Abstract
Symptomatic cerebrospinal fluid (CSF) viral escape (sCVE) is reported in people with HIV, who are on ritonavir-boosted protease inhibitor (PI/r) containing antiretroviral therapy (ART). Management of sCVE includes performing genotypic HIV-1 resistance testing (GRT) on CSF and plasma HIV and changing ART accordingly. Neither GRT nor newer drugs (Dolutegravir and Darunavir/ritonavir) are routinely available in India. As a result, management of sCVE includes 2 modalities: a) ART intensification by adding drugs that reach therapeutic concentrations in CSF, like Zidovudine, to existing ART or b) Changing to a regimen containing newer boosted PI/r and integrase strand transfer inhibitor (INSTI) as per GRT or expert opinion. In this retrospective study, we report the outcomes of above 2 modalities in treatment of sCVE in Pune, India.
Fifty-seven episodes of sCVE in 54 people with HIV taking PI/r-containing ART were identified. Clinical, demographic, laboratory and ART data were recorded. Forty-seven cases had follow-up data available after ART change including measurement of plasma and CSF viral load (VL).
Of the 47 cases, 23 received zidovudine intensification (Group A, median VL: plasma- 290, CSF- 5200 copies/mL) and 24 received PI/INSTI intensification (Group B, median VL: plasma- 265, CSF-4750 copies/mL). CSF GRT was performed in 16 participants: 8 had triple class resistance. After ART change, complete resolution of neurologic symptoms occurred in most participants (Group A: 18, Group B: 17). In Group A, follow-up plasma and CSF VL were available for 21 participants, most of whom achieved virologic suppression (VL < 20 copies/mL) in plasma (17) and CSF (15). Four participants were shifted to the PI/INSTI intensification group due to virologic failure (plasma or CSF VL > 200 copies/mL). In Group B, follow-up plasma and CSF VL were available for 23 participants, most of whom also achieved virologic suppression in plasma (21) and CSF (18). Four deaths were noted, 2 of which were in individuals who interrupted ART.
This is a unique sCVE cohort that was managed with 1 of 2 approaches based on treatment history and the availability of GRT. At least 75% of participants responded to either approach with virologic suppression and improvement in symptoms.
Keywords: cerebral penetration effectiveness score, CSF HIV escape, genotypic HIV-1 resistance testing, protease inhibitors, zidovudine
1. Introduction
Symptomatic cerebrospinal fluid (CSF) viral escape (sCVE) is defined as discordance in HIV ribonucleic acid (RNA) in plasma and CSF which is associated with new neurologic symptoms. This phenomenon was first described by Canestri et al in 2010.[1] It has been reported from higher income countries[2–15] and low and middle income countries (LMIC).[16–20] Several risk factors associated with sCVE have been described such as low nadir CD4 count (associated with HIV entry into the central nervous system (CNS) and productive infection of perivascular macrophages and other cells),[21,22] suboptimal adherence to antiretroviral therapy (ART, leading to low level plasma viremia and continual seeding of the CNS by infected cells that traffic into the brain),[23] treatment with drugs with limited CNS penetration (associated with low level replication of CNS HIV),[23–25] selection of drug resistant HIV strains[23–25] and chronic sustained immune activation.[25,26] In India, ritonavir boosted protease inhibitors (PI/r) are used as components of second or third line ART regimens [27]. Use of PI/r also increases the risk of sCVE.[28]
Management of sCVE appears to require ART regimen change to address these 2 elements (ie, drug resistance in CSF and subtherapeutic drug concentrations).[23] Based on genotypic HIV-1 resistance testing (GRT) and ART history, the new regimen should include at least 2 drugs to which HIV derived from CSF is sensitive. The ART drugs in the new regimen should also reach therapeutic concentrations in CSF.[23] However, neither GRT nor newer drugs like darunavir (DRV/r)[29] or dolutegravir (DTG)[30] are routinely available in LMIC like India. For this reason, our clinic in Pune, India has managed sCVE by 2 treatment strategies:
-
(1)
ART intensification, ie, adding to the existing regimen, drugs that reach high concentrations in CSF,[31] typically zidovudine (AZT) or
-
(2)
ART change, ie, changing to a new ART regimen that includes active drugs based on GRT or expert physician opinion. In this study, we report the outcomes of above 2 modalities used in treatment of sCVE developing in individuals on PI/r containing ART in Pune, India.
2. Methods
2.1. Study design
This cohort study was conducted between March 2009 and March 2019 at 3 private, tertiary level hospitals and research centers in Pune, Maharashtra (Ruby Hall Clinic, Poona Hospital and Noble Hospital). These 3 private hospitals provide clinical care, diagnostic and treatment services to people with HIV (PWH). All data, including demographic, clinical, laboratory and treatment are entered into a secure electronic database (Livehealth software solutions, Pune, India).
2.2. Diagnoses of CSF HIV escape (sCVE) and data collection
We retrospectively compiled data of all PWH who were taking PI/r-containing ART and were diagnosed with sCVE in our cohort. All participants consented to use of their data for research purposes and analyses were approved by the Institutional Review Board of all 3 hospitals. sCVE was defined as either a) CSF HIV RNA >20 copies/mL when plasma HIV RNA ≤20 copies/mL or b) when plasma HIV RNA was >20 copies/mL, CSF HIV RNA was ≥0.5 log10 higher than plasma HIV RNA. A subset of these cases has been previously described.[19] Neurologic symptoms included headache, imbalance, tremor, slurred speech, memory loss, seizures, limb weakness or paralysis, bowel or bladder incontinence, delirium, and coma. Demographic, laboratory, and imaging data were collected, including pre ART CD4+ T-cell count, nadir CD4+ T-cell count, CD4+ T-cell count at sCVE, paired plasma and CSF HIV RNA ((NucliSENS EasyQ, BioMérieux, France, lower limit of quantification 20 copies/ml), Magnetic resonance imaging reports (MRI, 1.5 Tesla), and CSF HIV GRT (ABI 3130, PE Applied Biosystems, minimum HIV RNA for successful sequencing: 1000 copies/mL). All CSF specimens had negative diagnostic tests for Treponema pallidum, Cryptococcus, Toxoplasma gondii, Mycobacterium tuberculosis, Herpes simplex virus, Varicella zoster virus and John Cunningham (JC) virus. Genotyping was interpreted according to the International Antiviral Society-USA guidelines.[32] CNS penetration effectiveness (CPE)[33] value of ART regimens was calculated. Genotypic susceptibility score (GSS) of each ART regimen was calculated in participants undergoing GRT by assigning a score of 0 (resistant), 0.5 (intermediate resistance), or 1 (susceptible) to each drug. GSS-adjusted CPE values were calculated by multiplying the CPE value by the GSS for each ART drug and summing scores.[28]
2.3. Management strategies used for CSF HIV escape
After diagnosis of sCVE, 1 of the 2 methods were followed as standard of care in our cohort (Fig. 1). A) AZT intensification- Criteria for inclusion were:
Figure 1.
