Abstract
To ascertain the relationship between the perimetric differences obtained between the limbs and the type of fluoroscopic pattern observed by Indocyanine green (ICG) lymphography in patients with upper limb lymphedema.
A correlational descriptive study was carried out in 19 patients with upper limb lymphedema secondary to breast cancer. The perimetric increase was recorded in 11 anatomical regions after ICG injection, fluoroscopic patterns were identified using an infrared camera. The ICG patterns were categorized into worse (stardust, diffuse) or better (linear, splash) patterns.
The pattern coincidence between the anterior and posterior regions of the edematous extremities was 45%. At the wrist level, a difference of 2 cm was associated with the presence of a worse fluoroscopic pattern, whereas perimeter differences of 4.25 cm in the elbow and 2.25 cm in the arm (12 cm from the epicondyle) were associated with the presence of a better fluoroscopic pattern.
The perimetric differences observed between the healthy and affected upper limbs in 4 specific anatomical areas allowed us to predict the type of fluoroscopic pattern. ICG lymphography has facilitated the study of the posterior regions of edema, which are difficult to visualize using other imaging techniques.
Keywords: indocyanine green, lymphatic vessels, lymphedema, lymphography
1. Introduction
The diagnosis of lymphedema is based on a detailed history,[1] skin observation, and measurement of the increase in limb volume,[2] which is the most characteristic aspect of this entity.[3] In the therapeutical approach of lymphedema, it is essential to find the appropriate tools to establish a correct evaluation[4] and to choose the most appropriate therapeutic strategy depending on the edema's stage.[5]
The qualitative evaluations carried out by the assessment of imaging tests can detect anomalies even before the edema is clinically evident,[1] which is fundamental for the early diagnosis of secondary lymphedema.[6]
The quantitative assessment allows for the staging of the severity of lymphedema.[7] It mainly uses direct and indirect methods to measure the difference in volume between the extremities and to establish the severity of the edema The indirect method is considered efficient for daily clinical practice due to its availability and high interobserver reliability. It calculates the volume from the measurements of the perimeters of several segments of the extremity[4] using a tailor's tape. A perimeter difference of 2 cm in 2 consecutive perimeter measurements of the affected limb with compared to the healthy 1 an increase of 150 to 400 mL between extremities or a difference of 10% to 20%[3,8,9,10] being are accepted as indicative of clinical lymphedema.
However, in the earliest stages of lymphedema, the perimeters and volumes may not have increased despite the subjective symptoms being observed.[11] In these cases, imaging tests such as indocyanine green (ICG)[6,7] provide useful information. ICG lymphography is becoming the method of choice for assessing and monitoring lymphedema.[12–13] It allows for early detection,[12–14] staging of lymphedema's severity,[15,16] and selection of the most appropriate therapeutic strategy according to the patient's situation.[12,17] In contrast to isotopic lymphography, which is considered the gold standard in the qualitative evaluation of lymphedema,[4] ICG lymphography does not pose a risk to radiation exposure, is less invasive,[18] costs less, and provides greater safety.[12,19]
Following Akita et al,[19] the images observed by ICG lymphography in the lymphatic system assessment can be categorized into the following: a normal (linear) pattern and abnormal pattern with signs of dermal reflux. The visualization of an abnormal ICG pattern is associated with the presence of lymphedema.[20] In the linear pattern, which is observed in normal cases and in mild or subclinical lymphedema, the images show superficial lymphatic channels, such as white, straight, and well-defined lines.[18,21]
The abnormal or reflux pattern is observed in patients with lymphatic system involvement and is classified into the following 3 categories, from lower to higher severity[15]:
-
(1)
The splash pattern is observed in the mildest cases of lymphedema and even in asymptomatic extremities.[6] It consists of the presence of scattered scintillation of the tracer around thin and tortuous lymphatic channels[18];
-
(2)
The stardust pattern which represents the progressive deterioration of lymphatic valves. The tracer appears as weakly luminous fluorescent signal spots[18]; and
-
(3)
The diffuse pattern represents the most serious form of lymphatic involvement; the valves and lymphatic channels are severely damaged. In ICG lymphography, the tracer is widely distributed, the spots are fused, and no scintillations or spots can be identified.[18] As the lymphedema progresses, the signs of dermal reflux progress from the splash pattern, to the stardust and diffuse patterns; thus, there seems to be a relationship between the observed pattern and the clinical severity of lymphedema.[6,19,22]
Generally, the reflux pattern initially appears in 1 part of the limb, but as the lymphedema progresses, the pattern extends to the entire extremity.[23] Similarly, as the duration of lymphedema increases, the prevalence of dermal reflux patterns increases as well.[18]
The study of dermal reflux patterns, as observed by ICG lymphography, would allow for the development of individualized therapeutic strategies[12,17] and the evaluation of the patient's response to treatment.[21,24] Yamamoto et al[6] reported that the presence of a splash pattern is the appropriate time for the initiation of lymphedema treatment. Akita et al[13] stated that the stardust pattern does not respond to conservative treatment, whereas Rasmussen et al.[21] showed that the presence of this pattern would indicate that surgical intervention could be the best therapeutic option.
Currently, there are studies that have described the relationship between the patterns observed by ICG lymphography and the severity of clinical symptoms.[12,13] However, the relationship between the increase in the perimeters of the edematous limb and the type of fluoroscopic pattern observed by ICG lymphography has not yet been analyzed.
Thus, this study aimed to determine, through ICG lymphography, if there is a relationship between the perimetric differences among the healthy and affected limbs, and to determine the type of fluoroscopic pattern present in the lymphedematous limb.
2. Methods
This descriptive correlational study was carried out at the DR Negrín University Hospital in Gran Canaria, Spain, between January and July 2017.
