Abstract
Background:
Acute respiratory tract infection (ARTI) should be deeply concerned all over the world. Panax ginseng (ginseng) as traditional Chinese medicine is widely used in the treatment and health care for respiratory diseases. However, only one similar systematic review based on common cold has been published in 2011. New studies have occurred and a new systematic evaluation which could describe ARTI is needed.
Methods and analysis:
We will search for randomized control trials of ginseng on preventing acute respiratory tract infection in the following 8 databases: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, AMED (via OVID) and 4 Chinese databases (Chinese Biomedical Literature Database, China National Knowledge Infrastructure, Chinese Science and Technology Periodical Database, and Wan fang Database). The time is limited from the construction of the library to April 2020. The selection of studies, data extraction and quality of assessment will be conducted independently by 2 reviewers. The morbidity of ARTI by assessing self-report, caregiver report or clinical confirmation will be considered as the primary outcome. ARTI-related death among children or adults, other adverse events, absenteeism, laboratory-confirmed infection will be regarded as secondary outcome. All reported side effects and adverse events will be included as safety outcomes. Standard meta-analysis will be performed using Rev Man software V5.3.
Results:
This study will provide a better understanding of the association between P ginseng and ARTI.
Conclusion:
This systematic review may offer stronger evidences for the clinicians to prevent the patients from ARTI and update the former one based on basic diseases and the safety.
PROSPERO registration number:
CRD42020181317.
Keywords: acute respiratory tract infection, panax ginseng, protocol, systematic review and meta-analysis
1. Introduction
Current stage, the whole world suffered from a health crisis, Corona Virus Disease 2019 (COVID-19), an acute respiratory tract infection (ARTI) with high mortality rate.[1] Influenza and other respiratory viral infections are the most common type of ARTI.[2] According to statistics, the number of deaths caused by ARTI in China is nearly 100,000 every year.[3] Meanwhile, ARTI also could increase the risk of acute attacks in patients with chronic obstructive pulmonary disease (COPD), cardiovascular disease and other chronic diseases.[4–6]
Medical technicians around the world have carried out many researches on different viruses and pathogens, but they still cannot effectively control the global pandemic such as COVID-19.[7] In the theory of traditional Chinese medicine, the Qi can protect the human body from pathogenic factors like virus is called Defensive Qi (Wei Qi in Chinese).[8] Especially patients suffering from chronic diseases will show a more significant trend of Qi Deficiency.[9,10] Therefore, benefiting Qi is helpful to strengthen the immunity and resist the invasion of exogenous evils to human health.[11,12]
Ginseng is a plant in the family Araliaceae and the genus Panax with the formal name of Panax ginseng C. A. Meyer and the treasure of traditional herbal medicine resources as the “King of Herbs.”[13,14] Ginseng is famous for its remarkable effect of benefiting Qi.[15] The application of benefiting Qi herb such as P ginseng (ginseng) is the cornerstone of Traditional Chinese Medicine (TCM) in the prevention of exogenous diseases, such as ARTI.[16] A series of clinical studies have found that ginseng has a preventive effect on ARTI, however, none of these studies could provide definitive evidence due to limited research design or small sample.[17–19] Therefore, a high-quality systematic review and meta-analysis to summarize current clinical evidence is urgently needed.
After preliminary search and database analysis, we have found that there has been no relevant systematic review and meta-analysis for many years. The most recent study was published in 2011 and only included 5 studies.[17] Therefore, we hope to evaluate the preventive effect of P ginseng or P ginseng extract on ARTI to provide sufficient evidence for medical personnel.
2. Methods
2.1. Study registration
The protocol of this systematic review and meta-analysis has been registered in the International Prospective Register of Systematic Reviews (PROSPERO), and the registration number is CRD42020181317. This systematic review and meta-analysis will be reported in accordance with the guidelines of the Cochrane handbook for systematic reviews of interventions and the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement. Ethical approval is not required for this study.
