Abstract
Purpose:
To demonstrate temporal lobe necrosis (TLN) rate and clinical/dose-volume factors associated with TLN in radiation-naïve patients with head and neck cancer treated with proton therapy where the field of radiation involved the skull base.
Materials and Methods:
Medical records and dosimetric data for radiation-naïve patients with head and neck cancer receiving proton therapy to the skull base were retrospectively reviewed. Patients with <3 months of follow-up, receiving <45 GyRBE or nonconventional fractionation, and/or no follow-up magnetic resonance imaging (MRI) were excluded. TLN was determined using MRI and graded using Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Clinical (gender, age, comorbidities, concurrent chemotherapy, smoking, radiation techniques) and dose-volume parameters were analyzed for TLN correlation. The receiver operating characteristic curve and area under the curve (AUC) were performed to determine the cutoff points of significant dose-volume parameters.
Results:
Between 2013 and 2019, 234 patients were included. The median follow-up time was 22.5 months (range = 3.2–69.3). Overall TLN rates of any grade, ≥ grade 2, and ≥ grade 3 were 5.6% (N = 13), 2.1%, and 0.9%, respectively. The estimated 2-year TLN rate was 4.6%, and the 2-year rate of any brain necrosis was 6.8%. The median time to TLN was 20.9 months from proton completion. Absolute volume receiving 40, 50, 60, and 70 GyRBE (absolute volume [aV]); mean and maximum dose received by the temporal lobe; and dose to the 0.5, 1, and 2 cm3 volume receiving the maximum dose (D0.5cm3, D1cm3, and D2cm3, respectively) of the temporal lobe were associated with greater TLN risk while clinical parameters showed no correlation. Among volume parameters, aV50 gave maximum AUC (0.921), and D2cm3 gave the highest AUC (0.935) among dose parameters. The 11-cm3 cutoff value for aV50 and 62 GyRBE for D2cm3 showed maximum specificity and sensitivity.
Conclusion:
The estimated 2-year TLN rate was 4.6% with a low rate of toxicities ≥grade 3; aV50 ≤11 cm3, D2cm3 ≤62 GyRBE and other cutoff values are suggested as constraints in proton therapy planning to minimize the risk of any grade TLN. Patients whose temporal lobe(s) unavoidably receive higher doses than these thresholds should be carefully followed with MRI after proton therapy.
Keywords: temporal lobe necrosis, proton therapy, head and neck cancer, toxicity
Introduction
Proton therapy in patients with head and neck cancer has the benefit of decreasing the total integral dose and the dose to organs at risk (OARs) compared with intensity-modulated radiation therapy (IMRT) [1]. Proton therapy has been shown to improve both clinician-reported and patient-reported toxicities [2]. Furthermore, in select disease sites within the head and neck, proton therapy offers improvement in oncologic outcomes in comparison to historical controls [3–5]. Although proton therapy can decrease the low to medium radiation dose spillage compared with IMRT [6, 7], in the high-dose region, it is similar, if not worse, in terms of tumor conformality. Thus, irradiating to the skull base region poses a substantial concern for toxicities in the adjacent healthy brain and temporal lobes, especially when using non–pencil beam 3-dimensional proton techniques. As a result, radiation-associated temporal lobe necrosis (TLN) can occur and result in devastating outcomes, which can compromise patients' quality of life and cause long-term morbidity. In the photon setting, many clinical factors have been found to be associated with development of TLN, such as age, chemotherapy, diabetes [8–10], total dose, and fractionation size [8, 11]. Multiple studies have suggested dose-volume constraints for the temporal lobes in patients with head and neck cancer receiving IMRT [9, 10, 12–16]. There is a paucity of literature on TLN in patients treated with proton therapy. In this study, we aimed to demonstrate the rate of TLN and identify risk factors and dose-volume parameters associated with TLN in radiation-naïve patients with head and neck cancer treated with proton therapy to the skull base. We report not only the rate of TLN but also the incidence of brain necrosis.
Materials and Methods
Patient Characteristics
This study was an institutional review board–approved (no.16-1648) retrospective analysis of patients with head and neck cancer who were treated with proton therapy to the skull base at our proton facility (Procure, Somerset, New Jersey). Metastatic patients who were irradiated with curative intent and dose to the skull base were eligible. Patients with prior radiation at the head and neck areas, less than 3 months follow-up time, who received less than 45 GyRBE of radiation dose, who received nonconventional fractionation and/or no follow-up magnetic resonance imaging (MRI) were excluded from the study. Primary brain tumors were not included. The electronic medical records and all available dosimetric data for eligible patients were reviewed.
Radiation Therapy
All patients underwent computed tomography (CT) simulation in the supine position with 3-point or 5-point head and neck masks. Positron emission tomography (PET)/CT and magnetic resonance (MR) simulation were co-registered to the CT simulation to facilitate target volume delineation. Gross tumor volume (GTV) was defined as any gross primary or nodal disease detected clinically or radiographically. Clinical target volume (CTV) was defined as GTV plus areas at risk of subclinical microscopic spread in both primary site and neck. In patients who were exclusively treated with proton therapy, CTV was used as a volume for plan evaluation of dose coverage. For those who were irradiated with IMRT followed by proton boost, planning target volume was generated by adding 3 to 5 mm margin to CTV to account for interfraction and intrafraction motion errors. Patients with definitive head and neck cancer received 70 to 76 GyRBE for those with intact tumor while postoperative patients were prescribed 60 to 66 GyRBE to tumor beds depending on pathologic risk factors in keeping with current standard of care. Subclinical areas at high risk, for example, adjacent sites in general head and neck cancers/whole nasopharyngeal area in the nasopharyngeal cases, were given a median dose of 60 GyRBE, while the areas at lower risk (elective nodal volume and perineural invasion coverage) were treated to 45 to 54 GyRBE. All patients received conventional fractionation defined as 1.8 to 2.12 GyRBE per fraction, once daily, 5 days a week. Most patients were treated with an initial irradiation to 45 to 54 GyRBE to the gross volume/tumor beds and areas at risk followed by a cone-down boost if any. We included metastatic patients who were treated to the skull base with curative intent (median dose 70 GyRBE, range 66-76) as their survival was comparable to that of nonmetastatic patients (87.5% vs 90.4%, P value .946), posing them at the same risk of developing TLN. The IMRT and proton dose summation was reported in GyRBE.
