Abstract
Background:
In the current literature, studies assessing the role of Helicobacter pylori (HP) infection in psoriasis have reported conflicting data. Therefore, we investigated the association between HP infection and psoriasis using a nationwide population-based longitudinal cohort study.
Methods:
We identified 41,539 patients with HP infection and 83,078 matched controls between 2000 and 2013 from the Longitudinal Health Insurance Research Database of the National Health Insurance Research Database in Taiwan. Propensity score analysis was used to match age, sex, comorbidities, and medical visits at a ratio of 1:2. Multiple Cox regression analysis was used to estimate the adjusted hazard ratio of psoriasis. Furthermore, sensitivity tests and a stratified analysis were conducted.
Results:
The incidence rates of psoriasis did not differ significantly between the HP and control cohorts (4.58 vs 4.20 per 100,000 person-months, crude relative risk: 1.092, 95% confidence interval: 0.917–1.302). After multivariate adjustment, no significant difference in psoriasis risk was observed in patients with HP infection (adjusted hazard ratio: 1.081, 95% confidence interval: 0.907–1.288). Risk of psoriasis was significantly higher in men and the elderly, and in those with diabetes, hyperlipidemia, chronic obstructive pulmonary disease, or tuberculosis. Stratified analysis also confirmed that HP infection was not correlated with an increased risk of psoriasis based on follow-up duration, sex, and age.
Conclusion:
This retrospective population-based longitudinal cohort study, conducted in Taiwan, found no association between HP infection and risk of psoriasis. Further research may be warranted.
Keywords: cohort study, Helicobacter pylori, population-based, psoriasis
1. Introduction
Psoriasis is a chronic inflammatory autoimmune disease with dermatological manifestations. It is typically characterized by erythematous papules and plaques with silvery scales, and is mediated by cytokine crosstalk between epidermal keratinocytes, dermal vascular cells, and immunocytes, such as antigen-presenting cells and T cells. An increased proliferation of endothelial cells and keratinocytes in conjunction with an increase in antigen-presenting cells, monocytes, macrophages, T cells, and inflammation may cause distinct epidermal and vascular hyperplasia, which are characteristics of psoriatic skin. No single genetic aberration has been identified as being responsible for the induction of psoriasis, which is believed to be multifactorial with numerous key components, including genetic susceptibility, and environmental triggers, skin barrier disruption, immune dysfunction, and infection, as well as microbial and complex cellular interactions.[1,2] Current therapies being used to manage psoriasis include traditional agents, such as methotrexate, cyclosporine, acitretin, and novel biologic agents, such as tumor necrosis factor alpha blockers, interleukin 17 blockers, and interleukin 23 blockers.[3]
Helicobacter pylori (HP) is a widely prevalent microbe which persists for multiple decades in infected individuals.[4] Epidemiological and experimental data now indicate the existence of a strong relationship between HP infection and the development of many extra-gastric diseases, including several allergic and autoimmune diseases.[5–7] Some findings support the hypothesis that HP can worsen psoriasis by interfering with and amplifying immune responses in genetically susceptible individuals.[8,9] Furthermore, HP infections are considerably more common in patients with psoriasis than in healthy controls.[10,11] A number of case studies have reported that psoriatic lesions cleared up following the eradication of HP infections.[9,12,13] However, there are conflicting results in the literature, and both the prevalence and role of HP infection in psoriasis remain topics of discussion. Many studies have speculated that an association between HP infection and psoriasis exists.[2,11,14–16] Whether HP infection exacerbates or triggers the pathogenetic mechanisms that lead to psoriasis remains debatable; however, the relationship between HP and psoriasis has never been investigated using data obtained from a large national database. Thus, the aim of our study was to assess the association between HP infection and psoriasis at a nationwide level.
2. Methods
2.1. Study design and population
We designed a retrospective cohort study to analyze the association between HP infection and psoriasis. The flowchart of the study design is depicted in Figure 1. We accessed the Longitudinal Health Insurance Research Database (LHIRD), which comprises 1 million individuals randomly sampled from Taiwan's National Health Insurance Research Database (NHIRD), a nationwide population-based insurance system that covers 99.6% of the nation's population and stores records of medical claims filed between 1997 and 2013.[17,18] Moreover, the LHIRD is one of the largest databases of the administrative medical care system.[19] Using this database, the prevalence, incidence, and correlations of selected factors can be determined. Patient diagnoses are recorded in accordance with the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Moreover, the database contains demographic data, inpatient and outpatient expenditure claims, and other clinical information. To prevent confounding bias, which often exists in observational studies, we controlled for differences by performing propensity score matching of selected variables. The database that was analyzed in this investigation has been used in thousands of previous studies in the literature.[17] This study was approved by the Institutional Review Board of Chung Shan Medical University Hospital (approval number CS15134).
