Abstract
Problem:
The effects of HIV on the gastrointestinal tract (GIT), including CD4 depletion, epithelial disruption, and collagen deposition, are well documented and only partially reversed by combination antiretroviral therapy (cART). However, the effects of HIV on the female reproductive tract (FRT) are poorly understood, and most studies have focused on ectocervix and vagina without assessing the upper tract. Here, we investigated CD4+ T-cell frequency, phenotype, and HIV-specific T-cell responses in the endocervix and endometrium of HIV-infected women, comparing these tissues to the GIT.
Method of Study:
Mucosal samples and blood were obtained from 18 women: 4 who were HIV positive and not on cART for at least three years prior to sampling, including 2 natural controllers (viral load [VL] undetectable and CD4 >350); 9 women on cART with low to undetectable VL; and 5 HIV-uninfected women. Mucosal samples included terminal ileum, sigmoid colon, endocervical cytobrush, endocervical curettage, and endometrial biopsy. T-cell frequency, phenotypes, and HIV-specific T-cell responses were analyzed by multiparameter flow cytometry.
Results:
T-cell activation, measured by CD38/HLA-DR co-expression, remained significantly elevated in endometrium following cART, but was lower in gastrointestinal tissues. HIV-specific CD8+ T-cell responses were detected in ileum, colon and endometrial tissues of women both on and off cART, and were of higher magnitude on those not on cART.
Conclusions:
Our findings reveal differences in CD4+ T-cell frequencies, immune activation and HIV-specific T-cell responses between the gastrointestinal and reproductive tracts, and highlight differences between HIV controllers and women on cART.
Keywords: T-cell, CTL, HIV, GALT, MALT, endometrium, endocervix
INTRODUCTION
The mucosal tissues of the reproductive and gastrointestinal tracts act as the major portals of HIV entry and are considered the first line of host defense against sexually transmitted pathogens. Both tissues house populations of CCR5-expressing, activated effector memory CD4+ T-cells that are highly susceptible to HIV infection1. Within days of infection gastrointestinal CD4+ T-cells are depleted, in striking contrast to the gradual decline of CD4+ T-cells in peripheral blood1,4,5. The emergence of HIV-specific cellular immune responses in the gut is considered to be “too little and too late” to prevent extensive early CD4+ T-cell depletion in this tissue2. Early depletion of gastrointestinal CD4+ T-cells, including Th17 cells, contributes to loss of epithelial barrier integrity; this in turn leads to translocation of microbial products into the systemic circulation, which is hypothesized to contribute to generalized immune activation3.
The extensive depletion of CD4+ T-cells from the gastrointestinal lamina propria is well described, and has been attributed in part to the increased availability of activated, CCR5+ T-cells, which constitute a primary target for HIV4, 5. Disruption of gut-associated lymphoid tissue (GALT) is strongly implicated as a contributor to HIV disease progression, and it has been suggested that maintaining a healthy GALT may be key to limiting HIV immunopathogenesis6–8. The dynamics of CD4+ T-cell depletion in the female reproductive tract (FRT) after HIV infection, and reconstitution of these cells following initiation of combination antiretroviral therapy (cART), have been less thoroughly studied. Like the gastrointestinal tract (GIT), the FRT houses numerous CCR5+ activated, effector memory CD4+ T-cells9, 10, and experimental infection of rhesus macaques with simian immunodeficiency virus (SIVmac) leads to rapid depletion of these cells11. The reproductive and gastrointestinal tracts share numerous immunological features1; however, few studies of HIV disease have explored both compartments simultaneously, likely due in part to the difficulties associated with tissue acquisition in humans.
Advances in the efficacy of cART regimens have resulted in dramatic increases in the life expectancy and survival of HIV-infected individuals12. Despite the success of cART in reducing plasma viremia and promoting recovery of CD4+ T-cells numbers in peripheral blood, immune reconstitution in the GIT is often incomplete or delayed13–15. The effects of cART on CD4+ T-cell recovery in the gut remain somewhat unclear, with reports ranging from minimal to complete restoration depending upon the area of the GIT studied, morbidity at the time of treatment initiation and duration of cART16–20. With few exceptions15, 21, most studies in humans have been limited to a single region of the GIT4, 14, 16–18, 22–26. Little information exists concerning the effects of cART on T-cell populations in the FRT; moreover, most studies of HIV pathogenesis in the FRT have focused on tissues of the lower (ectocervix and vagina) rather than the upper tract (endocervix and endometrium), and it has not been determined whether restoration of CD4+ T-cells and immune function in the FRT parallels restoration in the GIT27–31 or blood.
A small subset of HIV infected individuals who control plasma viremia in the absence of cART are termed elite or “natural” controllers32. These individuals frequently have higher CD4+ T-cell counts and less pronounced immune activation compared to typical progressors23, 33–35. Some individuals who meet virologic criteria as natural controllers nevertheless experience significant CD4+ T-cell loss36, 37, and demonstrate increased immune activation34, 38, 39 and a range of non-AIDS-defining morbidities40–43. Studies of mucosal tissues in these subjects have been limited; however, available data reveal that many individuals identified as controllers have strong, polyfunctional HIV-specific T-cell responses in rectal mucosa23. It is not known whether similarly robust HIV-specific responses are present in the upper female reproductive tract.
We now report the results of a study of CD4+ T-cell percentages, T-cell activation/maturation phenotypes and HIV-specific T-cell responses in small and large intestine, endocervix and endometrium, measured at a single time point in a group of long-term HIV-infected women. These women included natural controllers; women not taking cART despite progressive disease; recipients of cART and uninfected comparison participants (Table 1). To our knowledge, this study is the first to directly compare immune parameters in the GIT and the upper reproductive tract of HIV-positive women.
Table 1:
Characteristics of study participants.
| Patient Identification No./Group | Ethnicity | Age (yrs) | Time since first known pos HIV test (yrs) | CD4 count (Cells/μl) | Plasma Viral load (copies/mL)§ | Cervical viral load (copies/mL) |
|---|---|---|---|---|---|---|
| HIV uninfected (n=5) | ||||||
| W51 | AA | 35 | - | 905 | - | - |
| W52 | AA | 39 | - | 1051 | - | - |
| W53 | AA | 35 | - | 846 | - | - |
| W63 | AA | 40 | - | 1705 | - | - |
| W66 | AA | 43 | - | 1042 | - | - |
| HIV infected and off antiretroviral treatment (n=4) | ||||||
| S05 | AA | 47 | 16 | 1345 | <40 | <40 |
| W56** | C | 54* | 25 | 320 | 266 | NA |
| W57** | AA | 48* | 19 | 450 | <75 | NA |
| W58 | AA | 43 | 20 | 275 | 2500 | 1035 |
| HIV infected and receiving cART (n=9) | ||||||
| S04** | AA | 46 | 16 | 535 | 26 | <40 |
| S06 | C | 45 | 25 | 397 | <20 | <40 |
| S07 | C | 49 | 21 | 454 | <20 | <40 |
| S08** | C | 59* | 23 | 278 | <40 | <40 |
| W54• | AA | 42 | 18 | 1000 | <75 | <40 |
| W55 | AA | 38 | 9 | 896 | <75 | <40 |
| W59 | AA | 42 | 14 | 689 | 234 | <40 |
| W60 | AA | 44 | 12 | 720 | 100 | <40 |
| W62 | C | 48 | 21 | 559 | <40 | <40 |
Abbreviations:
Identification numbers beginning with “W” indicate WIHS participants; Numbers beginning with “S” are non-WIHS participants. AA-African American, C-Caucasian, NA-not available.
Post-menopausal;
No FRT tissues collected;
No ileum collected.
Plasma viral load data were obtained from WIHS or through self-reporting with medical record verification; accordingly, assay detection thresholds vary.
MATERIALS AND METHODS
Study Subjects and Specimen Collection.
Study participants included women recruited from the Women’s Interagency HIV Study (WIHS) and participants recruited in the San Francisco Bay area specifically for this study. The WIHS is a multisite observational cohort study of HIV among women which has been described previously44; all WIHS participants who contributed to this report were enrolled at the San Francisco Bay area WIHS site. Eight HIV infected and 5 HIV uninfected participants of WIHS enrolled in this study (Table 1). An additional 5 San Francisco Bay area women, who were not enrolled in WIHS, were recruited specifically for this study. We defined cART as described in the Department of Health and Human Services panel on Antiretroviral Guidelines for Adults and Adolescents45. HIV disease stage, HIV RNA viral loads and CD4+ T-cell counts over time were obtained from WIHS data, or through self-reporting with medical record verification for participants who were not recruited from WIHS. Of the 13 HIV seropositive participants, 9 reported receiving cART. Seven of the 9 demonstrated viral suppression (below 75 vRNA copies/mL); of these women, all but one had peripheral blood CD4+ T-cell counts >350 cells/μl (the outlier had a single CD4+ T-cell count of 278). Two women who reported using cART had detectable plasma HIV RNA (234 and 100 copies/ml, respectively); both had CD4+ T-cell counts >500 cells/ml. The other 4 HIV seropositive women reported that they had not received cART: these included two natural controllers with plasma HIV RNA <75 copies/mL and CD4+ T-cell counts >350 cells/μl; and two women with a past history of viremia >1,000 copies/ml and CD4+ T-cell counts between 250 and 350. We also studied 5 consistently HIV uninfected participants of WIHS whose peripheral blood CD4+ T-cell counts all exceeded 800 cells/ml. Median estimated duration of HIV infection was 19 years for both HIV-infected groups. The study protocol was approved by the Institutional Review Boards of the University of California, San Francisco (UCSF) and the University of California, Davis.
