Abstract
Objective:
To ascertain the efficacy and safety of daunorubicin combined with cytarabine comparing with idarubicin combined with cytarabine as a standard induction therapy for acute Myeloid leukemia by a meta-analysis.
Methods:
The randomized controlled trials included were retrieved from PubMed, Embase, and Cochrane library. We evaluated and cross-checked the randomized clinical trials (RCTs) comparing daunorubicin combined with cytarabine (DA) and idarubicin combined with cytarabine (IA) by two reviewers independently according to Cochrane Handbook for Systematic Reviewers of Interventions. The data of meta-analysis was conducted using Review Manager 5.3 and Stata 12.0 software.
Results:
A total of 6 studies containing 3140 patients were included. The primary outcomes were complete remission (CR), CR in one course (CR1), CR in two courses (CR2), overall survival (OS), and relapse rate. The secondary outcomes included adverse events and cytogenetic risk in subgroup analyses. IA showed a statistically significant in CR (RR = 1.05; 95%CI = 1.00–1.09, P = .03) and CR1 (RR = 1.11; 95%CI = 1.04–1.18, P = .003), but not in CR2 (RR = 0.97; 95%CI = 0.77–1.24, P = .83), and relapse rate (RR = 1.08; 95%CI = 0.98–1.43, P = .08). In high dose daunorubicin group, OS was significantly improved with IA compared to DA (HR = 0.89, 95%CI = 0.8–1.0, P = .041, I2 = 0). At grade 3/4 adverse events, the difference between IA and DA was not statistically significant (infection, P = .28; cardiac toxicity, P = .15; bleeding, P = .29). In the subgroup analysis, the genotypes of the IA and DA groups were not statistically significant for comparison of CR between the two groups (P = .07).
Conclusion:
This meta-analysis showed that IA had a better efficacy in the treatment of acute myeloid leukemia than DA, even with increased doses of DA. The OS of a standard dose of IA patients was longer than that of DA patients. Our research shows that anthracycline dose intensification of daunorubicin is of no clinically relevant benefit in AML patients comparing with a standard dose of IA. When it comes to adverse drug reactions, it is not a significant difference. Therefore, in clinical practice, IA should be the first choice for induction regimen in patients with acute myeloid leukemia.
Keywords: acute myeloid leukemia, cytarabine, daunorubicin, idarubicin, RCTs
1. Introduction
Acute myeloid leukemia (AML) is a malignant clonal proliferative tumor resulting from genetic alternation that increase immature hematopoietic cells in bone marrow and peripheral blood.[1] As the most common type of acute leukemias among adults. With clinical manifestations of anemia, bleeding, fever, and infection, AML could induce extremely complications resulting for the largest number of annual deaths from leukemias in the United States.[2]
Anthracycline antibiotic, especially daunorubicin and idarubicin, combined with cytarabine has been a classic induction regimen for treating AML in clinical guideline since 1980s.As the induction therapy treat for children and young adults, the complete response rate (CR) can reach 60% to 80%. The CR solution rate is about 50% in the elderly (>60 years old). However, the treatment of AML is still unsatisfactory. Only about 40% to 45% of AML patients achieve long-term disease-free survival (DFS). Unfortunately, most patients still die because of relapse of disease.[3]
In the past decades, a numerous randomized clinical trials (RCTs) compared daunorubicin with idarubicin respectively plus cytarabine as induction therapy in AML. In 1998, a review researching randomized trials comparing idarubicin with daunorubicin came to conclusion that idarubicin groups had higher complete remission (CR) rates (62% vs 53%; P = .002), fewer relapsed (P = .008) but slightly more died in remission.[4] A meta-analysis published in 2013 had showed idarubicin combined with cytarabine (IA) is more effective than daunorubicin combined with cytarabine (DA) in duration of overall survival (OS) in young patients, but the cytogenic risk in subgroup is unclear.[5] Similarly, the explicit reports have no conclusion in prevention of non-hematological toxicological reactions between DA and IA.
There is no evidence that whether IA/DA is better than the other in CR (CR), OS, relapse, and adverse events. In order to update the data, we include the latest literature to draw a serious conclusion. Based on the above studies, this meta-analysis mainly compares the efficacy and safety of daunorubicin and Idarubicin combined with cytarabine.
2. Materials and methods
2.1. Data sources
We searched the databases including PubMed, Embase, Cochrane library, and ClinicalTrials.gov (http://clinicaltrials.gov/) up to April 2019, combining the search terms (MeSH)
-
1.
daunorubicin;
-
2.
idarubicin;
-
3.
cytarabine;
-
4.
AML;
-
5.
RCTs.
We performed a manual search of abstracts from the annual meetings of the American Society of Hematology, the American Society of Clinical Oncology, and the European Hematology Association from 2000 to 2019. Studies identified underwent title and abstract review, discarded the clearly nonrelevant articles. Included language is English and only the last 20 years of literature were included.
2.2. Study selection
Clinical trials met the following predetermined criteria were eligible for inclusion:
-
1.
The patients in studies received either DA chemotherapy or IA chemotherapy as induction regimen;
-
2.
IA chemotherapy as an experimental group while DA as a control group;
-
3.
Randomized controlled trials with acute non-promyelocytic leukemia without age and sex restriction;
-
4.
Duplicated documents were selected with the latest and most complete data.
Excluded criteria were following:
-
1.
