Abstract
Altered mitochondrial respiration, morphology, and quality control collectively contribute to mitochondrial dysfunction in the aged heart. Because myocardial infarction remains the leading cause of death in aged women, the present study utilized a novel rodent model to recapitulate human menopause to interrogate the combination of age and estrogen-deficiency on mitochondrial ultrastructure and function with cardiac ischemia/reperfusion (I/R) injury. Female F344 rats were ovariectomized (OVX) at 15 mo and studied at 24 mo (MO OVX; n=41) vs adult ovary-intact (6 mo; n=41). Temporal declines in estrogen concomitant with increased visceral adipose tissue were observed in MO OVX vs adult. Following in vivo coronary artery ligation or sham surgery, state 3 mitochondrial respiration was selectively reduced by age in subsarcolemmal mitochondria (SSM), and by I/R in interfibrillar mitochondria (IFM); left ventricular maximum dP/dt was reduced in MO OVX (p<0.05). Elevated cyclophilin D and exacerbated I/R-induced mitochondrial acetylation in MO OVX suggest permeability transition pore involvement and reduced protection vs adult (p<0.05). Mitochondrial morphology by TEM revealed an altered time course of autophagy coordinate with attenuated Drp1 and LC3BII protein levels with age-associated estrogen loss (p<0.05). Here, reductions in both SSM and IFM function may play an additive role in enhanced susceptibility to regional I/R injury in aged estrogen-deficient female hearts. Moreover, novel insight into altered cardiac mitochondrial quality control garnered here begins to unravel the potentially important regulatory role of mitochondrial dynamics on sustaining respiratory function in the aged female heart.
Keywords: menopause, reperfusion injury, myocardial infarction, mitochondrial quality control, senescence
Introduction
Myocardial infarction is the leading cause of death in aged females [23], however mitochondrial mechanisms responsible for ischemic injury in the aged estrogen (E2)-deficient female heart remain incompletely characterized. The ability to clear damaged mitochondria through general autophagy and/or mitophagy following ischemia and reperfusion (I/R) injury has emerged as a critical mechanism impacting the maintenance of mitochondrial respiration with advancing age and disease [82, 61, 53]. Moreover, cardiac mitochondrial subpopulations contribute distinctly to cell death through regulation of energy metabolism and cardiac contraction dependent on cellular location [67, 62, 74, 29]. In contrast to previously published studies in males highlighting age-related defects in interfibrillar mitochondria (IFM) [67, 31, 73, 51, 50, 21, 59, 77], we have previously reported age-dependent functional declines in subsarcolemmal mitochondria (SSM) from females [36]. Importantly, the joint effects of aging and menopausal loss of E2 influence cardiovascular-related mortality in females, emphasizing the necessity of a more accurate model of female aging used herein [58, 57]. The interactive effects of altered mitochondrial function and morphology on age-associated vulnerability to cardiac cell death is largely unexplored in the female heart.
In the absence of ischemia (I), increased mitochondrial volume is observed with aging in male rodents [80, 16, 18]. Following ischemic periods of 30 min or longer, mitochondria undergo irreversible cell death, hallmarked by severe mitochondrial cristae and matrix disruption [27, 24]. Though the regulation of mitochondrial quality control during I/R injury is incompletely understood [55, 56], activation of mitophagy subsequent to dynamin-like GTPase related protein (Drp-1)-mediated fission is associated with cardioprotection [61, 37]. In this regard, skeletal muscle-specific E2 receptor (ERα) depletion is associated with enlarged dysfunctional mitochondria, ROS excess and fission/fusion imbalance, in part mediated by defective Drp-1 [72]. The impact of age-associated E2-deficiency on mitochondrial quality control and function with I/R injury in female animals is currently unknown.
Historically, achieving a precise animal model to recapitulate human menopause has been difficult, primarily due to variations in the onset of reproductive senescence in rodents [54, 48, 8]. Although middle-aged rats (12–14 mo) undergo ―estropause”, variable time to anestrous influences the temporal magnitude of E2 loss in aged ovary intact female rats [34, 35, 60, 33]. As such, the use of ovariectomy (OVX) to model human E2 loss has been widely used in adult rodents [11]. However, a proteomic study from our laboratory revealed a specific mitochondrial response of adult rats to OVX which fails to mimic age-associated E2-deficiency [45], as only 6 mitochondrial proteins changed similarly in adult OVX vs aged.
To inform mechanisms of cardiac cell death sensitivity in the aged female rat heart, the present study aimed to determine changes in mitochondrial subpopulation respiration and morphological responses following regional I/R injury using a novel, physiological model of age-associated E2-deficiency. Here, female rats were OVX at middle age (15 mo) and studied at 22–24 mo (MO OVX) to better simulate systemic effects of the human menopausal transition, standardize temporal declines in circulating E2 with age, and assess the combined physiological effect of age-associated E2 loss. We demonstrate for the first time, a selective cardiac mitochondrial subpopulation response (state 3 respiration) in aged female rats to ischemic insult not previously observed. Additionally, our data suggest that age-associated E2-deficiency may negatively impact mitochondrial quality control during I/R injury, thereby undermining protective reserves.
Materials and Methods
Animals.
Female Fischer 344 (F344) rats were obtained from the NIA colony at Charles River (Wilmington, MA). Rats were housed in an AAALAC certified facility, exposed to a 12-h light/dark cycle and received a standard laboratory rodent diet (LabDiet) and water ad libitum. All animal experimentation described was conducted with approval from the Institutional Animal Care and Use Committee of the Pennsylvania State University. All surgical procedures were carried out under anesthesia (40 mg/kg ketamine, 12 mg/kg xylazine, i.p.), the depth of which was determined by tail reflex prior to intubation and toe pinch during the procedure. If more anesthetic was warranted, 13 mg/kg of ketamine was delivered i.p.; the analgesic buprenorphine (0.3 mg/kg, i.m.) was administered upon anesthetic reversal. Groups studied were adult (5–6 mo, n=41) and MO OVX (OVX at 15 mo and aged to 22–24 mo, n=40). Out of a total number of 90 rats, 81 were included in the study. Criteria for exclusion were age-associated weight loss and morbidity (n=2), small AAR (<30%, n=2) or early death following coronary artery ligation (CAL; n=5; 12% in MO OVX and 2% in adult).
Surgical Ovariectomy.
All rats in the MO OVX group were subjected to bilateral OVX at 15 mo; surgeries were performed by the supplier (Charles River). Blood glucose levels were determined by tail stick (One Touch Ultra). Trunk blood prior to euthanasia was used to assess serum E2 concentrations by RIA of duplicate samples (Ultra-Sensitive E2 RIA) with an assay sensitivity of 5 pg/ml. Uterine weight was used to confirm E2-deficiency.
CAL procedure.
Following intubation, the chest was opened in the third rib space, the left coronary artery was visualized and ligated using 3–0 prolene suture (sham animals did not receive ligation). After 31 min of ischemia, the ligature was released and the myocardium was exposed to variable reperfusion (R) times (10 min, 6 hr, 24 hr). The heart was removed by midline thoracotomy, and perfused with Evan’s Blue dye (1%) to demarcate area at risk (AAR). Additionally, visceral adipose tissue (VAT) was removed at time of sacrifice and weighed.
Mitochondrial Isolation.
Mitochondria were isolated from left ventricular (LV) tissue displaying a 30–40% AAR from adult and MO OVX following 31 min ischemia and 10 min reperfusion [66, 28] or sham as previously described [67]. Briefly, the LV was roughly minced in sucrose, tris, EGTA (STE) buffer containing (in mM): 300 sucrose, 10 Tris, and 2 EGTA. The homogenate was centrifuged in STE containing 0.5% BSA. The supernatant contained the SSM, while the pellet contained the IFM. The pellet was subjected to nagarse digestion (5 mg/g wet weight of LV) and subsequently resuspended in STE following appropriate separation and washing. The final protein concentration of individual mitochondrial populations was determined using the Bradford method as previously described [7].
