Supplemental Digital Content is available in the text
Keywords: incidence, outcome, predictors, prosthesis-patient mismatch, transcatheter aortic valve replacement
Abstract
Background:
Prosthesis-patient mismatch (PPM) following transcatheter aortic valve replacement (TAVR) is common, but the incidence, predictors and outcome of PPM are still controversial.
Methods:
A total of 18 articles incorporating 72,016 patients were identified form PubMed and Embase online database.
Results:
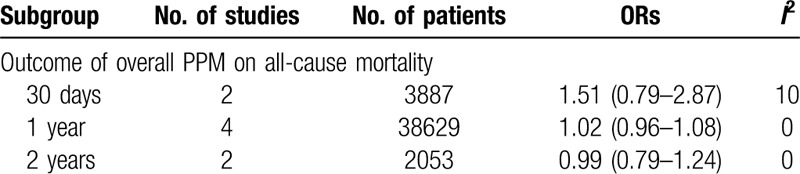
The pooled incidences of overall, and severe PPM following TAVR were 32.0% and 10.0% separately. Comparing to surgical aortic valve replacement (SAVR), TAVR had lower incidence of overall (OR, 0.31, 95% CI, 0.20–0.50) and severe PPM (OR, 0.38, 95% CI, 0.28–0.52). PPM was associated with a larger body surface area (BSA), larger body mass index (BMI) and previous myocardial infarction in comparison with those patients without PPM. Although PPM was not rare after TAVR, no significant differences were observed both in short- and mid-term all-cause mortality (30 day: OR: 1.51, 95% CI, 0.79–2.87, 1 year: OR: 1.02, 95% CI, 0.96–1.08, and 2 years: OR: 0.99, 95% CI, 0.79–1.24) between patients with PPM and those without PPM.
Conclusions:
Despite the fact that the incidence of PPM was lower than that of SAVR, PPM was not seen to have an impact on short- and mid-term survival.
1. Introduction
Aortic stenosis is the most prevalent of all valvular heart diseases in developed countries, especially among old patients. In the Cardiovascular Health Study, which included 5201 men and women older than 65 years, a clear increase in prevalence of aortic stenosis was seen with age: 1.3% in patients aged 65 to 75 years, 2.4% in those aged 75 to 85 years, and 4% in patients older than 85 years. Patients with severe aortic stenosis have a terrible prognosis, with three-quarters dying within 3 years of symptom onset. The mean survival of patients with symptoms of aortic stenosis was remarkably increased in patients treated with aortic valve replacement vs those not undergoing this procedure.[1] Initially, surgery was the only way for valve replacement and many patients who had been extremely ill from aortic valve stenosis and unresponsive to medical therapy were restored to good health by surgical aortic valve replacement (SAVR).[2] However, there are still many problems after the surgery, prosthesis-patient mismatch (PPM) is 1 of them.
PPM is an indicator of the intrinsic relationship of the implanted valve to the cardiac output requirements of the patient.[3] Prosthesis-patient mismatch occurs in the setting of a morphologically normal valve and is considered to be hemodynamically insignificant if the indexed EOA > 0.85 cm2/m2, moderate if between 0.65 and 0.85 cm2/m2, and severe if < 0.65 cm2/m2.[4] Some studies stated that severe PPM is associated with increased short- and long-term mortality, worse post perioperative heart function, and less regression of left ventricular (LV) hypertrophy.[5–10]
Apart from the PPM, for patients with severe aortic stenosis who are not suitable candidates for surgery, transcatheter aortic valve replacement (TAVR) should be considered and recommended. TAVR could effectively reduce the rates of death and hospitalization, with a decrease in symptoms and an improvement in valve hemodynamics.[11] With the prosperous development of techniques and prostheses, it is predictable that TAVR will be common among patients with severe aortic stenosis. Recently, more and more evidence also demonstrated that TAVR have comparable results in patients with intermediate surgical risk, compared with SAVR.[12,13]
Considering the potential damage of PPM, it is meaningful and important to study the PPM after TAVR. There are some studies that reported the relationship between PPM and TAVR, but the conclusions are controversial.[14,15] Hence, we aimed to offer a meta-analysis to comprehensively and quantitatively investigate the incidence, predictors, and outcome of PPM after TAVR.