2.1. Study sample
This study evaluated 19 upper limbs of 19 volunteers who had unilateral secondary lymphedema secondary to breast cancer, and who had undergone axillary lymph node resection. All patients provided written informed consent before study inclusion. The patients belonged to the waiting list for physical treatment within the Lymphatic Pathology Unit of the Rehabilitation Service at DR Negrin University Hospital in Gran Canaria, Spain. The exclusion criteria were as follows:
-
(1)
patients with clinical suspicion or with confirmed diagnosis of deep vein thrombosis;
-
(2)
patients with an allergy to iodine or some of its derivatives; and
-
(3)
those who did not sign the informed consent form.
The mean age of the patients was 59 (53 (percentile 25)-68 (percentile 75)) years, with 8 and 11 of the 19 volunteers presenting with stage IIa and IIb lymphedema, respectively, according to the classification proposed by the International Society of Lymphology.[25]
The percentage of severity calculated using the formula recommended by Ferrandez et al[26] was mild in 31.6% of the subjects (n = 6), moderate in 37% (n = 7) and most severe in 31.6% (n = 6).
2.2. Intervention
First, the physiotherapist marked the references for the measurement of the perimeters. A total of 11 measurements were recorded for each limb, as described below. In the hand, according to the recommendation of Villaverde et al,[3] the marking point was recorded behind the metacarpophalangeal joints. The joints of the wrist were marked by a line between the radial and ulnar styloid processes. At the elbow, the junction line between the epicondyle and the epitrochlea was marked. The ulnar styloid and lateral epicondyle were taken as reference points.[3] From these references, the authors drew lines on skin with a 4 cm[3,27] interval, registering a total of 4 measurement points on the forearm and 4 others on the arm.
Each reference point was identified on the anterior and posterior sides of the limb with adhesive tape, which was opaque to infrared light. Once the references were marked, the physiotherapist measured each point with tapestry tape while the patient was in the supine position. Given the high intra-observer reliability of this measurement system,[3,4] each patient was evaluated by a single physiotherapist. Three measurements were made for each of the points and the average value was recorded.
Next, 0.3 mL of a 25 mg solution of ICG (Verdye, Diagnostic Green GmbH, Aschheim-Dornach, Germany) in 5 mL of 5% glycated serum was injected by a doctor who specialized in physical medicine and rehabilitation into the second and fourth interdigital spaces of the hand of the affected limb.
After the injection, the patients were asked to remain motionless for 5 minutes. After this time, they performed isolated movements; specifically, flexion and extension of the fingers of the affected limb for another 5 minutes. At 90 minutes post-ICG injection, a team consisting of a specialized doctor in physical medicine and rehabilitation and a physiotherapist observed the presence of the tracer (ICG) by means of an infrared camera (Photodynamic Eye, Hamamatsu Photonics KK, Hamamatsu City, Shizuoka Pref, Japan). In addition, a third observer, a physiotherapist, verified the correct uptake and recording of the images by viewing them through the computer screen. The team, previously trained in the identification of the different patterns, agreed on the type of pattern in case there were any doubts.
The images were recorded for further analysis. The team checked and recorded the type of fluoroscopic pattern present in the same selected areas as for the perimeter measurement. The patterns visualized in the anterior and posterior regions of the affected limb were defined as linear (Fig. 1), splash (Fig. 2), stardust (Fig. 3), and diffuse (Fig. 4). In case the plotter was not displayed, the pattern was registered as ‘none’.
Figure 1.
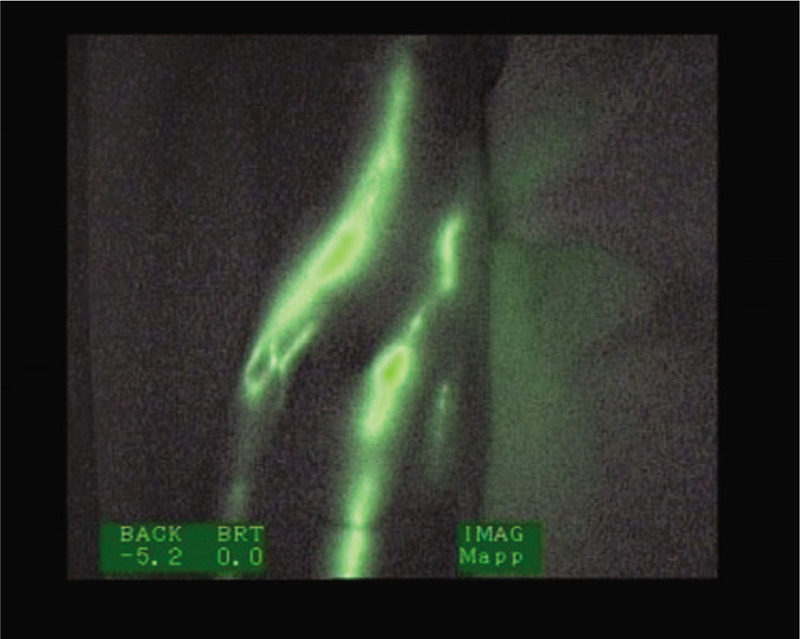
Linear fluoroscopic pattern.
Figure 2.
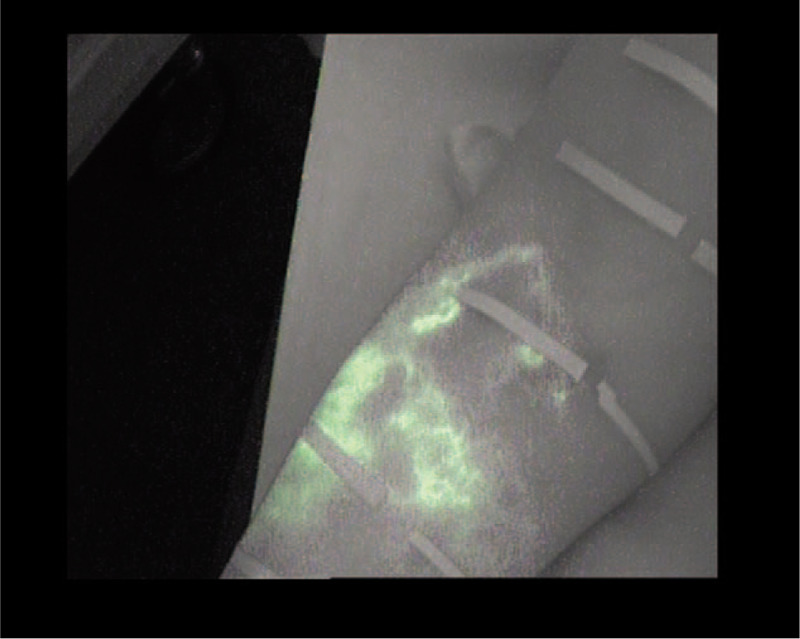
Splash fluoroscopic pattern.
