Abstract
Cell fusion is involved in the development of some adult organs, is implicated in the pathogenesis of specific types of cancer, and is known to participate in repair/regeneration processes mediated by bone-marrow-derived cells (BMDCs). Endometriosis is a disease characterized by growth of functional endometrial tissue outside of the uterine cavity. Endometriosis shares some molecular properties with cancer and BMDCs home to endometriosis lesions in a mouse model. Our objective was to determine if cell fusion can occur in endometriosis and establish whether bone-marrow-derived cells participate in cell fusion events in lesions. We employed a Cre-Lox system to identify cell fusion events in a mouse model of endometriosis. Fused cells were detected in endometriotic lesions, albeit at a low frequency (∼1 in 400 cells), localized to the stromal compartment, and displayed restricted proliferation. Using 5-fluorouracil-based nongonadotoxic bone marrow transplantation model, we demonstrate that bone marrow cells represent a principal cell source for fusion events in lesions. Cell fusion progeny uniformly lacked expression of selected markers of hematopoietic, endothelial, and epithelial markers, though they expressed the mesenchymal/stromal markers Sca-1 and CD29. This study is the first to describe the phenomenon of cell fusion in endometriosis and points to a mesenchymal population derived from cell fusion events with limited proliferative activity, properties previously attributed to endometrial stem cells. Their putative role in the pathogenesis of the disease remains to be elucidated.
Keywords: endometriosis, cell fusion, bone-marrow-derived cells
Cells of the endometriotic lesion fuse with cells of the host animal; bone-marrow-derived cells recruited to the endometriosis lesion contribute to this process.
Introduction
Cell fusion is a highly controlled process critical for mammalian development. It occurs as early as the merging of gametes during sexual reproduction and is implicated in the formation of diverse tissues and organs, including muscle fibers (myoblast fusion), bone (macrophage fusion), eye lens (fiber cell fusion), liver (hepatocyte fusion), and placenta [1, 2]. The latter process involves formation of a multinucleated syncytiotrophoblast layer through which most of the materno-fetal exchanges take place [3], and is catalyzed by syncytin—a proviral protein encoded by the human endogenous retrovirus envelope gene (HERV-W, rev. [4]). Cell fusion also occurs in postdevelopmental homeostatic processes of wound repair, tissue regeneration, and the inflammatory response. Whereas some of these processes require phagocyte fusion to form osteoclasts or giant cells, others involve fusion of stem cells with cells in the damaged tissue [5]. Cell fusion was also suggested to contribute to the pathogenesis of some cancers [6, 7], and the phenomenon has been shown to occur in bone marrow transplant (BMT) recipients [8, 9].
Endometriosis is a chronic, recurrent, and progressive disease characterized by the presence and growth of functional endometrial tissue, glands, and stroma outside of the uterine cavity. Its manifestations, including acute or chronic pelvic pain and infertility, occur in approximately 10% of reproductive age women (rev. [10]). Although it is a benign condition, it has molecular and cellular features in common with malignancy, including increased cell proliferation, re-expression of the pluripotent transcription factor OCT4 [11], epithelial-to-mesenchymal transition [12], acquisition of migratory phenotype [11, 13], development of distant foci, cell adhesion, invasion (rev. [14]), angiogenesis, and at times, treatment resistance [15]. These features raise the possibility that cell fusion may also be a shared phenomenon.
In support of this, the fusagen syncytin, which is highly expressed only in placental trophoblasts and in some cancers (including endometrial carcinoma [16, 17]), is upregulated in endometriosis lesions. Specifically, syncytin-1 expression is absent in the eutopic endometrium of endometriosis patients (concomitant with hypermethylation of the LTR promoter region of the gene), whereas in ectopic endometriotic lesions, syncytin-1 mRNA and protein are detected, concurrent with hypomethylation of the LTR region [18]. Furthermore, our group demonstrated homing of bone-marrow-derived cells (BMDCs) to the endometrium and endometriosis lesions [19–22]. Bone-marrow-derived cells were shown to fuse with cells in various organs they are recruited to, including cardiomyocytes, hepatocytes, intestinal cells, and Purkinje neurons. [8, 9, 23–32]. However, it is unknown whether fusion occurs in endometriosis and whether BMDCs may contribute to this process.
To investigate whether cell fusion can occur in endometriosis, we used an established mouse model of the disease together with a Cre-Lox system that enables detection of fusion events upon access of Cre recombinase to a floxed STOP cassette upstream of an enhanced green fluorescent protein (EGFP) reporter of the fusion partner. We utilized two experimental models; the first involved suturing of uterine explants from loxP-STOP-loxP-EGFP donors into the peritoneum of Cre recipients. The second employed nongonadotoxic BMT from loxP-STOP-loxP-EGFP donors into WT recipients, followed by endometriosis induction (EI) using Cre donor uterine explants, to explore whether cell fusion in the endometriosis lesion can originate from BM cells.
