Abstract
Di-(2-ethylhexyl) phthalate (DEHP) is a chemical that is widely used as a plasticizer. Exposure to DEHP has been shown to alter ovarian function in humans. Additionally, foods high in fat content, regularly found in the western diet, have been shown to be another potential disruptor of fetal ovarian function. Due to DEHP’s lipophilicity, high-fat foods can be easily contaminated. Therefore, exposure to DEHP and a high-fat diet are both health concerns, especially in pregnant women, and the effects of these exposures on fetal oocyte quality and quantity should be elucidated. In this study, our goal was to determine if there are synergistic effects of DEHP exposure at an environmentally relevant level (20 μg/kg body weight/day) and high-fat diet on oogenesis and folliculogenesis. Dams were fed with a high-fat diet (45 kcal% fat) or a control diet (10 kcal% fat) 1 week before mating and during pregnancy and lactation. The pregnant mice were dosed with DEHP (20 μg/kg body weight/day) or vehicle control from E10.5 to litter birth. We found that treatment with an environmentally relevant dosage of DEHP and consumption of high-fat diet significantly increases synapsis defects in meiosis and affects folliculogenesis in the F1 generation.
Keywords: meiosis, follicular development, fertility, synapsis, meiotic silencing of unsynapsed chromatin (MSUC), multigenerational effect
Prenatal exposure to DEHP + high-fat causes synaptic defects and affects folliculogenesis in F1 females.
Introduction
Phthalates are mainly used as plasticizers to increase the flexibility, transparency, durability, and longevity of plastics. They are classified as endocrine disrupting chemicals in both male and female reproductive systems [1,2]. Di-(2-ethylhexyl) phthalate (DEHP) is one of the most widely used phthalates around the world and is predominately used in the production of various building materials, medical devices, common household items, pesticides, and lubricants. On average, the United States produces nearly 300 million pounds of phthalates including DEHP each year [3]. Humans are readily exposed to DEHP because of its ability to migrate through the materials and leach out into the environment. Thus, people are continuously exposed to DEHP via inhalation, ingestion, and dermal absorption [4]. Indeed, the continuous exposure of DEHP has been evidenced by the fact that 100% of tested human urine samples contain DEHP and its metabolites [5]. The estimated volume of daily human exposure to DEHP is 3–30 μg/kg/day [6]. Furthermore, DEHP and its metabolites have been identified as top contaminants in various tissues, such as blood, umbilical cord blood, reproductive tissues, ovarian follicular fluid, amniotic fluid, and breast milk [7–10].
DEHP exposure during pregnancy can adversely affect fetal oocyte meiosis and meiotic defects can lead to infertility and birth defects. Previous studies have shown that DEHP exposure is linked to decreased synthesis of estradiol and a delay in the meiosis I progression in fetal oocytes [11]. Furthermore, fetal ovaries exposed to DEHP in vitro inhibited meiotic progression from pachytene to diplotene stage of prophase I [12]. DEHP impairs the repair of DNA double-stranded breaks and causes a delay of oogenesis via a checkpoint at pachynema [12]. Although previous studies show that in-vitro exposure of DEHP inhibits meiotic progression in ovarian tissue culture, they have not assessed the effects of prenatal DEHP exposure at an environmentally relevant dose in vivo. Thus, we tested the hypothesis that prenatal exposure to DEHP can disrupt female meiosis in vivo.
In addition to being exposed to environmental chemicals such as DEHP, pregnant women often consume high-fat foods. Maternal consumption of high-fat diet resulted in fetal oocyte reduction and impaired follicle growth in rats [13]. Interestingly, the abnormal spindle formation induced by an obesogenic diet could still be observed in mouse oocytes even after reversing the obesogenic diet back to a regular diet [14]. However, synergistic effects of DEHP + high-fat diet on fetal reproductive systems have not been studied. Thus, we investigated if the combination of DEHP exposure and consumption of high-fat diet can disrupt the female fetal meiosis and follicle development. We exposed pregnant mice to either vehicle or DEHP (20 μg/kg body weigh per day) starting from 10.5 days post coitum (dpc). The dams were treated with control diet or high-fat diet 7 days before they were paired for mating. We found that DEHP + high-fat diet synergistically disrupts the homologous chromosome synapsis in fetal oocytes and affects the development of follicles in the F1 offspring.
Materials and methods
Reagents
A stock solution of DEHP (99% purity, Sigma-Aldrich) was prepared using tocopherol-stripped corn oil (MP Bio Medicals) as the vehicle. The dose of DEHP selected in this study is 20 μg/kg/day. We used this dose because it is within the range of human exposure (3–30 μg/kg/day) and because the dose adversely affects ovarian morphology and function as well as reduces female fertility [15, 16].
Animals
CD-1 mice (Charles River Laboratories, Wilmington, MA) were used in this study and housed in the Animal Care Facility at the University of Illinois Urbana-Champaign (UIUC). Animal handling and procedures were approved by the UIUC Institutional Animal Care and Use Committee. Mice were housed under 12 h dark/12 h light cycles. Both high-fat food (D12451; 45 kcal% fat, 20 kcal% protein, 35 kcal% carbohydrate) and control food (D12450B; 10 kcal% fat, 20 kcal% protein, 70 kcal% carbohydrate) were purchased from Research Diets, Inc. (New Brunswick, NJ). In these experiments, dams were treated with vehicle control + control diet (n = 5), 20 μg/kg/day DEHP + control diet (n = 4), vehicle + high-fat diet (n = 4), or 20 μg/kg/day DEHP + high-fat diet (n = 5). The body weights of the female mice were measured before starting the special diet (Supplemental Figure S1). Female mice were paired with unexposed males to mate and checked regularly for the presence of a copulatory plug. The body weight of female mice was recorded when a copulatory plug was observed (Supplemental Figure S1). The day when the copulatory plug was detected was considered 0.5 dpc. All female mice were given access to either control diet or high-fat diet starting 7 days before the mating day. The food consumption of F0 females was measured from 0 dpc until 18.5 dpc (Supplemental Figure S2). Pregnant female mice were dosed with vehicle control or 20 μg/kg/day DEHP, starting at 10.5 dpc. The body weights of females were measured at 10.5 dpc as well (Supplemental Figure S1). Both treatments were continued until dams were euthanized by CO2 inhalation at 18.5 dpc or until the pups were born. Fetal ovaries were collected from the F1 fetus at 18.5 dpc and used to prepare oocyte chromosomal spreads as described below. The dams that give birth continued to have access to their special diet (control diet or high-fat diet) until the F1 pups were weaned at postnatal day (PND) 21. Body weights of F1 pups were measured at PND 8 (Supplemental Figure S3).