Clinical Laboratory Parameters.
Plasma HIV RNA quantification and determination of CD4+ T-cell counts were performed using standard assays in laboratories that participate in the NIAID DAIDS Laboratory Quality Assurance Program. Serological testing for HIV was performed via FDA-approved enzyme-linked immunoassays with Western blot confirmation.
Study Visits.
All participants attended a screening visit during which data on medical history, current medications and use of intravaginal products was assessed. Eligibility criteria included a history of regular menstrual cycles, or a history of menopausal cessation of bleeding, non-use of sex steroid treatments and IUDs, ability to follow study instructions and procedures, low risk for procedural bleeding (no use of anticoagulant treatments or history of bleeding dyscrasias) and no history of inflammatory bowel disease. For women enrolled in WIHS, screening occurred within a six-week window of a WIHS visit. If it occurred outside this time, or the woman was not enrolled in WIHS, CD4 counts and HIV RNA quantitation on peripheral blood and plasma were performed as described above. Routine pelvic examinations were performed to rule out acute reproductive tract infections, or other contraindication to endometrial biopsy. Cervical swab samples were obtained for nucleic acid amplification tests for infection with Neisseria gonorrhoeae and Chlamydia trachomatis. Participants were instructed in the use of urine LH surge tests (Clearblue brand, Proctor & Gamble, Cincinnati, OH) and colonoscopy bowel preparation procedures cessation of P.O intake and bowel cleansing (polyethylene glycol, GoLYTELY solution jug, Braintree Laboratories, Braintree, MA). Participants contacted study staff at the time of a positive LH surge results, or if no such result occurs. Colonoscopy visits were scheduled to occur 7–9 days after LH surge to coincide with the mid-luteal phase of the ovulatory cycle. If no LH surge was observed, colonoscopy was scheduled to occur at the estimated time of the mid-luteal ovulatory phase based on the participant’s prior menstrual record. Post-menopausal women were scheduled for colonoscopy at their convenience; reproductive mucosal samples were not collected from post-menopausal women. Participants were contacted prior to beginning the bowel preparation to determine if they were acutely ill (for example an upper respiratory infection or genital herpes recurrence) and if so, the procedure was rescheduled.
Approximately 20mL of blood was collected in vacutainer tubes coated with EDTA (BD Pharmingen, SA Jose, CA). Colonoscopy was performed in a hospital endoscopy suite using standard procedures under conscious sedation. Six to 10 endoscopic biopsies were obtained from healthy appearing tissues of each of the sigmoid colon and terminal ileum. After completion of colonoscopy, the participant, while sedated, was placed in the lithotomy position and reproductive tract specimens were collected. Following collection of endocervical wick samples, endometrial biopsies were obtained using 3 mm Miltex brand Softflex endometrial biopsy cannulas (1–2 passes). Subsequently, endocervical cytobrush and curettage specimens were collected using Cytobrush Plus® Cell collectors and Kevorkian curettes (both from CooperSurgical, Trumbull, CT), respectively. Tissue samples were placed in RPMI 1640 medium supplemented with 15% fetal calf serum, penicillin (100 U/mL), streptomycin (100ug/mL) and L-glutamine (2mM), designated as R15 medium. Specimens were immediately transported at ambient temperature to the laboratory at the University of California at Davis for processing and analysis.
Isolation of Mononuclear Cells.
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque density gradient centrifugation (Pfizer, New York, NY), and washed in phosphate-buffered saline (PBS). The mononuclear cells from cytobrush were isolated by rubbing the brushes together, rinsing the brushes with medium, passing the cell suspension through a 70-μM nylon cell strainer (Becton Dickinson Discovery Labware, Bedford, MA) and washing in R-15 medium. Mononuclear cells from endocervical curettage were isolated by repeated pipetting, followed by straining and washing, as described for cytobrushes. A previously published protocol, optimized for high leukocyte yield and viability without compromising the detection of most surface antigens, was followed for isolation of lymphocytes from mucosal biopsies9, 46, 47. Briefly, biopsies from endometrium, ileum and sigmoid colon were subjected to digestion with 0.5 mg/mL collagenase type II (0.5 mg/mL; Sigma-Aldrich, St. Louis, MO) in RPMI/5% fetal calf serum for 30 minutes in a shaking incubator at 37°C. After 30 minutes, the undigested pieces were passed through a 16-gauge blunt-end needle and a 70-μM nylon cell strainer (Becton Dickinson Discovery Labware, Bedford, MA); cells were then immediately washed in R15 medium and PBS. Remaining tissue pieces were digested an additional 1–2 times, as needed, until no observable fragments remained.
Antibodies and Peptide Pools.
Fluorochrome-labeled monoclonal antibodies used for the phenotypic and intracellular cytokine staining (ICS) assay included CD3 (UCHT1), CD8 (SK1), CCR7 (3D12), CXCR4 (12G5), CCR5 (2D7), IFNγ (B27), TNF-α (MAb11) and unlabeled co-stimulatory antibodies to CD28 (L293) and CD49d (L25) (purchased from Becton-Dickinson Pharmingen, San Diego, CA). CD45RA (2H4) and CD4 (T4D11) were purchased from Beckman Coulter (Fullerton, CA). CD38 (HB7), CD107a (H4A3) were obtained from BD Biosciences (San Jose, CA). HLADR (TU36), Aqua amino reactive dye from Invitrogen (Carlsbad, CA), and IL-2 (MQ1–17H12) were purchased from eBioscience (San Diego, CA). HIV Gag (p55, HXB2 sequence) peptide pools consisting of 15-mer peptides with an 11-amino acid overlap and CMV, EBV and Influenza (CEF) peptide pools were purchased from JPT Peptide Technologies (Berlin, Germany).
Phenotypic and ICS assay and Flow Cytometry.
Leukocytes from whole blood (WB), FRT and GIT were analyzed for cell surface phenotype on the day of collection46. The remaining mononuclear cells from PB, endometrium, ileum and sigmoid colon were rested overnight in R15 medium prior to ICS assay. GIT tissue cells suspended in R15 medium were supplemented with piperacillin-tazobactam (0.5 mg/mL) (Zosyn; Wyeth Pharmaceuticals, Philadelphia, PA) to limit bacterial growth during incubation. ICS assay was performed as described previously22, 26, 35. To measure antigen-specific immune responses, cells were stimulated with pooled peptides (3.5μg/mL) spanning the HIV Gag protein or a commercial peptide pool containing immunodominant peptides from cytomegalovirus, Epstein-Barr virus and influenza A virus (“CEF”). Medium containing the peptide vehicle (dimethyl sulfoxide) and co-stimulatory antibodies served as a negative control and staphylococcus enterotoxin B (SEB, 5μg/mL) was used as a positive control. During a 5-hr antigen stimulation that included co-stimulatory antibodies to CD28 and CD49d, cells were stained for CD107a. Cells were further stained for surface markers such as CD4, CD8 and for viability. Cells were fixed with 4% paraformaldehyde and permeabilized using FACS Perm 2 (BD Biosciences) prior to intracellular staining for CD3, IFNγ, TNFα and IL-2. Samples were read on an LSR II flow cytometer using FACSDiva software (BD Biosciences) within 24 hrs of assay completion. Data were analyzed using FlowJo software (TreeStar, Ashland, OR). Boolean gating was used to separate cells into functional categories. Response data were then graphed using SPICE software (version 5.35, provided by Mario Roederer, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD).
Data Analysis.
Examples of cell surface phenotyping data for CD4+ and CD8+ T-cells in all tissues are provided in Supplementary Figure S1. Comparisons of T-cell percentages and expression of activation, memory and chemokine markers between and within study groups were performed using the Mann-Whitney test and Wilcoxon matched pairs signed rank test, as appropriate, in Graph Pad Prism software (V5.0, Graph Pad, San Diego, CA). Correlations between variables were done using the Spearman correlation, and linear regression was used to graph a best-fit line to the data (GraphPad Prism).
Antigen-specific T-cell response data were analyzed as previously described22. Examples of CD8+ T-cell response data for all tissues are provided in Supplementary Figure S2. Briefly, we determined whether antigen-specific responses differed significantly from the negative control using a formula that assumed a Poisson distribution for both responses, accounting for the actual numbers of gated events in each case. Net antigen-specific responses were then calculated by subtracting the negative control values from antigen-specific responses. Statistical analysis of Boolean-gated data was performed in SPICE software using the Wilcoxon rank test to compare individual responses at a significance level of P <0.01, and a permutation test, based on χ2 statistics, to compare pie charts.
RESULTS
In women on cART, mean CD4+ T-cell percentages are lowest in endometrium.
Thirteen HIV infected and 5 uninfected women were included in the study. Characteristics of these women are detailed in Table 1 and summarized in Materials and Methods. We measured CD4+ T-cell percentages in blood and multiple sites of GIT and FRT collected from HIV-positive women and healthy controls (Supplementary Figure S1); comparisons between subject groups and between tissue sites are shown in Figure 1. In women on cART with viral suppression, we detected significantly lower percentages of CD4+ T-cells in blood, ileum, sigmoid colon and endometrium compared to HIV uninfected women. These findings suggest incomplete CD4+ T-cell reconstitution in the GIT and FRT tissues of women on cART. There were also some differences between tissues: in women on cART, CD4+ T-cell percentages in endometrium were significantly lower than in blood or sigmoid colon (Fig. 1). We also looked for strong associations between CD4+ T-cell percentages in different tissues in women on cART, and found two significant correlations: between blood and sigmoid CD4+ T-cell percentages (Spearman r = 0.73, P = 0.031); and between endometrial and endocervical cytobrush CD4+ T-cell percentages (Spearman r = 0.89, P = 0.033) (data not shown). Somewhat surprisingly, the three women on cART with the lowest blood CD4+ T-cell counts (participants S06, S07 and S08) did not consistently reveal lower CD4+ T-cell percentages in tissues compared to women on cART with blood CD4+ T-cell counts >500 (Fig. 1) (data not shown).
