada K, 2007. Dopamine D1 receptors regulate protein synthesis-dependent long-term recognition memory via extracellular signal-regulated kinase 1/2 in the prefrontal cortex. Learn Mem 14, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Nakamura NH, Sauvage MM, 2016. Encoding and reactivation patterns predictive of successful memory performance are topographically organized along the longitudinal axis of the hippocampus. Hippocampus 26, 67–75. [DOI] [PubMed] [Google Scholar]
- 295.Nasehi M, Rostam-Nezhad E, Ebrahimi-Ghiri M, Zarrindast MR, 2017. Interaction between hippocampal serotonin and cannabinoid systems in reactivity to spatial and object novelty detection. Behav Brain Res 317, 272–278. [DOI] [PubMed] [Google Scholar]
- 296.Nava-Mesa MO, Lamprea MR, Munera A, 2013. Divergent short- and long-term effects of acute stress in object recognition memory are mediated by endogenous opioid system activation. Neurobiol Learn Mem 106, 185–192. [DOI] [PubMed] [Google Scholar]
- 297.Naya Y, Chen H, Yang C, Suzuki WA, 2017. Contributions of primate prefrontal cortex and medial temporal lobe to temporal-order memory. Proc Natl Acad Sci U S A 114, 13555–13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Naya Y, Suzuki WA, 2011. Integrating what and when across the primate medial temporal lobe. Science 333, 773–776. [DOI] [PubMed] [Google Scholar]
- 299.Nelson AJ, Cooper MT, Thur KE, Marsden CA, Cassaday HJ, 2011. The effect of catecholaminergic depletion within the prelimbic and infralimbic medial prefrontal cortex on recognition memory for recency, location, and objects. Behav Neurosci 125, 396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Neugebauer NM, Miyauchi M, Sato T, Tadano J, Akal H, Ardehali H, Meltzer HY, 2018. Hippocampal GABAA antagonism reverses the novel object recognition deficit in sub-chronic phencyclidine-treated rats. Behav Brain Res 342, 11–18. [DOI] [PubMed] [Google Scholar]
- 301.Neunuebel JP, Yoganarasimha D, Rao G, Knierim JJ, 2013. Conflicts between local and global spatial frameworks dissociate neural representations of the lateral and medial entorhinal cortex. J Neurosci 33, 9246–9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Newcombe NS, Balcomb F, Ferrara K, Hansen M, Koski J, 2014. Two rooms, two representations? Episodic-like memory in toddlers and preschoolers. Dev Sci 17, 743–756. [DOI] [PubMed] [Google Scholar]
- 303.Nyakas C, Felszeghy K, Szabo R, Keijser JN, Luiten PG, Szombathelyi Z, Tihanyi K, 2009. Neuroprotective effects of vinpocetine and its major metabolite cis-apovincaminic acid on NMDA-induced neurotoxicity in a rat entorhinal cortex lesion model. CNS Neurosci Ther 15, 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.O’Brien J, Sutherland RJ, 2007. Evidence for episodic memory in a pavlovian conditioning procedure in rats. Hippocampus 17, 1149–1152. [DOI] [PubMed] [Google Scholar]
- 305.Okuda S, Roozendaal B, McGaugh JL, 2004. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci U S A 101, 853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Olarte-Sanchez CM, Kinnavane L, Amin E, Aggleton JP, 2014. Contrasting networks for recognition memory and recency memory revealed by immediate-early gene imaging in the rat. Behav Neurosci 128, 504–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Oliveira AM, Hawk JD, Abel T, Havekes R, 2010. Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn Mem 17, 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Orzel-Gryglewska J, Matulewicz P, Jurkowlaniec E, 2015. Brainstem system of hippocampal theta induction: the role of the ventral tegmental area. Synapse 69, 553–575. [DOI] [PubMed] [Google Scholar]
- 309.Ozawa T, Yamada K, Ichitani Y, 2011. Long-term object location memory in rats: effects of sample phase and delay length in spontaneous place recognition test. Neurosci Lett 497, 37–41. [DOI] [PubMed] [Google Scholar]
- 310.Ozawa T, Yamada K, Ichitani Y, 2014. Hippocampal BDNF treatment facilitates consolidation of spatial memory in spontaneous place recognition in rats. Behav Brain Res 263, 210–216. [DOI] [PubMed] [Google Scholar]