Methods
Animals
Mature (7–8 weeks old) C57BL/6J (wild type) and homozygous Ai6(RCL-ZsGreen) were purchased from Jackson Laboratories. Ai6 contains a targeted mutation of the Gt(ROSA)26Sor locus with a loxP-STOP-loxP-ZsGreen1 cassette, preventing transcription of EGFP. ZsGreen1 is expressed following Cre-mediated recombination. Mature β-actin-cre mice (expressing Cre recombinase directed by the human beta actin gene promoter) were obtained from the Yale Genome Editing Center. The following primers were used to screen for mice carrying the Cre transgene: 5΄-GCG GTC TGG CAG TAA AAA CTA TC-3΄, 5΄-GTG AAA CAG CAT TGC TGT CAC TT-3΄ (transgene forward and reverse, respectively), 5΄-CTA GGC CAC AGA ATT GAA AGA TCT-3΄, 5΄-GTA GGT GGA AAT TCT AGC ATC ATC C-3΄ (internal control forward and reverse, respectively). Both Ai6(RCL-ZsGreen) and β-actin-cre are syngenic with C57BL/6J.
Mice were maintained in environmentally controlled facilities in the Animal Facility at Yale School of Medicine in a room with a 12-h light, 12-h dark cycle (7 am to 7 pm) with ad libitum access to food and water. All animal procedures were performed according to an approved Yale University Institutional Animal Care and Use Committee protocol.
Endometriosis induction
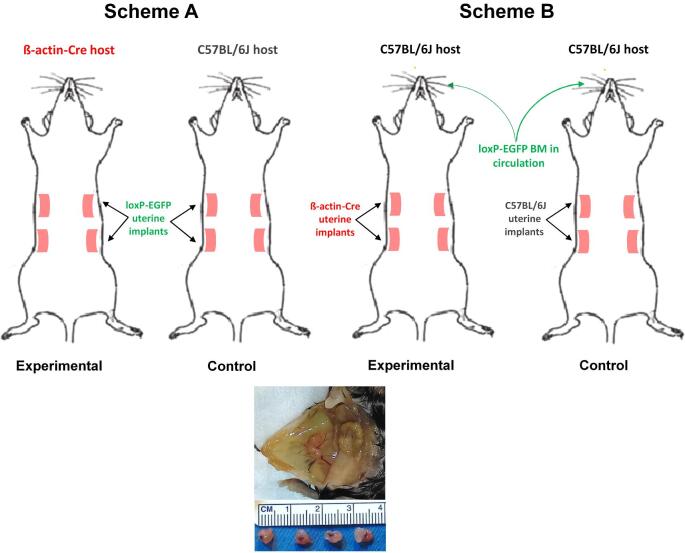
Endometriosis was induced as previously described [21] with modifications. Diestrous uteri from 7- to 8-week old cycling females were isolated, horns longitudinally dissected and the lumen exposed. Four 3-mm2 pieces were sutured onto the peritoneum (two on each side) of syngenic females, with the luminal side facing the peritoneum. 1 mg/kg meloxicam (Boehringer Ingelheim) was administered subcutaneously immediately following surgery. The diestrous stage was chosen for tissue harvesting as it is comparable to the human secretory phase preceding menstruation, and may therefore more closely resemble the endometrial cell population that is shed and deposited in the peritoneal cavity during retrograde menstruation. Females were kept cycling for 4 weeks by changing the cages’ bedding three times per week with fresh male bedding, and estrous cyclicity was verified by vaginal cytology. At 4 weeks postsurgery, endometriosis-like mouse lesions demonstrating increase in size and cyst formation (Figure 1, bottom panel) were isolated and analyzed by flow cytometry, immunohistochemistry (IHC), and immunofluorescence (IF).
Figure 1.
Experimental design examining the occurrence of cell fusion in a mouse model of endometriosis. (A) A schematic of the model used to assess fusion between cells of the endometriosis-like lesion and cells of the host. (B) A schematic of the model used to assess fusion between cells of the endometriosis-like lesion and circulating BMDCs recruited to the lesion. Hosts in this model underwent BMT 5 weeks prior to EI. Bottom: Development of mouse endometriosis lesions in vivo. Two adjacent vascularized endometriosis-like lesions (labeled with white arrows) forming ∼4–5 mm cyst-like structures in the peritoneum of female mice that underwent EI 4 weeks earlier.
Bone marrow transplant
Nongonadotoxic BMT was performed as described [33]. In brief, 125 mg/kg 5-fluorouracil (5-FU) was filtered and injected intraperitoneally on days 6 and 1 prior to BMT, and 75 μg/kg stem cell factor was injected intraperitoneally at 21 and 9 h before the second 5-FU dose. Donor whole BM was obtained by flushing the femur and tibia of 8–10 weeks old ZsGreen1 male mice with cold sterile Dulbecco modified eagle medium: nutrient mixture F-12 (DMEM-F12, Gibco, Thermo Fisher Scientific). The cell suspension was filtered through a 70-μm sterile nylon mesh cell strainer (BD Biosciences), and 3 × 107 unfractionated BMCs in 100 μL PBS were injected retro-orbitally into 8–9 weeks old female C57BL/6J recipients.