Documents with incomplete data, such as conference papers;
-
2.
Data from multiple publications of the same study at different time periods were excluded;
-
3.
Induction regimens with addition of other chemotherapeutic agents.
-
4.
Only phase II and III RCTs were included.
Study selection and data collection were conducted by two authors independently. All the data were checked with each other. Disagreement was resolved by discussion.
2.3. Data extraction
Two investigators independently searched the databases, any controversies between reviewers were discussed and resolved with consensus. The following data were extracted for each article: author's name, total number of patients, Mean/median age in years, DNR (daunorubicin)/IDR (idarubicin) ratio (mg/m2:mg/m2), dose of cytarabine (mg/m2), Median follow-up (month).
3. Methodological quality assessment
Each study was assessed by the Cochrane risk of bias assessment to identify the quality of included randomized trials. Evaluation criteria include the following components: random sequence generation, allocation concealment, blinding, incomplete outcome data, and other sources of bias. Risk of publication bias was assessed for each individual study by two reviewers independently examining the funnel plot.
3.1. Definition of outcomes
The primary outcome was CR, CR in one course (CR1), CR in two courses (CR2), OS, and relapse rate. The secondary outcomes included adverse events. Subgroup analyses were carried out for cytogenetic risks. All the outcomes were defined by the recommendation of International Working Group.[6]
3.2. Statistical analysis
All statistical analyses were performed using Review Manager ver.5.3 software and Stata ver.14.0 software. Dichotomous variables such as CR, adverse events and relapse rate were calculated as relative risks (RR). The survival outcomes such as OS were calculated as hazard ratio (HR). A P < .05 was considered to show a significant relationship.[7] The pooled results of each study were calculated by fixed-effects model (the Mantel–Haenszel method).[8] Respective 95% CIs were used as a fixed-effect model with the inverse variance approach unless moderate heterogeneity (I2 > 50% or P value < .1) was found.[9]
Heterogeneity pooled studies was calculated by using I2 of chi-square-based Q test and ranked as low (<30%), moderate (30–50%), or high (>50%). If I2 < 50% or P > .10, the study was considered no heterogeneity (According to Cochrane Handbook for Systematic Reviews of Interventions). If I2 > 50%, sensitivity test was used to examine the publication bias and there would be more discussion about the heterogeneity.
This work was performed in accordance with Quality of Reporting of Meta-analyses guidelines for meta-analysis of RCTs.
4. Results
4.1. Systematic review and qualitative assessment
According to the Inclusion and exclusion criteria, a total of 730 references were found. We read article titles and abstracts to exclude duplicate literature, irrelevant literature, and incomplete data. Six randomized controlled trials including 3140 patients fulfilled eligibility criteria (Table 1).[10–15] There were 1573 patients in the experimental group (idarubicin + cytarabine) and 1567 in the control group (daunorubicin + cytarabine). All the studies included were published between 2004 and 2017.
Table 1.
Characteristics of studies.
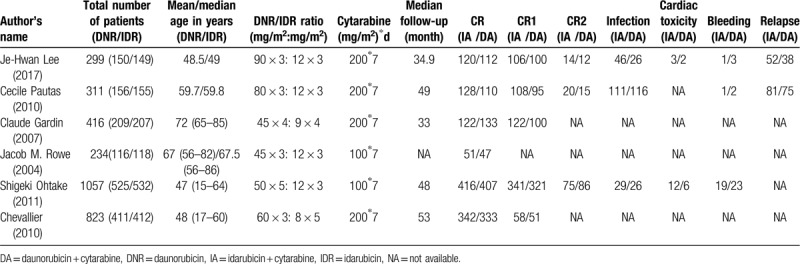
The RCTs compared daunorubicin at a dose of 45 to 90 mg/m2 per day with idarubicin at 8 to 12 mg/m2 per day for 3 to 5 days plus cytarabine at 100 or 200 mg/m2 per day for 7 days. The median age of patients ranged from 47 to 72 years old (Table 1). The CR rate (two period of treatment) ranged from 43% to 83% in IA groups and from 41% to 81% in DA groups. The median follow-up period ranged from 33 to 53 months. Only one trial had no reported median follow-up.
4.2. Risk of bias within studies
The risk bias was evaluated by Cochrane Handbook for Systematic Reviews of Interventions. It was formed using Review Manager 5.3 (Figs. 1–3). One trial had mentioned that it used computer for random sequence generation.[14] All the trials had explicit expression about incomplete outcome data and had no selective reporting. But allocation concealment and binding of participant and personnel were no reported in six studies.
Figure 1.
