Abstract
Acupuncture can provide therapeutic analgesic benefits but is limited by its cost and scheduling difficulties. Guided imagery is a commonly used method for treating many disorders, such as chronic pain. The present study examined a novel intervention for pain relief that integrates acupuncture with imagery called video-guided acupuncture imagery treatment (VGAIT). A total of 27 healthy subjects were recruited for a crossover-design study that included 5 sessions administered in a randomized order (i.e., baseline and 4 different interventions). We investigated changes in pain threshold and fMRI signals modulated by: 1) VGAIT, watching a video of acupuncture previously administered on the participant’s own body at baseline while imagining it being concurrently applied; 2) a VGAIT control condition, watching a video of a cotton swab touching the skin; 3) real acupuncture; and 4) sham acupuncture. Results demonstrated that real acupuncture and VGAIT significantly increased pain threshold compared with respective control groups. Imaging showed that real acupuncture produced greater activation of the insula compared with VGAIT. VGAIT produced greater deactivation at the rostral anterior cingulate cortex. Our findings demonstrate that VGAIT holds potential clinical value for pain management.
Keywords: acupuncture analgesia, chronic pain, fMRI, imagery, video-guided acupuncture imagery treatment
Introduction
Pain is a multidimensional experience associated with real or potential tissue damage (Loeser and Treede 2008). Substantial effort has been invested in the search for effective pain relievers. Unfortunately, available treatments are often unsatisfactory. Opioids are the most commonly prescribed class of drugs for relieving pain (Hudson et al. 2008; Ivanova et al. 2011). However, the addictive potential of opioids has increased their misuse and created a serious national crisis that affects public heath as well as social and economic welfare (Compton and Volkow 2006). There is a clear and urgent need for the development of new pain relief methods.
Guided imagery is a commonly used method for treating many disorders, such as chronic pain (Han 2011; Dasilva et al. 2012; Naylor et al. 2014) and stroke (Garcia-Larrea and Peyron 2007; Zhao 2008). Although its underlying mechanism of action remains unclear, research suggests that the brain responds to imagined experiences in a similar way to actual experiences (Kosslyn et al. 2001; Singer 2004; Ogino et al. 2007; Ochsner et al. 2008; Berna et al. 2012; Meier et al. 2012; Mochizuki et al. 2013; Christian et al. 2015). For example, the visualization of others experiencing pain can activate brain networks similar to those activated when one directly experiences pain, including the anterior insula, anterior cingulate cortex (ACC)/medial prefrontal cortex (mPFC), and secondary somatosensory cortex (S2) (Jackson et al. 2006; Singer et al. 2009; Lamm et al. 2011; Rütgen et al. 2015; Murphy et al. 2017).
Acupuncture is an invasive, nonpharmacological intervention characterized by the insertion and manipulation of needles at specific body sites. Its potential as a pain analgesic has been widely studied (Zhao 2008). Acupuncture has been found to induce the release of endogenous opioids in the brain stem, subcortical, and limbic structures (Pomeranz 1996; Han 2003; Dougherty et al. 2008). Neuroimaging studies of acupuncture stimulation in humans have shown immediate effects in limbic and basal forebrain areas related to somatosensory and affective functions that are involved in pain processing (Dhond et al. 2007). In particular, studies have shown that acupuncture needle stimulation (Kong et al. 2002; Kong, Gollub, Webb, et al. 2007; Huang et al. 2012; Chae et al. 2013) and the visualization of acupuncture needle stimulation (Cheng et al. 2007) can provoke overlapping activation of particular brain regions, including the insula, middle cingulate cortex (MCC), dorsal ACC (dACC), and periaqueductal gray (PAG). Thus, imagined acupuncture may activate brain regions that overlap with those activated by real acupuncture and may provide similar therapeutic benefits to real acupuncture without the associated cost and inconvenience.
The aim of the present study was to comparatively investigate the analgesic effects of acupuncture and video-guided acupuncture imagery treatment (VGAIT) as measured by pain threshold and underlying brain mechanisms using functional magnetic resonance imaging (fMRI). Specifically, acupuncture-naïve subjects were recruited and randomized to different treatment groups: 1) VGAIT, that is, watching a video of acupuncture previously administered on their own body while imagining it being concurrently applied; 2) a VGAIT control condition, that is, watching a video of a cotton swab touching their bodies and imagining it being concurrently applied; 3) real acupuncture, and 4) sham acupuncture (Streitberger needle; Streitberger and Kleinhenz 1998). We hypothesized that when compared with the VGAIT control and sham acupuncture, 1) both VGAIT and real acupuncture would produce analgesic effects as evidenced by pain threshold increases; 2) VGAIT and real acupuncture would induce fMRI changes in brain regions involved in pain modulation, including the insula, cingulate, and prefrontal cortices; and 3) fMRI activity in the brain regions associated with pain modulation evoked by acupuncture and VGAIT would be related to pain threshold changes following the interventions.
Material and Methods
Participants
A total of 27 healthy, right-handed, acupuncture-naïve participants were recruited for the study. Participants were asked not to change their usual daily activities for the duration of study involvement. The study was approved by the Partners Human Research Committee at Massachusetts General Hospital. All participants had normal or corrected-to-normal vision and gave written informed consent prior to participating in the study.
Of the 27 healthy subjects who participated in this study, 1 male and 2 female subjects finished the training session but did not complete the first fMRI session. Reasons for these 3 dropouts included scheduling difficulties, discomfort in the fMRI scanner, and report of a migraine before the scan. Thus, 24 participants were included in the final analysis.
Procedures
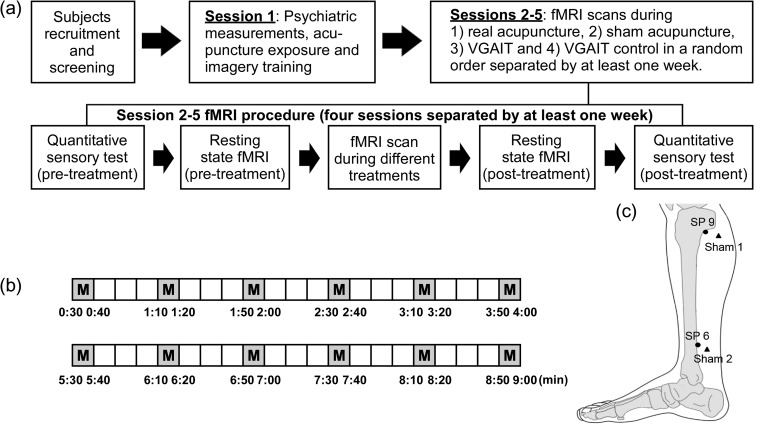
Subjects participated in 5 experimental sessions. Session 1 was a training and familiarity session designed to minimize anticipatory anxiety to acupuncture by exposing participants to acupuncture. Acupuncture in Session 1 was videotaped for use in a following session. Sessions 2–5 consisted of pain threshold assessments and fMRI recordings during which the participant received 1 of the 4 interventions: 1) VGAIT, 2) VGAIT control condition, 3) real acupuncture, or 4) sham acupuncture. Each participant received all interventions; order of interventions was randomized across participants. Each intervention session was separated by at least 7 days to avoid sensitization to the pain stimuli (Fig. 1a). During data acquisition, all study personnel, except the acupuncturist, were blinded with respect to the acupuncture intervention condition. Participants were also blinded to the acupuncture modality. At the end of the study, an investigator debriefed the participant and explained the reason for maintaining intervention blindness.
Figure 1.
Experimental procedure and manipulation points. (a) Study session timeline and description. (b) Two 9-min treatment scans were applied (M stands for needle manipulation). (c) Location of acupoints (dot) and sham points (triangle).
Session 1: Training and Testing
At the beginning of the session, we informed subjects that although acupuncture has been used to treat a number of disorders, including chronic pain, depression, and other disorders, its underlying mechanism remains unknown and this study aims to investigate the brain’s response to different interventions. We also explained that imagery of stimulation may produce sensations and brain activity changes in areas that may overlap with real stimulation. We provided this information so that the patients understood the aim and rationale of the study to facilitate their compliance and cooperation.