Proton Techniques
All patients were treated with proton therapy using Proteus 235 system (Ion Beam Applications, Louvain-la-Neuve, Belgium). Before April 2016, uniform scanning beam (US) using the match field technique was utilized for all patients. Apertures and compensators were custom-made to shape the lateral and distal edges of the target, respectively. After April 2016, patients were treated with either US or pencil beam scanning (PBS), depending on machine availability. The PBS was delivered in 2 ways; single-field uniform dose, with each beam angle delivering a uniform dose covering the whole volume, or multifield optimization, using each beam to collectively cover the volume. Monte Carlo algorithm was used as a PBS calculation model. Setup variations of 3 to 5 mm and range uncertainties of ±3.5% were accounted for in the planning optimization. A relative biological effectiveness (RBE) value of 1.1 was applied in planning and plan evaluation. Daily orthogonal x-ray imaging on a 6 df couch was performed for setup verification. Weekly quality assurance (QA) CT scans were implemented to assess any anatomic change. When significant changes were observed, a QA plan was calculated based on weekly QA CT to ensure dose to the target and the OARs. Any unacceptable dose coverage required adaptive radiotherapy planning.
Follow-up
Patients were evaluated weekly during radiation therapy by radiation oncologists and, if applicable, by a multidisciplinary team including medical oncologists, head and neck surgeons, nurses, and advanced practice providers. Patients were followed clinically and radiographically at approximate intervals of 1 to 3 months after treatment completion, every 3 months up to 2 years, and every 6 to 12 months thereafter. In general, MRI of the head and neck was performed 3 months after the end of radiotherapy then every 6 to 12 months or as clinically indicated.
TLN Evaluation
TLN was defined as radiographic evidence of temporal lobe injury on brain MRI. Some patients had pathologic confirmation. Radiographic TLN was defined as new contrast-enhanced lesions on postcontrast T1-weighted images and/or new T2-weighted signal consistent with edema [17–19] in location(s) corresponding with the prior radiation portals. Cerebral radionecrosis typically demonstrates some contrast enhancement on imaging; however, new edema without enhancement is evidence of radiation injury to the brain and can produce the same clinical issues as enhancing lesions. Therefore, for the purposes of our study we included both enhancing lesions with or without edema and non-enhancing edematous changes as radionecrosis. MR perfusion of the brain and/or PET/CT and/or follow-up MRI were subsequently performed after the diagnosis of TLN and to rule out recurrent tumor or new brain primary sites. TLN was graded with Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 [20] by 3 independent radiation oncologists. Any discordance was resolved by consensus. Time to TLN was calculated from the date of proton completion to the date of first TLN radiographic appearance. All other necrosis in non–temporal lobe regions were described but not further analyzed.
Dosimetric Parameters
Right and left temporal lobes were either prospectively or retrospectively contoured in patient's radiation plans. The incidence of brain necrosis and its dose-dependent relationship were well demonstrated in various studies using conventional radiation [8, 21]. However, necrosis can be seen in the brain area receiving a dose as moderate as 50 Gy [21]. For this study, absolute volume (aV) of the temporal lobe receiving 40, 50, 60, 70 GyRBE (aV40, aV50, aV60, aV70, respectively, in cm3); dose to the 0.5 cm3, 1 cm3, and 2 cm3 volume receiving the maximum dose (D0.5cm3, D1cm3, and D2cm3, respectively, in GyRBE); maximum dose received by the temporal lobe (Dmax in GyRBE); and mean dose received by the temporal lobe (Dmean in GyRBE) were investigated. When the tumor involved the temporal lobe (N = 1 patient), dose-volume parameters of a virtual organ of the temporal lobe subtracted by GTV was evaluated instead of the actual temporal lobe.
Both D2cm3 and Dmax were comprehensively documented during the planning process in 84.2% of patients. Other dose-volume parameters, not recorded at time points before the study, were subsequently extracted from the treatment planning system after proton therapy was completed. In summary, the data of 197 patients (394 temporal lobes) was available for D2cm3 and Dmax, and the data of 147 patients (294 temporal lobes) was available for other dose-volume parameters.
Statistical Analysis
Patient characteristics between patients with and without TLN were compared using the χ2 test (or Fisher exact test if indicated) for categorical data. All continuous data were tested for the normality and compared using the Mann-Whitney U test for the median(s). Logistic regression analysis was performed to identify predictive clinical parameters for TLN. For dose-volume parameters, right and left temporal lobes in each patient were considered separately. Generalized estimating equation, which adjusted for intrasubject repeated measures (right and left temporal lobes), was used for determining dose-volume variables and TLN correlation in the univariate analysis (UVA). Any factor with a P value less than .2 in the UVA was entered into the multivariate analysis (MVA). The receiver operating characteristic (ROC) curve and area under the curve (AUC) were used to identify the predictive performance of any statistically significant dose-volume parameters. Youden's index was performed to determine the cutoff points of each significant dose-volume predictors that gave maximum sensitivity and specificity. Time to events was calculated between the date of proton completion to date of target events. Overall TLN rate was reported in crude rate. The estimated actuarial rate was performed using Kaplan-Meier analysis. All tests were 2-sided. A P value less than 0.05 was considered statistically significant. All statistical analysis was performed using IBM SPSS statistics software version 26 (IBM Corporation, New York, USA).
Results
Between September 2013 and August 2019, 270 patients with head and neck cancer were treated with proton therapy to the skull base in the definitive primary setting. After excluding patients with inadequate follow-up time (N = 21), receiving dose <45 GyRBE (N = 8), or receiving nonconventional fractionation (N = 7), 234 patients were included. Twelve patients with metastatic disease who were treated to the skull base with a curative radiation dose (median = 70 GyRBE, range = 66-76) were included. Two-year survival was not statistically different between metastatic and nonmetastatic patients (87.5% vs 90.4%, P value .946). There was no statistical difference in overall survival of patients with and without TLN. The median follow-up time was 22.5 months (range = 3.2-69.3).