Figure 1.
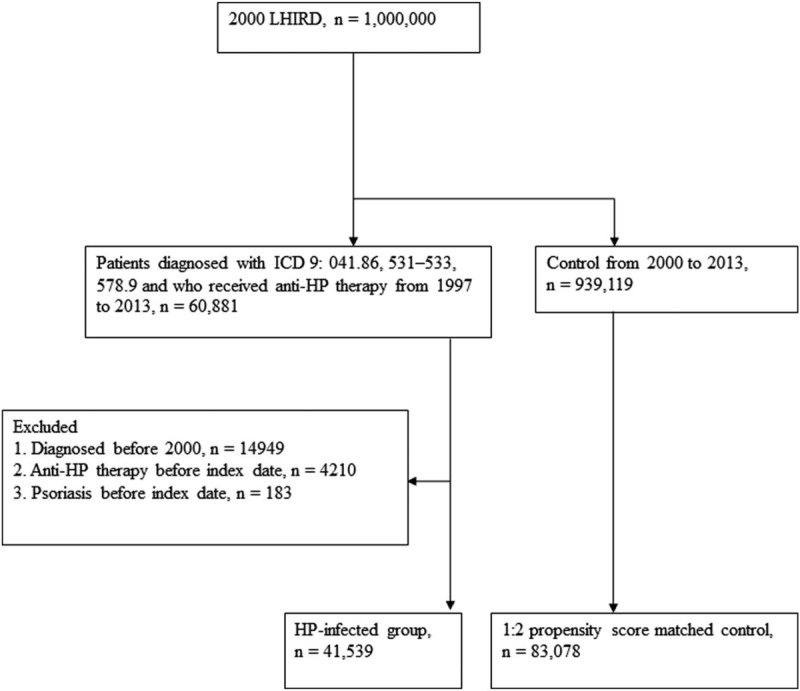
Flowchart of study.
2.2. Exposure definition of HP infection and controls
We identified patients who had diagnoses of HP infection (ICD-9-CM: 41.86), peptic ulcers (ICD-9-CM: 531–533), or hemorrhage of the gastrointestinal tract (ICD-9-CM: 578.9) and received anti-HP therapy from 1997 to 2013.[7] According to the reimbursement requirements of the National Health Insurance system, HP infection was confirmed by upper endoscopy with biopsy-based tests (such as a histological assessment, rapid urease test, or biopsy culture) and HP-related treatments were reimbursed based on biopsy-related tests. Anti-HP therapy with triple or quadruple therapy was defined as a proton pump inhibitor or H2 receptor antagonist plus clarithromycin or metronidazole and amoxicillin or tetracycline, with or without bismuth. These drug combinations were prescribed in the same order, and the duration of the therapy was 7 to 14 days. Details of all eligible HP-eradication regimens are described elsewhere.[7,20] The first date of diagnosis of HP infection, peptic ulcer, or hemorrhage of the gastrointestinal tract was defined as the index date. Patients were excluded if they had ever been diagnosed with psoriasis before the index date, were diagnosed before 2000, had undergone anti-HP therapy before the index date, or an appropriate propensity score-matched control could not be identified.