- 311.Ozawa T, Yamada K, Ichitani Y, 2017. Differential requirements of hippocampal de novo protein and mRNA synthesis in two long-term spatial memory tests: spontaneous place recognition and delay-interposed radial maze performance in rats. PLoS One 12, e0171629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Panoz-Brown D, Corbin HE, Dalecki SJ, Gentry M, Brotheridge S, Sluka CM, Wu JE, Crystal JD, 2016. Rats remember items in context using episodic memory. Curr Biol 26, 2821–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Panoz-Brown D, Iyer V, Carey LM, Sluka CM, Rajic G, Kestenman J, Gentry M, Brotheridge S, Somekh I, Corbin HE, Tucker KG, Almeida B, Hex SB, Garcia KD, Hohmann AG, Crystal JD, 2018. Replay of episodic memories in the rat. Curr Biol 28, 1628–1634 e1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Papp M, Gruca P, Lason-Tyburkiewicz M, Litwa E, Niemczyk M, Tota-Glowczyk K, Willner P, 2017. Dopaminergic mechanisms in memory consolidation and antidepressant reversal of a chronic mild stress-induced cognitive impairment`. Psychopharmacology (Berl) 234, 2571–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Parron C, Poucet B, Save E, 2004. Entorhinal cortex lesions impair the use of distal but not proximal landmarks during place navigation in the rat. Behav Brain Res 154, 345–352. [DOI] [PubMed] [Google Scholar]
- 316.Parron C, Save E, 2004. Comparison of the effects of entorhinal and retrosplenial cortical lesions on habituation, reaction to spatial and non-spatial changes during object exploration in the rat. Neurobiol Learn Mem 82, 1–11. [DOI] [PubMed] [Google Scholar]
- 317.Pastalkova E, Itskov V, Amarasingham A, Buzsaki G, 2008. Internally generated cell assembly sequences in the rat hippocampus. Science 321, 1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Pause BM, Jungbluth C, Adolph D, Pietrowsky R, Dere E, 2010. Induction and measurement of episodic memories in healthy adults. J Neurosci Methods 189, 88–96. [DOI] [PubMed] [Google Scholar]
- 319.Paylor JW, Wendlandt E, Freeman TS, Greba Q, Marks WN, Howland JG, Winship IR, 2018. Impaired cognitive function after perineuronal net degradation in the medial prefrontal cortex. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Petersen CC, Patton DF, Parfyonov M, Mistlberger RE, 2014. Circadian food anticipatory activity: entrainment limits and scalar properties re-examined. Behav Neurosci 128, 689–702. [DOI] [PubMed] [Google Scholar]
- 321.Pezze MA, Marshall HJ, Fone KC, Cassaday HJ, 2015. Dopamine D1 receptor stimulation modulates the formation and retrieval of novel object recognition memory: role of the prelimbic cortex. Eur Neuropsychopharmacol 25, 2145–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Pezze MA, Marshall HJ, Fone KC, Cassaday HJ, 2017. Role of the anterior cingulate cortex in the retrieval of novel object recognition memory after a long delay. Learn Mem 24, 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Pinto A, Fuentes C, Pare D, 2006. Feedforward inhibition regulates perirhinal transmission of neocortical inputs to the entorhinal cortex: ultrastructural study in guinea pigs. J Comp Neurol 495, 722–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Piterkin P, Cole E, Cossette MP, Gaskin S, Mumby DG, 2008. A limited role for the hippocampus in the modulation of novel-object preference by contextual cues. Learn Mem 15, 785–791. [DOI] [PubMed] [Google Scholar]
- 325.Place R, Farovik A, Brockmann M, Eichenbaum H, 2016. Bidirectional prefrontal-hippocampal interactions support context-guided memory. Nat Neurosci 19, 992–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Place R, Lykken C, Beer Z, Suh J, McHugh TJ, Tonegawa S, Eichenbaum H, Sauvage MM, 2012. NMDA signaling in CA1 mediates selectively the spatial component of episodic memory. Learn Mem 19, 164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Plescia F, Marino RA, Navarra M, Gambino G, Brancato A, Sardo P, Cannizzaro C, 2014. Early handling effect on female rat spatial and non-spatial learning and memory. Behav Processes 103, 9–16. [DOI] [PubMed] [Google Scholar]
- 328.Potier M, Georges F, Brayda-Bruno L, Ladepeche L, Lamothe V, Al Abed AS, Groc L, Marighetto A, 2016. Temporal memory and its enhancement by estradiol requires surface dynamics of hippocampal CA1 N-Methyl-D-Aspartate receptors. Biol Psychiatry 79, 735–745. [DOI] [PubMed] [Google Scholar]
- 329.Poulter SL, Kosaki Y, Easton A, McGregor A, 2013. Spontaneous object recognition memory is maintained following transformation of global geometric properties. J Exp Psychol Anim Behav Process 39, 93–98. [DOI] [PubMed] [Google Scholar]