Patient characteristics are summarized in Table 1. There was no statistically significant difference in hypertension, diabetes, smoking, and concurrent chemotherapy between the 2 groups. Overall, 86.3% of patients received proton therapy exclusively while 13.7% received IMRT with a median dose of 50 Gy followed by proton boost with a median dose of 20 GyRBE. Also, 35% received PBS while 65% received US. Patients with TLN were mostly T3-4 and had adenoid cystic carcinoma (ACC) histology. The most common primary sites for TLN patients were oral cavity (all were hard palate), nasopharynx, and sinonasal primary. Median prescribed doses were higher in patients with TLN than patients without TLN (proton only: 70 vs 66 GyRBE P value .001; IMRT+proton: 76 vs 70 GyRBE, P value .104), although not reaching statistical significance in the IMRT+proton group. An additional comparison between patients with and without any brain necrosis showed no statistical difference in types of radiation (proton only: 76.5% vs 87.1%; IMRT+proton: 23.5% vs 12.9%; P value .263). In patients treated exclusively with proton, the median prescribed dose was significantly higher in patients with any necrosis than in patients without necrosis (70 vs 66 GyRBE, P value < .001), similar to findings in the TLN groups. In patients treated with IMRT+proton, no statistical differences in median IMRT dose (50 vs 50 Gy, P value .755), proton boost dose (25 vs 20 GyRBE, P value .594), and summation dose (75 vs 70 GyRBE, P value .405) were found between patients with and without any brain necrosis.
Table 1.
Patient characteristics.
|
Patients with no TLN, N = 221 (%a) |
Patients with TLN, N = 13 (%a) |
P
Valueb |
|
| Age, median (range), years | 59 (14-88) | 54 (23-71) | .128 |
| Sex | 1.000 | ||
| Male | 55.20 | 53.80 | |
| Female | 44.80 | 46.20 | |
| Primary sites | <.001 | ||
| Major salivary glands | 35.30 | 0 | |
| Sinonasal | 31.20 | 23.10 | |
| Nasopharynx | 13.10 | 23.10 | |
| Ears and skin | 10.00 | 15.40 | |
| Other minor salivary glandsc | 1.80 | 0 | |
| Oral cavity | 4.10 | 38.50 | |
| Others | 4.50 | 0 | |
| Histology | .010 | ||
| SCC | 32.60 | 46.20 | |
| ACC | 19.90 | 53.80 | |
| Sarcoma | 5.00 | 0 | |
| Esthesioneuroblastoma | 2.30 | 0 | |
| Others | 40.30 | 0 | |
| T stage | .153 | ||
| T1-T2 | 42.50 | 23.10 | |
| T3-T4 | 50.20 | 76.90 | |
| Txd | 7.20 | 0 | |
| N stage | .414 | ||
| N0-N1 | 74.70 | 76.90 | |
| N2-N3 | 15.40 | 23.10 | |
| Nx | 10.00 | ||
| Comorbidities | |||
| Hypertension | 34.80 | 38.50 | .773 |
| Diabetes | 9.50 | 23.10 | .136 |
| Smoking | .780 | ||
| Yes | 43.90 | 38.50 | |
| No | 56.10 | 61.50 | |
| Concurrent chemotherapy | .252 | ||
| Yes | 43.00 | 61.50 | |
| No | 57.00 | 38.50 | |
| Type of radiation | .694 | ||
| Proton only | 86.40 | 84.60 | |
| IMRT + proton boost | 13.60 | 15.40 | |
| Proton technique | .773 | ||
| US | 65.20 | 61.50 | |
| PBS | 34.80 | 38.50 | |
| Radiation dose, median (range) | |||
| Proton only, GyRBE | 66 (45-77) | 70 (66-76) | .001 |
| Proton boost, GyRBE | 20 (10-48.8) | 26 | .145 |
| IMRT + proton boost, GyRBE | 70 (60-76) | 76 | .104 |
| Median dose per fraction, GyRBE | 2 (1.8-2.12) | 2 (2.0-2.12) | .518 |
| Temporal lobe dose-volume parameters, median (IQR)e | |||
| aV40 | 1.9 (0-8.7) | 20.0 (11.3-31.6) | <.001 |
| aV50 | 0.6 (0-5.0) | 16.3 (8.4-24.3) | <.001 |
| aV60 | 0 (0-1.3) | 7.3 (4.8-17.4) | <.001 |
| aV70 | 0 (0) | 1.8 (0.5-11.2) | <.001 |
| Dmean | 7.3 (0.4-16.7) | 24.5 (12.6-33.9) | <.001 |
| Dmax | 54.9 (15.8-68.6) | 76.0 (70.6-80.8) | <.001 |
| D0.5cm3 | 50.9 (8.3-63.2) | 74.9 (71.1-79.7) | <.001 |
| D1cm3 | 46.3 (5.6-61.0) | 74.0 (70.3-79.2) | <.001 |
| D2cm3 | 33.5 (1.8-55.9) | 70.0 (65.0-78.1) | <.001 |
Abbreviations: TLN, temporal lobe necrosis; SCC, squamous cell carcinoma; ACC, adenoid cystic carcinoma; IMRT, intensity-modulated radiation therapy; US, uniform scanning; PBS, pencil-beam scanning; IQR, interquartile range; aV40, aV50, aV60, and aV70, absolute volume of the temporal lobe receiving 40, 50, 60, 70 GyRBE, respectively, in cm3; D0.5cm3, D1cm3, and D2cm3, dose to the 0.5 cm3, 1 cm3, and 2 cm3 volume, respectively, receiving the maximum dose in GyRBE; Dmean, mean dose in GyRBE; Dmax, maximum dose in GyRBE.
Percentages are rounded to 1 decimal place.
P value <.05 was considered statistically significant.
Other minor salivary glands included primary sites of lacrimal duct, orbit, and parapharyngeal space.
Tx consisted of unknown primary cancers, schwannoma, paraganglioma and chordoma.
Left and right temporal lobes were considered separately.
