Abstract
Anti-programmed death-1 (PD-1) therapy has been extensively used to treat cancer. Recently, the combination of immunotherapy and anti-angiogenic therapy has emerged as a novel treatment approach. Therefore, we designed a study to evaluate the real-world benefit of the combination of anti-PD-1 and anti-angiogenesis therapy in patients with non-small cell lung cancer (NSCLC).
We obtained the medical records of patients at the Chinese People's Liberation Army General Hospital who received either nivolumab or pembrolizumab combined with anti-angiogenesis therapy from January 2015 to December 2018. The overall response rate (ORR), progression-free survival (PFS), and overall survival (OS) were evaluated for all patients.
Sixty-nine patients with NSCLC were included in our study. The ORR was 31.9% (95% CI: 20.6–43.2%) and the median PFS was 8.37 months (95% CI: 6.5–10.0 months). The subgroup analysis statistically revealed a significant difference in ORR for patients receiving first-line treatment vs other lines, and the values were 58.8% (95% CI: 32.7–84.9%) compared with 23.1% (95% CI: 11.2–34.9%). We also observed a significant improvement in PFS, with a median value of 10.5 months (95% CI: 7.4–13.1 months) for patients without EGFR mutations and 5.4 months (95% CI: 4.0–6.3 months) for patients with EGFR mutations.
The real-world ORR, PFS, and OS were comparable to previous clinical trials, despite the patients’ different baseline characteristics. Importantly, compared with patients having identified EGFR mutations, patients without EGFR mutations had a better PFS. Furthermore, these data support the use of anti-PD-1 combined with anti-angiogenesis therapy as a novel treatment approach for patients with NSCLC.
Keywords: anti-PD-1, epidermal growth factor receptor mutation, non-small cell lung cancer, overall response rate, progression-free survival
1. Introduction
Lung cancer is the most common cause of cancer-related death worldwide, causing 1.38 million deaths per year and accounting for 18.2% of total cancer-related deaths.[1] Lung cancer is also the cancer with the highest morbidity and mortality rates in China. In 2014, approximately 781,000 new cases and 626,000 deaths were reported.[2] The 5-year age-standardized survival rate for patients with lung cancer is 16.1%, and patients are usually diagnosed at an advanced stage. The burden of lung cancer in China remains high.[3] Non-small cell lung cancer (NSCLC) accounts for approximately 80% to 85% of lung cancer cases, and its clinical manifestations are complex and diverse. In recent years, with the continuous improvements in detection technology and treatment, important breakthroughs have been made in the treatment of non-small cell lung cancer. The treatment has developed from the era of traditional chemotherapy to precise molecular targeted therapy and, subsequently, to immunotherapy.[4,5] Compared with docetaxel, nivolumab is associated with a significantly longer median OS in patients with both squamous (CheckMate 017) and non-squamous lung cancer (CheckMate 057).[6,7] The phase III Keynote 024 and Keynote 042 trials also reported significant improvement of the PFS and OS using pembrolizumab, comparing to standard first-line platinum-based chemotherapy.[8,9] Based on these trials, anti-PD-1 monoclonal antibodies (mAbs), including nivolumab and pembrolizumab, have now been approved as a standard anticancer treatment for patients with NSCLC. Although anti-PD-1 monotherapy exhibits a significant improvement of therapeutic efficacy for non-small cell lung cancer, it fails to meet public expectations for long-term survival. Multi-drug treatment is a future trend.