- 330.Preston AR, Eichenbaum H, 2013. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol 23, R764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Prince TM, Wimmer M, Choi J, Havekes R, Aton S, Abel T, 2014. Sleep deprivation during a specific 3-hour time window post-training impairs hippocampal synaptic plasticity and memory. Neurobiol Learn Mem 109, 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Rajasethupathy P, Sankaran S, Marshel JH, Kim CK, Ferenczi E, Lee SY, Berndt A, Ramakrishnan C, Jaffe A, Lo M, Liston C, Deisseroth K, 2015. Projections from neocortex mediate top-down control of memory retrieval. Nature 526, 653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ, 2000. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci 3, 238–244. [DOI] [PubMed] [Google Scholar]
- 334.Rangel LM, Alexander AS, Aimone JB, Wiles J, Gage FH, Chiba AA, Quinn LK, 2014. Temporally selective contextual encoding in the dentate gyrus of the hippocampus. Nat Commun 5, 3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Rashid H, Ahmed T, 2019. Muscarinic activity in hippocampus and entorhinal cortex is crucial for spatial and fear memory retrieval. Pharmacol Rep 71, 449–456. [DOI] [PubMed] [Google Scholar]
- 336.Reid JM, Jacklin DL, Winters BD, 2012. Crossmodal object recognition in rats with and without multimodal object pre-exposure: no effect of hippocampal lesions. Neurobiol Learn Mem 98, 311–319. [DOI] [PubMed] [Google Scholar]
- 337.Reid JM, Jacklin DL, Winters BD, 2014. Delineating prefrontal cortex region contributions to crossmodal object recognition in rats. Cereb Cortex 24, 2108–2119. [DOI] [PubMed] [Google Scholar]
- 338.Riga D, Matos MR, Glas A, Smit AB, Spijker S, Van den Oever MC, 2014. Optogenetic dissection of medial prefrontal cortex circuitry. Front Syst Neurosci 8, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Roberts WA, Feeney MC, MacPherson K, Petter M, McMillan N, Musolino E, 2008. Episodic-like memory in rats: is it based on when or how long ago? Science 320, 113–115. [DOI] [PubMed] [Google Scholar]
- 340.Rodo C, Sargolini F, Save E, 2017. Processing of spatial and non-spatial information in rats with lesions of the medial and lateral entorhinal cortex: environmental complexity matters. Behav Brain Res 320, 200–209. [DOI] [PubMed] [Google Scholar]
- 341.Roozendaal B, Hernandez A, Cabrera SM, Hagewoud R, Malvaez M, Stefanko DP, Haettig J, Wood MA, 2010. Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. J Neurosci 30, 5037–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL, 2006. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci U S A 103, 6741–6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Rossato JI, Bevilaqua LR, Myskiw JC, Medina JH, Izquierdo I, Cammarota M, 2007. On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learn Mem 14, 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Rossato JI, Kohler CA, Radiske A, Lima RH, Bevilaqua LR, Cammarota M, 2015. State-dependent effect of dopamine D(1)/D(5) receptors inactivation on memory destabilization and reconsolidation. Behav Brain Res 285, 194–199. [DOI] [PubMed] [Google Scholar]
- 345.Rossato JI, Radiske A, Kohler CA, Gonzalez C, Bevilaqua LR, Medina JH, Cammarota M, 2013. Consolidation of object recognition memory requires simultaneous activation of dopamine D1/D5 receptors in the amygdala and medial prefrontal cortex but not in the hippocampus. Neurobiol Learn Mem 106, 66–70. [DOI] [PubMed] [Google Scholar]
- 346.Roy A, Svensson FP, Mazeh A, Kocsis B, 2017. Prefrontal-hippocampal coupling by theta rhythm and by 2–5 Hz oscillation in the delta band: the role of the nucleus reuniens of the thalamus. Brain Struct Funct 222, 2819–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Russell J, Cheke LG, Clayton NS, Meltzoff AN, 2011. What can What-When-Where (WWW) binding tasks tell us about young children’s episodic foresight? Theory and two experiments. Cognitive Dev 26, 356–370. [Google Scholar]
- 348.Sabec MH, Wonnacott S, Warburton EC, Bashir ZI, 2018. Nicotinic acetylcholine receptors control encoding and retrieval of associative recognition memory through plasticity in the medial prefrontal cortex. Cell Rep 22, 3409–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Salz DM, Tiganj Z, Khasnabish S, Kohley A, Sheehan D, Howard MW, Eichenbaum H, 2016. Time cells in hippocampal area CA3. J Neurosci 36, 7476–7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Sannino S, Russo F, Torromino G, Pendolino V, Calabresi P, De Leonibus E, 2012. Role of the dorsal hippocampus in object memory load. Learn Mem 19, 211–218. [DOI] [PubMed] [Google Scholar]