Mitochondrial Respiration.
Isolated mitochondria were respired using a Clarke-type electrode attached to a YSI oxygraph (Yellow Springs, OH) as described by us previously [36]. Briefly, mitochondria were incubated in a buffer containing (in mM): 125 KCl, 20 MOPS, 10 Tris, 2 MgCl2, 2 KH2PO4, 0.5 EGTA, pH 7.2. Complex I respiration was measured in the presence of 2.5 mM α-ketoglutarate + 1 mM malate, while Complex II respiration was measured in the presence of 1 μM rotenone + 2.5 mM succinate; state 3 respiration was initiated by addition of ADP (final concentration 1 mM). Groups studied for mitochondrial isolation and respiration were adult sham (n=8), adult I/R (n=10), MO OVX sham (n=8), and MO OVX I/R (n=7).
Tissue Homogenization.
LV tissue from adult and MO OVX following 31 min ischemia and 6 hr reperfusion [15, 41, 78, 2] or sham were frozen and homogenized by glass-glass grinding and subcellular separation of the cytosolic and mitochondrial fractions was performed using differential centrifugation as described by us previously [34, 83, 46, 65, 44, 35]. All protein concentrations were determined by the Bradford method [7].
Western Blot Analysis.
Western blotting was executed according to well-established procedures in our laboratory [43, 83, 46, 65, 44, 35]. Equal amounts of mitochondrial or cytosolic protein per lane were electrophoresed on Criterion SDS-polyacrylamide gels (Bio-Rad) and transferred to polyvinylidene difluoride membranes (Millipore). After blocking, samples were probed with primary antibodies against cyclophilin D (CypD; AbCam, 1:12,000, 5 μg), SIRT3 (1:12,000, 5 μg), acetylated-lysine (1:1,000, 20 μg), LC3B (1:1,000, 20 μg), and Drp1 (Santa Cruz, 1:1,000, 20 μg). Proteins were visualized using enhanced chemiluminescence (GE Amersham) and densitometry performed using ImageJ. SYPRO Ruby staining (Invitrogen, Grand Island, NY) of the membrane was performed and visually inspected for consistency of protein loading [70]. All antibodies were purchased from Cell Signaling unless otherwise specified. Groups studied for western blotting were adult sham (n=3), adult I/R (n=5), MO OVX sham (n=4), and MO OVX I/R (n=6).
Transmission Electron Microscopy (TEM) tissue sample preparation.
After injection with Evan’s blue dye, 1 mm3 samples were taken from the AAR of adult and MO OVX following 31 min ischemia and 10 min (n=3/group), 6 hr (n=4/group), 24 hr reperfusion (n=2/group) [2], or sham surgery (n=7 in adult, n=8 in MO OVX). Samples were immersed in fixative containing 0.1M cacodylate buffer, 16% paraformaldehyde, and 25% glutaraldehyde; post-fixation in 1% osmium tetroxide was followed by 2% uranyl acetate. Processed sample blocks were trimmed and sectioned (70 nm) using a Leica Ultracut UCT Microtome (Buffalo Grove, IL, USA) and placed on a copper TEM sample grid. Grids were double stained with uranyl acetate and lead citrate then samples were randomly imaged with a JEOL 1200 EXII TEM (Peabody, MA, USA) and Gatan camera. Mitochondrial area was determined in four randomly chosen longitudinal images per rat, using ImageJ (NIH).
Qualitative Analysis.
Qualitative analysis of mitochondrial ultrastructure was assessed according to a modified scoring system previously described [85] for electron densities, cristae disruption, and myofibrillar disarray. Cristae disruption is characterized by transition from dense tightly packed concentric cristae to the presence of tortuous cristae with matrix clearing. Myofibrillar disarray is characterized by Z-line streaming, increased spacing between myofilaments and distortion of the contractile elements. The following scale was constructed for each image: (+) present 0–25%, (++) 26–50%, (+++) 51–75%, or (++++) 76–100% of the image in 3–4 images/rat.
Left Ventricular Function.
A separate cohort of rats underwent CAL with 55 min ischemia. Following 6 hr of reperfusion, rats were intubated and the right carotid artery was isolated by blunt dissection. A small transverse slit was made and a Millar mikrotip pressure sensor (Colorado Springs, CO) was inserted and advanced into the LV. After a 15 min equilibration time, 15 min of data was collected for the calculation of maximum dP/dt (dP/dtmax) and heart rate measurements. Groups studied for LV function were adult sham (n=9), adult I/R (n=4), MO OVX sham (n=9), and MO OVX I/R (n=4).
Statistics.
All data are presented as means ± SEM and analyzed using Sigmastat. Data were compared using ANOVA: either one-way ANOVA (baseline measures only) or two-way ANOVA (age × I/R injury) as indicated. Significant interactions were analyzed with a Tukey post-hoc test. An α level of p≤0.05 was defined as statistically significant.
Results
Characteristics of adult and MO OVX rats upon euthanasia are displayed in Table 1. Body weight, left ventricular weight (LVW), and VAT weight were significantly increased with age, while uterine weight was significantly reduced (compared to adult; p<0.001). Normalized to body weight, uterine weight of MO OVX was 35% that of adult (0.74 vs. 2.09). Furthermore, serum E2 levels were significantly reduced in MO OVX compared to adult, (12.87±1.84 vs 5.61±1.72 pg/ml; p<0.01; n=15 in adult and n=21 in MO OVX) verifying E2-deficiency. VAT increased ~3-fold in MO OVX compared to adult representing a remarkable similarity to that seen in aging women [22]. Notably, blood glucose levels during the fed state were not different between groups (103.5±1.50 vs 104.8±3.74 mg/dL; n=6 per group).
Table 1.
Physiological characteristics of adult and MO OVX F344 raats that underwent CAL (31 min I and 10 min, 6 hr, or 24 hr R) or sham surgery.
| Characteristic | Adult | MO OVX | ||
|---|---|---|---|---|
| Sham (n=11) | I/R (n=17) | Sham (n=12) | I/R (n=15) | |
| Body Weight (g) | 203.3 ± 2.90 | 203.5 ± 2.08 | 313.5 ± 3.68 * | 321.9 ± 5.15 * |
| LVW (mg) | 456.1 ± 4.86 | 474.7 ± 8.77 | 617.0 ± 13.8 * | 652.5 ± 8.61 *† |
| Uterine Weight (g) | 0.463 ± 0.03 | 0.399 ± 0.04 | 0.282 ± 0.05 * | 0.213 ± 0.02 * |
| Uterine Weight/Body Weight (mg/g) | 2.280 ± 0.12 | 1.966 ± 0.21 | 0.840 ± 0.16 * | 0.665 ± 0.06 * |
| VAT (g) | 7.466 ± 0.68 | 6.664 ± 0.39 | 17.64 ± 0.77 * | 18.93 ± 0.64 * |
Results are presented as means ± SEM. MO OVX, 24 mo female rats ovariectomized at 15 mo; LVW, left ventricular weight; VAT, visceral adipose tissue.
p<0.001 effect of age-associated E2-loss.
p<0.05 effect of I/R within age status.
Protein yield of SSM and IFM following sham or CAL (31 min I / 10 min R) of adult vs. MO OVX is displayed in Fig 1. SSM yield was unaffected by age or I/R. In contrast, IFM yield was significantly reduced by 37% in the MO OVX compared to adult (p<0.001) with no additional effect of I/R, indicating a decline in the IFM subpopulation with age-associated E2 loss unrelated to an ischemic challenge. State 3 respiration rate in SSM (panel A) and IFM (panel B) is displayed in Fig 2. As expected, IFM respired at a higher rate compared to SSM (p<0.001). Statistically significant reductions in state 3 respiration energized by either Complex I or Complex II substrates were observed with age in SSM (8%) and with I/R in IFM (13–20%; p<0.05). Representative images of consistently obtained ~37% AAR are displayed in Fig 3. LV dP/dtmax (A) dP/dtmin (B) at baseline and following CAL (55 min I / 6 hr R) are displayed in Fig 4. Advancing age and I/R significantly reduce dP/dtmax by 21% and 27%, respectively and dP/dtmin by 27% and 30%, respectively (p<0.05). Group differences in HR were not observed (data not shown).
