Supplemental Digital Content is available in the text
Keywords: aphasia, meta-analysis, repetitive transcranial magnetic stimulation, stroke
Abstract
Backgrounds:
Previous studies indicated inconsistent results for the treatment effect of repetitive transcranial magnetic stimulation (rTMS) on post-stroke aphasics. The study conducted a meta-analysis to evaluate whether the rTMS with different frequencies demonstrated any effect in patients with post-stroke aphasia.
Methods:
Electronic databases (PubMed, Web of Science, Medline, EMBASE, and Google Scholar) were searched for articles published before July 2019. Statistical analyses were made using STATA 12.0 software. Standard mean difference (SMD) with 95% confidence intervals (CI) were calculated for the treatment effect of rTMS on post-stroke aphasia.
Results:
Meta-analysis indicated significant treatment effects on naming of rTMS in post-stroke aphasics (SMD 0.76, 95%CI 0.16 to 1.36, I2 = 76.9%, P < .001). Subgroup analyses showed significant treatment effects on naming of low frequency (LF)-rTMS (SMD 0.40, 95%CI 0.10 to 0.69, I2 = 0.0%, P = .671). However, the changes in repetition and comprehension following stimulation were not significant.
Conclusions:
In conclusion, we provide preliminary evidence that both LF-rTMS and high-frequency-rTMS might be relatively effective and safe treatment for post-stroke aphasics. However, LF-rTMS mainly plays a short-term role in subacute post-stroke aphasics. Longer-term and large-scale studies are essential to explore the effect of rTMS with different frequencies on post-stroke aphasia.
1. Introduction
Aphasia is common after stroke. Epidemiological evidence indicated that approximately 15% to 42% of stroke patients suffer aphasia in the acute phase of stroke.[1] In addition, more than 25% of stroke patients suffer from aphasia in rehabilitation or community (here after termed “chronic”) settings.[2] Extensive evidence suggests that aphasia negatively impacts quality of life.[3] In addition, 1 in 3 aphasia patients are estimated to suffer from depression 12 months post-stroke.[4] Thus, effective therapeutic strategies are essential to treat aphasia in stroke patients.
Repetitive transcranial magnetic stimulation (rTMS), a non-invasive brain stimulation method of stimulating selected regions of the brain, which may improve stroke rehabilitation.[5] rTMS plays a role by modulating the excitability of the cerebral cortex.[6,7] Specifically, the low frequency rTMS (LF-rTMS, ≤1 Hz) works via inhibiting cortical neural activity, whereas high frequency rTMS (HF-rTMS, >1 Hz) works via exciting cortical neural activity. In recent years, some studies provided inconsistent data for the treatment effect of rTMS on post-stroke aphasia.[8,9] The present study conducted a meta-analysis to evaluate whether the rTMS with different frequencies showed a feasible effect on aphasia after stroke.
2. Methods
This investigation conformed with Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.[10] The study is a meta-analysis. Thus, ethical approval was not applicable in the study.
2.1. Search strategy
The following databases (PubMed, Web of Science, Medline, EMBASE, and Google Scholar) were searched for articles published before July 2019. Search terms were showed as follows: (“transcranial magnetic stimulation” or “TMS”) and (“stroke”) and (“aphasia”). One hundred fifty-eight studies were screened after the exclusion of duplicates.
2.2. Selection criteria
In the present study, only randomized controlled trials (RCTs) were included. These studies reported data pertaining to the comparison of pre- and post-treatment naming, repetition, or comprehension scores between 2 groups of stroke patients with aphasia given rTMS and sham stimulation. We excluded articles as follows:
-
(1)
articles which did not provide sufficient data of pre- and post-treatment naming, repetition, or comprehension scores.
-
(2)
reviews, meta-analyses studies, and case reports.
2.3. Data collection
Data were independently extracted from these articles by 2 reviewers as follows: Author, publication year, study design, study location, information regard to included participants (sample size, gender, mean age), target area of rTMS, rTMS frequency, intensity of rTMS, rTMS stimulation parameters, sham stimulation, adverse effects, and stroke interval.