- 351.Sarfert KS, Knabe ML, Gunawansa NS, Blythe SN, 2017. Western-style diet induces object recognition deficits and alters complexity of dendritic arborization in the hippocampus and entorhinal cortex of male rats. Nutr Neurosci, 1–10. [DOI] [PubMed] [Google Scholar]
- 352.Sauvage MM, Beer Z, Ekovich M, Ho L, Eichenbaum H, 2010. The caudal medial entorhinal cortex: a selective role in recollection-based recognition memory. J Neurosci 30, 15695–15699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Savalli G, Bashir ZI, Warburton EC, 2015. Regionally selective requirement for D1/D5 dopaminergic neurotransmission in the medial prefrontal cortex in object-in-place associative recognition memory. Learn Mem 22, 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Save E, Poucet B, Foreman N, Buhot MC, 1992. Object exploration and reactions to spatial and nonspatial changes in hooded rats following damage to parietal cortex or hippocampal formation. Behav Neurosci 106, 447–456. [PubMed] [Google Scholar]
- 355.Save E, Sargolini F, 2017. Disentangling the role of the MEC and LEC in the processing of spatial and non-spatial information: contribution of lesion studies. Front Syst Neurosci 11, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Sawangjit A, Oyanedel CN, Niethard N, Salazar C, Born J, Inostroza M, 2018. The hippocampus is crucial for forming non-hippocampal long-term memory during sleep. Nature. [DOI] [PubMed] [Google Scholar]
- 357.Schildein S, Huston JP, Schwarting RK, 2002. Open field habituation learning is improved by nicotine and attenuated by mecamylamine administered posttrial into the nucleus accumbens. Neurobiol Learn Mem 77, 277–290. [DOI] [PubMed] [Google Scholar]
- 358.Schilstrom B, Konradsson-Geuken A, Ivanov V, Gertow J, Feltmann K, Marcus MM, Jardemark K, Svensson TH, 2011. Effects of S-citalopram, citalopram, and R-citalopram on the firing patterns of dopamine neurons in the ventral tegmental area, N-methyl-D-aspartate receptor-mediated transmission in the medial prefrontal cortex and cognitive function in the rat. Synapse 65, 357–367. [DOI] [PubMed] [Google Scholar]
- 359.Schott BH, Niklas C, Kaufmann J, Bodammer NC, Machts J, Schutze H, Duzel E, 2011. Fiber density between rhinal cortex and activated ventrolateral prefrontal regions predicts episodic memory performance in humans. Proc Natl Acad Sci U S A 108, 5408–5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Schott BH, Wustenberg T, Wimber M, Fenker DB, Zierhut KC, Seidenbecher CI, Heinze HJ, Walter H, Duzel E, Richardson-Klavehn A, 2013. The relationship between level of processing and hippocampal-cortical functional connectivity during episodic memory formation in humans. Hum Brain Mapp 34, 407–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Schultz H, Sommer T, Peters J, 2012. Direct evidence for domain-sensitive functional subregions in human entorhinal cortex. J Neurosci 32, 4716–4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Schwarz LA, Luo L, 2015. Organization of the locus coeruleus-norepinephrine system. Curr Biol 25, R1051–R1056. [DOI] [PubMed] [Google Scholar]
- 363.Scott H, Smith AE, Barker GR, Uney JB, Warburton EC, 2017. Contrasting roles for DNA methyltransferases and histone deacetylases in single-item and associative recognition memory. Neuroepigenetics 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Siapas AG, Lubenov EV, Wilson MA, 2005. Prefrontal phase locking to hippocampal theta oscillations. Neuron 46, 141–151. [DOI] [PubMed] [Google Scholar]
- 365.Smith EE, Jonides J, 1999. Storage and executive processes in the frontal lobes. Science 283, 1657–1661. [DOI] [PubMed] [Google Scholar]
- 366.Sofroniew NJ, Svoboda K, 2015. Whisking. Curr Biol 25, R137–140. [DOI] [PubMed] [Google Scholar]
- 367.Soule J, Penke Z, Kanhema T, Alme MN, Laroche S, Bramham CR, 2008. Object-place recognition learning triggers rapid induction of plasticity-related immediate early genes and synaptic proteins in the rat dentate gyrus. Neural Plast 2008, 269097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Spanswick SC, Dyck RH, 2012. Object/context specific memory deficits following medial frontal cortex damage in mice. PLoS One 7, e43698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Squire LR, Zola-Morgan S, 1991. The medial temporal lobe memory system. Science 253, 1380–1386. [DOI] [PubMed] [Google Scholar]
