Abstract
Although the positive correlation between serum uric acid (UA) levels and bone mineral density (BMD) has been reported in the general population, there are little data regarding the effect of serum UA levels on bone loss in patients with rheumatoid arthritis (RA).
We investigated whether increased serum UA levels were associated with a reduced risk of osteoporosis in postmenopausal women with RA.
In this retrospective cross-sectional study, 447 postmenopausal female patients with RA and 200 age-matched, postmenopausal healthy controls underwent BMD examination by dual energy x-ray absorptiometry and serum UA levels measurement. Osteoporosis was diagnosed when the T-score was <−2.5.
The median UA level in postmenopausal RA patients was found to be significantly lower than that in the healthy women (4 vs 4.1 mg/dL, P = .012) and the frequency of osteoporosis incidence in the lumbar spine, hip, and either site in RA patients was 25.5%, 15.9%, and 32.5%, respectively; the values were significantly higher than those of the controls. After adjusting for confounding factors, a significantly lower risk for osteoporosis of the hip in RA patients was observed within the highest quartile (odds ratio [OR] = 0.37, 95% confidence interval [CI] = 0.16–0.72, P = .021) and the second highest quartile (OR = 0.44, 95% CI = 0.2–0.95, P = .038) of serum UA levels as compared with the lowest quartile, but this association was not found to be consistent with respect to the lumbar spine. Serum UA levels also showed an independently positive correlation with femoral neck BMD (β = 0.0104, P = .01) and total hip BMD (β = 0.0102, P = .017), but not with lumbar BMD.
Our data suggest that UA may exert a protective effect on bone loss in RA, especially in the hip.
Keywords: bone density, osteoporosis, rheumatoid arthritis, uric acid
1. Introduction
Uric acid (UA) is the final metabolite of purine metabolism in humans. It is produced by xanthine oxidase and can crystallize into monosodium urate, which is a causative factor of gout and urinary stones. UA can act as a proinflammatory and pro-oxidative agent when its levels exceed the physiological range, and hyperuricemia is traditionally considered a risk factor for conditions like metabolic syndrome, chronic kidney diseases, and cardiovascular diseases.[1,2] Conversely, serum UA within the physiological range exerts anti-inflammatory and anti-oxidative effects, which play a defensive role in oxidative stress-induced diseases and in aging.[1] There is accumulating evidence that UA may also play a protective role in neurodegenerative disorders such as Alzheimer and Parkinson disease.[3,4] As oxidative stress has also been implicated in the development of osteoporosis,[5–7] the effect of UA on generalized bone loss is drawing increasing scrutiny. Recently, several observational studies have revealed that a higher serum UA level was associated with higher bone mineral density (BMD), and therefore, a reduced risk of osteoporosis and fragility fractures in men and post- and peri-menopausal women.[8–13] This indicates that UA may exert a protective effect against bone loss.
Rheumatoid arthritis (RA) is a chronic, autoimmune, and inflammatory arthritis of unknown etiology, characterized by progressive joint destruction, functional impairment, and various extra-articular manifestations. In addition to localized periarticular bone loss, systemic osteoporosis is a well-established complication of RA. Previous epidemiological studies found that the prevalence of osteoporosis and osteoporotic fractures in RA patients is approximately twice that of the general population, in both sexes,[14–16] which imposes a significant clinical and socioeconomic burden on patients with RA. RA-associated factors such as chronic inflammation, immobilization, disability, sarcopenia, and use of glucocorticoids (GCs), along with traditional risk factors, such as aging, low body mass, and the postmenopausal state, have been implicated in the development of secondary osteoporosis in RA.[17] However, it is necessary to identify additional risk factors inciting bone loss, to prevent fragility fractures in patients with RA. Although the relationship between serum UA levels and BMD has been evaluated in the general population,[8–13] in patients with type 2 diabetes,[18] and in those with ankylosing spondylitis (AS),[19] to our knowledge, data regarding the effect of UA levels on bone loss in patients with RA was lack. Therefore, in this study, we investigated the correlation between increased serum UA levels and the risk of osteoporosis in postmenopausal women with RA. We also analyzed the association of serum UA level with BMD of the lumbar spine and hip.
2. Methods
2.1. Study design and patients
This was a retrospective, cross-sectional study of medical records obtained from the rheumatology department of our university-affiliated tertiary referral center in South Korea. We collected data of 447 postmenopausal female patients with RA and 200 age-matched (± 2 years) postmenopausal healthy women. All the study patients underwent dual energy x-ray absorptiometry (DEXA) scanning for their BMD evaluation, as outpatients at the center, from January 2008 to December 2017. The “postmenopausal status" was defined as the history of cessation of menses for at least 1 year.[11] All patients with RA satisfied the 1987 American College of Rheumatology (formerly American Rheumatism Association) revised classification criteria for RA,[20] whereas those with rheumatoid diseases other than RA (except for secondary Sjogren syndrome) were excluded. The healthy controls were selected randomly from postmenopausal women who visited the health promotion center of the same hospital for comprehensive routine health examinations. This subset included patients with no history of rheumatic disease, including RA and also had no history of other chronic conditions that could affect bone metabolism, such as thyroid disorder or malignancy. The exclusion criteria for both groups were as follows: study subjects diagnosed with gout or urinary stones, study subjects with history of spine and/or hip surgery or with metal implants in-situ that could affect the BMD evaluation, study subjects who had been administered antiosteoporotic medications such as bisphosphonates, selective estrogen receptor modulators, teriparatide, or denosumab (excepting calcium and/or vitamin D), study subjects taking xanthine oxidase inhibitors such as allopurinol and febuxostat or receiving uricosuric agents such as benzbromarone and probenecid, study subjects with an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2. All patients with RA as well as the healthy controls included in our study were South Korean women. The study was approved by the Research and Ethical Review Board of the Pusan National University Hospital, which waived informed consent for study participation, considering the retrospective study design (IRB no. 1708-033-058).