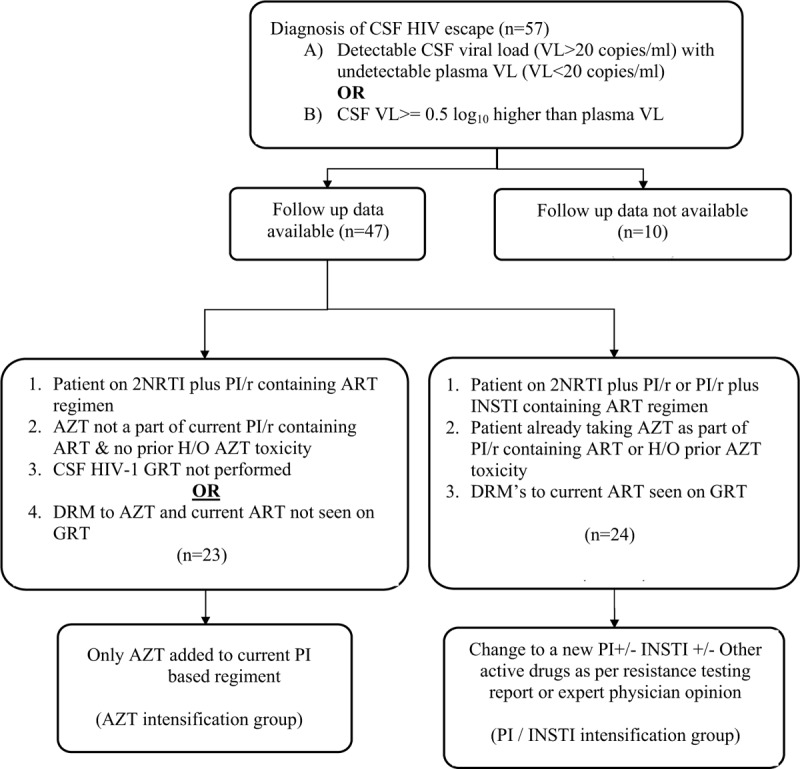
Flow chart illustrating the diagnostic criteria used for identifying CSF HIV escape and criteria used for changing ART regimen in patients. AZT = zidovudine, CSF = cerebrospinal fluid, DRM = drug resistance mutations, GRT = genotypic HIV-1 resistance testing, INSTI = integrase inhibitor, NRTI = nucleoside reverse transcriptase inhibitor, PI/r = boosted protease inhibitor.
-
(1)
Use of 2 nucleoside reverse transcriptase inhibitors (NRTI) plus PI/r at the time of sCVE,
-
(2)
not currently using AZT,
-
(3)
no prior history of AZT toxicity, and
-
(4)
CSF GRT not performed or no drug resistance mutations (DRMs) to AZT or current ART.
In this group, AZT was added to the current regimen. B) ART change or PI/INSTI intensification.
Criteria for inclusion were:
-
(1)
Individual on 2 NRTIs plus PI/r or PI/r plus Integrase inhibitor (INSTI) regimen at the time of sCVE,
-
(2)
history of prior AZT toxicity or current use of AZT,
-
(3)
DRMs to current ART drugs on CSF GRT.
This group was changed to a new ART regimen that included a new boosted PI/r, an INSTI, or both. Other drugs in the new regimen were guided by GRT or expert opinion.
2.4. Follow up of cases of CSF HIV escape
Patients with sCVE were followed up clinically at 2, 4, 12, and 24 weeks post ART change to look for resolution of neurologic symptoms. Plasma and CSF viral load (VL) analysis was done at 24 weeks post ART change and yearly thereafter. Follow up plasma and CSF HIV-1 VL was noted for both modalities. Total duration of follow up (in months) and CPE score of new ART regimen was recorded. Overall outcomes of these cases of sCVE (alive and well/ partial resolution of symptoms/ lost to follow up/ death) were noted. Patients in whom follow up plasma/CSF VL and outcome data was unavailable were excluded from analysis.
2.5. Statistical methods
Baseline characteristics for continuous variables were summarized using median and interquartile range (IQR), and for categorical variables using frequency and percentages. Continuous variables were compared using a median test. Categorical variables were compared using Chi-square test and Fisher exact test. All analyses were performed using STATA version 12.1.
3. Results
Of 513 PWH on PI/r containing ART (2 NRTI + 1 PI/r or 1 PI/r + 1 INSTI) in our cohort, 57 episodes of sCVE in 54 individuals (10.5%) were identified. Of these, 47/57 (82.5%) had follow-up data available and were included in our study (Fig. 1). Twenty-three cases underwent AZT intensification (Group A) and 24 underwent ART change (PI/INSTI intensification, Group B). Demographic data and neurologic symptoms are summarized in Table 1 and Figure 2. Median age was 42 years in Group A (IQR: 40–47) and 40 years in Group B (IQR: 34–45). Nadir CD4+ T-cell count was 65 cells/μL in Group A (IQR: 33–92) and 103 cells/μL in Group B (IQR: 75–122). At the time of sCVE, Group A had been on a stable PI/r containing ART regimen for a median of 33 months (IQR: 21–46) and Group B for 21 months (IQR: 15–36). Imbalance during walking, tremors of hands and memory loss were the most common neurologic symptoms (Fig. 2). Most symptoms had subacute onset (≥ 2 weeks) but 6 participants in each group had acute onset (< 2 weeks) of severe neurologic symptoms like seizures, delirium, or coma. Tenofovir disoproxil fumarate (TDF) plus lamivudine or emtricitabine (3TC, FTC) plus Atazanavir/ritonavir (ATV/r) was the most common ART regimen at the time of sCVE (32 of 47 episodes (68.1%), 21 in group A and 11 in Group B). Three individuals had 2 episodes of sCVE each (A9 and B22, A15 and B19, B9 and B15, Tables 2 and 3).