- 370.Stackman RW Jr., Cohen SJ, Lora JC, Rios LM, 2016. Temporary inactivation reveals that the CA1 region of the mouse dorsal hippocampus plays an equivalent role in the retrieval of long-term object memory and spatial memory. Neurobiol Learn Mem 133, 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Stanley EM, Wilson MA, Fadel JR, 2012. Hippocampal neurotransmitter efflux during one-trial novel object recognition in rats. Neurosci Lett 511, 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Stoiljkovic M, Leventhal L, Chen A, Chen T, Driscoll R, Flood D, Hodgdon H, Hurst R, Nagy D, Piser T, Tang C, Townsend M, Tu Z, Bertrand D, Koenig G, Hajos M, 2015. Concentration-response relationship of the alpha7 nicotinic acetylcholine receptor agonist FRM-17874 across multiple in vitro and in vivo assays. Biochem Pharmacol 97, 576–589. [DOI] [PubMed] [Google Scholar]
- 373.Stone SS, Teixeira CM, Devito LM, Zaslavsky K, Josselyn SA, Lozano AM, Frankland PW, 2011. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci 31, 13469–13484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Stranahan AM, 2011. Similarities and differences in spatial learning and object recognition between young male C57Bl/6J mice and Sprague-Dawley rats. Behav Neurosci 125, 791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Suarez-Pereira I, Carrion AM, 2015. Updating stored memory requires adult hippocampal neurogenesis. Sci Rep 5, 13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Suenaga T, Ichitani Y, 2008. Effects of hippocampal administration of a cannabinoid receptor agonist WIN 55,212–2 on spontaneous object and place recognition in rats. Behav Brain Res 190, 248–252. [DOI] [PubMed] [Google Scholar]
- 377.Sugita M, Yamada K, Iguchi N, Ichitani Y, 2015. Hippocampal NMDA receptors are involved in rats’ spontaneous object recognition only under high memory load condition. Brain Res 1624, 370–379. [DOI] [PubMed] [Google Scholar]
- 378.Suzuki M, Fujise Y, Tsuchiya Y, Tamano H, Takeda A, 2015. Excess influx of Zn(2+) into dentate granule cells affects object recognition memory via attenuated LTP. Neurochem Int 87, 60–65. [DOI] [PubMed] [Google Scholar]
- 379.Takahashi Y, Sawa K, Okada T, 2013. The diurnal variation of performance of the novel location recognition task in male rats. Behav Brain Res 256, 488–493. [DOI] [PubMed] [Google Scholar]
- 380.Takeuchi T, Duszkiewicz AJ, Sonneborn A, Spooner PA, Yamasaki M, Watanabe M, Smith CC, Fernandez G, Deisseroth K, Greene RW, Morris RG, 2016. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537, 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Talhati F, Patti CL, Zanin KA, Lopes-Silva LB, Ceccon LM, Hollais AW, Bizerra CS, Santos R, Tufik S, Frussa-Filho R, 2014. Food restriction increases long-term memory persistence in adult or aged mice. Prog Neuropsychopharmacol Biol Psychiatry 50, 125–136. [DOI] [PubMed] [Google Scholar]
- 382.Tam SK, Hasan S, Choi HM, Brown LA, Jagannath A, Hughes S, Hankins MW, Foster RG, Vyazovskiy VV, Bannerman DM, Peirson SN, 2017. Constant light desynchronizes olfactory versus object and visuospatial recognition memory performance. J Neurosci 37, 3555–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Tam SK, Hasan S, Hughes S, Hankins MW, Foster RG, Bannerman DM, Peirson SN, 2016. Modulation of recognition memory performance by light requires both melanopsin and classical photoreceptors. Proc Biol Sci 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Tam SK, Robinson J, Jennings DJ, Bonardi C, 2014. Dissociations in the effect of delay on object recognition: evidence for an associative model of recognition memory. J Exp Psychol Anim Learn Cogn 40, 106–115. [DOI] [PubMed] [Google Scholar]
- 385.Tanimizu T, Kono K, Kida S, 2018. Brain networks activated to form object recognition memory. Brain Res Bull 141, 27–34. [DOI] [PubMed] [Google Scholar]
- 386.Templer VL, Hampton RR, 2013. Episodic memory in nonhuman animals. Curr Biol 23, R801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Tennant SA, Fischer L, Garden DLF, Gerlei KZ, Martinez-Gonzalez C, McClure C, Wood ER, Nolan MF, 2018. Stellate cells in the medial entorhinal cortex are required for spatial learning. Cell Rep 22, 1313–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Thiel CM, Huston JP, Schwarting RK, 1998. Hippocampal acetylcholine and habituation learning. Neuroscience 85, 1253–1262. [DOI] [PubMed] [Google Scholar]