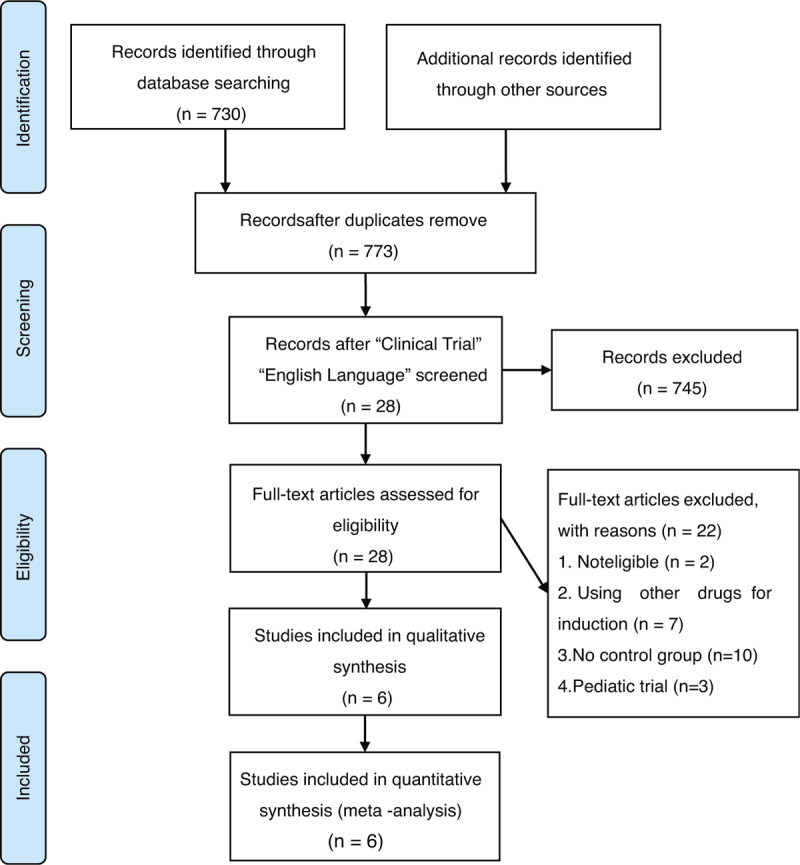
Flowchart of study screening and selection process.
Figure 3.
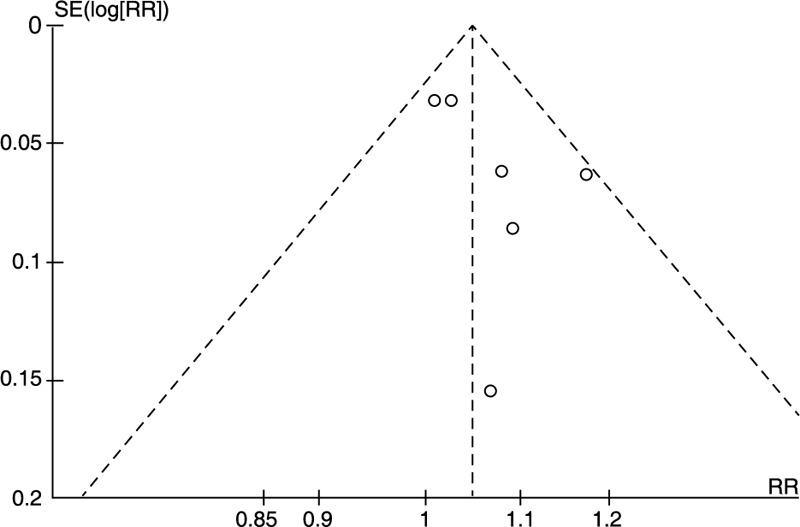
Funnel plot for publication bias test.
Figure 2.
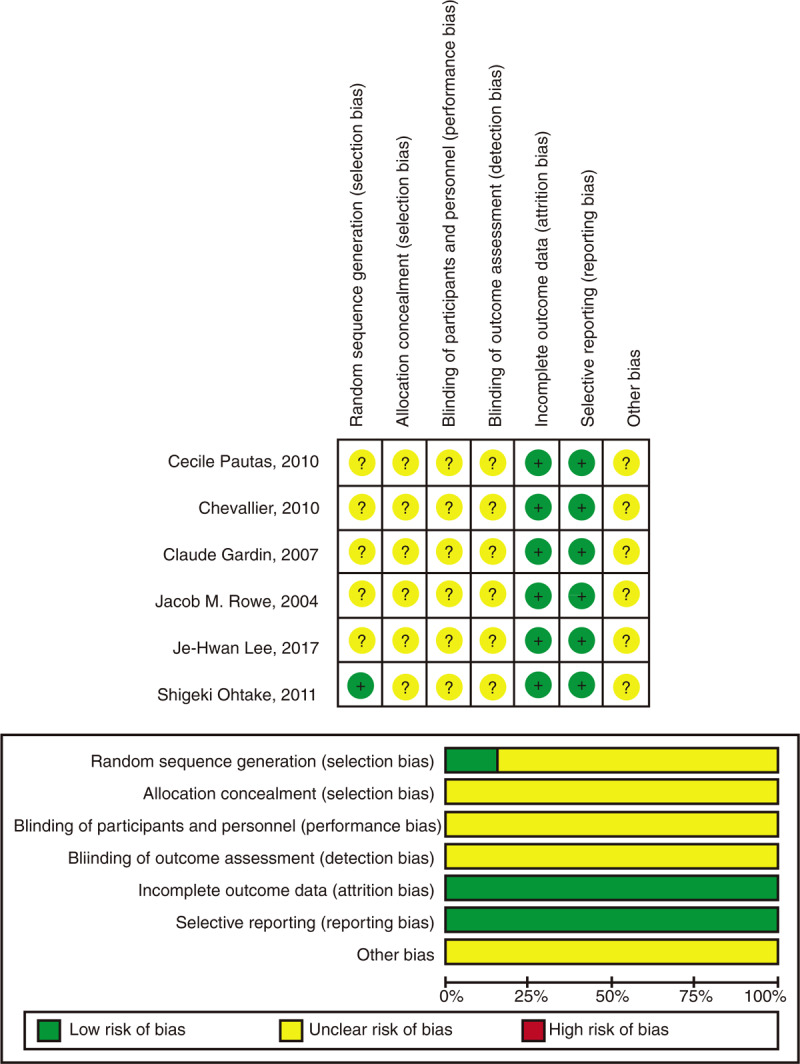
Risk of bias.
