Abstract
Background
The highest number of COVID-19 cases in Italy have been reported in Lombardy, a region in northern Italy. We aimed to analyse the course of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients with rheumatic and musculoskeletal diseases living in a district of Lombardy with a high prevalence of COVID-19.
Methods
We did a single-centre observational study at the Azienda Socio Sanitaria Territoriale (ASST) Spedali Civili of Brescia, Italy. We collected data from patients with rheumatic and musculoskeletal diseases enrolled in our outpatient clinic to identify confirmed or possible cases of SARS-CoV-2 infection. Data were collected through a survey that was administered via telephone or in the outpatient clinic by rheumatologists. We also did a case–control study of all patients with confirmed COVID-19 pneumonia and rheumatic and musculoskeletal diseases who were admitted to the ASST Spedali Civili of Brescia during the study period. Cases were matched by age, sex, and month of hospital admission to at least two controls admitted to the same hospital for COVID-19 pneumonia during the study period.
Findings
Between Feb 24 and May 1, 2020, we collected data from 1525 patients with rheumatic and musculoskeletal diseases: 117 (8%) presented with symptoms that were compatible with COVID-19. 65 patients had a swab confirmation of SARS-CoV-2 infection, whereas 52 presented with a spectrum of symptoms indicative of COVID-19 but were not swab tested. Patients with confirmed COVID-19 were older than those with suspected COVID-19 (median age 68 [IQR 55–76] years vs 57 [49–67] years; p=0·0010) and more likely to have arterial hypertension (33 [51%] vs 14 [27%] patients; odds ratio [OR] 2·8 [95% CI 1·3–6·1]; p=0·031) and obesity (11 [17%] vs 1 [2%]; OR 11·0 [1·3–83·4]; p=0·0059). We found no differences in rheumatological disease or background therapy between confirmed and suspected COVID-19 cases. 47 (72%) of the 65 patients with confirmed COVID-19 developed pneumonia that required admission to hospital. 12 (10%) deaths occurred among the 117 patients with confirmed or suspected COVID-19 (ten in those with confirmed COVID-19 and two in those with suspected COVID-19). Deceased patients with confirmed COVID-19 were older than survivors (median age 78·8 years [IQR 75·3–81·3] vs 65·5 years [53·3–74·0]; p=0·0002). We observed no differences in sex, comorbidities, or therapies between the deceased patients and survivors. The case–control study comprised 26 patients with rheumatic and musculoskeletal diseases and COVID-19 pneumonia and 62 matched controls. We found no significant differences between cases and controls in duration of COVID-19 symptoms before admission, duration of stay in hospital, or the local chest X-ray scoring system. Glucocorticoids were used for severe respiratory manifestations related to lung involvement in 17 (65%) of 26 cases and tocilizumab in six (23%) of 26; thrombotic events occurred in four (15%) of 26 cases. Four (15%) of 26 cases and six (10%) of 62 controls died during the study period.
Interpretation
In this cohort of patients with rheumatic and musculoskeletal diseases in a geographical region with a high prevalence of COVID-19, a poor outcome from COVID-19 seems to be associated with older age and the presence of comorbidities rather than the type of rheumatic disease or the degree of pharmacological immunosuppression.
Funding
None.
Introduction
The Lombardy region in northern Italy has reported the highest incidence of COVID-19 cases in the country, with more than 90 581 swab-confirmed cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and more than 16 349 deaths as of June 10, 2020.1 Our rheumatology outpatient clinic at the Azienda Socio Sanitaria Territoriale (ASST) Spedali Civili of Brescia follows up patients living in the province of Brescia, and is located in a geographical region where more than 15 100 swab-positive cases of SARS-CoV-2 infection and more than 2600 deaths have been reported during the ongoing COVID-19 pandemic, as of June 10, 2020.1 Given the quick escalation in hospital admissions, the majority of patients with mild symptoms of SARS-CoV-2 infection were not confirmed by swab assays and patients were followed up at home; therefore, the true number of infected patients is likely to be much higher.
As COVID-19 is a novel infectious disease, it is particularly important to assess its clinical characteristics in patients who have rheumatic and musculoskeletal diseases. Poorer outcomes from COVID-19 have been reported in older patients, especially those with comorbidities, such as coronary heart disease or cerebrovascular disease.2 Many patients with rheumatic and musculoskeletal diseases are considered to be at risk of developing serious infections, because of their lowered immunity resulting from underlying conditions and chronic use of glucocorticoids or targeted immune-modulating therapies such as biologics. However, many of the drugs used for treating rheumatic and musculoskeletal diseases, such as hydroxychloroquine and biologics targeting interleukin (IL)-6 and IL-1, are being used in patients with COVID-19, especially in subgroups that have subsequently developed abnormal immune responses, including cytokine storm syndrome.3
Research in context.
Evidence before this study
Data about the disease course of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients with rheumatic diseases are scarce. At the time we undertook this study, no case–control studies were available to assess the prognosis and outcome of SARS-CoV-2 infection in patients with rheumatic diseases compared with non-affected individuals.