Fig 1:

Protein yield of subsarcolemmal mitoschondria (SSM) and interfibrillar mitochondria (IFM) following isolation from adult and MO OVX female F344 rat left ventricle (LV) following sham or CAL (31 min I/10 min R). Data are presented as a mean ± SEM (n=7–10 per group). * p<.001 main effect of age-associated E2-loss within IFM.
Fig 2.
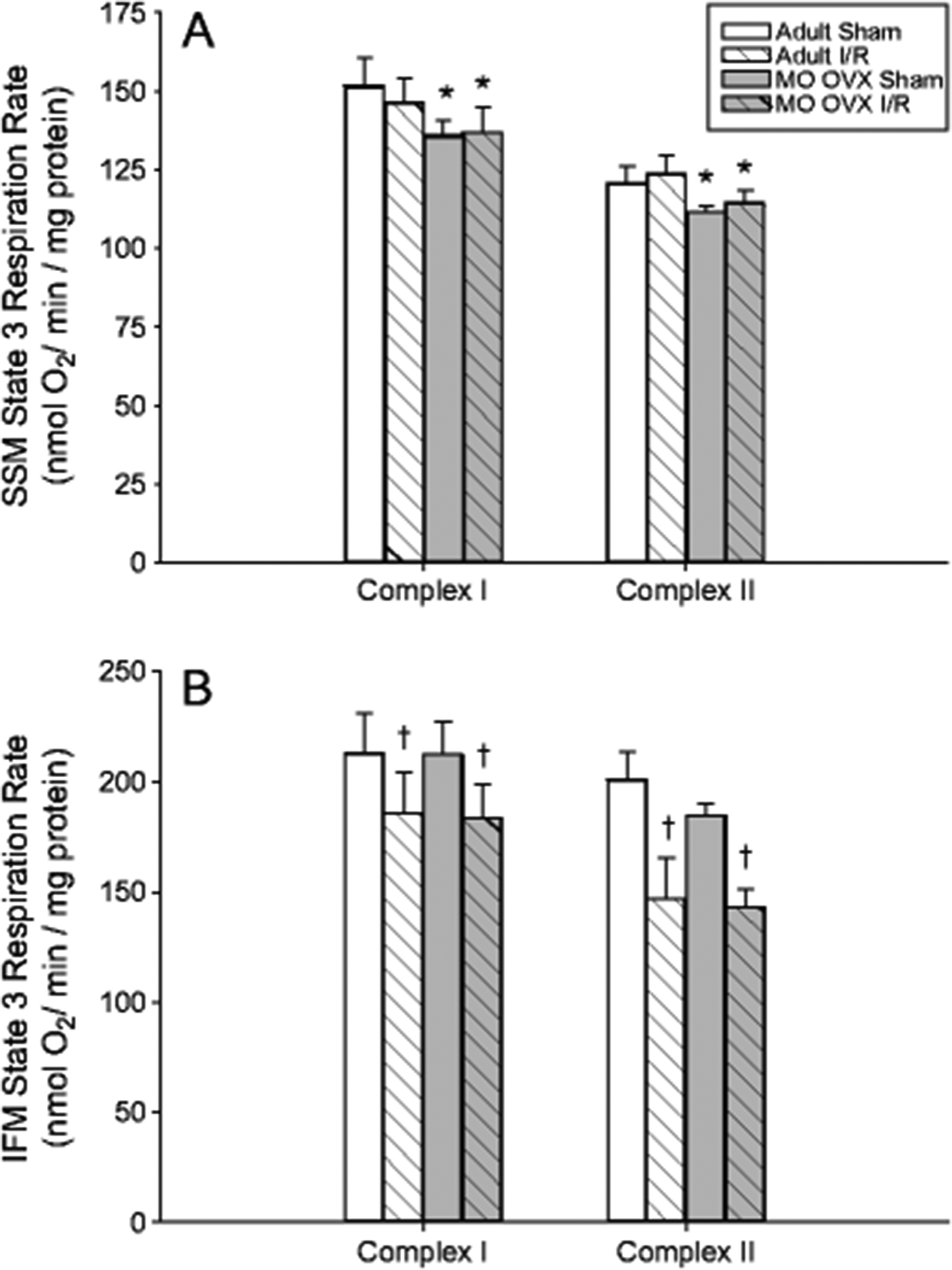
State 3 mitochondrial respiration rate in adult and MO OVX female F344 rat LV tissue following sham or CAL (31 min I/10 min R). State 3 respiration at Complex I or Complex II in SSM (panel A) and IFM (panel B). Data are presented as a mean ± SEM (n=7–10 per group). * p<0.05 main effect of age-associated E2-loss; † p<0.01 main effect of I/R; ‡ p<.001 main effect of population.
Fig 3.

Representative Evan’s Blue staining of adult (A) and MO OVX (B) hearts following CAL demonstrating ~37% AAR.
Fig 4.

LV maximum dP/dt measured in vivo in sham vs CAL-operated animals (55 min I/6 hr R). Data are presented as a mean ± SEM (n=4–9/group). * p<0.05 effect of age-associated E2-loss; † p<0.05 effect of I/R.
Mitochondrial protein levels of cyclophilin D (CypD), SIRT3, and acetylated-lysine following CAL (31 min I / 6 hr R) are displayed in Fig 5. CypD protein was significantly increased by 42% with age independent of I/R injury (panel A; p<0.001). SIRT3 was significantly reduced by I/R in adult (panel B; p<0.05) and increased by age following I/R (p<0.001) leading to an interaction between age and I/R (p<0.02). Mitochondrial protein acetylation was also significantly increased in MO OVX by ~200% compared to adult following I/R (panel C; p<0.01).
Fig 5.

Mitochondrial protein levels of cyclophilin D (CypD; A), SIRT3 (B) and acetylated-lysine residues (C) in adult and MO OVX F344 rat LV tissue following sham or CAL (31 min I/6 hr R) with representative immunoblots. Data are presented as a mean ± SEM (n=3–6 per group); * p<0.001 effect of age-associated E2-loss within I/R status; † p<0.05 effect of I/R within age status; age × I/R interaction in SIRT3 (p<0.05).
Mitochondrial morphological analysis is displayed in Fig 6. Following CAL with 10 min reperfusion and relative to sham baseline control responses, mitochondrial area increased by 26% in adult (p<0.001) but not MO OVX. Increases in mitochondrial area persisted at 6 hr reperfusion in adult, and a 29% increase was observed in MO OVX compared to sham (p<0.01). Mitochondrial area decreased in adult at 24 hr reperfusion, reaching similar values observed for the sham only condition. In contrast, mitochondrial area remained increased (16%) in MO OVX at 24 hr reperfusion (p<0.05). Qualitative characteristics of electron densities, disrupted cristae, and myofibrillar disarray are displayed in Fig 7 (panel E). Increased prevalence of electron densities began at 10 min and 6 hr reperfusion in adult and MO OVX, respectively. Disrupted cristae were more prevalent and severe in MO OVX under conditions of sham and reperfusion at 10 min and 24 hr post ischemic insult vs adult. Greater myofibrillar disarray was also observed at 6 and 24 hr reperfusion in MO OVX compared to adult. Representative examples of electron densities (panel A), disrupted cristae (panel B), and myofibrillar disarray (panel C) from adult I/R samples are presented in Fig 7. Interestingly, autophagasomes (panel D) were detected in adult I/R, but not MO OVX I/R at 6 hr reperfusion, suggesting altered autophagic temporal responses in aged. Accordingly, mitochondrial Drp1 and cytosolic LC3BII were assessed and are displayed in Fig 8. Drp1, a marker of fission-mediated mitophagy, was significantly increased by I/R in adult (panel A; p<0.05). No age effect was observed in sham animals, but the response to I/R resulted in a significant decrease of 39% in MO OVX compared to adult (p<0.05) exposing a blunted activation of Drp1 upon I/R with age. Greater increases in LC3BII, a marker of general autophagy, were observed in adult (78%) compared to MO OVX during reperfusion following ischemia (panel B; p<0.05). LC3BII is undetectable in sham groups.