2.4. Meta-analysis
The treatment effect was estimated by calculating the standard mean difference (SMD) and a 95% confidence interval (CI) in naming, repetition or auditory comprehension scores with STATA 12.0 software. I2 statistic was used to evaluate the heterogeneity among studies. Random effects meta-analysis was used when I2 was equal to or more than 50%; otherwise, fixed effects model was employed. Subgroup analyses (for different frequencies of rTMS) were employed to explore the effect of HF-rTMS and LF-rTMS on the aphasia after stroke, respectively. In addition, meta-regression analyses were performed to explore source of the heterogeneity. In addition, we applied sensitivity analysis to evaluate the stability of the meta-analysis by removing 1 individual study each time. Moreover, publication bias was evaluated with Begg test, Egger test, and funnel plots.
3. Results
3.1. Search and selection results
Figure 1 shows the results of search and selection procedure. Characteristics of 28 eligible included studies can be found in supplementary content (see supplementary Table 1, which illustrates the characteristics of studies).[8,9,11–19] Among them, 1 study explored the effect of HF-rTMS (including 10 patients receiving HF-rTMS and 10 patients receiving sham stimulation), 1 study explored the effect of combined HF-rTMS and LF-rTMS (including 19 patients receiving combined HF-rTMS and LF-rTMS and 10 patients receiving sham stimulation), 10 studies investigated the effect of LF-rTMS (including 181 patients receiving LF-rTMS and 154 patients receiving sham stimulation). In included studies, 7 studies explored naming function (including 125 patients in experimental arms and 105 patients in control arms). Six studies explored repetition function (including 110 patients in experimental arms and 81 patients in control arms). Six studies explored auditory comprehension function (including 120 patients in experimental arms and 100 patients in control arms).
Figure 1.
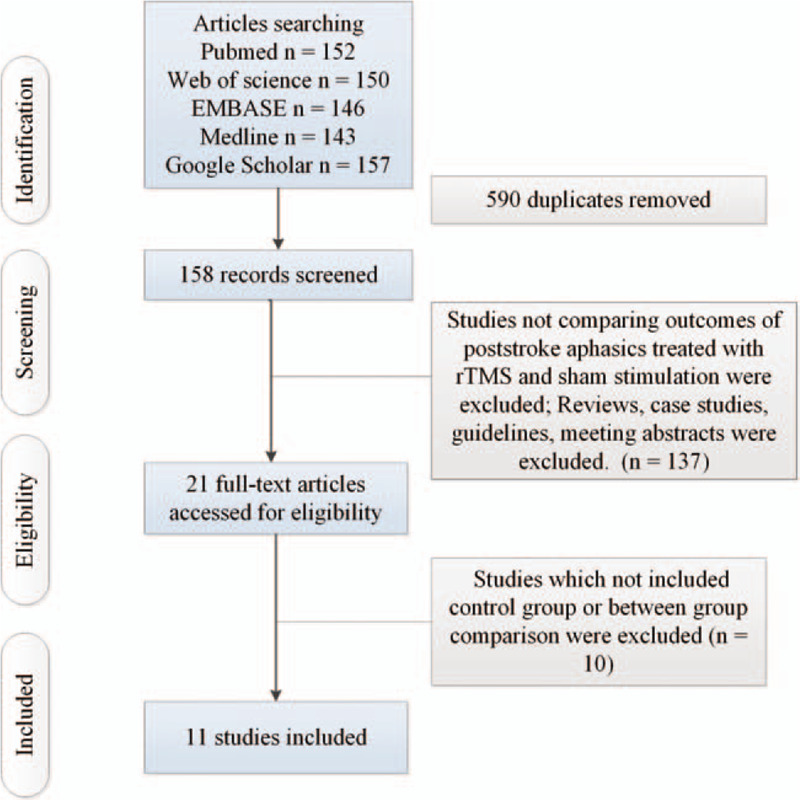
Flow of information through the different phases of a systematic review.