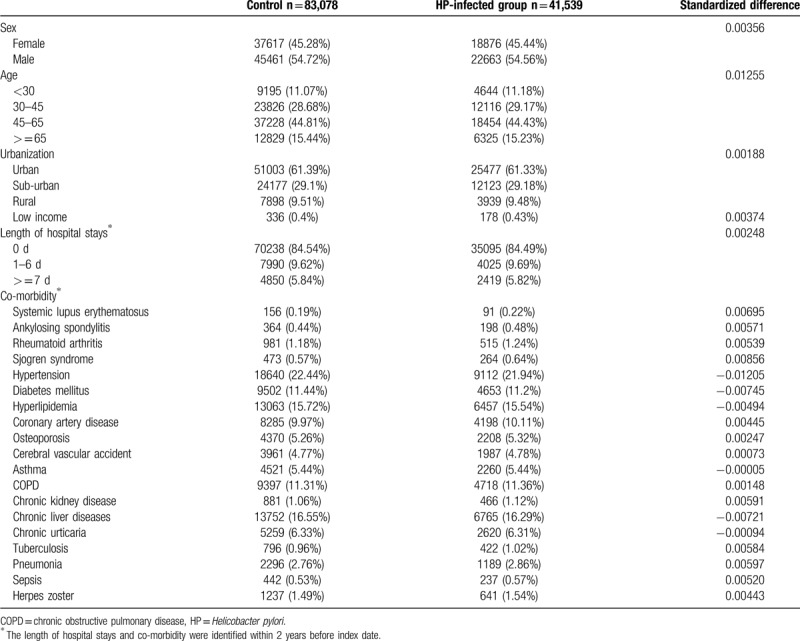
For the control group, individuals registered in the LHIRD who did not receive anti-HP therapy were selected as candidates. To resolve the possible effect of confounding bias of comorbidities on incidence of psoriasis, control participants were 2:1 propensity score-matched with HP-infected patients using an 8-to-1 digit greedy matching algorithm.[21] The index date for the controls was determined according to the respective matched cases. In this procedure, the probability was estimated by logistic regression model using the predictors, including age, gender, urbanization level, income level, length of hospital stay (within 2 years before the index date), and comorbidities listed in Table 1. The standardized differences between the covariates of these 2 groups after propensity score matching were <10%, which indicated a good balance between the 2 cohorts. Finally, 41,539 patients who received anti-HP therapy and 83,078 propensity score-matched controls were included in the analysis.
Table 1.
Baseline characteristics of study groups after propensity score matching.
2.3. Outcome and comorbidities
The patients with psoriasis were identified using ICD-9-CM codes 696.0 and 696.1. To ensure that the diagnoses of psoriasis were accurate, only patients who had been diagnosed with psoriasis at least 3 times at outpatient clinics, or were admitted at least once, were eligible for inclusion in the final analysis. These codes have been utilized in previous epidemiologic studies employing the NHIRD.[22,23] In addition, we identified comorbidities related to HP infection and psoriasis, namely systemic lupus erythematosus (ICD-9-CM: 710.0), ankylosing spondylitis (ICD-9-CM: 720.0), rheumatoid arthritis (ICD-9-CM: 714.0), Sjogren syndrome (ICD-9-CM: 710.2), hypertension (ICD-9-CM: 401–405), diabetes mellitus (ICD-9-CM: 250), hyperlipidemia (ICD-9-CM: 272), coronary artery disease (ICD-9-CM: 410–414), osteoporosis (ICD-9-CM: 733), cerebral vascular accident (ICD-9-CM: 430–438), asthma (ICD-9-CM: 493), chronic obstructive pulmonary disease (COPD) (ICD-9-CM: 490–492 and 493–496), chronic kidney disease (ICD-9-CM: 585), chronic liver diseases (ICD-9-CM: 571 and 573), chronic urticaria (ICD-9-CM: 708.8 and 708.9), tuberculosis (ICD-9-CM: 011–018 and 1370), pneumonia (ICD-9-CM: 480–486), sepsis (ICD-9-CM: 038), and herpes zoster (ICD-9-CM: 053). Comorbidities were defined, using the relevant diagnostic codes, as at least 1 hospital admission or 2 outpatient visits of a given disease within 2 years before the index date. These comorbidities were considered covariates in the multivariate analysis.
2.4. Statistical analysis
We used the χ2 test to analyze the demographic differences between the HP and control groups. The univariate and multiple Cox proportional hazard regression models were used to calculate the hazard ratios. P < .05 was considered statistically significant. For evaluating the precision of measurements, the 95% confidence interval (CI) was used. The cumulative incidence probability curves of psoriasis were generated using the Kaplan–Meier method, and the log-rank test was performed to test the difference between curves. A landmark analysis was conducted to determine the psoriasis risk at 0 to 12, 13 to 36, and ≥36 months from the index date. The age and sex subgroup analysis evaluated the potential interaction effects of age, sex, and HP infection on psoriasis risk. All data were processed using SAS software (version 9.4; SAS Institute, Cary, NC).