5. Meta-analysis
5.1. Complete remission
All studies reported CR rates of AML treated with IA and DA. The CR was performed within two induction cycles for all trials. Five studies reported CR1 (CR in one induction course) and three reported CR2 (CR in two induction courses).
IA treatment arms significantly improved the CR (RR = 1.05; 95%CI = 1.00–1.09, P = .03, Fig. 4A) and CR1 (RR = 1.11; 95%CI = 1.04–1.18, P = .003, Fig. 4B) than DA arms, but not in CR2 (RR = 0.97; 95%CI = 0.77–1.24, P = .83, Fig. 4C). Heterogeneity of all results was not statistically significant (I2 < 50), which demonstrated as same as the sensitivity analysis of CR results (Fig. 5).
Figure 4.
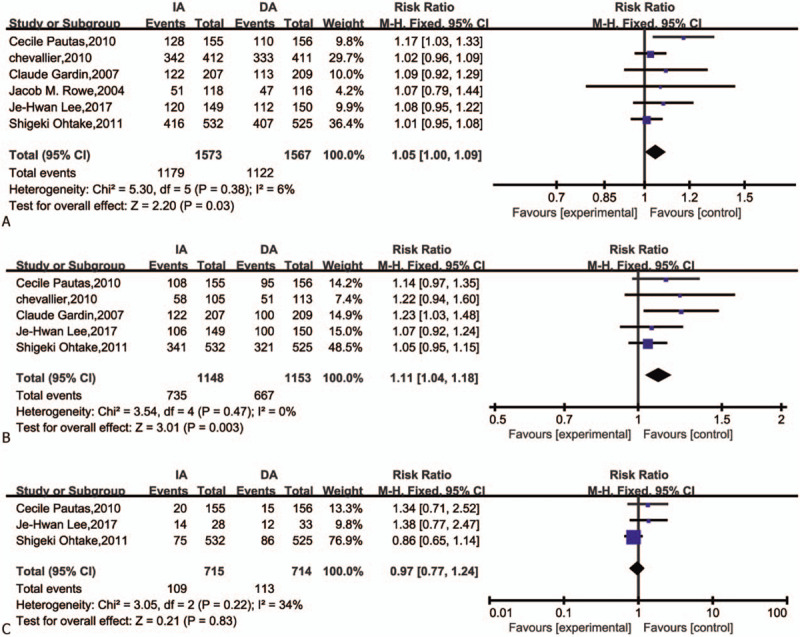
Forest plots of RR for CR, CR1, CR2 (CI = confidence interval, Fixed = fixed-effect model, M–H = Mantel–Haenszel method, RR = risk ratio). (A) CR complete remission; (B) CR1 complete remission in one course; (C) CR2 complete remission in two courses).
Figure 5.
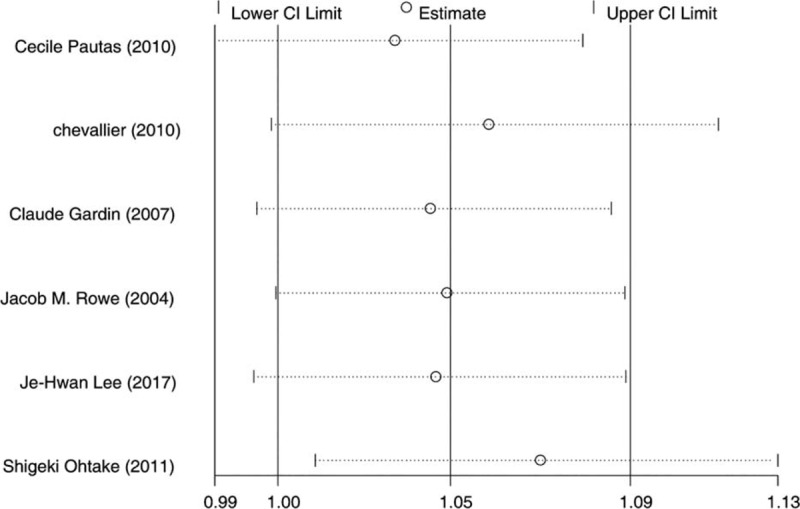
Sensitivity test for complete remission.
5.2. Toxicities
We included the following grade 3/4 adverse events: infection, cardiac toxicity, and bleeding, because they were reported comprehensively in these studies. The data for infection (Fig. 6A) was extracted from three studies. The results indicated that no meaningful difference between IA and DA in infection (RR = 1.08, 95%CI = 0.94–1.25, P = .29). Because of I2 > 50% (I2 = 73%), a sensitivity analysis by Stata 12.0 showed that one trial might has heterogeneity (Fig. 7). However, the result was no reversal after removed this article and carried out RR analysis (Fig. 8). There was also no difference in risk of grade 3/4 cardiac toxicity and bleeding between the IA and DA groups (Fig. 6B and C).
Figure 6.