Fig 6.

Mitochondrial area garnered from TEM images from adult and MO OVX LV following sham or CAL (31 min I) and varying R times (10 min, 6 hr, 24 hr). Data are presented as a mean ± SEM (n=7–8 for sham groups and n=2–4 for I/R groups); † p<.001 effect of I/R vs sham in adult and p<0.05 effect of I/R vs sham in MO OVX.
Fig 7.

Representative TEM images of mitochondrial ultrastructure in an adult heart. Electron densities (black arrows, A), disrupted cristae and matrix clearing (white arrows, B), myofibrillar disarray (C), and autophagic vesicles (black arrows, D). E) The occurrence of ultrastructural components was assessed qualitatively in adult and MO OVX mitochondria at varying reperfusion times; +, observed in 25%; ++, observed in 50%; +++, observed in 75% (n=2–8 per group).
Fig 8.

Protein levels of mitochondrial Drp1 (A) and cytosolic LC3BII (B) in adult and MO OVX F344 rat LV tissue following sham or CAL (31 min I/6 hr R) with representative immunoblots. Data are presented as a mean ± SEM (n=3–6 per group); * p<0.05 effect of age-associated E2-loss within I/R status; † p<0.05 effect of I/R within age status.
Discussion
The current study utilized a novel model to recapitulate human menopause in aged female rats and aimed to characterize, for the first time, cardiac mitochondrial subpopulation respiratory behavior and morphological responses to ischemic stimuli. Our results suggest a phenotype where reductions in both SSM and IFM function may play an additive role in the enhanced susceptibility to cardiac I/R injury in aged E2-deficient females compared to adults. The main findings are as follows: 1) OVX at 15 mo in rats produced a physiologically timed decline in E2 similar to that observed in postmenopausal women, 2) age-associated E2-deficiency significantly reduced state 3 respiration in SSM while IFM were affected more by I/R injury, and 3) enlarged mitochondria with blunted activation of quality control proteins and greater protein acetylation were observed following I/R injury in aged vs adult females. Collectively these results are suggestive of decreased mitophagy and delayed resolution of mitochondrial damage following infarction, providing the first insight into cardiac mitochondrial health following an ischemic insult in a physiological model of age-associated E2-deficiency.
Previous models used by our lab to evaluate age-associated E2-deficiency include adult OVX (6 mo), aged (24 mo; ovary intact) or aged OVX (OVX at 23 mo and studied at 24 mo) female rats. Here, we further refine our approach using the MO OVX rat to more closely model human menopause, including increased VAT [47, 84, 81, 22, 42], which directly correlates with increased acute myocardial infarction (AMI) [64]. Over time, increases in abdominal fat area of 3.3-fold are observed in older (70 yrs) vs younger (20 yrs) women [22], which is nearly identical to the magnitude of increase in VAT growth observed here in MO OVX vs adult rats within a similar age context (i.e. 5–6 mo vs 23–24 mo). Previous results from our laboratory indicate a significantly lower increase in VAT with aged ovary intact vs adult (1.7-fold). Therefore, the MO OVX rat may represent more accurate timing of E2 loss over time and allow for the development of a metabolic environment more consistent with human female aging. Yet, importantly, blood glucose level in the MO OVX rat is normal.
In the absence of ischemia, previous work in male rats is suggestive of IFM-selective dysfunction with senescence [51, 50, 59, 21, 40]. In contradistinction, we provide evidence for a decline in SSM with age-associated E2 loss, but not IFM respiratory function in females. These findings extend our previous results indicating exacerbated swelling in SSM from the aged female heart [36], and are complimented by unchanged SSM protein content. Following I/R injury, respiratory dysfunction was specific to IFM in adult and aged females, but the long term functional impact in vivo is likely greater in aged due to significant reductions in IFM yield in MO OVX. Indeed, greater declines in LV dP/dtmax and dP/dtmin were observed in MO OVX vs adult following I/R injury. Given our observations that mortality in MO OVX increases by 15%, 20%, and 40% following 6 hr, 24 hr, and 3 days of reperfusion, respectively, compared to adult (data not shown), we speculate that LV function may worsen throughout reperfusion in aged vs adult in part due to IFM loss. Because ischemic injury in males has been reported to disrupt both SSM and IFM subpopulations [52, 12, 14, 13, 49, 39], our findings of an IFM specific-defect in females may also support a previously uncharacterized gender associated phenotype in ischemia-induced mitochondrial dysfunction. Alternatively, differences amongst studies may arise from use of global vs regional ischemic injury. Here, I/R insult was induced by CAL in vivo whereby the full impact of the systemic inflammatory response is captured. To the best of our knowledge, all prior investigations of SSM and IFM respiration following I/R in males utilized a model of global ischemia within the context of the isolated heart [52, 12, 14, 13, 49, 39]. Indeed, unpublished observations from our laboratory indicate that global ischemia in the isolated adult female heart reduces state 3 respiration of both SSM and IFM. An issue deserving of future study includes the physiological relevance of different ischemic models to evaluate the contribution of mitochondrial dysfunction to the natural history of cardiac AMI with aging. Coordinate assessment of SSM and IFM function at varying time points during reperfusion is also indicated.
I/R injury induces calcium overload, ATP depletion, and permeability transition pore (PTP) opening [4, 30] leading to mitochondrial destruction and cell death. Because PTP opening is known to be regulated by CypD [3, 63, 20], I/R-induced reductions in IFM respiration may also be related to the observation that IFM express significantly more CypD compared to SSM in rat heart [5]. Indeed, we observed elevated CypD levels in aged which likely impact PTP opening and overall mitochondrial function in vivo. Additionally, deacetylation by SIRT3 can activate complex I [1] and inhibit CypD [76], fostering a cardioprotective milieu. Here, though SIRT3 protein levels were increased by age following I/R, significantly elevated mitochondrial acetylation presumably reflects reduced SIRT3 activity in the aged. Given known reductions in complex I subunits, and therefore reduced NAD+ availability with age [45], we speculate that diminished SIRT3 activity in the presence of elevated CypD may contribute to reduced mitochondrial function in IFM following an ischemic insult. The mechanisms of estrogenic action on mitochondrial function, particularly on SSM vs IFM, remain speculative. Mitochondrial respiration and ATP production are reduced by OVX in both cardiac and skeletal muscle of adult rodents, and normalized by in vivo E2 supplementation [10, 71, 9]. We, among others, have previously demonstrated localization of ERα to cardiac mitochondria [65, 68, 32, 87], with significant reductions in aged vs adult females. Moreover, acute ERα agonism can ameliorate age-associated defects in cardiac SSM respiration [36]. Recently, knock down of skeletal muscle ERα levels was associated with profound reductions in mitochondrial respiratory capacity and aberrations in mitochondrial ultrastructure and dynamics [72], providing compelling evidence for a regulatory role for ER signaling on mitochondrial function. ERα activation is also involved in the transcription of major components of the electron transport chain including subunits of complex I, complex IV, and the ATP synthase [10]. Indeed, a recent proteomic screen conducted by our laboratory illustrated a mechanistic link between age-associated E2 loss and mitochondrial oxidative phosphorylation proteins [45]. Thus, observed decrements in SSM respiration with age may be influenced by the loss of estrogenic effects on mitochondrial protein expression. Given known structural and functional differences in SSM and IFM [29], it is conceivable that the effects of E2 are also subpopulation specific.