Overall, there were 21 sites of brain necrosis (13 in the temporal lobes and 8 in other brain parts) in 17 patients. Of the 13 patients who developed TLN, 1 patient had synchronous grade 2 cerebellar necrosis. Radiographic findings of TLN showed new contrast-enhanced lesions in 12 patients and non-enhancing edema in 1 grade 1 patient. The other 4 patients developed frontal lobe necrosis: 2 patients with grade 1 (1 with bilateral necrosis) and 2 patients with bilateral grade 2. Overall, TLN rates of any grade, ≥grade 2, and ≥grade 3 were 5.6% (N = 13), 2.1% (N = 5), and 0.9% (N = 2), respectively. The estimated 2-year actuarial TLN rate was 4.6%, and the 2-year rate of any brain necrosis was 6.8%. All grade 1 patients had radiographic TLN without clinical symptoms. Of 3 grade 2 patients, 2 patients had radiographic TLN before their clinical presentation of nausea and intermittent headache, respectively. The other grade-2 patient presented with confusion preceding her radiographic TLN. One patient developed grade 3 TLN at 14.4 months with cognitive impairment and hemiparesis. Brain MRI showed a right temporal lobe mass requiring craniotomy and removal of the mass. Histopathology tests confirmed the diagnosis of TLN. At 30.7 months, another patient presented with seizure at an outside hospital. Brain MRI showed a right temporal lobe mass with uncal herniation resulting in an urgent craniotomy with resection of the mass. The pathology report later confirmed brain necrosis. Due to a life-threatening condition and urgent procedure, this patient was scored grade 4. The median time to TLN was 20.9 months (range = 4.4-47, interquartile range = 14.1-32.7). The median time to any brain necrosis was 20.3 months (range = 4.4-47, interquartile range = 14.1-32.7). Findings for all patients with TLN are summarized in Table 2. All dose-volume parameters in the TLN group were statistically significantly higher than in those without TLN as shown in Table 1.
Table 2.
Summary of characteristics of patients with temporal lobe necrosis.
|
Case |
Laterality |
CTCAE v5.0 grade |
Time to TLN (months) |
aV50 (cm3) |
D2cm3 (GyRBE) |
Types of RT |
Proton technique |
| 1 | Left | 1 | 25.1 | 14.4 | 61.8 | Proton | US |
| 2 | Right | 1 | 38.5 | 18.0 | 68.2 | Proton | US |
| 3 | Left | 1 | 34.8 | 22.0 | 75.2 | Proton | PBS |
| 4 | Right | 1 | 4.4 | 11.0 | 69.8 | Proton | PBS |
| 5a | Right | 1 | 20.9 | 28.2 | 78.0 | Proton | US |
| 6 | Right | 1 | 13.7 | 12.6 | 69.4 | Proton | PBS |
| 7 | Left | 1 | 27.6 | 0.8 | 61.2 | Proton | US |
| 8 | Left | 1 | 46.0 | 4.1 | 54.2 | Proton | US |
| 9 | Right | 2 | 14.5 | 12.9 | 77.6 | IMRT+proton | US |
| 10 | Right | 2 | 20.3 | 50.7 | 83.7 | Proton | PBS |
| 11 | Left | 2 | 12.4 | 5.7 | 70.0 | Proton | PBS |
| 12 | Right | 3 | 14.4 | 16.3 | 78.3 | IMRT+proton | US |
| 13 | Right | 4 | 30.7 | 26.5 | 79.2 | Proton | US |
Abbreviations: CTCAE, Common Terminology Criteria for Adverse events; TLN, temporal lobe necrosis; aV50, absolute volume of the temporal lobe receiving 50 GyRBE; D2cm3, dose to the 2 cm3 volume receiving the maximum dose; RT, radiation; IMRT, intensity-modulated radiation therapy; US, uniform scanning; PBS, pencil-beam scanning.
Patient with synchronous cerebellar necrosis.
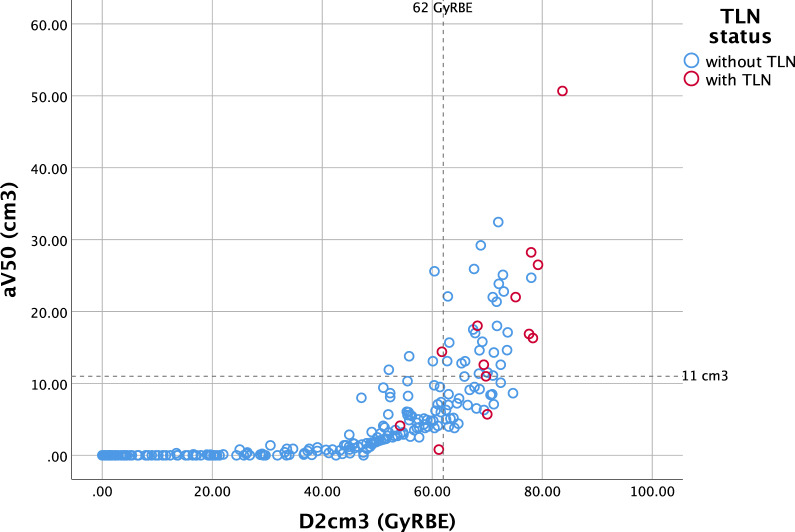
The UVA showed that higher values of all dose-volume parameters were associated with greater TLN incidence. All clinical parameters (gender, age, comorbidities, concurrent chemotherapy, and smoking) as well as proton techniques (PBS vs US) and types of RT showed no correlation with TLN (Table 3) and with any brain necrosis in an additional analysis. To prevent multicollinearity, 1 temporal lobe dose-volume covariate was entered at a time in the MVA. Higher dose in D0.5cm3, D1cm3, D2cm3, Dmean, Dmax, and higher volume of aV40, aV50, aV60, and aV70 were identified as risk factors for TLN in the MVA. Diabetes and concurrent chemotherapy were found in higher proportion of patients with TLN than in patients without TLN; however, there was no statistical significance in the UVA and MVA. In the ROC analysis, all dose-volume parameters gave high AUC values as shown in Table 4. Among volume parameters, aV50 gave maximum AUC (0.921), and D2cm3 gave the highest AUC (0.935) among dose parameters indicating the maximum predictive power for TLN. The cutoff values for all temporal lobe covariates that gave maximum Youden's Index results are shown in Table 4. The 11-cm3 cutoff for aV50 showed 83.3% sensitivity and 89.3% specificity, while the 62-GyRBE cutoff dose for D2cm3 gave 91.7% sensitivity and 82.6% specificity. The dose-volume relationship among aV50, D2cm3, and TLN status is shown in Figure 1. In our population, temporal lobes receiving aV50 >11 cm3 had TLN in 23.1% compared with 1.6% in those with aV50 ≤11 cm3. TLN developed in approximately 14% of temporal lobes receiving D2cm3 >62 GyRBE and in 0.9% of those with D2cm3 ≤62 GyRBE.
Table 3.