Table 1.
Baseline demographic, clinical and laboratory characteristics of patients with Neurosymptomatic CSF HIV escape.
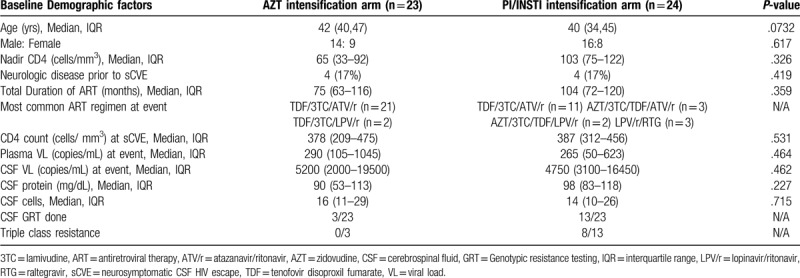
Figure 2.
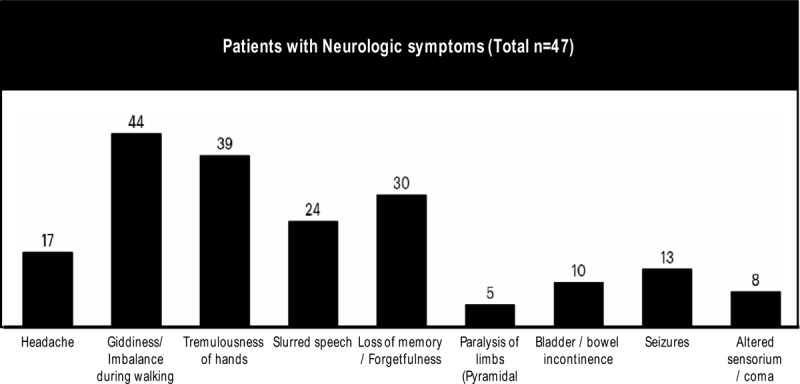
Major neurologic symptoms seen in patients with CSF HIV escape in Pune Cohort and their relative frequency. CSF = cerebrospinal fluid.
Table 2.
Follow-up plasma and CSF HIV-1 viral load values in AZT intensification arm (Group A).

Table 3.
Follow up plasma and CSF HIV-1 VL values in PI/INSTI intensification arm (Group B).

CD4+ T-cell count at sCVE was 378/μL in Group A (IQR: 209–475) and 387/μL (IQR: 312–456) in Group B. Twenty of 23 cases in Group A and all 24 cases in Group B had elevated CSF protein levels (median, 90 mg/dL; IQR: 53–113 mg/dL versus median, 98 mg/dL; IQR: 83–118 mg/dL). CSF pleocytosis (CSF leukocytes > 5 cells/μL) was observed in 19/23 (82.6%) cases in Group A (median 16; IQR: 11–29) and 19/24 (79.2%) cases in Group B (median 14; IQR: 10–26). Median plasma HIV RNA at the time of developing sCVE was 290 copies/mL in Group A (IQR: 105–1045) and 265 copies/mL in Group B (IQR: 50–623). Median CSF HIV RNA was 5,200 copies/mL in Group A (IQR: 2000–19500) and 4,750 copies/mL in Group B (IQR: 3100–16450). CSF GRT was performed in 3 participants in Group A (13.0%) and 13 participants in Group B (54.2%). Triple class resistance (NRTI, non-NRTI [NNRTI] and PI DRMs) was seen in no participants in Group A and 8 (33.3%) in Group B. Median CPE at the time of sCVE was 5 in Group A (IQR: 5–6) and 6 in Group B (IQR: 6–7). GSS-adjusted CPE values was <5 in all 16 individuals in whom CSF GRT was performed.
3.1. AZT intensification group (Group A)
Follow up data of Group A is summarized in Table 2. At the time of sCVE, none of the 23 PWH were on thymidine analogues. TDF plus 3TC or FTC plus ATV/r was the ART regimen used by 21 (91.3%) participants. Duration of follow up after AZT intensification was 14 months (IQR: 9–19). Median CPE value after AZT intensification was 9 (IQR: 9–9). After AZT intensification, 18/23 (78.3%) cases had complete resolution of symptoms. All 6 individuals with severe neurologic symptoms like seizures and altered sensorium had neurologic recovery and could resume all activities of daily living. Follow up plasma HIV-1 RNA was available for 21/23 (91.3%, Table 2) patients, of whom 17 had viral suppression (VL < 20 copies/mL), 1 had VL between 20 and 200 copies/ml, and 3 had VL > 200 copies/ml. Follow up CSF HIV-1 RNA was also available for 21/23 (91.3%) patients, of whom 15 had VL < 20 copies/ml, 2 had VL between 20 and 200 copies/ml while 4 had VL > 200 copies/ml. In 16/21 (76.2%) cases, discordance between CSF and plasma HIV RNA replication completely resolved at follow-up. In 3 cases, discordance persisted but at a lower level (A8, A9 and A18, Table 2). In 2 cases, discordance worsened (A15 and A17). Virologic failure of CNS active ART (VL > 200 copies/ml in either of the compartments) was seen in 4 cases (A9, A15, A17 and A18, Table 2). CSF GRT was performed in 2 cases (A9 and A15) both of whom had triple class resistance. All 4 were subjected to ART change with PI/INSTI intensification (Group B). Incomplete efficacy of CNS active ART (either plasma or CSF HIV RNA or both > 20 copies/ml) was seen in 6/21 (28.6%, Fig. 3) cases.
Figure 3.

Flow chart depicting follow up of patients with CSF HIV escape after change of ART in Pune, India. Majority of patients had neurologic improvement at follow-up due to plasma and CSF HIV-1 RNA suppression (HIV RNA < 20 copies/ml). Incomplete efficacy was defined as either plasma or CSF HIV RNA > 20 copies/ml despite change of ART regimen. There were 4 deaths during follow-up in entire cohort. ART = antiretroviral therapy, AZT = zidovudine, CSF = cerebrospinal fluid, INSTI = integrase inhibitors, PI = protease inhibitors, RNA = ribonucleic acid, T1 = follow up plasma and CSF viral loads.