Figure 3.
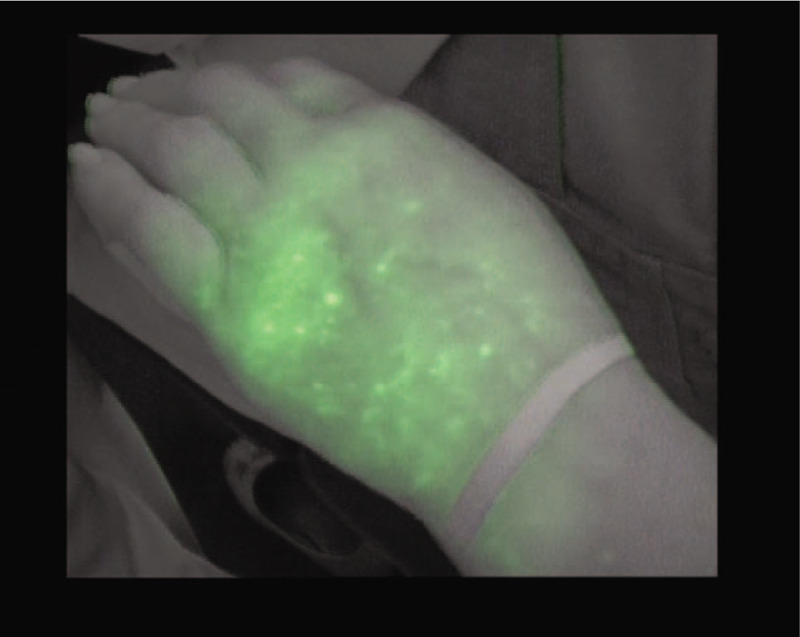
Stardust fluoroscopic pattern.
Figure 4.
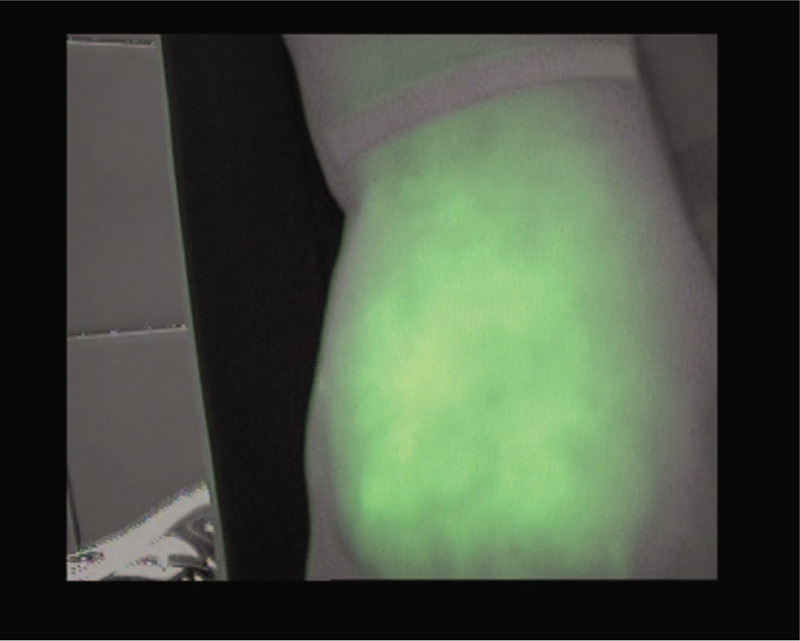
Diffuse fluoroscopic pattern.
The secondary objective was to determine if there was a relationship in the areas of marking, between the perimetric difference, the edematous limb, and the visualized pattern. To simplify the study and subsequent analysis, the observable patterns were grouped into the following 2 types: better and worse patterns. The worse patterns included the most evolved pathological patterns, such as stardust and diffuse. To avoid contamination of the patient's skin with ICG, all individuals (participants, physicians, and physiotherapists) used latex gloves.
The splash pattern already appears in the latent stage[28,29] or in subclinical lymphedema, preceding even its clinical manifestation.[6] In a normal situation or in the case of a subclinical or mild pathology, there would be no perimetric difference between the extremities. For this reason, the linear (Fig. 2) and splash patterns (Fig. 3) were considered as better patterns.
2.3. Statistical analysis
The mean, standard deviation, and quartiles were calculated to describe the quantitative variables. The Shapiro-Wilk test was used to analyze the distributions, while the Mann-Whitney U test was used to examine if there were any differences between groups. For the qualitative variables, the frequency and percentage were calculated. The type of pattern was classified as better or worse (dichotomous variable), considering the perimetric differences of the edema (numerical variable). To verify the relationship between the continuous variable “perimetric difference” with the probability of presenting a worse pattern; positive predictive value was calculated. A negative predictive value (NPV) was used to verify the relationship between the continuous variable “perimetric difference” with the probability of presenting a better pattern. To obtain the best cut-off point, receiver operating characteristic (ROC) curves, and area under the curve (AUC) were used. P- values < .05 were considered statistically significant. R Core Team (2018) (R Foundation for Statistical Computing, Vienna) was used for statistical analyses.