Anti-angiogenic drugs experimentally can improve the immune microenvironment of tumor tissue, thereby enhancing the efficacy of immunotherapy.[10–12] In the IMPOWER150 study, atezolizumab joint with bevacizumab and chemotherapy prolonged PFS and OS, providing evidence for the effectiveness of combination medications.[13,14]
However, clinical trials have certain limitations due to their strict entry requirements. Patients with an older age and EGFR mutations are often excluded from studies because of the lower expectation regarding the treatment benefits. Thus, differences in the curative effects are often observed when clinical trials tested drugs are widely applied in the clinic. In addition, the same treatment pattern may exert various effects on different populations. Real-world research can, to a certain extent, compensate for the limitations of clinical trials to better guide clinical applications. As nivolumab and pembrolizumab became available in China in July 2018, lung cancer experts need to address the immunotherapy demands of patients in various situations, which requires real data obtained from the field.[15] Due to the lack of clinical data for the effectiveness of anti-PD-1 combined with anti-angiogenesis therapy in patients with non-small cell lung cancer, a real-world evidence-based retrospective clinical study was conducting to analyze the actual effect of the treatment pattern. This study collected data from all patients who received anti-PD-1 combined with anti-angiogenesis therapy at the General Hospital of PLA from January 2015 to December 2018 and analyzed clinical factors that may affect the prognosis.
2. Methods
2.1. Data source
We conducted a study of 69 patients with NSCLC who received anti-PD-1 combined with anti-angiogenesis therapy at the General Hospital of PLA from January 2015 to December 2018. The baseline characteristics, PD-L1 expression, EGFR mutation status and prior treatment lines were retrospectively analyzed. All data were obtained from follow-up visits and medical records. This study was approved by the Ethics Committee of Chinese People's Liberation Army General Hospital.
2.2. Patient selection
The target sample included patients who received either nivolumab or pembrolizumab combined with anti-angiogenesis therapy at the General Hospital of PLA from January 2015 to December 2018 and had a definite histological or cytological diagnosis of non-small cell lung cancer. Exclusion criteria included patients with a history of autoimmune disease and the need for steroids at a dose equivalent to more than 10 mg prednisone daily or other immunosuppressive drugs.
2.3. Study variables
According to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (Eisenhauer et al, 2009), the clinical response to anti-PD-1 combined with anti-angiogenesis therapy was defined as follows: complete response (CR; the disappearance of all target lesions), partial response (PR; at least a 30.0% reduction in the sum of the diameters of the target lesions), progressive disease (PD; at least a 20.0% increase in the sum of the diameters of the target lesions), and stable disease (SD; cannot be classified as PR or PD).[16]
Short-term efficacy was chosen to evaluate the treatment effect, which is usually defined as 2 or 3 cycles after the combined therapy is established. The overall response rate (ORR) is defined as the percentage of patients having achieved a complete response (CR) or partial response (PR) recorded in the medical system. The disease control rate (DCR) is defined as the percentage of patients with CR, PR or SD (stable disease). Progression-free survival (PFS) was calculated as the time from the initiation of treatment with anti-PD-1 combined with anti-angiogenesis therapy to PD (progressive disease) or death. Overall survival (OS) referred to the time from the start of treatment with anti-PD-1 combined with anti-angiogenesis therapy to death from any cause.
2.4. Statistical analysis
Descriptive statistics (percentages, means, medians, and standard deviations [SDs]) were used to describe baseline characteristics and clinical features of the sample of patients with NSCLC. ORR was calculated by using the Chi-square test and Fisher exact test to analyze the relationship between short-term efficacy and clinical features.
PFS and OS were analyzed by using the Kaplan–Meier method, and subgroups were compared with the log-rank test for the total number of patients. P < .05 was considered statistically significant. Statistical analyses were performed by using SPSS statistical software (version 25.0; SPSS, IBM Corporation).
3. Results
From January 2015 to December 2018, 69 patients with NSCLC were enrolled in this real-world study. Baseline demographics and clinical characteristics are displayed in Table 1.
Table 1.
Population characteristics.