Added value of this study
To our knowledge, this is the largest single-centre cohort of COVID-19 cases in patients with rheumatic and musculoskeletal diseases. Moreover, this is the first case–control study to evaluate possible differences in disease course and outcomes of COVID-19 in patients with rheumatic and musculoskeletal diseases compared with the general population. We found that patients with rheumatic and musculoskeletal diseases do not appear to have a milder form of COVID-19 than age-matched and sex-matched controls. Age and comorbidities appear to substantially affect the prognosis of these patients.
Implications of all the available evidence
Having a rheumatic or musculoskeletal disease and receiving immunosuppressive treatment does not seem to be protective against a severe disease course of COVID-19. However, there is no indication that stopping immunosuppressive drugs could prevent COVID-19 or other infections, as patients with rheumatic and musculoskeletal diseases who were on disease-modifying anti-rheumatic drugs (DMARDs) did not have a poorer outcome than controls. In patients with rheumatic and musculoskeletal diseases, older age and comorbidities should be regarded as risk factors for a more severe course of COVID-19.
No data about the prevalence of COVID-19 among patients with rheumatic and musculoskeletal diseases were provided in the initial report of 72 314 cases from the Chinese Center for Disease Control and Prevention.4 Since then, small case series of SARS-CoV-2 infection in patients with rheumatic and musculoskeletal diseases5, 6, 7, 8, 9, 10 have been published and several collaborative databases are collecting reports from patients with rheumatic and musculoskeletal diseases, such as the EULAR COVID-19 database (2149 cases up to June 9) and the COVID-19 Global Rheumatology Alliance (1032 cases up to June 4).11, 12, 13 Based on these limited data, rheumatic and musculoskeletal diseases do not seem to affect the disease course of COVID-19.
We aimed to report the occurrence of SARS-CoV-2 infection in a single tertiary outpatient centre located in a geographical region of northern Italy with a high prevalence of COVID-19, to provide more information about the course of SARS-CoV-2 infection in patients with rheumatic diseases.
Methods
Study design and overview
This observational and case–control study was done at the ASST Spedali Civili of Brescia, a public health-care and research institution in the province of Brescia and one of the largest hospitals in Lombardy, Italy. This study was done according to the principles of the Declaration of Helsinki. The study protocol was approved by the local ethics committee (NP 4080). Patients signed a written informed consent form upon admission to hospital, and specific written informed consent for participation in this study was obtained from patients when possible, in agreement with local regulations for retrospective studies.
Between Feb 24 and May 1, 2020, we collected data from patients scheduled for a visit at our outpatient clinic. From Feb 24, all outpatient visits, except for urgent cases, were cancelled. Data were therefore collected through a survey that was administered by telephone or in the outpatient clinic by rheumatologists who were not directly involved in first-line medical care (figure ). In all patients, the survey (in the form of a pre-established interview) aimed to investigate the occurrence of symptoms possibly related to SARS-CoV-2 infection since the beginning of the COVID-19 pandemic.
Figure.
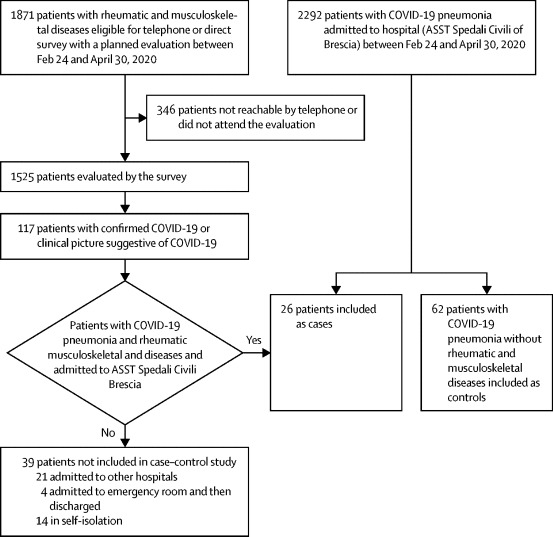
Selection of patients for observational and case–control study
ASST=Azienda Socio Sanitaria Territoriale.
As part of the survey, the presence of the following clinical symptoms was investigated: persistent fever higher than 37·5°C, non-productive cough, rhinorrhoea, fatigue, myalgias, arthralgias, anosmia, ageusia, headache, nausea, vomiting, diarrhoea, and dyspnoea.14 Onset date and duration of these symptoms, contact with the general practitioner, prescribed medications, and management of background medications were also evaluated. Finally, data on employment status, the presence of COVID-19 symptoms or confirmed SARS-CoV-2 infection among family members, and compliance with infection prevention and quarantine measures were also recorded.
The presence of any of the aforementioned clinical symptoms was defined as a “clinical picture suggestive of COVID-19”, especially if patients reported a close contact with a confirmed case (by swab), in accordance with the definition of the European Centre for Disease Prevention and Control (ECDC),15 and after exclusion of other diagnoses explaining the clinical picture. Patients were diagnosed with confirmed COVID-19 if rhinopharyngeal swabs were positive for SARS-CoV-2.