2.2. Inclusion and exclusion criteria
2.2.1. Types of studies
Parallel-group randomized controlled trials (RCT) will be included. No restriction will be put on the language, publication date or status of the study.
2.2.2. Types of patients
The target population is adult or children irrespective of gender and ethnicity with symptoms of ARTI. A clinical diagnosis of ARTI was the main inclusion criteria. Diagnoses of upper or lower ARTI include acute common cold, influenza, rhino sinusitis, laryngitis, tonsillitis, pharyngitis, croup, acute otitis media, bronchitis, pneumonia, and acute exacerbations of COPD.
2.2.3. Types of interventions
The experimental interventions include a ginseng alone and a combination of ginseng and another active treatment (pharmacological or nonpharmacological intervention).
No restrictions will be made on the types of control groups
2.2.4. Types of outcome measures
The primary efficacy end point is the frequency rate of ARTI.
2.2.4.1. Primary outcomes
The primary outcome will be the morbidity of ARTI by assessing self-report, caregiver report or clinical confirmation.
2.2.4.2. Secondary outcomes
ARTI-related death among children or adults, other adverse events, absenteeism, laboratory-confirmed infection will be regarded as additional outcome. And all reported side effects and adverse events will be included as safety outcomes.
2.3. Search methods for the identification of studies
2.3.1. Electronic searches