Figure 1. Percentages of CD4+ T-cells in GIT, FRT and WB.
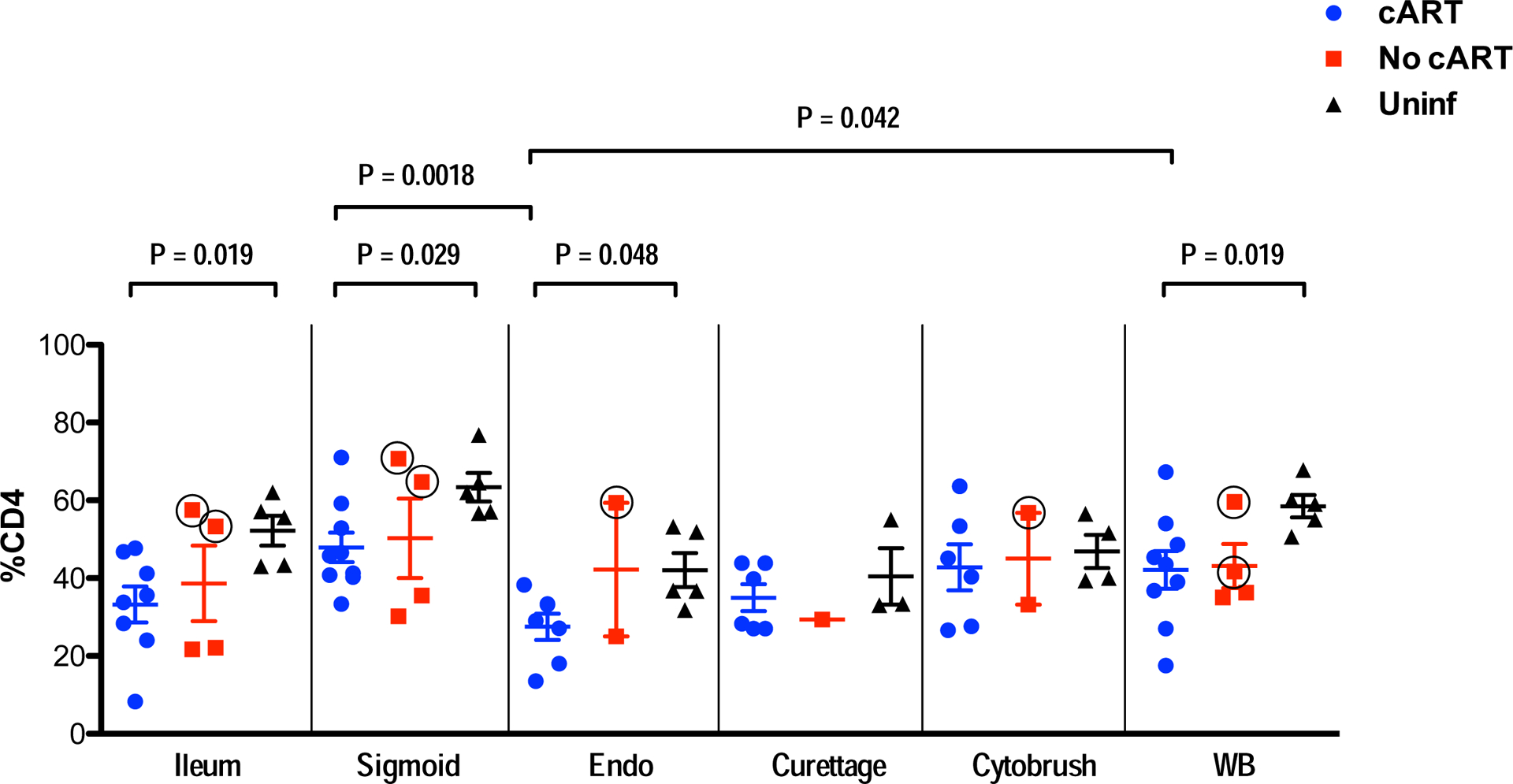
CD4+ T-cell percentage, relative to all viable CD3+ cells, is indicated on the y-axis. Tissue types are indicated on the x-axis. After initial gating based on scatter characteristics and doublet discrimination, viable cells (based on Aqua dye exclusion) were gated for expression of CD3, followed by CD49. As described in the text, the No cART group included two HIV controllers; data points for these two participants are identified as red squares with black circles. Abbreviations: Endo, endometrium; Uninf, uninfected; WB, whole blood. Comparisons between groups and tissues were performed with Mann-Whitney and Wilcoxon matched pairs signed rank tests, respectively. Horizontal and vertical bars represent mean and standard deviation, respectively. Significant differences, either between participant groups within a single tissue type or between tissues within a participant group, are indicated by horizontal brackets.
HIV controllers maintain CD4+ T-cell percentages in FRT and GIT similar to healthy controls.
Within the group of participants who were not receiving cART at the time of sampling, two HIV positive women (S05 and W57) met virologic and immunologic criteria as HIV controllers (i.e., CD4+ >350 cells/μL and VL <75 copies/mL). Strikingly, these women maintained tissue CD4+ T-cell percentages similar to those of HIV uninfected women (Figure 1). In contrast, two other participants who were not receiving cART (W56 and W58) had low but detectable viremia and blood CD4 counts below 350 cells/μL. These women had noticeably lower percentages of CD4+ T-cells in mucosal tissues compared to S05 and W57 (Figure 1). Although the sample size was too small to establish statistically significant differences between groups, these findings indicate differential maintenance of mucosal CD4+ T-cell populations in individuals with different HIV disease progression phenotypes.
High percentages of activated T-cells in endometrium of women on cART.
To assess mucosal T-cell activation status, we measured coexpression of HLA-DR and CD38 on CD4+ and CD8+ T-cells from blood and tissues (Supplementary Figure S1). Previous studies have shown that T-cell activation is relatively low in GIT of controllers and individuals on cART as compared to untreated progressors15, 23; however, to our knowledge T-cell activation in the upper FRT of HIV-infected women has not been studied, and comparisons between GIT and FRT have not been previously reported. In the present study, the percentage of activated T-cells in the GIT was generally comparable between subject groups. However, we observed a significantly higher percentage of activated CD4+ and CD8+ T-cells in the endometrium of participants on cART compared to uninfected women (Figures 2A, 2B). Comparison between tissues within the cART-treated group also indicated a significantly higher percentage of activated CD8+ T-cells in endometrium than in endocervix (curettage and cytobrush), GIT tissues (ileum and sigmoid), or blood (Figure 2B). In the uninfected group, T-cell activation marker expression was also greater in endometrial CD8+ T-cells compared to ileal CD8+ T-cells.
Figure 2. Percentages of activated T-cells.
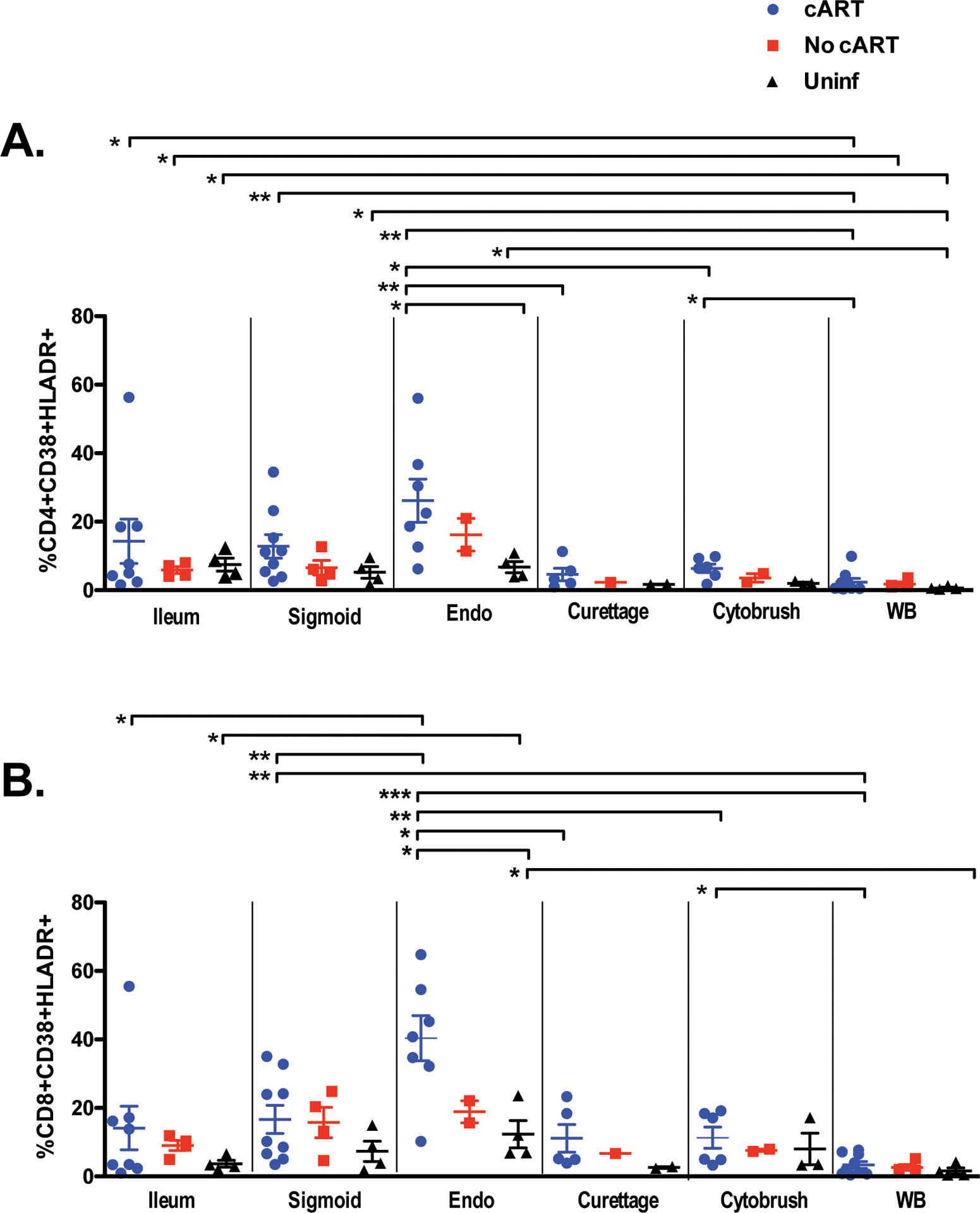
Shown are the percentages of (A) CD4+ and (B) CD8+ T-cells co-expressing HLA-DR and CD38. After initial gating based on scatter characteristics and doublet discrimination, viable cells (based on Aqua dye exclusion) were gated for expression of CD3, followed by CD4 and/or CD8, and finally HLA-DR and/or CD389. bbreviations: Endo, endometrium; Uninf, uninfected; WB, whole blood. Comparisons between participant groups and tissues were performed with Mann-Whitney and Wilcoxon matched pairs signed rank test, respectively. The horizontal and vertical bars represent mean and standard deviation, respectively. Significant differences, either between participant groups within a single tissue type or between tissues within a participant group, are indicated by horizontal brackets with asterisks to indicate P values as follows: *P < 0.05; **P < 0.01; ***P <0.001.