2. Methods
2.1. Literature search and study selection
Ethical approval and participants informed consent were not necessary because all data were extracted from previously published studies. The process of study selection was illustrated in Figure 1. The search strategy was described in supplementary material. The Articles were included if they
Figure 1.
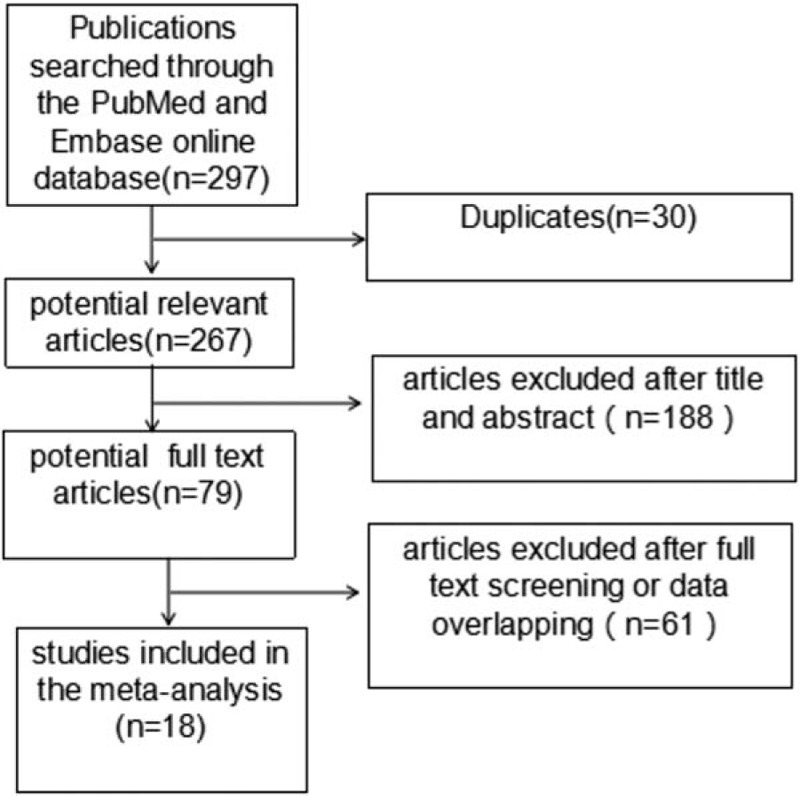
Flow diagram of citation research and selection.
-
1.
included the exact number or incidence of PPM;
-
2.
defined the PPM as insignificant if the indexed EOA > 0.85 cm2/m2, moderate if between 0.65 and 0.85 cm2/m2, and severe if <0.65 cm2/m2;
-
3.
indicated the predictive factors of PPM;
-
4.
displayed the all-cause mortality of PPM;
-
5.
were human adult studies and published in English.
The exclusion criteria were editorials, reviews, and case reports. There were 79 studies left after screening the titles and abstracts. Following full text screening and overlapped data removing, a total of 18 studies,[14–31] incorporating 72,016 patients were eligible.
2.2. Data extraction
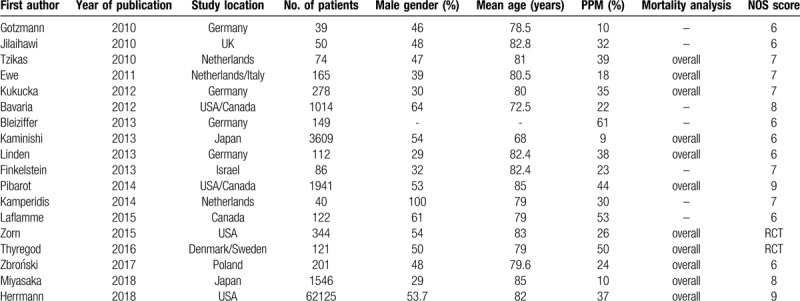
The 2 authors (Shixin He and Zhenfei Fang) independently extracted the data. The basic characteristics from eligible studies including author, year of publication, study location, patient baseline characteristics, the prevalence of PPM, and mortality analysis (Table 1). PPM in our meta-analysis was defined: moderate PPM (indexed EOA ≥ 0.65 cm2/m2 and ≤0.85 cm2/m2); severe PPM (index EOA < 0.65 cm2/m2).