3. Results
At baseline, the frequencies of selected factors, including age, sex, urbanization, monthly income, length of hospital stays, and comorbidities, were approximately the same in the 2 cohorts after propensity score matching (Table 1). The incidence rate of psoriasis revealed no significant difference between the HP cohort and the control cohort (4.58 vs 4.20 per 100,000 person-months, crude relative risk: 1.092, 95% CI: 0.917–1.302, Table 2). After multivariate adjustment, psoriasis risk did not differ significantly between patients with HP infection and the controls (adjusted hazard ratio [aHR]: 1.081, 95% CI: 0.907–1.288, Table 3). Furthermore, psoriasis risk was higher in male patients (aHR: 1.821; 95% CI: 1.515–2.188) than in female patients. Patients aged older than 45 years were at a significantly higher risk of psoriasis (aHR: 1.273; 95% CI: 1.023–1.584 for ages 45–65 years; aHR: 1.520; 95% CI: 1.141–2.025 for ages ≥ 65 years; Table 3). In terms of comorbidities, patients with diabetes mellitus (aHR: 1.268; 95% CI, 1.014–1.586), hyperlipidemia (aHR: 1.494; 95% CI, 1.161–1.922), COPD (aHR: 1.451; 95% CI, 1.061–1.984) and tuberculosis (aHR: 1.627; 95% CI, 1.215–2.179) were also at a significantly higher risk of psoriasis (Table 3). We further examined the association between HP infection and psoriasis risk after stratifying by follow-up time, sex, and age (Table 4), and the results showed the risk of psoriasis was similar in the HP-infected group and control group. The Kaplan–Meier curves of the cumulative psoriasis rate are shown in Figure 2 (Log-rank P = .32).
Table 2.
Psoriasis incidence in patients with HP infection and controls.
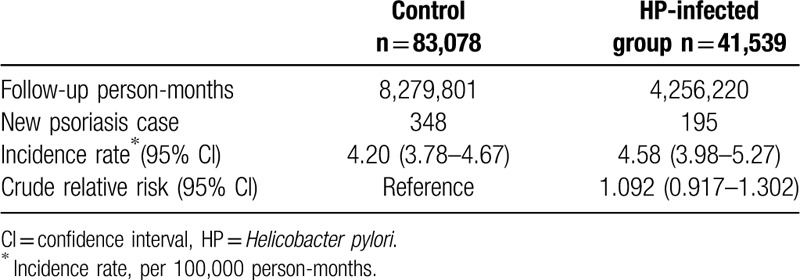
Table 3.
Multiple Cox proportional hazard regression analysis for estimation of adjusted hazard ratios on psoriasis.
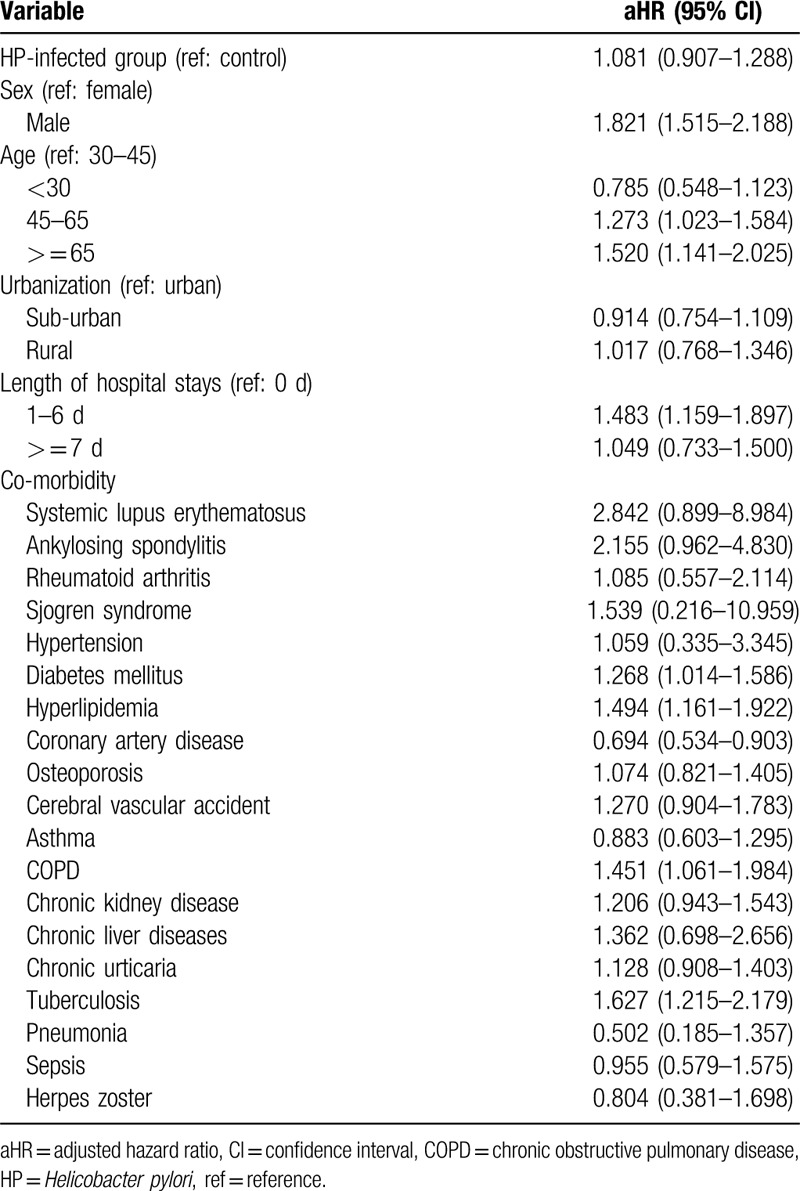
Table 4.