For all reported cases of COVID-19, demographic data as well as data about concomitant disease, ongoing therapy, and comorbidities were obtained from clinical charts. Patients who were admitted to hospital with confirmed COVID-19 and bilateral pneumonitis at chest X-ray were considered to have COVID-19-related pneumonia, with or without substantially decreased peripheral oxygen saturation. The severity of COVID-19 pneumonia was assessed by the Brescia-COVID Respiratory Severity Scale (BCRSS) algorithm,16 a stepwise approach for management of patients with COVID-19 according to clinical severity: a BCRSS score of 3 or greater means that a patient requires high flow oxygen, continuous positive airway pressure, or biphasic positive airway pressure.
The case–control study comprised all patients admitted to the ASST Spedali Civili of Brescia with confirmed COVID-19 pneumonia and rheumatic and musculoskeletal diseases (ie, cases), who were matched by age (± 1 year), sex, and month of hospital admission to at least two controls. Controls were defined as patients who were admitted to the same hospital for COVID-19 pneumonia, but without any rheumatic and musculoskeletal disease. Cases and controls were analysed for differences in disease course, radiological findings, presence of biomarkers of hyperinflammation, and treatments.
Radiographic score
The hospital proposed its own interstitial pneumonia chest X-ray scoring system,17 named the Brixia score, in which the lungs were divided into six zones on frontal chest projection and a score (from 0 to 3) was assigned to each zone on the basis of lung abnormalities, as follows: score 0 for no lung abnormalities; score 1 for interstitial infiltrates; score 2 for interstitial and alveolar infiltrates (interstitial predominance); and score 3 for interstitial and alveolar infiltrates (alveolar predominance). The overall score was between 0 and 18.
Hyperinflammation score
The presence of hyperinflammation was defined as lymphocyte counts lower than 1000 cells per mL, and two among the following three criteria: serum ferritin higher than 500 ng/mL, lactate dehydrogenase higher than 300 U/L, and D-dimers higher than 1000 ng/mL. The cutoffs were the same as those used in an ongoing clinical trial that is exploring the efficacy and safety of emapalumab and anakinra in reducing hyperinflammation in patients with COVID-19 (NCT04324021).
Statistical analysis
Categorical variables were reported as proportions or percentages, whereas continuous variables were expressed as median and IQR values. Quantitative variables were compared by use of the non-parametric Mann-Whitney test. Categorical variables were compared by use of contingency tables and p values were calculated with χ2 or Fisher's exact tests, when appropriate. p values less than 0·05 were considered significant. The effect size for retrospective studies was then evaluated with odds ratios (ORs) with 95% CIs by the use of the Woolf logit method, as indicated by GraphPad Prism program.
Role of the funding source
There was no funding source for this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Results are reported in accordance with STROBE guidelines.18 Between Feb 24 and May 1, 2020, we collected data from 1525 patients enrolled in our outpatient clinic. By May 1, 2020, 117 (8%) confirmed or suspected cases of COVID-19 were reported in this cohort (figure): 65 patients with confirmed COVID-19 and 52 with a clinical picture suggestive of COVID-19 (table 1 ). All patients stopped their background therapies following onset of COVID-19 symptoms. Confirmed cases of COVID-19 were more frequently observed in female patients than in male patients (63% vs 37%). The highest number of cases was reported in patients with rheumatoid arthritis (37%), followed by spondyloarthritis or psoriatic arthritis (15%).
Table 1.
Clinical features of 117 patients with rheumatic and musculoskeletal disease and confirmed or suspected COVID-19
| Patients with confirmed COVID-19 (n=65) | Patients with clinical picture suggestive of COVID-19 (n=52) | OR (95% CI) | p value | |||
|---|---|---|---|---|---|---|
| Age, years | 68 (55–76) | 57 (49–67) | .. | 0·0010 | ||
| Age >65 years | 37 (57%) | 12 (23%) | 4·4 (1·9–9·9) | 0·0003 | ||
| Sex | ||||||
| Male | 24 (37%) | 13 (25%) | .. | 0·23 | ||
| Female | 41 (63%) | 39 (75%) | .. | .. | ||
| Rheumatological diagnosis | ||||||
| Rheumatoid arthritis | 24 (37%) | 13 (25%) | .. | 0·23 | ||
| Spondyloarthritis or psoriatic arthritis | 10 (15%) | 10 (19%) | .. | 0·58 | ||
| SLE (with or without APS) | 5 (8%) | 7 (13%) | .. | 0·55 | ||