Mouse models of cell fusion in endometriosis
To study cell fusion in endometriosis, we employed two models. The first examines cell fusion between uterine cells of the endometriosis lesion and cells of the host. For this whole body fusion model, uteri from ZsGreen1 homozygous females were sutured in the peritoneal cavity of transgenic Cre females (Figure 1, scheme A). In this model, only fused cells should express GFP. Control animals had a similar configuration, with the exception that the host for the ZsGreen1-uterine implants was a wild-type C57BL/6J female mouse. This controlled for potential leaky EGFP expression in the mouse lesion from the ZsGreen1 locus.
The second model tested specifically for cell fusion occurring between cells of the endometriosis lesion and circulating BMDCs. In this BMDC fusion model, whole BM was isolated from ZsGreen1 homozygous males and transplanted into age-matched cycling C57BL/6J female recipients that underwent nongonadotoxic submyeloablation. This protocol was chosen over whole body irradiation as it was shown to preserve ovarian function, estrus cyclicity, and fertility [33]. Since development of endometriosis-like lesions is estrogen-dependent, nongonadotoxic submyeloablation allowed for more physiological modeling of the contribution of BM by avoiding the requirement of exogenous estrogen to support lesion growth. Five weeks post-BMT (allowing adequate time for recovery), EI was performed as before, with the donor uterus derived from cycling age-matched Cre females (Figure 1, scheme B). Control animals were wild-type, received BMT from ZsGreen1 males, and were subsequently the recipients of uterine implants derived from a wild-type mouse. This design controlled for potential leaky EGFP expression originating in the BM.
Flow cytometry
Flow cytometry of mouse endometriosis lesions was performed as previously described [33]. In brief, mouse endometriosis lesions were finely minced and digested in a solution of Hanks balanced salt solution containing 25 mM HEPES (Life Technologies), 1 mg/mL collagenase B (Roche Diagnostics), and 0.1 mg/mL deoxyribonuclease I (Sigma-Aldrich) for 45 min at 37°C with periodic pipetting. Samples were filtered using 70-μm mesh, centrifuged at 2000 rpm at 4°C for 8 min and resuspended in PBS. After a washing step, flow cytometry was performed on FACS MoFlo (Beckman Coulter). Gates were applied to forward-scatter/side-scatter dot plots to exclude nonviable cells and cell debris. Data were analyzed using FlowJo V10.
Immunostaining
Mouse endometriosis lesions were fixed in 4% paraformaldehyde, paraffin-embedded, and cut into 5-μm thin sections. Antigen retrieval was accomplished by boiling in sodium citrate (pH 6) for 10 min. For IHC, blocking was performed by incubating sections in PBS containing 0.3% Triton X-100 (Sigma) and 5% normal rabbit serum at room temperature for 30 min. Sections were then incubated with goat anti-GFP antibody (1 μg/mL: Abcam (ab6673) overnight at 4°C). Secondary antibody and detection reagents were supplied by the Vectastain Elite ABC HRP kit (Peroxidase, goat IgG) and ImmPACT DAB (Vector Laboratories), and carried out according to manufacturer's instructions. Tissue sections were counterstained with hematoxylin (Sigma-Aldrich). Images of stained sections were captured using Nikon Eclipse 80i microscope (Nikon).
For IF, blocking was performed with 10% donkey serum (Vector Laboratories) for 1 h. Sections were then incubated with the following primary antibodies (Abcam) at 4°C overnight: goat anti-GFP antibody (2.5 μg/mL; ab6673), rat anti-CD45 (2.5 μg/mL; ab25386), rabbit anti-CD31 (4.5 μg/mL; ab28634), rabbit anti-vimentin (0.6 μg/mL; ab92547), rabbit anti-pan cytokeratin (2.5 μg/mL; ab9377), rabbit anti-PCNA (2.5 μg/mL; ab18197), rabbit anti-Sca-1 (0.5 μg/mL; ab109211), rabbit anti-CD29 (0.6 μg/mL; ab179471). The secondary antibodies (ThermoFisher Scientific): Alexa Fluor 568-conjugated donkey anti-goat (A-11057), Alexa Fluor 488-conjugated donkey anti-rabbit (A-21206), or Alexa Fluor 488-conjugated donkey anti-rat (A-21208), were all used at a concentration of 10 μg/mL. Sections were DAPI stained and cover-slipped using Vectashield fluorescent mounting media with DAPI (Vector Laboratories). Sections were imaged using laser scanning confocal microscope (LSM 710; Zeiss) and captured using ZEN software (Carl Zeiss).
Quantification of GFP+ cells in lesions and statistical analysis
The frequency of fusion-derived (GFP+) cells in immunohistochemical sections was assessed by counting cells in three high power (20×) fields imaged using NIS-Elements D 3.1 software. At least 3000 cells were counted in each uterine section, and three sections were counted per mouse uterus. Uteri from a total of 10 mice were analyzed by cell counting. Data were analyzed using GraphPad Prism 6 software (GraphPad Software). An unpaired t-test was used to compare cell fusion frequency in the whole and BMT models.