Univariate and multivariate analysis for temporal lobe necrosis.
|
Parameter |
Variable |
Univariate analysis |
Multivariate analysis |
||
|
OR (95% CI) |
P
Valuea |
OR (95% CI) |
P
Valuea |
||
| Gender | Male vs female | 0.95 (0.31-2.91) | .924 | ||
| Age | Continuous | 0.98 (0.95-1.01) | .209 | ||
| Concurrent chemotherapy | Yes vs no | 2.12 (0.67-6.69) | .199 | 2.18 (0.72-6.54) | .166 |
| Hypertension | Yes vs no | 1.17 (0.37-3.70) | .791 | ||
| Diabetes | Yes vs no | 2.86 (0.73-11.20) | .132 | 2.91 (0.80-10.61) | .105 |
| Smoking | Yes vs no | 0.80 (0.25-2.52) | .702 | ||
| Type of RT | IMRT+proton vs proton only | 1.16 (0.24-5.48) | .854 | ||
| Proton technique | PBS vs US | 1.17 (0.37-3.70) | .791 | ||
| aV40 | Continuous | 1.12 (1.08-1.17) | <.001 | 1.13 (1.10-1.18) | <.001 |
| aV50 | Continuous | 1.16 (1.10-1.22) | <.001 | 1.16 (1.10-1.24) | <.001 |
| aV60 | Continuous | 1.20 (1.10-1.31) | <.001 | 1.21 (1.10-1.33) | <.001 |
| aV70 | Continuous | 1.41 (1.20-1.65) | <.001 | 1.44 (1.22-1.70) | <.001 |
| Dmean | Continuous | 1.08 (1.03-1.13) | .001 | 1.08 (1.04-1.13) | .001 |
| Dmax | Continuous | 1.20 (1.06-1.34) | .003 | 1.21 (1.06-1.37) | .005 |
| D0.5cm3 | Continuous | 1.22 (1.06-1.40) | .006 | 1.24 (1.06-1.44) | .006 |
| D1cm3 | Continuous | 1.22 (1.07-1.39) | .003 | 1.25 (1.07-1.44) | .004 |
| D2cm3 | Continuous | 1.22 (1.10-1.36) | <.001 | 1.25 (1.10-1.42) | <.001 |
Abbreviations: OR, odds ratio; CI, confidence interval, RT, radiation; IMRT, intensity-modulated radiation therapy, PBS, pencil-beam scanning; US, uniform scanning; aV40, aV50, aV60, and aV70, absolute volume of the temporal lobe receiving 40, 50, 60, 70 GyRBE, respectively, in cm3; Dmean, mean dose in GyRBE; Dmax, maximum dose in GyRBE; D0.5cm3, D1cm3, and D2cm3, dose to the 0.5 cm3, 1 cm3, and 2 cm3 volume, respectively, receiving the maximum dose in GyRBE.
P value < .05 was considered statistically significant.
Table 4.
AUC of each dose-volume parameter.
|
Parameter |
AUC |
SE |
P
valuea |
95% CI |
Cutoff values |
Sensitivity, % |
Specificity, % |
|
|
Lower |
Upper |
|||||||
| aV40 | 0.904 | 0.030 | <.001 | 0.845 | 0.964 | 14.2 | 83.3 | 85.8 |
| aV50 | 0.921 | 0.026 | <.001 | 0.870 | 0.972 | 11.0 | 83.3 | 89.3 |
| aV60 | 0.896 | 0.056 | <.001 | 0.787 | 1.000 | 3.7 | 91.7 | 86.1 |
| aV70 | 0.868 | 0.068 | <.001 | 0.735 | 1.000 | 0.9 | 83.3 | 90.7 |
| Dmean | 0.857 | 0.040 | <.001 | 0.778 | 0.936 | 11.0 | 100 | 62.3 |
| Dmax | 0.888 | 0.041 | <.001 | 0.808 | 0.967 | 72.3 | 83.3 | 83.6 |
| D0.5cm3 | 0.913 | 0.036 | <.001 | 0.843 | 0.984 | 70.8 | 83.3 | 89.3 |
| D1cm3 | 0.923 | 0.031 | <.001 | 0.861 | 0.984 | 70.0 | 83.3 | 90.7 |
| D2cm3 | 0.935 | 0.027 | <.001 | 0.882 | 0.987 | 62.0 | 91.7 | 82.6 |
Abbreviations: AUC, area under the curve; SE, standard error; CI, confidence interval; aV40, aV50, aV60, and aV70, absolute volume of the temporal lobe receiving 40, 50, 60, 70 GyRBE, respectively, in cm3; Dmean, mean dose in GyRBE; Dmax, maximum dose in GyRBE; D0.5cm3, D1cm3, and D2cm3, dose to the 0.5 cm3, 1 cm3, and 2 cm3 volume, respectively, receiving the maximum dose in GyRBE.
P value < .05 was considered statistically significant.
Figure 1.
Demonstrated temporal lobe necrosis (TLN) and its relationship to aV50 (an absolute volume of the temporal lobe receiving 50 GyRBE), and D2cm3 (dose to the 2 cm3 volume receiving the maximum dose). The dash lines demonstrate the cutoff values of each parameter.
Discussion
With a median follow-up time of 22.5 months, the observed rate of any TLN in this study was 5.6%, with an estimated 2-year actuarial rate of 4.6%. This finding is similar to the rate reported by Santoni et al at the same follow-up period in which the TLN rate was 7.6% [22]. For other proton studies with approximately 3-year follow-up time, overall TLN rates ranged from 12.4% to 17.1% [23–25]. In IMRT studies, researchers reported crude TLN rates of 3.48% to 8.1% at 3 to 4 years [16, 26, 27], 7.5% to 13.2% at 5 years [10, 12], and 8.5% to 15% at >5 years of follow-up time [14, 28]. In comparison, the rate of TLN was more frequent in patients receiving proton therapy than IMRT at the same follow-up time. The lack of conformality at the high-dose region with older proton techniques versus IMRT may account for the higher rates of TLN. Secondly, another plausible explanation was different dose prescription. Proton dosing prescription tended to be higher than IMRT (median prescribed dose 66-70.2 Gy in IMRT [10, 13–16, 26, 27, 29], 65-75.6 GyRBE in proton [22–25, 30]). Moreover, the total dose delivered with proton may in fact be higher than anticipated if the RBE is higher than 1.1 at the distal edge. Thirdly, the increasing availability of MRI as a follow-up modality facilitated early detection of radiographic changes, hence, as expected, increasing the overall TLN rate in modern studies.