Chromosomal spreads and immunofluorescent staining
To prepare chromosomal spreads, pregnant dams (n = 4–5 dams/treatment) were euthanized at 18.5 dpc. The female pups were removed from the dams, and their ovaries were collected and placed in PBS. Hypotonic extraction buffer was used to incubate the ovaries for 20 min. Each ovary was placed in 10 μl of sucrose on a depression slide and then minced with scalpel blades. Then, an additional 20 μl of sucrose was added and mixed to make a cell suspension. The cell suspension (6 μl) was added into 30 μl of 1% PFA with 0.1% Triton X-100 on a microscope slide where the oocytes were spread out. At least two different slides were made using one ovary from a fetus. Each slide contained a considerable amount of oocyte nuclei. The slides containing the spread oocyte chromosomes were first subjected to blocking by adding antibody dilution buffer (ADB) (0.3% BSA, 10% normal goat serum, and 0.005% Triton-X-100 in TBS) twice (15 min each). The blocked slides were incubated in two primary antibodies, rabbit anti-SYCP3 (Santa Cruz Biotechnology, sc-33195) at a dilution of 1:300 and mouse anti-γH2AX (Millipore-Sigma, Cat. # 05–636)) at a dilution of 1:500 overnight. The slides were further blocked twice with ADB. The slides were subsequently incubated 1 h at 37°C in a pair of goat secondary antibodies: anti-rabbit 488 (Molecular Probes, A11070; 1:1000 dilution) and anti-mouse 594 (Molecular Probes, A11020; 1:1000 dilution). After immunostaining of meiotic cells on the glass slides, images were taken with a Nikon A1R confocal microscope and processed using NIS-Elements software. The total number of oocytes varies in each treatment groups because F0 dams often do not have the same number of female fetuses. Also from each fetus, we imaged different numbers of oocyte nuclei. A total number of 232 oocytes were imaged from corn oil + control diet group. A total number of 178 oocytes were imaged from control diet + DEHP group. A total number of 151 oocytes were imaged from corn oil + high-fat diet group. A total number of 351 oocytes were imaged from high-fat diet + DEHP group.
Ovarian follicle counts in F1 females
The ovaries were collected from PND 8 and PND 21 F1 females and fixed in Dietrich's fixative. The fixed ovaries were embedded in paraffin wax, the PND 8 ovaries were sectioned at 5 μm, and the PND 21 ovaries were sectioned at 8 μm. Sections were mounted on glass slides and stained with hematoxylin and eosin. EVOS XL Core Imaging System was used to image every fifth section of the PND 8 ovaries, and the images were used to count primordial, primary, and preantral follicles in a blind fashion by two individuals. Every 10th section of the PND 21 ovaries was directly used to count the number of primordial, primary, preantral, and antral follicles under the microscope. The proportion of each follicle type was calculated by dividing the number of each type of follicles from the total number of follicles per ovary. The following criteria were used to classify each type of follicle: primordial follicles contained a single layer of squamous granulosa cells and an oocyte can be observed within the granulosa cell circle; primary follicles contained an oocyte with a single surrounding layer of cuboidal granulosa cells; preantral follicles had at least two layers of cuboidal granulosa cells and theca cell layers with an oocyte inside the cell layers; and antral follicles contained multiple layers of cuboidal granulosa cells with fluid-filled antral space(s), theca cell layers and an oocyte inside the cell layers [17,18]. Preantral and antral follicles without a clear oocyte nucleus were not counted. The follicle counting was conducted without the knowledge of the treatment groups.
Fertility tests of F1 females
F1 female mice were mated with unexposed CD-1 male mice and monitored for the presence of copulatory vaginal sperm plug. After confirming the presence of copulatory vaginal sperm plug, the females were separated from the male, weighed, and individually housed. These female mice were weighed twice a week to confirm successful pregnancy. Further, we assessed the mating index, pregnancy rate, and gestational index by mating female F1 pups at PND 90 from each treatment group with untreated males. We calculated the mating and gestational indexes as well as the pregnancy rate by using the following equations [15]
Mating index = number of females with copulatory vaginal sperm plugs/number of total females × 100
Pregnancy rate = number of pregnant females/number of total females × 100
Gestational index = number of females who delivered/number of pregnant females × 100
Gene expression analysis
Total RNA was isolated from PND 21 F1 ovaries (three ovaries from three different animals per treatment) using the RNeasy Micro Kit (Qiagen, Inc., Valencia, CA). Isolated RNA was reverse transcribed to complementary DNA (cDNA) using the SuperScriptIII kit based on the manufacturer's protocol. The mRNA expression levels of genes encoding for enzymes in estradiol biosynthetic pathway were analyzed for each treatment group via quantitative real-time polymerase chain reaction (qPCR) using SsoFast™EvaGreen® Supermixes according to the manufacturer's protocol (BioRad). A standard curve was generated to calculate the efficiencies of each primer set using six serial dilutions of a combination of samples. The gene expression data obtained from each sample were normalized to the corresponding values of housekeeping gene beta-actin (Actb). Beta-actin was chosen as the reference gene because this housekeeping gene is typically used for normalizing gene expression data in mouse studies [19,20], and the expression of beta-actin did not differ among treatments. The genes we tested participate in synthesizing estradiol and estradiol precursor sex steroid hormones in the ovary (Supplemental Figure S4) [21], including steroidogenic acute regulatory protein (Star), 17α-hydroxylase-17,20-desmolase (Cyp17a1), aromatase (Cyp19a1), cytochrome P450 cholesterol side-chain cleavage enzyme (Cyp11a1), 17β-hydroxysteroid dehydrogenase 1 (Hsd17b1), and 3β-hydroxysteroid dehydrogenase 1 (Hsd3b1). The qPCR primer sequences of the tested genes were listed in Table 1. Pfaffl method was used to calculate individual relative fold changes [22]. The relative mRNA expression level was expressed as the mean fold change ± SD from three independent samples.