Results
Cell fusion between cells of the endometriosis lesion and host cells is a rare event restricted to the stromal compartment of the lesion
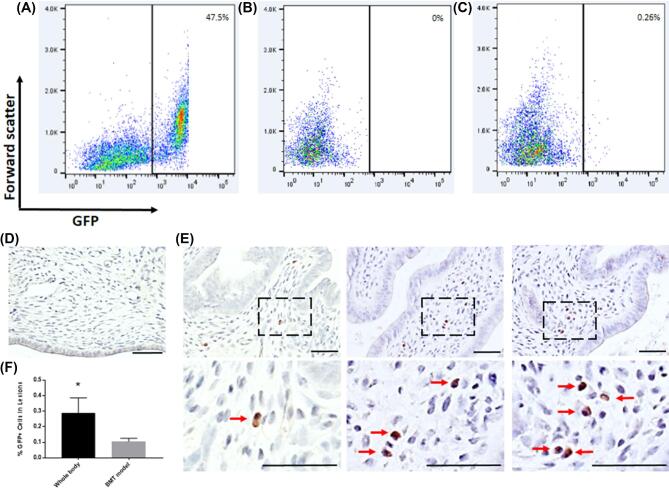
To quantitate cell fusion between endometrial cells of the endometriosis-like lesion and host cells from the whole body model, mouse endometriosis lesions were subjected to flow cytometry. The incidence of GFP+ events was approximately 2.5 in 1000, with a mean of 0.26% ± 0.07% (n = 10 lesions) (Figures 2A–C). These GFP+ events represent cells arising from fusion between cells of the ZsGreen1-derived uterine implant and cells in the β-actin-cre host. IHC using GFP antibody demonstrated GFP-positive fused cells in the β-actin-cre host but not in wild-type control host mice (Figures 2D and E). IHC further revealed that GFP+ cells localized specifically to the stromal compartment of the mouse lesion and appeared invariably mononucleated (Figure 2E). Quantification of the GFP+ cells in the endometriosis tissue by cell counting showed that the frequency of GFP+ cells was 0.29% ± 0.09% within the lesion (n = 6) (Figure 2F, left column), consistent with our flow cytometry data.
Figure 2.
Cell fusion in endometriosis-like lesions in the whole body model. (A–C) Flow cytometric analysis of GFP+ cells (A) in the uterus of a female offspring resulting from the cross β-actin-Cre X ZsGreen1 (positive control), (B) in ZsGreen1 lesions implanted within a wild-type mouse (negative control) at 4 weeks post EI, and in (C) ZsGreen1 lesions implanted within β-actin-Cre mouse at 4 weeks post EI. n = 10 mice in each group. (D) Representative image of GFP immunostaining in control endometriosis lesions at 4 weeks post EI. (E) Representative images of GFP immunostaining in experimental lesions showing GFP+ cells (brown) at 4 weeks post EI. Bottom panel: higher magnification images of dashed areas. Red arrows point to fusion-derived cells. n = 6–12 mice in each group. *P < 0.02. Scale bar = 50 μm. (F) Quantification of GFP+ cells in mouse lesions in immunohistochemical sections from the whole body model (left column) and BMT model (right column). n = 5 mice in each group.
BM-derived cells participate in fusion events within endometriosis lesions
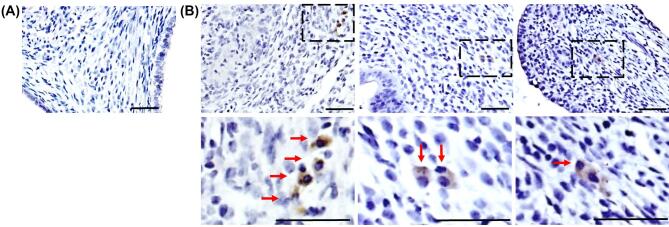
Our published observations of BMDCs homing to endometriosis lesions following BMT [19–21] led us to investigate whether BM cells recruited to the lesion can contribute to the observed cell fusion events. BMT was performed as described above. In this model, GFP+ mononucleated fusion-derived cells were again detected in the stromal compartment of the endometriosis-like lesion 4 weeks following EI (Figure 3B), albeit at about one-third the frequency observed in the whole body model (mean 0.11% ± 0.02%, n = 12, P = 0.02) (Figure 2F, right column). Fusion-derived cells in this model were also clustered more closely together, at times appearing binucleated by IHC (Figure 3B, right image). No GFP+ cells were detected in control mice (Figure 3A).
Figure 3.
Cell fusion in endometriosis-like lesions in the BMT model. IHC was performed using GFP antibody to detect fused cells. (A) Representative image of GFP immunostaining in control endometriosis lesions (Scheme B) at 4 weeks post EI. (B) Representative images of GFP staining in experimental lesions, showing GFP+ cells (brown) at 4 weeks post EI. Bottom panel: higher magnification images of the dashed areas. Red arrows point to fusion-derived cells. Scale bar = 50 μm. n = 12.