- 389.Tierney PL, Degenetais E, Thierry AM, Glowinski J, Gioanni Y, 2004. Influence of the hippocampus on interneurons of the rat prefrontal cortex. Eur J Neurosci 20, 514–524. [DOI] [PubMed] [Google Scholar]
- 390.Tsao A, Moser MB, Moser EI, 2013. Traces of experience in the lateral entorhinal cortex. Curr Biol 23, 399–405. [DOI] [PubMed] [Google Scholar]
- 391.Tsao A, Sugar J, Lu L, Wang C, Knierim JJ, Moser MB, Moser EI, 2018. Integrating time from experience in the lateral entorhinal cortex. Nature 561, 57–62. [DOI] [PubMed] [Google Scholar]
- 392.Tse D, Takeuchi T, Kakeyama M, Kajii Y, Okuno H, Tohyama C, Bito H, Morris RG, 2011. Schema-dependent gene activation and memory encoding in neocortex. Science 333, 891–895. [DOI] [PubMed] [Google Scholar]
- 393.Tubridy S, Davachi L, 2011. Medial temporal lobe contributions to episodic sequence encoding. Cereb Cortex 21, 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Tulving E, 1983. Elements of episodic memory. Clarendon Press; Oxford University Press, Oxford Oxfordshire, New York. [Google Scholar]
- 395.Tulving E, 2002. Episodic memory: from mind to brain. Annu Rev Psychol 53, 1–25. [DOI] [PubMed] [Google Scholar]
- 396.Tulving E, Markowitsch HJ, 1998. Episodic and declarative memory: role of the hippocampus. Hippocampus 8, 198–204. [DOI] [PubMed] [Google Scholar]
- 397.Tuscher JJ, Taxier LR, Fortress AM, Frick KM, 2018. Chemogenetic inactivation of the dorsal hippocampus and medial prefrontal cortex, individually and concurrently, impairs object recognition and spatial memory consolidation in female mice. Neurobiol Learn Mem 156, 103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Vago DR, Kesner RP, 2008. Disruption of the direct perforant path input to the CA1 subregion of the dorsal hippocampus interferes with spatial working memory and novelty detection. Behav Brain Res 189, 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Valenti O, Grace AA, 2009. Entorhinal cortex inhibits medial prefrontal cortex and modulates the activity states of electrophysiologically characterized pyramidal neurons in vivo. Cereb Cortex 19, 658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Van Cauter T, Camon J, Alvernhe A, Elduayen C, Sargolini F, Save E, 2013. Distinct roles of medial and lateral entorhinal cortex in spatial cognition. Cereb Cortex 23, 451–459. [DOI] [PubMed] [Google Scholar]
- 401.Van Cauter T, Poucet B, Save E, 2008a. Delay-dependent involvement of the rat entorhinal cortex in habituation to a novel environment. Neurobiol Learn Mem 90, 192–199. [DOI] [PubMed] [Google Scholar]
- 402.Van Cauter T, Poucet B, Save E, 2008b. Unstable CA1 place cell representation in rats with entorhinal cortex lesions. Eur J Neurosci 27, 1933–1946. [DOI] [PubMed] [Google Scholar]
- 403.van Goethem NP, Rutten K, van der Staay FJ, Jans LA, Akkerman S, Steinbusch HW, Blokland A, van’t Klooster J, Prickaerts J, 2012. Object recognition testing: rodent species, strains, housing conditions, and estrous cycle. Behav Brain Res 232, 323–334. [DOI] [PubMed] [Google Scholar]
- 404.van Strien NM, Cappaert NL, Witter MP, 2009. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci 10, 272–282. [DOI] [PubMed] [Google Scholar]
- 405.Vanmierlo T, Creemers P, Akkerman S, van Duinen M, Sambeth A, De Vry J, Uz T, Blokland A, Prickaerts J, 2016. The PDE4 inhibitor roflumilast improves memory in rodents at non-emetic doses. Behav Brain Res 303, 26–33. [DOI] [PubMed] [Google Scholar]
- 406.Varela C, Kumar S, Yang JY, Wilson MA, 2014. Anatomical substrates for direct interactions between hippocampus, medial prefrontal cortex, and the thalamic nucleus reuniens. Brain Struct Funct 219, 911–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Veyrac A, Allerborn M, Gros A, Michon F, Raguet L, Kenney J, Godinot F, Thevenet M, Garcia S, Messaoudi B, Laroche S, Ravel N, 2015. Memory of occasional events in rats: individual episodic memory profiles, flexibility, and neural substrate. J Neurosci 35, 7575–7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Vieira PA, Korzus E, 2015. CBP-Dependent memory consolidation in the prefrontal cortex supports object-location learning. Hippocampus 25, 1532–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.von Ziegler LM, Selevsek N, Tweedie-Cullen RY, Kremer E, Mansuy IM, 2018. Subregion-specific proteomic signature in the hippocampus for recognition processes in adult mice. Cell Rep 22, 3362–3374. [DOI] [PubMed] [Google Scholar]