| Systemic sclerosis | 4 (6%) | 4 (8%) | .. | 1 | ||
| Adult-onset Still's disease | 3 (5%) | 2 (4%) | .. | 1 | ||
| Giant cell arteritis | 2 (3%) | 2 (4%) | .. | 1 | ||
| Polymyalgia rheumatica | 4 (6%) | 2 (4%) | .. | 0·69 | ||
| Other vasculitis* | 8 (12%) | 3 (6%) | .. | 0·34 | ||
| Other† | 5 (8%) | 9 (17%) | .. | 0·11 | ||
| Rheumatological treatment | ||||||
| Glucocorticoids | 43 (66%) | 26 (50%) | 1·9 (0·9–4·1) | 0·078 | ||
| Weekly dosage glucocorticoids, mg | 35 (21·2–48·7) | 35 (20–49·9) | .. | 0·97 | ||
| Colchicine | 1 (2%) | 1 (2%) | .. | 1 | ||
| Conventional synthetic DMARDs (csDMARDs) | 39 (60%) | 29 (56%) | .. | 0·64 | ||
| Methotrexate | 24 (37%) | 15 (29%) | .. | 0·43 | ||
| Hydroxychloroquine | 10 (15%) | 12 (23%) | .. | 0·34 | ||
| Leflunomide | 3 (5%) | 1 (2%) | .. | 0·62 | ||
| Azathioprine | 2 (3%) | 2 (4%) | .. | 1 | ||
| Cyclosporine | 2 (3%) | 1 (2%) | .. | 0·98 | ||
| Mycophenolate mofetil | 2 (3%) | 1 (2%) | .. | 0·98 | ||
| Sulfasalazine | 1 (2%) | 2 (4%) | .. | 0·58 | ||
| Biological DMARDs | 27 (42%) | 24 (46%) | .. | 0·70 | ||
| Infliximab | 2 (3%) | 2 (4%) | .. | 1 | ||
| Etanercept | 3 (5%) | 2 (4%) | .. | 1 | ||
| Adalimumab | 8 (12%) | 4 (8%) | .. | 0·36 | ||
| Golimumab | 0 | 2 (4%) | .. | 0·19 | ||
| Certolizumab pegol | 2 (3%) | 1 (2%) | .. | 0·98 | ||
| Tocilizumab | 3 (5%) | 3 (6%) | .. | 1 | ||
| Secukinumab | 1 (2%) | 1 (2%) | .. | 1 | ||
| Anakinra | 1 (2%) | 0 | .. | 1 | ||
| Belimumab | 1 (2%) | 0 | .. | 1 | ||
| Abatacept | 3 (5%) | 7 (13%) | .. | 0·11 | ||
| Previous or ongoing rituximab | 4 (6%) | 2 (4%) | .. | 0·62 | ||
| Apremilast | 1 (2%) | 0 | .. | 1 | ||
| Baricitinib or tofacitinib | 0 | 0 | .. | NA | ||
| csDMARD plus bDMARD | 13 (20%) | 10 (19%) | .. | 0·91 | ||
| Comorbidities | ||||||
| Arterial hypertension | 33 (51%) | 14 (27%) | 2·8 (1·3 to 6·1) | 0·031 | ||
| Cardiovascular disease | 8 (12%) | 3 (6%) | .. | 0·23 | ||
| Obesity (BMI >30 kg/m2) | 11 (17%) | 1 (2%) | 11·0 (1·3 to 83·4) | 0·0059 | ||
| Diabetes | 9 (14%) | 3 (6%) | .. | 0·15 | ||
| Lung disease‡ | 7 (11%) | 2 (4%) | .. | 0·30 | ||
| Chronic renal insufficiency or ESRD | 4 (6%) | 0 | .. | 0·13 | ||
| Characteristics related to SARS-CoV-2 infection | ||||||
| Hospital admission for COVID-19 pneumonia | 47 (72%)§ | 0 | 269 (15·8 to >1000) | <0·0001 | ||
| Death | 10 (15%) | 2 (4%) | .. | 0·063 | ||
| Close contact, defined according to ECDC | NA | 8 (15%) | .. | NA | ||
Data are median (IQR) or n (%). SLE=systemic lupus erythematosus. APS=antiphospholipid antibody syndrome. DMARDs=disease-modifying anti-rheumatic drugs. NA=not applicable. BMI=body-mass index. ESRD=end-stage renal disorder. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. ECDC=European Center for Disease Control and Prevention.
Other vasculitis (ie, Behçet disease, polyarteritis nodosa, Takayasu's arteritis, eosinophilic granulomatosis with polyangiitis, cryoglobulinaemia, Birdshot chorioretinopathy, or other vasculitis).
Other (ie, immune-mediated myopathies, Sjögren's syndrome, undifferentiated connective tissue disease, or immunodeficiencies).
Asthma, chronic obstructive pulmonary disease, or interstitial lung disease.
Four patients who were admitted to the emergency room and had a diagnosis of interstitial pneumonia but were not admitted to hospital or were discharged after an overnight stay, were not included.
Patients who were not tested for SARS-CoV-2 infection were treated at home by their general practitioners because they presented with milder symptoms of COVID-19. Confirmed cases of COVID-19 were older than suspected cases (median age 68 years vs 57 years, p=0·001), with a higher rate of comorbidities such as arterial hypertension (51% vs 27% of patients, OR 2·8 [95% CI 1·3–6·1]; p=0·031) and obesity (17% vs 2% of patients, OR 11·0 [95% CI 1·3–83·4]; p=0·0059). We observed no differences in the use of glucocorticoids, conventional synthetic disease-modifying antirheumatic drugs (DMARDs), or biological DMARDs, including hydroxychloroquine, between confirmed and suspected COVID-19 cases. COVID-19 pneumonia requiring admission to hospital occurred in 47 (72%) of the 65 confirmed cases of COVID-19. When comparing confirmed COVID-19 cases that required admission to hospital versus those that were not admitted to hospital, the only significant difference was in age; patients admitted to hospital were older than those who were not (median age 70 years [IQR 60·5–76·0] vs 54 years [47·0–73·8], p=0·036). We found no differences in background therapy or comorbidities (appendix 2).