One death occurred in Group A (A5) and 1 participant was lost to follow up (A14). Both had complete resolution of neurologic symptoms after AZT addition. A5 developed severe anemia (Hemoglobin < 6 g/dL) after 12 weeks and discontinued AZT. After AZT withdrawal, neurologic symptoms recurred followed by status epilepticus and rapid clinical deterioration, culminating in coma and death. After availability of generic DTG in India in August 2018, 11 of the 23 participants in Group A substituted AZT with DTG and have not had a relapse of neurologic symptoms till date.
3.2. ART change or PI/INSTI intensification group (Group B)
Follow up data of participants managed in Group B are summarized in Table 3. In this group, median duration of exposure to thymidine analogues during first line ART was 47 months (IQR: 15–66). At the time of development of sCVE, 5 patients were already taking AZT and 9 had a history of AZT toxicity. Twenty of 24 (87.5%) were taking 2 NRTIs and a PI/r at the time of sCVE. Four were taking a PI/r and an INSTI. CSF GRT was performed in 13 (Table 4) and all had M184 V present, which confers high level resistance to 3TC and emtricitabine. Ten patients also had intermediate to high level resistance to AZT with T215Y/V/F being the most common thymidine analog mutation (TAM). M46I and V82A were the most common protease mutations seen in 3 participants each. Integrase mutations were identified in CSF GRT in 2 patients (B7 and B13)
Table 4.
CSF HIV-1 Genotypic resistance testing performed in patients with neurosymptomatic CSF HIV escape.

The 2 most common ART changes occurring in Group B were a) Replacing current PI/r (mostly Atazanavir) with a better CNS penetrating PI/r like lopinavir/ritonavir or DRV/r plus addition of an INSTI (9 cases, B1–B7, B11 and B17, Table 5) or b) Replacing current PI/r with a better CNS penetrating PI/r plus addition of AZT/3TC plus continuation of TDF (4 cases, B10, B14, B15 and B24). Median CPE score of ART regimens after PI/INSTI intensification was 8 (IQR: 6–10). After modification of ART regimen, median GSS improved from 1 (IQR: 1–1) to 2 (IQR: 2–2). Total duration of follow up in Group B was 32 (IQR: 14–45) months. After PI/INSTI intensification, 17/24 (70.83%) patients had complete resolution of symptoms while 6 had partial resolution. Five out of 6 individuals developing acute onset, severe neurologic symptoms like seizures and altered sensorium had neurologic recovery with resumption of activities of daily living. Follow up plasma VL were available for 23/24 (95.8%) patients, out of which 21 (91.3%) had VL suppression (VL < 20 copies/mL), 1 had VL between 20 and 200 copies/ml while 1 had VL > 200 copies/mL. Follow up CSF VL were available for 23/24 (95.8%) patients, out of which 18 had VL < 20 copies/ml, 4 had VL between 20 and 200 copies/ml while 1 had VL > 200 copies/ml. In 18/23 (78.3%) cases, discordance between CSF and plasma HIV-1 replication completely resolved at follow-up. In 5 cases, discordance persisted but at a lower level (B2, B14–16, B24 Table 3). Discordance did not worsen at follow up in any patient. Virologic failure on CNS active ART (VL > 200 copies/ml in either compartment) was seen in 1 individual (B24, Table 3). B24 had CSF VL > 1000 copies/mL despite controlled plasma VL at follow up. CSF GRT was attempted but DRM could not be amplified in CNS HIV. B24 was then shifted to a new regimen of DRV/r plus DTG. Incomplete efficacy of CNS active ART (either plasma or CSF VL or both > 20 copies/mL) after PI/INSTI intensification was seen in 6/23 (26. 1%, Fig. 3) patients.
Table 5.
New antiretroviral treatment strategy prescribed to patients in PI/INSTI intensification arm (Group B).

There were 3 deaths in the cohort (B9, B13 and B14). B9 and B14 had attained plasma and CSF HIV suppression (VL< 20 copies/mL) on follow up. B9 stopped CNS active ART after 16 months of follow up resulting in reappearance of neurologic symptoms over a period of 1 month for which he refused medical care and died at home. B14 had partial recovery of neurologic symptoms with persistence of imbalance during walking and aggressive anti-social behavior. He died in a road traffic accident. B13 had developed sCVE on a regimen consisting of Lopinavir/r and RTG (raltegravir). We changed her ART to a new regimen containing AZT/3TC plus DRV/r plus DTG. But there was no improvement in neurologic symptoms. B13 continued to remain comatose and died of aspiration pneumonia. After availability of low cost generic DTG in India in August 2018, 5 out of the 24 cases in Group B substituted RTG with DTG, keeping rest of the regimen intact.