2.4. Ethical approval
The Committee of Ethics in Biomedical Research of the DR Negrin University Hospital of Gran Canaria (Code CEIC Negrín 170022) and the Spanish Agency of Medicines, which classified the present work as a non-observational study without medication, approved the study protocol. The ethical principles for medical research in humans included in the latest revision of the Declaration of Helsinki were adhered to, and the confidentiality of the information collected was guaranteed in accordance with the current legislation on the protection of personal data.
3. Results
The perimetric differences between the healthy and lymphedematous limbs, as well as the distribution of the patterns in the anterior and posterior regions of the hand, wrist, forearm, elbow, and arm are shown in Table 1. The perimetric differences between the anatomical regions of the healthy and affected extremities are shown in this Table as the mean and standard deviation and percentiles. This perimetric differences were expressed in centimeters. The type of ICG patterns (none, linear, splash, stardust or diffuse) present in each anatomical region of the affected arm, has been shown as absolute frequencies and percentages in Table 1.
Table 1.
Perimetric differences between the anatomical regions of the affected and unaffected extremities, and types of ICG patterns showed on the anterior and posterior side.
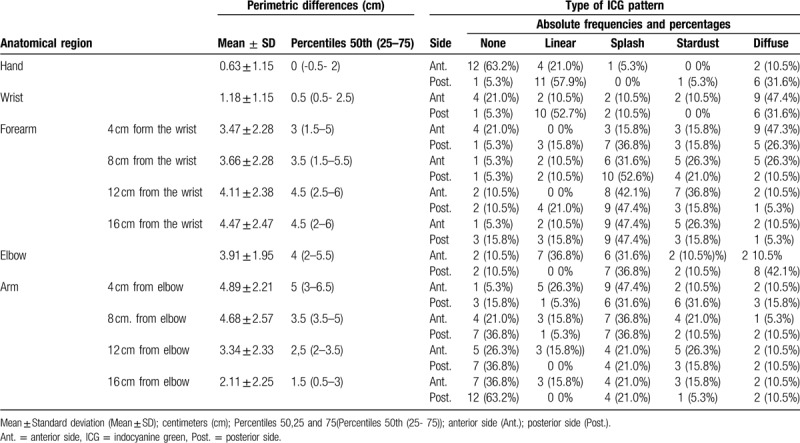
The relationship between the perimetric differences and the possibility of finding a better or worse ICG pattern, as well as the determination of the cut-off points, were analyzed using the ROC curves (Table 2).
Table 2.
Association between the perimetric difference and the probability of presenting a worse or a better pattern.
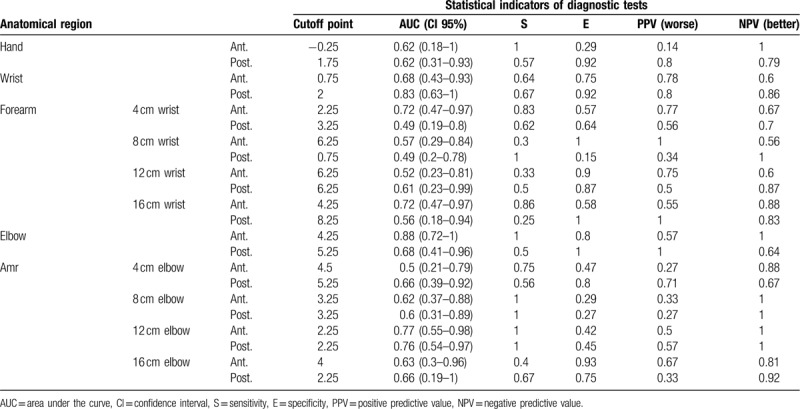
The data from the ROC curves where significant cut-off points were found are presented graphically in Figures 5–8.
Figure 5.
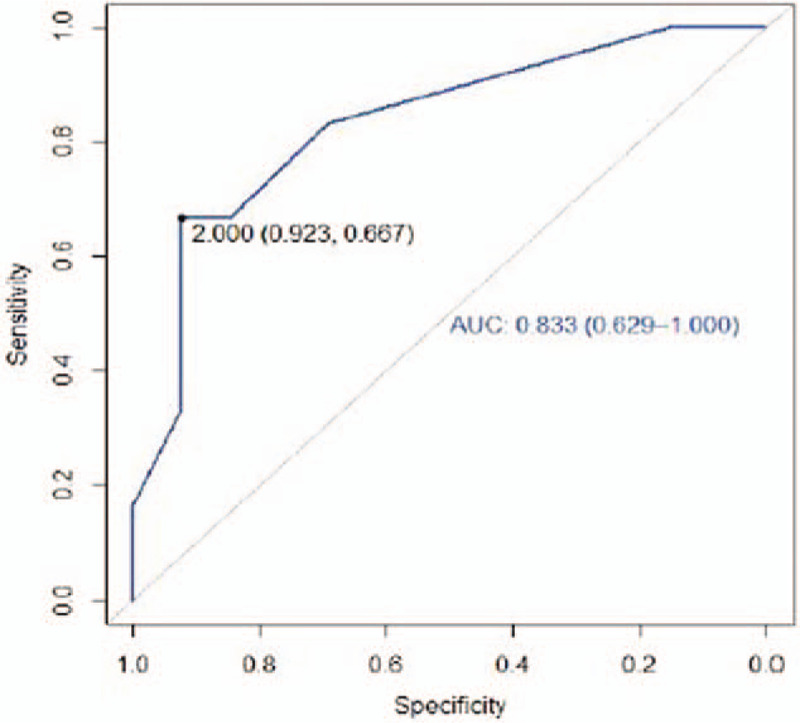
Receiver operating characteristic curve posterior wrist.
Figure 8.