Higher percentages of effector memory T-cells in ileum of women not on cART, including controllers, compared to uninfected women.
To assess the preservation and restoration of memory T-cell subsets in mucosal tissues, we determined the breakdown of memory/effector T-cell subsets based on expression of surface markers CCR7 and CD45RA (Supplementary Figure S1; Figure 3A–D). Using these standard markers, naïve T-cells are defined as CCR7+/CD45RA+; central memory cells as CCR7+/CD45RA-; effector memory cells as CCR7-/CD45RA-, and terminally differentiated effector cells as CCR7-/CD45RA+ 48, 49. We found large variation in expression of memory/effector markers on mucosal T-cells, particularly among women on cART (Figure 3). Also, as compared to uninfected participants, women not on cART had significantly higher percentages of effector memory CD4+ and CD8+ T-cells in ileal mucosa (Figs. 3B, 3D), with correspondingly lower percentages of central memory T-cells (Figs. 3A, 3C). Similar trends were observed in sigmoid mucosa, although they did not reach significance. These trends were not observed in either blood or FRT tissues; however, fewer reproductive tract samples were available than GIT samples for these analyses.
Figure 3. Memory T-cell subsets.
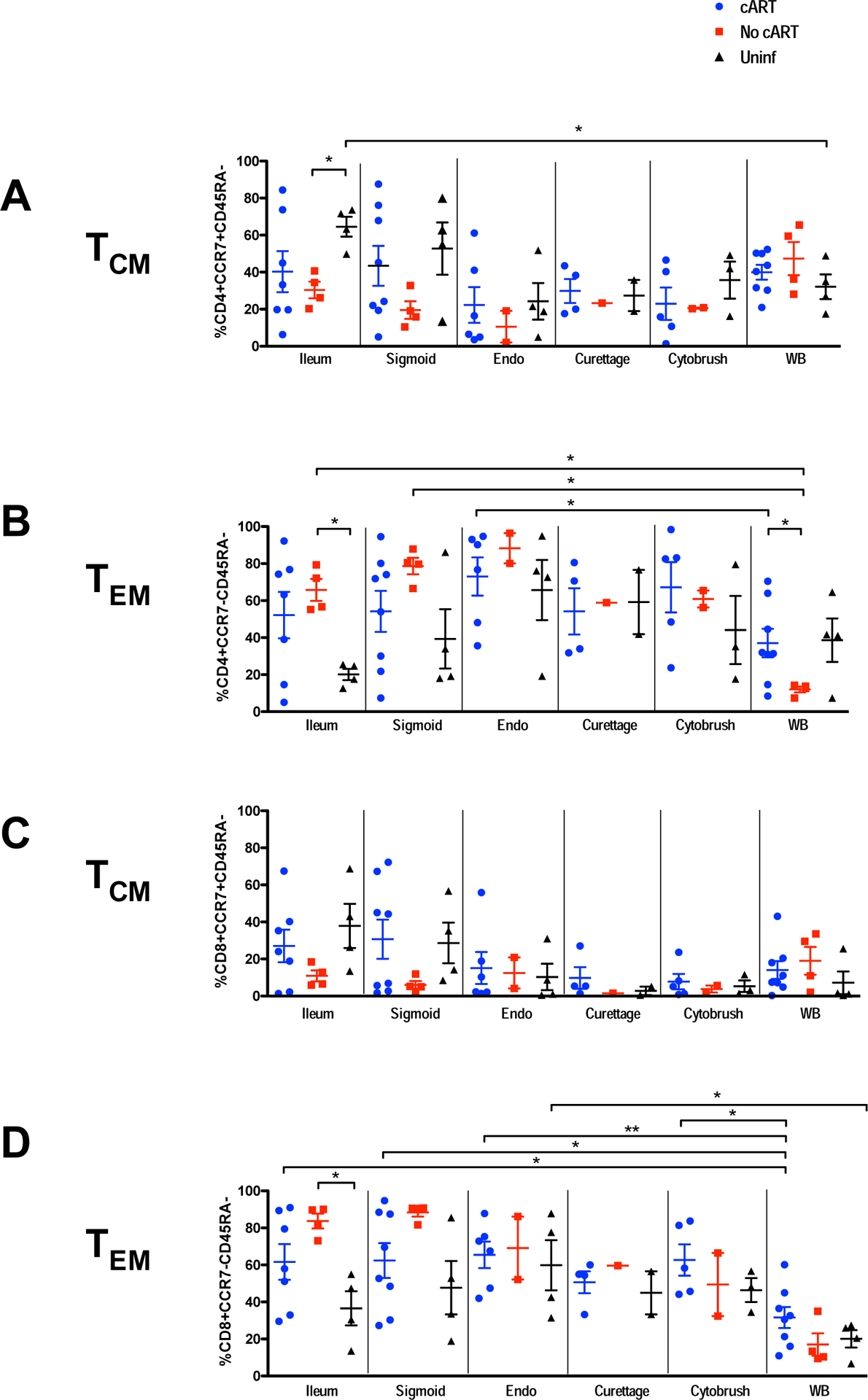
Percentages of central (A, C) and effector memory (B, D) T-cells. Panels A and B correspond to CD4+ T-cells; C and D are CD8+ T-cells. After initial gating based on scatter characteristics and doublet discrimination, viable cells (based on Aqua dye exclusion) were gated for expression of CD3, followed by CD4 and/or CD8, and finally CD45RA and/or CCR79. Abbreviations: Endo, endometrium; Uninf, uninfected; WB, whole blood. Comparisons between groups and tissues were performed with Mann-Whitney and Wilcoxon matched pairs signed rank test, respectively. The horizontal and vertical bars represent mean and standard deviation, respectively. Significant differences, either between participant groups within a single tissue type or between tissues within a participant group, are indicated by horizontal brackets with asterisks to indicate P values as follows: *P < 0.05; **P < 0.01.
HIV Gag-specific T-cell responses in tissues are consistently lower in women on cART compared to those not on cART.
To measure HIV-specific T-cell responses, lymphocytes freshly isolated from FRT, GIT and blood were stimulated with pooled peptides spanning HIV Gag; CD8+ T-cells producing IL-2, IFNγ, TNFα and CD107a were measured by flow cytometry (Supplementary Figure S2). The total percentage of responding T-cells (i.e., exhibiting any response or combination of responses), and the percentages of cells producing individual factors, were determined after background subtraction. These results are summarized in Figures 4A–4D. The percentage of CD8+ T-cells expressing either CD107 or IFNγ in response to HIV Gag stimulation was greater in sigmoid colon of women not on cART compared to those on cART; similar trends were observed in ileum, but did not reach significance due to small sample size (Fig. 4B, C). Production of IL-2 was generally weak in mucosal samples (Fig. 5) and was not included in Figure 4. Together, these findings are consistent with our previous reports of strong HIV Gag-specific T-cell responses in gastrointestinal mucosa of individuals not on antiretroviral therapy, including HIV controllers15, 22, 23, 35, 50. HIV-specific T-cell responses in mid-luteal endometrial tissues were generally comparable in magnitude and range to those detected in blood (Figure 4). Unfortunately, insufficient T-cells (<1×105) were obtained from endocervical curettage and cytobrush to reliably perform antigen-specific T-cell response assays by flow cytometry using these samples.
Figure 4. HIV Gag-specific CD8+ T-cell responses.