Table 1.
The study characteristics.
2.3. Quality assessment
The quality of eligible studies were assessed using the NOS scale (NOS score was listed in Table 1). Overall quality of these eligible studies was good.
2.4. Statistical analysis
Pooled incidences, odds ratios (OR), mean difference and risk difference were acquired using the Review Manager version 5.3. A random-effects model was used to obtain the pooled OR. Heterogeneity was assessed by calculating the I2 statistic. Publication bias was assessed by the Egger test in the meta-analysis. If the P value was less than .05, then publication bias existed.
3. Results
3.1. Incidence of PPM
The pooled incidences of overall, and severe PPM after TAVR were 32.0%, and 10.0% separately.
3.2. TAVR vs SAVR
TAVR had lower incidence of overall (41% vs 61%, OR: 0.31, 95% CI, 0.20–0.50, I2 = 84, P < .001, Fig. 2), and severe PPM (13% vs 26%, OR: 0.38, 95% CI, 0.28–0.52, I2 = 48, P < .001, Fig. 3) than SAVR. The Egger regression test suggested that significant publication bias was not observed in this meta-analysis (P = .062 for overall PPM, P = .308 for severe PPM) (Table 2). The Egger funnel plots were provided in supplementary Figures.
Figure 2.
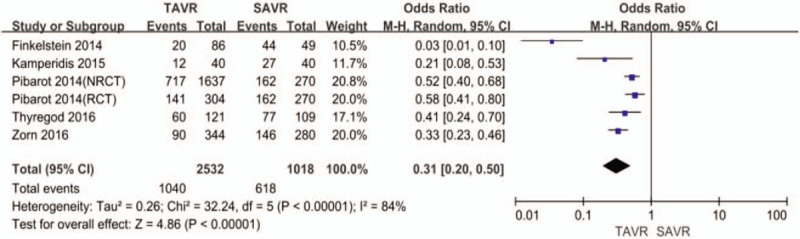
Odds ratio for overall prosthesis-patient mismatch comparing transcatheter aortic valve replacement with surgical aortic valve replacement.
Figure 3.
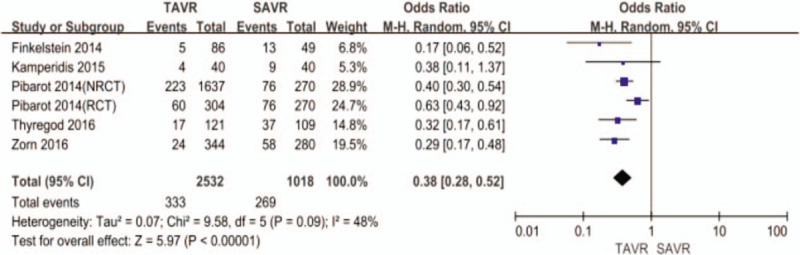
Odds ratio for severe prosthesis-patient mismatch comparing transcatheter aortic valve replacement with surgical aortic valve replacement.
Table 2.
The Egger test of publication bias.

3.3. Predictive factors
In order to investigate the predictors of PPM, we pooled some of the included studies using the univariate analysis method (Figs. 4–6). The differences of BSA, BMI, and previous myocardial infarction are statistically significant between PPM group and No PPM group. The PPM group was associated with larger body surface area (BSA), larger body mass index (BMI), and previous myocardial infarction.
Figure 4.
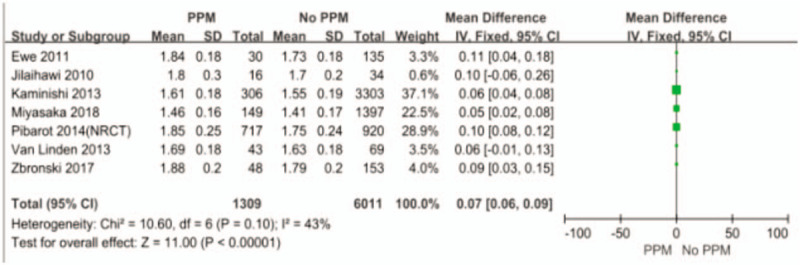
The difference of BSA between PPM and No PPM.