In the final eligible sample, the mean age of the patients was 59 years. Notably, 28% of patients were 65 years or older, and 65% had a KPS score ≥90 at the time of diagnosis. Current or former smokers accounted for the majority of patients. Patients with non-squamous histology predominated: 55 (80%) had adenocarcinoma, 13 (19%) had squamous cell carcinoma and 1 (1%) had adenosquamous carcinoma. Most patients had grade IV NSCLC. Among patients with a known EGFR mutation status (n = 59), 16 patients carried mutations (16/59, 27%). Bevacizumab was the most frequently used anti-angiogenic drug, as 45 patients received this drug alone. Apatinib, anlotinib, and endostar were used by the remaining patients. Thirty patients used anti-PD-1 combined with anti-angiogenesis therapy, while the remaining 39 patients took anti-PD-1 combined with anti-angiogenesis therapy and chemotherapy.
After a median follow-up period of 12.4 months (range, 5.63–39.47 months), 20 patients had died, and 18 patients still remained at the progression-free stage. The median PFS was 8.37 months (95% CI: 6.5–10.0 months), while the median OS was not reached (Fig. 1).
Figure 1.

Kaplan-Meier survival curve of progression-free survival (A) and overall survival (B) in 69 non-small cell lung cancer patients. PFS = progression-free survival, CI = confidence interval.
Of the 69 patients with NSCLC who received anti-PD-1 inhibitors combined with anti-angiogenesis therapy, the short-term efficacy resulted in 22 patients achieving PR and 40 patients presenting stable disease. The ORR was 31.9%, and the DCR was 89.9%. Only 7 patients experienced disease progression. Subgroup analyses were mainly performed for short-term efficacy, and PFS provided evidence of subgroup differences as well. No significant differences in PFS or ORR were observed in subgroups stratified according to age (<65 years: 8.3 months vs ≥65 years: 10.2 months, P = .854), KPS score (<90 points: 6.5 months vs ≥90 points: 8.8 months, P = .968), histological subtype (adenocarcinoma: 8.3 months vs squamous cell carcinoma: 8.4 months, P = .728), or disease stage (III: 15.6 months vs IV: 8.3 months, P = .752). A detailed description of the results of the statistical is presented in Table 2 (Fig. 2).
Table 2.
Correlations between the clinical features with overall response rate and progression-free survival.
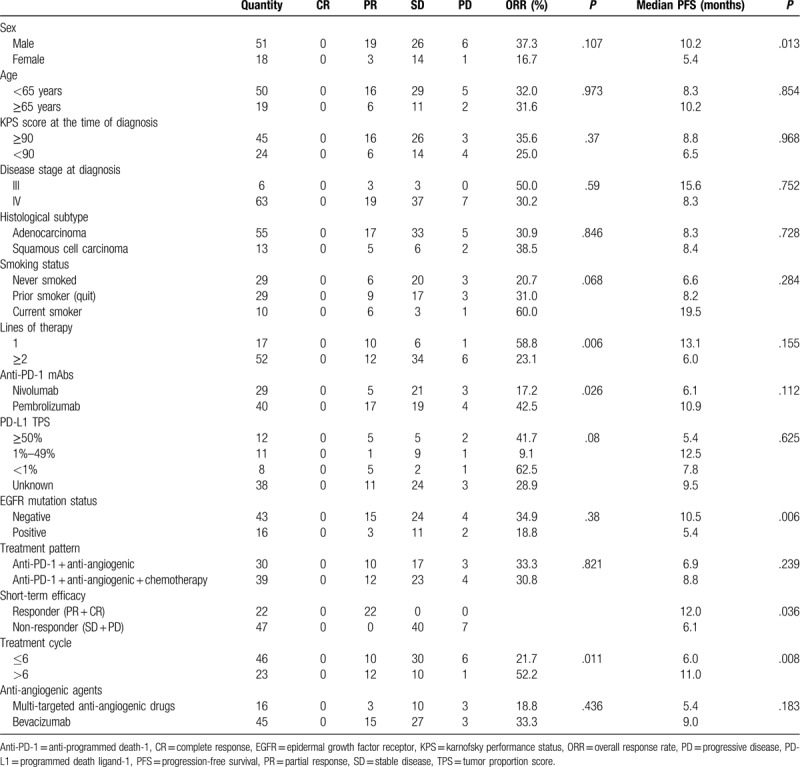
Figure 2.