As of May 1, 12 (10%) of 117 patients with rheumatic and musculoskeletal diseases who were being followed up by our rheumatology unit had died: ten patients had confirmed SARS-CoV-2 infection, whereas two were not tested by swab. Clinical features of the deceased patients are reported in table 2 .
Table 2.
Clinical features of 12 deceased patients with confirmed or suspected COVID-19 and rheumatic and musculoskeletal diseases
| Sex and age | Rheumatic and musculoskeletal disease | Ongoing therapy | Comorbidities | |
|---|---|---|---|---|
| Patient 1 | Male, age 74 years | Immune-mediated myopathy | Oral prednisone 50 mg weekly | Arterial hypertension, Cardiovascular disease, obesity, lung disease |
| Patient 2 | Male, age 80 years | Rheumatoid arthritis | Oral prednisone 50 mg weekly, methotrexate 7·5 mg weekly, etanercept 50 mg weekly | Cardiovascular disease, obesity, lung disease |
| Patient 3 | Female, age 86 years | Psoriatic arthritis | Adalimumab 40mg every other week | Arterial hypertension, obesity |
| Patient 4 | Female, age 76 years | Rheumatoid arthritis | Oral prednisone 17·5 mg weekly, methotrexate 10 mg weekly | Arterial hypertension |
| Patient 5 | Male, age 81 years | Systemic lupus erythematosus | Mycophenolate mofetil 500 mg daily | Arterial hypertension, cardiovascular disease, lung disease |
| Patient 6 | Male, age 75 years | Psoriatic arthritis | Methotrexate 7·5 mg weekly | Arterial hypertension, chronic renal insufficiency |
| Patient 7 | Male, age 73 years | Rheumatoid arthritis | Oral prednisone 35 mg weekly, colchicine 1 mg daily | Obesity, diabetes |
| Patient 8 | Male, age 86 years | Systemic sclerosis | Oral prednisone 35 mg weekly, methotrexate 12·5 mg weekly | Arterial hypertension, cardiovascular disease, lung disease, renal cancer |
| Patient 9 | Female, age 76 years | Psoriatic arthritis | Oral prednisone 35 mg weekly, adalimumab 40 mg every other week | None |
| Patient 10 | Female, age 82 years | Rheumatoid arthritis | Oral prednisone 52·5 mg weekly, hydroxychloroquine 300 mg daily, methotrexate 7·5 mg weekly | Arterial hypertension, cardiovascular disease |
| Patient 11* | Female, age 93 years | Polymyalgia rheumatica | Methotrexate 5 mg weekly | None |
| Patient 12* | Female, age 61 years | Rheumatoid arthritis | Oral prednisone 35 mg weekly | Metastatic breast cancer |
Patients with suspected COVID-19.
When analysing the 65 patients with confirmed SARS-CoV-2 infection, we found no differences in sex, comorbidities, or therapies between the ten deceased and 55 alive patients, although the median age of deceased patients was older than that of alive patients (78·8 years [IQR 75·3–81·3] vs 65·5 years [53·3–74·0]; p=0·0002).
During the study period, 2292 patients with COVID-19 were admitted to our hospital, of whom 26 (1%) had a rheumatic or musculoskeletal disease. Of the 47 patients with a rheumatic or musculoskeletal disease requiring admission to hospital for COVID-19, the other 21 patients were admitted to other hospitals in Brescia and nearby cities. The 26 patients admitted to our hospital were matched with 62 patients admitted to the same hospital for COVID-19 but without a concomitant rheumatic or musculoskeletal disease (table 3 ). The most frequent rheumatological diagnosis was rheumatoid arthritis in nine (35%) of 26 patients, followed by adult-onset Still's disease in three (12%), and polymyalgia rheumatica in three (12%). Background therapy consisted of glucocorticoids in 18 (69%) of 26 patients, conventional synthetic DMARDs in 11 (42%), biological DMARDs in nine (35%), and conventional synthetic plus biological DMARDs in three (12%). When comparing the disease course between cases and controls, we found no significant differences in duration of COVID-19 symptoms before admission, duration of hospital stay, or chest X-ray Brixia score (table 3).
Table 3.