Figure 6.
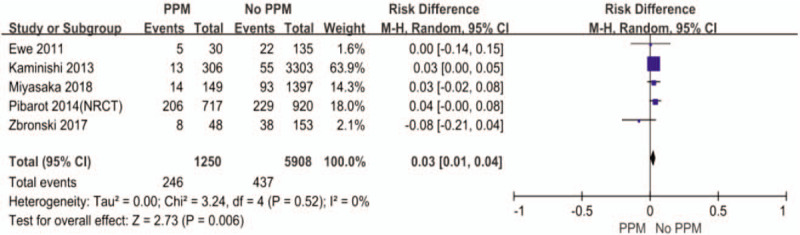
The difference of previous myocardial infarction between PPM and No PPM.
Figure 5.
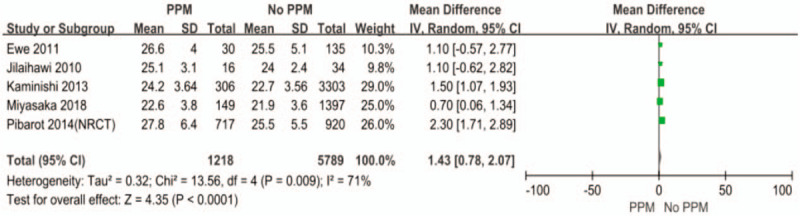
The difference of BMI between PPM and No PPM.
3.4. Outcome of PPM
There was no significant difference between patients with PPM and those without PPM in both short-term and mid-term all-cause mortality (PPM vs No-PPM: 30 day: OR: 1.51, 95% CI, 0.79–2.87, 1 year: OR: 1.02, 95% CI, 0.96–1.08, and 2 years: OR: 0.99, 95% CI, 0.79–1.24) (Table 3).
Table 3.
The outcome of PPM on all-cause mortality.
4. Discussion
The reported incidence of PPM after SAVR is diverse and ranging from 20% to 70%.[26,32] The impact of PPM on patients prognosis is still controversial.[3,33,34] There are some explanations that explain these discrepancies, for example:
-
1.
different parameters used to define PPM and different methods used to estimate the EOA;
-
2.
diverse types and sizes of prosthesis;
-
3.
population heterogeneity.[33]
To overcome the above limitations of studies, meta-analysis is necessary. Promisingly, comparing to SAVR, TAVR was associated with lower risk in the prevalence of overall, moderate and severe PPM in our meta-analysis.
The pooled incidence of PPM following TAVR was 32%, while the prevalence of severe PPM was 10% in our meta-analysis. The definition of PPM in our eligible studies was based on measured EOA indexed to BSA. To evaluate the influence of PPM after TVAR more precisely, it is indispensable to standardize the measure of EOA (the data from in vivo, in vitro or by Doppler echocardiography). There is no doubt that invasive micromanometer catheter assessment of valves is the most accurate, but the application would be medically inappropriate after TAVR. In addition, considering the correlation between left ventricular output tract diameter (LVOTd) and EOA, the precise measurements of LVOTd is also vital for the reporting prevalence of PPM.
Now that PPM does exit in many patients after aortic valve replacement, we want to know the exact predictors of PPM, which may facilitate the clinical work. Larger BSA and BMI, previous myocardial infarction were the significant predictors in our meta-analysis. BSA and BMI are closely related to the choice of proper prosthesis and the calculation of PPM. Previous myocardial infarction is associated with poor vascular condition and increased risk of calcification of aortic valve, which may restrict the doctors from implanting a larger valve. Moreover, Dayan et al reported that female sex, older age, hypertension, diabetes, and renal failure were the main predictors for PPM.[33] Therefore, to exactly determine the predictors of PPM, more precise and comprehensive patients information are needed.