Phenotype of fusion-derived cells in the endometriosis-like lesion
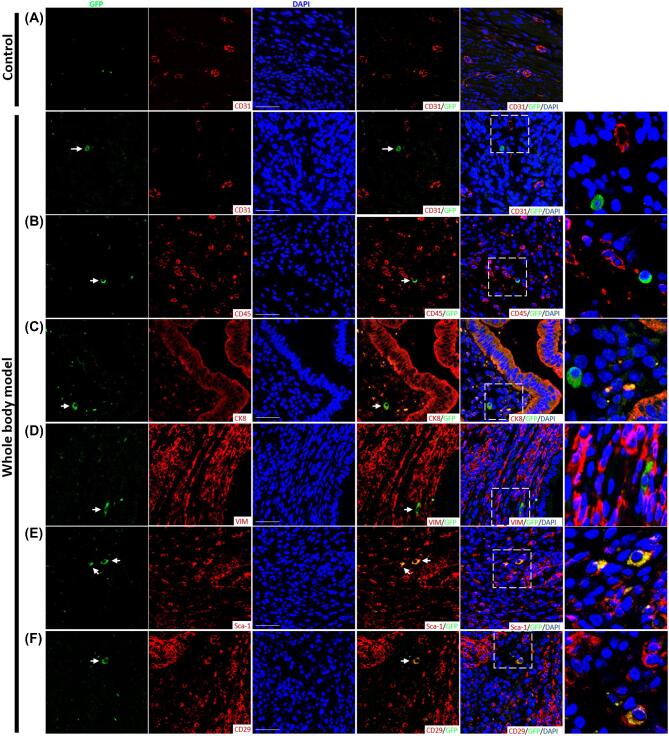
We next sought to characterize fused cells using selected markers commonly found in endometriosis-like lesions. In both whole animal and BMT fusion models, CD45 (pan-hematopoietic), CD31 (endothelial), cytokeratin (epithelial), and vimentin (stromal) stainings were uniformly absent in fusion-derived cells in all sections/lesions examined. GFP+ cells were consistently positive for both mesenchymal/stromal markers Sca-1 and CD29. Figure 4 shows representative images from the whole animal fusion model.
Figure 4.
Characterization of fusion-derived cells in mouse endometriosis lesions. Single-stain and merged immunofluorescent photomicrographs of endometriosis lesions 4 weeks post EI. Sections from control and experimental lesions from the whole body fusion model are shown. Costaining of GFP-positive fusion-derived cells (green) with (A) CD31, (B) CD45, (C) CK8, (D) Vimentin, (E) Sca-1, and (F) CD29 (red). Sections were counterstained with DAPI showing nuclei (blue). Arrowheads point to GFP+ fusion-derived cells. Right column: higher magnification images of dashed areas. Scale bar = 50 μm. n = 12.
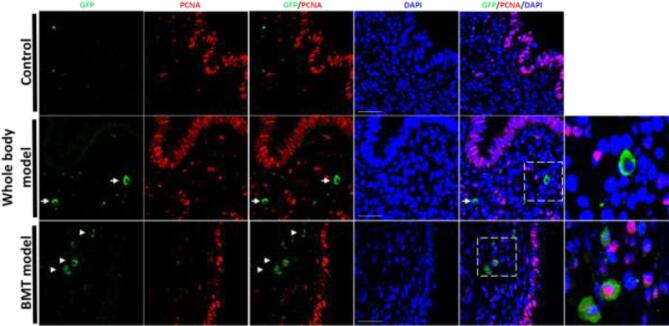
To study the proliferative nature of the fused cells, we used PCNA as a marker of proliferation. In the whole animal fusion model, none of the fusion-derived cells expressed the proliferation marker PCNA (Figure 5), though in the BMT model these cells were PCNA positive when they were in very close proximity (Figure 5, bottom panel), potentially representing earlier stages of the physical separation of the original fused cell.
Figure 5.
Proliferation status of fusion-derived cells in mouse endometriosis lesions. PCNA immunostaining of endometriosis lesion sections from control (upper panel), whole body model (middle panel), and BMT model (lower panel). IF photomicrographs demonstrate costaining of GFP-positive fusion-derived cells (green) with PCNA (red). Sections were counterstained with DAPI showing nuclei (blue). White arrows point to GFP-positive cells. Right column: higher magnification of the dashed areas showing GFP-positive cells. Scale bar = 50 μm. n = 12.
Discussion
We describe herein a novel phenomenon in a mouse model of endometriosis, namely, cell fusion. The relatively rare frequency of the fusion events we observed is consistent with that reported for fusion between BMDCs and diseased pneumocytes [32] and for fusion between BMDCs and noninjured hepatocytes [34] in BM-transplanted mice. We show that fusion occurs between endometriosis lesion cells and host cells, some of which are of BM origin.