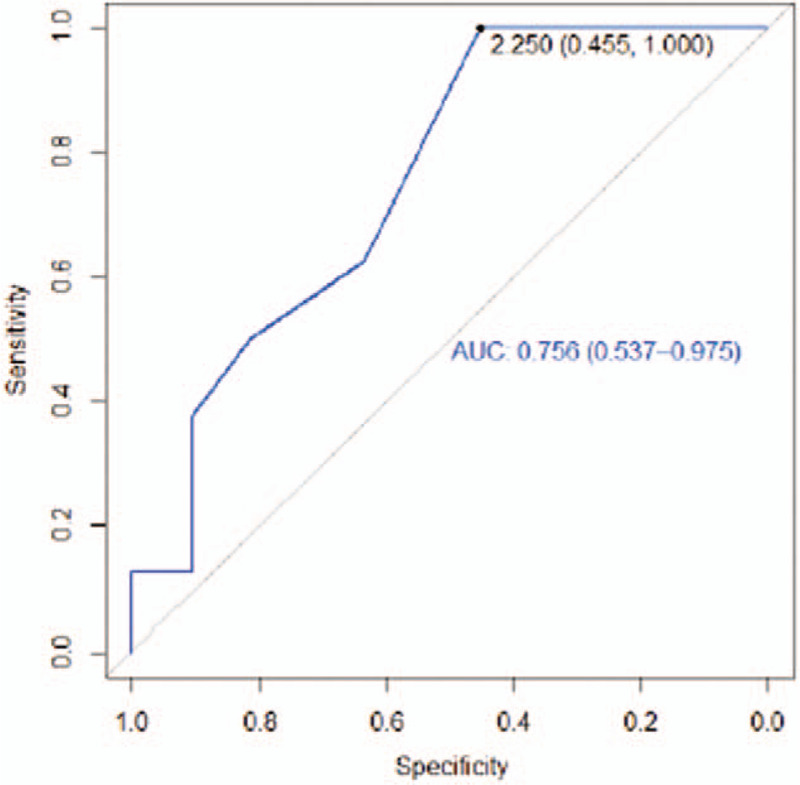
Receiver operating characteristic curve 12 cm from the posterior elbow.
The perimeter cut- off point (cut-off point) which discriminated between the possibility of finding a better or worse pattern in the posterior (dorsal) wrist was 2 cm (Table 2). As the positive predictive value shows, a perimetric difference between the limbs at the wrist level greater than 2 cm shows that the probability of finding a worse pattern, stardust or diffuse, is 80%. The result of the AUC at the posterior wrist level was 0.83; close to 1 (Fig. 5). In the forearm, a mild pattern was observed more frequently. Table 1 shows the same pattern frequencies in the anterior and posterior areas. Cut-off points were not found for the perimetric differences of the forearm areas (Table 2).
In the elbow region and in the region located 12 cm above this, we found cut-off points in the perimetric differences between the limbs, which could be associated with a greater probability of presenting a better or worse ICG pattern (Table 2).
At the elbow level, subjects with a difference between their limbs, healthy and lymphedematous, less than 4.25 cm showed a better ICG pattern in the anterior region of this area. Based on our results, there is a 100% probability (NPV) that the pattern displayed in the elbow region is linear or splash (Table 2). The result of the AUC at the anterior elbow was 0. 88; close to 1 (Fig. 6). The value of 0. 88 can be considered a good estimator using the cutoff point of 4.25 cm. Similarly, in the region located 12 cm above the elbow, a perimetric difference less than 2.25 cm presented a 100% probability (NPV) of showing a normal or mild pattern (Table 2).
Figure 6.
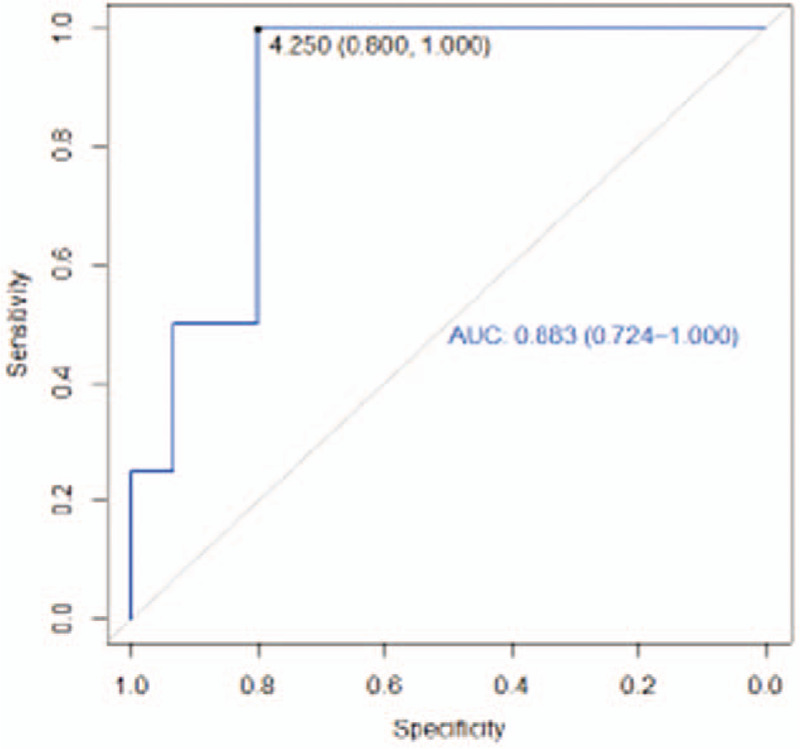
Receiver operating characteristic curve anterior elbow.
In the arm, the most frequent pattern was the mild 1 (splash). In the regions located 12 and 16 cm from the elbow, it was not possible to define the type of pattern as a result of the absence of the visualization of the ICG tracer (Table 1).
The AUCs for the regions 12 cm anterior and posterior from the elbow were greater than 0.5 (0.77 and 0.76 respectively) (Figs. 7 and 8). These values, which are close to 1, can be considered a good estimator using the cut-off point (2.25 cm).
Figure 7.