2.2. BMD measurements
The BMD of the lumbar spine (Levels L1–4) and the left hip (femoral neck and total hip) was measured, using DEXA equipment (GE-Lunar Prodigy, GE, Madison, MA). All measurements were taken by experienced operators on the same machine using standardized positioning and scanning protocols. The BMD was expressed in grams per square centimeter (g/cm2) along with the standard deviation (SD) measured from the healthy young population (T-score). For the BMD reference values, we used baseline data applicable to South Korean women, as provided by the equipment manufacturers. The diagnosis of osteoporosis in the postmenopausal women was determined based on the T-score criteria (≤−2.5 SD) established by the World Health Organization (WHO).[21]
2.3. Clinical and laboratory data
The relevant clinical data including demographic parameters such as age, height, weight, and body mass index (BMI) and the laboratory data including serum UA levels, serum creatinine, and eGFR were collated from the records of the patients with RA and the healthy controls. The BMI was calculated by dividing each subject's weight in kilograms, by the square of their height in meters (kg/m2). The serum UA and creatinine levels were assessed using overnight fasting blood samples collected from all subjects. Serum UA levels of all the study patients were estimated within 2 weeks of the DEXA scan. The samples were assessed via an enzyme colorimetric assay using the Roche-Hitachi Cobas 8000 c702 chemistry autoanalyzer (Cobas 8000, Roche Diagnostics, Switzerland). The eGFR in all patients was calculated using the Modification of Diet in Renal Disease formula, that is, eGFR = 186 × (serum creatinine)−1.154 × (age)−0.203 × 0.742 (if female).[22]
The following disease-related data were collected from the records of the RA patients: disease duration, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) level, disease activity score assessed using the 28-joint count for swelling and tenderness with ESR (DAS28-ESR), rheumatoid factor (RF) level, anti-cyclic citrullinated peptide antibody (anti-CCP Ab) level, and details of current medications such as calcium, vitamin D3, disease modifying anti-rheumatic drugs (DMRADs) and GCs. The DAS28-ESR score was calculated using the formula- .[23].
.[23].
The disease activity was classified into 4 groups as follows- high disease activity was defined as DAS28-ESR of >5.1, moderate disease activity was defined as 3.2 < DAS28-ESR ≤ 5.1, low disease activity was defined as 2.6 < DAS28-ESR≤ 3.2, and remission was defined as DAS28-ESR ≤2.6. The serum RF titer was assessed using a particle-enhanced immunoturbidimetric assay (range 0–14 IU/ml) and the serum anti-CCP Ab titer was measured using a chemiluminescent micro-particle immunoassay (range 0–5 U/mL). The DMARDs found in current use in this study included methotrexate, sulfasalazine, hydroxychloroquine, leflunomide, tacrolimus, and biologic DMARDs. The cumulative dose (in grams) of GCs was expressed in the form of “prednisone equivalents" and was determined by multiplying the current daily dose by the number of days for which patient with RA had been treated with GCs (calculated from the date of the first prescription).
2.4. Statistical analyses
Continuous variables were expressed as mean ± SD or median (with interquartile range [IQR]), as appropriate, whereas the categorical variables were expressed as number of cases with the percentage. The Kolmogorov-Smirnov test was utilized to assess the normality of distribution of the continuous variables. The group comparisons between RA patients and the healthy subjects for the continuous variables were performed using the Student t test or the Mann-Whitney U test and for the discrete variables, by using the χ2 test or Fisher exact test, as indicated. The patients with RA were divided into 4 quartiles as per their serum UA levels, and comparisons between the groups were conducted using the analysis of variance test with the least significant difference post-hoc test or the Kruskal-Wallis test for the continuous variables and by the χ2 test or Fisher exact test for the categorical variables, as appropriate. The association of serum UA levels with BMD and with other clinical parameters such as BMI, eGFR, and age, were studied using Spearman correlation analyses. To estimate the statistical power of the association between serum UA levels and BMD in patients with RA, we applied stepwise multivariable linear regression models, including variables with P < .1 in univariable analyses and clinically relevant variables such as BMI and eGFR. In addition, the correlation between serum UA levels and the occurrence of osteoporosis in RA patients was assessed by applying backward multivariable logistic regression models, which included covariates with P < .1 in univariable analyses and other variables with clinical relevance such as BMI and eGFR. The results were calculated in the form of odds ratios (ORs) with 95% confidence intervals (CIs), and the odds for osteoporosis in the RA patients in each of higher 3 quartiles were compared to the odds for those in the lowest quartile. A 2-sided P < .05 was considered statistically significant. All statistical analyses were conducted using the STATA version 15.0 of Windows software (StataCorp LP, College Station, TX) and GraphPad Prism software (PRISM 7.0; GraphPAD Software Inc., San Diego, CA).