Subjects were told that they would receive 2 modalities of acupuncture treatment (real and sham) in random order during the following sessions. We also informed subjects that some investigators think sham acupuncture is more like a nontraditional acupuncture treatment and that they may not be able to tell the difference between the two. Further, we disclosed that many clinical trials have found no significant difference between real and sham acupuncture. To avoid the potential confounding of expectancy, we neither suggested that subjects link the pain threshold assessments with acupuncture analgesia nor told them how long the effects would last.
Participants were then trained to understand and complete the heat and pressure pain threshold assessments. Next, participants received real acupuncture treatment, which lasted approximately minutes and was videotaped for the VGAIT intervention. In addition, a cotton swab was used to repeatedly touch nonacupuncture sites located adjacent to real acupuncture points, which was videotaped for the VGAIT control condition. Finally, participants were introduced to the Massachusetts General Hospital Acupuncture Sensation Scale (MASS), which was used to report sensations experienced during the interventions. Participants were also asked to complete the State–Trait Anxiety Inventory (STAI) to measure changes in anxiety levels before acupuncture exposure in Session 1 and interventions in Sessions 2–5 (we used STAI–trait in Session 1, and STAI–state in Sessions 2–5) (Spielberger et al. 1970). We also used the Betts’ Questionnaire upon Mental Imagery (BQMI, (Sheehan 1967)) to measure imagery vividness during VGAIT.
Sessions 2–5: Administration of Treatment Interventions
Acupuncture procedures were carried out by a licensed acupuncturist. During fMRI scanning, needles were inserted and adjusted to obtain deqi before scanning began. Deqi is an acupuncture term used to describe needle sensation, which is assumed to be associated with the therapeutic benefit of acupuncture. During the course of each intervention, 2 fMRI scans, 9-min each, were performed with a 5-min break between scans during which participants completed the MASS (Kong, Gollub, Huang, et al. 2007; Spaeth et al. 2013). Subjects were told to close their eyes and focus on the sensation around the needle during acupuncture. At the onset of the real and sham acupuncture interventions and prior to the fMRI scan, we specifically asked subjects whether they could feel the needle sensations. We continued manipulation until subjects confirmed that they felt the needle sensation.
Real acupuncture procedure
Real acupuncture was applied on the right SP6 and SP9 (Fig. 1c). For each participant, leg position, acupoint location, and needling parameters (1–2 cm depth, 120 rotations/min, 90° insertion angle, moderate deqi sensations on a 0–10 scale) were kept constant. Needles were rotated at one point and then the other in 10-s rotations with 30-s breaks (Fig. 1b). The starting acupoint was randomized across participants but remained the same between the two 9-min fMRI scans.
Sham acupuncture procedure
Sham/placebo acupuncture was applied at 2 sham acupuncture points using a specially designed needle, which has a blunt and retractable tip (Streitberger and Kleinhenz 1998; White et al. 2003; Kong, Gollub, et al. 2006). Instead of penetrating the skin, the point of the Streitberger needle retracts up the handle shaft when the acupuncturist presses it against the skin. This sham needle has been validated in studies demonstrating that subjects cannot distinguish between genuine and sham needling (Streitberger and Kleinhenz 1998; Kong et al. 2005). Two sham points were used during placebo acupuncture: sham point 1, which is located about 1 cun (“cun” is a traditional Chinese unit of length used to locate the acupoints; the width of a person’s thumb at the knuckle represents 1 cun) posterior to the superior 1/3 of K9 and K10; and sham point 2, which is located 1 cun posterior to K8 (Fig. 1c). Both sham points are located on the leg where there is no meridian (a pathway in the body through which Qi (life energy) flows). Otherwise, sham acupuncture treatment was applied by gently rotating the sham needle using the same procedure as for real acupuncture.
Video-guided acupuncture imagery treatment
At the beginning of the VGAIT session, participants were trained to imagine the acupuncture treatment outside of the fMRI scanner. They were given text to read that introduced the imagery acupuncture treatment as follows: “You will see a video of acupuncture treatment being applied on your leg. Please focus on the needle manipulation and try to imagine there is an actual needle being placed into your leg at the same spot. You will find that you can actually feel the needle manipulation on your leg at the same spot as in the video, as well as some soreness and an aching, dull pain along with other sensations. It is very important that you stay focused and try to imagine the sensation of receiving acupuncture as vividly as you can. After the treatment, we will provide a scale to measure the sensations you felt during the video.” Following these instructions, VGAIT was applied while the participant underwent fMRI scanning. There were 2 fMRI scans during VGAIT, each lasting 9 min, as was done during the acupuncture treatment (Fig. 1b). Once the treatment had been completed, participants assessed the sensations felt using the MASS.
VGAIT control condition
The VGAIT control condition was the same as VGAIT, except that cotton swabs were used to repeatedly touch nonacupoints and were gently rotated using the same procedure as the real and sham acupuncture treatments (Fig. 1c). Participants were told, “You will see a video of a swab touching your leg. Please focus on the cotton swab and try to imagine there is an actual swab being placed on your leg at the same spot. You will find that you can actually feel the cotton swab on your leg at the same spot as in the video. It is very important that you stay focused and try to imagine the sensation of the swab as vividly as you can. After the scan, we will provide a scale to measure the sensations you felt during the video.” Following these instructions, the VGAIT control was applied during fMRI scanning. Once the control treatment had been completed, participants reported the sensations felt using the MASS.
Brief Quantitative Sensory Testing of Responses to Thermal and Pressure Pain Stimuli
Two pain modalities were assessed using quantitative sensory testing (QST) before and after each treatment intervention. Pain threshold assessments of 2 locations (leg and arm/thumbnail) were conducted 3 times, with the thermode (heat) and algometer (pressure) repositioned between each threshold assessment. We chose 2 pain modalities because heat-evoked pain is predominantly mediated by small, nonmyelinated peripheral nociceptive nerve fibers (C-fibers), whereas pressure-evoked pain is predominantly mediated by small, myelinated peripheral nociceptive nerve fibers (A-delta fibers) (Angst et al. 2009). We tested both local and distal pain thresholds so that we could measure segmental and suprasegmental analgesic effects produced by the different interventions (Coronado et al. 2011).
Contact heat stimuli were delivered using a PATHWAY CHEPS (Contact Heat-Evoked Potential Stimulator, Medoc Advanced Medical Systems) with pain thresholds measured on the medial side of the right knee and left volar forearm. An ascending method, with a rate of increase of 0.5 °C/s from 32 °C was applied (Kong et al. 2013). A study staff member held the thermode lightly on the skin. Participants were required to press a button to indicate when the heat stimulus first became painful, thereby indicating the heat pain threshold. Pressure pain thresholds were assessed using an algometer applied at the medial side of the right knee and left thumbnail. Pressure was gradually increased at a constant rate of 1 kg/s. The participant was instructed to say “stop” to indicate when the sensation first became painful (Schabrun et al. 2014).
Questionnaires
Immediately following each intervention, participants quantified the sensations they felt around the stimulated acupoint using the MASS (Kong, Gollub, Huang, et al. 2007). After each fMRI scan, the participant rated, using the Expectations for Relief Scale (ERS) (De Pascalis et al. 2002; Wager et al. 2004; Kong, Gollub, et al. 2006), the amount of heat or pressure pain relief that was anticipated from the just-received intervention. The ERS uses a 0–10 scale, with 0 indicating a very negative expectation of “does not work at all” and 10 indicating a very positive expectation of “complete pain relief” (De Pascalis et al. 2002; Kong, Gollub, et al. 2006; Gollub et al. 2018; Kong, Wang, et al. 2018). The MASS was administered at the midpoint and end of each intervention.
fMRI Data Acquisition
Brain imaging was performed with a 3-axis gradient head coil in a 3 T Siemens MRI System equipped for echo planar imaging. A high-resolution T1-weighted structural image was acquired by an isotropic multi-echo MPRAGE pulse sequence, which was collected for anatomic localization of significant signal changes. fMRI images were acquired using a gradient echo T2*-weighted pulse sequence (time repetition [TR]/time echo [TE] = 2000/30 ms, flip angle [FA] = 90˚, field of view [FOV] = 192 × 192 mm2, 48 AC-PC aligned slices, slice thickness = 3.0 mm with 0.6 mm interslice gap, 90 image volumes per slice, matrix = 96 × 96) and a 32-channel multiarray coil. fMRI data were collected while subjects completed two 9-min fMRI scans, during which intermittent acupuncture, VGAIT, and the corresponding control conditions were applied.