Sensitivity analysis for the adjusted hazard ratios stratified by follow-up time, sex, and age.
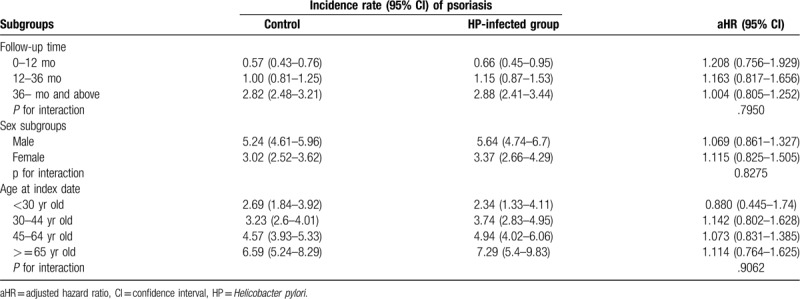
Figure 2.
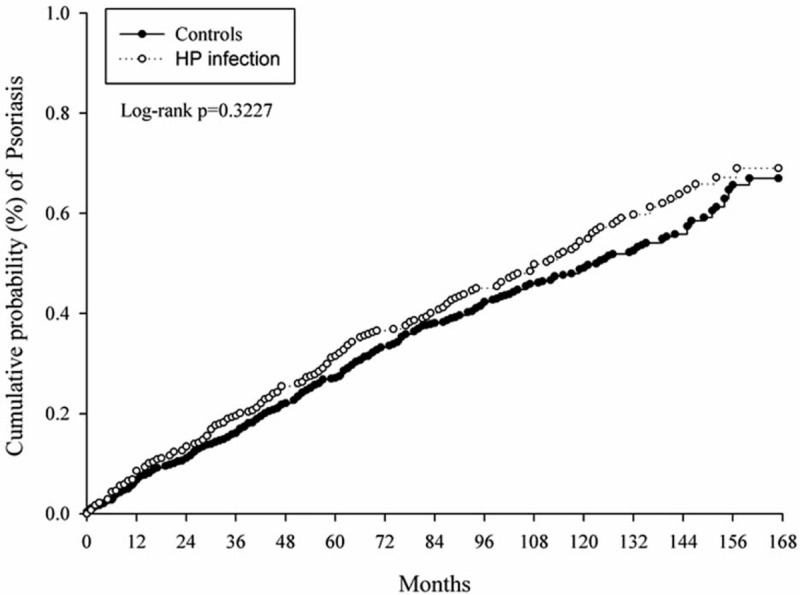
Kaplan–Meier curves of the cumulative probability of psoriasis in the study groups.
4. Discussion
The relationship between HP infection and psoriasis is an intriguing and challenging field of research. Although some studies have shown that HP has a role in the pathogenesis of some dermatological diseases,[16,24,25] our study found no significant relationship between HP infection and psoriasis. To the best of our knowledge, this is the first and largest epidemiological study to use a nationwide longitudinal population-based dataset to clarify the relationship between HP infection and psoriasis. According to our findings, we cannot recommend the routine HP test-and-treat strategy for patients with psoriasis. We also found that male gender, old age, diabetes, hyperlipidemia, COPD, and tuberculosis were associated with greater prevalence of psoriasis. There are few studies in the literature on factors affecting the risk of psoriasis and thus further investigation of these possible risk factors should be taken.