Kaplan-Meier survival curves for progression-free survival were compared among patients with different lines of therapy (A), EGFR mutation status (B), short-term efficacy (C) and treatment cycle (D). PFS = progression-free survival, EGFR = epidermal growth factor receptor.
The subgroup analysis revealed a statistically significant difference in the ORR of patients receiving first-line treatments vs patients receiving other lines of treatment, and the values were 58.8% (95% CI: 32.7–84.9%) and 23.1% (95% CI: 11.2–34.9%), respectively, P = .006. Moreover, the median PFS of patients receiving first-line treatment with anti-PD-1 combined with anti-angiogenesis therapy was 13.1 months (95% CI: 9.0–17.2 months), which was better than the result of patients receiving other lines of treatment, with a median PFS of 6.0 months (95% CI: 3.1–9.0 months). However, the difference in PFS was not statistically significant, P = .155. We then further analyzed the differences in PFS between the first-line therapy and other lines of therapy, and the results were as follows: compared with the second-line therapy (13.1 vs 8.2 months, 95% CI: 3.9–12.5 months, P = .304), third-line (13.1 vs 9.8 months, 95% CI: 8.5–11.1 months, P = .497), more than third-line (13.1 vs 5.9 months, 95% CI: 4.6–7.2 months, P = .075). No statistical difference was found in PFS between the 2 groups, probably because 7 of the 17 patients receiving first-line treatments had not reached PFS. Perhaps, as follow-up continues, we can further discover whether there is a difference in PFS or OS between the first-line therapy and other lines.
We also observed a statistically significant improvement in the median PFS of 10.5 months (95% CI: 7.7–13.4 months) in patients without EGFR mutations, and 5.4 months (95% CI: 4.3–6.6 months) in patients with EGFR mutations, P = .006. For further analysis, we divided the patients with EGFR mutations into different groups, of which 11 were sensitive mutations and 5 were non-sensitive mutations. The median PFS was 5.9 months with sensitive mutations versus 3.5 months with non-sensitive mutations (5.9 months, 95% CI: 4.8–7.0 months vs 3.5 months, 95% CI: 1.0–6.0 months, P = .066). However, too few samples may have an impact on the statistical analysis, and we will continue to collect samples and extend the follow-up time to obtain more reliable results.
To identify patients who might receive greater benefits from immunotherapy combined with anti-angiogenesis therapy, the short-term efficacy of CR or PR is defined as responders among patients treated with this pattern. Patients experiencing better short-term efficacy exhibited a significantly longer PFS of 12.0 months (95% CI: 9.5–14.6 months) vs 6.1 months for non-responders (95% CI: 5.0–7.1 months), P = .036.
Due to the tailing phenomenon of immunotherapy, we also analyzed the treatment outcomes of patients based on the cumulative treatment cycle. Patients who received more than six cumulative treatment cycles experienced a significantly longer PFS (11.0 months, 95% CI: 8.3–13.8 months vs 6.0 months, 95% CI: 4.4–7.6 months, P = .008) and higher ORR (52.2%, 95% CI: 30.1–74.3% vs 21.7%, 95% CI: 9.4–34.1%, P = .011) than patients who received 6 or fewer cycles of the treatment.
Treatment-related AEs appeared in 62% of patients, as shown in Table 3. General side effects (63.8%) were grade 1–2, and 2 (2.9%) had events of grade 3, with no grade 4 or 5 events. The most common adverse effects were fatigue 14 (20.3%), decreased appetite 6 (8.7%), and nausea 5 (7.2%), which did not have much effect on the progress of treatment. The most serious adverse event was grade-3 pneumonitis and diarrhea, which occurred in 2 patients (2.9%), recovered with endovenous topical corticosteroids and liquids. Two patients had hypertension and another two had proteinuria, which may be related to the use of anti-angiogenic agents. In general, three (4.3%) patients discontinued due to treatment-related AEs in our study, which indicates that anti-PD-1 combined with anti-angiogenesis therapy is tolerable in the real world.
