In March, 2020, WHO declared COVID-19 a global pandemic. At the time of writing, the UK has the highest number of recorded fatalities in Europe, with London regarded as the epicentre of infection in the UK.
Physiological stress from critical illness and elective surgery increases serum cortisol concentrations and bioavailability by activation of the hypothalamic–pituitary–adrenal axis, decreased metabolism of cortisol, and a reduction in the amount of binding proteins (eg, cortisol-binding globulin).1, 2 The increase in cortisol is an essential part of the body's stress response, triggering adaptive changes in metabolism, cardiovascular function, and immune regulation.1
The effects of COVID-19 on cortisol are currently unknown. It has been suggested that severe acute respiratory syndrome coronavirus (SARS-CoV), the predecessor of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), might trigger an immunogenic response to adrenocorticotropic hormone because of mimicry. Similar mechanisms might apply to SARS-CoV-2, theoretically amplifying morbidity and mortality by inducing a cortisol insufficiency related to critical illness.1, 3 To understand whether this process might be a contributor to the pathophysiology of COVID-19, we did a cohort study describing the acute cortisol concentrations observed in patients with COVID-19.
Patients admitted to three large teaching hospitals (Charing Cross, Hammersmith, and St Mary's) in London, UK, with a clinical suspicion of COVID-19 were included in this series (appendix p 1). Patients who were suspected to have SARS-CoV-2 infection had a standard set of blood samples drawn, including a full blood count, creatinine, C-reactive protein (CRP), D-dimer, and serum total cortisol measurement. During the study period (admissions from March 9 to April 22, 2020; follow-up to May 8, 2020), a total of 621 patients were admitted with suspected COVID-19 who had at least one cortisol measurement during their admission. We included only baseline cortisol measurements made within 48 h of admission for suspected COVID-19 or diagnosis of COVID-19 during a hospital admission. We excluded patients with pre-existing hypoadrenalism, concurrent systemic glucocorticoid treatment, or who had cortisol measured as part of a diagnostic test (eg, a synacthen test). After these exclusions, a cohort of 535 patients with cortisol measurements were available for analysis. 403 patients were diagnosed with COVID-19 on the basis of either a positive result from real-time RT-PCR testing of a nasopharyngeal swab (356 [88%] patients) or a strong clinical and radiological suspicion of COVID-19, despite negative swab testing (47 [12%] patients). 132 (25%) individuals in this cohort were not diagnosed with COVID-19 (appendix p 2). In the group of patients with COVID-19, the mean age of the patients was 66·3 years (SD 15·7) and 240 (59.6%) were men (appendix p 2). The most frequent comorbidities in the cohort of patients with COVID-19 were hypertension (191 [47·4%] patients), diabetes (160 [39·7%] patients), cardiovascular disease (94 [23·3%] patients), chronic kidney disease (50 [12·4%] patients), and a current diagnosis of cancer (38 [9·4%] patients). 112 (27·8%) of patients with COVID-19 died during the study period, compared with 9 (6·8%) of patients without COVID-19 (p<0·0001) (appendix p 2). Median cortisol concentration in the group of patients with COVID-19 was 619 nmol/L [IQR 456–833] versus 519 nmol/L [378–684] in the patient group who did not have COVID-19 (p<0·0001) (appendix p 2).
Univariable analysis of the group of patients with COVID-19 by Cox proportional hazards regression modelling showed that age 75 years and older had the highest risk of acute mortality, and age younger than 75 years was associated with a reduced relative risk of acute mortality (appendix p 5). The presence of diabetes, hypertension, current diagnosis of cancer, chronic kidney disease, or cardiovascular disease was significantly associated with acute mortality. Increased cortisol, CRP, neutrophil to leukocyte ratio, and creatinine were predictive of acute mortality (appendix p 5).
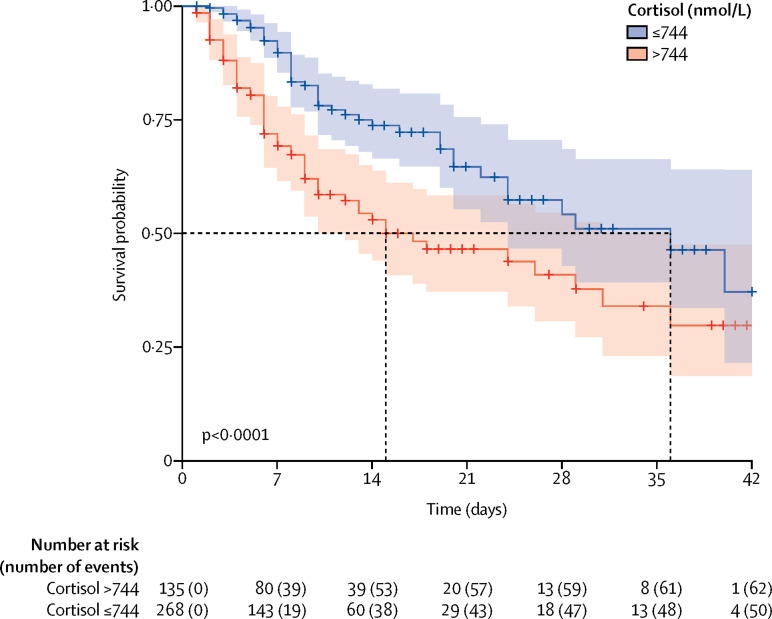
Multivariable analysis showed that a doubling of cortisol concentration was associated with a significant 42% increase in the hazard of mortality, after adjustment for age, the presence of comorbidities, and laboratory tests (appendix p 3). An optimal cutoff for cortisol was selected by use of maximally selected rank statistics. Patients with COVID-19 whose baseline cortisol concentration was equal to or less than 744 nmol/L (268 patients [67%]) had a median survival of 36 days [95% CI 24–not determined]; whereas, patients with COVID-19 whose cortisol value was more than 744 nmol/L (135 patients [33%]) had a median survival of 15 days [10–36] (log-rank test p<0·0001; figure ).