Our findings conflict with previous studies that reported HP plays a causative role in psoriasis. Halasz et al were the first to investigate serum immunoglobulin G (IgG) antibody titers against HP in 33 patients with psoriasis and established that a causative relationship exists linking this pathogen with psoriasis. Qayoom et al demonstrated that HP infection is more common in patients with psoriasis than in healthy controls.[10] Mesquita et al found that seroprevalence of HP infection was higher in patients with severe psoriasis (79%) than in those with moderate (69.5%) or mild (46.2%) disease and controls (33.3%).[11] Recent meta-analyses reported by Yong et al[2] and Yu et al[26] revealed a higher prevalence of HP infection in patients with psoriasis compared with the control group (pooled odds ratio = 1.58; 95% CI: 1.02–2.46 and odds ratio = 1.7; 95% CI: 1.15–2.52). They found that a previous HP infection played roles in the autoimmune cascade that leads to the development of psoriasis. However, the positive correlation was mainly driven by infections detected using the HP IgG serology test and not by ongoing infections detected using the stool antigen test and urea breath test.[2,26]
Consistent with our finding that there was no significant correlation of HP infection with psoriasis, Turkmen et al found that the results of the urea breath test did not differ significantly between patients with psoriasis and controls (67.9% vs 66.7%, respectively).[27] Onsun et al used the stool antigen test and found the prevalence of HP infection was 61.3% in patients with psoriasis versus 59.3% in the control group (P > .05).[8] In addition, Azizzadeh et al did not find a significant relationship between HP infection and psoriasis or a correlation between HP IgG level and psoriasis area and severity index (PASI) score.[28] Daudén et al were unable to find any differences in terms of cytotoxin-associated gene A seropositivity between psoriatic patients and patients with nonulcer dysplasia (54.5% vs 68.1%, respectively).[29] Recently, Patrikiou et al also found that prevalence rates of anti-HP seropositivity were similar in patients with psoriasis and psoriatic arthritis.[30]
Campanati et al[14] and Onsun et al[8] reported a significant improvement in PASI score in HP-infected patients with psoriasis receiving a combination of anti-HP therapy and psoralen plus ultraviolet A radiation (PUVA),[14] or anti-HP therapy and acitretin.[8] Furthermore, HP-infected patients with psoriasis who received HP eradication alone demonstrated greater improvement than those who received acitretin alone. Thus, taken together, the aforementioned studies indicate that HP contributes to the severity of psoriasis.[8] By contrast, although Campanati et al reported a significant improvement in PASI score in the infected group compared with the non-infected group after anti-HP therapy, the authors observed that it is possible the improvement in PASI score could be solely attributable to PUVA rather than to the effect of anti-HP treatment. Nevertheless, a few case reports of HP-infected patients have reported improvement in psoriasis after treatment.[9,12,13] However, Daudén et al did not find any improvement in psoriasis after eradicating HP infection.[31]
In summary, based on the studies published to date, there does not appear to be a clearly established consensus of opinion regarding the role of HP infection in psoriasis. The immunopathological process by which HP increases the risk of psoriasis remains uncertain. In theory, HP bacterial toxins could act as super-antigens, promoting a local inflammatory response, involving release of inflammatory mediators including interleukin 1, tumor necrosis factor alpha, Interferon gamma, leukotriene, and activation of T cells and monocytes.[16,32] The reported increased incidence of HP in psoriatic patients may be due to the use of topical or systemic immunosuppressive drugs leading to a greater susceptibility to HP infection. The current controversy concerning the relationship between psoriasis and HP infection may be due to several methodological limitations. First, most studies were conducted with small study populations and mainly employed HP serological testing. Studies based on serological tests provided information attributing the patient's immune status to bacteria or a marker of HP exposure and not necessarily to “true infection”. This difference may be explained by the diversity in seropositive HP infections in different countries worldwide. Second, most studies adopted case-control methods rather than the cohort approach. Studies that use a case-control design are not able to confirm causal relationships. Furthermore, most of these studies conducted analyses of unmatched groups and were prone to potential confounding bias. Third, in some studies, patients did not receive the same psoriasis treatment during the study period; therefore, understanding the contribution of eradication in alleviating disease was extremely difficult. Finally, some authors have reported clinical improvement in psoriasis without using a validated and reproducible severity scale.