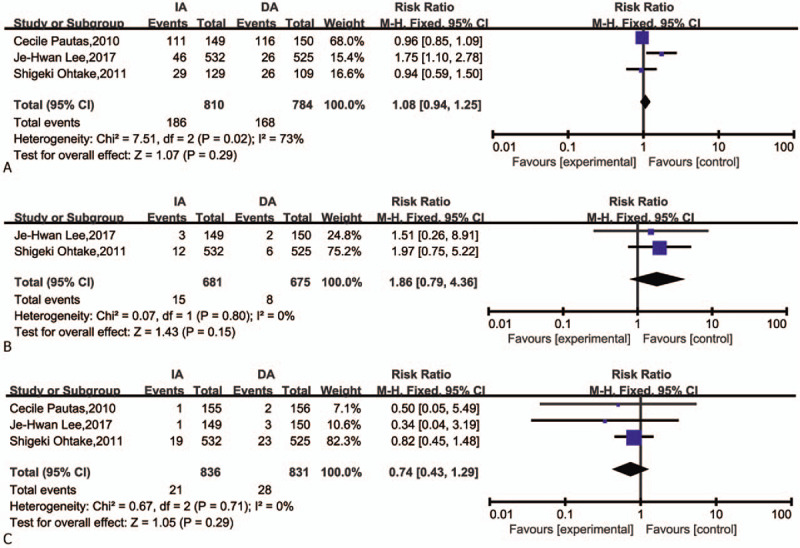
Forest plots of RR for infection, cardiac toxicity, and bleeding. (CI = confidence interval, Fixed = fixed-effect model, M–H = Mantel–Haenszel method, RR = risk ratio. Grade 3/4 adverse events). (A) Infection; (B) cardiac toxicity; (C) bleeding.
Figure 7.
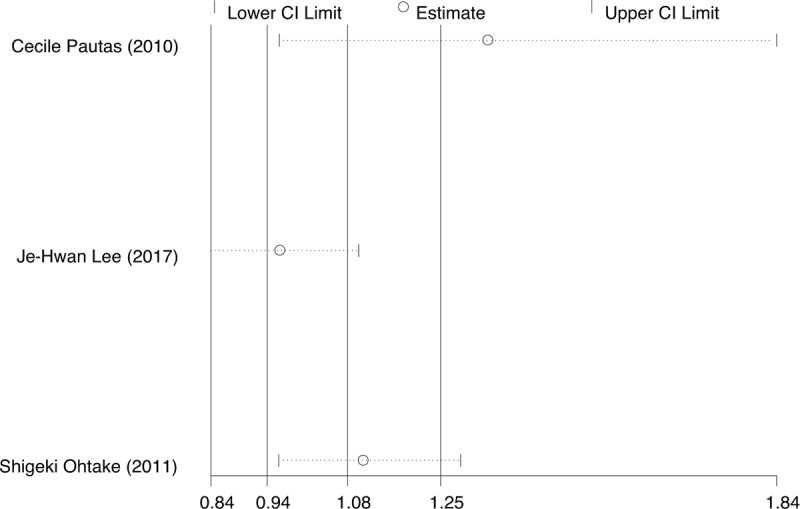
Sensitivity analysis for infection.
Figure 8.
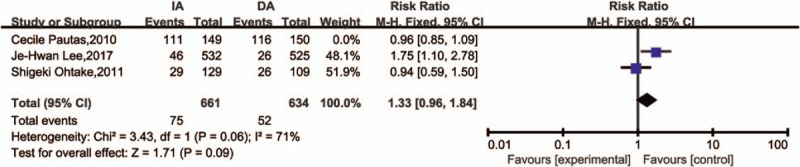
Forest plots of RR for infection excluded the one trial that might has heterogeneity (CI = confidence interval, Fixed = fixed-effect model, M–H = Mantel–Haenszel method, RR = risk ratio).
5.3. Relapse
Only two trials reported relapse rate. The result showed that no significant difference between IA and DA groups in relapse (RR = 1.18, 95%CI = 0.98–1.43, P = .08, Fig. 9).
Figure 9.

Forest plots of RR for relapse (CI = confidence interval, Fixed = fixed-effect model, M–H = Mantel–Haenszel method, RR = risk ratio).
5.4. Overall survival
We focused on high-dose DA regimens versus a standard dose of IA regimens in OS. High-dose DA regimen was defined as total daunorubicin dose ≥180 mg/m2 and daily dose ≥50 mg/m2 on the basis of the NCCN recommendations. The four included articles met the requirements of high dose DA. The total dose went from 180 to 270 mg/m2. The statistical results showed that the OS (HR = 0.89, 95%CI = 0.8–1.0, P = .041, I2 = 0) of IA was higher than that of DA, which was statistically significant (Fig. 10).
Figure 10.