To inform the relationship between changes in mitochondrial function and ultrastructure with cardiac female aging, we next examined mitochondrial morphology prior to and at varying time points following ischemia. In the absence of ischemia, mitochondrial area did not change with age, and contrasts known volume increases reported previously in aged male mouse hearts [80, 16, 18]. Increased prevalence of disrupted cristae in aged prior to ischemia is consistent with findings in adult OVX rats [89] and ERα knockout mice [88], raising interesting speculation regarding the role of E2 signaling on mitochondrial ultrastructure. Here, I/R injury differentially affected the temporal response of mitochondrial morphology in aged vs adult. Though IFM function was similarly reduced in adult and aged following 10 min of reperfusion, increased mitochondrial size was observed in adult only, and likely associated with calcium-induced swelling [6, 69]. At 6 hr of reperfusion, enlarged mitochondria were accompanied by a notable absence of autophagic vacuoles in aged, perhaps indicating dysregulated mitochondrial quality control response in aged females [25, 17, 86]. At 24 hr of reperfusion, adult mitochondria returned to pre-ischemic size while increased mitochondrial area persisted in aged along with a greater prevalence of disrupted cristae. That Drp1-related mitophagy is associated with reduced mitochondrial size in mice under pathological conditions [75] provides an interesting mechanistic link between mitochondrial dynamism and observed changes in mitochondrial area described herein. Alternatively, prolonged mitochondrial enlargement combined with attenuated I/R-related Drp1 and LC3BII expression in aged may reflect impaired fission and aberrant autophagic flux known to foster dysfunctional mitochondrial populations [19, 38, 79, 53]. Future studies are necessary to characterize the potential influence of delayed mitophagy on mitochondrial respiration ultrastructure, and turnover in the aged female heart [82].
Conclusions
Here we utilize a unique model of age-associated E2 loss in an attempt to better recapitulate the impact of human reproductive senescence and declines in circulating E2 on cardiac I/R injury in aged females. The findings herein examine effects of the unique milieu produced by aging and estrogen-deficiency on the female heart. Ischemic heart disease remains the number one killer of aging postmenopausal women, and it is well known that strategies such as estrogen replacement have been insufficient to limit cardiac cell death in this population and are in fact, contraindicated [26]. Thus, use of female-specific models that are inclusive of advanced age and diminished estrogen in tandem are pathologically relevant and necessary to characterize the full mechanistic spectrum of ischemic cardiac cell death. Here, the MO OVX model shows promise as a platform to investigate the cellular processes involved in age-related vulnerability to cardiac ischemia/ reperfusion injury, and provide insight into how mechanisms differ from previous studies conducted in aged males. The data presented here also employ a physiological model of regional coronary I/R injury to induce myocardial infarction. Novel findings include adaptive functional responses in SSM and IFM to age and I/R injury in females. Though infarct size was not directly assessed, increases in mitochondrial acetylation, CypD, blunted markers of mitophagy, and altered mitochondrial ultrastructure in MO OVX rats are likely to enhance susceptibility to I/R injury and AMI in the post-menopausal female heart. These results may have important implications for therapeutic interventions and treatment of ischemic heart disease in aged women.
Acknowledgements:
The authors acknowledge the TEM analysis provided by Jessica Henry
Funding: National Heart, Lung, and Blood Institute [HL091097 to DHK])
National Institute on Aging [AG044132 to AMG, DHK]
Biological Seed Grant mechanism from the College of Health and Human Development and Huck Institutes of the Life Sciences [001 to DHK], Pennsylvania State University.
Footnotes
Conflict of Interest: The authors declare that they have not conflict of interest.
References
- 1.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T (2008) A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A 105:14447–14452. doi:0803790105 [pii] 10.1073/pnas.0803790105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azhar G, Gao W, Liu LX, Wei JY (1999) Ischemia-reperfusion in the adult mouse heart Influence of age. Experimental Gerontology 34:699–714. doi:Doi 10.1016/S0531-5565(99)00031-5 [DOI] [PubMed] [Google Scholar]
- 3.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD (2005) Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434:658–662. doi:nature03434 [pii] 10.1038/nature03434 [DOI] [PubMed] [Google Scholar]
- 4.Bers DM, Barry WH, Despa S (2003) Intracellular Na+ regulation in cardiac myocytes. Cardiovasc Res 57:897–912. doi:S0008636302006569 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Boengler K, Stahlhofen S, van de Sand A, Gres P, Ruiz-Meana M, Garcia-Dorado D, Heusch G, Schulz R (2009) Presence of connexin 43 in subsarcolemmal, but not in interfibrillar cardiomyocyte mitochondria. Basic Research in Cardiology 104:141–147. doi:DOI 10.1007/s00395-009-0007-5 [DOI] [PubMed] [Google Scholar]
- 6.Bosetti F, Baracca A, Lenaz G, Solaini G (2004) Increased state 4 mitochondrial respiration and swelling in early post-ischemic reperfusion of rat heart. FEBS Lett 563:161–164. doi: 10.1016/S0014-5793(04)00294-7S0014579304002947 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:S0003269776699996 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Broekmans FJ, Soules MR, Fauser BC (2009) Ovarian aging: mechanisms and clinical consequences. Endocr Rev 30:465–493. doi:er.2009-0006 [pii] 10.1210/er.2009-0006 [DOI] [PubMed] [Google Scholar]
- 9.Capllonch-Amer G, Sbert-Roig M, Galmes-Pascual BM, Proenza AM, Llado I, Gianotti M, Garcia-Palmer FJ (2014) Estradiol stimulates mitochondrial biogenesis and adiponectin expression in skeletal muscle. J Endocrinol 221:391–403. doi:JOE-14-0008 [pii] 10.1530/JOE-14-0008 [DOI] [PubMed] [Google Scholar]
- 10.Cavalcanti-de-Albuquerque JP, Salvador IC, Martins EL, Jardim-Messeder D, Werneck-de-Castro JP, Galina A, Carvalho DP (2014) Role of estrogen on skeletal muscle mitochondrial function in ovariectomized rats: a time course study in different fiber types. J Appl Physiol (1985) 116:779–789. doi:japplphysiol.00121.2013 [pii] 10.1152/japplphysiol.00121.2013 [DOI] [PubMed] [Google Scholar]
- 11.Chakraborty TR, Gore AC (2004) Aging-related changes in ovarian hormones, their receptors, and neuroendocrine function. Exp Biol Med (Maywood) 229:977–987. doi:229/10/977 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Chen Q, Hoppel CL, Lesnefsky EJ (2006) Blockade of Electron Transport before Cardiac Ischemia with the Reversible Inhibitor Amobarbital Protects Rat Heart Mitochondria. Journal of Pharmacology and Experimental Therapeutics 316:200–207. doi: 10.1124/jpet.105.091702 [DOI] [PubMed] [Google Scholar]