3.2. Meta-analysis results
The present study indicated significant treatment effects on naming of rTMS in post-stroke aphasics (SMD 0.76, 95%CI 0.16 to 1.36, I2 = 76.9%, P < .001; Fig. 2). Subgroup analyses showed significant treatment effects on naming of LF-rTMS (SMD 0.40, 95%CI 0.10 to 0.69, I2 = 0.0%, P = .671; Fig. 2). Additionally, Khedr et al reported that combining dual-hemisphere rTMS might be an effective treatment for naming in post-stroke aphasia, which remained elevated 2 months following the final treatment sessions,[13] whereas Hu et al found no significant differences in change in naming scores between post-stroke aphasics receiving HF-rTMS and those receiving sham stimulation.[16] Meta-regression analysis reported that publication years, ages, numbers of pulses, and numbers of sessions were not responsible for heterogeneity between studies regarding effects of LF-rTMS on naming in post-stroke aphasics (publication years: P = .795; ages: P = .924; numbers of pulses: P = .596; numbers of sessions: P = .577). Subgroup meta-analysis for LF-rTMS demonstrated significant treatment effects on naming in subacute post-stroke aphasics, whereas there was no significant effect of LF-rTMS on naming in chronic post-stroke aphasics (subacute: SMD 0.43, 95%CI 0.01 to 0.84, I2 = 32.2%, P = .229; chronic: SMD 0.36, 95%CI –0.06 to 0.79, I2 = 0.0%, P = .906; see supplementary Fig. 1, presenting subgroup analyses results for change in naming scores between post-stroke aphasics receiving LF-rTMS and those receiving sham stimulation in subacute and chronic post-stroke aphasics). Additionally, subgroup meta-analysis for LF-rTMS offered no significantly long-lasting treatment effects on naming in post-stroke aphasics (SMD 0.31, 95%CI –0.01 to 0.63; see supplementary Fig. 2, presenting subgroup analyses results for long-term and immediate change in naming scores between post-stroke aphasics receiving LF-rTMS treatment compared with sham stimulation in post-stroke aphasics), whereas Heiss et al[18] reported significantly immediate effect of LF-rTMS on naming in post-stroke aphasics.
Figure 2.
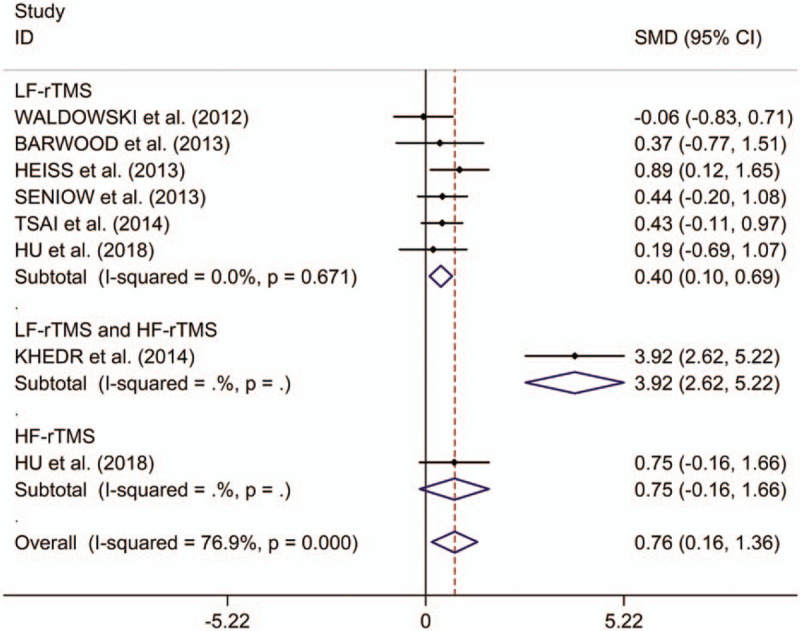
Forest plots of change in naming scores between post-stroke aphasics receiving repetitive transcranial magnetic stimulation and those receiving sham stimulation. HF = high frequency, LF = low frequency, rTMS = repetitive transcranial magnetic stimulation.
This study observed no significant differences in change in repetition scores between post-stroke aphasics treated with rTMS compared with those receiving sham stimulation (SMD 0.55, 95%CI –0.37 to 1.48, I2 = 88.2%, P < .001; Fig. 3). No significant treatment effects on repetition of LF-rTMS were detected in post-stroke aphasics by subgroup analysis (SMD 0.15, 95%CI –0.28 to 0.57, I2 = 37.2%, P = .173; Fig. 3). In addition, Hu et al offered that HF-rTMS showed no significant treatment effects on repetition.[16] However, Khedr et al[13] identified that that combining dual-hemisphere rTMS had feasible treatment effects on repetition in post-stroke aphasia even at 2 months following the final treatment session. Meta-regression analysis showed that publication years, ages, numbers of pulses, and numbers of sessions were not responsible for heterogeneity across studies regarding effects of LF-rTMS on repetition in post-stroke aphasics (publication years: P = .777; ages: P = .574; numbers of pulses: P = .526; numbers of sessions: P = .803). Subgroup meta-analysis for LF-rTMS showed no significant treatment effects on repetition in both subacute and chronic post-stroke aphasics (subacute: SMD 0.42, 95%CI –0.06 to 0.91, I2 = 0.0%, P = .455; chronic: SMD –0.03, 95%CI –0.47 to 0.40, I2 = 49.3%, P = .139; see supplementary Fig. 3, for subgroup analysis results for change in repetition scores between post-stroke aphasics receiving LF-rTMS and those receiving sham stimulation in subacute and chronic post-stroke aphasics). Subgroup meta-analysis for LF-rTMS showed no significantly long-lasting treatment effects on repetition in post-stroke aphasics (SMD 0.06, 95%CI –0.30 to 0.42; see supplementary Fig. 4, which illustrates subgroup analysis results for long-term and immediate change in repetition scores between post-stroke aphasics receiving LF-rTMS and those receiving sham stimulation in post-stroke aphasics). Additionally, Heiss et al[18] found no significantly immediate effect of LF-rTMS on repetition in post-stroke aphasics.