Table 1.
Sequences of primer sets used for gene expression analyses.
| Transcript | Species | Forward sequence | Reverse sequence |
|---|---|---|---|
| Actb | Mouse | 5′-AGC ACA GCT TCT TTG CAG | 5′-CAG CGC AGC GAT ATC GTC |
| CTC CTT-3′ | ATC CAT-3′ | ||
| StAR | Mouse | 5′-CAG GGA GAG GTG GCT ATG | 5′-CCG TGT CTT TTC CAA TCC |
| CA-3′ | TCT G-3′ | ||
| Cyp17a1 | Mouse | 5′-CCA GGA CCC AAG TGT GTT | 5′-CCT GAT ACG AAG CAC TTC |
| CT-3′ | TCG-3′ | ||
| Cyp19a1 | Mouse | 5′-CAT GGT CCC GGA AAC TGT | 5′-GTA GTA GTT GCA GGC ACT |
| GA-3′ | TC-3′ | ||
| Cyp11a1 | Mouse | 5′-AGA TCC CTT CCC CTG GTG | 5′-CGC ATG AGA AGA GTA TCG |
| ACA ATG-3′ | ACG CAT C-3′ | ||
| Hsd17b1 | Mouse | 5′-AAG CGG TTC GTG GAG AAG | 5′-ACT GTG CCA GCA AGT TTG |
| TAG-3′ | CG-3′ | ||
| Hsd3b1 | Mouse | 5′-CAG GAG AAA GAA CTG CAG | 5′-GCA CAC TTG CTT GAA CAC |
| GAG GTC-3′ | AGG C-3′ |
Statistical analysis
Differences among groups were statistically analyzed by unpaired two-sample independent t-test or one-way analysis of variance. Mating and gestational indexes and the pregnancy rate were analyzed by using the one-tailed Fisher exact test. A Mann–Whitney U test was used to analyze the differences among groups when normality cannot be assumed. Raw data were used to statistically analyze follicle counts at PND 8 and PND 21. All tests were conducted using the Graph-Pad Prism analysis software. Comparisons were considered significant at *P < 0.05.
Results
Exposure to DEHP + high-fat diet increases meiotic silencing of unsynapsed chromatin in fetal oocytes
During meiotic prophase I, homologous chromosomes pair up along their length and are stabilized via synapsis (the assembly of a proteinaceous complex called synaptonemal complex (SC)). The process of synapsis and desynapsis can divide prophase I into several substages: leptonema, zygonema, pachynema, and diplonema/diakinesis. The axial elements of the SC are composed of cohesin proteins and meiosis-specific proteins, such as synaptonemal complex protein 3 (SYCP3) [23]. Immunostaining of oocyte chromosomal spreads for SYCP3 can be used to stage meiotic prophase I [24,25]. To further assess the condition of meiotic chromosomes, immunostaining of gamma-histone 2AX (γH2AX) can be used. γH2AX represents DNA damage signaling that detects DNA double-strand breaks and asynapsis of homologous chromosomes [26,27]. Asynapsis of autosomal chromosomes can be identified as γH2AX-rich chromatin domains known as meiotic silencing of unsynapsed chromatin (MSUC) [28,29].
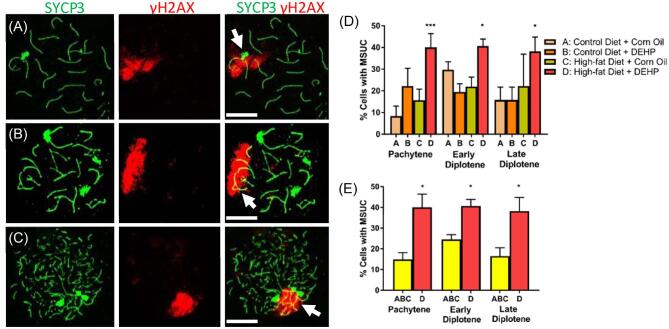
We found that some oocytes have large sections of γH2AX staining, resembling the γH2AX staining of MSUC in the control group and treatment groups (Figure 1). Analysis of MSUC revealed that at least 20% of the fetal oocytes contained MSUC in the three treatment groups: (A) the group dosed with corn oil and fed with control diet; (B) the group dosed with DEHP and treated with control diet; (C) the group dosed with corn oil and treated with high-fat diet (Figure 1D). However, approximately 40% of fetal oocytes from dams treated with DEHP + high-fat diet contained MSUC. The data from control group, DEHP only group, and high-fat diet only group were further combined to form a “combined control” group to compare with the DEHP + high-fat diet group (Figure 1E). F-test for the equality of variances was used to verify that the variances of the three groups did not differ significantly. Counted cells from each group were treated as identical after verification of equality of means and variances, and the combined total cells were used as the “sample” for the combined control. Oocytes in the DEHP + high-fat group contained a significantly greater percentage of cells with MSUC compared to the combined control group. The increased percentage of cells with MSUC held true for DEHP + high-fat group oocytes at pachynema, early diplonema, and late diplonema. These results indicate that the exposure of pregnant dams to a high-fat diet coupled with DEHP results in significant disruption of synapsis.
Figure 1.
Representative images for fetal oocytes at the pachynema (A), early diplonema (B), and late diplonema (C) containing MSUC (arrows). (D) Percentage of pachytene, early diplotene, and late diplotene fetal oocytes with MSUC in A group (control diet 460 + corn oil) (n = 232 oocytes), B group (control diet + DEHP) (n = 178 oocytes), C group (high-fat diet + corn oil) (n = 151 oocytes), and D group (high-fat diet + DEHP) (n = 351 oocytes). Differences within treatment groups in % MSUC at each stage were evaluated using the A group as a reference (*P < 0.05, ***P < 0.001). (E) Comparison of MSUC percentage of D group with combined A, B, and C controls (*P < 0.05). Graphs represent means ± SEM. Scale bars = 10 μm.