Two investigators search the following databases from construction of databases to April 2020: MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), the Cumulative Index to Nursing and Allied Health Literature (CINAHL), Web of Science and three Chinese databases (China BioMedical Literature, China National Knowledge Infrastructure, and Wan Fang database).
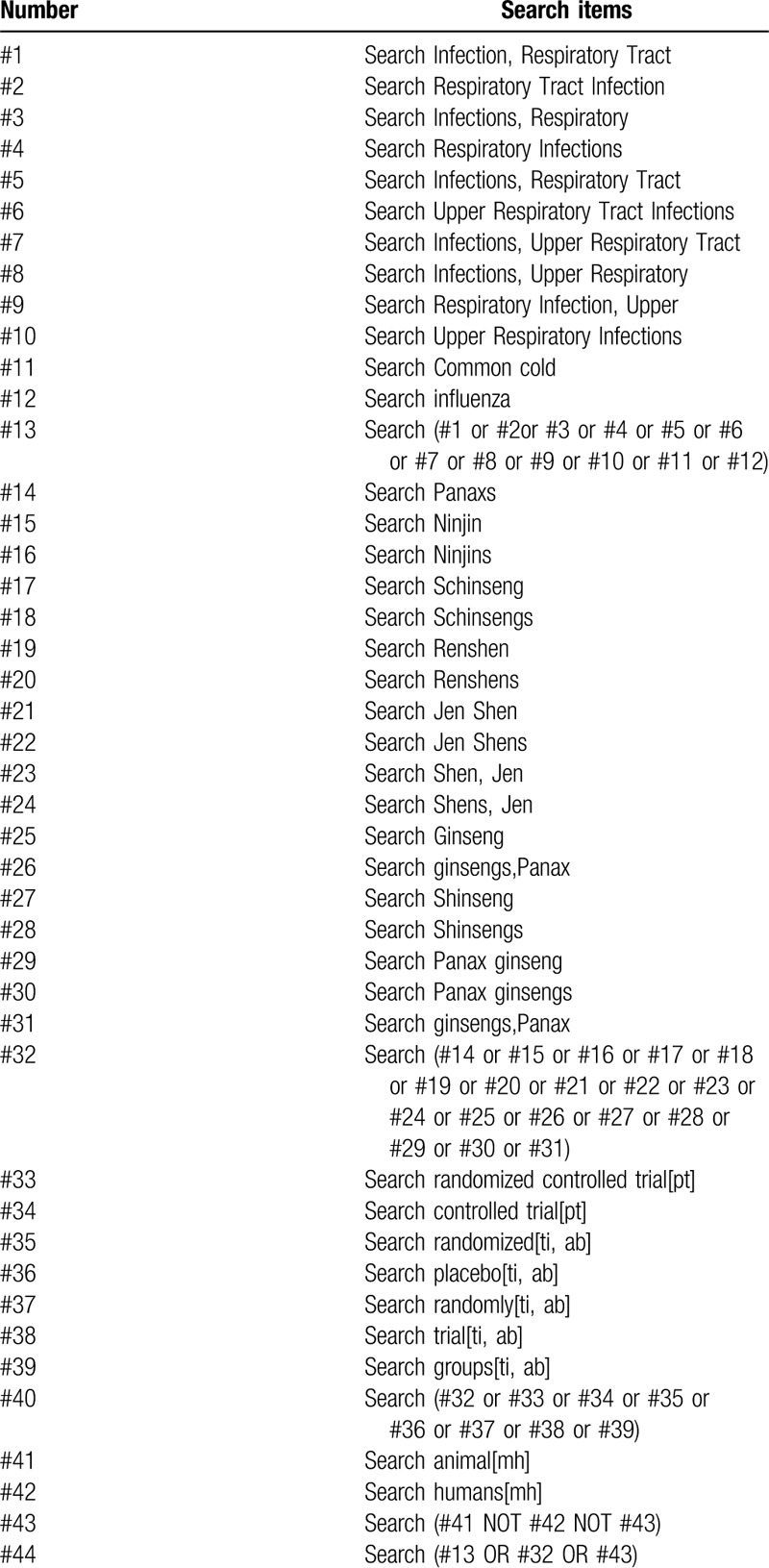
We will conduct search strategy by using Medical Subject Headings/Emtree headings combined with free text words. The search strategy for PubMed is shown in Table 1. The equivalent search words will be used in the Chinese databases.
Table 1.
Search strategy used in PubMed database.
2.3.2. Searching other resources
In addition, Chinese Clinical Trial Registry, Clinical Trials.gov, International Clinical Trials Registry Platform (WHO-ICTRP) will be searched to identify additional ongoing or unpublished studies.
2.4. Data collection and analysis
2.4.1. Selection of studies
The titles and abstracts of studies retrieved using the search strategy and those from additional sources will be screened independently by 2 review authors to identify the studies that potentially meet the predetermined inclusion criteria. Disagreement in this and all following steps of the systematic review process will be resolved by discussion or adjudication by a third reviewer, when necessary. The authors will the record reasons for exclusion of each study and report the results of the screening, according to PRISMA flow diagram.[20] The flow diagram of study selection was shown in Figure 1.
Figure 1.
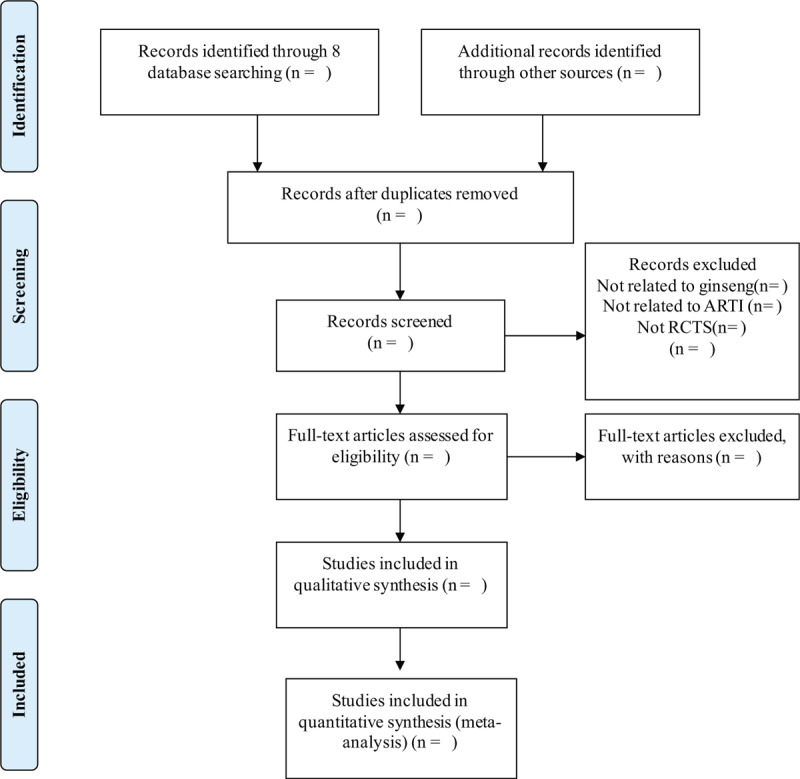
Flow chart of study selection. ARTI = acute respiratory tract infection, RCT = randomized controlled trials.