- 410.Wang AL, Fazari B, Chao OY, Nikolaus S, Trossbach SV, Korth C, Sialana FJ, Lubec G, Huston JP, Mattern C, de Souza Silva MA, 2017a. Intra-nasal dopamine alleviates cognitive deficits in tgDISC1 rats which overexpress the human DISC1 gene. Neurobiol Learn Mem 146, 12–20. [DOI] [PubMed] [Google Scholar]
- 411.Wang AL, Liou YM, Pawlak CR, Ho YJ, 2010. Involvement of NMDA receptors in both MPTP-induced neuroinflammation and deficits in episodic-like memory in Wistar rats. Behav Brain Res 208, 38–46. [DOI] [PubMed] [Google Scholar]
- 412.Wang M, Li D, Yun D, Zhuang Y, Repunte-Canonigo V, Sanna PP, Behnisch T, 2017b. Translation of BDNF-gene transcripts with short 3’ UTR in hippocampal CA1 neurons improves memory formation and enhances synaptic plasticity-relevant signaling pathways. Neurobiol Learn Mem 138, 121–134. [DOI] [PubMed] [Google Scholar]
- 413.Warburton EC, Barker GR, Brown MW, 2013. Investigations into the involvement of NMDA mechanisms in recognition memory. Neuropharmacology 74, 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Watrous AJ, Tandon N, Conner CR, Pieters T, Ekstrom AD, 2013. Frequency-specific network connectivity increases underlie accurate spatiotemporal memory retrieval. Nat Neurosci 16, 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Watson DJ, Loiseau F, Ingallinesi M, Millan MJ, Marsden CA, Fone KC, 2012. Selective blockade of dopamine D3 receptors enhances while D2 receptor antagonism impairs social novelty discrimination and novel object recognition in rats: a key role for the prefrontal cortex. Neuropsychopharmacol 37, 770–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Weber FD, Wang JY, Born J, Inostroza M, 2014. Sleep benefits in parallel implicit and explicit measures of episodic memory. Learn Mem 21, 190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Weible AP, Rowland DC, Monaghan CK, Wolfgang NT, Kentros CG, 2012. Neural correlates of long-term object memory in the mouse anterior cingulate cortex. J Neurosci 32, 5598–5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Weible AP, Rowland DC, Pang R, Kentros C, 2009. Neural correlates of novel object and novel location recognition behavior in the mouse anterior cingulate cortex. J Neurophysiol 102, 2055–2068. [DOI] [PubMed] [Google Scholar]
- 419.Wiescholleck V, Manahan-Vaughan D, 2013. Persistent deficits in hippocampal synaptic plasticity accompany losses of hippocampus-dependent memory in a rodent model of psychosis. Front Integr Neurosci 7, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Willems JGP, Wadman WJ, Cappaert NLM, 2018. Parvalbumin interneuron mediated feedforward inhibition controls signal output in the deep layers of the perirhinal-entorhinal cortex. Hippocampus 28, 281–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Williams Avram SK, Lee HJ, Fastman J, Cymerblit-Sabba A, Smith A, Vincent M, Song J, Granovetter MC, Lee SH, Cilz NI, Stackmann M, Chaturvedi R, Young WS, 2019. NMDA receptor in vasopressin 1b neurons is not required for short-term social memory, object memory or aggression. Front Behav Neurosci 13, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Wilson AG, Crystal JD, 2012. Prospective memory in the rat. Anim Cogn 15, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Wilson AG, Pizzo MJ, Crystal JD, 2013a. Event-based prospective memory in the rat. Curr Biol 23, 1089–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Wilson DI, Langston RF, Schlesiger MI, Wagner M, Watanabe S, Ainge JA, 2013b. Lateral entorhinal cortex is critical for novel object-context recognition. Hippocampus 23, 352–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Wilson DI, Watanabe S, Milner H, Ainge JA, 2013c. Lateral entorhinal cortex is necessary for associative but not nonassociative recognition memory. Hippocampus 23, 1280–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ, 2004. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci 24, 5901–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Winters BD, Reid JM, 2010. A distributed cortical representation underlies crossmodal object recognition in rats. J Neurosci 30, 6253–6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Witter MP, Wouterlood FG, Naber PA, Van Haeften T, 2000. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci 911, 1–24. [DOI] [PubMed] [Google Scholar]