DEHP + high-fat diet treatment does not alter the follicle number of F1 pups at PND 8
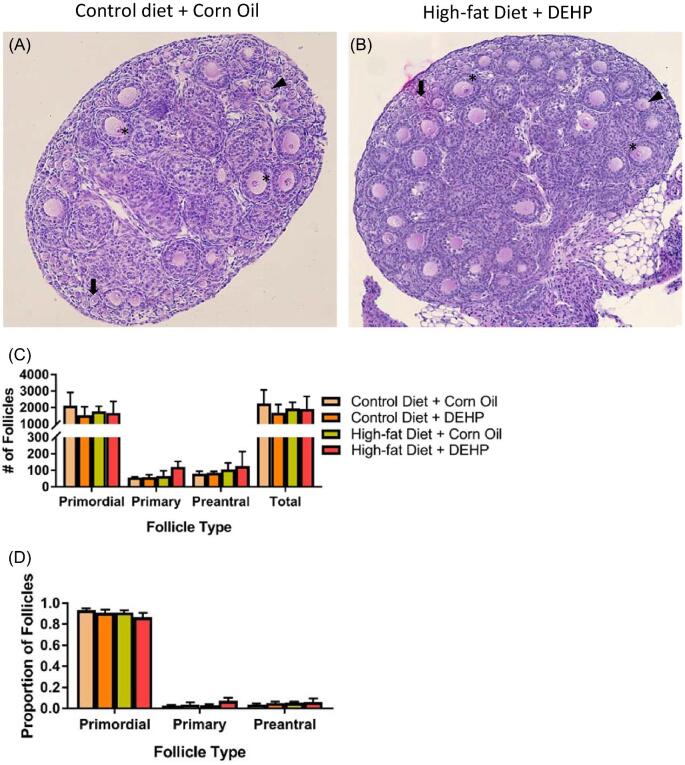
Asynapsis-induced gene silencing in MSUC regions could result in oocyte loss [30]. To examine whether the elevated MSUC found in DEHP + high-fat diet treated group led to a smaller oocyte pool compared to other three treatment groups, we counted the numbers of primordial, primary, and preantral follicles at PND 8. Ovaries in all four treatment groups contained similar numbers of primordial, primary, and preantral follicles at PND 8 (Figure 2C). Calculating the proportion of each follicle type in the ovaries of all four groups yielded no significant differences (Figure 2D). These results indicate that, in our study, increase in MSUC did not result in significant oocyte loss.
Figure 2.
PND 8 ovarian section in A group (control diet + corn oil) (A) and B group (high-fat diet + DEHP) (B) stained with hematoxylin and eosin. Some primordial (arrows), primary (arrow heads), and preantral (asterisks) follicles have been demarcated. (C) Counts of primordial, primary, and preantral follicles for each treatment group at PND 8 (n = 3 dams/treatment group). (D) Follicular progression of PND 8 ovaries, as measured by the proportions of primordial, primary, and preantral follicles in each treatment group. Proportions were gathered separately for each ovary. Graphs represent means ± SD.
DEHP + high-fat diet treatment affected preantral follicle development in F1 ovaries at PND 21
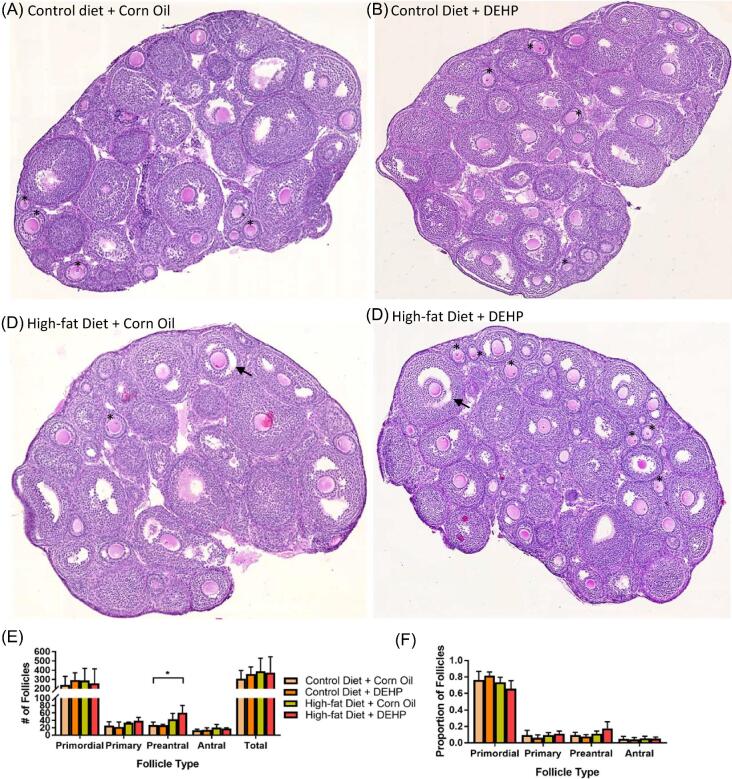
Ovaries in all four treatment groups at PND 21 contained primordial, primary, preantral, and antral follicles, with majority of follicles being at the primordial stage (Figure 3E). The percentage of each follicle type present in the ovaries did not vary among the four groups (Figure 3 F). However, ovaries of F1 pups treated with DEHP + high-fat diet had a significantly higher number of preantral follicles than the ovaries of F1 pups in the other three groups at PND 21 (Figure 3D). These results suggest that the exposure to DEHP + high-fat diet affects preantral follicle development in F1 ovaries at PND 21.
Figure 3.
PND 21 F1 ovarian sections stained with hematoxylin and eosin in four treatment groups: A group (control diet + corn oil) (A), B group (control diet + DEHP) (B), C group (high-fat diet + corn oil) (C), and D group (high-fat diet + DEHP) (D). Some antral follicles (arrows) and preantral follicles (asterisks) have been demarcated. (E) Counts of primordial, primary, and preantral follicles for each treatment group at PND 21 (*P < 0.05) (n = 4 dams/treatment group). (F) Follicular progression of PND 21 ovaries, as measured by the proportions of primordial, primary, and preantral follicles in each treatment group. Proportions were gathered separately for each ovary. Graphs represent means ± SD.