2.4.2. Data extraction and management
A standard data extraction form will be created before data extraction. Two reviewers (JL and QH) will independently extract the following information:
-
1.
General information (title, first author, year of publication, funding).
-
2.
Study characteristics (design, randomization, allocation, blinding, inclusion and exclusion criteria, sample size).
-
3.
Participant characteristics (age, ethnicity, diagnosis criteria, number in each group).
-
4.
Intervention characteristics (intervention, comparator intervention, dos-age, frequency, and duration).
-
5.
Outcomes (primary and secondary outcomes, time points, methods of outcome assessments, blinding of outcome assessment, adverse events).
Only the latest report will be included when a same trial was described by multiple publications. Data not available in the publications will be obtained by contacting corresponding authors for more information.
2.4.3. Date synthesis
We will perform the meta-analysis when more than one trial examines the same intervention and outcomes with comparable methods in similar populations. If the statistical heterogeneity is not identified, the fixed-effect model will be built to estimate the overall intervention effects.[21] Otherwise, the random-effect model will be used to provide more conservative results. When multiple intervention groups are used in a study, we will make pair-wise comparisons by combining groups if possible. All statistical analyses will be performed by the RevMan V.5.3 software. The statistical significance is defined as P < .05. If the meta-analysis is not feasible, we will provide a narrative description of the results.
2.4.4. Risk of bias assessment
The methodological quality of each individual study will be independently assessed by 2 reviewers (ZW and DZ) according to the Cochrane ROB tool.[22] The following seven domains will be assessed: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other potential sources of bias. The risk of bias for each domain will be graded as low, high or unclear for each included study. The consistency will be checked by a third reviewer (LS) and the disagreements were resolved by discussion with methodologists (JW, XL).
2.4.5. Assessment of heterogeneity
Statistical heterogeneity across the studies included will be tested using χ2 test and I2 statistic. The heterogeneity is significant statistically when the P value based on χ2 test less than 0.10 or I2 more than 50%.[23] If so, exploratory sensitivity or subgroup analyses will be performed to identify possible reasons.[24]
2.4.6. Assessment of reporting biases
The reporting bias will be investigated using visual funnel plots if more than 10 RCTs are included in a meta-analysis. If the reporting bias is identified, we will explore possible reasons using the subgroup analysis or meta-regression analysis.[25]
2.4.7. Assessment of evidence quality
The overall quality of the evidence will be assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach on the efficacy and safety of P ginseng for acute respiratory tract infection. The quality of RCT evidence will be classified into “high,” “‘moderate,” “low,” or “very low” quality evidence, depending on the presence of these 5 factors:
-
(1)
limitations in the design and implementation;
-
(2)
indirectness of evidence;
-
(3)
unexplained heterogeneity or inconsistency of results;
-
(4)
imprecision of results; and
-
(5)
high probability of publication bias.[25]
2.5. Analysis of subgroups or subsets
Subgroup analyses will be divided by basic disease, literature quality and type of outcome reported.