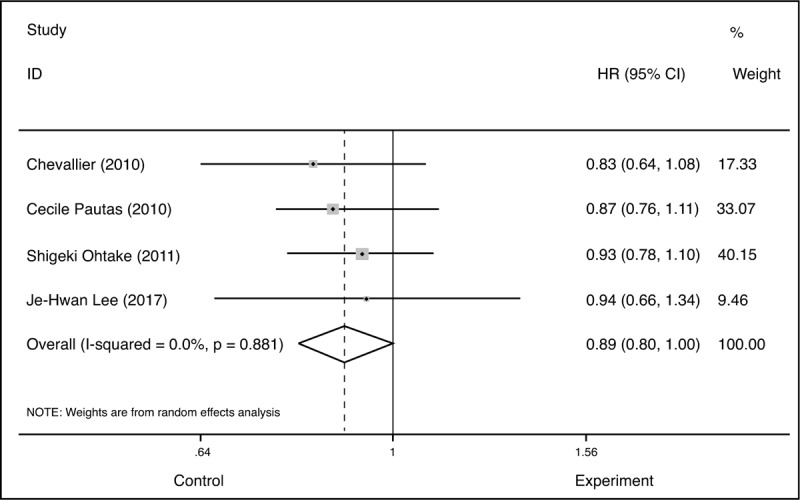
Forest plots of HR for overall survival (CI = confidence interval, Fixed = fixed-effect model, HR = hazard ratio, M–H = Mantel–Haenszel method).
5.5. Subgroup analysis
5.5.1. Cytogenetic risk
There were three measures for cytogenetic risk, favorable (RR = 0.95, 95%CI = 0.89–1.00, P = .07), intermediate (RR = 1.06, 95%CI = 1.00–1.13, P = .06), and adverse (RR = 1.19, 95%CI = 0.97–1.48, P = .10). The subgroup analysis for CR in accordance with cytogenetic risk group suggested the CR between IA and DA had no difference (Fig. 11). The heterogeneity was not significant (I2 < 50).
Figure 11.
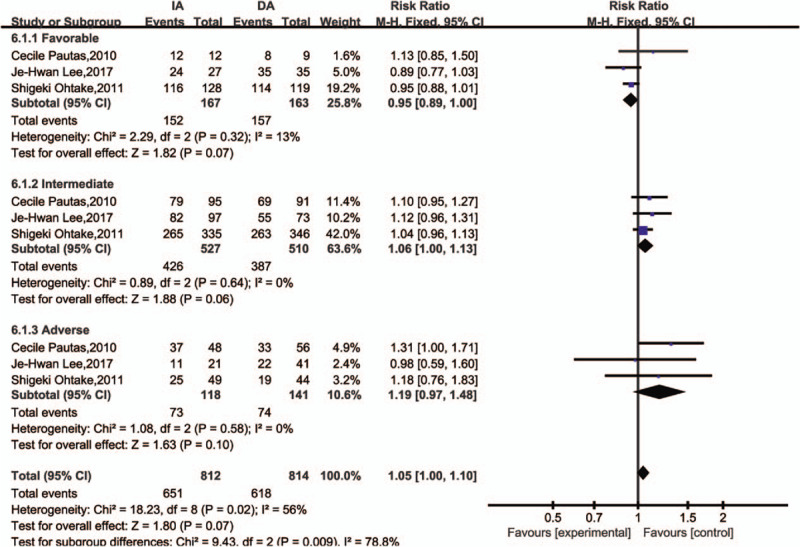
Forest plots of -RR for cytogenetic risks for complete remission (subgroup) (CI = confidence interval, Fixed = fixed-effect model, M–H = Mantel–Haenszel method, RR = risk ratio).
5.5.2. Age
The included articles were divided into two groups, with median age of two articles12,13 >60 as the elderly group (RR = 1.08, 95%CI = 0.94–1.26, P = .28) and another four articles were considered the younger group (RR = 1.05, 95%CI = 0.99–1.11, P = .09). We analyzed the CR in two subgroups, the results suggested no difference in the two groups (Fig. 12).
Figure 12.

Forest plots of RR for age for complete remission (subgroup) (CI = confidence interval, Fixed = fixed-effect model, M–H = Mantel–Haenszel method, RR = risk ratio).
6. Discussion
In this systematic review and meta-analysis, we assessed the efficacy and safety of daunorubicin versus idarubicin combined with cytarabine for induction therapy in AML.
Our analysis showed that IA treatment can increase CR (RR = 1.05; 95%CI = 1.00–1.09, P = .03) and OS (HR = 0.89, 95%CI = 0.8–1.0, P = .04) than DA treatment, but not in relapse (RR = 1.18, 95%CI = 0.98–1.43, P = .08). Our results were consistent with previous meta-analysis.[16] The difference between our analysis and previous meta-analysis lies in the included studies: we excluded old clinical studies, and result of one head-to-head research study was added.
Furthermore, our analysis showed that IA was associated with higher CR1 (RR = 1.11; 95%CI = 1.04–1.18, P = .003), but no significant differences in CR2 (RR = 0.97; 95%CI = 0.77–1.24, P = .83) compared with DA.
According to the NCCN guidelines, the best indicator of efficacy for AML is CR. But in our meta-analysis, only three of the included articles reported CR about the two courses of treatment. The results were not statistically significant, perhaps there were some deaths before the second course.
Therefore, we further investigated the effect of cytogenetic risk subgroup on prognosis because Genotype had the most significant effect on prognosis. Leukemia can be differentiated into multiple subtypes based on leukemogenic history and etiology. After the synthesis of the included literatures, we stratified cytogenetic risk into three levels, favorable, intermediate and adverse according to standard International System for Human Cytogenetic Nomenclature (ISCN) criteria .[17] As a result, the genotypes of the IA and DA groups were not statistically significant for comparison of CR between the two groups. The results have low heterogeneity and high reliability. We also analyzed the subgroups of age and stratified the subgroups according to the median age. The results suggested no difference in the two groups which was in accordance to other researches. But there was an age bias in how daunorubicin they used, with elderly people using low doses and younger people using high doses. We were curious about whether the use of high dose daunorubicin in the elderly would have unexpected results. In the actual clinical application, the elderly still uses individualized treatment, and the dose is determined according to the assessment of the elderly patients’ own status. We look forward to more clinical trials.