Arguably, PPM after TAVR was not associated with increased short- and mid-term all-cause mortality in our meta-analysis, which was in accordance with the previous study.[26] However, in some studies, severe PPM predicted higher mid-term mortality in a multivariable analysis.[35,36] Several published studies, Takagi et al,[37] Chen et al,[38] and Head et al,[3] reported a risk increase of 31%, 34%, and 42%, respectively, in mid and late all-cause mortality in patients with any degree of PPM. This paradox may be related to the absence of severe PPM subgroup in our analysis of outcome, the influence of individual preoperative characteristics and baseline comorbidities. Furthermore, our analysis included some newest large studies, which made it different from the others. Nonetheless, the influence of PPM on TAVR would be changeable with the development of new techniques and studies.
5. Limitations
There were several limitations that must be taken into account while interpreting the conclusions of the present meta-analysis. First, the included studies were small and mainly from America and Europe, so it would be more representative if patients from different continents are included. Second, studies focusing on severe PPM are still rare, therefore it is difficult to determine severe PPMs effect after TAVR. Third, although we tried our best to accomplish this meta-analysis, incomplete retrieval of identified research and reporting bias may be present.
6. Conclusion
TAVR in this study was associated with a significantly lower risk of overall, and severe PPM compared with SAVR. Although PPM after TAVR did not display a significant harmful effect on short- and mid-term all-cause mortality, it still seems reasonable to struggle to optimize TAVR hemodynamic performance and reduce the occurrence of PPM.
Author contributions
Conceptualization: Zhenfei Fang.
Data curation: Shixin He, Zhenfei Fang.
Methodology: Shixin He, Zhenfei Fang.
Supervision: Zhenfei Fang.
Writing – original draft: Shixin He and Zhenfei Fang.
Writing – review and editing: Shixin He and Zhenfei Fang.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: BSA = body surface area, BMI = body mass index, EOA = effective orifice area, LVOTd = left ventricular output tract diameter, OR = odds ratio, PPM = prosthesis-patient mismatch, SAVR = surgical aortic valve replacement, TAVR = transcatheter aortic valve replacement.
How to cite this article: He S, Fang Z. Incidence, predictors and outcome of prosthesis-patient mismatch after transcatheter aortic valve replacement: a meta-analysis. Medicine. 2020;99:24(e20717).
There is no funding to state. We declare that there was no commercial interest or conflict of interest for this study.
Supplemental Digital Content is available for this article.
The datasets generated during and/or analyzed during the current study are publicly available.
References
- [1].Carabello BA, Paulus WJ. Aortic stenosis. Lancet 2009;373:956–66. [DOI] [PubMed] [Google Scholar]
- [2].Lawrie GM. Role of transcatheter aortic valve implantation (TAVI) versus conventional aortic valve replacement in the treatment of aortic valve disease. Methodist Debakey Cardiovasc J 2012;8:4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Head SJ, Mokhles MM, Osnabrugge RL, et al. The impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: a systematic review and meta-analysis of 34 observational studies comprising 27 186 patients with 133 141 patient-years. Eur Heart J 2012;12:1518–29. [DOI] [PubMed] [Google Scholar]
- [4].Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol 2012;15:1438–54. [DOI] [PubMed] [Google Scholar]
- [5].Rao V, Jamieson WR, Ivanov J, et al. Prosthesis patient mismatch affects survival after aortic valve replacement. Circulation 2000;102:1115–9. [DOI] [PubMed] [Google Scholar]
- [6].Blais C, Dumesnil JG, Baillot R, et al. Impact of valve prosthesispatient mismatch on short-term mortality after aortic valve replacement. Circulation 2003;108:983–8. [DOI] [PubMed] [Google Scholar]