- 13.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ (2006) Reversible Blockade of Electron Transport during Ischemia Protects Mitochondria and Decreases Myocardial Injury following Reperfusion. Journal of Pharmacology and Experimental Therapeutics 319:1405–1412. doi: 10.1124/jpet.106.110262 [DOI] [PubMed] [Google Scholar]
- 14.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ (2008) Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol 294:C460–466. doi:00211.2007 [pii] 10.1152/ajpcell.00211.2007 [DOI] [PubMed] [Google Scholar]
- 15.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK (2009) Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137:1112–1123. doi:S0092-8674(09)00642-4 [pii] 10.1016/j.cell.2009.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corsetti G, Pasini E, D’Antona G, Nisoli E, Flati V, Assanelli D, Dioguardi FS, Bianchi R (2008) Morphometric changes induced by amino acid supplementation in skeletal and cardiac muscles of old mice. Am J Cardiol 101:26E–34E. doi:S0002-9149(08)00393-7 [pii] 10.1016/j.amjcard.2008.02.078 [DOI] [PubMed] [Google Scholar]
- 17.Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A (2005) Autophagy and aging: the importance of maintaining “clean” cells. Autophagy 1:131–140. doi:2017 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Cury DP, Dias FJ, Sosthenes MC, Dos Santos Haemmerle CA, Ogawa K, Da Silva MC, Mardegan Issa JP, Iyomasa MM, Watanabe IS (2013) Morphometric, quantitative, and three-dimensional analysis of the heart muscle fibers of old rats: transmission electron microscopy and high-resolution scanning electron microscopy methods. Microsc Res Tech 76:184–195. doi: 10.1002/jemt.22151 [DOI] [PubMed] [Google Scholar]
- 19.Dorn GW 2nd (2015) Gone fission…: diverse consequences of cardiac Drp1 deficiency. Circ Res 116:225–228. doi: 10.1161/CIRCRESAHA.114.305672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, Goonasekera SA, Karch J, Gabel S, Farber J, Force T, Heller Brown J, Murphy E, Molkentin JD (2010) Cyclophilin D controls mitochondrial pore–dependent Ca2+ exchange, metabolic flexibility, and propensity for heart failure in mice. The Journal of Clinical Investigation 120:3680–3687. doi: 10.1172/jci43171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fannin SW, Lesnefsky EJ, Slabe TJ, Hassan MO, Hoppel CL (1999) Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch Biochem Biophys 372:399–407. doi:S0003-9861(99)91508-4 [pii] 10.1006/abbi.1999.1508 [DOI] [PubMed] [Google Scholar]
- 22.Gába A, Přidalová M (2014) Age-related changes in body composition in a sample of Czech women aged 18–89 years: a cross-sectional study. European journal of nutrition 53:167–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB (2014) Heart Disease and Stroke Statistics—2014 Update: A Report From the American Heart Association. Circulation 129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldstein MA, Murphy DL (1983) A Morphometric Analysis of Ischemic Canine Myocardium with and without Reperfusion. Journal of Molecular and Cellular Cardiology 15:325–334. doi:Doi 10.1016/0022-2828(83)91344-5 [DOI] [PubMed] [Google Scholar]
- 25.Gottlieb RA, Carreira RS (2010) Autophagy in health and disease. 5. Mitophagy as a way of life. Am J Physiol Cell Physiol 299:C203–210. doi:ajpcell.00097.2010 [pii] 10.1152/ajpcell.00097.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harman SM, Naftolin F, Brinton EA, Judelson DR (2005) Is the estrogen controversy over? Deconstructing the Women’s Health Initiative study: a critical evaluation of the evidence. Ann N Y Acad Sci 1052:43–56. doi: 10.1196/annals.1347.004 [DOI] [PubMed] [Google Scholar]
- 27.Herdson PB, Sommers HM, Jennings RB (1965) A Comparative Study of the Fine Structure of Normal and Ischemic Dog Myocardium with Special Reference to Early Changes Following Temporary Occlusion of a Coronary Artery. Am J Pathol 46:367–386 [PMC free article] [PubMed] [Google Scholar]
- 28.Heusch G, Musiolik J, Gedik N, Skyschally A Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res 109:1302–1308. doi:CIRCRESAHA.111.255604 [pii] 10.1161/CIRCRESAHA.111.255604 [DOI] [PubMed] [Google Scholar]
- 29.Hollander JM, Thapa D, Shepherd DL (2014) Physiological and structural differences in spatially distinct subpopulations of cardiac mitochondria: influence of cardiac pathologies. Am J Physiol Heart Circ Physiol 307:H1–14. doi:ajpheart.00747.2013 [pii] 10.1152/ajpheart.00747.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hool LC (2007) What Cardiologists Should Know About Calcium Ion Channels and Their Regulation by Reactive Oxygen Species. Heart, Lung and Circulation 16:361–372. doi: 10.1016/j.hlc.2007.01.005 [DOI] [PubMed] [Google Scholar]
- 31.Hoppel CL, Tandler B, Fujioka H, Riva A (2009) Dynamic organization of mitochondria in human heart and in myocardial disease. Int J Biochem Cell Biol 41:1949–1956. doi:S1357-2725(09)00160-5 [pii] 10.1016/j.biocel.2009.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsieh YC, Yu HP, Suzuki T, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH (2006) Upregulation of mitochondrial respiratory complex IV by estrogen receptor-beta is critical for inhibiting mitochondrial apoptotic signaling and restoring cardiac functions following trauma-hemorrhage. J Mol Cell Cardiol 41:511–521. doi:S0022-2828(06)00589-X [pii] 10.1016/j.yjmcc.2006.06.001 [DOI] [PubMed] [Google Scholar]
- 33.Huang HH, Steger RW, Bruni JF, Meites J (1978) Patterns of sex steroid and gonadotropin secretion in aging female rats. Endocrinology 103:1855–1859. doi: 10.1210/endo-103-5-1855 [DOI] [PubMed] [Google Scholar]
- 34.Hunter JC, Korzick DH (2005) Age- and sex-dependent alterations in protein kinase C (PKC) and extracellular regulated kinase 1/2 (ERK1/2) in rat myocardium. Mech Ageing Dev 126:535–550. doi:S0047-6374(04)00296-9 [pii] 10.1016/j.mad.2004.11.003 [DOI] [PubMed] [Google Scholar]
- 35.Hunter JC, Kostyak JC, Novotny JL, Simpson AM, Korzick DH (2007) Estrogen deficiency decreases ischemic tolerance in the aged rat heart: Roles of PKCdelta, PKCepsilon, Akt, and GSK3beta. Am J Physiol Regul Integr Comp Physiol 292:R800–809. doi:00374.2006 [pii] 10.1152/ajpregu.00374.2006 [DOI] [PubMed] [Google Scholar]
- 36.Hunter JC, Machikas AM, Korzick DH (2012) Age-Dependent Reductions in Mitochondrial Respiration are Exacerbated by Calcium in the Female Rat Heart. Gend Med 9:197–206. doi: 10.1016/j.genm.2012.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ikeda Y, Sciarretta S, Nagarajan N, Rubattu S, Volpe M, Frati G, Sadoshima J (2014) New Insights into the Role of Mitochondrial Dynamics and Autophagy during Oxidative Stress and Aging in the Heart. Oxidative Medicine and Cellular Longevity 2014:13. doi: 10.1155/2014/210934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, Abdellatif M, Sadoshima J (2015) Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res 116:264–278. doi:CIRCRESAHA.116.303356 [pii] 10.1161/CIRCRESAHA.116.303356 [DOI] [PubMed] [Google Scholar]