3. Discussion
ARTI induced by COVID-19, influenza or chronic diseases causes the horrible threat to human health in the whole world. Based on the effect of benefiting Qi, ginseng can strengthen human immunity against ARTI, but the relationship between ginseng and the prevention of ARTI is still unclear. Some new studies have been conducted since 2011, it is necessary to update previous systemic review about the preventive effect of ginseng on ARTI. This study will obtain the evidences of ginseng or ginseng extract on ARTI prevention by summarizing previous clinical evidences.
The advantages of this review will be:
-
(1)
this review will include more clinical studies than the former one, since the last study only extracted the proportion and symptoms of a cold or ARTI in only 5 research reports;
-
(2)
this study will also try to analyze the research reports of ARTI from COPD, cardiovascular disease, and other chronic diseases and get more comprehensive and practical results;
-
(3)
To avoid bias as much as possible, we will collect all relevant documents as comprehensively as possible. As to the exploration of heterogeneity, post hoc subgroup analysis should be avoided as much as possible.
Despite these efforts, the limitations in this systematic review will still exist:
-
(1)
Only the studies for conventional treatment with ginseng will be included, the formula or others combined with ginseng will not be included;
-
(2)
the origin and age of ginseng in included studies are not mentioned and should be considered for the analysis.
Author contributions
Conceptualization: Zepeng Zhang, Peng Xu, Jian Wang, Xiangyan Li.
Data curation: Peng Xu.
Formal analysis: Qingxia Huang, Jing Lu.
Funding acquisition: Daqing Zhao.
Investigation: Zhihong Wang.
Methodology: Peng Xu, Liwei Sun, Xiangyan Li.
Project administration: Zepeng Zhang.
Resources: Zepeng Zhang, Peng Xu.
Software: Zepeng Zhang, Peng Xu.
Supervision: Jian Wang, Xiangyan Li.
Writing – original draft: Zepeng Zhang, Peng Xu.
Writing – review & editing: Jian Wang, Xiangyan Li.
Footnotes
Abbreviations: ARTI = acute respiratory tract infection, COPD = chronic obstructive pulmonary disease, COVID-19 = Corona Virus Disease 2019, CNKI = China National Knowledge Infrastructure database, Ginseng = Panax ginseng, GRADE = Grading of Recommendations Assessment, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analysis, PROSPERO = the International Prospective Register of Systematic Reviews, RCT = randomized controlled trials, WHO = World Health Organization.
How to cite this article: Zhang Z, Xu P, Wang Z, Zhao D, Huang Q, Lu J, Sun L, Wang J, Li X. Effect of Panax ginseng on preventing acute respiratory tract infection: a protocol for systematic review and meta-analysis. Medicine. 2020;99:24(e20690).
ZZ and PX contributed equally and are co-first authors.
This work was supported by the National Key Research and Development Program of China (2017YFC1702103, 2018YFC170203), the National Natural Science Foundation of China (U19A2013), the National Administration of Traditional Chinese Medicine (No.2019XZZX-NB005), the Science and Technology Development Plan Project of Jilin Province (No. 20190101010JH, 20180623041TC). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Desai AN, Patel P. Stopping the spread of COVID-19. JAMA 2020;323:1516. [DOI] [PubMed] [Google Scholar]
- [2].Hanada S, Pirzadeh M, Carver KY, et al. Respiratory viral infection-induced microbiome alterations and secondary bacterial pneumonia. Front Immunol 2018;9:2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Li L, Liu Y, Wu P, et al. Influenza-associated excess respiratory mortality in China, 2010-15: a population-based study. Lancet Public Health 2019;4:e473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Linden D, Guo-Parke H, Coyle PV, et al. Respiratory viral infection: a potential “missing link” in the pathogenesis of COPD. Eur Respir Rev 2019;28: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fountoulaki K, Tsiodras S, Polyzogopoulou E, et al. Beneficial effects of vaccination on cardiovascular events: myocardial infarction, stroke. Heart Failure Cardiol 2018;141:98–106. [DOI] [PubMed] [Google Scholar]