DEHP + high-fat diet do not alter the expression levels of major steroidogenic enzymes in PND 21 ovaries
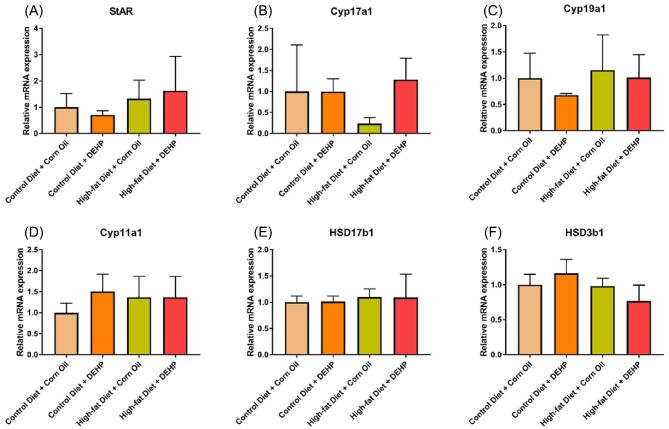
Preantral and antral follicles are steroidogenically active. The increase of preantral follicle number in DEHP + high-fat diet ovaries may elevate the expression levels of estradiol and its precursor steroid hormones in the ovaries with the combined treatment compared to the controls. Thus, we examined the gene expression of the steroidogenic enzymes in PND 21 ovaries (Table 1). We focused on these genes because previous studies have shown that DEHP and its primary metabolite, MEHP, alter the expression of these genes in the estradiol biosynthesis pathway [7,31–33]. However, we did not observe any significant differences in expression of the tested genes among the four treatment groups (Figure 4). Our results differ from the previous studies because the amount of MEHP reaching the ovaries may be different between our study and the previous studies.
Figure 4.
DEHP and high-fat diet do not change the mRNA expression levels of steroid enzymes in PND 21 F1 ovaries. After each treatment, ovaries were collected and subjected to qPCR analysis for (A) StAR, (B) Cyp17a1, (C) Cyp19a1, (D) Cyp11a1, (E) HSD17b1, and (F) HSD3b1 mRNA expression levels. All gene expression values were normalized to the expression of β-actin (n = 3). Graphs represent means ± SD.
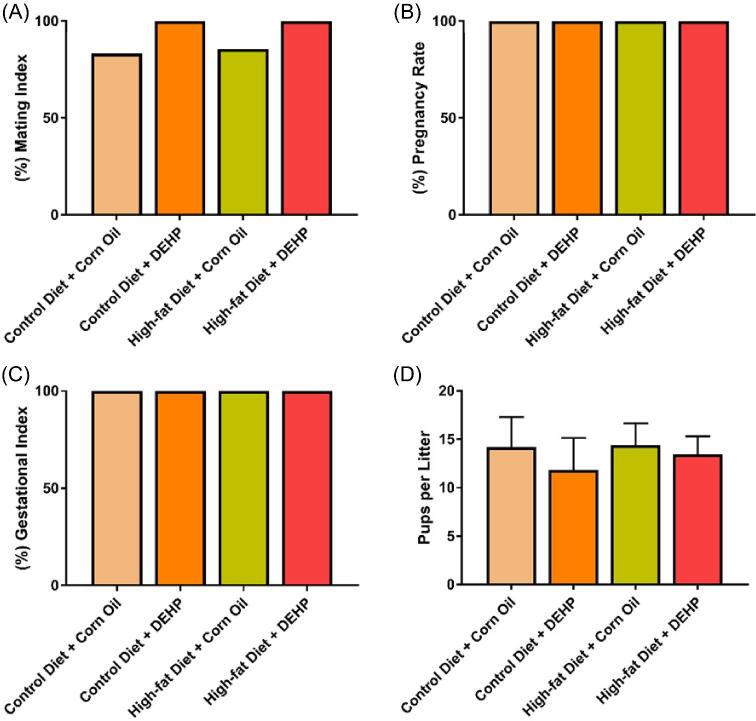
Female F1 pups exposed to DEHP + high-fat diet do not experience a decline in the mating index, pregnancy rate, and gestational index at PND 90
The disrupted oogenesis and affected folliculogenesis caused by DEHP + high-fat diet led us to test several fertility-related indices. None of the treatments affected mating index compared to the control group. In fact, the mating index in groups ranged from 83.33% to 100% (Figure 5A). Similarly, none of the treatments affected pregnancy rates and gestational indices of F1 females in each group. F1 dams from all four groups were become pregnant and gave birth successfully (Figure 5B and C). Litter sizes among the F1 pups also did not differ significantly (P > 0.40), with each mother giving birth to 10–15 pups on average (Figure 5D). These data suggest that the effects of DEHP + high-fat diet on F1 oocytes during meiosis ultimately do not impact the fertility of F1 females at an early age.
Figure 5.
(A) Mating indices of female F1 pups at PND 90 in the control diet + corn oil, control diet + DEHP, high-fat diet + corn oil, and high-fat diet + DEHP groups. (B) Pregnancy rates of female F1 pups at PND 90 in the control diet + corn oil, control diet + DEHP, high-fat diet + corn oil, and high-fat diet + DEHP groups. (C) Gestational indices of female F1 pups at PND 90 in the control diet + corn oil, control diet + DEHP, high-fat diet + corn oil, and high-fat diet + DEHP groups. (D) Average litter size of F1 dams in the control diet + corn oil, control diet + DEHP, high-fat diet + corn oil and high-fat diet + DEHP groups. Control diet + corn oil (n = 5 F1 females), control diet + DEHP (n = 7 F1 females), high-fat diet + corn oil (n = 7 F1 females), and high-fat diet + DEHP (n = 7 F1 females). Graphs represent means ± SD.