Figure 3.
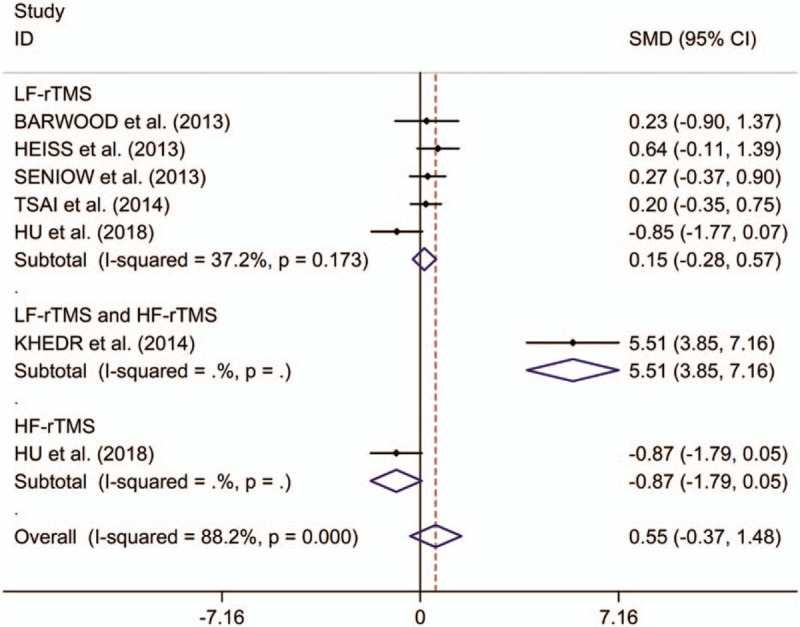
Forest plots of change in repetition scores between post-stroke aphasics receiving repetitive transcranial magnetic stimulation and those receiving sham stimulation. HF = high frequency, LF = low frequency, rTMS = repetitive transcranial magnetic stimulation.
This meta-analytical review found no significant differences in change in auditory comprehension scores between post-stroke aphasics receiving rTMS and those given sham stimulation (SMD 0.45, 95%CI –0.42 to 1.33, I2 = 88.0%, P < .001; Fig. 4). Subgroup analysis showed no significant treatment effects of LF-rTMS on auditory comprehension in post-stroke aphasics (SMD 0.01, 95%CI –1.09 to 1.12, I2 = 90.4%, P < .001; Fig. 4). However, Khedr et al reported that combining dual-hemisphere rTMS showed significant treatment effects on auditory comprehension of rTMS in post-stroke aphasics.[13] Additionally, Hu et al reported that HF-rTMS showed significant treatment effects on comprehension in post-stroke aphasia.[16] In these included articles, patients tolerated rTMS well, and no adverse effects were observed. Meta-regression analysis showed that publication years, ages, numbers of pulses, and numbers of sessions were not responsible for heterogeneity across studies regarding effects of LF-rTMS on comprehension in post-stroke aphasia (publication years: P = .245; ages: P = .069; numbers of pulses: P = .209; numbers of sessions: P = .260). Subgroup meta-analysis for LF-rTMS showed no significant treatment effects on comprehension in both subacute and chronic post-stroke aphasics (subacute: SMD 0.37, 95%CI –0.20 to 0.95, I2 = 27.7%, P = .240; chronic: SMD –0.35, 95%CI –2.74 to 2.03, I2 = 94.8%, P < .001; see supplementary Fig. 5, which illustrates subgroup analysis results for change in comprehension scores between post-stroke aphasics receiving LF-rTMS and those receiving sham stimulation in subacute and chronic post-stroke aphasics). Subgroup meta-analysis for LF-rTMS showed no significantly long-lasting treatment effects on comprehension in post-stroke aphasics (SMD –0.19, 95%CI –1.66 to 1.27; see supplementary Fig. 6, which illustrates subgroup analysis results for long-term and immediate change in comprehension scores between post-stroke aphasics receiving LF-rTMS and those receiving sham stimulation in post-stroke aphasics). Additionally, Heiss et al[18] reported no significantly immediate effect of LF-rTMS on comprehension in post-stroke aphasics.