Data Analysis
Analysis of Demographic and Neuropsychological Rating Data
In order to compare the treatment efficacy across the 4 treatments, the perceived pain threshold changes for each treatment were analyzed using a one-way repeated-measures analysis of variance (ANOVA). A separate ANOVA was conducted for each of the 4 pain threshold measures. When the treatment main effect was significant, post hoc (Bonferroni-corrected) pairwise comparisons were performed. Demographic and questionnaire data analyses were conducted using the r program incorporated in JASP software (Version 0.8.1, http://www.jasp-stats.org).
fMRI Data Analysis
fMRI data processing and statistical analysis were carried out using MATLAB (version 2013b; the MathWorks, Inc.; Natick, Mass) and Statistical Parametric Mapping software (SPM12; Wellcome Trust Centre for Neuroimaging, London, UK). Preprocessing included coregistration, motion correction, normalization to Montreal Neurological Institute stereotactic space, and spatial smoothing with a 6-mm, full-width-at-half-maximum Gaussian kernel.
For each participant, the contrast between needle manipulation versus no needle manipulation during acupuncture and sham acupuncture was calculated using a general linear model (GLM). The same procedure was followed for VGAIT and VGAIT control. For VGAIT, the contrast was between watching-and-imagining needle manipulation versus no manipulation. For VGAIT control, the contrast was between cotton-swab-touching versus no manipulation. Group analyses were performed using a random-effects model. A one-sample t-test was performed to compare the fMRI signal changes during manipulation versus no manipulation within each treatment. Thresholds of P < 0.005 uncorrected and P < 0.05 false discovery rate (FDR) corrected were used. We also compared brain activations between the following treatment interventions: real versus sham acupuncture, real acupuncture versus VGAIT, and VGAIT versus VGAIT control.
Multivariate Pattern Analysis
If participants exhibited different analgesic effects from the various interventions, it would be of interest to explore the relationship between the brain activations and analgesic effects across the different interventions. Because pain is related to sensory, affective, and cognitive brain systems (Price 2000; Wiech et al. 2008; Wager et al. 2013), we defined 10 brain regions based on the automated anatomical labeling (AAL) brain atlas as regions of interest (ROIs), including the ACC, MCC, posterior cingulate cortex (PCC), inferior frontal gyrus (IFG), mPFC, middle frontal gyrus (MFG), bilateral insula, bilateral postcentral gyrus (S1), supplementary motor area (SMA), and bilateral thalamus. Previous studies have found these regions to be involved in pain modulation (Bornhövd et al. 2002; Naglatzki et al. 2012; Egorova et al. 2015; Wilcox et al. 2015). We used the beta contrast estimates of all voxels (manipulation vs. no manipulation within the 10 predefined ROIs based on the AAL brain atlas) from GLM analyses as features to associate with the analgesic effects.
Because the 2 acupuncture treatments and VGAIT produced significant analgesic effects, as indicated by the pressure pain threshold change (see Results for details), we used the percentage changes of pressure pain thresholds on the leg and thumbnail as measures of analgesic effects for each subject. For all subjects, the relationship between beta contrast estimates for voxels (independent variables) and changes in pain threshold (dependent variable) was described using a multivariate linear regression (MVLR) model (Wager et al. 2011, 2013; Lindquist et al. 2017). The model was decoded using partial least square regression (PLSR) (implemented by Nonlinear Iterative Partial Least Squares [NIPALS] algorithm) since the number of voxels was much larger than the number of subjects (Tu, Tan, et al. 2016; Tu, Zhang, et al. 2016; Tu et al. 2018). The result was a spatial pattern of regression weights across all voxels within 10 ROIs, and the significance of each voxel was assessed by bootstrap testing with a threshold of voxel level uncorrected P < 0.005 (see Statistical Analysis for details) and a small volume correction (within each predefined ROI) with a threshold of cluster level P < 0.05.
We also attempted to predict analgesic effects based on brain activities evoked by acupuncture and VGAIT. Please see Supplementary Material for details of the method and results.
Statistical Analysis
To threshold and select the voxels associated with analgesic effects, we constructed 1000 bootstrap samples (with replacement) consisting of paired beta contrast estimates and changes in pain threshold, and ran PLSR decoding analysis on each. A one-sample t-test was performed for each voxel based on the proportion of weights below or above zero and was subjected to small volume correction within 10 predefined ROIs.
Results
Demographic and Neuropsychologic Rating Data
In total, 24 subjects (mean age = 25.2 years, standard error (SE) = 0.77 years, 8 males) completed the study and were included in the data analyses. The mean STAI-trait and BQMI ratings (mean ± SE) were 46.33 ± 0.72 and 81.13 ± 4.13, respectively.
Changes in Pain Threshold Between Postintervention and Preintervention
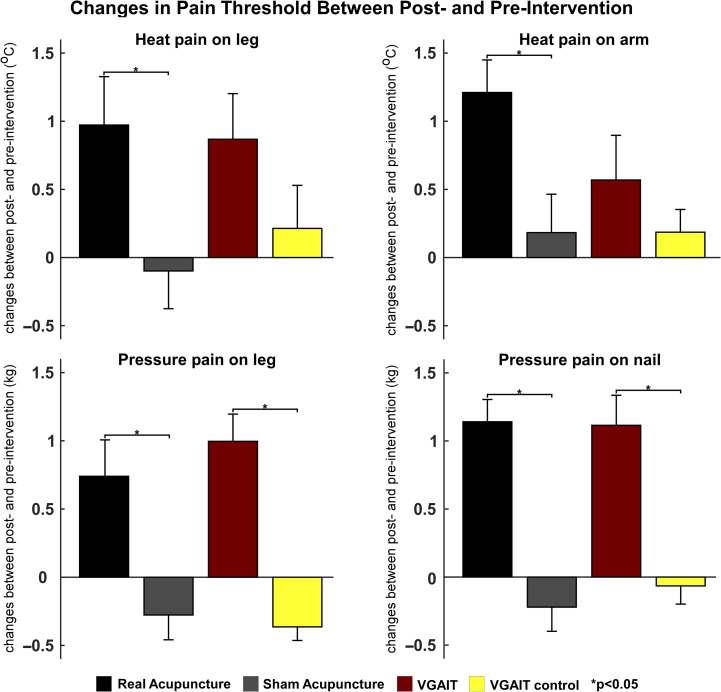
One-way repeated-measures ANOVA revealed that the heat and pressure threshold changes were significantly different among the 4 interventions (heat pain threshold changes on the leg, F(3,69) = 2.819, P = 0.045; heat pain threshold changes on the arm, F(3,69) = 3.435, P = 0.022; pressure pain threshold changes on the leg, F(3,69) = 15.07, P < 0.001; pressure pain threshold changes on the thumbnail, F(3,69) = 15.67, P < 0.001). Post hoc (Bonferroni-corrected) analyses showed a significant difference in pressure pain threshold changes between real and sham acupuncture (P = 0.003 for pressure pain on the leg and P < 0.001 for pressure pain on the thumbnail), as well as between VGAIT and VGAIT control (P < 0.001 for pressure pain on the leg and P = 0.005 for pressure pain on the thumbnail). There was no significant difference between real acupuncture and VGAIT (P > 0.05 for all 4 pain threshold changes).
Pre- and post-treatment comparisons within each group showed that Real acupuncture significantly increased subject’s pain threshold for all 4 pain threshold measures (P < 0.05). VGAIT significantly increased subject’s pain threshold for heat pain on the leg (P = 0.017), pressure pain on the leg (P < 0.001), and pressure pain on the thumbnail (P < 0.001). Sham acupuncture and VGAIT control conditions did not significantly modulate subjects’ pain thresholds (P > 0.05).
Paired Student’s t-test analyses revealed that: 1) real acupuncture increased subject’s pain threshold significantly more than sham acupuncture for heat pain administered to the arm (P = 0.015), heat pain administered to the leg (P = 0.023), pressure pain administered to the leg (P < 0.001), and pressure pain administered to the thumbnail (P < 0.001); 2) VGAIT increased subject’s pain threshold more than VGAIT control for pressure pain administered to the leg (P < 0.001) and pressure pain administered to the thumbnail (P < 0.001), but not for heat pain administered to the leg (P = 0.087) or heat pain administered on the forearm (P = 0.348); 3) no significant analgesic effect difference was observed between VGAIT and real acupuncture across all measures (P > 0.05). A summary of pain threshold values is shown in Figure 2.