In this study, we used two models to investigate the occurrence of cell fusion in endometriosis: a whole body model and a BMT model. In both models, we employed a genetic Cre-Lox approach in which Cre-mediated recombination only occurs in hybrid cells, thus minimizing the risk of false positives. Still, some limitations of the system may mask expression of the gene encoded by ZsGreen1, including inadequate Cre expression, inaccessibility of the transgene to Cre recombinase, and loss of genomic DNA containing the transgene in the process of fusion [34]. These inherent limitations may underestimate fusion rates. Importantly, both models used in this study had intact ovarian function. For the BMT model, we used our previously described 5-FU-based nongonadotoxic submyeloablation [33] in order to preserve ovarian function and estrus cyclicity, as estrogen is well-known to be a driving force of endometriosis.
The models we employed to study cell fusion between cells originating in the BM and transplanted tissue demonstrate that BMDCs recruited to the endometriosis lesion can fuse with uterine cells as they do in processes of tissue regeneration and cancer [8, 9, 24, 26, 28, 29]. As 50% BM chimerism is typical in the submyoablation protocol we employed [33], the adjusted number of fused cells detected in the BM transplant model is similar to the number of cells obtained in the whole animal Cre host model. This suggests that the BMDCs are likely the principal contributors to fusion events in this endometriosis model, identifying a novel mechanism by which BMDCs potentially contribute to this disease.
Multinucleated hybrids are often chromosomally unstable and eliminate supernumerary nuclei by extrusion (i.e., nuclear shedding) or reductive division [23, 35]. This may explain the difficulty to detect numerous multinucleated GFP+ cells. Furthermore, the limited proliferation evident in fusion-derived cells points to a quiescent state. Preferential proliferation of the surrounding stroma may effectively disperse these cells in lesion space. Limited proliferation was previously attributed to endometrial stem cells [36, 37]. In ovarian carcinoma, fusion-derived quiescent cells were suggested to play a role in maintaining stem-cell-like phenotypes [13]. We were limited in our capacity to expand fused cells in vitro due to their scarcity and restricted proliferation, precluding a more comprehensive phenotyping such as evaluation of the stem cell potential of fused cells.
The fusion-derived cells detected in the lesions were negative for pan-hematopoietic (CD45), endothelial (CD31), epithelial (CK8), and stromal (vimentin) markers. These cells were, however, positive for Sca-1 and CD29, which are known to be expressed on mesenchymal/stromal cells [38, 39]. Taken together, this suggests that fusion occurs between host/BM-derived nonhematopoietic/nonendothelial cells and a resident endometrial mesenchymal/stromal/stem cell in the implanted uterine tissue. Alternatively, as loss of expression of hematopoietic markers was often reported in various adult cell types in mouse studies employing BMT [24, 26, 28, 29], fusion with BMDCs in our endometriosis model may similarly be associated with epigenetic reprogramming that silences genes originally expressed in the fusion donors. The lack of vimentin staining in fusion-derived cells in our model is interesting in light of the reduced vimentin staining reported in ectopic endometrium of women with endometriosis, attributed to lower degree of cell differentiation [40].
Endometriosis is frequently resistant to standard progestin-based therapies and ovarian endometriosis is known to be associated with development of malignancy [41–43]. Additionally, the reduced proliferation rate of cancer stem cells was proposed to confer drug resistance and to result from stem cell fusion [44]. The fused cells we observed in endometriosis lesions in our experimental model may be related to the pathogenesis of both these phenomena: fusion-derived cells may represent quiescent cells with the potential to become treatment resistant or malignant under selective pressure or in the presence of mutations in kras and p53 [45]. However, these possibilities were not explored in the current study and some limitations of our study prevent extrapolating into the causative role of fusion events in endometriosis. These limitations include their low numbers and proliferative potential, as well as the lack of markers usually found in lesions. Moreover, the occurrence of cell fusion was not yet investigated in human lesions, and the evidence gathered so far is circumstantial (i.e., expression of syncytin-1). With the advent of mouse models of endometriosis progression, the fate and role of cells derived from cell fusion events in the pathogenesis of this disease may be further investigated.
In conclusion, we describe the phenomenon of cell fusion occurring in an experimental mouse model of endometriosis. Our data point to a mesenchymal population derived from cell fusion events with limited proliferative activity in lesions, and that many of these cells may originate in the bone marrow. Their putative role in the pathogenesis of the disease remains to be elucidated.
Supplementary Material
Notes
Edited by Dr. Romana Nowak, PhD, University of Illinois Urbana-Champaign
Footnotes
Grant Support: Institutional support NIH R01 HD076422.
Author contributions statement
A.T. conceived and designed the study, performed the experiments, analyzed the data, and drafted the manuscript. R.T. helped perform experiments, analyze the data, and revise the manuscript. S.S. and S.G. helped perform the experiments. R.M. helped perform the experiments and revise the final manuscript. H.S.T. contributed to study design and revised the final manuscript.
Conflict of Interest: The authors have declared that no conflict of interest exists.