Figure.
Kaplan-Meier plot of survival probability over time.
The plot is categorised by baseline cortisol concentration above or equal to and below the cutoff of 744 nmol/L. Shading indicates 95% CI for each curve.
To our knowledge, our analyses show for the first time that patients with COVID-19 mount a marked and appropriate acute cortisol stress response and that this response is significantly higher in this patient cohort than in individuals without COVID-19. In other words, our cohort did not obviously exhibit an adrenal insufficiency with SARS-CoV-2 infection in the acute setting. However, it is possible that patients might exhibit a relative adrenal insufficiency later on in the course of their disease, as has been observed for SARS-CoV infection3 and in the context of an extended stay in intensive care.1 The cortisol stress responses observed in this cohort range up to 3241 nmol/L. Despite the non-linearity of the cortisol assay at this high range, these values indicate a marked cortisol stress response, perhaps higher than is observed in patients undergoing major surgery.2 Until now, data were not available to guide an evidence-based approach to tailor glucocorticoid stress regimens in patients with adrenal insufficiency and SARS-CoV-2 infection.4 Our data suggest that it is appropriate for patients with hypoadrenalism—a situation quite commonly encountered in the 3% of the population taking systemic glucocorticoid therapy5— to take or be given supplemental glucocorticoids at a high dose to prevent an acute adrenal crisis if they acquire a SARS-CoV-2 infection. Furthermore, we found that high cortisol concentrations were associated with increased mortality and a reduced median survival, probably because this is a marker of the severity of illness.6 In our cohort, cortisol seemed to be a better independent predictor than were other laboratory markers associated with COVID-19, such as CRP, D-dimer, and neutrophil to leukocyte ratio. Nonetheless, we note the following caveats. First, for simplicity's sake, this study confined itself to the analysis of a single baseline cortisol concentration measured within 48 h of hospital admission for COVID-19. Consequently, this analysis does not consider variations within and between individuals in the dynamics of cortisol response to stress, as we observed in our surgical series.2 Second, although we found that a cortisol concentration cutoff of more than 744 nmol/L was predictive of a reduced median survival, this finding is likely to differ with other cortisol assays. Third, any potential role for cortisol measurement at baseline and later during an inpatient stay with COVID-19 as a prognostic biomarker, either by itself or in combination with other biomarkers, will require validation in a prospective study.
Acknowledgments
We declare no competing interests. TT and BK were joint first authors of this Correspondence.
Supplementary Material
References
- 1.Teblick A, Peeters B, Langouche L, Van den Berghe G. Adrenal function and dysfunction in critically ill patients. Nat Rev Endocrinol. 2019;15:417–427. doi: 10.1038/s41574-019-0185-7. [DOI] [PubMed] [Google Scholar]
- 2.Khoo B, Boshier PR, Freethy A. Redefining the stress cortisol response to surgery. Clin Endocrinol (Oxf) 2017;87:451–458. doi: 10.1111/cen.13439. [DOI] [PubMed] [Google Scholar]
- 3.Pal R. COVID-19, hypothalamo–pituitary–adrenal axis and clinical implications. Endocrine. 2020;68:251–252. doi: 10.1007/s12020-020-02325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isidori AM, Pofi R, Hasenmajer V, Lenzi A, Pivonello R. Use of glucocorticoids in patients with adrenal insufficiency and COVID-19 infection. Lancet Diabetes Endocrinol. 2020;8:472–473. doi: 10.1016/S2213-8587(20)30149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laugesen K, Jorgensen JOL, Petersen I, Sorensen HT. Fifteen-year nationwide trends in systemic glucocorticoid drug use in Denmark. Eur J Endocrinol. 2019;181:267–273. doi: 10.1530/EJE-19-0305. [DOI] [PubMed] [Google Scholar]
- 6.Christ-Crain M, Stolz D, Jutla S. Free and total cortisol levels as predictors of severity and outcome in community-acquired pneumonia. Am J Respir Crit Care Med. 2007;176:913–920. doi: 10.1164/rccm.200702-307OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.