4. Discussion
In India, first-line ART comprises of a NRTI backbone, preferably Non-Thymidine (Tenofovir plus 3TC (TDF/3TC) or AZT plus 3TC (AZT/3TC)) and 1 NNRTI, preferably efavirenz.[34] Virologic failure under the national ART programme (National AIDS control organization) is defined as a Plasma VL of 1,000 or more copies/mL after 6 months of ART, with individual being treatment adherent by >95%. A low-level viremia (LLV, VL< 1000 copies/mL), does not require switch in therapy.[34] Second-line ART is based on use of a Ritonavir boosted PI (ATV/r (preferred) or Lopinavir/ritonavir) supported by at least 1 new and unused NRTI (AZT or Tenofovir) or in an inevitable situation an integrase inhibitor (RTG, Alternate second line ART).[27,34] In cases of virologic failure on second line ART, individual is shifted to third line ART which consists of INSTI (RTG) and a new PI/r (Darunavir/ritonavir).[27,34] Implementation of World health organization[35] and National AIDS control organization ART[34] guidelines may result in ideal conditions for compartmental replication of HIV.[16] Use of first-line ART with low genetic barrier to resistance,[16,19,34] delayed switch to second or third line ART due to infrequent VL monitoring,[19,36] higher cut off (1000 copies/mL) for ART switch which encourages low level viremia and accumulation of DRM,[16,19] recycling of NRTI for second line ART favoring functional PI monotherapy[16,19] and Use of ATV/r which does not consistently achieve in vitro 50% inhibitory concentrations (IC50) for wild-type HIV-1 in CSF[37,38] are factors which contribute to higher prevalence of sCVE in LMIC like India.[39,40] In addition, drugs required to combat multidrug resistant CNS HIV like second generation PI/r (DRV/r), newer integrase inhibitors (DTG), entry inhibitors (Maraviroc) and second generation NNRTI (Etravirine) are scarcely available in India. In such circumstances, we describe 47 cases of Neurosymptomatic CSF HIV escape (sCVE) identified during a 10 year period at our cohort in Pune, India and their follow up virologic and outcome data. Two treatment modalities, AZT intensification (to improve CNS penetrability of ART) and ART change with PI/INSTI intensification (to tackle CNS HIV drug resistance) were used in our cohort for management of sCVE. Both treatment modalities were effective in resolution of neurologic symptoms. Almost 75% cases in Group A and B had plasma and CSF VL <20 copies/mL after change to CNS active ART. To the best of our knowledge, our study is 1 of the first reports of follow up plasma and CSF VL and outcome data of a large number of sCVE cases from a LMIC. We also believe that recent World health organization guidelines recommending initiating ART irrespective of baseline CD4 count (test and treat strategy) and use of DTG plus 2NRTI as preferred ART regimen will go a long way in reducing incidence of HIV associated neurocognitive disease in LMIC like India.[35]
In AZT intensification group (group A), 20/23 (87%) cases had a past history of virologic failure on Thymidine analogue (AZT or Stavudine (d4T) as NRTI backbone) containing first line ART. The median exposure to Thymidine analogs was 4 years. These drugs carry well-recognized risks of side-effects like Anemia (AZT), Dyslipidemia and Lipodystrophy syndrome (AZT, Stavudine). Twenty 1 patients in Group A were on TDF plus 3TC/FTC plus ATV/r while 2 used TDF plus 3TC/FTC plus lopinavir/ritonavir at the time of sCVE. Due to inability to access newer potent drugs, we were forced to reuse AZT due to its excellent CNS penetration in spite of knowledge about its toxicity and compromised antiviral activity. Despite all the drawbacks, addition of AZT to current PI/r containing ART led to complete resolution of symptoms in more than 70% cases in cohort. Plasma viral suppression (VL < 20 copies/ml) improved from 17.9% at sCVE to 81% at follow up (Fig. 4). CSF viral suppression (VL < 20 copies/mL) improved from 0% at sCVE to 71.4% at follow up. This suggests that adding AZT increased the antiviral potency of PI/r containing ART in plasma and CNS compartments leading to resolution of sCVE in majority of cases.
Figure 4.
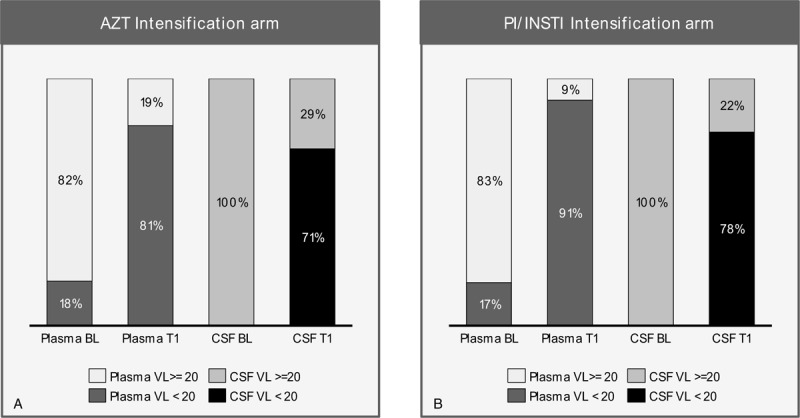
Significant increase in plasma and CSF HIV-1 viral load suppression (VL < 20 copies/mL) after change of ART in both groups of patients with CSF HIV escape. ART = antiretroviral therapy, AZT = zidovudine, BL = baseline, CSF = cerebrospinal fluid, PI/INSTI = boosted protease inhibitor and/or integrase inhibitor, T1 = follow up visit, VL = viral load.
One explanation could be that there was low level or no resistance to Thymidine analogs after virologic failure of first line ART because of which AZT was effective in VL suppression when used again. This might be the case for A6, A7 and A8 in whom CSF GRT showed resistance only to 3TC (Table 4). However, in all these 3 cases, Thymidine analogs were not a part of ART at the time of sampling of CSF for GRT. As a result, TAM's could have been archived in the past but not seen on current CSF HIV-1 GRT. Second explanation could be that TAM's were generated due to long duration of exposure to Thymidine analogs. However the power of TAM's to compromise efficacy of NRTI component of PI/r containing ART in vivo was overestimated. As a result, AZT and TDF along with PI/r were successful in VL suppression in both compartments in majority of cases. Unfortunately, this could not be confirmed as GRT was not done for rest of the 20 individuals in Group A. Virologic efficacy of NRTI component in a 2NRTI plus PI/r containing ART regimen was well elucidated in EARNEST trial[41] which was done in LMIC with late diagnosis of first-line ART failure and no resistance testing to inform decisions about drug choice. In this trial, PI/r plus RTG offered no advantage over PI/r plus 2 NRTI in virologic efficacy or safety in individuals failing 1st line NNRTI containing ART. The trial confirmed the contribution that NRTIs make to the virologic efficacy of a PI/r containing regimen, even when their activity is predicted to be substantially, or even completely, compromised by cross-resistance. Contribution of NRTI's to a PI/r containing regimen can also be judged from the fact that in patients who have developed sCVE on PI/r monotherapy, CSF HIV-1 RNA returned to undetectable levels along with complete resolution of neurologic symptoms after reintroduction of baseline NRTI's.[42,43] Some patients in Group A may have had functional PI monotherapy which was reversed by AZT intensification. A third explanation could be that Epstein Barr Virus (EBV) de-oxyribonucleic acid (DNA) is frequently detected in CSF of PWH on ART. CSF EBV DNA is associated with higher CSF HIV RNA, higher CSF pleocytosis, higher levels of biomarkers of neuronal damage/inflammation and could be responsible for HIV-associated CNS disorders like sCVE.[44] AZT effectively inhibits Epstein-Barr virus (EBV) DNA replication along with HIV RNA suppression.[45] This could be another reason for symptomatic improvement in PWH with sCVE. However, EBV DNA PCR was not performed on any of the CSF samples in our cohort. AZT is freely available in LMIC like India and it can help clinicians to successfully manage majority of cases of sCVE developing on a regimen containing TDF and PI/r. It will serve as a bridge till individuals with sCVE get access to newer drugs like DRV/r and DTG.