Discussion
The purpose of this study was to determine the synergistic effects of high-fat consumption and environmentally relevant DEHP exposure on female fetal meiosis. Although a previous in vitro study has shown that prenatal DEHP exposure (10 and 100 μM) results in delay of meiotic progression and disruption of DNA damage repair [12], studies have not examined the effect of DEHP on meiosis in vivo and they have not examined high-fat diet as a combined factor. In our in-vivo study, prenatal DEHP exposure alone did not cause an observable delay in meiotic progression of F1 oocytes from DEHP-exposed dams (S.M. and H.Q., unpublished data). Our results differ from this in-vitro study probably because we used a different dose of DEHP (20 μg/kg/day), and because DEHP exposure is direct to the fetal ovaries in the previous in-vitro study [12] but indirect in our in-vivo study. Since DEHP is quickly metabolized to MEHP in animals’ GI tracts, fetal ovaries were exposed to not only DEHP but also MEHP in our in-vivo study. The bioactivity differences between DEHP and its metabolite MEHP may explain why we cannot recapitulate the meiotic arrest found by Liu et al. [12].
In this study, we also observed that the exposure of dams to a high-fat diet (45% calories from fat: relevant to a western diet) [34] and environmentally relevant levels of DEHP results in disruption of synapsis during prophase I of meiosis, as evidenced by a significantly high number of γH2AX-stained MSUC in the fetal oocytes. Due to the failure of synapsis, the chromatin in the unsynapsed region may trigger MSUC, which is known to transcriptionally silence genes [29,35]. The gene silencing caused by MSUC can trigger meiotic checkpoints and induce germ cell death [27]. Thus, if majority of the oocytes in the ovary experience asynapsis, increased levels of MSUC may potentially affect the size of the oocyte pool by eliminating the affected oocytes via the DNA damage checkpoint [36]. This may lead to a decreased reproductive lifespan in affected individuals [37].
To investigate later stage oocyte development and to examine if the presence of MSUC is associated with increased oocyte death, we evaluated folliculogenesis by counting the total number of follicles in F1 ovaries at PND 8 and PND 21. Previous studies in adult mice have reported that acute exposure to 200 and 500 mg/kg/day DEHP for 10 days resulted in decreased total follicle numbers after 9 months [3]. Surprisingly, even though we observed increased levels of MSUC from the DEHP + high-fat diet group (Figure 1D), we did not see a decrease in the size of oocyte pool at both PND 8 and PND 21 (Figures 2C and 3E). A size reduction of the oocyte pool and infertility are rarely observed unless most oocytes in the ovaries are extensively affected [37]. If the damage induced by DEHP + high-fat diet treatment is not severe enough to kill the defective oocytes, the oocyte pool reduction compared to control mice may not be observed. However, future research should investigate if there are any size differences of 9-month-old oocyte pools among the four treatment groups.
Interestingly, when we counted the number of each follicle type (primordial, primary, preantral, and antral follicles) in F1 ovaries, we observed an increase in the average number of preantral follicles in the high-fat diet + DEHP-treated group at PND 21. This result demonstrates the synergistic effect of DEHP + high-fat diet on folliculogenesis. This result correlates with a previous study where the PND 21 preantral follicle counts increased in two in utero DEHP exposed groups (200 and 500 mg/kg/day) [38]. It is possible that the combination of DEHP and high-fat diet allows more DEHP and its metabolites to reach the F1 fetal ovaries, thus producing the same phenotype despite the lower concentrations. The interaction between DEHP and high-fat diet may be explained by the lipophilicity of DEHP [39]. Consumption of a high-fat diet during the administration of DEHP may facilitate the biotransformation of this chemical.
DEHP and its major metabolite, MEHP, are both endocrine disruptors [40]. To investigate whether DEHP affects steroidogenesis and folliculogenesis through the disruption of endocrine system, we conducted qPCRs to measure relative expression of various genes in estradiol biosynthetic pathways. Our results did not show any significant differences in expression of the tested genes (Figure 4), suggesting that the higher number of preantral follicles may not have been caused by disruption of the estradiol biosynthetic pathway, at least at the level of gene expression.
It is still unclear why preantral follicle number significantly increased in F1 ovaries at PND 21. It is possible that the combination of DEHP and high-fat diet accelerates the maturation of primordial and primary follicles into preantral follicles. However, we did not observe a corresponding decrease in the number of primordial and primary follicles in DEHP + high-fat diet treated ovaries. It is possible that accelerated follicle maturation does not significantly decrease the number of primordial and primary follicles due to the higher number and variation of these follicles as compared to preantral and antral follicles. Another potential explanation for our findings is that prenatal exposure to DEHP + high-fat diet inhibits the natural follicular atresia of preantral follicles but promotes antral-follicle atresia in the ovaries.
To analyze the effects of DEHP + high-fat diet on reproductive capacity of the F1 generation, we recorded the mating and gestational indices, litter size, and pregnancy rate of PND 90 F1 female mice. In a previous study in F1 female mice, prenatal exposure to 20 and 200 μg/kg/day of DEHP resulted in slightly lower pregnancy rate without significant differences compared to the controls [15]. In our study, no significant differences were observed in any of the four treatment groups. This suggests the synapsis disruption observed in defective oocytes was likely screened and removed by perinatal and/or later-stage checkpoints [18].
Future studies should investigate the effects of higher levels of occupational [41] and medical [42] DEHP exposure and/or consumption of fat during pregnancy that are above the levels we have tested, because high levels of DEHP and high-fat diet exposure may still potentially cause adverse effects on F1 fertility. Since phthalate exposure is unavoidable and present worldwide, our study stresses the importance and need for future studies to elucidate the effects of phthalates during prenatal, postnatal, and multigenerational development, especially at higher doses.
Supplementary data
Supplemental Figure S1. Body weight of F0 females at the initiation of the diet treatment, at 0 dpc, and at 10.5 dpc. Control diet + corn oil (n = 5 F0 females), control diet + DEHP (n = 4 F0 females), high-fat diet + corn oil (n = 4 F0 females), and high-fat diet + DEHP (n = 5 F0 females). Graph represents means ± SD.
Supplemental Figure S2. Food consumption of F0 females from 0 to 18.5 dpc. Control diet + corn oil (n = 5 F0 females), control diet + DEHP (n = 4 F0 females), high-fat diet + corn oil (n = 4 F0 females), and high-fat diet + DEHP (n = 5 F0 females). Graph represents means ± SD.