Table 3.
Treatment-related adverse events.
3.1. Follow-up
The interval from the diagnosis to the initiation of immunotherapy combined with anti-angiogenesis therapy ranged from 0.1 to 79.9 months. Since the interval between initial diagnosis and combination therapy initiation spans a large range, we divided the patients into two groups at the dividing line of 6 months. Patients who started the combined therapy within 6 months had a better ORR compared with the others (59.1%, 95% CI: 36.8–81.4% vs 19.1%, 95% CI: 7.5–30.8%, P = .001). This suggests that the appropriate time for applying immunotherapy combined with anti-angiogenesis therapy may be the early stage after the initial diagnosis. At the completion of follow-up, 49 patients were still alive. A follow-up figure (Fig. 3) shows the temporal distribution of treatment with anti-PD-1 combined with anti-angiogenesis therapy and the subsequent survival time for each patient.
Figure 3.
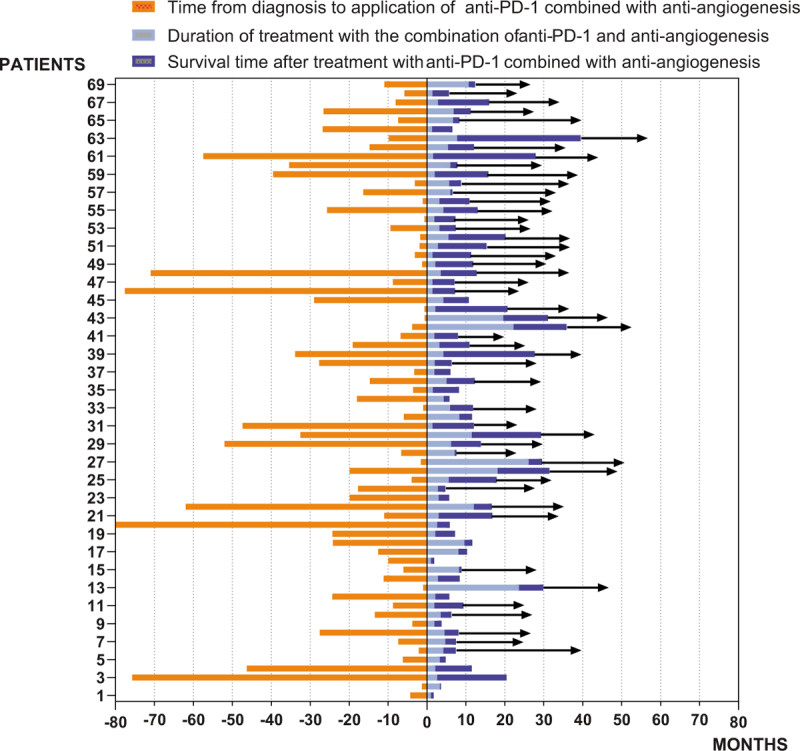
Follow-up of 69 patients (→indicates still living). Anti-PD-1 = anti-programmed death-1.