3. Results
3.1. Baseline characteristics
The comparison of clinical and laboratory characteristics of the postmenopausal RA patients with the healthy controls is shown in Table 1. Although there was no significant difference with respect to factors such as age, eGFR, and BMI between the 2 groups, the median (IQR) UA levels of postmenopausal RA patients were found to be significantly lower than those of the healthy controls (4 [3.3–4.8] vs 4.1 [3.6–4.8] mg/dL, P = .012). Patients with RA had a significantly lower lumbar spine, femoral neck, and total hip BMD as compared to that of the healthy controls. In addition, the overall occurrence of osteoporosis in patients with RA was significantly higher than that in the control group (lumbar spine: 25.5% vs. 7%, P < .001; hip: 15.9% vs 1.5%, P < .001; either site: 32.5% vs 8%, P < .001, respectively).
Table 1.
Comparisons of clinical and demographic features between postmenopausal women with rheumatoid arthritis and healthy subjects.
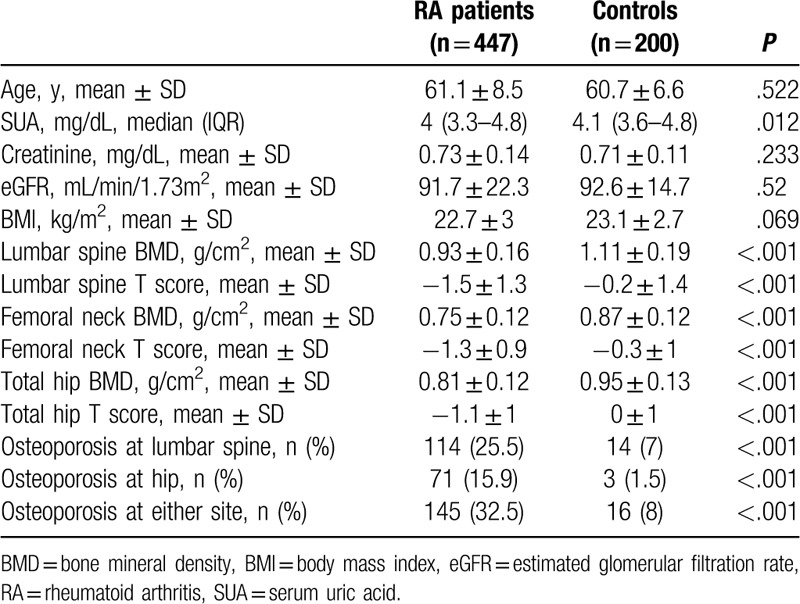
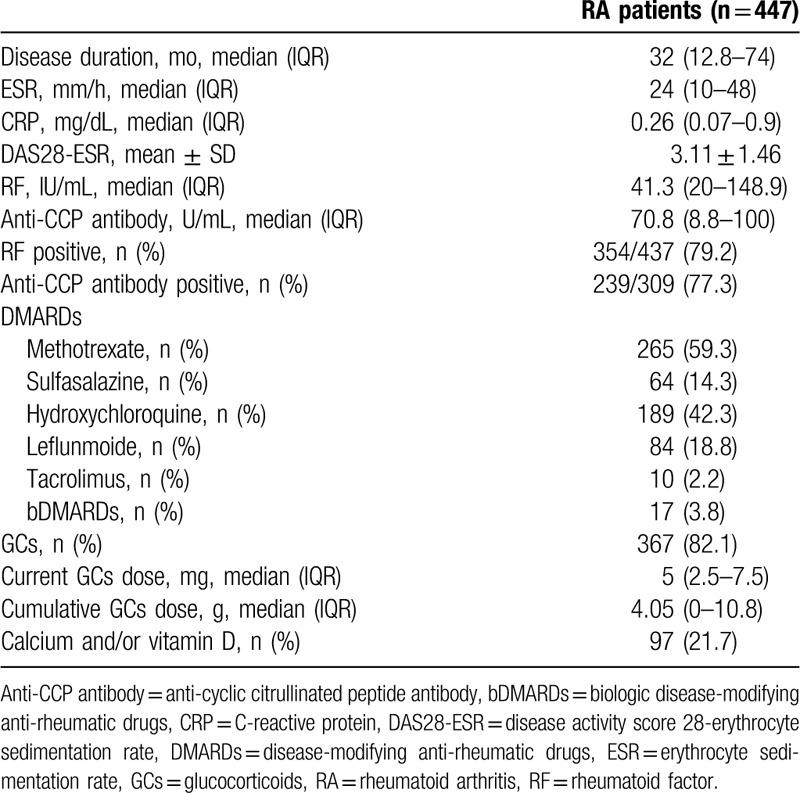
Table 2 details the baseline clinical features in postmenopausal patients with RA. The median (IQR) disease duration was 32 (12.8–74) months and the mean ± SD DAS28-ESR was 3.11 ± 1.46. The proportion of patients with RA having positive results for RF and anti-CCP Ab was 79.2% and 77.3%, respectively. The most common concomitant DMARD in use was methotrexate (59.3%), followed by hydroxychloroquine (42.3%) and leflunomide (18.8%). Most patients with RA (82.1%) were receiving GCs and the median (IQR) cumulative prednisone-equivalent dose of GCs was 4.05 (0–10.8) g, and 97 RA patients (21.7%) were being treated with calcium and/or vitamin D supplementation as well.