Supplemental Figure S3. Body weight of F1 pups at PND 8. Control diet + corn oil (n = 10 F1 pups), control diet + DEHP (n = 15 F1 pups), high-fat diet + corn oil (n = 5 F1 pups), and high-fat diet + DEHP (n = 17 F1 pups). Graph represents means ± SD.
Supplemental Figure S4. Steroid-hormone biosynthetic pathways in the ovary. Theca cells and granulosa cells in antral follicles participate in steroidogenesis. Metabolism of cholesterol to steroid hormones is regulated by steroidogenic enzymes STAR, CYP11A1, CYP17A1, CYP19A1, HSD3B1, and HSD17B1.
Acknowledgments
We would like to thank the members of Dr Flaws’ Laboratory for their help in gathering data on F1 generation fertility. We would also like to thank Benjamin Tobias, Kelly Fahey, Qingyi Lan, and Pranathi Karumanchi for their help in data collection and Dr Ko's Laboratory for their help in imaging ovarian sections.
Footnotes
† Grant Support: This work was supported by the National Institutes of Health [R00 HD082375, P01 ES022848, and T32 ES007326] and the Environmental Protection Agency [RD 83459301].
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1. Richardson KA, Hannon PR, Johnson-Walker YJ, Myint MS, Flaws JA, Nowak RA. Di (2-ethylhexyl) phthalate (DEHP) alters proliferation and uterine gland numbers in the uteri of adult exposed mice. Reprod Toxicol 2018; 77:70–79. [DOI] [PubMed] [Google Scholar]
- 2. Zhang X-F, Zhang T, Wang L, Zhang H-Y, Chen Y-D, Qin X-S, Feng Y-M, Feng Y-N, Shen W, Li L. Effects of diethylhexyl phthalate (DEHP) given neonatally on spermatogenesis of mice. Mol Biol Rep 2013; 40:6509–6517. [DOI] [PubMed] [Google Scholar]
- 3. Hannon PR, Niermann S, Flaws JA. Acute exposure to di(2-ethylhexyl) phthalate in adulthood causes adverse reproductive outcomes later in life and accelerates reproductive aging in female mice. Toxicol Sci 2016; 150:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Erythropel HC, Maric M, Nicell JA, Leask RL, Yargeau V. Leaching of the plasticizer di(2-ethylhexyl)phthalate (DEHP) from plastic containers and the question of human exposure. Appl Microbiol Biotechnol 2014; 98:9967–9981. [DOI] [PubMed] [Google Scholar]
- 5. Silva MJ, Wong L-Y, Samandar E, Preau JL, Calafat AM, Ye X. Exposure to di-2-ethylhexyl terephthalate in a convenience sample of U.S. adults from 2000 to 2016. Arch Toxicol 2017; 91:3287–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doull J, Cattley R, Elcombe C, Lake BG, Swenberg J, Wilkinson C, Williams G, van Gemert M. A cancer risk assessment of di(2-ethylhexyl)phthalate: application of the new U.S. EPA Risk Assessment Guidelines. Regul Toxicol Pharmacol 1999; 29:327–357. [DOI] [PubMed] [Google Scholar]
- 7. Hannon PR, Flaws JA.. The effects of phthalates on the ovary. Front Endocrinol (Lausanne) 2015; 6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Högberg J, Hanberg A, Berglund M, Skerfving S, Remberger M, Calafat AM, Filipsson AF, Jansson B, Johansson N, Appelgren M, Håkansson H. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ Health Perspect 2008; 116:334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, Mazzeo P. Exposure to di(2-ethylhexyl)phthalate in humans during pregnancy. Neonatology 2003; 83:22–24. [DOI] [PubMed] [Google Scholar]
- 10. Silva MJ, Reidy JA, Herbert AR, Preau JL, Needham LL, Calafat AM. Detection of phthalate metabolites in human amniotic fluid. Bull Environ Contam Toxicol 2004; 72:1226–1231. [DOI] [PubMed] [Google Scholar]
- 11. Zhang X-F, Zhang T, Han Z, Liu J-C, Liu Y-P, Ma J-Y, Li L, Shen W. Transgenerational inheritance of ovarian development deficiency induced by maternal diethylhexyl phthalate exposure. Reprod Fertil Dev 2015; 27:1213. [DOI] [PubMed] [Google Scholar]
- 12. Liu J-C, Lai F-N, Li L, Sun X-F, Cheng S-F, Ge W, Wang Y-F, Li L, Zhang X-F, De Felici M, Dyce PW, Shen W. Di (2-ethylhexyl) phthalate exposure impairs meiotic progression and DNA damage repair in fetal mouse oocytes in vitro. Cell Death Dis 2017; 8:e2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsoulis MW, Chang PE, Moore CJ, Chan KA, Gohir W, Petrik JJ, Vickers MH, Connor KL, Sloboda DM. Maternal high-fat diet-induced loss of fetal oocytes is associated with compromised follicle growth in adult rat offspring. Biol Reprod 2016; 94:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reynolds KA, Boudoures AL, Chi MM-Y, Wang Q, Moley KH. Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice. Reprod Fertil Dev 2015; 27:716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rattan S, Brehm E, Gao L, Flaws JA. Di(2-Ethylhexyl) phthalate exposure during prenatal development causes adverse transgenerational effects on female fertility in mice. Toxicol Sci 2018; 163:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rattan S, Brehm E, Gao L, Niermann S, Flaws JA. Prenatal exposure to di(2-ethylhexyl) phthalate disrupts ovarian function in a transgenerational manner in female mice†. Biol Reprod 2018; 98:130–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol 1991; 124:43–101. [DOI] [PubMed] [Google Scholar]