In this study, we used a nationwide longitudinal population-based cohort database to overcome the limitations of previously reported analyses. The major strength of this study was the large sample size, in which a complete history of medical service use was available for all cases and controls. Therefore, our study was free from selection and recall bias, which made testing our hypothesis feasible. Furthermore, we attempted to use propensity score matching to achieve more complete control of the potential confounders. However, several limitations must be noted. First, the NHIRD does not disclose information regarding the patients’ socioeconomic status, family history, and personal lifestyle habits, such as smoking, alcohol use, body weight index, and inflammatory biomarkers. We accounted for lifestyle-related comorbidities that were potential risk factors for psoriasis. For example, COPD might be associated with smoking habits and hyperlipidemia, which in turn could be associated with obesity. Although we adjusted for various comorbidities and performed propensity score-matching, these unmeasured confounders might have affected our results. Second, the NHIRD database only contained data on patients who had received therapy for HP infection; therefore, the severity and duration of HP infection could not be evaluated in this study. Data on the virulence factors of HP strains were unavailable. Because symptoms were difficult to detect, the HP infection prevalence could have been underestimated. Third, whether anti-HP therapy reduced psoriasis risk could not be confirmed in this study. According to the general practice pattern and guidelines of Taiwan's National Health Insurance system, almost all patients with HP infection receive anti-HP therapy, and therefore, there were very few patients diagnosed with HP infection who did not receive anti-HP therapy. Finally, whether the findings of our study are generalizable to other ethnic groups remains uncertain because the majority of the patients were Taiwanese. Our results are likely more generalizable to Asian populations rather than to other ethnic populations. Large-scale clinical studies using population-based datasets should be conducted to elucidate the relationship between HP infection and psoriasis in patients from other ethnic backgrounds and countries.
5. Conclusion
HP infection was not associated with risk of psoriasis in this nationwide, population-based longitudinal cohort study. Further research is warranted.
Acknowledgments
We thank Hsing-Ju Wu, Peter Wilds, and Wallace Academic Editing for the editing of the manuscript.
Author contributions
All authors provided a substantial contribution to the conception, design, and interpretation of the work. MCW and KSKM drafted the manuscript. JCCW revised the manuscript critically. All authors approved the final version of the manuscript.
Conceptualization: Meng-Che Wu, Kevin Sheng-Kai Ma, Huang-Hsi Chen, Jing-Yang Huang, James Cheng-Chung Wei.
Data curation: James Cheng-Chung Wei.
Formal analysis: Meng-Che Wu, Kevin Sheng-Kai Ma, Huang-Hsi Chen, Jing-Yang Huang, James Cheng-Chung Wei.
Funding acquisition: Meng-Che Wu.
Writing – original draft: Meng-Che Wu, Kevin Sheng-Kai Ma.
Writing – review & editing: James Cheng-Chung Wei.
Footnotes
Abbreviations: aHR = adjusted hazard ratio, CI = confidence interval, COPD = chronic obstructive pulmonary disease, HP = Helicobacter pylori, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, IgG = immunoglobulin G, LHIRD = Longitudinal Health Insurance Research Database, NHIRD = National Health Insurance Research Database, PASI = psoriasis area and severity index.
How to cite this article: Wu M-C, Ma K-K, Chen H-H, Huang J-Y, Wei JC. Relationship between Helicobacter pylori infection and psoriasis: a nationwide population-based longitudinal cohort study. Medicine. 2020;99:24(e20632).
This study was funded by a grant (TCVGH-1096505B) from the Taichung Veterans General Hospital Research Foundation.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article.
References
- [1].Ayala-Fontánez N, Soler DC, McCormick TS. Current knowledge on psoriasis and autoimmune diseases. Psoriasis (Auckland, N Z) 2016;6:7–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yong WC, Upala S, Sanguankeo A. Association between psoriasis and helicobacter pylori infection: a systematic review and meta-analysis. Indian J Dermatol 2018;63:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kaushik SB, Lebwohl MG. Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol 2019;58:649–58. [DOI] [PubMed] [Google Scholar]
- [4].Linz B, Balloux F, Moodley Y, et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature 2007;445:915–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ram M, Barzilai O, Shapira Y, et al. Helicobacter pylori serology in autoimmune diseases – fact or fiction? Clin Chem Lab Med 2013;51:1075–82. [DOI] [PubMed] [Google Scholar]