4. Discussion
Currently, immunotherapy is considered as a new revolution in cancer therapy. The blockade of immunological checkpoint pathways has become an important paradigm shift in the treatment of non-small cell lung cancer (NSCLC).[17] Anti-PD-1 antibodies (nivolumab and pembrolizumab) and anti-PD-L1 antibodies (atezolizumab) are approved by the US Food and Drug Administration and have become a new standard treatment for advanced NSCLC in recent years due to the enduring anti-tumor responses and survival advantage when compares with chemotherapy.[18] However, the results of immunotherapy monotherapy in clinical trials fails to meet our expectations, so we tried to explore the possibility of administering immunotherapy in combination with other medications.[19,20] Indeed, the combination of immunotherapy and anti-angiogenic therapy has recently emerged as a novel treatment pattern. This pattern is based on the observation that anti-angiogenic therapy enhances the transport of immune effector cells to tumor sites, thereby enhancing the efficacy of immunotherapy.[21,22] In the IMPOWER150 study, the combination of atezolizumab, bevacizumab and chemotherapy prolonged PFS and OS, providing evidence for the effectiveness of combination therapy. However, data on the real-world clinical effects of immunotherapy combined with anti-angiogenic therapy remain scarce.
Sixty-nine eligible patients with NSCLC were included in the real-world study. All patients received anti-PD-1 combined with anti-angiogenesis therapy. All data were collected in a real-world setting. Follow-up was performed until June 18, 2019, with a median follow-up time of 12.4 months. Fifty-one patients had achieved PFS, and the median PFS was 8.37 months. Only 20 patients reached the OS endpoint, while the median OS was not reached. From the perspective of short-term efficacy, the ORR was 31.9%, and the DCR was 89.9%.
The administration of anti-PD-1 combined with anti-angiogenesis therapy exerted a good therapeutic effect on real-world NSCLC patients. Additionally, this treatment pattern has promising prospects of clinical applications. Several clinical trials, such as CheckMate 057 and KEYNOTE-010, show that patients with NSCLC carrying EGFR mutations are not sensitive to immunotherapy.[23,24] A recognized explanation is that patients with mutations in genes such as EGFR, ALK, and BRAF do not readily produce new tumor antigens; thus, the tumor mutation burden is low.[25,26] Among the 69 patients with NSCLC, 16 patients had EGFR mutations, 43 patients had a negative EGFR mutation status, and information about the EGFR mutation status was not available for 10 patients. We divided the patients into two groups based on the EGFR mutation status and found that the median PFS of the EGFR mutation-positive group was 5.4 months, while the median PFS of the EGFR mutation-negative group was 10.5 months, P = .006. This result corresponds with previous clinical trials and provides a basis for the clinical application of immunotherapy combined with anti-angiogenic drugs. Subsequently, we analyzed whether there were differences in PFS within the EGFR mutation group. However, a reliable conclusion cannot be given due to the limited number of samples, which requires additional investigation.
Regarding the most appropriate time for the administration of anti-PD-1 combined with anti-angiogenesis therapy, first-line therapy was superior to other lines of therapy in terms of the ORR. Here 7 of the 17 patients receiving first-line treatment had not reached their PFS endpoint, and a significant difference in PFS was not observed, P = .155. Surprisingly, this treatment pattern resulted in a median PFS of 5.9 months for 24 patients who used this regimen after the third line, which has important implications for clinical practice.
In addition, 30 patients only used anti-PD-1 combined with anti-angiogenesis therapy, while other 39 patients received a combination therapy of anti-PD-1, chemotherapy and anti-angiogenesis in our study. Yet, significant differences in the ORR and PFS indicators were not observed between two groups. Of the 69 patients, 31 patients clearly displayed PD-L1 TPS (12 patients≥ 50%, 11 patients 1%–49%, and 8 patients< 1%), and correlation between the TPS of PD-L1 and the effect of this treatment regimen was not observed.