Figure 2.
Changes in heat and pressure pain thresholds between postintervention and preintervention (postintervention minus preintervention, mean ± SE) on the arm and leg, *P < 0.05.
To explore the association between the analgesic effect produced by real acupuncture and VGAIT, we also performed a correlation analysis of pain threshold changes between real acupuncture and VGAIT. We found there was a marginally significant correlation between real acupuncture and VGAIT in pain threshold changes for pressure pain administered to the thumbnail (R = 0.396, P = 0.055). There were no other significant associations in pain threshold changes between the real acupuncture and VGAIT intervention.
The STAI-state was administered before each fMRI scan to provide a measure of the participant’s current anxiety level. The mean state anxiety level in the study was 47.79; there were no significant differences for STAI-state scores across the 4 intervention sessions (F(3,69) = 1.252, P = 0.298).
We also assessed the sensations evoked by the various interventions using the MASS. The mean MASS ratings (mean ± SE) for each of the 4 interventions were: 1.46 ± 0.22 for real acupuncture, 0.76 ± 0.12 for sham acupuncture, 0.49 ± 0.10 for VGAIT, and 0.19 ± 0.05 for VGAIT control. Paired Student’s t-test analyses revealed that real acupuncture produced a significantly higher acupuncture sensation compared with sham acupuncture (P < 0.001), and there was a significant difference for MASS scores between VGAIT and VGAIT control (P = 0.026). There were no significant correlations between MASS, BQMI scores, and threshold change induced by VGAIT or VGAIT control (P > 0.05). (Detailed comparisons for each acupuncture sensation for different intervention conditions can be found in Supplementary Fig. S2.)
Following each treatment, the expected relief for that treatment was measured using the ERS. The mean ERS ratings (mean ± SE) for heat and pressure pain, respectively, for each of the 4 interventions were: 3.29 ± 0.34 and 3.83 ± 0.34 for real acupuncture, 3.38 ± 0.41 and 3.29 ± 0.39 for sham acupuncture, 2.79 ± 0.45 and 2.67 ± 0.40 for VGAIT, and 1.63 ± 0.36 and 1.83 ± 0.38 for VGAIT control. For heat pain, ANOVA showed that there was a significant difference in expectancy ratings across the 4 interventions (F(3,69) = 14.70, P < 0.001). A post hoc t-test (Bonferroni-corrected) revealed that there was a significant difference between VGAIT and VGAIT control (P = 0.003). For pressure pain, ANOVA revealed that there was a significant difference in relief expectancy ratings across the 4 interventions (F(3,69) = 13.51, P < 0.001). A post hoc t-test (Bonferroni-corrected) indicated a significant difference between real acupuncture and VGAIT (P = 0.007). For real acupuncture, there was a significant correlation between relief expectancy ratings and pain threshold changes for pressure pain administered to the leg (R = 0.461, P = 0.023); this was the only significant correlation observed.
Intervention-Evoked Blood Oxygen Level Dependent Responses
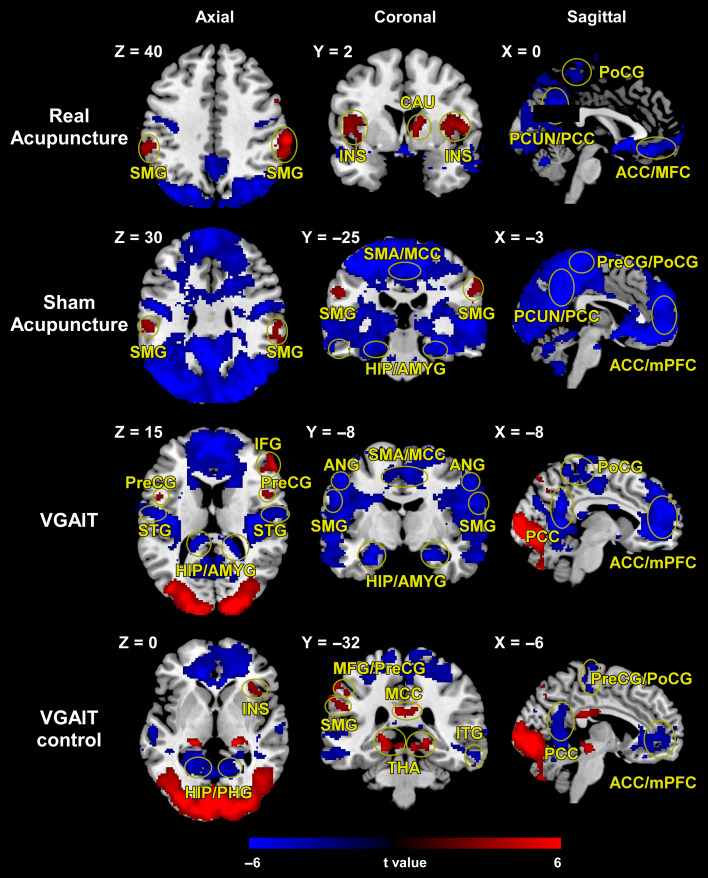
As expected, real acupuncture elicited blood oxygen level dependent (BOLD) activations within a wide range of brain regions, specifically the bilateral insula (INS), bilateral supramarginal gyrus (SMG), right caudate (CAU), and BOLD deactivations in the ACC/medial frontal cortex (MFC), right postcentral gyrus (PoCG), and precuneus (PCUN)/PCC. Sham acupuncture evoked BOLD activation in the bilateral SMG and BOLD deactivation in the SMA/MCC, bilateral hippocampus (HIP)/amygdala (AMYG), PCUN/PCC, bilateral precentral gyrus (PreCG)/PoCG, and ACC/mPFC.
Aside from BOLD increases in brain regions associated with visual activity, VGAIT produced BOLD activation in the bilateral PreCG and right IFG and BOLD decreases in the bilateral superior temporal gyrus (STG), HIP/AMYG, SMG, angular gyrus (ANG), SMA/MCC, PCC, PoCG, and ACC/mPFC. During the VGAIT control condition, there were BOLD activations in the right INS, left MFG/PreCG, left SMG, MCC, and bilateral thalamus (THA) and BOLD deactivations in the bilateral HIP/parahippocampal gyrus (PHG), right inferior temporal gyrus (ITG), PCC, PreCG/PoCG, and ACC/mPFC (Fig. 3). (Detailed activations and deactivations for the different intervention conditions can be found in Supplementary Tables S1–S4.)
Figure 3.
Brain activations (red color)/deactivation (blue color) evoked by 4 interventions. Brain regions indicated in Figure 3: insula (INS), supramarginal gyrus (SMG), caudate (CAU), anterior cingulate cortex (ACC), medial frontal cortex (MFC), postcentral gyrus (PoCG), precuneus (PCUN), posterior cingulate cortex (PCC), supplementary motor area (SMA), midcingulate cortex (MCC), hippocampus (HIP), amygdala (AMYG), precentral gyrus (PreCG), medial prefrontal cortex (mPFC), inferior frontal gyrus (IFG), superior temporal gyrus (STG), angular gyrus (ANG), middle frontal gyrus (MFG), thalamus (THA), parahippocampal gyrus (PHG), inferior temporal gyrus (ITG).
Because the insula has been widely reported to be modulated by acupuncture and plays an important role during the visualization of others experiencing pain (Jackson et al. 2006; Singer et al. 2009; Rütgen et al. 2015; Murphy et al. 2017), we provided additional results for insula activity during each intervention (Supplementary Fig. S3). All interventions activated the insula, but they varied in level and region of activation: 1) real acupuncture produced the strongest activations in the bilateral insula (cluster level: PFDR < 0.05); 2) sham acupuncture and VGAIT produced significant activation in the left anterior insula (voxel level: P < 0.05; cluster level: P < 0.005 small volume correction); and 3) VGAIT control also significantly activated the bilateral anterior insula (voxel level: P < 0.005; cluster level: P < 0.05 small volume correction).