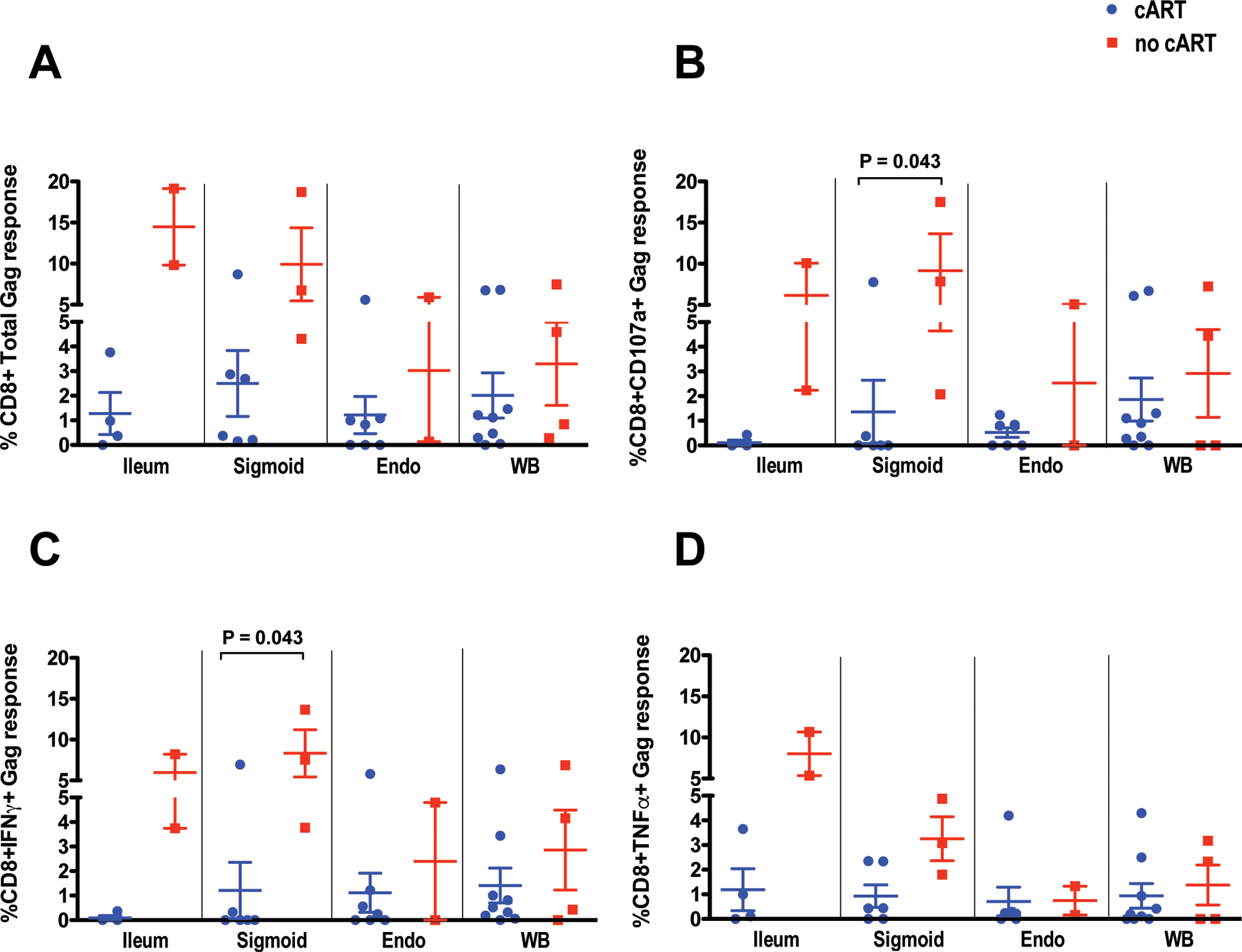
Panel (A) summarizes the total percentage of Gag-specific T-cells in each tissue based on cytokine flow cytometry results; cells producing multiple analytes are only counted once. Panels B thru D show the percentages of cells producing (B) CD107a, (C) IFNγ, or (D) TNFα, respectively, in response to HIV Gag stimulation. After initial gating based on scatter characteristics and doublet discrimination, viable cells (based on Aqua dye exclusion) were gated for expression of CD3, followed by CD4 and/or CD8, and finally Boolean gating for production of each analyte9. Abbreviations: Endo, endometrium; WB, whole blood. Comparisons between groups and tissues were performed with Mann-Whitney and Wilcoxon matched pairs signed rank test, respectively. The horizontal and vertical bars represent mean and standard deviation, respectively. Significant differences between participant groups within a single tissue type are indicated by horizontal brackets.
Figure 5. Polyfunctional analysis of HIV Gag-specific CD8+ T-cell responses.
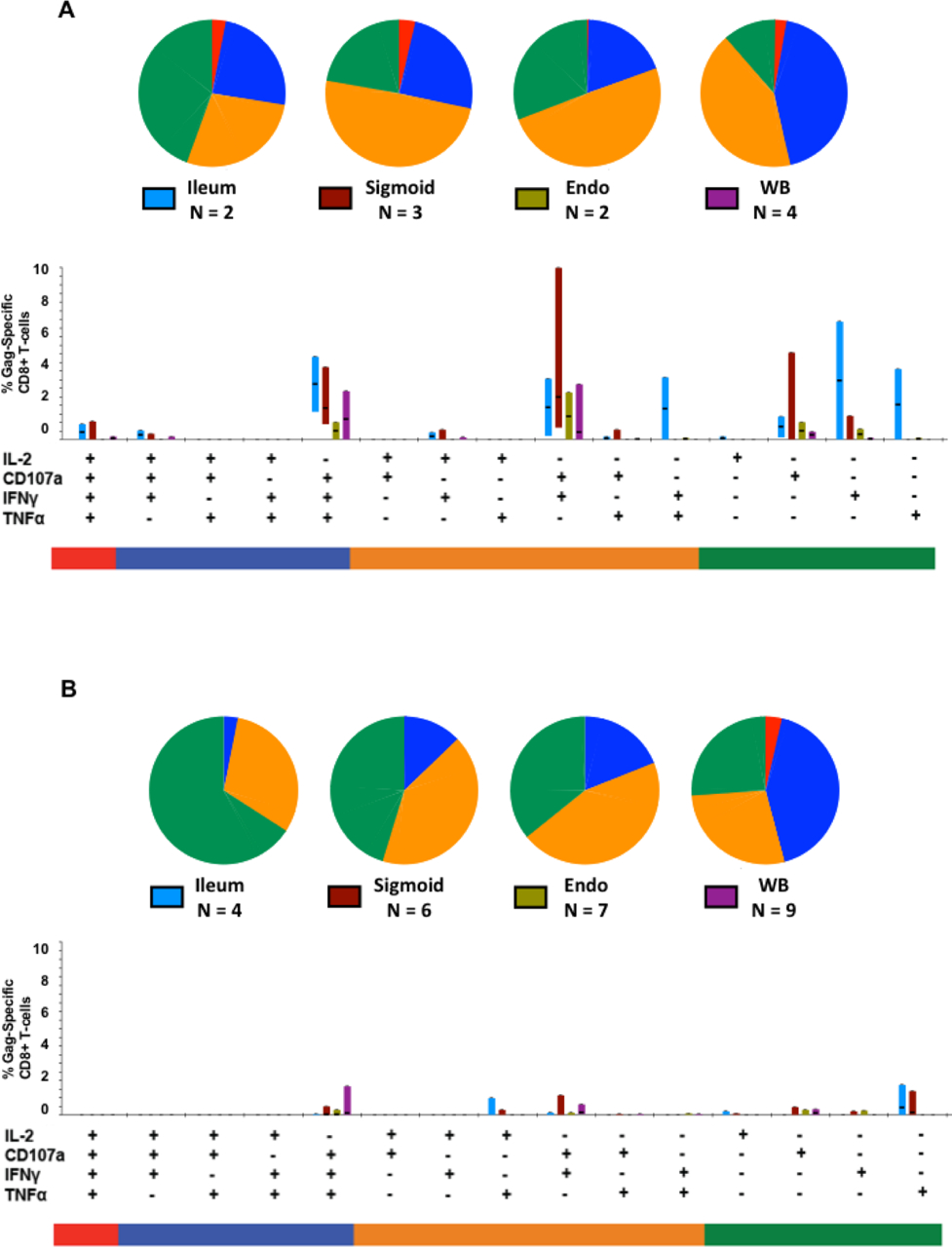
The Figure summarizes responses in terminal ileum, sigmoid colon, endometrium (Endo), and whole blood (WB). As described in the legend to Figure 4, after initial gating based on scatter characteristics and doublet discrimination, viable cells (based on Aqua dye exclusion) were gated for expression of CD3, followed by CD4 and/or CD8, and finally Boolean gating for production of each analyte9. Figure (5A) shows responses in women not on cART; Figure (5B) includes only responses from women on cART. The total HIV Gag-specific response was broken down into 15 individual categories, as indicated by plus and minus signs. Vertical bars show the percentage of HIV Gag-specific CD8+ T-cells from each tissue (which are color coded and indicated in the chart legend) after subtraction of background counts. The median response for each functional category is indicated by a horizontal black line; bar height spans inter-quartile ranges (25th thru 75th percentile). Pie charts are color-coded to indicate responses comprised of a single function (green), two functions (orange), three functions (blue) or four functions (red). Responses involving 3 or 4 functions are considered as polyfunctional.