Immunotherapy based on anti-PD-1 has led to a new era in the treatment of cancer. We will only be able to better screen potentially responsive patients and maximize the clinical benefits of patients with cancer by identifying the appropriate predictors. The TPS of PD-L1, tumor mutational burden (TMB), and microsatellite instability play a vital role in predicting the efficacy of immunotherapy in many clinical trials.[27,28] KEYNOTE-010 was the first prospective study to confirm that PD-L1 expression is a useful biomarker for predicting the efficacy of pembrolizumab, while the use of TMB, microsatellite instability and other biomarkers as predictors of the efficacy of immunological checkpoint inhibitors is attracting increasing attention.[29,30] However, immunotherapy is often used in combination with other drugs in clinical practice. Few clinical studies have identified predictors of the efficacy of immunotherapy combined with anti-angiogenic therapy, an increasingly widespread treatment pattern. Due to the relatively small number of patients, we have not yet determined whether the TPS of PD-L1 predicts the efficacy of this scheme.[31] With the wide application of precision medical molecular detection technology such as genetic testing, cancer treatment has gradually evolved into an individualized and precise treatment. The identification of specific genetic targets for immunotherapy combined with anti-angiogenic therapy is an important goal for clinicians.[32]
The limitations of this observational study still exist. The total number of patients is not enough, especially for some subgroup analysis, and the follow-up time was short; thus, the OS was not available for our analysis. Follow-up will be continued in future work.
In conclusion, we examined the real-world use of anti-PD-1 combined with anti-angiogenesis therapy in patients with NSCLC. Our cohort had a more diverse background than patients in previous clinical trials. These clinically relevant data support the use of anti-PD-1 combined with anti-angiogenesis therapy as a new treatment approach for patients with NSCLC, particularly for the aforementioned groups of patients who might receive a benefit.
Author contributions
Conceptualization: Lupeng Qiu, Xiao Zhao, Shunchang Jiao.
Data curation: Lupeng Qiu, Xiao Zhao, Weiwei Shi, Shengjie Sun, Guoqing Zhang, Qiong Sun, Boyu Qin.
Formal analysis: Lupeng Qiu, Xiao Zhao, Shengjie Sun, Shunchang Jiao.
Funding acquisition: Guoqing Zhang.
Investigation: Jing Meng, Boyu Qin.
Methodology: Lupeng Qiu, Xiao Zhao, Weiwei Shi, Shengjie Sun, Qi Xiong, Shunchang Jiao.
Resources: Weiwei Shi, Qi Xiong.
Software: Lupeng Qiu, Xiao Zhao, Guoqing Zhang, Jing Meng.
Supervision: Qiong Sun, Shunchang Jiao.
Writing – original draft: Lupeng Qiu, Shunchang Jiao.
Writing – review & editing: Lupeng Qiu, Xiao Zhao, Shunchang Jiao.
Footnotes
Abbreviations: DCR = disease control rate, EGFR = epidermal growth factor receptor, KPS = karnofsky performance status, NSCLC = non-small cell lung cancer, OS = overall survival, ORR = overall response rate, PD-1 = programmed death 1, PD-L1 = programmed death-1 ligand, PD = progression of disease, PFS = progression free survival, PR = partial response, RECIST = response evaluation criteria in solid tumors, SD = stable disease.
How to cite this article: Qiu L, Zhao X, Shi W, Sun S, Zhang G, Sun Q, Meng J, Xiong Q, Qin B, Jiao S. Real-world treatment efficacy of anti-programmed death-1 combined with anti-angiogenesis therapy in non-small cell lung cancer patients. Medicine. 2020;99:24(e20545).
LQ and XZ contributed equally to this work.
This study was approved by the Ethics Committee of Chinese People's Liberation Army General Hospital [S2018-203-01]. Informed consent was waived due to the retrospective nature of this study, approved by the institutional review board.
Editorial and medical writing support was provided by Haiying Yang, Medical Affairs, LinkDoc Technology Co, Ltd, Beijing, China.