Clinical features of cases and matched controls
| Cases (n=26) | Controls (n=62) | p value | ||
|---|---|---|---|---|
| Age | 69 (59·5–78·2) | 70 (59–78) | 0·92 | |
| Age >65 years | 16 (62%) | 37 (60%) | 1 | |
| Sex | ||||
| Male | 13 (50%) | 30 (48%) | 1 | |
| Female | 13 (50%) | 32 (52%) | .. | |
| Duration of symptoms before hospital admission, days | 7 (5–10) | 7 (8–10) | 0·14 | |
| Chest X-ray Brixia score at admission* | 7 (4–10) | 7 (4–11) | 0·56 | |
| Highest chest X-ray Brixia score† | 12 (9–14) | 11 (7–13) | 0·16 | |
| Chest X-ray Brixia score at discharge‡ | 9 (8–11) | 7 (5–10) | 0·13 | |
| Hyperinflammation score (within 1 week of admission) | 11/23 (48%) | 26/56 (46%) | 0·78 | |
| Lymphopenia (<1000 per mm3) | 21/25 (84%) | 40/61 (66%) | 0·11 | |
| Lowest lymphocyte count | 560 (515–860) | 810 (540–1110) | 0·021 | |
| Elevated ferritin (>500 ug/L) | 16/21 (76%) | 36/54 (67%) | 0·57 | |
| Highest value of ferritin (ug/L) | 789 (478–1561) | 1039 (596–1624) | 0·25 | |
| Lactate dehydrogenase (>300 U/L) | 16/25 (64%) | 37/56 (66%) | 1 | |
| Highest value of lactate dehydrogenase (U/L) | 375 (244–548·5) | 369 (267–476) | 0·87 | |
| D-dimer (>1000 ng/mL) | 8/23 (35%) | 15/55 (27%) | 0·55 | |
| Highest value of D-dimer (ng/mL) | 491 (267–1622) | 530 (314–1039) | 0·84 | |
| Duration of hospital stay, days | 15 (11·5–27·5) | 14 (9–20) | 0·21 | |
| Death | 4 (15%) | 6 (10%) | 0·47 | |
| Discharged from hospital | 21 (81%) | 55 (89%) | 0·32 | |
| Patients still in the hospital as of May 27, 2020 | 1 (4%) | 1 (2%) | 0·50 | |
| Thrombotic events§ | 4 (15%) | 4 (6%) | 0·22 | |
| COVID-19 therapy (described for discharged or deceased patients) | ||||
| Antiviral drugs | 19 (73%) | 55 (89%) | 0·067 | |
| Hydroxychloroquine | 24 (92%) | 58 (94%) | 1 | |
| High-dose glucocorticoids (intravenous dexamethasone or oral prednisone) | 17 (65%) | 30 (48%) | 0·14 | |
| Tocilizumab | 6 (23%) | 11 (18%) | 0·55 | |
| Canakinumab or anakinra | 1 (4%) | 1 (2%) | 0·50 | |
| Oxygen | 25 (96%) | 58 (94%) | 1 | |
| High flow oxygen, continuous positive airway pressure, biphasic positive airway pressure | 16 (62%) | 39 (63%) | 1 | |
| Comorbidities | ||||
| Arterial hypertension | 15 (58%) | 38 (61%) | 0·81 | |
| Cardiovascular disease | 8 (31%) | 13 (21%) | 0·41 | |
| Cancer | 2 (8%) | 7 (11%) | 1 | |
| Obesity (BMI >30 kg/m2) | 4 (15%) | 12 (19%) | 0·76 | |
| Chronic renal insufficiency or ESRD | 2 (8%) | 4 (6%) | 1 | |
| Diabetes | 2 (8%) | 12 (19%) | 0·21 | |
| Lung disease¶ | 5 (19%) | 4 (6%) | 0·11 | |
Data are median (IQR), n (%), or n/N; denominators are provided where data were not available for all 26 cases and 62 controls. Cases are patients with rheumatic and musculoskeletal diseases and COVID-19 pneumonia; controls are patients with COVID-19 pneumonia. BMI=body-mass index. ESRD=end-stage renal disorder.
Chest X-ray was available on admission for 20 cases and 53 controls.
More than 1 chest X-ray was available for 19 cases and 52 controls.
Chest X-ray at discharge was calculated on alive discharged patients (ten cases and 47 controls).
Cases: pulmonary embolism (n=1), ischaemic stroke (n=1), acute coronary syndrome (n=1), and deep venous thrombosis (n=1). Controls: pulmonary embolism (n=3) and ischaemic stroke (n=1).
Asthma, chronic obstructive pulmonary disease, and interstitial lung disease.
The presence of biomarkers of hyperinflammation (lymphocyte count, lactate dehydrogenase, ferritin, and D-dimer concentrations), evaluated within the first 7 days of hospital admission, did not differ significantly between cases and controls. However, we observed a significant difference in the lowest number of lymphocytes, with a more profound lymphopenia in cases than in controls (560 cells per mm3 vs 810 cells per mm3, p=0·021). No cases presented with leukopenia or lymphopenia at the last rheumatological evaluation.
A thrombotic event during hospital admission occurred in four cases (15%) and four controls (6%). Among the four cases, one presented with a venous thrombosis and three had an arterial thrombotic event. None of these patients had a known positivity for antiphospholipid antibodies. No difference in death rate was reported between four (15%) of 26 cases and six (10%) of 62 controls.
During hospital stay, high-dose intravenous or oral glucocorticoids for respiratory disease related to COVID-19 pneumonitis were administered to 17 (65%) of 26 cases: intravenous dexamethasone was started in 13 of 17, whereas oral prednisone was increased or introduced in the other four cases. Additionally, six (23%) of 26 cases were treated with tocilizumab for worsening of respiratory condition: five had a diagnosis of rheumatoid arthritis and one had a diagnosis of polymyalgia rheumatica. As background therapy, two cases were chronically treated with anti-tumour necrosis factor (TNF) therapy, three with methotrexate, and one with low-dose glucocorticoids. Both anti-TNF therapy and methotrexate were suspended during hospital admission.
Hyperinflammation is generally considered as a marker of poor prognosis and as a predictor of cytokine storm syndrome or admission to the intensive care unit (ICU). Among 11 cases who had hyperinflammation and 12 cases who had no hyperinflammation, we found no differences in the duration of hospital admission, chest X-ray Brixia score, or therapies administered during hospital admission.