As a control, we also measured CD8+ T-cell responses to a cocktail of immunodominant peptides from CMV, EBV and influenza (abbreviated CEF) in blood, endometrium, and GIT tissues. CEF-specific CD8+ T-cell responses ranging from 1–3% of CD8+ T-cells were detected in all three subject groups, including uninfected women, indicating the presence of memory T-cell responses to other pathogens in gastrointestinal and reproductive tissues (data not shown)51.
Boolean gating reveals fewer polyfunctional HIV-specific CD8+ T-cells in women on cART.
Using Boolean gating and SPICE analysis, HIV-specific T-cells were assigned to 15 different functional categories (excluding the non-responding subset) based upon patterns of co-expression of the four analytes: IFNγ, TNFα, IL2 and CD107. Figures 5A and 5B show the breakdown of CD8+ T-cell responses by analyte(s) expressed by mucosal CD8+ T-cells from women who were not on cART (Figure 5A) or on cART (Figure 5B). In women not on cART, polyfunctional HIV-specific T-cells, defined as those cells co-expressing 3 or 4 analytes in response to stimulation, were more abundant in ileum and sigmoid colon than in endometrium (Figure 5A), although this finding is limited by the small number of samples in each group. A comparison of Figures 5A and 5B reveals that generally, HIV Gag-specific T-cell responses in women on cART (Fig. 5B) were lower in magnitude across all functional groups, in agreement with previous work15, 22, 23, 35, 50.
DISCUSSION
The effects of HIV infection on the FRT remain to be fully characterized, and most previous studies evaluating mucosal tissues in HIV disease have focused on a single tissue site14, 17, 18. Similar to the gastrointestinal tract, tissues of the lower (ectocervix, vagina) and upper (endocervix) FRT contain partially activated, memory CD4+ T-cells expressing CCR5 and/or CXCR4, which can serve as targets for HIV52. In this study, we compared T-cell frequency, phenotype, immune activation and HIV-specific T-cell responses in multiple regions of the GIT and upper FRT in a group of long-term HIV-positive women, both on and off cART.
To the best of our knowledge, this is the first study to address the effects of cART on CD4+ T-cell recovery, immune activation and HIV-specific CD8+ T-cell responses in parallel in both the GIT and upper FRT of HIV-infected women. Wide variation in the degree of CD4+ T-cell reconstitution in the gut mucosa, ranging from minimal to near complete, has been reported in response to cART, and initiation of cART during chronic HIV infection generally does not lead to complete CD4+ T-cell restoration14, 17, 19, 20, 53. Consistent with previous reports, we observed incomplete CD4+ T-cell recovery in mucosal tissues of cART-treated women compared to uninfected participants. There were subtle differences in CD4+ T-cell percentages between mucosal sites, reaching significance only for the comparison between endometrium (with the lowest mean percentages) and sigmoid colon (with the highest). It should be noted that in the absence of data on the number of CD4+ T-cells per unit area in each tissue, it remains unclear the extent to which observed differences in T-cell percentage reflect differences in absolute T-cell numbers.
Although the mechanisms controlling CD4+ T-cell recovery during cART are not fully defined, potential contributing factors include chronic immune activation, residual viral replication, altered expression of mucosal homing and retention molecules and inflammation-associated fibrosis54–57. Chronic immune activation is a better predictor of survival in HIV disease than either HIV RNA or CD4+ T-cell count8, 58, 59, and the efficacy of cART in reconstituting the immune system is strongly correlated with its ability to reduce this activation60–63. Notably, in this study, the highest levels of co-expression of T-cell activation markers CD38 and HLA-DR were found on endometrial CD8+ and CD4+ T-cells in women on cART. The specific mechanisms underlying the maintenance of chronic T-cell activation in this tissue following cART are unclear and will require further study. HIV replication may induce immune activation by directly stimulating innate and adaptive immune responses64; female reproductive hormones also influence immune activation in the FRT65–67. Age may also contribute to these differences, as the mean/median ages of women in this study were 38/39, 48/47.5, and 45.8/45 for HIV-negative, HIV+ without cART, and HIV+ with cART groups, respectively, although the three post-menopausal participants did not contribute reproductive mucosal samples.
An effective CD8+ T-cell response contributes to reduction in peak viremia during acute HIV infection68, 69. In many HIV controllers, strong and polyfunctional HIV Gag-specific T-cell responses are strongly associated with viral control22, 23, 26, 35, 70, 71. cART is associated with decreased HIV-specific T-cell frequency and breadth, largely due to reduced antigen load15, 22, 23, 35, 72, 73. Polyfunctional HIV-specific T-cells have been reported at individual mucosal sites including bronchoalveolar tissues, the gastrointestinal tract and the lower reproductive tract15, 23, 28, 35, 74. However, very little information is available on HIV specific CD8+ T-cell responses in the upper FRT75. In the present study, HIV Gag-specific CD8+ T-cells were detected in parallel in gastrointestinal and reproductive tissues of the same individuals. While the number of endometrial samples obtained from women not on cART was unfortunately too low to draw general conclusions regarding the relative abundance of these cells compared to other mucosal tissues, we were able to demonstrate HIV-specific CD8+ T-cells expressing multiple cytokines in luteal phase endometrium of women both on and off cART.
Cyclic changes in female sex hormones influence CD4+ and CD8+ T-cell abundance, CCR5 expression and cytotoxic T-cell activity in the upper FRT76, 77, with potentially important consequences for HIV susceptibility and disease progression78. CD8+ T-cell responses in the upper FRT are suppressed by high levels of estrogen and progesterone secreted during the luteal phase (days 14 to 28) of the menstrual cycle77. In contrast, such hormonal fluctuations have minimal effects on T-cells in the lower reproductive tract, which remain constant throughout the menstrual cycle79. In the present study, it was not possible to obtain samples at multiple time points; however, given the reported effects of female sex hormones on T-cell responses in the upper FRT, it would be of interest to directly compare T-cell activation and HIV-specific T-cell responses in samples acquired during the proliferative and luteal phases.
In conclusion, these studies reveal important parallels as well as significant differences in CD4+ T-cell frequencies, immune activation and HIV-specific T-cell responses between two major mucosal tissues: the gastrointestinal and reproductive tracts. Importantly, T-cell phenotypes and HIV-specific response patterns differed between blood and mucosal tissues. This finding underscores the importance of studying lymphocyte distribution and antigen-specific responses in tissues, as blood is not always an accurate surrogate marker. This study also calls attention to the potential role of the upper FRT, currently an under-studied tissue, in HIV disease. Future work should attempt to further elucidate the ability of this tissue to serve as a potential reservoir for replicating virus, and to address the role of hormonally regulated, cyclic variation of immune responses in the host’s susceptibility to HIV infection and disease.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the study volunteers for their participation in this work. We thank Jane Pannell RN, ACRN; Carol Thuman RN, MSN, NP; and Jonathan Terdiman MD, for assistance with study coordination, reproductive tract and endoscopic sample collection; and Warner C. Greene MD, PhD for scientific advice and encouragement. This research was supported by NIH/NIAID P01-AI083050 and by the Connie Wofsy Women’s HIV Study (WIHS) (NIH/NIAID U01-AI034989). US, JWC and BLS were supported in part by NIH/NIAID R01-AI057020. This project was supported by the University of California Davis Flow Cytometry Shared Resource with funding from the NCI P30-CA0933730, and NIH NCRR C06-RR012088, S10-RR012964 and S10-RR026825 grants and the James B. Pendleton Charitable Trust, and technical assistance from Ms. Bridget McLaughlin and Mr. Jonathan Van Dyke. The authors declare no conflicts of interest that could be perceived to influence this work.