The authors report no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Pierce BA. CA: A Cancer Journal for Clinicians. Biomedical Market Newsletter. 2012. [Google Scholar]
- [2].Chen W, Sun K, Zheng R, et al. Cancer incidence and mortality in China, 2014. Chin J Cancer Res 2018;30:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zheng R, Zeng H, Zhang S, et al. Estimates of cancer incidence and mortality in China, 2013. Chin J Cancer 2017;36:384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (Anti–Programmed Death 1 Antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non–small-cell lung cancer. J Clin Oncol 2016;33:2004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Reck M, Rodríguezabreu D, Robinson AG, et al. KEYNOTE-024: Pembrolizumab (pembro) vs platinum-based chemotherapy (chemo) as first-line therapy for advanced NSCLC with a PD-L1 tumor proportion score (TPS) ≥50%. Ann Oncol 2016;27(suppl_6): [Google Scholar]
- [9].Reck M, Rodríguezabreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823. [DOI] [PubMed] [Google Scholar]
- [10].Yasuda S, Sho M, Yamato I, et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin Exp Immunol 2013;172:500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wu FTH, Xu P, Chow A, et al. Pre- and post-operative anti-PD-L1 plus anti-angiogenic therapies in mouse breast or renal cancer models of micro- or macro-metastatic disease. Br J Cancer 2019;120:196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol 2013;31:2205–U210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018;378: NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- [14].Santini FC, Rudin CM. Atezolizumab for the treatment of non-small cell lung cancer. Expert Rev Clin Pharmacol 2017;10:935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Song P, Zhang J, Shang C, et al. Real-world evidenceand clinical observations of the treatment of advanced non-small cell lung cancer with PD-1/PD-L1 inhibitors. Sci Rep 2019;9:4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- [17].Huang Q, Zhang H, Hai J, et al. Impact of PD-L1 Expression, Driver Mutations and Clinical Characteristics on Survival after Anti-PD-1/PD-L1 Immunotherapy versus Chemotherapy in Non-Small-Cell Lung Cancer: a Meta-analysis of Randomized Trials. Oncoimmunology. 2017:00–00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Brahmer JR, Drake CI, Powderly JD, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yang JC, Leah H, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003;349:427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sun X, Roudi R, Chen S, et al. Immune-related adverse events associated with PD-1 and PD-L1 inhibitors for nonsmall cell lung cancer: Protocol for a systematic review and meta-analysis. Medicine 2017;96:e8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yi M, Jiao D, Qin S, et al. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer 2019;18:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gamerith G, Kocher F, Rudzki J, et al. ASCO 2018 NSCLC highlights—combination therapy is key. Memo 2018;11:266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017;35:3924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540–50. [DOI] [PubMed] [Google Scholar]
- [25].Barnfield PC, Ellis PM. Second-LINE TREATMENT OF NON-SMALL CELL LUNG CANCER: NEW DEVELOPMENTS FOR TUMOURS NOT HARBOURING TARGETABLE ONCOGENIC DRIVER MUTATIONS. Drugs 2016;76:1321–36. [DOI] [PubMed] [Google Scholar]
- [26].Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69-74. [DOI] [PubMed] [Google Scholar]
- [27].Davis AA, Chae YK, Agte S, et al. Abstract 658: Association of tumor mutational burden (TMB) with DNA repair mutations and response to anti-PD-1/PD-L1 therapy in non-small cell lung cancer (NSCLC). Cancer Research. 2017;77:658-658. [DOI] [PubMed] [Google Scholar]
- [28].Chae YK, Pan A, Davis AA, et al. Biomarkers for PD-1/PD-L1 blockade therapy innon–small-cell lung cancer: is PD-L1 expression a good marker for patient selection? Clin Lung Cancer 2016;17:350–61. [DOI] [PubMed] [Google Scholar]
- [29].Dirk S, F, Stephen H, Caroline R, et al. Pooled analysis of long-term survival data from phase II and Phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015;33:1889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Seruga B, Ocana A, Amir E, et al. Failures in phase III: causes and consequences. Clin Cancer Res 2015;21:4552–60. [DOI] [PubMed] [Google Scholar]
- [31].Rizvi NA, Hellmann MD, Alexandra S, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dai Y, Grant S. New insights into checkpoint kinase 1 in the DNA damage response signaling network. Clin Cancer Res 2010;16:376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