Discussion
COVID-19 is a novel infectious disease caused by SARS-CoV-2, with a wide-ranging disease course. The impact of SARS-CoV-2 infection on patients with chronic rheumatic disease is currently unclear. Here we have presented data on the largest (to the best of our knowledge) single-centre cohort of patients with confirmed or suspected COVID-19, all of whom were followed up in a rheumatology outpatient unit. The data reported here indicate that COVID-19 in patients with a rheumatic and musculoskeletal disease is not rare in a district with a high dissemination of SARS-CoV-2 infection.
Until now, the largest cohort of patients with COVID-19 and underlying rheumatic disease was reported by the COVID-19 Global Rheumatology Alliance, which recently described 110 patients and then updated their results with data from 600 cases.11, 19 Compared to that cohort, patients in the present study were more likely to be aged older than 65 years, with a higher rate of comorbidities, and with a more severe disease course.
To the best of our knowledge, this is the first case–control study of patients with COVID-19 and rheumatic and musculoskeletal diseases. The findings indicate that patients with rheumatic and musculoskeletal diseases do not appear to have a milder form of COVID-19 pneumonia than controls. In fact, we observed the same rate of hyperinflammation or need for respiratory support, assessed by the BCRSS,16 which required the use of dexamethasone or tocilizumab, or both, in cases and controls.16 In our hospital, the use of dexamethasone or tocilizumab, or both, was based on the severity of respiratory symptoms, in accordance with the BCRSS.16 Tocilizumab was used in cases of acute respiratory failure requiring ventilatory support (BCRSS ≥3) after the absence of improvement with the use of dexamethasone.20
In a recent report of patients with psoriasis treated with biological therapies in Italy, Damiani and colleagues21 reported a higher rate of SARS-CoV-2 infection in patients with psoriasis than in the general population. However, no cases of ICU admission or death occurred in these patients, and the authors concluded that the use of biological therapies could have prevented more severe disease. In our cohort, we were not able to confirm this hypothesis. In fact, we found no differences in background therapies when comparing confirmed COVID-19 cases with suspected cases and when comparing patients who had COVID-19 pneumonia and hyperinflammation with those who did not show laboratory features of hyperinflammation.
These data reinforce the hypothesis that the prognosis of SARS-CoV-2 infection is more likely to be related to the presence of other risk factors rather than the rheumatic and musculoskeletal disease itself or the background therapy. In fact, in our cohort, older age represented the main risk factor for hospital admission and death, in line with what has been reported in the American College of Rheumatology Guidelines.22 In the present study, we also observed an increased frequency of hypertension and obesity in patients with virologically confirmed SARS-CoV-2 infection, and these risk factors were also found to be associated with more severe symptoms of COVID-19.
This study had some limitations. First, full data were not available for all patients with rheumatic diseases admitted to hospital during the study period, therefore the case–control study was done only in patients admitted to a single hospital. This restriction could limit the generalisability of our findings. Second, we were unable to analyse and compare the effect of SARS-CoV-2 infection on individual rheumatic and musculoskeletal diseases because of the small numbers of patients with each condition. Third, we were unable to do a multivariate analysis because of the small study size.
The cases presented in this report have been notified to the CONTROL-19 registry of the Italian Society of Rheumatology,23 as such collaborative efforts will improve our understanding of the effect of SARS-CoV-2 infection in patients with rheumatic and musculoskeletal diseases. Nevertheless, our study provides some additional information: our cohort was from a small geographical area, and consistently treated for both rheumatic conditions and SARS-CoV-2 infection. Moreover we were able to include a control group in our study, which is not feasible for ongoing collaborative registries.
We believe that the findings from this single-centre study, done in a region in northern Italy with a high number of COVID-19 cases, highlight some considerations that could guide the work of rheumatologists in this period of uncertainty, while we wait for conclusive data from international collaborative registries and clinical trials. Based on the current findings, pre-existing systemic autoimmune disease and ongoing immunosuppressive treatment do not appear to represent the most important risk factors for SARS-CoV-2 infection and for its final outcome. Instead, mortality seems to be associated more with older age and the presence of comorbidities rather than the degree of pharmacological immunosuppression.
Acknowledgments
Acknowledgments
No specific grant was required for this research from any funding agency in the public, commercial or not-for-profit sectors. This Article was written on behalf of all the clinicians included in the Brescia Rheumatology COVID-19 Study Group of the Rheumatology and Clinical Immunology Unit who contributed to this work by collecting the data presented here. Moreover, we thank every single health-care worker at the Azienda Socio Sanitaria Territoriale (ASST) Spedali Civili of Brescia during the COVID-19 emergency, especially Marco Trivelli, General Manager, and Camillo Rossi, Medical Director, of ASST Spedali Civili of Brescia, for their support and collaboration in facilitating the prompt start of this study.
Contributors
MF was responsible for the literature search, study design, data collection, data analysis, data interpretation, and writing of the manuscript. IC was responsible for study design, data analysis, data interpretation, writing of the manuscript. LM was responsible for data collection, data analysis, and data interpretation. LA was responsible for data interpretation and writing of the manuscript. FF was responsible for study design, data interpretation, and writing of the manuscript.