Table 2.
Baseline clinical features in postmenopausal patients with rheumatoid arthritis.
3.2. Comparisons clinical and laboratory characteristics of the postmenopausal women with RA according to serum UA levels
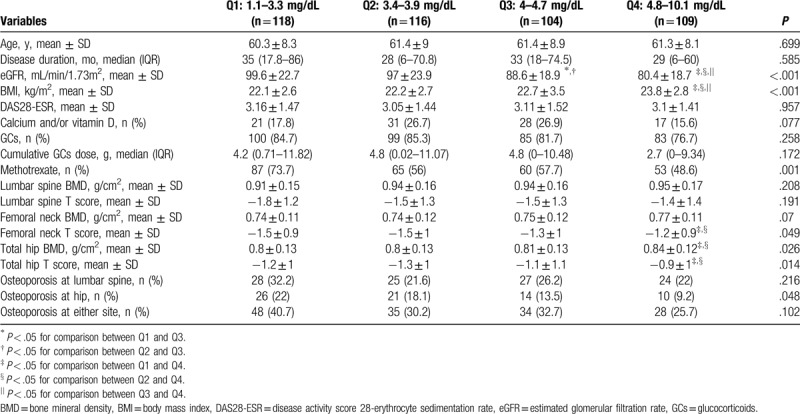
Table 3 presents the clinical and laboratory characteristics of the postmenopausal women with RA, subdivided into 4 quartiles according to their serum UA levels (Q1: 1.1–3.3 mg/dL, Q2: 3.4–3.9 mg/dL, Q3: 4–4.7 mg/dL, Q4: 4.8–10.1 mg/dL). The femoral neck T-score, total hip BMD, and total hip T-score of the patients in the highest quartile (Q4) were all significantly higher than those of the patients in the lowest and second lowest quartiles (Q1 and Q2, respectively). The frequency of osteoporosis incidence at the hip significantly differs between the patients in the different quartiles, whereas no significant difference was observed in the frequency of osteoporosis incidence at the lumbar spine and at either site according to the quartiles of serum UA levels. In addition, RA patients within the Q4 group showed significantly lower eGFR and higher BMIs than those within the remaining 3 groups and there was a significant difference in the frequency of methotrexate use according to the quartiles of serum UA levels (Table 3).
Table 3.
Comparisons of clinical and laboratory characteristics in patients with rheumatoid arthritis according to the quartiles of serum uric acid levels.
3.3. Correlations between serum UA levels and clinical parameters in the postmenopausal women with RA
As depicted in Figure 1, serum UA concentrations in postmenopausal patients with RA were found to have a positive correlation with the lumbar spine BMD (ρ = 0.102, P = .032), femoral neck BMD (ρ = 0.123, P = .01), and total hip BMD (ρ = 0.146, P = .002). In addition, serum UA levels correlated positively with the BMI (ρ = 0.231, P < .001), and inversely with the eGFR (ρ = -0.363, P < .001), whereas no significant association was found between serum UA levels and age in RA patient.
Figure 1.
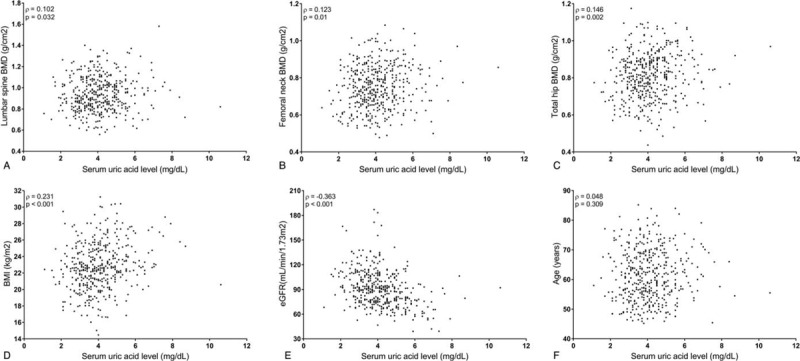
Correlation plots between serum uric level and clinical parameters in postmenopausal women with rheumatoid arthritis.