Comparison of Brain Activations Between Different Interventions
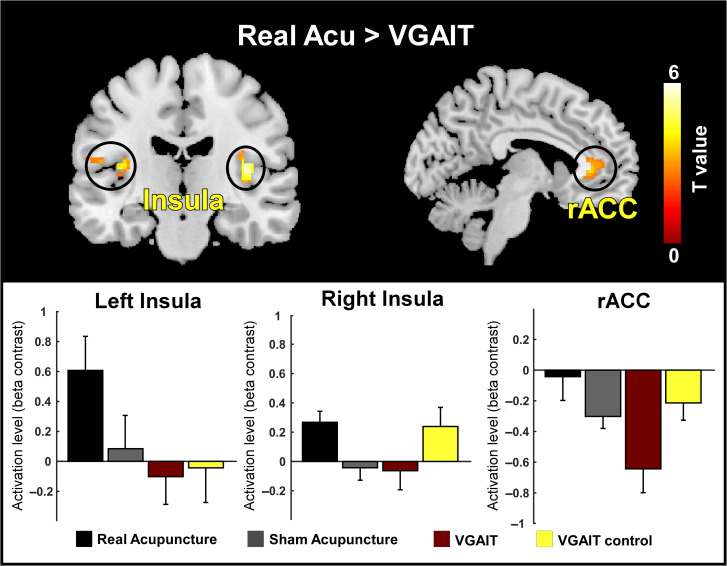
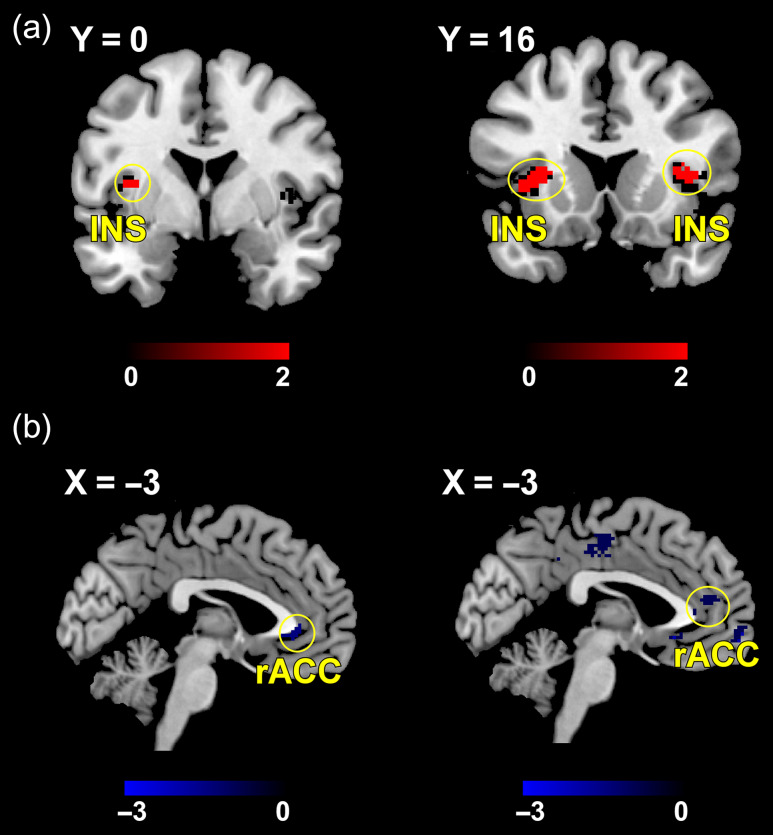
Real acupuncture produced significantly greater BOLD activation in the bilateral insula compared with VGAIT (voxel level: P < 0.005; cluster level: PFDR < 0.05; Fig. 4, upper panel); this activation was significant but less strong when compared with sham acupuncture (voxel level: P < 0.005; cluster level: P < 0.05 small volume correction; Supplementary Fig. S4). VGAIT produced significant BOLD deactivation in the rostral ACC (rACC) compared with real acupuncture and VGAIT control (cluster level: PFDR < 0.05; Fig. 4, upper panel and Supplementary Fig. S4). To elucidate the activation/deactivation levels between different interventions within the clusters identified in the upper panel of Figure 4, we extracted the mean of beta contrast estimates within the left insula, right insula and rACC for each intervention. The results showed strongest activation in the left insula for real acupuncture (contralateral to the stimulation side) and strongest deactivation in the rACC for VGAIT (Fig. 4, lower panel).
Figure 4.
Comparison of brain activations between different interventions. Upper panel: Real acupuncture produced significantly greater BOLD activation in the bilateral insula, whereas VGAIT produced significant BOLD deactivation in the rACC. Lower panel: illustration of group-level BOLD changes in the bilateral insula and rACC for the 4 interventions.
Brain Regions Related to Analgesic Effects
BOLD responses in the insula were related to pain threshold changes measured on the leg and thumbnail for real acupuncture (voxel level: P < 0.005; cluster level: P < 0.05 small volume correction), while BOLD responses in the rACC were associated with pain threshold changes for VGAIT (voxel level: P < 0.05; cluster level: P < 0.05 small volume correction) (Fig. 5).
Figure 5.
Brain responses evoked by real acupuncture and VGAIT were related to their analgesic effects. (a) For real acupuncture, BOLD responses in the left insula were related to changes in pressure pain threshold on the leg (left panel), while in the bilateral insula were related to changes of pressure pain threshold in the thumbnail (right panel). (b) For VGAIT, BOLD deactivations in the rACC were related to changes of pressure pain threshold in the leg (left panel) and thumbnail (right panel).
Discussion
In this study, we tested a complementary procedure for producing analgesia using video-guided acupuncture imagery and examined the underlying brain activations associated with acupuncture and VGAIT. Behavioral findings demonstrate that both real acupuncture and VGAIT significantly increased pain threshold, thereby achieving analgesic effects, in contrast to sham and VGAIT control interventions. Brain imaging results indicate that: 1) real acupuncture, compared with sham acupuncture, was associated with BOLD activation in the bilateral insula and (2) VGAIT, compared with VGAIT control, was associated with BOLD deactivation in the rACC. Our results suggest that acupuncture and VGAIT could be a promising complimentary therapeutic approach for relieving pain.
In this study, we found real acupuncture produced segmental (leg) and suprasegmental (arm/thumbnail) heat and mechanical pain threshold increases, while VGAIT produced segmental (leg) and suprasegmental (thumbnail) mechanical pain threshold increases and only a trend (P = 0.087) for segmental heat pain threshold. Previous studies suggest that heat-evoked pain is predominantly mediated by small, nonmyelinated peripheral nociceptive nerve fibers (C-fibers), whereas pressure-evoked pain is predominantly mediated by small, myelinated peripheral nociceptive nerve fibers (A-delta fibers) (Angst et al. 2009; Beissner et al. 2010). Our results suggest that VGAIT may be more effective on the A-delta fibers. However, further research is needed to confirm these findings.
The present study found that real acupuncture increased fMRI BOLD signals in the insula. This result is consistent with our previous studies (Kong et al. 2002; Kong, Gollub, Webb, et al. 2007; Dougherty et al. 2008; Chen et al. 2015), in which we investigated fMRI BOLD changes evoked by acupuncture needle manipulation in both healthy individuals and patients with knee osteoarthritis (OA) and found that acupuncture stimulation produced widespread brain activations (insula, parietal operculum [S2]) and deactivations (mPFC, PCC, hippocampus, basal ganglia). Our finding is also consistent with 2 meta-analyses (Huang et al. 2012; Chae et al. 2013) of fMRI signal changes evoked by acupuncture needle stimulation.
The insula is a brain region that integrates sensory and affective information. There is substantial evidence supporting a prominent role for the insula in pain processing (Ogino et al. 2007; Kong, Kaptchuk, et al. 2009; Krishnan et al. 2016). The insula has been found to be the most frequently activated brain region in pain studies using fMRI (Apkarian et al. 2005). Stimulation of the insula, but not other pain regions, has been shown to induce pain perception (Isnard et al. 2011). Brain connectivity between the insula and sensorimotor areas has been found to be disrupted in chronic pain patients (Flodin et al. 2014). Given the well-documented role of the insula in coding experiences and modulating pain perception as well as the findings from our lab (Kong et al. 2002; Kong, Gollub, et al. 2006; Kong, White, et al. 2006) and others (Wager et al. 2004; Diers et al. 2015; Segerdahl et al. 2015), we believe the analgesic effect produced by acupuncture is achieved by the modulation of brain activity in the insula.