- 39.Javadov S, Choi A, Rajapurohitam V, Zeidan A, Basnakian AG, Karmazyn M (2008) NHE-1 inhibition-induced cardioprotection against ischaemia/reperfusion is associated with attenuation of the mitochondrial permeability transition. Cardiovasc Res 77:416–424. doi:cvm039 [pii] 10.1093/cvr/cvm039 [DOI] [PubMed] [Google Scholar]
- 40.Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C (2005) Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. FASEB J 19:419–421. doi:04-2622fje [pii] 10.1096/fj.04-2622fje [DOI] [PubMed] [Google Scholar]
- 41.Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH, Krajewski S, Reed JC, Olivetti G, Anversa P (1996) Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest 74:86–107 [PubMed] [Google Scholar]
- 42.Kanaley J, Sames C, Swisher L, Swick A, Ploutz-Snyder L, Steppan C, Sagendorf K, Feiglin D, Jaynes E, Meyer R (2001) Abdominal fat distribution in pre-and postmenopausal women: the impact of physical activity, age, and menopausal status. Metabolism 50:976–982 [DOI] [PubMed] [Google Scholar]
- 43.Korzick DH, Hunter JC, McDowell MK, Delp MD, Tickerhoof MM, Carson LD (2004) Chronic exercise improves myocardial inotropic reserve capacity through alpha1-adrenergic and protein kinase C-dependent effects in Senescent rats. J Gerontol A Biol Sci Med Sci 59:1089–1098. doi:59/11/1089 [pii] [DOI] [PubMed] [Google Scholar]
- 44.Korzick DH, Kostyak JC, Hunter JC, Saupe KW (2007) Local delivery of PKCepsilon-activating peptide mimics ischemic preconditioning in aged hearts through GSK-3beta but not F1-ATPase inactivation. Am J Physiol Heart Circ Physiol 293:H2056–2063. doi:00403.2007 [pii] 10.1152/ajpheart.00403.2007 [DOI] [PubMed] [Google Scholar]
- 45.Lancaster TS, Jefferson SJ, Hunter JC, Lopez V, Van Eyk JE, Lakatta EG, Korzick DH (2012) Quantitative proteomic analysis reveals novel mitochondrial targets of estrogen deficiency in the aged female rat heart. Physiol Genomics 44:957–969. doi:physiolgenomics.00184.2011 [pii] 10.1152/physiolgenomics.00184.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lancaster TS, Jefferson SJ, Korzick DH (2011) Local delivery of a PKCepsilon-activating peptide limits ischemia reperfusion injury in the aged female rat heart. Am J Physiol Regul Integr Comp Physiol 301:R1242–1249. doi:ajpregu.00851.2010 [pii] 10.1152/ajpregu.00851.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee CG, Carr MC, Murdoch SJ, Mitchell E, Woods NF, Wener MH, Chandler WL, Boyko EJ, Brunzell JD (2009) Adipokines, inflammation, and visceral adiposity across the menopausal transition: a prospective study. The Journal of clinical endocrinology and metabolism 94:1104–1110. doi:jc.2008-0701 [pii] 10.1210/jc.2008-0701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LeFevre J, McClintock MK (1988) Reproductive senescence in female rats: a longitudinal study of individual differences in estrous cycles and behavior. Biol Reprod 38:780–789 [DOI] [PubMed] [Google Scholar]
- 49.Lesnefsky EJ, Gudz TI, Migita CT, Ikeda-Saito M, Hassan MO, Turkaly PJ, Hoppel CL (2001) Ischemic injury to mitochondrial electron transport in the aging heart: damage to the iron-sulfur protein subunit of electron transport complex III. Arch Biochem Biophys 385:117–128. doi:S0003-9861(00)92066-6 [pii] 10.1006/abbi.2000.2066 [DOI] [PubMed] [Google Scholar]
- 50.Lesnefsky EJ, Gudz TI, Moghaddas S, Migita CT, Ikeda-Saito M, Turkaly PJ, Hoppel CL (2001) Aging decreases electron transport complex III activity in heart interfibrillar mitochondria by alteration of the cytochrome c binding site. Journal of Molecular and Cellular Cardiology 33:37–47. doi:DOI 10.1006/jmcc.2000.1273 [DOI] [PubMed] [Google Scholar]
- 51.Lesnefsky EJ, He DC, Moghaddas S, Hoppel CL (2006) Reversal of mitochondrial defects before ischemia protects the aged heart. Faseb Journal 20:1543–+. doi:DOI 10.1096/fj.05-4535fje [DOI] [PubMed] [Google Scholar]
- 52.Lesnefsky EJ, Tandler B, Ye JA, Slabe TJ, Turkaly J, Hoppel CL (1997) Myocardial ischemia decreases oxidative phosphorylation through cytochrome oxidase in subsarcolemmal mitochondria. Am J Physiol-Heart C 273:H1544–H1554 [DOI] [PubMed] [Google Scholar]
- 53.Linton PJ, Gurney M, Sengstock D, Mentzer RM, Gottlieb RA (2015) This old heart: Cardiac aging and autophagy. Journal of Molecular and Cellular Cardiology 83:44–54. doi: 10.1016/j.yjmcc.2014.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu KH, Hopper BR, Vargo TM, Yen SS (1979) Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. Biol Reprod 21:193–203 [DOI] [PubMed] [Google Scholar]
- 55.Matsui Y, Kyoi S, Takagi H, Hsu CP, Hariharan N, Ago T, Vatner SF, Sadoshima J (2008) Molecular mechanisms and physiological significance of autophagy during myocardial ischemia and reperfusion. Autophagy 4:409–415. doi:5638 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J (2007) Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res 100:914–922. doi:01.RES.0000261924.76669.36 [pii] 10.1161/01.RES.0000261924.76669.36 [DOI] [PubMed] [Google Scholar]
- 57.Miller LR, Marks C, Becker JB, Hurn PD, Chen W-J, Woodruff T, McCarthy MM, Sohrabji F, Schiebinger L, Wetherington CL, Makris S, Arnold AP, Einstein G, Miller VM, Sandberg K, Maier S, Cornelison TL, Clayton JA (2017) Considering sex as a biological variable in preclinical research. The FASEB Journal 31:29–34. doi: 10.1096/fj.201600781R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller VM, Garovic VD, Kantarci K, Barnes JN, Jayachandran M, Mielke MM, Joyner MJ, Shuster LT, Rocca WA (2013) Sex-specific risk of cardiovascular disease and cognitive decline: pregnancy and menopause. Biol Sex Differ 4:6. doi: 10.1186/2042-6410-4-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moghaddas S, Stoll MS, Minkler PE, Salomon RG, Hoppel CL, Lesnefsky EJ (2002) Preservation of cardiolipin content during aging in rat heart interfibrillar mitochondria. J Gerontol A Biol Sci Med Sci 57:B22–28 [DOI] [PubMed] [Google Scholar]
- 60.Moorthy K, Sharma D, Basir SF, Baquer NZ (2005) Administration of estradiol and progesterone modulate the activities of antioxidant enzyme and aminotransferases in naturally menopausal rats. Exp Gerontol 40:295–302. doi:S0531-5565(05)00005-7 [pii] 10.1016/j.exger.2005.01.004 [DOI] [PubMed] [Google Scholar]
- 61.Moyzis AG, Sadoshima J, Gustafsson AB (2015) Mending a broken heart: the role of mitophagy in cardioprotection. Am J Physiol Heart Circ Physiol 308:H183–192. doi:ajpheart.00708.2014 [pii] 10.1152/ajpheart.00708.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muller W (1976) Subsarcolemmal mitochondria and capillarization of soleus muscle fibers in young rats subjected to an endurance training. A morphometric study of semithin sections. Cell Tissue Res 174:367–389 [DOI] [PubMed] [Google Scholar]
- 63.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y (2005) Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434:652–658. doi:nature03317 [pii] 10.1038/nature03317 [DOI] [PubMed] [Google Scholar]
- 64.Nicklas BJ, Penninx BW, Cesari M, Kritchevsky SB, Newman AB, Kanaya AM, Pahor M, Jingzhong D, Harris TB, Health A, Body Composition S (2004) Association of visceral adipose tissue with incident myocardial infarction in older men and women: the Health, Aging and Body Composition Study. Am J Epidemiol 160:741–749. doi: 10.1093/aje/kwh281 [DOI] [PubMed] [Google Scholar]