Most patients who developed brain necrosis in our study had grade 1 disease. Three studies also reported higher rates of grade 1-2 than grade 3-4 of proton-associated TLN [23–25]. For grade 1 patients, the literature suggests the latent nature with subsequent stability or regression in the majority of lesions after following up [17, 23]. However, 1 of our grade 1 TLN patients showed enlargement of an enhancing temporal lobe lesion 1 year after TLN diagnosis but remained asymptomatic. For grade 2 TLN, 2 of 3 grade 2 patients in our cohort had progression in sizes of enhancing temporal lobe lesions on the follow-up dedicated MRI/MR perfusion of the brain; 1 patient also had new enhancing lesion despite steroid intervention. Therefore, careful evaluation of the temporal lobes is essential in all patients undergoing proton therapy for early TLN detection and for studying TLN evolution. Incidence of grade 3-5 TLN was reportedly low at a rate of 1.9% to 6.8% in IMRT cohorts. Our rate of ≥grade 3 TLN was less than the rate of approximately 6% (4/66 patients) reported by McDonald et al [24]. Note that 10/15 (66.7%) TLN patients in our study received US and 5 (33.3%) received PBS, while all TLN patients in the study by McDonald et al [24] received double scattering, which is effectively identical to US. Both patients who developed grade 3 and 4 TLN in our study were treated with US. Although we could not demonstrate US as a TLN risk factor, this observation emphasizes the possibility that newer proton techniques allowing advanced optimization might result in lower high-dose complications and warrant further exploration.
Our study identified a median time to TLN of 20.9 months, but the onset can occur as early as 4.4 months, as seen in 1 patient with grade 1 TLN. Other proton studies showed similar median time to radiographic TLN of 17 to 24 months with the earliest onset seen at 6 months after proton initiation [25] and 8 to 9 months after proton completion [23, 24]. A study in patients with central nervous system tumor receiving proton therapy reported a median time to TLN at 12 months [31]. The median time to TLN was earlier in proton cohorts (≤24 months) compared with conventional radiation and IMRT with median time to onset of ≥24 months (range = 24-38) [10, 13–16, 28, 29].This early onset could be partly a result of more frequent use of follow-up MRI in recent series as discussed earlier.
Age [9], concurrent chemotherapy [9, 10], and male gender [22] were previously identified as risk factors for TLN but showed no association with TLN in our study. Diabetes and concurrent chemotherapy were found more frequent in TLN patients, although not statistically significant as risk factors. Nevertheless, caution of possible TLN and more stringent dose constraint should be considered when giving these patients proton therapy. There were higher proportions of ACC and T3-T4 stage in the TLN group as the dose used to treat these patients at the skull base in the entire population was escalated compared with other histologies and other T stages. (median dose in GyRBE: ACC vs non-ACC 70 vs 66, P value .094; T3-4 vs others 70 vs 66, P value < .001).
We identified that higher values of all dose-volume parameters were correlated with higher risk of TLN in proton therapy patients. While patients with head and neck cancer inevitably received high radiation dose, minimizing the dose as low as possible to the OARs remains the basic principle. In proton therapy planning, aV50 ≤11 cm3, D2cm3 ≤ 62 GyRBE, and other cutoff values can be applied as strict dose constraints to prevent any grade TLN and can be used to define patients who are at higher risk of developing TLN. All patients whose temporal lobe(s) unavoidably received higher doses than these thresholds should be carefully monitored with MRI after proton therapy. The Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) study did not specify any dose constraints to the brain but stated a risk of brain necrosis of 5% at 5 years when partial brain was irradiated to 72 Gy, and in some cases, at as low as 60 Gy [8]. However, most data were derived from 2-dimensional or 3-dimensional radiation era. Multiple dose-volume parameters for temporal lobes have been suggested in the IMRT literature [9, 10, 12–14, 16] (supplementary table A). Limited numbers of studies have explored the dose-volume relationship associated with TLN in proton therapy [22–25, 30, 32]. Most studies used non–pencil beam 3-dimensional technique as shown in supplementary table B. A study by McDonald et al [24] found a sharply increased risk of TLN when aV60 exceeded 5.5 cm3 or aV70 exceeded 1.7 cm3. It is still unclear whether incorporating the same dose-volume constraints for OARs from photon to proton therapy planning will yield the comparable clinical outcomes. Despite the same endpoints, the cutoff values were different across IMRT and proton studies. In a proton study by McDonald et al [24], aV40 cut point of 16.5 cm3 was reported to predict 15% TLN risk at 3 years, while an IMRT study by Su et al [14], presenting an actual crude TLN rate of 15% at 3 years, used aV40Gy of 5 cm3. The cutoff point for aV40 in our study was 14.175 cm3 to give 83.3% sensitivity and 85.8% specificity. Our result was in accordance with those of McDonald et al [24]. Hence, more proton-specific studies are needed to ensure result homogeneity. We encourage reporting an absolute volume (aV) of temporal lobe receiving radiation dose rather than relative volume allowing variety in temporal lobe contouring and boundary definition.
Despite the small number of toxicities ≥grade 3, any means to avoid these complications should be taken by the treating physicians. Utilization of dose-volume constraints for the temporal lobes and beam angle optimization to avoid RBE uncertainty near the critical organs should be strongly considered. There is still an ongoing debate about the increased linear energy transfer (LET) at the end and distal to the proton Bragg peak, which leads to RBE variation of more than the conventional value of 1.1 [33, 34]. In patients with head and neck cancer involving the skull base, this effect could lead to a substantial increase in biological response if the uncertainty is placed near the adjacent brain tissue [35]. Further, as tumors respond to proton therapy during treatment, the delivered dose to the normal structure may differ from the planned dose due to the uncertainty of beam path as a result of changes in filling of air cavities in the sinuses. Some studies showed the correlation between increased LET and imaging changes of the brain in patients after proton therapy [36, 37]. Thus, incorporating LET distribution to the planning optimization may not be negligible, as historically suggested, but may be important for further investigation.
To our knowledge, this study is the largest analysis reporting TLN in radiation-naïve patients with head and neck cancer treated with proton therapy in conventional fractionation where the radiation field involved the skull base. While our population was heterogeneous, the findings emphasize that every patient treated to the skull base are at risk of TLN, regardless of primary site and histology. Limitations of this study included its retrospective nature, the heterogeneity of primary sites and histology with various treatment approaches, no routine MRI surveillance after 3 months, and relatively short follow-up time.