Figure 4.
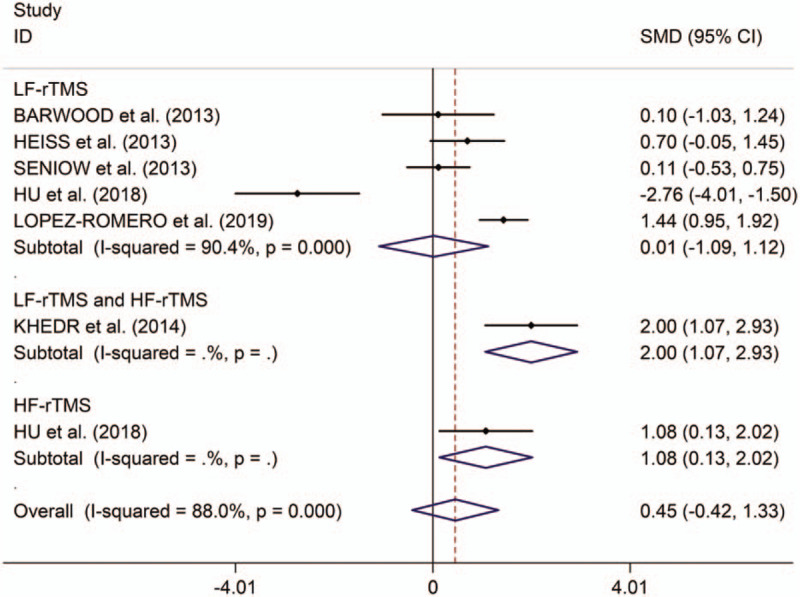
Forest plots of change in comprehension scores between post-stroke aphasics receiving repetitive transcranial magnetic stimulation and those receiving sham stimulation. HF = high frequency, LF = low frequency, rTMS = repetitive transcranial magnetic stimulation.
Sensitivity analyses found no changes in the direction of effect when any 1 study was excluded for all meta-analyses (see supplementary Fig. 7, which presents sensitivity analyses results for change in naming, repetition and comparison scores between post-stroke aphasics given LF-rTMS and those given sham stimulation in post-stroke aphasics). In addition, Begg test, Egger tests, and funnel plots did not show significant risk of publication bias for all meta-analyses (see supplementary Table 2, which illustrates results of Begg test and Egger tests; see supplementary Fig. 8 which illustrates funnel plots results for change in naming, repetition, and comparison scores between post-stroke aphasics given LF-rTMS and those given sham stimulation in post-stroke aphasics).
4. Discussion
The present study presents preliminary data for LF-rTMS induced benefit on naming in post-stroke aphasia, in the absence of any significant effect of LF-rTMS on repetition and comprehension or recorded adverse effects. In addition, Hu et al reported no significant differences in change in naming and repetition scores between post-stroke aphasics given HF-rTMS and those given sham stimulation, whereas they observed significant treatment effects of HF-rTMS on comprehension in post-stroke aphasics.[16]
In all included studies, only a study explored the effects of HF-rTMS and LF-rTMS on post-stroke aphasia at the same time.[16] Hu et al[16] reported the superior therapeutic effect of LF-rTMS over HF-rTMS on naming in post-stroke aphasics. The LF-rTMS works via inhibiting excitability by the contra-lesional hemisphere to the lesioned hemisphere, which resumes the balance to enable the undamaged parts of the language area to function properly.[20] Conversely, HF-rTMS works via facilitating the excitation of the targeted area, and thus promote, post-stroke recovery.[21] Regarding LF-rTMS, the result was corresponding to a recent meta-analysis, which indicated that LF-rTMS stimulation is an effective therapy for recovery of naming.[22] Regarding HF-rTMS, Belin et al[23] found that post-stroke activation of the mirror area on the right hemisphere might be unrelated to the restoration from aphasia by using positron emission tomography. However, Hu et al[16] reported that the HF-rTMS group showed significantly improved repetition scores compared to sham stimulation group. The result was corresponding to a case study by Dammekens et al[24] who reported that HF-rTMS (10 Hz) conducted on the left inferior frontal region improved naming and comprehension, in non-fluent patients. Taken together, the present study indicated that both LF-rTMS and HF-rTMS showed significant therapeutic effect on post-stroke aphasia. However, more large-scale RCTs should be made to explore the effect of HF-rTMS on post-stroke aphasia.