3.4. Association of serum UA levels with BMD and osteoporosis in the postmenopausal women with RA
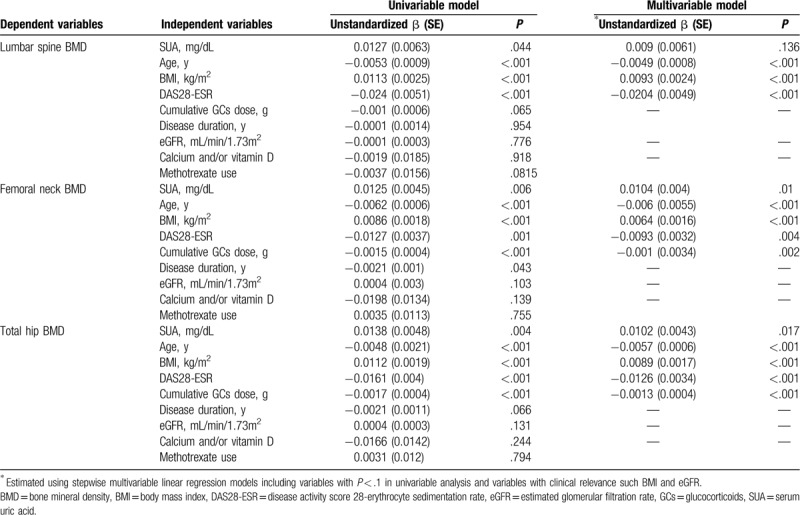
Table 4 shows the results of the linear regression analyses of associated factors for BMD in postmenopausal patients with RA. The serum UA levels had a significantly higher positive association with the BMD of all three sites in the univariable analyses, but this relationship was no longer significant with respect to the lumbar spine BMD when the multivariable linear regression model was applied (β = 0.009, P = .136). However, serum UA levels showed independent positive associations with femoral neck BMD (β = 0.0104, P = .01) and total hip BMD (β = 0.0102, P = .017) after adjusting for confounding factors. In addition, factors like older age, higher DAS28-ESR, and lower BMI were found to be significantly associated with a lower BMD at all sites, after adjusting for confounding factors. A higher cumulative dosage of GCs had a significantly positive correlation with lower BMD at the femoral neck and at the total hip in multivariable analysis.
Table 4.
Linear regression models for bone mineral density in postmenopausal women with rheumatoid arthritis.
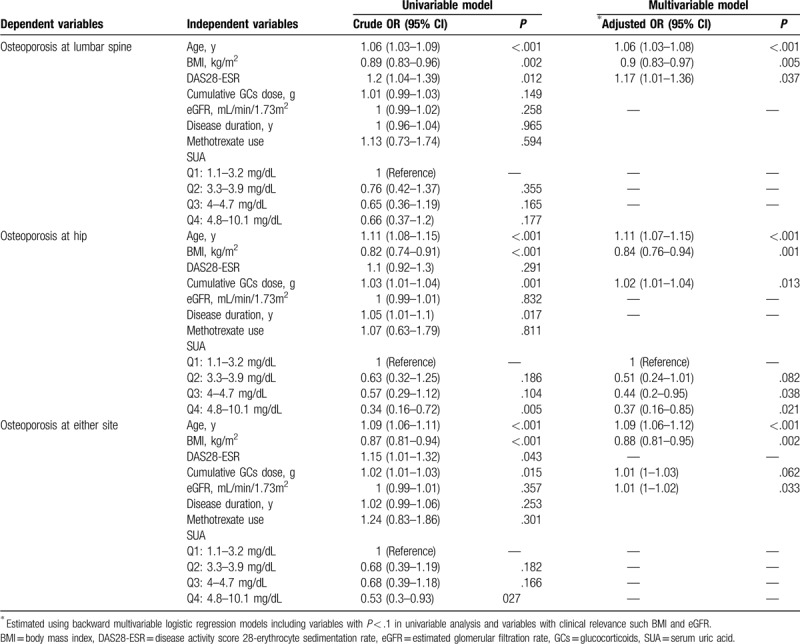
Associated factors for osteoporosis in postmenopausal women with RA evaluated by logistic regression models are summarized in Table 5. After adjusting for confounding factors, a significantly lower risk for osteoporosis at the hip in RA patients within the highest (Q4) and second highest (Q3) quartiles was observed, as compared to the lowest quartile (Q1), (Q4: OR = 0.37, 95% CI = 0.16–0.72, P = .021, Q3: OR = 0.44, 95% CI = 0.2–0.95, P = .038). The RA patients within the second lowest quartile (Q2) showed a trend of lower risk for hip osteoporosis as compared within those in Q1, but the finding did not achieve statistical significance (OR = 0.51, 95% CI = 0.24–1.01, P = .082). However, this association between serum UA levels and osteoporosis was not found at the lumbar spine and at either site in postmenopausal RA patients. In addition, application of the multivariable logistic regression models revealed that a higher DAS28-ESR value was associated with a higher risk for osteoporosis at the lumbar spine but not at hip and at either site. A higher cumulative GC dosage correlated with an increased risk for lumbar spine and hip osteoporosis, while advanced age and lower BMI were found to be independent risk factors for osteoporosis at all evaluated sites, after adjusting for confounding factors (Table 5).
Table 5.
Associated factors for osteoporosis in postmenopausal women with rheumatoid arthritis evaluated by logistic regression models.
4. Discussion
In the present study, we found that serum UA levels in postmenopausal women with RA were significantly lower than those in healthy controls, despite no significant differences in age, eGFR, and BMI between the 2 groups. In postmenopausal women with RA, increased serum UA levels were independently associated with a lower risk of osteoporosis at the hip, but this relationship was not observed with respect to osteoporosis at the lumbar spine. In addition, serum UA levels in RA patients showed a significant positive correlation with femoral and total hip BMD, but not with the BMD of the lumbar spine, after adjusting for potential confounding factors. Our data provide evidence that UA may have a protective effect on bone loss at the hip, but not at the lumbar spine in postmenopausal patients with RA.