- 429.Xu W, Sudhof TC, 2013. A neural circuit for memory specificity and generalization. Science 339, 1290–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Yamada K, Arai M, Suenaga T, Ichitani Y, 2017. Involvement of hippocampal NMDA receptors in encoding and consolidation, but not retrieval, processes of spontaneous object location memory in rats. Behav Brain Res 331, 14–19. [DOI] [PubMed] [Google Scholar]
- 431.Yang K, Broussard JI, Levine AT, Jenson D, Arenkiel BR, Dani JA, 2017. Dopamine receptor activity participates in hippocampal synaptic plasticity associated with novel object recognition. Eur J Neurosci 45, 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Yi JH, Park HJ, Kim BC, Kim DH, Ryu JH, 2016. Evidences of the role of the rodent hippocampus in the non-spatial recognition memory. Behav Brain Res 297, 141–149. [DOI] [PubMed] [Google Scholar]
- 433.Yi JH, Zhang J, Ko SY, Kwon H, Jeon SJ, Park SJ, Jung J, Kim BC, Lee YC, Kim DH, Ryu JH, 2018. Fluoxetine inhibits natural decay of long-term memory via Akt/GSK-3beta signaling. Mol Neurobiol 55, 7453–7462. [DOI] [PubMed] [Google Scholar]
- 434.Yu JY, Fang P, Wang C, Wang XX, Li K, Gong Q, Luo BY, Wang XD, 2018. Dorsal CA1 interneurons contribute to acute stress-induced spatial memory deficits. Neuropharmacology 135, 474–486. [DOI] [PubMed] [Google Scholar]
- 435.Zalcman G, Federman N, de la Fuente V, Romano A, 2015. Nuclear factor kappa B-dependent Zif268 expression in hippocampus is required for recognition memory in mice. Neurobiol Learn Mem 119, 10–17. [DOI] [PubMed] [Google Scholar]
- 436.Zhang C, Hu WH, Wu DL, Zhang K, Zhang JG, 2015. Behavioral effects of deep brain stimulation of the anterior nucleus of thalamus, entorhinal cortex and fornix in a rat model of Alzheimer’s disease. Chin Med J (Engl) 128, 1190–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Zhang G, Cinalli D, Cohen SJ, Knapp KD, Rios LM, Martinez-Hernandez J, Lujan R, Stackman RW Jr., 2016. Examination of the hippocampal contribution to serotonin 5-HT2A receptor-mediated facilitation of object memory in C57BL/6J mice. Neuropharmacology 109, 332–340. [DOI] [PubMed] [Google Scholar]
- 438.Zhao Z, Fan L, Fortress AM, Boulware MI, Frick KM, 2012. Hippocampal histone acetylation regulates object recognition and the estradiol-induced enhancement of object recognition. J Neurosci 32, 2344–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Zheng C, Bieri KW, Hwaun E, Colgin LL, 2016. Fast gamma rhythms in the hippocampus promote encoding of novel object-place pairings. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Zhou LYY, Wright TE, Clarkson AN, 2016. Prefrontal cortex stroke induces delayed impairment in spatial memory. Behav Brain Res 296, 373–378. [DOI] [PubMed] [Google Scholar]
- 441.Zhou W, Crystal JD, 2009. Evidence for remembering when events occurred in a rodent model of episodic memory. Proc Natl Acad Sci U S A 106, 9525–9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Zhou W, Hohmann AG, Crystal JD, 2012. Rats answer an unexpected question after incidental encoding. Curr Biol 22, 1149–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Zhu XO, Brown MW, McCabe BJ, Aggleton JP, 1995. Effects of the novelty or familiarity of visual stimuli on the expression of the immediate early gene c-fos in rat brain. Neuroscience 69, 821–829. [DOI] [PubMed] [Google Scholar]
- 444.Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, El Gamal A, Schnitzer MJ, 2013. Long-term dynamics of CA1 hippocampal place codes. Nat Neurosci 16, 264–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Zlomuzica A, Preusser F, Totzeck C, Dere E, Margraf J, 2016. The impact of different emotional states on the memory for what, where and when features of specific events. Behav Brain Res 298, 181–187. [DOI] [PubMed] [Google Scholar]
- 446.Zou D, Chen L, Deng D, Jiang D, Dong F, McSweeney C, Zhou Y, Liu L, Chen G, Wu Y, Mao Y, 2016. DREADD in parvalbumin interneurons of the dentate gyrus modulates anxiety, social interaction and memory extinction. Curr Mol Med 16, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]