- [7].Pibarot P, Dumesnil JG, Lemieux M, et al. Impact of prosthesis-patient mismatch on hemodynamic and symptomatic status, morbidity and mortality after aortic valve replacement with a bioprosthetic heart valve. J Heart Valve Dis 1998;7:211–8. [PubMed] [Google Scholar]
- [8].Fuster RG, Montero Argudo JA, Albarova OG. Patient-prosthesis mismatch in aortic valve replacement: really tolerable? Eur J Cardiothorac Surg 2005;27:441–9. [DOI] [PubMed] [Google Scholar]
- [9].Fuster RG, Estevez V, Rodríguez I. Prosthesis-patient mismatch with latest generation supra-annular prostheses: the beginning of the end? Interact Cardiovasc Thorac Surg 2007;6:462–9. [DOI] [PubMed] [Google Scholar]
- [10].Kandler K, Møller CH, Hassager C, et al. Patient-prosthesis mismatch and reduction in left ventricular mass after aortic valve replacement. Ann Thorac Surg 2013;96:66–71. [DOI] [PubMed] [Google Scholar]
- [11].Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;17:1609–20. [DOI] [PubMed] [Google Scholar]
- [12].Makkar RR, Fontana GP, Jilaihawi H, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012;18:1696–704. [DOI] [PubMed] [Google Scholar]
- [13].Sondergaard L, Steinbruchel DA, Ihlemann N, et al. Two-year outcomes in patients with severe aortic valve stenosis randomized to transcatheter versus surgical aortic valve replacement: the all-comers nordic aortic valve intervention randomized clinical trial. Circ Cardiovasc Interv 2016;6. [DOI] [PubMed] [Google Scholar]
- [14].Bleiziffer S, Hettich I, Hutter A, et al. Incidence and impact of prosthesis-patient mismatch after transcatheter aortic valve implantation. J Heart Valve Dis 2013;3:309–16. [PubMed] [Google Scholar]
- [15].Kukucka M, Pasic M, Unbehaun A, et al. Patient-prosthesis mismatch after transapical aortic valve implantation: Incidence and impact on survival. Eur Heart J Cardiovasc Imaging 2011;i133–4. [DOI] [PubMed] [Google Scholar]
- [16].Bavaria JE, Desai ND, Cheung A, et al. The St Jude Medical Trifecta aortic pericardial valve: results from a global, multicenter, prospective clinical study. J Thorac Cardiovasc Surg 2014;2:590–7. [DOI] [PubMed] [Google Scholar]
- [17].Ewe SH, Delgado V, Tamborini G, et al. Impact of prosthesis-patient mismatch on hemodynamic and clinical outcomes after transcatheter aortic valve implantation with balloon-expandable valves. Eur Heart J 2011;904. [DOI] [PubMed] [Google Scholar]
- [18].Finkelstein A, Schwartz AL, Uretzky G, et al. Hemodynamic performance and outcome of percutaneous versus surgical stentless bioprostheses for aortic stenosis with anticipated patient-prosthesis mismatch. J Thorac Cardiovasc Surg 2014;6:1892–9. [DOI] [PubMed] [Google Scholar]
- [19].Gotzmann M, Lindstaedt M, Bojara W, et al. Hemodynamic results and changes in myocardial function after transcatheter aortic valve implantation. Am Heart J 2010;5:926–32. [DOI] [PubMed] [Google Scholar]
- [20].Herrmann HC, Daneshvar SA, Fonarow GC, et al. Prosthesis-Patient Mismatch in 62,125 Patients Following Transcatheter Aortic Valve Replacement: From the STS/ACC TVT Registry. J Am Coll Cardiol 2018;72:2701–11. [DOI] [PubMed] [Google Scholar]
- [21].Jilaihawi H, Chin D, Spyt T, et al. Prosthesis-patient mismatch after transcatheter aortic valve implantation with the Medtronic-Corevalve bioprosthesis. Eur Heart J 2010;7:857–64. [DOI] [PubMed] [Google Scholar]
- [22].Kaminishi Y, Misawa Y, Kobayashi J, et al. Patient-prosthesis mismatch in patients with aortic valve replacement. Gen Thorac Cardiovasc Surg 2013;5:274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kamperidis V, van Rosendael PJ, de Weger A, et al. Surgical sutureless and transcatheter aortic valves: hemodynamic performance and clinical outcomes in propensity score-matched high-risk populations with severe aortic stenosis. JACC Cardiovasc Interv 2015;5:670–7. [DOI] [PubMed] [Google Scholar]