To our knowledge, this is the first study to demonstrate an association of serum UA levels with osteoporosis and BMD in postmenopausal women with RA. Since Nabipour et al[8] first reported a relationship between serum UA concentrations and bone health in older men, observational studies have consistently reported that a higher serum UA level had a significantly positively correlation with higher BMD in peri- and postmenopausal women[10–13,24,25] and men[26,27] and was associated with a lower risk of osteoporotic fractures,[9,27,28] although conflicting data on the topic has also been reported.[29,30] Most previous studies assessing the relationship between UA and bone metabolism have been performed in the healthy general population. Kang et al[19] also reported a positive association between serum UA concentrations and BMD in young male patients with AS. When the existing literature is considered in its entirety, it can be theorized that serum UA may have an inhibitory effect on bone loss, not only in the general population, but also in patients with rheumatic joint diseases such as RA and AS.
The protective effect of UA in osteoporosis may be mediated through its antioxidant effect on bone metabolism. An excess of reactive oxygen species (ROS) produced by oxidative stress have been identified in RA,[31] which contribute to the pathological process of osteoporosis by stimulating osteoclast differentiation and inhibiting osteoblast activity, a process which can be countered by antioxidants.[32] As mentioned above, at physiologic level, UA is considered an important endogenous antioxidant that scavenges ROS and mitigates cellular and vascular damage caused by oxidative stress,[1] which is suggestive of its potential in preventing bone loss. This notion is supported by an in-vitro study which showed how UA significantly suppressed osteoclastogenesis in a dose-dependent manner, by reducing ROS production in mice osteoclast-precursor cells[11] Alternately, UA can also stimulate inflammatory reactions by inducing oxidative stress and producing proinflammatory cytokines,[1] which can suppress 1-α hydroxylase activity and subsequently lead to a reduced production of active vitamin D.[33] This may result in a detrimental effect on bone metabolism. Gout, a chronic inflammatory arthritis caused by hyperuricemia, was reported to be associated with an increased risk of hip fractures, in a recent epidemiologic study.[34] Therefore, the net effect of UA on bone mass may be dependent on its levels in the human body or on other specific conditions.
More importantly, our study showed that the serum UA level was an independent protective factor against osteoporosis and a decreasing BMD of the hip, but not of the lumbar spine. Given that bone loss induced by oxidative stress was reported be more prominent in trabecular bone than in cortical bone[35] and that the beneficial effect of UA on bone metabolism could be related to its antioxidative action, this finding was unexpected. One of the possible explanations for this result may be the potential inaccuracy of lumbar spine BMD in patients with RA, in our study. In previous studies, a substantial proportion of RA patients have been documented with abnormal radiologic findings of the lumbar spine such as endplate erosion, spondylolisthesis and osteophytes,[36,37] which could compromise the accuracy of DEXA measurements in the lumbar spine. As we did not evaluate abnormal lumbar spine lesions on an x-ray, RA patients with these lesions were not excluded from this study, which may have contributed to the lack of association of UA level with the lumbar spine BMD and osteoporosis. However, the role of UA level on bone metabolism has not been fully elucidated and further research is required to clarify whether UA has a differential effect on cortical and trabecular bone in RA patients.
Significantly lower serum UA levels were observed in postmenopausal women with RA as compared to those of the controls, in this study. Factors such as aging, renal impairment, and obesity are known to be major determinants of serum UA levels.[38] As age, eGFR, and BMI were comparable across the 2 groups, these parameters were not responsible for the lower serum UA levels of the RA patients in our study. Although the underlying mechanisms are not entirely known, we can conjecture that medications for RA treatment might impact serum UA levels. A previous study showed that treatment with methotrexate decreased serum UA levels in patients with early RA.[39] In addition, RA patients with lower quartiles of serum UA levels tended to have higher frequency of methotrexate use in our data (Table 3), which supports this notion. More than half of the RA patients were taking methotrexate in our study, which may have attributed to the lower serum UA levels in the group. In addition, there might be other causes for the difference of serum UA levels between the RA patients and controls. Serum UA levels are also affected by intake of alcohol, fructose, red meat, caffeine, vitamin C, and with drugs such as aspirin, thiazide, and losartan, all of which were not fully accounted for, in this study. However, the difference in serum UA levels between RA patients and controls was only 0.1 mg/dL as per our data. Thus, the underlying mechanisms and the clinical significance of this difference are needed to be determined in further studies.
The present study has several limitations. First, due to its cross-sectional study design, we could not determine whether a causal relationship exists between serum UA levels and osteoporosis in patients with RA. In particular, we could not fully adjust the effect of disease activity or RA medications such as methotrexate and GCs on the association of serum UA levels with BMD and osteoporosis because this was not a randomized clinical trial. GCs and disease activity were reported to be significant risk factors for osteoporosis in RA.[14,15] Thus, further longitudinal studies adjusting potential confounding factors are indicated to confirm our findings. Secondly, our study population only consisted of postmenopausal women with RA and the role of serum UA in bone metabolism in their male counterparts also needs to be determined through further studies. Thirdly, we did not measure bone turnover markers such as parathyroid hormone and vitamin D, which may also affect BMD and act as residual confounding factors. Finally, we could not analyze the effects of habits such as smoking and alcohol-intake on BMD, because this information was not available and could not be elicited due to the retrospective nature of our study.