REFERENCES
- 1.Shacklett BL, Greenblatt RM: Immune responses to HIV in the female reproductive tract, immunologic parallels with the gastrointestinal tract, and research implications. Am J Reprod Immunol 2011;65:230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reynolds MR, Rakasz E, Skinner PJ, White C, Abel K, Ma ZM, Compton L, Napoe G, Wilson N, Miller CJ, Haase A, Watkins DI: CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. J Virol 2005;79:9228–9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenchley JM, Price DA, Douek DC: HIV disease: fallout from a mucosal catastrophe? Nat Immunol 2006;7:235–239. [DOI] [PubMed] [Google Scholar]
- 4.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC: CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 2004;200:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M: Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med 2004;200:761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersson J, Fehniger TE, Patterson BK, Pottage J, Agnoli M, Jones P, Behbahani H, Landay A: Early reduction of immune activation in lymphoid tissue following highly active HIV therapy. AIDS 1998;12:F123–129. [DOI] [PubMed] [Google Scholar]
- 7.Haase AT: Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu Rev Immunol 1999;17:625–656. [DOI] [PubMed] [Google Scholar]
- 8.Hofer U, Speck RF: Disturbance of the gut-associated lymphoid tissue is associated with disease progression in chronic HIV infection. Semin Immunopathol 2009;31:257–266. [DOI] [PubMed] [Google Scholar]
- 9.Shanmugasundaram U, Critchfield JW, Pannell J, Perry J, Giudice LC, Smith-McCune K, Greenblatt RM, Shacklett BL: Phenotype and functionality of CD4+ and CD8+ T cells in the upper reproductive tract of healthy premenopausal women. Am J Reprod Immunol 2014;71:95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poonia B, Wang X, Veazey RS: Distribution of simian immunodeficiency virus target cells in vaginal tissues of normal rhesus macaques: implications for virus transmission. Journal of reproductive immunology 2006;72:74–84. [DOI] [PubMed] [Google Scholar]
- 11.Veazey RS, Marx PA, Lackner AA: Vaginal CD4+ T cells express high levels of CCR5 and are rapidly depleted in simian immunodeficiency virus infection. The Journal of infectious diseases 2003;187:769–776. [DOI] [PubMed] [Google Scholar]
- 12.Lederman MM, Valdez H: Immune restoration with antiretroviral therapies: implications for clinical management. JAMA 2000;284:223–228. [DOI] [PubMed] [Google Scholar]
- 13.Smith K, Aga E, Bosch RJ, Valdez H, Connick E, Landay A, Kuritzkes D, Gross BH, Francis IR, McCune JM, Kessler H, Lederman M: Long-term changes in circulating CD4 T lymphocytes in virologically suppressed patients after 6 years of highly active antiretroviral therapy. AIDS 2004;18:1953–1956. [DOI] [PubMed] [Google Scholar]
- 14.Mehandru S, Poles MA, Tenner-Racz K, Jean-Pierre P, Manuelli V, Lopez P, Shet A, Low A, Mohri H, Boden D, Racz P, Markowitz M: Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med 2006;3:e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayes TL, Asmuth DM, Critchfield JW, Knight TH, McLaughlin BE, Yotter T, McConnell DH, Garcia JC, Pollard RB, Shacklett BL: Impact of highly active antiretroviral therapy initiation on CD4(+) T-cell repopulation in duodenal and rectal mucosa. AIDS 2013;27:867–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheth PM, Chege D, Shin LY, Huibner S, Yue FY, Loutfy M, Halpenny R, Persad D, Kovacs C, Chun TW, Kandel G, Ostrowski M, Kaul R: Immune reconstitution in the sigmoid colon after long-term HIV therapy. Mucosal immunology 2008;1:382–388. [DOI] [PubMed] [Google Scholar]
- 17.Chun TW, Nickle DC, Justement JS, Meyers JH, Roby G, Hallahan CW, Kottilil S, Moir S, Mican JM, Mullins JI, Ward DJ, Kovacs JA, Mannon PJ, Fauci AS: Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. The Journal of infectious diseases 2008;197:714–720. [DOI] [PubMed] [Google Scholar]
- 18.Macal M, Sankaran S, Chun TW, Reay E, Flamm J, Prindiville TJ, Dandekar S: Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal immunology 2008;1:475–488. [DOI] [PubMed] [Google Scholar]
- 19.Gordon SN, Cervasi B, Odorizzi P, Silverman R, Aberra F, Ginsberg G, Estes JD, Paiardini M, Frank I, Silvestri G: Disruption of intestinal CD4+ T cell homeostasis is a key marker of systemic CD4+ T cell activation in HIV-infected individuals. J Immunol 2010;185:5169–5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guadalupe M, Sankaran S, George MD, Reay E, Verhoeven D, Shacklett BL, Flamm J, Wegelin J, Prindiville T, Dandekar S: Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J Virol 2006;80:8236–8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cassol E, Malfeld S, Mahasha P, Bond R, Slavik T, Seebregts C, Poli G, Cassol S, van der Merwe SW, Rossouw T: Impaired CD4+ T-cell restoration in the small versus large intestine of HIV-1-positive South Africans receiving combination antiretroviral therapy. The Journal of infectious diseases 2013;208:1113–1122. [DOI] [PubMed] [Google Scholar]
- 22.Critchfield JW, Young DH, Hayes TL, Braun JV, Garcia JC, Pollard RB, Shacklett BL: Magnitude and complexity of rectal mucosa HIV-1-specific CD8+ T-cell responses during chronic infection reflect clinical status. PLoS One 2008;3:e3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferre AL, Hunt PW, Critchfield JW, Young DH, Morris MM, Garcia JC, Pollard RB, Yee HF Jr., Martin JN, Deeks SG, Shacklett BL: Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood 2009;113:3978–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciccone EJ, Read SW, Mannon PJ, Yao MD, Hodge JN, Dewar R, Chairez CL, Proschan MA, Kovacs JA, Sereti I: Cycling of gut mucosal CD4+ T cells decreases after prolonged anti-retroviral therapy and is associated with plasma LPS levels. Mucosal immunology 2010;3:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George MD, Reay E, Sankaran S, Dandekar S: Early antiretroviral therapy for simian immunodeficiency virus infection leads to mucosal CD4+ T-cell restoration and enhanced gene expression regulating mucosal repair and regeneration. Journal of virology 2005;79:2709–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA: HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 2006;107:4781–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirbod T, Kimani J, Tjernlund A, Cheruiyot J, Petrova A, Ball TB, Mugo N, Jaoko W, Plummer FA, Kaul R, Broliden K: Stable CD4 expression and local immune activation in the ectocervical mucosa of HIV-infected women. J Immunol 2013;191:3948–3954. [DOI] [PubMed] [Google Scholar]
- 28.Bere A, Denny L, Naicker P, Burgers WA, Passmore JA: HIV-specific T-cell responses detected in the genital tract of chronically HIV-infected women are largely monofunctional. Immunology 2013;139:342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKinnon LR, Nyanga B, Kim CJ, Izulla P, Kwatampora J, Kimani M, Shahabi K, Mugo N, Smith JS, Anzala AO, Kimani J, Kaul R: Early HIV-1 infection is associated with reduced frequencies of cervical Th17 cells. J Acquir Immune Defic Syndr 2015;68:6–12. [DOI] [PubMed] [Google Scholar]
- 30.Mkhize NN, Gumbi PP, Liebenberg LJ, Ren Y, Smith P, Denny L, Passmore JA: Persistence of genital tract T cell responses in HIV-infected women on highly active antiretroviral therapy. J Virol 2010;84:10765–10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shacklett BL, Cu-Uvin S, Beadle TJ, Pace CA, Fast NM, Donahue SM, Caliendo AM, Flanigan TP, Carpenter CC, Nixon DF: Quantification of HIV-1-specific T-cell responses at the mucosal cervicovaginal surface. AIDS 2000;14:1911–1915. [DOI] [PubMed] [Google Scholar]
- 32.Carrington M, Walker BD: Immunogenetics of spontaneous control of HIV. Annu Rev Med 2012;63:131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owen RE, Heitman JW, Hirschkorn DF, Lanteri MC, Biswas HH, Martin JN, Krone MR, Deeks SG, Norris PJ: HIV+ elite controllers have low HIV-specific T-cell activation yet maintain strong, polyfunctional T-cell responses. AIDS 2010;24:1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K, Hsue P, Emu B, Krone M, Lampiris H, Douek D, Martin JN, Deeks SG: Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. The Journal of infectious diseases 2008;197:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Critchfield JW, Lemongello D, Walker DH, Garcia JC, Asmuth DM, Pollard RB, Shacklett BL: Multifunctional human immunodeficiency virus (HIV) gag-specific CD8+ T-cell responses in rectal mucosa and peripheral blood mononuclear cells during chronic HIV type 1 infection. J Virol 2007;81:5460–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunt PW: Natural control of HIV-1 replication and long-term nonprogression: overlapping but distinct phenotypes. The Journal of infectious diseases 2009;200:1636–1638. [DOI] [PubMed] [Google Scholar]
- 37.Okulicz JF, Marconi VC, Landrum ML, Wegner S, Weintrob A, Ganesan A, Hale B, Crum-Cianflone N, Delmar J, Barthel V, Quinnan G, Agan BK, Dolan MJ: Clinical outcomes of elite controllers, viremic controllers, and long-term nonprogressors in the US Department of Defense HIV natural history study. The Journal of infectious diseases 2009;200:1714–1723. [DOI] [PubMed] [Google Scholar]
- 38.Bello G, Velasco-de-Castro CA, Bongertz V, Rodrigues CA, Giacoia-Gripp CB, Pilotto JH, Grinsztejn B, Veloso VG, Morgado MG: Immune activation and antibody responses in non-progressing elite controller individuals infected with HIV-1. Journal of medical virology 2009;81:1681–1690. [DOI] [PubMed] [Google Scholar]
- 39.Krishnan S, Wilson EM, Sheikh V, Rupert A, Mendoza D, Yang J, Lempicki R, Migueles SA, Sereti I: Evidence for innate immune system activation in HIV type 1-infected elite controllers. The Journal of infectious diseases 2014;209:931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsue PY, Hunt PW, Schnell A, Kalapus SC, Hoh R, Ganz P, Martin JN, Deeks SG: Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS 2009;23:1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, Nasir K, Grinspoon SK: Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS 2010;24:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLeod KE, Omar T, Tiemessen CT, Tshabangu N, Martinson NA: Prevalence of premalignant cervical lesions in women with a long-term nonprogressor or HIV controller phenotype. J Acquir Immune Defic Syndr 2014;65:e29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pereyra F, Lo J, Triant VA, Wei J, Buzon MJ, Fitch KV, Hwang J, Campbell JH, Burdo TH, Williams KC, Abbara S, Grinspoon SK: Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS 2012;26:2409–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, Young M, Greenblatt R, Sacks H, Feldman J: The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology 1998;9:117–125. [PubMed] [Google Scholar]
- 45.Services DoHaH: Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. . In, 2011, pp 1–166.