References
- 1. Aguilar PS, Baylies MK, Fleissner A, Helming L, Inoue N, Podbilewicz B, Wang H, Wong M. Genetic basis of cell-cell fusion mechanisms. Trends Genet 2013; 29:427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Duncan AW, Taylor MH, Hickey RD, Hanlon Newell AE, Lenzi ML, Olson SB, Finegold MJ, Grompe M. The ploidy conveyor of mature hepatocytes as a source of genetic variation. Nature 2010; 467:707–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holder BS, Tower CL, Abrahams VM, Aplin JD. Syncytin 1 in the human placenta. Placenta 2012; 33:460–466. [DOI] [PubMed] [Google Scholar]
- 4. Shemer G, Podbilewicz B. Fusomorphogenesis: cell fusion in organ formation. Dev Dyn 2000; 218:30–51. [DOI] [PubMed] [Google Scholar]
- 5. Alvarez-Dolado M, Martinez-Losa M. Cell fusion and tissue regeneration. Adv Exp Med Biol 2011; 713:161–175. [DOI] [PubMed] [Google Scholar]
- 6. Platt JL, Zhou X, Lefferts AR, Cascalho M. Cell fusion in the war on cancer: a perspective on the inception of malignancy. Int J Mol Sci 2016; 17:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duelli D, Lazebnik Y. Cell fusion: a hidden enemy? Cancer Cell 2003; 3:445–448. [DOI] [PubMed] [Google Scholar]
- 8. Yilmaz Y, Lazova R, Qumsiyeh M, Cooper D, Pawelek J. Donor Y chromosome in renal carcinoma cells of a female BMT recipient: visualization of putative BMT-tumor hybrids by FISH. Bone Marrow Transplant 2005; 35:1021–1024. [DOI] [PubMed] [Google Scholar]
- 9. Lazova R, Laberge GS, Duvall E, Spoelstra N, Klump V, Sznol M, Cooper D, Spritz RA, Chang JT, Pawelek JM. A melanoma brain metastasis with a donor-patient hybrid genome following bone marrow transplantation: first evidence for fusion in human cancer. PLoS One 2013; 8:e66731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greene AD, Lang SA, Kendziorski JA, Sroga-Rios JM, Herzog TJ, Burns KA. Endometriosis: where are we and where are we going? Reproduction 2016; 152:R63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Au HK, Chang JH, Wu YC, Kuo YC, Chen YH, Lee WC, Chang TS, Lan PC, Kuo HC, Lee KL, Lee MT, Tzeng CR et al. . TGF-betaI regulates cell migration through pluripotent transcription factor OCT4 in endometriosis. PLoS One 2015; 10:e0145256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bartley J, Julicher A, Hotz B, Mechsner S, Hotz H. Epithelial to mesenchymal transition (EMT) seems to be regulated differently in endometriosis and the endometrium. Arch Gynecol Obstet 2014; 289:871–881. [DOI] [PubMed] [Google Scholar]
- 13. Ramakrishnan M, Mathur SR, Mukhopadhyay A. Fusion-derived epithelial cancer cells express hematopoietic markers and contribute to stem cell and migratory phenotype in ovarian carcinoma. Cancer Res 2013; 73:5360–5370. [DOI] [PubMed] [Google Scholar]
- 14. Young VJ, Brown JK, Saunders PT, Horne AW. The role of the peritoneum in the pathogenesis of endometriosis. Hum Reprod Update 2013; 19:558–569. [DOI] [PubMed] [Google Scholar]
- 15. Cakmak H, Taylor HS. Molecular mechanisms of treatment resistance in endometriosis: the role of progesterone-hox gene interactions. Semin Reprod Med 2010; 28:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang Q, Chen H, Li J, Oliver M, Ma X, Byck D, Gao Y, Jiang SW. Epigenetic and non-epigenetic regulation of syncytin-1 expression in human placenta and cancer tissues. Cell Signal 2014; 26:648–656. [DOI] [PubMed] [Google Scholar]
- 17. Strick R, Ackermann S, Langbein M, Swiatek J, Schubert SW, Hashemolhosseini S, Koscheck T, Fasching PA, Schild RL, Beckmann MW, Strissel PL. Proliferation and cell-cell fusion of endometrial carcinoma are induced by the human endogenous retroviral Syncytin-1 and regulated by TGF-beta. J Mol Med (Berl) 2007; 85:23–38. [DOI] [PubMed] [Google Scholar]
- 18. Zhou H, Li J, Podratz KC, Tipton T, Marzolf S, Chen HB, Jiang SW. Hypomethylation and activation of syncytin-1 gene in endometriotic tissue. Curr Pharm Des 2014; 20:1786–1795. [DOI] [PubMed] [Google Scholar]
- 19. Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells 2007; 25:2082–2086. [DOI] [PubMed] [Google Scholar]
- 20. Sakr S, Naqvi H, Komm B, Taylor HS. Endometriosis impairs bone marrow-derived stem cell recruitment to the uterus whereas bazedoxifene treatment leads to endometriosis regression and improved uterine stem cell engraftment. Endocrinology 2014; 155:1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ersoy GS, Zolbin MM, Cosar E, Mamillapalli R, Taylor HS. Medical therapies for endometriosis differentially inhibit stem cell recruitment. Reprod Sci 2017; 24:818–823. [DOI] [PubMed] [Google Scholar]
- 22. Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA 2004; 292:81–85. [DOI] [PubMed] [Google Scholar]