- [6].Gravina AG, Zagari RM, De Musis C, et al. Helicobacter pylori and extragastric diseases: a review. World J Gastroenterol 2018;24:3204–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wu M-C, Leong P-Y, Chiou J-Y, et al. Increased risk of systemic lupus erythematosus in patients with helicobacter pylori infection: a nationwide population-based cohort study. Front Med 2020;6:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Onsun N, Arda Ulusal H, Su O, et al. Impact of Helicobacter pylori infection on severity of psoriasis and response to treatment. Eur J Dermatol 2012;22:117–20. [DOI] [PubMed] [Google Scholar]
- [9].Saez-Rodriguez M, Noda-Cabrera A, Garcia-Bustinduy M, et al. Palmoplantar pustulosis associated with gastric Helicobacter pylori infection. Clin Exp Dermatol 2002;27:720. [DOI] [PubMed] [Google Scholar]
- [10].Qayoom S, Ahmad QM. Psoriasis and Helicobacter pylori. Indian J Dermatol Venereol Leprol 2003;69:133–4. [PubMed] [Google Scholar]
- [11].Mesquita PM, Diogo AF, Jorge MT, et al. Relationship of Helicobacter pylori seroprevalence with the occurrence and severity of psoriasis. An Bras Dermatol 2017;92:52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ali M, Whitehead M. Clearance of chronic psoriasis after eradication therapy for Helicobacter pylori infection. J Eur Acad Dermatol Venereol 2008;22:753–4. [DOI] [PubMed] [Google Scholar]
- [13].Martin Hubner A, Tenbaum SP. Complete remission of palmoplantar psoriasis through Helicobacter pylori eradication: a case report. Clin Exp Dermatol 2008;33:339–40. [DOI] [PubMed] [Google Scholar]
- [14].Campanati A, Ganzetti G, Martina E, et al. Helicobacter pylori infection in psoriasis: results of a clinical study and review of the literature. Int J Dermatol 2015;54:e109–14. [DOI] [PubMed] [Google Scholar]
- [15].Hernando-Harder AC, Booken N, Goerdt S, et al. Helicobacter pylori infection and dermatologic diseases. Eur J Dermatol 2009;19:431–44. [DOI] [PubMed] [Google Scholar]
- [16].Magen E, Delgado JS. Helicobacter pylori and skin autoimmune diseases. World J Gastroenterol 2014;20:1510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lin LY, Warren-Gash C, Smeeth L, et al. Data resource profile: the National Health Insurance Research Database (NHIRD). Epidemiol Health 2018;40:e2018062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hsing AW, Ioannidis JP. Nationwide population science: lessons from the Taiwan National Health Insurance Research Database. JAMA Intern Med 2015;175:1527–9. [DOI] [PubMed] [Google Scholar]
- [19].Chen Y-C, Yeh H-Y, Wu J-C, et al. Taiwan's National Health Insurance Research Database: administrative health care database as study object in bibliometrics. Scientometrics 2011;86:365–80. [Google Scholar]
- [20].Wu CY, Kuo KN, Wu MS, et al. Early Helicobacter pylori eradication decreases risk of gastric cancer in patients with peptic ulcer disease. Gastroenterology 2009;137:1641–8. [DOI] [PubMed] [Google Scholar]
- [21].Parsons LS. Performing a 1:N case-control match on propensity score. SUGI 29 Montréal, Canada 2004;(Paper): 165-29. [Google Scholar]
- [22].Tsai TF, Wang T S, Hung S T, et al. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J Dermatol Sci 2011;63:40–6. [DOI] [PubMed] [Google Scholar]
- [23].Wu CY, Hu HY, Li CP, et al. Comorbidity profiles of psoriasis in Taiwan: a latent class analysis. PLoS One 2018;13:e0192537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tuzun Y, Keskin S, Kote E. The role of Helicobacter pylori infection in skin diseases: facts and controversies. Clin Dermatol 2010;28:478–82. [DOI] [PubMed] [Google Scholar]
- [25].Campanati A, Gesuita R, Giannoni M, et al. Role of small intestinal bacterial overgrowth and Helicobacter pylori infection in chronic spontaneous urticaria: a prospective analysis. Acta Derm Venereol 2013;93:161–4. [DOI] [PubMed] [Google Scholar]
- [26].Yu M, Zhang R, Ni P, et al. Helicobacter pylori infection and psoriasis: a systematic review and meta-analysis. Medicina (Kaunas) 2019;55:645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Turkmen D, Ozcan H, Kekilli E. Relation between Psoriasis and Helicobacter pylori. Turk J Dermatol 2011;5:39–42. [Google Scholar]
- [28].Azizzadeh M, Nejad ZV, Ghorbani R, et al. Relationship between Helicobacter pylori infection and psoriasis. Ann Saudi Med 2014;34:241–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Smyk DS, Koutsoumpas AL, Mytilinaiou MG, et al. Helicobacter pylori and autoimmune disease: cause or bystander. World J Gastroenterol 2014;20:613–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Patrikiou E, Liaskos C, Zafiriou E, et al. AB0932 Helicobacter pylori antigen specific antibodies in psoriatic arthritis. Ann Rheum Dis 2018;77:1591. [Google Scholar]
- [31].Dauden E, Vazquez-Carrasco MA, Penas PF, et al. Association of Helicobacter pylori infection with psoriasis and lichen planus: prevalence and effect of eradication therapy. Arch Dermatol 2000;136:1275–6. [DOI] [PubMed] [Google Scholar]
- [32].Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med 2002;347:1175–86. [DOI] [PubMed] [Google Scholar]