We found VGAIT, compared with the VGAIT control and real acupuncture, decreased brain activity in the rACC, and this decrease was related to the analgesic effect of VGAIT. The rACC, along with the mPFC, is a key region of the default mode network (DMN). Studies have suggested that the DMN is the neurological basis for selfhood, which includes memories of events and facts about oneself, traits and descriptions of oneself, and reflections about one’s own emotional state (Andrews-Hanna 2012). The DMN has been shown to deactivate during external goal-oriented tasks such as visual attention or cognitive working memory tasks (Fox et al. 2005; Baliki et al. 2008). In addition, the ACC is a key region for interoception (defined as the sense of the internal state of the body) (Khalsa and Lapidus 2016). Interoception encompasses the brain’s process of integrating signals relayed from the body, plays an important role in maintaining homeostatic conditions (Barrett and Simmons 2015), and potentially aids in self-awareness (Craig 2009).
In addition, the rACC/mPFC plays an indispensable role in learning and encoding from experience, suggesting that higher-order areas of the brain process forthcoming information based on personal experience (Wager et al. 2004; Freeman et al. 2015). In the present study, subjects were required to watch the video of first-person acupuncture manipulation and imagine/recall the acupuncture needle stimulation experience. Our results demonstrate that this unique experience can significantly enhance DMN activity as indicated by greater deactivation of the rACC.
Finally, rACC is a key region in the descending pain modulatory system (Bingel et al. 2006; Kong, Tu, et al. 2010; Li et al. 2016; Kong, Wang, et al. 2018). Previous imaging studies found that the rACC can be activated by opioid analgesia (Adler et al. 1997; Casey et al. 2000). Moreover, findings from studies investigating stimulus-induced analgesia (García-Larrea et al. 1999; Davis et al. 2000), nitrous oxide-induced analgesia (Gyulai et al. 1997), hypnosis-induced change in pain perception (Faymonville et al. 2000), placebo analgesia (Wager et al. 2004; Kong, Gollub, et al. 2006), and acupuncture (Chen et al. 2015; Li et al. 2016) and mind–body (Kong, Wolcott, et al. 2018) treatment of chronic pain support the role of the rACC in pain modulation. Petrovic et al. (2002) suggested that placebo and opioid analgesia may share the same mechanism. The rACC plays a key role in the cortical control of the brainstem through fiber tracts projecting directly to the PAG. From our previous studies, we found that chronic pain patients have abnormal functional connectivity between the rACC and PAG and that this connectivity can be modulated by acupuncture treatment (Egorova et al. 2015; Li et al. 2016). Taken together, these findings suggest that VGAIT may share a similar mechanism with opioid and placebo analgesia.
The different mechanisms underlying motor tasks and imagery, such as dancer’s movements or the thumb-finger movements and actions shown in video clips, have been widely studied using fMRI (Cross et al. 2006; Macuga and Frey 2012; Nedelko et al. 2012). Findings from previous fMRI studies have shown that imagined and observed actions produce similar activation patterns. To our best knowledge, the present study is the first study to have individuals observe an actual acupuncture treatment being performed on themselves and then subsequently imagine this treatment using video-guided imagery for the purpose of reducing pain. Comparing the brain mechanisms that underlie real and imagined treatments may provide insight for the development of new treatment regimens for pain.
In recent years, acupuncture has gained increasing popularity in Western countries, particularly for the treatment of chronic pain. For example, acupuncture has been recommended for the treatment of chronic low back pain in the most recent guidelines from the American College of Physicians (Qaseem et al. 2017). Our findings suggest that VGAIT has an analgesic effect that is comparable to real acupuncture in healthy subjects. VGAIT would seem to have substantial clinical value due to its advantages over real acupuncture, such as its low cost and flexible application. It could provide a low-cost and efficacious adjunctive treatment that could be combined with other conventional or complementary treatments or used independently of other treatments. This may be particularly advantageous for elderly or disabled patients who have limited access to acupuncture treatment and medical care and who could self-administer the treatment at home after completing a real acupuncture treatment session. Furthermore, VGAIT minimizes potential adverse side-effects and contraindications associated with acupuncture. Such contraindications include clotting and bleeding disorders (e.g., hemophilia and advanced liver disease), warfarin use, severe psychiatric conditions (e.g., psychosis), and local skin infections or trauma to the skin.
There are several limitations to the present study. First, the study was conducted with a relatively modest sample of healthy individuals and it is not yet clear whether the findings will extend to patient populations. Future studies are needed to examine the efficacy of VGAIT in chronic pain patients. Second, the present study examined only a single administration of VGAIT and did not examine the extent to which the pain relief persisted. Future work should examine whether multiple VGAIT sessions may enhance pain relief and/or extend its benefits. In addition, the sample size for the multivariate pattern analysis analyses we conducted is small; thus, these results must be interpreted with caution. Further research using a larger sample size is needed to validate our findings. Finally, we did not include a final questionnaire that assessed whether subjects could differentiate the sham from real acupuncture intervention. However, we emphasized to participants at the beginning of the study that sham acupuncture is a nontraditional way to perform acupuncture, and that they may not be able to tell the difference between the real and sham acupuncture. Also, we used a Streitberger placebo needle for the sham acupuncture intervention, which has a blunt and retractable tip. Participants could feel the needle press against their skin but could not tell whether the needle tip penetrated. This sham acupuncture has been validated in many studies (Streitberger and Kleinhenz 1998; White et al. 2003; Kong, Gollub, et al. 2006).
In summary, we found that both real acupuncture and VGAIT can significantly increase pain threshold compared with their respective control groups in healthy subjects. Brain imaging results revealed that real acupuncture modulated the insula, whereas VGAIT modulated the rACC. The development of VGAIT holds potential as a noninvasive treatment for chronic pain.
Supplementary Material
Funding
National Institutes of Health/National Center for Complementary and Integrative Health (R61AT009310, R01AT008563, and R21AT008707).
Notes
Conflict of interest: J.K. has a disclosure to report (holding equity in a startup company (MNT) and pending patents to develop new neuromodulation tools), but declares no conflict of interest. All other authors declare no conflict of interest.