- 23. Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 2003; 422:897–901. [DOI] [PubMed] [Google Scholar]
- 24. Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature 2003; 422:901–904. [DOI] [PubMed] [Google Scholar]
- 25. Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 2003; 425:968–973. [DOI] [PubMed] [Google Scholar]
- 26. Nygren JM, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med 2004; 10:494–501. [DOI] [PubMed] [Google Scholar]
- 27. Willenbring H, Bailey AS, Foster M, Akkari Y, Dorrell C, Olson S, Finegold M, Fleming WH, Grompe M. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nat Med 2004; 10:744–748. [DOI] [PubMed] [Google Scholar]
- 28. Rizvi AZ, Swain JR, Davies PS, Bailey AS, Decker AD, Willenbring H, Grompe M, Fleming WH, Wong MH. Bone marrow-derived cells fuse with normal and transformed intestinal stem cells. Proc Natl Acad Sci USA 2006; 103:6321–6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johansson CB, Youssef S, Koleckar K, Holbrook C, Doyonnas R, Corbel SY, Steinman L, Rossi FM, Blau HM. Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat Cell Biol 2008; 10:575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davies PS, Powell AE, Swain JR, Wong MH. Inflammation and proliferation act together to mediate intestinal cell fusion. PLoS One 2009; 4:e6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weimann JM, Johansson CB, Trejo A, Blau HM. Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat Cell Biol 2003; 5:959–966. [DOI] [PubMed] [Google Scholar]
- 32. Herzog EL, Van Arnam J, Hu B, Zhang J, Chen Q, Haberman AM, Krause DS. Lung-specific nuclear reprogramming is accompanied by heterokaryon formation and Y chromosome loss following bone marrow transplantation and secondary inflammation. FASEB J 2007; 21:2592–2601. [DOI] [PubMed] [Google Scholar]
- 33. Tal R, Liu Y, Pluchino N, Shaikh S, Mamillapalli R, Taylor HS. A murine 5-fluorouracil-based submyeloablation model for the study of bone marrow-derived cell trafficking in reproduction. Endocrinology 2016; 157:3749–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS, Krause DS. Lack of a fusion requirement for development of bone marrow-derived epithelia. Science 2004; 305:90–93. [DOI] [PubMed] [Google Scholar]
- 35. Ogle BM, Cascalho M, Platt JL. Biological implications of cell fusion. Nat Rev Mol Cell Biol 2005; 6:567–575. [DOI] [PubMed] [Google Scholar]
- 36. Cervello I, Martinez-Conejero JA, Horcajadas JA, Pellicer A, Simon C. Identification, characterization and co-localization of label-retaining cell population in mouse endometrium with typical undifferentiated markers. Hum Reprod 2007; 22:45–51. [DOI] [PubMed] [Google Scholar]
- 37. Wang Y, Sacchetti A, van Dijk MR, van der Zee M, van der Horst PH, Joosten R, Burger CW, Grootegoed JA, Blok LJ, Fodde R. Identification of quiescent, stem-like cells in the distal female reproductive tract. PLoS One 2012; 7:e40691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gunjal P, Bhartiya D, Metkari S, Manjramkar D, Patel H. Very small embryonic-like stem cells are the elusive mouse endometrial stem cells–a pilot study. J Ovarian Res 2015; 8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Indumathi S, Harikrishnan R, Rajkumar JS, Sudarsanam D, Dhanasekaran M. Prospective biomarkers of stem cells of human endometrium and fallopian tube compared with bone marrow. Cell Tissue Res 2013; 352:537–549. [DOI] [PubMed] [Google Scholar]
- 40. Nisolle M, Casanas-Roux F, Donnez J. Coexpression of cytokeratin and vimentin in eutopic endometrium and endometriosis throughout the menstrual cycle: evaluation by a computerized method. Fertil Steril 1995; 64:69–75. [PubMed] [Google Scholar]
- 41. Thomsen LH, Schnack TH, Buchardi K, Hummelshoj L, Missmer SA, Forman A, Blaakaer J. Risk factors of epithelial ovarian carcinomas among women with endometriosis: a systematic review. Acta Obstet Gynecol Scand 2017; 96:761–778. [DOI] [PubMed] [Google Scholar]
- 42. Anglesio MS, Bashashati A, Wang YK, Senz J, Ha G, Yang W, Aniba MR, Prentice LM, Farahani H, Li Chang H, Karnezis AN, Marra MA et al. . Multifocal endometriotic lesions associated with cancer are clonal and carry a high mutation burden. J Pathol 2015; 236:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand 2017; 96:623–632. [DOI] [PubMed] [Google Scholar]
- 44. Lu X, Kang Y. Cell fusion hypothesis of the cancer stem cell. Adv Exp Med Biol 2011; 714:129–140. [DOI] [PubMed] [Google Scholar]
- 45. Tang FH, Hsieh TH, Hsu CY, Lin HY, Long CY, Cheng KH, Tsai EM. KRAS mutation coupled with p53 loss is sufficient to induce ovarian carcinosarcomas in mice. Int J Cancer 2017; 140:1860–1869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.