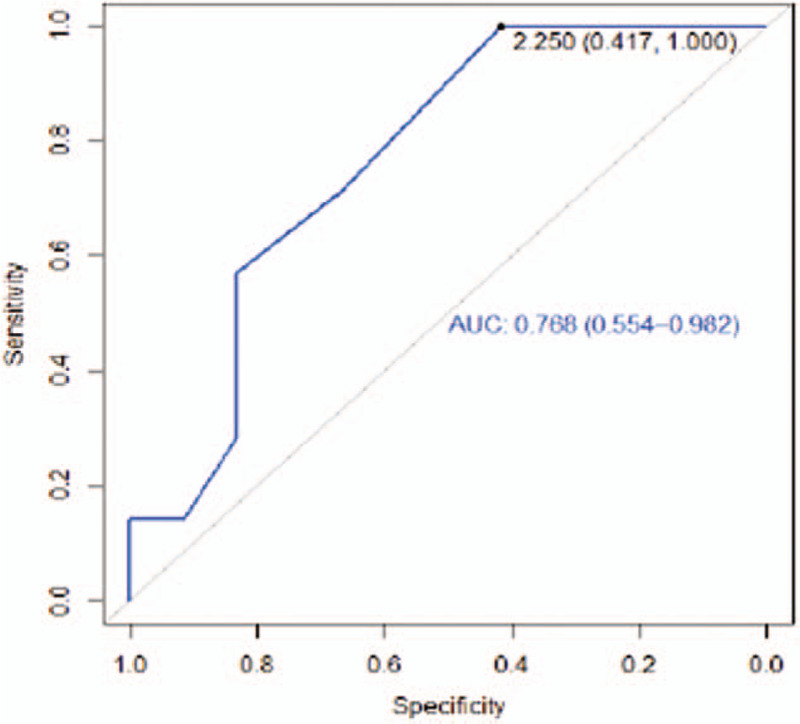
Receiver operating characteristic curve 12 cm from the anterior elbow.
4. Discussion
In this study, ICG lymphography was shown to be a comfortable to use and portable method of exploration that is advantageous for the examination of the lymphatic system. ICG lymphography allows us to observe the lymphatic flow in all directions, unlike isotopic lymphography, which does not facilitate the visualization of the posterior or lateral regions of the extremities, where the edema usually begins, as reported by Mihara et al.[11]
According to findings of a study by Akita et al,[19] the presence of several patterns was verified in the same patient in the current study. Regarding the forms of presentation of the patterns in the anterior and posterior regions of the edematous limb, they coincided in 45% of the reference areas of study. The pattern that coincided most frequently was the splash pattern.
Narushima et al[18] reported that lymphatic channels were not found in the palm, and in most cases, we did not identify any ICG pattern in our study.
The vessels in the region of the dorsum of the hand and wrist ascended linearly, as described by Yamamoto et al[12] Although they did not describe the position of the limb during the examination; they could be referring to the region of the hand not supported on the stretcher, the dorsal region. This linear arrangement of the dorsal regions, which is a rectilinear trajectory converging towards the wrist, coincides with the anatomical organization of the superficial dorsal vessels of the hand described by Jean Claude Ferrandez.[30]
The vessels of the posterior region of the forearm converge towards the anterior region, almost in their entirety, before reaching the flexure of the elbow.[30] However, in the present study, this disposition could not be observed, as the anterior and posterior forearm regions coincided in a mild pathological splash fluoroscopic pattern.
Secondary lymphatic edema classifications have been published that consider the extent and severity of fluoroscopic patterns.[22,29] The splash pattern has been observed in the more proximal regions of more recent edema. Considering that the splash pattern is a representation of an earlier and less severe dysfunction,[6,18,22] the finding of this pattern in the more distal regions of the forearm could indicate a shorter time of evolution of the edema in these areas and in consequence, a better prognosis of its response to conventional treatment. We have hypothesized, based on the idea that secondary lymphedema progresses from proximal to distal zones,[19] that the distal regions could present less evolved fluoroscopic patterns than the proximal ones, in which the dysfunction has been established for a longer period of time. The hypothesis that reflux patterns are present in the most proximal regions is in accordance with the findings of Yamamoto.[12]
At the level of the elbow joint, as in the wrist, the anterior vessels were disposed more frequently, following a normal linear physiological pattern, coinciding with that described by Yamamoto et al.[12] Immediately above the elbow joint, at 4 and 8 cm, the most frequent pattern observed in both the anterior and posterior regions was splashing.
Nevertheless, as the observation approached the most proximal regions of the limb, 12 and 16 cm above the elbow, it was increasingly difficult to visualize the presence of the tracer and to define a pattern. Regarding the main objective of this study (ie, determining the association between the perimetric difference and the presence of a better or worse pattern), the results show that there are 4 anatomical areas (wrist, elbow, and arm regions located 12 cm anterior and posterior above the epicondyle) showing perimetric differences between the healthy sides, which would allow for the discrimination between the probability of presenting a better or worse fluoroscopic pattern.
Therefore, in practical exploration, it is expected that those patients who present a difference of >2 cm at the level of the wrist will more frequently present a more pathological pattern (stardust or diffuse).
A perimeter difference of up to 4.25 in the elbow and of up 2.25 cm in the region located 12 cm above the elbow, are associated with the visualization of a better fluoroscopic pattern.
Lymphedema of the upper limb is 1 of the main complications experienced by patients undergoing breast cancer treatment. It is a chronic condition, in which unnecessary or excessive treatments should be avoided as Yamamoto et al argued.[22] The best choice between the 2 currently possible strategies (conservative or surgical) should be made based on the knowledge of the clinical situation of edema. ICG lymphography allows us to know the functional situation of the lymphatic system of the limb from the analysis of the fluoroscopic patterns and to decide the most suitable therapeutic approach. The quantitative data of the perimetric increase in the extremity, a sign of system malfunction, could be extrapolated in certain edematous areas and related to the type of fluoroscopic pattern. There is a possibility of associating quantitative with qualitative information in the diagnosis of the clinical situation with the evolution or response of edema to treatment.