In theory, publication years, ages, numbers of pulses, numbers of sessions, stroke intervals, and follow-up times were important source of heterogeneity. However, meta-regression analysis showed that publication years, ages, numbers of pulses, numbers of sessions were not responsible for heterogeneity across studies. However, regarding effect of LF-rTMS on naming, subgroup analysis indicated that the effective therapy only appeared in subacute post-stroke aphasics. In addition, no significantly long-lasting treatment effects were found on naming in post-stroke aphasics. Thus, more large-scale RCTs were essential to make to explore the effect of LF-rTMS on post-stroke aphasia.
rTMS could produce potential adverse effects, such as headaches and seizures. Thus, safety is a fatal consideration. These included studies did not report severe adverse effects. The study indicated that rTMS is a safe treatment in the short term. However, long-term follow-up was essential to further explore the safety of rTMS. In addition, researchers should follow safety guidelines and examine the potential risk of post-stroke seizure related to rTMS carefully.
There were some limitations in the present study. Firstly, regarding the effect of HF-rTMS post-stroke aphasia, there were a limited number of studies, potentially limiting statistical power. Secondly, regarding LF-rTMS, the amount of included studies was limited to explore the sources of heterogeneities.
5. Conclusions
In conclusion, both LF-rTMS and HF-rTMS might be relatively effective and safe treatment in post-stroke aphasics. However, LF-rTMS mainly plays a short-term role in post-stroke aphasics. Longer-term and large-scale studies are essential to explore the effect of rTMS with different frequencies on post-stroke aphasia.
Author contributions
Data curation: Xiaoxiang Zeng, Lijuan Lin, Tingting Xian, Zhuoming Chen.
Funding acquisition: Zhuoming Chen.
Investigation: Tiao Li.
Methodology: Tiao Li, Xiaoxiang Zeng, Lijuan Lin, Tingting Xian.
Supervision: Zhuoming Chen.
Writing – original draft: Tiao Li.
Writing – review & editing: Zhuoming Chen.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CI = confidence intervals, HF = high frequency, LF = low frequency, RCTs = randomized controlled trials, rTMS = repetitive transcranial magnetic stimulation, SMD = standard mean difference.
How to cite this article: Li T, Zeng X, Lin L, Xian T, Chen Z. Effects of repetitive transcranial magnetic stimulation with different frequencies on post-stroke aphasia: a PRISMA-compliant meta-analysis. Medicine. 2020;99:24(e20439).
The study was supported by the Guangdong Science and Technology Basic Conditions Support Project (No. 2018A030321015).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Inatomi Y, Yonehara T, Omiya S, et al. Aphasia during the acute phase in ischemic stroke. Cerebrovasc Dis 2008;25:316–23. [DOI] [PubMed] [Google Scholar]
- [2].Gialanella B, Bertolinelli M, Lissi M, et al. Predicting outcome after stroke: the role of aphasia. Disabil Rehabil 2011;33:122–9. [DOI] [PubMed] [Google Scholar]
- [3].Cruice M, Worrall L, Hickson L. Health-related quality of life in people with aphasia: implications for fluency disorders quality of life research. J Fluency Disord 2010;35:173–89. [DOI] [PubMed] [Google Scholar]
- [4].Hilari K, Northcott S, Roy P, et al. Psychological distress after stroke and aphasia: the first six months. Clin Rehabil 2010;24:181–90. [DOI] [PubMed] [Google Scholar]
- [5].Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol 2006;5:708–12. [DOI] [PubMed] [Google Scholar]
- [6].Hoffman RE, Cavus I. Slow transcranial magnetic stimulation, long-term depotentiation, and brain hyperexcitability disorders. Am J Psychiatry 2002;159:1093–102. [DOI] [PubMed] [Google Scholar]
- [7].Cirillo G, di Pino G, Capone F, et al. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul 2017;10:1–8. [DOI] [PubMed] [Google Scholar]