5. Conclusion
In conclusion, this study revealed that a higher serum UA level is independently associated with a reduced risk of osteoporosis and lower bone mass at the hip but not at the lumbar spine, in postmenopausal women with RA. This finding suggests that UA may exert a protective effect on bone health in RA, especially at the hip joint, probably through its antioxidative effect against oxidative stress-induced bone loss. Our data may provide a novel insight into the role of UA in the bone metabolism of patients with RA. However, due to its retrospective cross-sectional design, further experimental and prospective longitudinal studies are necessary to validate our results.
Acknowledgments
The authors specially thank the late Professor Sung-Il Kim who devoted himself to education, research, and patient care in Division of Rheumatology, Department of Internal Medicine, Pusan National University School of Medicine (1963–2011).
Author contributions
HN Lee: study design, data collection and analysis and writing manuscript, YK Kim: data interpretation, GT Kim: data interpretation and revision of manuscript, DH Sohn: data interpretation and revision of manuscript, SG Lee: study design, data analysis and interpretation, writing manuscript and coordination of entire study.
Footnotes
Abbreviations: anti-CCP Ab = anti-cyclic citrullinated peptide antibody, AS = ankylosing spondylitis, BMD = bone mineral density, BMI = body mass index, CI = confidence intervals, CRP = C-reactive protein, DAS28-ESR = disease activity score assessed using the 28-joint count for swelling and tenderness with ESR, DEXA = dual energy X-ray absorptiometry, DMARDs = disease-modifying antirheumatic drugs, eGFR = estimated glomerular filtration rate, ESR = erythrocyte sedimentation rate, GCs = glucocorticoids, IQR = interquartile range, OR = odds ratios, RA = rheumatoid arthritis, RF = rheumatoid factor, SD = standard deviation, UA = uric acid, WHO = World Health Organization.
How to cite this article: Lee HN, Kim A, Kim Y, Kim GT, Sohn DH, Lee SG. Higher serum uric acid levels are associated with reduced risk of hip osteoporosis in postmenopausal women with rheumatoid arthritis. Medicine. 2020;99:24(e20633).
Data Availability: The raw data used to support the results of this study are available from the corresponding author upon reasonable request.
Data Availability: The data used to support the findings of this study are included within the article and the supplementary materials.
The authors report no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Vassalle C, Mazzone A, Sabatino L, et al. Uric acid for cardiovascular risk: Dr. Jekyll or Mr. Hide? Diseases 2016;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sharaf El Din UAA, Salem MM, Abdulazim DO. Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: a review. J Adv Res 2017;8:537–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Latourte A, Bardin T, Richette P. Uric acid and cognitive decline: a double-edge sword? Curr Opin Rheumatol 2018;30:183–7. [DOI] [PubMed] [Google Scholar]
- [4].Yu Z, Zhang S, Wang D, et al. The significance of uric acid in the diagnosis and treatment of Parkinson disease: an updated systemic review. Medicine (Baltimore) 2017;96:e8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhou Q, Zhu L, Zhang D, et al. Oxidative stress-related biomarkers in postmenopausal osteoporosis: a systematic review and meta-analyses. Dis Markers 2016;2016:7067984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bai XC, Lu D, Liu AL, et al. Reactive oxygen species stimulates receptor activator of NF-kappaB ligand expression in osteoblast. J Biol Chem 2005;280:17497–506. [DOI] [PubMed] [Google Scholar]
- [7].Cornelius C, Koverech G, Crupi R, et al. Osteoporosis and alzheimer pathology: role of cellular stress response and hormetic redox signaling in aging and bone remodeling. Front Pharmacol 2014;5:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nabipour I, Sambrook PN, Blyth FM, et al. Serum uric acid is associated with bone health in older men: a cross-sectional population-based study. J Bone Miner Res 2011;26:955–64. [DOI] [PubMed] [Google Scholar]
- [9].Kim BJ, Baek S, Ahn SH, et al. Higher serum uric acid as a protective factor against incident osteoporotic fractures in Korean men: a longitudinal study using the National Claim Registry. Osteoporos Int 2014;25:1837–44. [DOI] [PubMed] [Google Scholar]
- [10].Ishii S, Miyao M, Mizuno Y, et al. Association between serum uric acid and lumbar spine bone mineral density in peri- and postmenopausal Japanese women. Osteoporos Int 2014;25:1099–105. [DOI] [PubMed] [Google Scholar]
- [11].Ahn SH, Lee SH, Kim BJ, et al. Higher serum uric acid is associated with higher bone mass, lower bone turnover, and lower prevalence of vertebral fracture in healthy postmenopausal women. Osteoporos Int 2013;24:2961–70. [DOI] [PubMed] [Google Scholar]
- [12].Makovey J, Macara M, Chen JS, et al. Serum uric acid plays a protective role for bone loss in peri- and postmenopausal women: a longitudinal study. Bone 2013;52:400–6. [DOI] [PubMed] [Google Scholar]
- [13].Han W, Bai X, Wang N, et al. Association between lumbar bone mineral density and serum uric acid in postmenopausal women: a cross-sectional study of healthy Chinese population. Arch Osteoporos 2017;12:50. [DOI] [PubMed] [Google Scholar]