- [6].Ginde AA, Blatchford P, Breese K, et al. High-dose monthly vitamin D for prevention of acute respiratory infection in older long-term care residents: a randomized clinical trial. J Am Geriatr Soc 2017;65:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Petrosillo N, Viceconte G, Ergonul O, et al. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect 2020;26:729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang XM, Li XB, Peng Y. Impact of Qi-invigorating traditional Chinese medicines on intestinal flora: a basis for rational choice of prebiotics. Chin J Nat Med 2017;15:241–54. [DOI] [PubMed] [Google Scholar]
- [9].Shen DD, Yang ZH, Huang J, et al. Liuweibuqi capsules improve pulmonary function in stable chronic obstructive pulmonary disease with lung-qi deficiency syndrome by regulating STAT4/STAT6 and MMP-9/TIMP-1. Pharm Biol 2019;57:744–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhou L, Guo SN, Gao Y. Effects and perspectives of Chinese patent medicines for Tonifying Qi and promoting blood circulation on patients with cerebral infarction. Curr Vasc Pharmacol 2015;13:475–91. [DOI] [PubMed] [Google Scholar]
- [11].Xu YY, Liu JH, Ding H, et al. Clinical research of auricular gold-needle therapy in treatment of chronic fatigue syndrome of qi deficiency constitution. Chin Acupunct Moxibust 2019;39:128–32. [DOI] [PubMed] [Google Scholar]
- [12].Sheng W, Wang Y, Li JB, et al. Clinical and basic research on Renshen Yangrong decoction. Front Nutr 2019;6:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Park HJ, Kim DH, Park SJ, et al. Ginseng in traditional herbal prescriptions. J Ginseng Res 2012;36:225–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Li XY, Sun LW, Zhao DQ. Current status and problem-solving strategies for ginseng industry. Chin J Integr Med 2019;25:883–6. [DOI] [PubMed] [Google Scholar]
- [15].Nguyen NH, Nguyen CT. Pharmacological effects of ginseng on infectious diseases. Inflammopharmacology 2019;27:871–83. [DOI] [PubMed] [Google Scholar]
- [16].Wu T, Yang X, Zeng X, et al. Traditional Chinese medicine in the treatment of acute respiratory tract infections. Respir Med 2008;102:1093–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Seida JK, Durec T, Kuhle S. North American (Panax quinquefolius) and Asian Ginseng (Panax ginseng) preparations for prevention of the common cold in healthy adults: a systematic review. Evid Based Complement Alternat Med 2011;2011: 282151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Saboori S, Falahi E, Yousefi Rad E, et al. Effects of ginseng on C-reactive protein level: a systematic review and meta-analysis of clinical trials. Compl Therap Med 2019;45:98–103. [DOI] [PubMed] [Google Scholar]
- [19].Vohra S, Johnston BC, Laycock KL, et al. Safety and tolerability of North American ginseng extract in the treatment of pediatric upper respiratory tract infection: a phase II randomized, controlled trial of 2 dosing schedules. Pediatrics 2008;122:e402–10. [DOI] [PubMed] [Google Scholar]
- [20].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. [DOI] [PubMed] [Google Scholar]
- [21].Hopp L. Risk of bias reporting in Cochrane systematic reviews. Int J Nurs Pract 2015;21:683–6. [DOI] [PubMed] [Google Scholar]
- [22].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical Research ed) 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Weng H, Zeng XT, Li S, et al. Intrafascial versus interfascial nerve sparing in radical prostatectomy for localized prostate cancer: a systematic review and meta-analysis. Sci Rep 2017;7:11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Baicus C, Purcarea A, von Elm E, et al. Alpha-lipoic acid for diabetic peripheral neuropathy. Cochrane Database of Syst Rev 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Higgins J, Green S, Collaboration C. Cochrane handbook for systematic reviews for interventions. Cochrane Database Syst Rev 2011;2011:S38. [Google Scholar]