In conclusion, we report an estimated 2-year TLN rate of 4.6% with a low rate of toxicities ≥grade 3 after proton therapy to the skull base. We propose aV50 ≤11 cm3, D2cm3 ≤62 GyRBE, and other cutoff values as temporal lobe constraints in proton therapy planning to minimize the risk of any grade TLN. All patients whose temporal lobe(s) unavoidably received higher doses than these thresholds should be carefully monitored with MRI after proton therapy.
Supplementary Material
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: The authors have no relevant conflicts of interest to disclose.
Funding: The research is funded by P30 Cancer Center Support Grant (P30 CA008748).
Ethical Approval: All patient data has been collected under internal review board–approved protocol.
References
- 1.van de Water TA, Bijl HP, Schilstra C, Pijls-Johannesma M, Langendijk JA. The potential benefit of radiotherapy with protons in head and neck cancer with respect to normal tissue sparing: a systematic review of literature. Oncologist. 2011;16:366–77. doi: 10.1634/theoncologist.2010-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanchard P, Garden AS, Gunn GB, Rosenthal DI, Morrison WH, Hernandez M, Crutison J, Lee JJ, Ye R, Fuller CD, Mohamed AS, Hutcheson KA, Holliday EB, Thaker NG, Sturgis EM, Kies MS, Zhu XR, Mohan R, Frank SJ. Intensity-modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for patients with oropharynx cancer—a case matched analysis. Radiother Oncol. 2016;120:48–55. doi: 10.1016/j.radonc.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanchard P, Gunn GB, Lin A, Foote RL, Lee NY, Frank SJ. Proton therapy for head and neck cancers. Semin Radiat Oncol. 2018;28:53–63. doi: 10.1016/j.semradonc.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Yu NY, Gamez ME, Hartsell WF, Tsai HK, Laramore GE, Larson GL, Simone CB, II, Rossi C, Katz SR, Buras MR, Golafshar MA, Vargas CE, Patel SH. A. Multi-institutional experience of proton beam therapy for sinonasal tumors. Adv Radiat Oncol. 2019;4:689–98. doi: 10.1016/j.adro.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel SH, Wang Z, Wong WW, Murad MH, Buckey CR, Mohammed K, Alahdab F, Altayar O, Nabhan M, Schild SE, Foote RL. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: a systematic review and meta-analysis. Lancet Oncol. 2014;15:1027–38. doi: 10.1016/S1470-2045(14)70268-2. [DOI] [PubMed] [Google Scholar]
- 6.Lomax AJ, Bortfeld T, Goitein G, Debus J, Dykstra C, Tercier PA, Coucke PA, Mirimanoff RO. A treatment planning inter-comparison of proton and intensity modulated photon radiotherapy. Radiother Oncol. 1999;51:257–71. doi: 10.1016/s0167-8140(99)00036-5. [DOI] [PubMed] [Google Scholar]
- 7.Mock U, Georg D, Bogner J, Auberger T, Potter R. Treatment planning comparison of conventional, 3D conformal, and intensity-modulated photon (IMRT) and proton therapy for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys. 2004;58:147–54. doi: 10.1016/s0360-3016(03)01452-4. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence YR, Li XA, el Naqa I, Hahn CA, Marks LB, Merchant TE, Dicker AP. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76:S20–7. doi: 10.1016/j.ijrobp.2009.02.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee AW, Ng WT, Hung WM, Choi CW, Tung R, Ling YH, Cheng PT, Yau TK, Chang AT, Leung SK, Lee MC, Bentzen SM. Major late toxicities after conformal radiotherapy for nasopharyngeal carcinoma-patient- and treatment-related risk factors. Int J Radiat Oncol Biol Phys. 2009;73:1121–8. doi: 10.1016/j.ijrobp.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 10.Zeng L, Tian YM, Sun XM, Chen CY, Han F, Xiao WW, Deng XW, Lu TX. Late toxicities after intensity-modulated radiotherapy for nasopharyngeal carcinoma: patient and treatment-related risk factors. Br J Cancer. 2014;110:49–54. doi: 10.1038/bjc.2013.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee AW, Foo W, Chappell R, Fowler JF, Sze WM, Poon YF, Law SC, Ng SH, O SK, Tung SY, Lau WH, Ho JH. Effect of time, dose, and fractionation on temporal lobe necrosis following radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1998;40:35–42. doi: 10.1016/s0360-3016(97)00580-4. [DOI] [PubMed] [Google Scholar]
- 12.Huang J, Kong FF, Oei RW, Zhai RP, Hu CS, Ying HM. Dosimetric predictors of temporal lobe injury after intensity-modulated radiotherapy for T4 nasopharyngeal carcinoma: a competing risk study. Radiat Oncol. 2019;14:31. doi: 10.1186/s13014-019-1229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu L, Sheng Y, Zhang G, Li Y, OuYang PY, Ge Y, Xie T, Chang H, Deng X, Wu JQ. Temporal lobe injury patterns following intensity modulated radiotherapy in a large cohort of nasopharyngeal carcinoma patients. Oral Oncol. 2018;85:8–14. doi: 10.1016/j.oraloncology.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Su SF, Huang SM, Han F, Huang Y, Chen CY, Xiao WW, Sun XM, Lu TX. Analysis of dosimetric factors associated with temporal lobe necrosis (TLN) in patients with nasopharyngeal carcinoma (NPC) after intensity modulated radiotherapy. Radiat Oncol. 2013;8:17. doi: 10.1186/1748-717X-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y, Zhou GQ, Qi ZY, Zhang L, Huang SM, Liu LZ, Li L, Lin AH, Ma J. Radiation-induced temporal lobe injury after intensity modulated radiotherapy in nasopharyngeal carcinoma patients: a dose-volume-outcome analysis. BMC Cancer. 2013;13:397. doi: 10.1186/1471-2407-13-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou X, Ou X, Xu T, Wang X, Shen C, Ding J, Hu C. Effect of dosimetric factors on occurrence and volume of temporal lobe necrosis following intensity modulated radiation therapy for nasopharyngeal carcinoma: a case-control study. Int J Radiat Oncol Biol Phys. 2014;90:261–9. doi: 10.1016/j.ijrobp.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 17.Wang YX, King AD, Zhou H, Leung SF, Abrigo J, Chan YL, Hu CW, Yeung DK, Ahuja AT. Evolution of radiation-induced brain injury: MR imaging-based study. Radiology. 2010;254:210–8. doi: 10.1148/radiol.09090428. [DOI] [PubMed] [Google Scholar]
- 18.Shah R, Vattoth S, Jacob R, Manzil FF, O'Malley JP, Borghei P, Patel BN, Cure JK. Radiation necrosis in the brain: imaging features and differentiation from tumor recurrence. Radiographics. 2012;32:1343–59. doi: 10.1148/rg.325125002. [DOI] [PubMed] [Google Scholar]
- 19.LENT SOMA scales for all anatomic sites. Int J Radiat Oncol Biol Phys. 1995;31:1049–91. doi: 10.1016/0360-3016(95)90159-0. [DOI] [PubMed] [Google Scholar]
- 20.Common Terminology Criteria for Adverse Events (CTCAE) v5.0. 2017 https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5.0.xlsx Accessed Dec 30, 2019.