- [14].Lee SG, Park YE, Park SH, et al. Increased frequency of osteoporosis and BMD below the expected range for age among South Korean women with rheumatoid arthritis. Int J Rheum Dis 2012;15:289–96. [DOI] [PubMed] [Google Scholar]
- [15].Kweon SM, Sohn DH, Park JH, et al. Male patients with rheumatoid arthritis have an increased risk of osteoporosis: frequency and risk factors. Medicine (Baltimore) 2018;97:e11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xue AL, Wu SY, Jiang L, et al. Bone fracture risk in patients with rheumatoid arthritis: a meta-analysis. Medicine (Baltimore) 2017;96:e6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vis M, Guler-Yuksel M, Lems WF. Can bone loss in rheumatoid arthritis be prevented? Osteoporos Int 2013;24:2541–53. [DOI] [PubMed] [Google Scholar]
- [18].Zhao DD, Jiao PL, Yu JJ, et al. Higher serum uric acid is associated with higher bone mineral density in chinese men with type 2 diabetes mellitus. Int J Endocrinol 2016;2016:2528956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kang KY, Hong YS, Park SH, et al. Low levels of serum uric Acid increase the risk of low bone mineral density in young male patients with ankylosing spondylitis. J Rheumatol 2015;42:968–74. [DOI] [PubMed] [Google Scholar]
- [20].Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- [21].Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 1994;4:368–81. [DOI] [PubMed] [Google Scholar]
- [22].Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007;53:766–72. [DOI] [PubMed] [Google Scholar]
- [23].Prevoo ML, van ’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- [24].Pirro M, Mannarino MR, Bianconi V, et al. Uric acid and bone mineral density in postmenopausal osteoporotic women: the link lies within the fat. Osteoporos Int 2017;28:973–81. [DOI] [PubMed] [Google Scholar]
- [25].Dong XW, Tian HY, He J, et al. Elevated serum uric acid is associated with greater bone mineral density and skeletal muscle mass in middle-aged and older adults. PLoS One 2016;11:e0154692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sritara C, Ongphiphadhanakul B, Chailurkit L, et al. Serum uric acid levels in relation to bone-related phenotypes in men and women. J Clin Densitom 2013;16:336–40. [DOI] [PubMed] [Google Scholar]
- [27].Muka T, de Jonge EA, Kiefte-de Jong JC, et al. The influence of serum uric acid on bone mineral density, hip geometry, and fracture risk: The Rotterdam Study. J Clin Endocrinol Metab 2016;101:1113–22. [DOI] [PubMed] [Google Scholar]
- [28].Lane NE, Parimi N, Lui LY, et al. Association of serum uric acid and incident nonspine fractures in elderly men: the Osteoporotic Fractures in Men (MrOS) study. J Bone Miner Res 2014;29:1701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mehta T, Buzkova P, Sarnak MJ, et al. Serum urate levels and the risk of hip fractures: data from the Cardiovascular Health Study. Metabolism 2015;64:438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Veronese N, Bolzetta F, De Rui M, et al. Serum uric acid and incident osteoporotic fractures in old people: The PRO.V. A study Bone 2015;79:183–9. [DOI] [PubMed] [Google Scholar]
- [31].Phull AR, Nasir B, Haq IU, et al. Oxidative stress, consequences and ROS mediated cellular signaling in rheumatoid arthritis. Chem Biol Interact 2018;281:121–36. [DOI] [PubMed] [Google Scholar]
- [32].Domazetovic V, Marcucci G, Iantomasi T, et al. Oxidative stress in bone remodeling: role of antioxidants. Clin Cases Miner Bone Metab 2017;14:209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chen W, Roncal-Jimenez C, Lanaspa M, et al. Uric acid suppresses 1 alpha hydroxylase in vitro and in vivo. Metabolism 2014;63:150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Paik JM, Kim SC, Feskanich D, et al. Gout and risk of fracture in women: a prospective cohort study. Arthritis Rheumatol 2017;69:422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Brzoska MM, Rogalska J, Kupraszewicz E. The involvement of oxidative stress in the mechanisms of damaging cadmium action in bone tissue: a study in a rat model of moderate and relatively high human exposure. Toxicol Appl Pharmacol 2011;250:327–35. [DOI] [PubMed] [Google Scholar]
- [36].Sakai T, Sairyo K, Hamada D, et al. Radiological features of lumbar spinal lesions in patients with rheumatoid arthritis with special reference to the changes around intervertebral discs. Spine J 2008;8:605–11. [DOI] [PubMed] [Google Scholar]
- [37].Kawaguchi Y, Matsuno H, Kanamori M, et al. Radiologic findings of the lumbar spine in patients with rheumatoid arthritis, and a review of pathologic mechanisms. J Spinal Disord Tech 2003;16:38–43. [DOI] [PubMed] [Google Scholar]
- [38].Kim Y, Kang J, Kim GT. Prevalence of hyperuricemia and its associated factors in the general Korean population: an analysis of a population-based nationally representative sample. Clin Rheumatol 2018;37:2529–38. [DOI] [PubMed] [Google Scholar]
- [39].Lee JJ, Bykerk VP, Dresser GK, et al. Reduction in serum uric acid may be related to methotrexate efficacy in early rheumatoid arthritis: data from the Canadian Early Arthritis Cohort (CATCH). Clin Med Insights Arthritis Musculoskelet Disord 2016;9:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]