In the survival analysis, we focused on OS. It has been reported in previous literature that high-dose DA regimen can achieve the same therapeutic effect and survival time as IA. In 2019, Sunil Adige reported that in the US study, 90 mg/m2, compared with 45 mg/m2, DNR led to a significantly higher CR rate (71% vs 57%, P < .001) and improved OS (median, 23.7 vs. 15.7 months, P = .003).[18] There is a similar case published by another meta-analysis and the results suggest that comparing HD-DNR with LD-DNR, there were significant differences in CR, OS, and EFS.[16] So, we only focused on high-dose DA regimens versus a standard dose of IA regimens in OS. On the basis of the NCCN recommendations, we set a total of more than 180 mg/m2 and a daily dose of more than 50 mg/m2 as the high dose boundaries. The dose of IA regimen was set as the standard dose of 8 to 12 mg/m2 per day.
However, a total of 2490 patients were reported in this paper based on the four literatures, suggesting that even with high doses of DA, the OS time of patients with IA was relatively long and statistically significant (HR = 0.89, 95%CI = 0.8–1.0, P = .04, I2 = 0).
The toxic and side effects of chemotherapeutic drugs are worth discussing. Comparing the adverse reactions of IA and DA regimens, we observed infection, cardiac toxicity bleeding, and relapse rates. In clinical use, if there are severe adverse reactions to anthracyclines, they may be considered for reduction or withdrawal or, in some cases, for replacement with a less toxic drug. Our statistical results showed that there was no significant statistical difference in the adverse reaction results after the use of IA and DA regimens.
In recent years, many clinical trials for AML have innovated the new regimens on the basis of idarubicin combined with cytarabine. On the basis of IA, we can explore the new treatment plan for patients with AML who are newly diagnosed or refractory. For example, Fengqi Liu reported that DAC (a DNA methylated transferase) combined with IA represented a new option of induction therapy for newly diagnosed AML patients with MDS features and the results demonstrated that the rate of CR was higher in the DAC+IA group than in the IA group (85.2% vs 68.5%, P = .04) after the first course, and toxicities were comparable in both groups.[19] In another phase I/II randomized trial,[20] the authors aimed to evaluate the effect of IA plus clofarabine or fludarabine for adults with relapsed or refractory AML. Although there is no explicit difference between the two groups, both of these outcomes were prior to the previously reported relapsed or refractory cases in response rates and survival.
Therefore, future associations with improved IA schemes could be an interesting option to treatment of AML.
This study conducted a systematic and comprehensive evaluation and analysis of the randomized controlled literature comparing IA and DA chemotherapy regimens for AML, which improved the statistical effectiveness of the study results and made the study results more reliable and comprehensive.
There are some limitations in this meta-analysis. Although the quality of literature included in this paper is very high, all of which are from authoritative magazines, only 6 articles were included. Unclear or inappropriate random methods in the included literature may increase the selectivity bias and affect the stability of the results. Deaths or withdrawal from clinical trials during induction may increase reporting bias. Compared with the meta-analysis published before, this paper only updated lee's article of 2017. The indicators of survival analysis, such as event free survival (EFS), disease free survival (DFS), etc., could not obtain complete data, so the analysis was not carried out in the study. These all affect the accuracy of the results. We only considered the dose of daunorubicin and did not study the impact of cytarabine dose changes on the results because there were no head-to-head clinical trials. Therefore, in future studies, large sample randomized controlled studies, especially prospective ones, should be emphasized to provide more systematic and specific analysis and more powerful evidence for future clinical work.
7. Conclusion
This study compared the efficacy and safety of IA regimen and DA regimen on inducing remission of AML in RCTs. The IA regimen has better effect of inducing remission than the DA regimen, which is consistent with the recommendations of NCCN and the results reported in previous literatures. The OS was better at standard dose IA regimen than at high dose DA regimen, and the results were statistically significant. Adverse events and cytogenetic subgroup analysis of the IA and DA groups were not statistically significant for comparison of CR between the two groups. Therefore, it is concluded that IA regimen can be given priority in clinical application as an induction regimen for AML.
Author contributions
Conceptualization: Hanyu Wang.
Data curation: Hanyu Wang.
Formal analysis: Hanyu Wang, Qirong Xiao.
Investigation: Hanyu Wang, Yanhong Lu.
Methodology: Hanyu Wang, Qirong Xiao, Yanhong Lu.
Project administration: Hanyu Wang, Qirong Xiao.
Resources: Hanyu Wang.
Software: Hanyu Wang, Yanhong Lu.
Supervision: Hanyu Wang, Qirong Xiao, Yong Wu.
Validation: Hanyu Wang.
Visualization: Hanyu Wang, Yong Wu.
Writing – original draft: Hanyu Wang, Qirong Xiao, Yong Wu.
Writing – review & editing: Hanyu Wang, Qirong Xiao, Yong Wu.