The Mind Exchange Consensus Report[46] suggests that for patients with persistent or worsening cognitive impairment and detectable HIV RNA in CSF, doctors should consider patient adherence to ART, co-morbidities, plasma and CSF resistance profiles and possibly the CPE score of ART regimen prior to modification of therapy. European AIDS clinical society (EACS) guidelines for the treatment of cognitive impairment in HIV-seropositive individuals have made similar recommendations.[47] Management of patients in PI/INSTI intensification group (group B) followed these guidelines. We could tackle both the aspects causing sCVE i.e. CSF HIV drug resistance and CNS ART penetration as majority of patients could perform a CSF HIV-1 GRT and also had access to DRV/r and DTG. In group B, 20/24 (87.5%) cases had a past history of virologic failure on Thymidine analogue containing first line ART with a median exposure of 4 years. 58.3% cases either had AZT as a part of current PI/r containing ART regimen or had past history of AZT toxicity. In addition, out of the 13 CSF GRT reports, TAM's were seen in 10 while triple class resistance was seen in 8 patients. Despite the lack of GRT in Group A, it is safe to conclude that individuals in Group B had a much more drug resistant CSF HIV as compared to Group A. PI/INSTI intensification led to complete resolution of symptoms in more than 75% cases in cohort. Plasma viral suppression (VL < 20 copies/mL) improved from 17.4% at sCVE to 91.3% at follow up while CSF viral suppression (VL < 20 copies/mL) improved from 0% at sCVE to 78.3% at follow up (Fig. 4).
Out of the 4 deaths in entire cohort, 2 occurred in individuals who interrupted CNS active ART leading to rapid viral rebound in plasma and CNS compartment (A5 and B9). This led to incident neurologic symptoms which were much more severe than the first instance. sCVE relapse on ART simplification has also been described by Zan et al in their CSF HIV escape case series from Italy.[48] This reinforces the fact that strict adherence to neuroactive ART maintains plasma and CSF HIV suppression, prevents relapse of sCVE and prevents death.
4.1. Limitations
Our study has several limitations. First, as for all retrospective studies, some episodes of sCVE may be unreported leading to measurement bias and underestimation of prevalence. Second, milder neurologic symptoms such as headache may not have triggered lumbar puncture and measurement of CSF HIV-1 RNA, leading to unaccounted cases of mildly symptomatic CSF escape. Third, neuropsychological testing for cognitive impairment was not performed at baseline or follow-up in our cohort and hence milder forms of HIV-associated neurocognitive disease[49] could not be identified and sCVE in these patients could not be studied. Screening for functional impairment and mood disorders[50] was also not performed. Fourth, GRT of CNS virus was not routinely performed in Group A leading to potential selection bias. Fifth, different follow up duration in both treatment modalities (32 months in Group B versus 14 months in Group A) may have influenced our results. Virologic suppression in protected compartments like CNS may require extra time. Sixth, our follow-up data is limited in some cases because further studies were not pursued as individuals had declined repeated CSF examination after the first follow up lumbar puncture and once symptoms resolved. Seventh, EBV DNA was not studied in CSF samples in our cohort and hence contribution of EBV to sCVE could not be identified. Despite these limitations, this is a unique cohort of individuals with CSF HIV escape with homogeneous treatment interventions leading to successful resolution in a majority of cases.
5. Conclusions
Physicians in resource limited settings like India should perform CSF HIV-1 VL analysis in patients who develop neurologic symptoms while on PI/r containing ART with well controlled plasma HIV. After noting GRT of CSF HIV and history of prior treatments, the new regimen should include at least 2 drugs that penetrate the CNS well and to whom the CSF HIV is sensitive. In majority of cases it involves using better CNS penetrating PI/r like Lopinavir or Darunavir (DRV/r) and Integrase inhibitor like DTG. In absence of GRT and newer drugs like DRV/r and DTG, AZT intensification of PI/r containing regimen serves as a practical, effective and easily implementable therapeutic modality in a subset of cases. Both modalities, PI/INSTI intensification and AZT intensification were successful in resolving neurologic symptoms and suppressing plasma/CSF HIV in majority of cases in our cohort. These cases add to the growing literature on Neurosymptomatic CSF HIV escape that underscores the need for further investigation into the mechanism of establishment of CNS HIV reservoir and long term consequences of HIV replication and persistence in the CNS.
Author contributions
Ameet Dravid – Writing Manuscript, Study concept and design, acquisition of data.
Kartik Natrajan – Study concept and design, acquisition of data.
Mahenderkumar Medisetty- Study concept and design, acquisition of data.
Avadesh K Sharma- Study concept and design, acquisition of data.
Tarun Betha- Study concept and design, acquisition of data.
Raviraj Gawali- Study concept and design, acquisition of data.
Milind Kulkarni – Study concept and design, acquisition of data.
Chinmay Saraf – Study concept and design, acquisition of data.
Uma Mahajan – Analysis and Interpretation of Data.
Sachin Kore – Study concept and design, acquisition of data.
Niranjan Rathod – Study concept and design, acquisition of data.
Rustom Wadia – Critical review of manuscript for intellectual content.
Andrea Calcagno – Writing Manuscript, Study concept and design
Scott Letendre – Writing Manuscript, Study concept and design
Conceptualization: Ameet N Dravid, Milind M Kulkarni, Chinmay K Saraf, Uma S Mahajan, Sachin D Kore, Niranjan M Rathod, Andrea Calcagno.
Data curation: Ameet N Dravid, Raviraj Gawali, Tarun P Betha, Avadesh K Sharma, Mahenderkumar Medisetty, Kartik Natrajan, Milind M Kulkarni, Uma S Mahajan, Sachin D Kore, Niranjan M Rathod.
Formal analysis: Ameet N Dravid, Uma S Mahajan.
Investigation: Ameet N Dravid, Chinmay K Saraf, Umakant S Mahajan.
Methodology: Milind M Kulkarni, Uma S Mahajan, Sachin D Kore.
Project administration: Ameet N Dravid, Kartik Natrajan, Milind M Kulkarni, Chinmay K Saraf, Uma S Mahajan, Sachin D Kore, Niranjan M Rathod.
Resources: Uma S Mahajan.
Supervision: Niranjan M Rathod.