References
- Adler LJ, Gyulai FE, Diehl DJ, Mintun MA, Winter PM, Firestone LL. 1997. Regional brain activity changes associated with fentanyl analgesia elucidated by positron emission tomography. Anesth Analg. 84:120–126. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR. 2012. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 18:251–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst MS, Tingle M, Phillips NG, Carvalho B. 2009. Determining heat and mechanical pain threshold in inflamed skin of human subjects. J Vis Exp. 23:1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede R-D, Zubieta J-K. 2005. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 9:463. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV, Chialvo DR. 2008. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 28:1398–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Simmons WK. 2015. Interoceptive predictions in the brain. Nat Rev Neurosci. 16:419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissner F, Brandau A, Henke C, Felden L, Baumgärtner U, Treede R-D, Oertel BG, Lötsch J. 2010. Quick discrimination of A(delta) and C fiber mediated pain based on three verbal descriptors. PLoS One. 5:e12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berna C, Tracey I, Holmes E. 2012. How a better understanding of spontaneous mental imagery linked to pain could enhance imagery-based therapy in chronic pain. J Exp Psychopathol. 3:258–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Schoell E, Weiller C, Büchel C. 2006. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 120:8–15. [DOI] [PubMed] [Google Scholar]
- Bornhövd K, Quante M, Glauche V, Bromm B, Weiller C, Büchel C. 2002. Painful stimuli evoke different stimulus–response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single‐trial fMRI study. Brain. 125:1326–1336. [DOI] [PubMed] [Google Scholar]
- Casey KL, Svensson P, Morrow TJ, Raz J, Jone C, Minoshima S. 2000. Selective opiate modulation of nociceptive processing in the human brain. J Neurophysiol. 84:525–533. [DOI] [PubMed] [Google Scholar]
- Chae Y, Chang D-S, Lee S-H, Jung W-M, Lee I-S, Jackson S, Kong J, Lee H, Park H-J, Lee H, et al. 2013. Inserting needles into the body: a meta-analysis of brain activity associated with acupuncture needle stimulation. J Pain. 14:215–222. [DOI] [PubMed] [Google Scholar]
- Chen X, Spaeth RB, Freeman SG, Scarborough DM, Hashmi JA, Wey H-Y, Egorova N, Vangel M, Mao J, Wasan AD, et al. 2015. The modulation effect of longitudinal acupuncture on resting state functional connectivity in knee osteoarthritis patients. Mol Pain. 11:s12990–015-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Lin CP, Liu HL, Hsu YY, Lim KE, Hung D, Decety J. 2007. Expertise modulates the perception of pain in others. Curr Biol. 17:1708–1713. [DOI] [PubMed] [Google Scholar]
- Christian BM, Parkinson C, Macrae CN, Miles LK, Wheatley T. 2015. When imagining yourself in pain, visual perspective matters: the neural and behavioral correlates of simulated sensory experiences. J Cogn Neurosci. 27:866–875. [DOI] [PubMed] [Google Scholar]
- Compton WM, Volkow ND. 2006. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 81:103–107. [DOI] [PubMed] [Google Scholar]
- Coronado RA, Kindler LL, Valencia C, George SZ. 2011. Thermal and pressure pain sensitivity in patients with unilateral shoulder pain: comparison of involved and uninvolved sides. J Orthop Sports Phys Ther. 41:165–173. [DOI] [PubMed] [Google Scholar]
- Craig ADB. 2009. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 10:59–70. [DOI] [PubMed] [Google Scholar]
- Cross ES, Hamilton AF, Grafton ST. 2006. Building a motor simulation de novo: observation of dance by dancers. Neuroimage. 31:1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasilva AF, Mendonca ME, Zaghi S, Lopes M, Dossantos MF, Spierings EL, Bajwa Z, Datta A, Bikson M, Fregni F. 2012. tDCS-induced analgesia and electrical fields in pain-related neural networks in chronic migraine. Headache. 52:1283–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Taub E, Duffner F, Lozano AM, Tasker RR, Houle S, Dostrovsky JO. 2000. Activation of the anterior cingulate cortex by thalamic stimulation in patients with chronic pain: a positron emission tomography study. J Neurosurg. 92:64–69. [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Chiaradia C, Carotenuto E. 2002. The contribution of suggestibility and expectation to placebo analgesia phenomenon in an experimental setting. Pain. 96:393–402. [DOI] [PubMed] [Google Scholar]
- Dhond RP, Kettner N, Napadow V. 2007. Neuroimaging acupuncture effects in the human brain. J Altern Complement Med. 13:603–616. [DOI] [PubMed] [Google Scholar]
- Diers M, Kamping S, Kirsch P, Rance M, Bekrater-Bodmann R, Foell J, Trojan J, Fuchs X, Bach F, Maaß H, et al. 2015. Illusion-related brain activations: a new virtual reality mirror box system for use during functional magnetic resonance imaging. Brain Res. 1594:173–182. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Kong J, Webb M, Bonab AA, Fischman AJ, Gollub RL. 2008. A combined [11C]diprenorphine PET study and fMRI study of acupuncture analgesia. Behav Brain Res. 193:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorova N, Gollub RL, Kong J. 2015. Repeated verum but not placebo acupuncture normalizes connectivity in brain regions dysregulated in chronic pain. NeuroImage Clin. 9:430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faymonville ME, Laureys S, Degueldre C, DelFiore G, Luxen A, Franck G, Lamy M, Maquet P. 2000. Neural mechanisms of antinociceptive effects of hypnosis. Anesthesiology. 92:1257–1267. [DOI] [PubMed] [Google Scholar]
- Flodin P, Martinsen S, Löfgren M, Bileviciute-Ljungar I, Kosek E, Fransson P. 2014. Fibromyalgia is associated with decreased connectivity between pain- and sensorimotor brain areas. Brain Connect. 4:587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman S, Yu R, Egorova N, Chen X, Kirsch I, Claggett B, Kaptchuk TJ, Gollub RL, Kong J. 2015. Distinct neural representations of placebo and nocebo effects. Neuroimage. 112:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Larrea L, Peyron R. 2007. Motor cortex stimulation for neuropathic pain: from phenomenology to mechanisms. Neuroimage. 37(Suppl 1):S71–S79. [DOI] [PubMed] [Google Scholar]
- García-Larrea L, Peyron R, Mertens P, Gregoire MC, Lavenne F, Le Bars D, Convers P, Mauguière F, Sindou M, Laurent B. 1999. Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. Pain. 83:259–273. [DOI] [PubMed] [Google Scholar]
- Gollub RL, Kirsch I, Maleki N, Wasan AD, Edwards RR, Tu Y, Kaptchuk TJ, Kong J. 2018. A functional neuroimaging study of expectancy effects on pain response in patients with knee osteoarthritis. J Pain. 19:515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyulai FE, Firestone LL, Mintun MA, Winter PM. 1997. In vivo imaging of nitrous oxide-induced changes in cerebral activation during noxious heat stimuli. Anesthesiology. 86:538–548. [DOI] [PubMed] [Google Scholar]
- Han J-S. 2003. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 26:17–22. [DOI] [PubMed] [Google Scholar]
- Han J-S. 2011. Acupuncture analgesia: areas of consensus and controversy. Pain. 152:S41–S48. [DOI] [PubMed] [Google Scholar]
- Huang W, Pach D, Napadow V, Park K, Long X, Neumann J, Maeda Y, Nierhaus T, Liang F, Witt CM. 2012. Characterizing acupuncture stimuli using brain imaging with FMRI—a systematic review and meta-analysis of the literature. PLoS One. 7:e32960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson TJ, Edlund MJ, Steffick DE, Tripathi SP, Sullivan MD. 2008. Epidemiology of regular prescribed opioid use: results from a national, population-based survey. J Pain Symptom Manage. 36:280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isnard J, Magnin M, Jung J, Mauguière F, Garcia-Larrea L. 2011. Does the insula tell our brain that we are in pain? Pain. 152:946–951. [DOI] [PubMed] [Google Scholar]
- Ivanova JI, Birnbaum HG, Schiller M, Kantor E, Johnstone BM, Swindle RW. 2011. Real-world practice patterns, health-care utilization, and costs in patients with low back pain: the long road to guideline-concordant care. Spine J. 11:622–632. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J. 2006. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 44:752–761. [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Lapidus RC. 2016. Can interoception improve the pragmatic search for biomarkers in psychiatry? Front Psychiatry. 7:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Fufa DT, Gerber AJ, Rosman IS, Vangel MG, Gracely RH, Gollub RL. 2005. Psychophysical outcomes from a randomized pilot study of manual, electro, and sham acupuncture treatment on experimentally induced thermal pain. J Pain. 6:55–64. [DOI] [PubMed] [Google Scholar]