- 18. Qiao H, Rao HBDP, Yun Y, Sandhu S, Fong JH, Sapre M, Nguyen M, Tham A, Van BW, Chng TYH, Lee A, Hunter N. Impeding DNA break repair enables oocyte quality control. Mol Cell 2018; 0:211–221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mahalingam S, Ther L, Gao L, Wang W, Ziv-Gal A, Flaws JA. The effects of in utero bisphenol A exposure on ovarian follicle numbers and steroidogenesis in the F1 and F2 generations of mice. Reprod Toxicol 2017; 74:150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peretz J, Neese SL, Flaws JA. Mouse strain does not influence the overall effects of bisphenol a-induced toxicity in adult antral follicles. Biol Reprod 2013; 89:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Craig ZR, Leslie TC, Hatfield KP, Gupta RK, Flaws JA. Mono-hydroxy methoxychlor alters levels of key sex steroids and steroidogenic enzymes in cultured mouse antral follicles. Toxicol Appl Pharmacol 2010; 249:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baier A, Alsheimer M, Benavente R. Synaptonemal complex protein SYCP3: Conserved polymerization properties among vertebrates. Biochim Biophys Acta - Proteins Proteomics 2007; 1774:595–602. [DOI] [PubMed] [Google Scholar]
- 24. Qiao H, Rao HBDP, Yun Y, Sandhu S, Fong JH, Sapre M, Nguyen M, Tham A, Van BW, Chng TYH, Lee A, Hunter N. Impeding DNA break repair enables oocyte quality control. Mol Cell 2018; 72:211–221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alavattam KG, Abe H, Sakashita A, Namekawa SH. Chromosome Spread Analyses of Meiotic Sex Chromosome Inactivation. New York, NY:Humana Press; 2018:113–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jiang H, Gao Q, Zheng W, Yin S, Wang L, Zhong L, Ali A, Khan T, Hao Q, Fang H, Sun X, Xu P et al.. MOF influences meiotic expansion of H2AX phosphorylation and spermatogenesis in mice. PLOS Genet 2018; 14:e1007300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Turner JMA, Mahadevaiah SK, Fernandez-Capetillo O, Nussenzweig A, Xu X, Deng CX, Burgoyne PS. Silencing of unsynapsed meiotic chromosomes in the mouse. Nat Genet 2005; 37:41–47. [DOI] [PubMed] [Google Scholar]
- 28. Turner JMA, Mahadevaiah SK, Fernandez-Capetillo O, Nussenzweig A, Xu X, Deng C-X, Burgoyne PS. Silencing of unsynapsed meiotic chromosomes in the mouse. Nat Genet 2005; 37:41–47. [DOI] [PubMed] [Google Scholar]
- 29. Baarends WM, Wassenaar E, Van Der Laan R, Hoogerbrugge J, Sleddens-Linkels E, Hoeijmakers JHJ, De Boer P, Grootegoed JA. Silencing of unpaired chromatin and histone H2A ubiquitination in mammalian meiosis. Mol Cell Biol 2005; 25:1041–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cloutier JM, Mahadevaiah SK, ElInati E, Nussenzweig A, Tóth A, Turner JMA. Histone H2AFX links meiotic chromosome asynapsis to prophase I oocyte loss in mammals. PLoS Genet 2015; 11:e1005462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hannon PR, Brannick KE, Wang W, Flaws JA. Mono(2-ethylhexyl) phthalate accelerates early folliculogenesis and inhibits steroidogenesis in cultured mouse whole ovaries and antral follicles. Biol Reprod 2015; 92:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moyer B, Hixon ML.. Reproductive effects in F1 adult females exposed in utero to moderate to high doses of mono-2-ethylhexylphthalate (MEHP). Reprod Toxicol 2012; 34:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pocar P, Fiandanese N, Secchi C, Berrini A, Fischer B, Schmidt JS, Schaedlich K, Borromeo V. Exposure to di(2-ethyl-hexyl) phthalate (DEHP) in utero and during lactation causes long-term pituitary-gonadal axis disruption in male and female mouse offspring. Endocrinology 2012; 153:937–948. [DOI] [PubMed] [Google Scholar]
- 34. Wilson CR, Tran MK, Salazar KL, Young ME, Taegtmeyer H. Western diet, but not high fat diet, causes derangements of fatty acid metabolism and contractile dysfunction in the heart of Wistar rats. Biochem J 2007; 406:457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Manterola M, Page J, Vasco C, Berríos S, Parra MT, Viera A, Rufas JS, Zuccotti M, Garagna S, Fernández-Donoso R. A high incidence of meiotic silencing of unsynapsed chromatin is not associated with substantial pachytene loss in heterozygous male mice carrying multiple simple robertsonian translocations. PLoS Genet 2009; 5:e1000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rinaldi VD, Bolcun-Filas E, Kogo H, Kurahashi H, Schimenti JC. The DNA damage checkpoint eliminates mouse oocytes with chromosome synapsis failure. Mol Cell 2017; 67:1026–1036.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Burgoyne PS, Mahadevaiah SK, Turner JMA. The consequences of asynapsis for mammalian meiosis. Nat Rev Genet 2009; 10:207–216. [DOI] [PubMed] [Google Scholar]
- 38. Niermann S, Rattan S, Brehm E, Flaws JA. Prenatal exposure to di-(2-ethylhexyl) phthalate (DEHP) affects reproductive outcomes in female mice. Reprod Toxicol 2015; 53:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rose RJ, Priston MJ, Rigby-Jones AE, Sneyd JR. The effect of temperature on di(2-ethylhexyl) phthalate leaching from PVC infusion sets exposed to lipid emulsions. Anaesthesia 2012; 67:514–520. [DOI] [PubMed] [Google Scholar]
- 40. Feige JN, Gelman L, Rossi D, Zoete V, Métivier R, Tudor C, Anghel SI, Grosdidier A, Lathion C, Engelborghs Y, Michielin O, Wahli W et al.. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor γ modulator that promotes adipogenesis. J Biol Chem 2007; 282:19152–19166. [DOI] [PubMed] [Google Scholar]
- 41. Fong J-P, Lee F-J, Lu I-S, Uang S-N, Lee C-C. Estimating the contribution of inhalation exposure to di-2-ethylhexyl phthalate (DEHP) for PVC production workers, using personal air sampling and urinary metabolite monitoring. Int J Hyg Environ Health 2014; 217:102–109. [DOI] [PubMed] [Google Scholar]
- 42. Tickner JA, Schettler ÃT, Guidotti T, Mccally M, Rossi M. Health risks posed by use of di-2-ethylhexyl phthalate (DEHP) in PVC medical devices: a critical review. Am J Ind Med 2001; 111:100–111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.