Validation: Uma S Mahajan, Niranjan M Rathod.
Visualization: Niranjan M Rathod.
Writing – original draft: Ameet N Dravid.
Writing – review & editing: Ameet N Dravid, Milind M Kulkarni, Scott L Letendre, Rustom S Wadia, Andrea Calcagno.
Manisha Ghate MD, PhD (National AIDS research institute (NARI), Pune, India) - edited the manuscript for non-intellectual content.
Gaurav Arun Joshi, MBA (Director, AArete LLC) – helped in data analysis, and preparing figures and tables
Footnotes
Abbreviations: 3TC = lamivudine, ART = antiretroviral therapy, ATV/r = atazanavir/ritonavir, AZT = zidovudine, CNS = central nervous system, CPE = central nervous system penetration effectiveness score, CSF = cerebrospinal fluid, DNA = de-oxyribonucleic acid, DRM = drug resistance mutations, DRV/r = darunavir/ritonavir, DTG = dolutegravir, EBV = Epstein barr virus, FTC = emtricitabine, GRT = genotypic HIV-1 resistance testing, GSS = genotypic susceptibility score, INSTI = integrase strand transfer inhibitor, IQR = interquartile range, LMIC = low and middle income countries, NNRTI = non-nucleoside reverse transcriptase inhibitor, NRTI = nucleoside reverse transcriptase inhibitor, PI/r = protease inhibitor, PWH = people with HIV, RNA = ribonucleic acid, RTG = raltegravir, sCVE = symptomatic CSF HIV escape, TAM = thymidine analog mutation, TDF = tenofovir disoproxyl fumarate, VL = viral load.
How to cite this article: Dravid AN, Gawali R, Betha TP, Sharma AK, Medisetty M, Natrajan K, Kulkarni MM, Saraf CK, Mahajan US, Kore SD, Rathod NM, Mahajan US, Letendre SL, Wadia RS, Calcagno A. Two treatment strategies for management of Neurosymptomatic CSF HIV escape in Pune, India. Medicine. 2020;99:24(e20516).
The current study did not receive any funding from any agency.
The authors have no funding and no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Canestri A, Lescure FX, Jaureguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis 2010;50:773–8. [DOI] [PubMed] [Google Scholar]
- [2].Peluso MJ, Ferretti F, Peterson J, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS 2012;26:1765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rawson T, Muir D, Mackie NE, et al. Factors associated with cerebrospinal fluid HIV RNA in HIV infected subjects undergoing lumbar puncture examination in a clinical setting. J Infect 2012;65:239–45. [DOI] [PubMed] [Google Scholar]
- [4].Nightingale S, Geretti AM, Beloukas A, et al. Discordant CSF/plasma HIV-1 RNA in patients with unexplained low-level viremia. J Neurovirol 2016;22:852–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mukerji S, Misra V, Lorenz D, et al. Temporal patterns and drug resistance in CSF viral escape among ART-experienced HIV-1 infected adults. J Acquir Immune Defic Syndr 2017;75:246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pérez-Valero I, Ellis R, Heaton R, et al. Cerebrospinal fluid viral escape in aviremic HIV-infected patients receiving antiretroviral therapy: prevalence, risk factors and neurocognitive effects. AIDS 2019;33:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wendell KA, McArthur JC. Acute meningoencephalitis in chronic human immunodeficiency virus (HIV) infection: putative central nervous system escape of HIV replication. Clin Infect Dis 2003;37:1107–11. [DOI] [PubMed] [Google Scholar]
- [8].Garvey LJ, Everitt A, Winston A, et al. Detectable cerebrospinal fluid HIV RNA with associated neurological deficits, despite suppression of HIV replication in the plasma compartment. AIDS 2009;23:1443–4. [DOI] [PubMed] [Google Scholar]
- [9].Bingham R, Ahmed N, Rangi P, et al. HIV encephalitis despite suppressed viremia: a case of compartmentalized viral escape. Int J STD AIDS 2011;22:608–9. [DOI] [PubMed] [Google Scholar]
- [10].Bogoch II, Davis BT, Venna N. Reversible dementia in a patient with central nervous system escape of human immunodeficiency virus. J Infect 2011;63:236–9. [DOI] [PubMed] [Google Scholar]
- [11].Khoury MN, Tan CS, Peaslee M, et al. CSF viral escape in a patient with HIV-associated neurocognitive disorder. J Neurovirol 2013;19:402–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tamarit Mdel P, Quereda C, Gonzalez-Rozas M, et al. HIV type 1 viral encephalitis after development of viral resistance to plasma suppressive antiretroviral therapy. AIDS Res Hum Retrovir 2012;28:83–6. [DOI] [PubMed] [Google Scholar]
- [13].Imaz A, Cayuela N, Niubo J, et al. Short communication: focal encephalitis related to viral escape and resistance emergence in cerebrospinal fluid in a patient on lopinavir/ritonavir monotherapy with plasma HIV-1 RNA suppression. AIDS Res Hum Retrovir 2014;30:984–7. [DOI] [PubMed] [Google Scholar]
- [14].Bierhoff M, Boucher CA, Fibriani A, et al. Ongoing HIV replication in cerebrospinal fluid under successful monotherapy. Antivir Ther 2013;18:641–3. [DOI] [PubMed] [Google Scholar]
- [15].Mangioni D, Muscatello A, Sabbatini F, et al. A case of cerebrospinal fluid viral escape on a dual antiretroviral regimen: worth the risk? Clin Infect Dis Off Publ Infect Dis Soc Am 2014;59:1655–6. [DOI] [PubMed] [Google Scholar]
- [16].Ssebambulidde K, Segawa I, Laker E, et al. Symptomatic cerebrospinal fluid HIV- 1 escape in two patients on second - line antiretroviral therapy in Uganda. Oxf Med Case Reports 2019;2019:omy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Patel AK, Patel KK, Gohel S, et al. Incidence of symptomatic CSF viral escape in HIV infected patients receiving atazanavir/ritonavir (ATV/r)-containing ART: a tertiary care cohort in western India. J Neurovirol 2018;4:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kulkarni V, Kulkarni R, Parchure R. Neurosymptomatic cerebrospinal fluid escape in HIV-2: a case report. Int J STD AIDS 2018;29:726–8. [DOI] [PubMed] [Google Scholar]
- [19].Dravid AN, Natrajan K, Kulkarni MM, et al. Discordant CSF/plasma HIV-1 RNA in individuals on virologically suppressive antiretroviral therapy in Western India. Medicine 2018;97:e9969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Manesh A, Barnabas R, Mani S, et al. Symptomatic HIV CNS viral escape among patients on effective cART. Int J Infect Dis 2019;84:39–43. [DOI] [PubMed] [Google Scholar]