- [24].Laflamme J, Puri R, Urena M, et al. Incidence and risk factors of hemolysis after transcatheter aortic valve implantation with a balloon-expandable valve. Am J Cardiol 2015;11:1574–9. [DOI] [PubMed] [Google Scholar]
- [25].Miyasaka M, Tada N, Taguri M, et al. Incidence, predictors, and clinical impact of prosthesis-patient mismatch following transcatheter aortic valve replacement in asian patients: the OCEAN-TAVI registry. JACC Cardiovasc Interv 2018;8:771–80. [DOI] [PubMed] [Google Scholar]
- [26].Pibarot P, Weissman NJ, Stewart WJ, et al. Incidence and sequelae of prosthesis-patient mismatch in transcatheter versus surgical valve replacement in high-risk patients with severe aortic stenosis: a PARTNER trial cohort--a analysis. J Am Coll Cardiol 2014;13:1323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Thyregod HG, Steinbruchel DA, Ihlemann N, et al. No clinical effect of prosthesis-patient mismatch after transcatheter versus surgical aortic valve replacement in intermediate- and low-risk patients with severe aortic valve stenosis at mid-term follow-up: an analysis from the NOTION trial. Eur J Cardiothorac Surg 2016;4:721–8. [DOI] [PubMed] [Google Scholar]
- [28].Tzikas A, Piazza N, Geleijnse ML, et al. Prosthesis-patient mismatch after transcatheter aortic valve implantation with the medtronic CoreValve system in patients with aortic stenosis. Am J Cardiol 2010;2:255–60. [DOI] [PubMed] [Google Scholar]
- [29].Van Linden A, Kempfert J, Blumenstein J, et al. Prosthesis-patient mismatch after transcatheter aortic valve implantation using the Edwards SAPIEN prosthesis. Thorac Cardiovasc Surg 2013;5:414–20. [DOI] [PubMed] [Google Scholar]
- [30].Zbronski K, Rymuza B, Scislo P, et al. Patient-prosthesis mismatch in patients treated with transcatheter aortic valve implantation - predictors, incidence and impact on clinical efficacy. A preliminary study. Postepy Kardiol Interwencyjnej 2017;4:281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zorn GR, Little SH, Tadros P, et al. Prosthesis-patient mismatch in high-risk patients with severe aortic stenosis: a randomized trial of a self-expanding prosthesis. J Thorac Cardiovasc Surg 2016;4:1014–22. 1021-1023. [DOI] [PubMed] [Google Scholar]
- [32].Dumesnil JG, Pibarot P. Prosthesis-patient mismatch: an update. Curr Cardiol Rep 2011;3:250–7. [DOI] [PubMed] [Google Scholar]
- [33].Dayan V, Vignolo G, Soca G, et al. Predictors and outcomes of prosthesis-patient mismatch after aortic valve replacement. JACC Cardiovasc Imaging 2016;8:924–33. [DOI] [PubMed] [Google Scholar]
- [34].Price J, Toeg H, Lam BK, et al. The impact of prosthesis-patient mismatch after aortic valve replacement varies according to age at operation. Heart 2014;14:1099–106. [DOI] [PubMed] [Google Scholar]
- [35].Munoz-Garcia AJ, Munoz-Garcia M, Carrasco-Chinchilla F, et al. Incidence and clinical outcome of prosthesis-patient mismatch after transcatheter aortic valve implantation with the CoreValve prosthesis. Int J Cardiol 2013;3:1074–6. [DOI] [PubMed] [Google Scholar]
- [36].Utsunomiya H, Mihara H, Itabashi Y, et al. Geometric changes in ventriculoaortic complex after transcatheter aortic valve replacement and its association with post-procedural prosthesis-patient mismatch: an intraprocedural 3D-TEE study. Eur Heart J Cardiovasc Imaging 2017;1:1–0. [DOI] [PubMed] [Google Scholar]
- [37].Takagi H, Yamamoto H, Iwata K, et al. A meta-analysis of effects of prosthesis-patient mismatch after aortic valve replacement on late mortality. Int J Cardiol 2012;159:150–4. [DOI] [PubMed] [Google Scholar]
- [38].Chen J, Lin Y, Kang B, et al. Indexed effective orifice area is a significant predictor of higher mid- and long-term mortality rates following aortic valve replacement in patients with prosthesis-patient mismatch. Eur J Cardiothorac Surg 2014;45:234–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