- 65.Novotny JL, Simpson AM, Tomicek NJ, Lancaster TS, Korzick DH (2009) Rapid estrogen receptor-alpha activation improves ischemic tolerance in aged female rats through a novel protein kinase C epsilon-dependent mechanism. Endocrinology 150:889–896. doi:en.2008-0708 [pii] 10.1210/en.2008-0708 [DOI] [PubMed] [Google Scholar]
- 66.Paillard M, Gomez L, Augeul L, Loufouat J, Lesnefsky EJ, Ovize M (2009) Postconditioning inhibits mPTP opening independent of oxidative phosphorylation and membrane potential. J Mol Cell Cardiol 46:902–909. doi:S0022-2828(09)00093-5 [pii] 10.1016/j.yjmcc.2009.02.017 [DOI] [PubMed] [Google Scholar]
- 67.Palmer JW, Tandler B, Hoppel CL (1977) Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem 252:8731–8739 [PubMed] [Google Scholar]
- 68.Pavon N, Martinez-Abundis E, Hernandez L, Gallardo-Perez JC, Alvarez-Delgado C, Cerbon M, Perez-Torres I, Aranda A, Chavez E (2012) Sexual hormones: effects on cardiac and mitochondrial activity after ischemia-reperfusion in adult rats. Gender difference. J Steroid Biochem Mol Biol 132:135–146. doi:S0960-0760(12)00099-4 [pii] 10.1016/j.jsbmb.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 69.Petrosillo G, Colantuono G, Moro N, Ruggiero FM, Tiravanti E, Di Venosa N, Fiore T, Paradies G (2009) Melatonin protects against heart ischemia-reperfusion injury by inhibiting mitochondrial permeability transition pore opening. Am J Physiol Heart Circ Physiol 297:H1487–1493. doi:00163.2009 [pii] 10.1152/ajpheart.00163.2009 [DOI] [PubMed] [Google Scholar]
- 70.Ping P, Zhang J, Qiu Y, Tang XL, Manchikalapudi S, Cao X, Bolli R (1997) Ischemic preconditioning induces selective translocation of protein kinase C isoforms epsilon and eta in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res 81:404–414 [DOI] [PubMed] [Google Scholar]
- 71.Rattanasopa C, Phungphong S, Wattanapermpool J, Bupha-Intr T (2015) Significant role of estrogen in maintaining cardiac mitochondrial functions. J Steroid Biochem Mol Biol 147:1–9. doi: 10.1016/j.jsbmb.2014.11.009 [DOI] [PubMed] [Google Scholar]
- 72.Ribas V, Drew BG, Zhou Z, Phun J, Kalajian NY, Soleymani T, Daraei P, Widjaja K, Wanagat J, de Aguiar Vallim TQ, Fluitt AH, Bensinger S, Le T, Radu C, Whitelegge JP, Beaven SW, Tontonoz P, Lusis AJ, Parks BW, Vergnes L, Reue K, Singh H, Bopassa JC, Toro L, Stefani E, Watt MJ, Schenk S, Akerstrom T, Kelly M, Pedersen BK, Hewitt SC, Korach KS, Hevener AL (2016) Skeletal muscle action of estrogen receptor alpha is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci Transl Med 8:334ra354. doi:8/334/334ra54 [pii] 10.1126/scitranslmed.aad3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Riva A, Tandler B, Loffredo F, Vazquez E, Hoppel C (2005) Structural differences in two biochemically defined populations of cardiac mitochondria. Am J Physiol-Heart C 289:H868–H872. doi:DOI 10.1152/ajpheart.00866.2004 [DOI] [PubMed] [Google Scholar]
- 74.Shimada T, Horita K, Murakami M, Ogura R (1984) Morphological studies of different mitochondrial populations in monkey myocardial cells. Cell Tissue Res 238:577–582 [DOI] [PubMed] [Google Scholar]
- 75.Shirakabe A, Zhai P, Ikeda Y, Saito T, Maejima Y, Hsu C-P, Nomura M, Egashira K, Levine B, Sadoshima J (2016) Drp1-Dependent Mitochondrial Autophagy Plays a Protective Role Against Pressure Overload–Induced Mitochondrial Dysfunction and Heart Failure. Circulation 133:1249–1263. doi: 10.1161/circulationaha.115.020502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shulga N, Wilson-Smith R, Pastorino JG (2010) Sirtuin-3 deacetylation of cyclophilin D induces dissociation of hexokinase II from the mitochondria. J Cell Sci 123:894–902. doi:jcs.061846 [pii] 10.1242/jcs.061846 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Suh JH, Heath SH, Hagen TM (2003) Two subpopulations of mitochondria in the aging rat heart display heterogenous levels of oxidative stress. Free Radic Biol Med 35:1064–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suzuki K, Kostin S, Person V, Elsasser A, Schaper J (2001) Time course of the apoptotic cascade and effects of caspase inhibitors in adult rat ventricular cardiomyocytes. J Mol Cell Cardiol 33:983–994. doi: 10.1006/jmcc.2001.1364 S0022-2828(01)91364-1 [pii] [DOI] [PubMed] [Google Scholar]
- 79.Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, Oka T, Tamai T, Oyabu J, Murakawa T, Nishida K, Shimizu T, Hori M, Komuro I, Takuji Shirasawa TS, Mizushima N, Otsu K (2010) Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy 6:600–606. doi:11947 [pii] 10.4161/auto.6.5.11947 [DOI] [PubMed] [Google Scholar]
- 80.Tate EL, Herbener GH (1976) A morphometric study of the density of mitochondrial cristae in heart and liver of aging mice. J Gerontol 31:129–134 [DOI] [PubMed] [Google Scholar]
- 81.Tchernof A, Poehlman ET (1998) Effects of the menopause transition on body fatness and body fat distribution. Obesity research 6:246–254 [DOI] [PubMed] [Google Scholar]
- 82.Terman A, Kurz T, Navratil M, Arriaga EA, Brunk UT (2010) Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging. Antioxid Redox Signal 12:503–535. doi: 10.1089/ars.2009.2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tomicek NJ, Miller-Lee JL, Hunter JC, Korzick DH (2011) Estrogen Receptor Beta Does Not Influence Ischemic Tolerance in the Aged Female Rat Heart. Cardiovascular Therapeutics:no-no. doi: 10.1111/j.1755-5922.2011.00288.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toth M, Tchernof A, Sites C, Poehlman E (2000) Effect of menopausal status on body composition and abdominal fat distribution. International journal of obesity 24:226–231 [DOI] [PubMed] [Google Scholar]
- 85.Vetterlein F, Schrader C, Volkmann R, Neckel M, Ochs M, Schmidt G, Hellige G (2003) Extent of damage in ischemic, nonreperfused, and reperfused myocardium of anesthetized rats. Am J Physiol Heart Circ Physiol 285:H755–765. doi: 10.1152/ajpheart.00269.2002 00269.2002 [pii] [DOI] [PubMed] [Google Scholar]
- 86.Wohlgemuth SE, Calvani R, Marzetti E (2014) The interplay between autophagy and mitochondrial dysfunction in oxidative stress-induced cardiac aging and pathology. Journal of Molecular and Cellular Cardiology 71:62–70. doi: 10.1016/j.yjmcc.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 87.Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM Jr., Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW (2004) Mitochondrial localization of estrogen receptor beta. Proc Natl Acad Sci U S A 101:4130–4135. doi: 10.1073/pnas.03069481010306948101 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhai P, Eurell TE, Cooke PS, Lubahn DB, Gross DR (2000) Myocardial ischemia-reperfusion injury in estrogen receptor-alpha knockout and wild-type mice. Am J Physiol Heart Circ Physiol 278:H1640–1647 [DOI] [PubMed] [Google Scholar]
- 89.Zhai P, Eurell TE, Cotthaus R, Jeffery EH, Bahr JM, Gross DR (2000) Effect of estrogen on global myocardial ischemia-reperfusion injury in female rats. American Journal of Physiology -Heart and Circulatory Physiology 279:H2766–H2775 [DOI] [PubMed] [Google Scholar]