- Kong J, Gollub R, Huang T, Polich G, Napadow V, Hui K, Vangel M, Rosen B, Kaptchuk TJ. 2007. Acupuncture de qi, from qualitative history to quantitative measurement. J Altern Complement Med. 13:1059–1070. [DOI] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ. 2006. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 26:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Webb JM, Kong J-T, Vangel MG, Kwong K. 2007. Test-retest study of fMRI signal change evoked by electroacupuncture stimulation. Neuroimage. 34:1171–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Kaptchuk TJ, Polich G, Kirsch I, Vangel M, Rosen B, Gollub R. 2009. Expectancy and treatment interactions: a dissociation between acupuncture analgesia and expectancy evoked placebo analgesia. Neuroimage. 45:940–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Ma L, Gollub RL, Wei J, Yang X, Li D, Weng X, Jia F, Wang C, Li F, et al. 2002. A pilot study of functional magnetic resonance imaging of the brain during manual and electroacupuncture stimulation of acupuncture point (LI-4 Hegu) in normal subjects reveals differential brain activation between methods. J Altern Complement Med. 8:411–419. [DOI] [PubMed] [Google Scholar]
- Kong J, Spaeth R, Cook A, Kirsch I, Claggett B, Vangel M, Gollub RL, Smoller JW, Kaptchuk TJ. 2013. Are all placebo effects equal? Placebo pills, sham acupuncture, cue conditioning and their association. PLoS One. 8:e67485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Tu P, Zyloney C, Su T. 2010. Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav Brain Res. 211:215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Wang Z, Leiser J, Minicucci D, Edwards R, Kirsch I, Wasan AD, Lang C, Gerber J, Yu S, et al. 2018. Enhancing treatment of osteoarthritis knee pain by boosting expectancy: a functional neuroimaging study. NeuroImage Clin. 18:325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, Gollub RL. 2006. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp. 27:715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Wolcott E, Wang Z, Jorgenson K, Harvey WF, Tao J, Rones R, Wang C. 2018. Altered resting state functional connectivity of the cognitive control network in fibromyalgia and the modulation effect of mind-body intervention. Brain Imaging Behav. doi:10.1007/s11682-018-9875-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslyn SM, Ganis G, Thompson WL. 2001. Neural foundations of imagery. Nat Rev Neurosci. 2:635–642. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Woo CW, Chang LJ, Ruzic L, Gu X, López-Solà M, Jackson PL, Pujo J, Fan J, Wager TD. 2016. Somatic and vicarious pain are represented by dissociable multivariate brain patterns. Elife. 5:1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. 2011. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 54:2492–2502. [DOI] [PubMed] [Google Scholar]
- Li Z, Liu M, Lan L, Zeng F, Makris N, Liang Y, Guo T, Wu F, Gao Y, Dong M, et al. 2016. Altered periaqueductal gray resting state functional connectivity in migraine and the modulation effect of treatment. Sci Rep. 6:20298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist MA, Krishnan A, Lopez-Sola M, Jepma M, Woo CW, Koban L, Roy M, Atlas LY, Schmidt L, Chang LJ, et al. 2017. Group-regularized individual prediction: theory and application to pain. Neuroimage. 145(Pt B):274–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser JD, Treede R-D. 2008. The Kyoto protocol of IASP basic pain terminology. Pain. 137:473–477. [DOI] [PubMed] [Google Scholar]
- Macuga KL, Frey SH. 2012. Neural representations involved in observed, imagined, and imitated actions are dissociable and hierarchically organized. Neuroimage. 59:2798–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier BP, Schnall S, Schwarz N, Bargh JA. 2012. Embodiment in social psychology. Top Cogn Sci. 4:705–716. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Baumgärtner U, Kamping S, Ruttorf M, Schad LR, Flor H, Kakigi R, Treede R-D. 2013. Cortico-subcortical activation patterns for itch and pain imagery. Pain. 154:1989–1998. [DOI] [PubMed] [Google Scholar]
- Murphy SE, O’Donoghue MC, Blackwell SE, Nobre AC, Browning M, Holmes EA. 2017. Increased rostral anterior cingulate activity following positive imagery training in healthy older adults. Soc Cogn Affect Neurosci. 12:1905–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naglatzki RP, Schlamann M, Gasser T, Ladd ME, Sure U, Forsting M, Gizewski ER. 2012. Cerebral somatic pain modulation during autogenic training in fMRI. Eur J Pain. 16:1293–1301. [DOI] [PubMed] [Google Scholar]
- Naylor JC, Borckardt JJ, Marx CE, Hamer RM, Fredrich S, Reeves ST, George MS. 2014. Cathodal and anodal left prefrontal tDCS and the perception of control over pain. Clin J Pain. 30:693–700. [DOI] [PubMed] [Google Scholar]
- Nedelko V, Hassa T, Hamzei F, Schoenfeld MA, Dettmers C. 2012. Action imagery combined with action observation activates more corticomotor regions than action observation alone. J Neurol Phys Ther. 36:182–188. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Zaki J, Hanelin J, Ludlow DH, Knierim K, Ramachandran T, Glover GH, Mackey SC. 2008. Your pain or mine? Common and distinct neural systems supporting the perception of pain in self and other. Soc Cogn Affect Neurosci. 3:144–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino Y, Nemoto H, Inui K, Saito S, Kakigi R, Goto F. 2007. Inner experience of pain: imagination of pain while viewing images showing painful events forms subjective pain representation in human brain. Cereb Cortex. 17:1139–1146. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. 2002. Placebo and opioid analgesia—imaging a shared neuronal network. Science. 295:1737–1740. [DOI] [PubMed] [Google Scholar]
- Pomeranz B. 1996. Scientific research into acupuncture for the relief of pain. J Altern Complement Med. 2:53–60. discussion 73-5. [DOI] [PubMed] [Google Scholar]
- Price DD. 2000. Psychological and neural mechanisms of the affective dimension of pain. Science. 288:1769–1772. [DOI] [PubMed] [Google Scholar]
- Qaseem A, Wilt TJ, McLean RM, Forciea MA. 2017. Noninvasive treatments for acute, subacute, and chronic low back pain: a Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 166:514. [DOI] [PubMed] [Google Scholar]
- Rütgen M, Seidel E-M, Silani G, Riečanský I, Hummer A, Windischberger C, Petrovic P, Lamm C. 2015. Placebo analgesia and its opioidergic regulation suggest that empathy for pain is grounded in self pain. Proc Natl Acad Sci USA. 112:E5638–E5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabrun SM, Jones E, Elgueta Cancino EL, Hodges PW. 2014. Targeting chronic recurrent low back pain from the top-down and the bottom-up: a combined transcranial direct current stimulation and peripheral electrical stimulation intervention. Brain Stimul. 7:451–459. [DOI] [PubMed] [Google Scholar]
- Segerdahl AR, Mezue M, Okell TW, Farrar JT, Tracey I. 2015. The dorsal posterior insula subserves a fundamental role in human pain. Nat Neurosci. 18:499–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan PW. 1967. A shortened form of Betts’ questionnaire upon mental imagery. J Clin Psychol. 23:386–389. [DOI] [PubMed] [Google Scholar]
- Singer T. 2004. Empathy for pain involves the affective but not sensory components of pain. Science. 303:1157–1162. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. 2009. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 13:334–340. [DOI] [PubMed] [Google Scholar]
- Spaeth RB, Camhi S, Hashmi JA, Vangel M, Wasan AD, Edwards RR, Gollub RL, Kong J. 2013. A longitudinal study of the reliability of acupuncture deqi sensations in knee osteoarthritis. Evid Based Complement Alternat Med. 2013:204259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. 1970. Manual for the state-trait anxiety inventory. Palo Alto, Calif: Consulting Psychologists Press. [Google Scholar]
- Streitberger K, Kleinhenz J. 1998. Introducing a placebo needle into acupuncture research. Lancet. 352:364–365. [DOI] [PubMed] [Google Scholar]
- Tu Y, Fu Z, Tan A, Huang G, Hu L, Hung Y, Zhang Z. 2018. A novel and effective fMRI decoding approach based on sliced inverse regression and its application to pain prediction. Neurocomputing. 273:373–384. [Google Scholar]
- Tu Y, Tan A, Bai Y, Hung Y, Zhang Z. 2016. Decoding subjective intensity of nociceptive pain from pre-stimulus and post-stimulus brain activities. Front Comput Neurosci. 10:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y, Zhang Z, Tan A, Peng W, Hung Y, Moayedi M, Iannetti GD, Hu L. 2016. b. Alpha and gamma oscillation amplitudes synergistically predict the perception of forthcoming nociceptive stimuli. Hum Brain Mapp. 37:501–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Leotti LA, Rilling JK. 2011. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J Neurosci. 31:439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo C-W, Kross E. 2013. An fMRI-based neurologic signature of physical pain. N Engl J Med. 368:1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. 2004. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science (80-). 303:1162–1167. [DOI] [PubMed] [Google Scholar]
- White P, Lewith G, Hopwood V, Prescott P. 2003. The placebo needle, is it a valid and convincing placebo for use in acupuncture trials? A randomised, single-blind, cross-over pilot trial. Pain. 106:401–409. [DOI] [PubMed] [Google Scholar]
- Wiech K, Ploner M, Tracey I. 2008. Neurocognitive aspects of pain perception. Trends Cogn Sci. 12:306–313. [DOI] [PubMed] [Google Scholar]
- Wilcox CE, Mayer AR, Teshiba TM, Ling J, Smith BW, Wilcox GL, Mullins PG. 2015. The subjective experience of pain: an FMRI study of percept-related models and functional connectivity. Pain Med. 16:2121–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z-Q. 2008. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 85:355–375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.