- [21].Ellis RJ, Badiee J, Vaida F, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS 2011;25:1747–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].McArthur JC, Steiner J, Sacktor N, et al. Human immunodeficiency virus-associated neurocognitive disorders: mind the gap. Ann Neurol 2010;67:699–714. [DOI] [PubMed] [Google Scholar]
- [23].Ferretti F, Gisslen M, Cinque P, et al. Fluid HIV escape from antiretroviral therapy. Curr HIV/AIDS Rep 2015;12:280–8. [DOI] [PubMed] [Google Scholar]
- [24].Letendre S, MD Central nervous system complications in HIV Disease: HIV-associated neurocognitive disorder. Top Antivir Med 2011;19:137–42. [PMC free article] [PubMed] [Google Scholar]
- [25].Nightingale S, Winston A, Letendre S, et al. Controversies in HIV-associated neurocognitive disorders Lancet Neurology NIH Public Access. XXX 2014;13:1139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Edén A, Marcotte TD, Heaton RK, et al. Increased intrathecal immune activation in virally suppressed HIV-1 infected patients with neurocognitive impairment. PLoS One 2016;11:e0157160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].National AIDS control organization (NACO). National Technical guidelines on Antiretroviral therapy_ October 2018. Available at: http://naco.gov.in/sites/default/files/NACO%20-%20National%20Technical%20Guidelines%20on%20ART_October%202018%20%281%29.pdf. [Google Scholar]
- [28].Mukerji SS, Misra V, Lorenz DR, et al. Impact of antiretroviral regimens on CSF viral escape in a prospective multicohort study of ART experienced HIV-1 infected adults in the United States. Clin Infect Dis 2018;67:1182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Deeks ED. Darunavir: a review of its use in the management of HIV-1 infection. Drugs 2014;74:99–125. [DOI] [PubMed] [Google Scholar]
- [30].McCormack PL. Dolutegravir: a review of its use in the management of HIV-1 infection in adolescents and adults. Drugs 2014;74:1241–52. [DOI] [PubMed] [Google Scholar]
- [31].Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 2008;65:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA 2010;304:321–33. [DOI] [PubMed] [Google Scholar]
- [33].Letendre S, Ellis RJ, Deutsch R, et al. 17th Conference on Retroviruses and Opportunistic Infections. San Fransisco, CA, USA: 2010. Correlates of time-to-loss-of-viral-response in CSF and plasma in the CHARTER cohort.; p. 430. [Google Scholar]
- [34].National AIDS control organization (NACO): Policy and Guidelines. Available at: http://naco.gov.in/documents/policy-guidelines. [Google Scholar]
- [35].World Health Organization (WHO): Guidelines and policy briefs on HIV. Available at: http://www.who.int/hiv/pub/guidelines/en/. [Google Scholar]
- [36].National AIDS control organization. National operational guidelines for viral load testing. March 2018. Available at: http://naco.gov.in/sites/default/files/National%20Operational%20Guidelines%20for%20Viral%20Load%20Testing%20Mar%2718.pdf. [Google Scholar]
- [37].Best BM, Letendre SL, Brigid E, et al. Low atazanavir concentrations in cerebrospinal fluid. AIDS 2009;23:83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Calcagno A, Simiele M, Alberione MC, et al. Cerebrospinal fluid inhibitory quotients of antiretroviral drugs in HIV infected patients are associated with compartmental viral control. Clin Infect Dis 2015;60:311–7. [DOI] [PubMed] [Google Scholar]
- [39].Joseph J, Cinque P, Colosi D, et al. Highlights of the Global HIV-1 CSF escape consortium meeting, 9 June 2016, Bethesda, MD, USA. J virus Erad 2016;2:243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lewin SR, Mellors JW. HIV persistence in the CNS: the final frontier for a cure? J Virus Erad 2016;2:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hakim JG, Thompson J, Kityo C, et al. Lopinavir plus nucleoside reverse-transcriptase inhibitors, lopinavir plus raltegravir, or lopinavir monotherapy for second-line treatment of HIV (EARNEST): 144-week follow-up results from a randomised controlled trial. Lancet Infect Dis 2018;18:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gutmann C, Cusini A, Gunthard HF, et al. Randomized controlled study demonstrating failure of LPV/r monotherapy in HIV: the role of compartment and CD4-nadir. AIDS 2010;24:2347–54. [DOI] [PubMed] [Google Scholar]
- [43].Arenas-Pinto A, Stöhr W, Clarke A, et al. Evaluation of cerebrospinal fluid virological escape in patients on long-term protease inhibitor monotherapy. Antivir Ther 2017;22:535–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lupia T, Milia MG, Atzori C, et al. Presence of EBV DNA in cerebrospinal fluid is associated with greater HIV RNA and inflammation. AIDS 2019;34:373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lin JC, Zhang ZX, Smith MC, et al. Anti-human immunodeficiency virus agent 3′-azido-3′-deoxythymidine inhibits replication of Epstein-Barr virus. Antimicrob Agents Chemother 1988;32:265–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mind Exchange Working Group. Assessment, diagnosis, and treatment of HIV-associated neurocognitive disorder: a consensus report of the mind exchange program. Clinical Infectious Diseases 2013;56:1004–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].European AIDS Clinical Society. [July 3, 2014] Guidelines version 7.02. Available at: http://www.eacsociety.org/Portals/0/140601_EACEN7.02.pdf. [Google Scholar]
- [48].Zan VD, Calcagno A, Trunfio M, et al. Clinical and Laboratory Characterization of Neurosymptomatic Cerebrospinal fluid (CSF) Viral escape in a Large Multicentre survey. Presented as a poster at European AIDS Clinical society (EACS) conference 2017, Milan, Italy. [Google Scholar]
- [49].Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007;69:1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Savard J, Laberge B, Gauthier JG, et al. Screening clinical depression in HIV-seropositive patients using the Hospital Anxiety and Depression Scale. AIDS Behav 1999;3:167–75. [Google Scholar]