Brescia Rheumatology COVID-19 Study Group
Paolo Airò,* Chiara Bazzani,* Francesca Crisafulli,*† Matteo Filippini,* Micol Frassi,* Maria Chiara Gerardi,*† Roberto Gorla,* Maria Grazia Lazzaroni,*† Daniele Lini,*† Cecilia Nalli,* Salvatore Panaro,*† Silvia Piantoni,*† Francesca Regola,*† Marco Taglietti,* Angela Tincani,*† Paola Toniati,* Tamara Vojinovic,* and Stefania Zingarelli*. *Rheumatology and Clinical Immunology Unit, ASST Spedali Civili of Brescia, Brescia, Italy. †Department of Clinical and Experimental Sciences, University of Brescia, Brescia, Italy.
Declaration of interests
We declare no competing interests.
Contributor Information
Brescia Rheumatology COVID-19 Study Group:
Paolo Airò, Chiara Bazzani, Francesca Crisafulli, Matteo Filippini, Micol Frassi, Maria Chiara Gerardi, Roberto Gorla, Maria Grazia Lazzaroni, Daniele Lini, Cecilia Nalli, Salvatore Panaro, Silvia Piantoni, Francesca Regola, Marco Taglietti, Angela Tincani, Paola Toniati, Tamara Vojinovic, and Stefania Zingarelli
Supplementary Materials
References
- 1.EpiCentro—Higher Institute of Health Integrated surveillance COVID-19: the main national data. https://www.epicentro.iss.it/coronavirus/sars-cov-2-sorveglianza-dati
- 2.Chen R, Liang W, Jiang M. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020 doi: 10.1016/j.chest.2020.04.010. published online April 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henderson LA, Canna SW, Schulert GS. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheumatol. 2020 doi: 10.1002/art.41285. published online April 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Monti S, Balduzzi S, Delvino P, Bellis E, Quadrelli VS, Montecucco C. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis. 2020;79:667–668. doi: 10.1136/annrheumdis-2020-217424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Favalli EG, Ingegnoli F, Cimaz R, Caporali R. What is the true incidence of COVID-19 in patients with rheumatic diseases? Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217615. published online April 22. [DOI] [PubMed] [Google Scholar]
- 7.Mathian A, Mahevas M, Rohmer J. Clinical course of coronavirus disease 2019 (COVID-19) in a series of 17 patients with systemic lupus erythematosus under long-term treatment with hydroxychloroquine. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217566. published online April 24. [DOI] [PubMed] [Google Scholar]
- 8.Emmi G, Bettiol A, Mattioli I. SARS-CoV-2 infection among patients with systemic autoimmune diseases. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gartshteyn Y, Askanase AD, Schmidt NM. COVID-19 and systemic lupus erythematosus: a case series. Lancet Rheumatol. 2020 doi: 10.1016/52665-9913(20)30161-2. published online May 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haberman R, Axelrad J, Chen A. Covid-19 in immune-mediated inflammatory diseases-case series from New York. N Engl J Med. 2020 doi: 10.1056/NEJMc2009567. published online April 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gianfrancesco MA, Hyrich KL, Gossec L. Rheumatic disease and COVID-19: initial data from the COVID-19 Global Rheumatology Alliance provider registries. Lancet Rheumatol. 2020;2:e250–e253. doi: 10.1016/S2665-9913(20)30095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eular EULAR-COVID-19 database. https://www.eular.org/eular_covid19_database.cfm
- 13.COVID-19 Global Rheumatology Alliance The global rheumatology community's response to the worldwide COVID-19 pandemic. Healthcare provider entered registries. https://rheum-covid.org/provider-registry-gate/
- 14.Chen N, Zhou M, Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Centre for Disease Prevention and Control Case definition for coronavirus disease 2019 (COVID-19), as of 29 May 2020. https://www.ecdc.europa.eu/en/case-definition-and-european-surveillance-human-infection-novel-coronavirus-2019-ncov
- 16.Piva S, Filippini M, Turla F. Clinical presentation and initial management of critically ill patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Brescia, Italy. J Crit Care. 2020;58:29–33. doi: 10.1016/j.jcrc.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borghesi A, Maroldi R. COVID-19 outbreak in Italy: experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol Med. 2020;125:509–513. doi: 10.1007/s11547-020-01200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 19.Gianfrancesco M, Hyrich KL, Al-Adely S. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217871. published online May 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toniati P, Piva S, Cattalini M. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Damiani G, Pacifico A, Bragazzi NL, Malagoli P. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: real-life data from a large cohort during RED-ZONE declaration. Dermatol Ther. 2020; May 1 doi: 10.1111/dth.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mikuls TR, Johnson SR, Fraenkel L. American College of Rheumatology Guidance for the management of adult patients with rheumatic disease during the COVID-19 pandemic. Arthritis Rheumatol. 2020 doi: 10.1002/art.41301. published online April 29. [DOI] [PubMed] [Google Scholar]
- 23.Italian Society of Rheumatology COVID-19 monitoring in patients with rheumatic and musculoskeletal diseases. https://www.reumatologia.it/cmsx.asp?IDPg=1107
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