- 46.Shacklett BL, Yang O, Hausner MA, Elliott J, Hultin L, Price C, Fuerst M, Matud J, Hultin P, Cox C, Ibarrondo J, Wong JT, Nixon DF, Anton PA, Jamieson BD: Optimization of methods to assess human mucosal T-cell responses to HIV infection. J Immunol Methods 2003;279:17–31. [DOI] [PubMed] [Google Scholar]
- 47.Shacklett BL, Critchfield JW, Lemongello D: Isolating mucosal lymphocytes from biopsy tissue for cellular immunology assays. Methods Mol Biol 2009;485:347–356. [DOI] [PubMed] [Google Scholar]
- 48.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A: Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999;401:708–712. [DOI] [PubMed] [Google Scholar]
- 49.Michie CA, McLean A, Alcock C, Beverley PC: Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature 1992;360:264–265. [DOI] [PubMed] [Google Scholar]
- 50.Ferre AL, Lemongello D, Hunt PW, Morris MM, Garcia JC, Pollard RB, Yee HF Jr., Martin JN, Deeks SG, Shacklett BL: Immunodominant HIV-specific CD8+ T-cell responses are common to blood and gastrointestinal mucosa, and Gag-specific responses dominate in rectal mucosa of HIV controllers. J Virol 2010;84:10354–10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lange CG, Lederman MM, Madero JS, Medvik K, Asaad R, Pacheko C, Carranza C, Valdez H: Impact of suppression of viral replication by highly active antiretroviral therapy on immune function and phenotype in chronic HIV-1 infection. J Acquir Immune Defic Syndr 2002;30:33–40. [DOI] [PubMed] [Google Scholar]
- 52.Stieh DJ, Maric D, Kelley ZL, Anderson MR, Hattaway HZ, Beilfuss BA, Rothwangl KB, Veazey RS, Hope TJ: Vaginal challenge with an SIV-based dual reporter system reveals that infection can occur throughout the upper and lower female reproductive tract. PLoS Pathog 2014;10:e1004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kok A, Hocqueloux L, Hocini H, Carriere M, Lefrou L, Guguin A, Tisserand P, Bonnabau H, Avettand-Fenoel V, Prazuck T, Katsahian S, Gaulard P, Thiebaut R, Levy Y, Hue S: Early initiation of combined antiretroviral therapy preserves immune function in the gut of HIV-infected patients. Mucosal immunology 2014. [DOI] [PubMed] [Google Scholar]
- 54.Hatano H, Somsouk M, Sinclair E, Harvill K, Gilman L, Cohen M, Hoh R, Hunt PW, Martin JN, Wong JK, Deeks SG, Yukl SA: Comparison of HIV DNA and RNA in gut-associated lymphoid tissue of HIV-infected controllers and noncontrollers. AIDS 2013;27:2255–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rueda CM, Velilla PA, Chougnet CA, Montoya CJ, Rugeles MT: HIV-induced T-cell activation/exhaustion in rectal mucosa is controlled only partially by antiretroviral treatment. PloS one 2012;7:e30307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mavigner M, Cazabat M, Dubois M, L’Faqihi FE, Requena M, Pasquier C, Klopp P, Amar J, Alric L, Barange K, Vinel JP, Marchou B, Massip P, Izopet J, Delobel P: Altered CD4+ T cell homing to the gut impairs mucosal immune reconstitution in treated HIV-infected individuals. J Clin Invest 2012;122:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Estes J, Baker JV, Brenchley JM, Khoruts A, Barthold JL, Bantle A, Reilly CS, Beilman GJ, George ME, Douek DC, Haase AT, Schacker TW: Collagen deposition limits immune reconstitution in the gut. The Journal of infectious diseases 2008;198:456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hazenberg MD, Otto SA, van Benthem BH, Roos MT, Coutinho RA, Lange JM, Hamann D, Prins M, Miedema F: Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS 2003;17:1881–1888. [DOI] [PubMed] [Google Scholar]
- 59.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, Shih R, Lewis J, Wiley DJ, Phair JP, Wolinsky SM, Detels R: Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. The Journal of infectious diseases 1999;179:859–870. [DOI] [PubMed] [Google Scholar]
- 60.Ensoli B, Bellino S, Tripiciano A, Longo O, Francavilla V, Marcotullio S, Cafaro A, Picconi O, Paniccia G, Scoglio A, Arancio A, Ariola C, Ruiz Alvarez MJ, Campagna M, Scaramuzzi D, Iori C, Esposito R, Mussini C, Ghinelli F, Sighinolfi L, Palamara G, Latini A, Angarano G, Ladisa N, Soscia F, Mercurio VS, Lazzarin A, Tambussi G, Visintini R, Mazzotta F, Di Pietro M, Galli M, Rusconi S, Carosi G, Torti C, Di Perri G, Bonora S, Ensoli F, Garaci E: Therapeutic immunization with HIV-1 Tat reduces immune activation and loss of regulatory T-cells and improves immune function in subjects on HAART. PloS one 2010;5:e13540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng L, Taiwo B, Gandhi RT, Hunt PW, Collier AC, Flexner C, Bosch RJ: Factors associated with CD8+ T-cell activation in HIV-1-infected patients on long-term antiretroviral therapy. J Acquir Immune Defic Syndr 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hunt PW, Cao HL, Muzoora C, Ssewanyana I, Bennett J, Emenyonu N, Kembabazi A, Neilands TB, Bangsberg DR, Deeks SG, Martin JN: Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS 2011;25:2123–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang X, Hunt PW, Hammer SM, Cespedes MS, Patterson KB, Bosch RJ: Immune Activation While on Potent Antiretroviral Therapy Can Predict Subsequent CD4+ T-Cell Increases Through 15 Years of Treatment. HIV Clin Trials 2013;14:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.d’Ettorre G, Paiardini M, Ceccarelli G, Silvestri G, Vullo V: HIV-associated immune activation: from bench to bedside. AIDS research and human retroviruses 2011;27:355–364. [DOI] [PubMed] [Google Scholar]
- 65.Ferreira VH, Kafka JK, Kaushic C: Influence of common mucosal co-factors on HIV infection in the female genital tract. Am J Reprod Immunol 2014;71:543–554. [DOI] [PubMed] [Google Scholar]
- 66.Chandra N, Thurman AR, Anderson S, Cunningham TD, Yousefieh N, Mauck C, Doncel GF: Depot medroxyprogesterone acetate increases immune cell numbers and activation markers in human vaginal mucosal tissues. AIDS research and human retroviruses 2013;29:592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wira CR, Rodriguez-Garcia M, Patel MV: The role of sex hormones in immune protection of the female reproductive tract. Nature reviews Immunology 2015;15:217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maenetje P, Riou C, Casazza JP, Ambrozak D, Hill B, Gray G, Koup RA, de Bruyn G, Gray CM: A steady state of CD4+ T cell memory maturation and activation is established during primary subtype C HIV-1 infection. J Immunol 2010;184:4926–4935. [DOI] [PubMed] [Google Scholar]
- 69.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF: The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol 2010;10:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Migueles SA, Weeks KA, Nou E, Berkley AM, Rood JE, Osborne CM, Hallahan CW, Cogliano-Shutta NA, Metcalf JA, McLaughlin M, Kwan R, Mican JM, Davey RT Jr., Connors M: Defective human immunodeficiency virus-specific CD8+ T-cell polyfunctionality, proliferation, and cytotoxicity are not restored by antiretroviral therapy. Journal of virology 2009;83:11876–11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Julg B, Williams KL, Reddy S, Bishop K, Qi Y, Carrington M, Goulder PJ, Ndung’u T, Walker BD: Enhanced anti-HIV functional activity associated with Gag-specific CD8 T-cell responses. Journal of virology 2010;84:5540–5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kalams SA, Goulder PJ, Shea AK, Jones NG, Trocha AK, Ogg GS, Walker BD: Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. Journal of virology 1999;73:6721–6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ogg GS, Jin X, Bonhoeffer S, Moss P, Nowak MA, Monard S, Segal JP, Cao Y, Rowland-Jones SL, Hurley A, Markowitz M, Ho DD, McMichael AJ, Nixon DF: Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J Virol 1999;73:797–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brenchley JM, Knox KS, Asher AI, Price DA, Kohli LM, Gostick E, Hill BJ, Hage CA, Brahmi Z, Khoruts A, Twigg HL, 3rd, Schacker TW, Douek DC: High frequencies of polyfunctional HIV-specific T cells are associated with preservation of mucosal CD4 T cells in bronchoalveolar lavage. Mucosal immunology 2008;1:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.White HD, Musey LK, Andrews MM, Yeaman GR, DeMars LR, Manganiello PD, Howell AL, Wira CR, Green WR, McElrath MJ: Human immunodeficiency virus-specific and CD3-redirected cytotoxic T lymphocyte activity in the human female reproductive tract: lack of correlation between mucosa and peripheral blood. The Journal of infectious diseases 2001;183:977–983. [DOI] [PubMed] [Google Scholar]
- 76.Yeaman GR, Guyre PM, Fanger MW, Collins JE, White HD, Rathbun W, Orndorff KA, Gonzalez J, Stern JE, Wira CR: Unique CD8+ T cell-rich lymphoid aggregates in human uterine endometrium. J Leukoc Biol 1997;61:427–435. [PubMed] [Google Scholar]
- 77.White HD, Crassi KM, Givan AL, Stern JE, Gonzalez JL, Memoli VA, Green WR, Wira CR: CD3+ CD8+ CTL activity within the human female reproductive tract: influence of stage of the menstrual cycle and menopause. J Immunol 1997;158:3017–3027. [PubMed] [Google Scholar]
- 78.Kaushic C, Roth KL, Anipindi V, Xiu F: Increased prevalence of sexually transmitted viral infections in women: the role of female sex hormones in regulating susceptibility and immune responses. Journal of reproductive immunology 2011;88:204–209. [DOI] [PubMed] [Google Scholar]
- 79.Pudney J, Quayle AJ, Anderson DJ: Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod 2005;73:1253–1263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