The advantages offered by ICG lymphography, such as safety,[21] speed,[11] a lower cost,[19] and a lack of exposure to radiation,[18] make this technique useful in the routine assessment of lymphedema and in the assessment of patients’ response to physical treatment. The changes in the severity and extension of the fluoroscopic patterns would be considered positive indicators of the response to conventional treatment which are useful not only in the decision making based on the evidence (regarding the stabilization moment and treatment discharge), but also in choosing the timing and the clinical situation to explore other treatment options, such as a surgical approach.
Given that lymphatic reconstructive surgery can sometimes reverse the stardust fluoroscopic pattern,[18] it would be interesting to know the changes that conventional therapy induces in fluoroscopic patterns. The portable handling of this tool would also allow for the verification of the changes in the distal regions, which are hardly observable with other imaging techniques. This would be of great help in the design of the lymphatic drainage sequence of the limb and gaining a better understanding of areas that need a more concrete and intensive intervention.
4.1. Limitations
The identification of patterns in the most proximal regions of the upper limb was limited. It is probable that a longer observation is required for the tracer to reach the more distal regions and to describe a pattern by ICG lymphography. In the present study, the observation was conducted at 90 minutes after the injection of the tracer, which seems to be insufficient to detect and describe the more proximal patterns of the axilla.
In addition, the sample size of our study was limited to 19 subjects as a result of technical difficulties during the time of the study. This size could seem of little statistical power, but the results obtained have been shown to be statistically significant, suggesting that there is a relationship between the perimetric increase and the ICG pattern. Other studies[12,28] have been published, with the aim of verifying the relationship between the presence of ICG patterns and lymphedema assessment systems; but their sample sizes were also limited. New studies with larger sample sizes are needed to verify the results.
5. Conclusions
The type of fluoroscopic pattern observed by ICG lymphography in the anterior and posterior regions of the edematous extremities coincided in 45% of the cases. Moreover, 4 key anatomical areas (wrist, elbow, and the anterior and posterior arm regions, located 12 cm above the epicondyle) showed a relationship between the perimetric increase of the affected limb and the type of fluoroscopic pattern. The regions described would allow us to ascertain the probability of presenting a more or less affected fluoroscopic pattern, and to know the severity of lymphatic dysfunction.
Acknowledgments
This research team is grateful to the Management of the Gran Canaria Hospital Negrín, and the department heads of the Rehabilitation Service, Victor Manuel Sierra González and of the Vascular Surgery Service, Guido Volo Pérez. We would like to thank Editage (www.editage.com) for English language editing.
Author contributions
Conceptualization: M.E.M.-R. and E.M.M.-S.
Data curation: E.M.M-R. and M.E.M-S.
Formal Analysis: J.M.G-M. and M.E.M.-R.
Investigation: M.E.M.-R and A.M.-R.
Methodology: M.E.M.-R. and M.d.-l.-C.-A.
Project administration: M.E.M.-R.
Resources: J.M.G-M. and M.E.M-R.
Software: M.E.M.-R and E.M.M.-S.
Supervision: M.E.M.-R and M.d.-l.-C.-A.
Validation: E.M.M.-S. and M.d.-l.-C.-A.
Visualization: M.E.M.-R and A.M.-R.
Writing – review & editing: M.E.M.-R, E.M.M.-S, and M.d.-l.-C.-A.
Writing original draft preparation: M.E.M.-R., E.M.M.-S, and M.d.-l.-C.-A.
All authors have read and agreed to the published version of the manuscript.
Footnotes
Abbreviations: AUC = area under the curve, ICG = indocyanine green, NPV = negative predictive value, ROC = receiver operating characteristic.
How to cite this article: Medina-Rodríguez ME, de-la-Casa-Almeida M, Mena-Rodriguez A, Gonzalez-Martin JM, Medrano-Sánchez EM. Relationship between perimetric increase and fluoroscopic pattern type in secondary upper limb lymphedema observed by Indocyanine green lymphography. Medicine. 2020;99:24(e20432).
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors have no funding and no conflicts of interest to disclose.
Details of any previous presentation of the research, manuscript, or abstract in any form: The authors have declared that this study has not been presented elsewhere.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Unno N, Inuzuka K, Suzuki M, et al. Preliminary experience with a novel fluorescence lymphography using indocyanine green in patients with secondary lymphedema. J Vasc Surg 2007;45:1016–21. [DOI] [PubMed] [Google Scholar]
- [2].Arias A, Alvarez MJ. (2008) Tratamiento del linfedema. (Xunta de Galicia. Hospital Comarcal de Valdeorras). Available at: https://studylib.es/doc/4516561/rehabilitaci%C3%B3n-del-linfedema Accessed August, 15 2019. [Google Scholar]
- [3].Cuello-Villaverde E, Forner-Cordero I, Forner-Cordero A. Linfedema: métodos de medición y criterios diagnósticos. Rehabilitación (Madr) 2010;44:21–8. [Google Scholar]
- [4].Belgrado JP, Bracale P, Bates J, et al. Lymphoedema: what can be measured and how overview. Eur J Lymphology Relat Probl 2010;21:3. [Google Scholar]
- [5].Tassenoy A, De Mey J, De Ridder F, et al. Postmastectomy lymphoedema: different patterns of fluid distribution visualised by ultrasound imaging compared with magnetic resonance imaging. Physiotherapy 2011;97:234–43. [DOI] [PubMed] [Google Scholar]