Footnotes
Abbreviations: CR = complete remission, CR1 = complete remission in one course, CR2 = complete remission in two courses, DA = daunorubicin combined with cytarabine, DFS = disease-free survival, DNR = daunorubicin, IA = idarubicin combined with cytarabine, IDR = idarubicin, OS = overall survival, RCTs = randomized clinical trials.
How to cite this article: Wang H, Xiao X, Xiao Q, Lu Y, Wu Y. The efficacy and safety of daunorubicin versus idarubicin combined with cytarabine for induction therapy in acute myeloid leukemia: A meta-analysis of randomized clinical trials. Medicine. 2020;99:24(e20094).
HW, XX, and QX contributed equally to this work (1st set of equal contributors).
YL also contributed equally to this work (2nd set of equal contributors).
Construction project of Fujian Medical Center of Hematology (Min201704); Sponsored by National and Fujian Provincial Key Clinical Specialty Discipline Construction Program, P.R.C.
Ethical approval: This study is a meta-analysis of published data and does not contain any studies with human participants or animals performed.
The authors have no conflicts of interest to disclose.
References
- [1].Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med 1999;341:1051–62. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- [3].Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol 2011;29:487–94. [DOI] [PubMed] [Google Scholar]
- [4].AML Collaborative Group. A systematic collaborative overview of randomized trials comparing idarubicin with daunorubicin (or other anthracyclines) as induction therapy for acute myeloid leukaemia. Br J Haematol 1998;103:100–9. [PubMed] [Google Scholar]
- [5].Wang J, Yang YG, Zhou M, et al. Meta-analysis of randomised clinical trials comparing idarubicin + cytarabine with daunorubicin + cytarabine as the induction chemotherapy in patients with newly diagnosed acute myeloid leukaemia. PLoS One 2013;8:e60699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 2003;21:4642–9. [DOI] [PubMed] [Google Scholar]
- [7].Li X, Wang C, Chen G, et al. Combined chemotherapy for acute promyelocytic leukemia: a meta-analysis. Hematology 2017;22:450–9. [DOI] [PubMed] [Google Scholar]
- [8].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [9].Zhu Y, Gao Q, Du J, et al. Effects of post-remission chemotherapy before allo-HSCT for acute myeloid leukemia during first complete remission: a meta-analysis. Ann Hematol 2018;97:1519–26. [DOI] [PubMed] [Google Scholar]
- [10].Lee JH, Kim H, Joo YD, et al. Prospective randomized comparison of idarubicin and high-dose daunorubicin in induction chemotherapy for newly diagnosed acute myeloid leukemia. J Clin Oncol 2017;35:2754–63. [DOI] [PubMed] [Google Scholar]
- [11].Pautas C, Merabet F, Thomas X, et al. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: results of the ALFA-9801 study. J Clin Oncol 2010;28:808–14. [DOI] [PubMed] [Google Scholar]
- [12].Gardin C, Turlure P, Fagot T, et al. Postremission treatment of elderly patients with acute myeloid leukemia in first complete remission after intensive induction chemotherapy: results of the multicenter randomized Acute Leukemia French Association (ALFA) 9803 trial. Blood 2007;109:5129–35. [DOI] [PubMed] [Google Scholar]
- [13].Rowe JM, Neuberg D, Fridenberg W, et al. A phase 3 study of three induction regimens and of priming with GM-CSF in older adults with acute myeloid leukemia: a trial by the Eastern Cooperative Oncology Group. Blood 2004;103:479–85. [DOI] [PubMed] [Google Scholar]
- [14].Ohtake S, Miyawaki S, Fujita H, et al. Randomized study of induction therapy comparing standard-dose idarubicin with high-dose daunorubicin in adult patients with previously untreated acute myeloid leukemia: the JALSG AML201 Study. Blood 2011;117:2358–65. [DOI] [PubMed] [Google Scholar]
- [15].Chevallier P, Fornecker L, Lioure B, et al. Tandem versus single autologous peripheral blood stem cell transplantation as post-remission therapy in adult acute myeloid leukemia patients under 60 in first complete remission: results of the multicenter prospective phase III GOELAMS LAM-2001 trial. Leukemia 2010;24:1380–5. [DOI] [PubMed] [Google Scholar]
- [16].Gong Q, Zhou L, Xu S, et al. High doses of daunorubicin during induction therapy of newly diagnosed acute myeloid leukemia: a systematic review and meta-analysis of prospective clinical trials. PLoS One 2015;10:e0125612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fernandez S, Desplat V, Villacreces A, et al. Targeting tyrosine kinases in acute myeloid leukemia: why, who and how? Int J Mol Sci 2019;20: undefined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Adige S, Lapidus RG, Carter-Cooper BA, et al. Equipotent doses of daunorubicin and idarubicin for AML: a meta-analysis of clinical trials versus in vitro estimation. Cancer Chemother Pharmacol 2019;83:1105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu F, Wang H, Liu J, et al. A favorable inductive remission rate for decitabine combined with chemotherapy as a first course in <60-year-old acute myeloid leukemia patients with myelodysplasia syndrome features. Cancer Med 2019;8:5108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Short NJ, Kantarjian H, Ravandi F, et al. A phase I/II randomized trial of clofarabine or fludarabine added to idarubicin and cytarabine for adults with relapsed or refractory acute myeloid leukemia. Leuk Lymphoma 2018;59:813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]