- [8].Medina J, Norise C, Faseyitan O, et al. Finding the right words: transcranial magnetic stimulation improves discourse productivity in non-fluent aphasia after stroke. Aphasiology 2012;26:1153–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lopez-Romero LA, Riano-Carreno DM, Pachon-Poveda MY, et al. Efficacy and safety of transcranial magnetic stimulation in patients with non-fluent aphasia, following an ischaemic stroke. A controlled, randomised and double-blind clinical trial. Rev Neurol 2019;68:241–9. [DOI] [PubMed] [Google Scholar]
- [10].Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Revista Española De Nutrición Humana Y Dietética 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Waldowski K, Seniow J, Lesniak M, et al. Effect of low-frequency repetitive transcranial magnetic stimulation on naming abilities in early-stroke aphasic patients: a prospective, randomized, double-blind sham-controlled study. ScientificWorldJournal 2012;2012:518568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Barwood CH, Murdoch BE, Riek S, et al. Long term language recovery subsequent to low frequency rTMS in chronic non-fluent aphasia. NeuroRehabilitation 2013;32:915–28. [DOI] [PubMed] [Google Scholar]
- [13].Khedr EM, Abo El-Fetoh N, Ali AM, et al. Dual-hemisphere repetitive transcranial magnetic stimulation for rehabilitation of poststroke aphasia: a randomized, double-blind clinical trial. Neurorehabil Neural Repair 2014;28:740–50. [DOI] [PubMed] [Google Scholar]
- [14].Tsai PY, Wang CP, Ko JS, et al. The persistent and broadly modulating effect of inhibitory rTMS in nonfluent aphasic patients: a sham-controlled, double-blind study. Neurorehabil Neural Repair 2014;28:779–87. [DOI] [PubMed] [Google Scholar]
- [15].Wang CP, Hsieh CY, Tsai PY, et al. Efficacy of synchronous verbal training during repetitive transcranial magnetic stimulation in patients with chronic aphasia. Stroke 2014;45:3656–62. [DOI] [PubMed] [Google Scholar]
- [16].Hu XY, Zhang T, Rajah GB, et al. Effects of different frequencies of repetitive transcranial magnetic stimulation in stroke patients with non-fluent aphasia: a randomized, sham-controlled study. Neurol Res 2018;40:459–65. [DOI] [PubMed] [Google Scholar]
- [17].Seniow J, Waldowski K, Lesniak M, et al. Transcranial magnetic stimulation combined with speech and language training in early aphasia rehabilitation: a randomized double-blind controlled pilot study. Top Stroke Rehabil 2013;20:250–61. [DOI] [PubMed] [Google Scholar]
- [18].Heiss WD, Hartmann A, Rubi-Fessen I, et al. Noninvasive brain stimulation for treatment of right- and left-handed poststroke aphasics. Cerebrovasc Dis 2013;36:363–72. [DOI] [PubMed] [Google Scholar]
- [19].Heikkinen PH, Pulvermuller F, Makela JP, et al. Combining rTMS with intensive language-action therapy in chronic aphasia: a randomized controlled trial. Front Neurosci 2018;12:1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Norise C, Hamilton RH. Non-invasive brain stimulation in the treatment of post-stroke and neurodegenerative aphasia: parallels, differences, and lessons learned. Front Hum Neurosci 2016;10:675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jaillard A, Martin CD, Garambois K, et al. Vicarious function within the human primary motor cortex? A longitudinal fMRI stroke study. Brain 2005;128(Pt 5):1122–38. [DOI] [PubMed] [Google Scholar]
- [22].Li Y, Qu Y, Yuan M, et al. Low-frequency repetitive transcranial magnetic stimulation for patients with aphasia after stoke: a meta-analysis. J Rehabil Med 2015;47:675–81. [DOI] [PubMed] [Google Scholar]
- [23].Belin P, van Eeckhout P, Zilbovicius M, et al. Recovery from nonfluent aphasia after melodic intonation therapy: a PET study. Neurology 1996;47:1504–11. [DOI] [PubMed] [Google Scholar]
- [24].Dammekens E, Vanneste S, Ost J, et al. Neural correlates of high frequency repetitive transcranial magnetic stimulation improvement in post-stroke non-fluent aphasia: a case study. Neurocase 2014;20:1–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