- [6].Yamamoto T, Matsuda N, Doi K, et al. The earliest finding of indocyanine green lymphography in asymptomatic limbs of lower extremity lymphedema patients secondary to cancer treatment: the modified dermal backflow stage and concept of subclinical lymphedema. Plast Reconstr Surg 2011;128:314e–21e. [DOI] [PubMed] [Google Scholar]
- [7].Mihara M, Hara H, Kikuchi K, et al. Scarless lymphatic venous anastomosis for latent and early stage lymphoedema using indocyanine green lymphography and non-invasive instruments for visualizing subcutaneous vein. J Plast Reconstr Aesthet Surg 2012;65:1551–8. [DOI] [PubMed] [Google Scholar]
- [8].Hayes S, Cornish B, Newman B. Comparison of methods to diagnose lymphoedema among breast cancer survivors: 6-month follow-up. Breast Cancer Res Treat 2005;89:221–6. [DOI] [PubMed] [Google Scholar]
- [9].Ozaslan C, Kuru B. Lymphedema after treatment of breast cancer. Am J Surg 2004;187:69–72. [DOI] [PubMed] [Google Scholar]
- [10].Kissin MW, Querci della Rovere G, Easton D, et al. Risk of lymphoedema following the treatment of breast cancer. Br J Surg 1986;73:580–4. [DOI] [PubMed] [Google Scholar]
- [11].Mihara M, Hara H, Narushima M, et al. Indocyanine green lymphography is superior to lymphoscintigraphy in imaging diagnosis of secondary lymphedema of the lower limbs. J Vasc Surg Venous Lymphat Disord 2013;1:194–201. [DOI] [PubMed] [Google Scholar]
- [12].Yamamoto T, Yamamoto N, Doi K, et al. Indocyanine green enhanced lymphography for upper extremity lymphedema: a novel severity staging system using dermal backflow patterns. Plast Reconstr Surg 2011;128:941–7. [DOI] [PubMed] [Google Scholar]
- [13].Akita S, Mitsukawa N, Rikihisa N, et al. Early diagnosis and risk factors for lymphedema following lymph node dissection for gynecologic cancer. Plast Reconstr Surg 2013;131:283–90. [DOI] [PubMed] [Google Scholar]
- [14].Shaitelman SF, Cromwell KD, Rasmussen JC, et al. Recent progress in the treatment and prevention of cancer-related lymphedema: lymphedema treatment and prevention. CA Cancer J Clin 2015;65:55–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mihara M, Seki Y, Hara H, et al. Predictive lymphatic mapping: a method for mapping lymphatic channels in patients with advanced unilateral lymphedema using indocyanine green lymphography. Ann Plast Surg 2014;72:706–10. [DOI] [PubMed] [Google Scholar]
- [16].Tashiro K, Yamashita S, Saito T, et al. Proximal and distal patterns: different spreading patterns of indocyanine green lymphography in secondary lower extremity lymphedema. J Plast Reconstr Aesthet Surg 2016;69:368–75. [DOI] [PubMed] [Google Scholar]
- [17].Chowdhry M, Rozen WM, Griffiths M. Lymphatic mapping and preoperative imaging in the management of post-mastectomy lymphoedema. Gland Surg 2016;5:187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Narushima M, Yamamoto T, Ogata F, et al. Indocyanine green lymphography findings in limb lymphedema. J Reconstr Microsurg 2015;32:72–9. [DOI] [PubMed] [Google Scholar]
- [19].Akita S, Mitsukawa N, Kazama T, et al. Comparison of lymphoscintigraphy and indocyanine green lymphography for the diagnosis of extremity lymphoedema. J Plast Reconstr Aesthet Surg 2013;66:792–8. [DOI] [PubMed] [Google Scholar]
- [20].Tashiro K, Shibata T, Mito D, et al. Indocyanine green lymphographic signs of lymphatic collateral formation in lower extremity lymphedema after cancer resection. Ann Plast Surg 2016;77(2.): [DOI] [PubMed] [Google Scholar]
- [21].Rasmussen JC, Kwon S, Sevick-Muraca EM, et al. The role of lymphatics in cancer as assessed by near-infrared fluorescence imaging. Ann Biomed Eng 2012;40:408–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yamamoto T, Narushima M, Doi K, et al. Characteristic indocyanine green lymphography findings in lower extremity lymphedema: the generation of a novel lymphedema severity staging system using dermal backflow patterns. Plast Reconstr Surg 2011;127:1979–86. [DOI] [PubMed] [Google Scholar]
- [23].Liu H-L, Pang S-Y, Chan Y-W. The use of a microscope with near-infrared imaging function in indocyanine green lymphography and lymphaticovenous anastomosis. J Plast Reconstr Aesthet Surg 2014;67:231–6. [DOI] [PubMed] [Google Scholar]
- [24].Tan I-C, Maus EA, Rasmussen JC, et al. Assessment of lymphatic contractile function after manual lymphatic drainage using near-infrared fluorescence imaging. Arch Phys Med Rehabil 2011;92:756–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bernas MJ, Witte CL, Witte MH. The Diagnosis and treatment of peripheral lymphedema draft revision of the 1995 consensus document of the International Society of Lymphology Executive Committee for Discussion at the September 3-7, 2001, XVIII International Congress of Lymphology in Geno. Lymphology 2001;34:84–91. [PubMed] [Google Scholar]
- [26].Ferrandez JC, Theys S, Bouchet JY. Reeducación de Los Edemas de Los Miembros Inferiores, 1st ed.; Barcelona (Spain): Masson; 2002; 116–121. [Google Scholar]
- [27].Mortimer PS. Investigation and management of lymphoedema. Vasc Med Rev 1990;1:1–20. [Google Scholar]
- [28].Unno N, Nishiyama M, Suzuki M, et al. Quantitative lymph imaging for assessment of lymph function using indocyanine green fluorescence lymphography. Eur J Vasc Endovasc Surg 2008;36:230–6. [DOI] [PubMed] [Google Scholar]
- [29].Ogata F, Azuma R, Kikuchi M, et al. Novel lymphography using indocyanine green dye for near-infrared fluorescence labeling. Ann Plast Surg 2007;58:652–5. [DOI] [PubMed] [Google Scholar]
- [30].Ferrandez JC. El Sistema Linfático: Historia, Iconografía e Implicaciones Fisioterapéuticas; Madrid (Spain): Panamericana; 2006; 39-70. [Google Scholar]