- 21.Emami B, Lyman J, Brown A, Cola L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–22. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 22.Santoni R, Liebsch N, Finkelstein DM, Hug E, Hanssens P, Goitein M, Smith AR, O'Farrell D, Efird JT, Fullerton B, Munzenrider JE. Temporal lobe (TL) damage following surgery and high-dose photon and proton irradiation in 96 patients affected by chordomas and chondrosarcomas of the base of the skull. Int J Radiat Oncol Biol Phys. 1998;41:59–68. doi: 10.1016/s0360-3016(98)00031-5. [DOI] [PubMed] [Google Scholar]
- 23.Engeseth GM, Mohamed AS, Stieb S, Fuller CD, Garden AS, Rosenthal DI, Phan J, Morrison WH, Reddy JP, Brydøy M, Stokkevåg CH, Wu RY, Frank SJ, Gunn GB. Radiation associated brain necrosis following proton therapy for head and neck skull base andiIntracranial tumors. Int J Radiat Oncol Biol Phys. 2019;105:S5–S6. [Google Scholar]
- 24.McDonald MW, Linton OR, Calley CS. Dose-volume relationships associated with temporal lobe radiation necrosis after skull base proton beam therapy. Int J Radiat Oncol Biol Phys. 2015;91:261–7. doi: 10.1016/j.ijrobp.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Miyawaki D, Murakami M, Demizu Y, Sasaki R, Niwa Y, Terashima K, Nishimura H, Hishikawa Y, Sugimura K. Brain injury after proton therapy or carbon ion therapy for head-and-neck cancer and skull base tumors. Int J Radiat Oncol Biol Phys. 2009;75:378–84. doi: 10.1016/j.ijrobp.2008.12.092. [DOI] [PubMed] [Google Scholar]
- 26.Lertbutsayanukul C, Prayongrat A, Kannarunimit D, Chakkabat C, Netsawang B, Kitpanit S. A randomized phase III study between sequential versus simultaneous integrated boost intensity-modulated radiation therapy in nasopharyngeal carcinoma. Strahlenther Onkol. 2018;194:375–85. doi: 10.1007/s00066-017-1251-5. [DOI] [PubMed] [Google Scholar]
- 27.Liang SB, Wang Y, Hu XF, He SS, Yang XL, Liu LZ, Cui CY, Chen Y, Fu LW. Survival and toxicities of IMRT based on the RTOG protocols in patients with nasopharyngeal carcinoma from the endemic regions of China. J Cancer. 2017;8:3718–24. doi: 10.7150/jca.20351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng M, Huang Y, Fan X, Xu P, Lang J, Wang D. Prognostic variables for temporal lobe injury after intensity modulated-radiotherapy of nasopharyngeal carcinoma. Cancer Med. 2018;7:557–64. doi: 10.1002/cam4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bakst RL, Lee N, Pfister DG, Zelefsky MJ, Hunt MA, Kraus DH, Wolden SL. Hypofractionated dose-painting intensity modulated radiation therapy with chemotherapy for nasopharyngeal carcinoma: a prospective trial. Int J Radiat Oncol Biol Phys. 2011;80:148–53. doi: 10.1016/j.ijrobp.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Huo W, Adams JA, Sanford NN, Lam MB, Lu Y, Goldberg SI, Paganetti H, Lu HM, Chan AW. Temporal lobe necrosis after proton for nasopharyngeal carcinoma: predictive factors and clinical RBE estimation. Int J Radiat Oncol Biol Phys. 2017;99(supp):E386. [Google Scholar]
- 31.Harrabi S, Gudden C, Adeberg S, Bougatf N, Haberer T, Rieken S, Debus J, Herfarth K. OC-0514: radiation necrosis after proton beam therapy—when and where does it happen? Radiother Oncol. 2017;123:S271. [Google Scholar]
- 32.Merchant TE, Hua CH, Sabin ND, Ezell SE, Madey MA, Wu S, Khan RB, Indelicato DJ. Necrosis, vasculopathy, and neurological complications after proton therapy for childhood craniopharyngioma: Results From a Prospective Trial and a Photon Cohort Comparison. Int J Radiat Oncol Biol Phys. 2016;96:S120–S1. [Google Scholar]
- 33.Grassberger C, Trofimov A, Lomax A, Paganetti H. Variations in linear energy transfer within clinical proton therapy fields and the potential for biological treatment planning. Int J Radiat Oncol Biol Phys. 2011;80:1559–66. doi: 10.1016/j.ijrobp.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuaron JJ, Chang C, Lovelock M, Higginson DS, Mah D, Cahlon O, Powell S. Exponential increase in relative biological effectiveness along distal edge of a proton Bragg peak as measured by deoxyribonucleic acid double-strand breaks. Int J Radiat Oncol Biol Phys. 2016;95:62–9. doi: 10.1016/j.ijrobp.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grosshans DR, Duman JG, Gaber MW, Sawakuchi G. Particle radiation induced neurotoxicity in the central nervous system. Int J Part Ther. 2018;5:74–83. doi: 10.14338/IJPT-18-00026.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peeler CR, Mirkovic D, Titt U, Blanchard P, Gunther JR, Mahajan A, Mohan R, Grosshans DR. Clinical evidence of variable proton biological effectiveness in pediatric patients treated for ependymoma. Radiother Oncol. 2016;121:395–401. doi: 10.1016/j.radonc.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuemann JP, Giantsoudi D, Niemierko A, Maquilan G, Shih HA, Busse PM, Niyazi M, Paganetti H. Brain Necrosis in adult proton therapy patients. Do necrotic regions have elevated linear energy transfer? Int J Radiat Oncol Biol Phys. 2019;105:S230. doi: 10.1016/j.ijrobp.2020.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.