Abstract
Myocardial energy deprivation plays a causal role in the development of heart failure. A cardiac protection blend (CPB) of nutrients including medium chain triglycerides, fish oil and other key nutrients was developed to slow the progression of canine myxomatous mitral valve disease (MMVD). A six-month dietary intervention demonstrated efficacy of CPB in slowing MMVD progression. Untargeted metabolomic analysis of serum from these dogs identified 102 differential metabolites (adjusted P < 0.05). The ratios of omega-6 to omega-3 fatty acid (FA) changed from 2.41 and 1.46 in control and CPB groups at baseline to 4.30 and 0.46 at 6 months respectively. A 2.7-fold increase of α-aminobutyrate, a myocardial modulator of glutathione homeostasis, was found in CPB dogs compared to 1.3-fold increase in control dogs. Arginine and citrulline, precursors of nitric oxide biosynthesis, were both increased 2-fold; caprate, a medium chain FA, was increased 3-fold; and deoxycarnitine, precursor of carnitine biosynthesis, was increased 2.5-fold in CPB dogs. Margarate and methylpalmitate decreased in response to CPB, a potential benefit in MMVD dogs as positive correlations were found between changes in both these FAs and left atrial diameter (r = 0.69, r = 0.87 respectively, adjusted P < 0.05). Sphingomyelins with very long chain saturated FAs associated with decreased risk of heart failure in humans were increased in MMVD dogs fed the CPB diet. Our data supports the hypothesis that CPB improves FA utilization and energetics, reduces oxidative stress and inflammation in MMVD dogs. More studies are needed to understand the roles of specific metabolites in MMVD.
Introduction
The adult mammalian heart requires a large quantity of ATP produced through mitochondrial fatty acid (FA) oxidation in order to support its normal contractile work [1]. Perturbations in myocardial energy metabolism play a key role in the development of heart failure [2–4]. A shift from long chain fatty acids (LCFAs) as the main energy source to other energy substrates has been documented in the failing heart in both humans and animals [5–7]. Chronic myxomatous mitral valve disease (MMVD) is the most common naturally-occurring heart disease in dogs affecting approximately 10–15% of the canine population, with greater frequency in geriatric, small, or medium breed dogs [8]. It is characterized by a slowly progressive valvular degeneration that can cause mitral regurgitation and ultimately lead to congestive heart failure.
Gene expression and metabolomic profiling studies comparing healthy dogs and dogs with MMVD revealed alterations in energy metabolism, oxidative stress, inflammation and extracellular matrix homeostasis [9–11]. Similar findings were documented in human MMVD, which afflicts 2–3% of the general population [12–16]. Canine MMVD is considered a model for MMVD in man [13, 16]. To address those metabolic changes in dogs, a cardiac protection blend (CPB) of nutrients was formulated to include medium chain triglycerides (MCT) as an alternative energy source, carnitine precursors to enhance FA oxidation, fish oil to reduce inflammatory mediators, antioxidants to reduce oxidative stress, and magnesium to support myocardial function [17].
Medium chain fatty acids (MCFAs) from MCTs do not require transporters or carnitine-mediated transport pathway to reach mitochondria and thus can be rapidly oxidized for energy [18, 19]. A MCT-supplemented diet has been shown to prevent progressive cardiac remodeling in spontaneously hypertensive rats, possibly by maintenance of myocardial energy and reduction in oxidative stress [20]. MCTs have been proposed for potential clinical application in the management of cardiovascular diseases in humans [21, 22]. In dogs, a 6-month blinded, randomized placebo-controlled dietary intervention study with this CPB demonstrated beneficial effects in reducing left atrial enlargement and in slowing or preventing the progression of early stage MMVD [17]. The objective of the study presented here was to evaluate the serum metabolomic changes associated with this dietary supplement and with the clinical improvement observed in these dogs.
The metabolome encompasses a large variety of metabolites, the small intermediary molecules in metabolism. Metabolomics has been increasingly used to investigate metabolic pathways in cardiovascular diseases [23–27], including mitral valve disease in both dogs [9] and humans [15]. Emerging evidence points to derangements in cardiac metabolism including perturbations in mitochondrial bioenergetics and reactive oxygen species (ROS) homeostasis in failing hearts [1]. The hypothesis of this study was that serum metabolomic changes in response to the CPB diet would provide markers reflecting improved metabolic states in mitochondrial bioenergetics, oxidative stress and inflammation in dogs with early stage MMVD. These findings would advance understanding in MMVD pathogenesis and modulation of cardiac metabolism could provide potential alternative therapies for MMVD in both humans and dogs.
Materials and methods
Diets, animals, and study design
The study design of this blinded, randomized, placebo-controlled study, including detail on the diets, animals, husbandry and sampling protocols, was previously published [17]. The two study diets (control (CON) and CPB) were similar in protein, total fat, and carbohydrate contents: the MCTs and fish oil in the CPB diet were balanced with animal fats in the CON diet [17]. Additional analysis of dietary amino acids and fatty acids is provided in S1 Table. Nineteen small to medium-sized dogs (age range: 7.9–13.7 years) with naturally-occurring preclinical MMVD were enrolled from among the permanent canine population of a Nestlé Purina PetCare Center. All dogs were fed the same commercial diet, with a nutrient profile similar to the CON diet, during the baseline period; then received either the CON (n = 9) or CPB (n = 10) diet for the duration of the 6-month study. One dog in the CON group developed an illness unrelated to the diet after 3 months and was excluded from this study. After completion of this study, dogs were returned to the general canine population in the Nestlé Purina PetCare Center. The study protocol was approved by the Institutional Animal Care and Use Committee of the Nestlé Purina PetCare Company.
Serum samples and metabolomics assay
Venous blood samples were collected from each dog after overnight fasting at baseline and 6 months. Six milliliters of blood were drawn by jugular venipuncture into serum separator tubes and allowed to separate for 30 minutes in room temperature. The tubes were then centrifuged at 3500 RPM for 9 minutes. Aliquots of 500 microliters serum from the clear top layer were transferred into small Cryovials and stored in -80°C until analyzed. Serum samples were shipped on dry ice to Metabolon, Inc. (Durham, NC) for sample processing and untargeted metabolomics assays. Sample preparation and extraction, liquid chromatography and mass spectrometry methods, and compound detection and identification were performed by Metabolon Inc. using Metabolon’s standard protocols and software [28] (S1 File). A total of 759 metabolites were identified, including 619 known and 140 unknown.
Pre-processing of metabolomics data
The raw data was based on area-under-the-curve generated using ion counts that provide relative quantification (S2 File). Metabolites with a constant value across all samples or with more than 80% of missing data were removed. Under the assumption that missing data were values below the detection limit, the remaining missing values were estimated and replaced by a value equal to half of the minimal positive value in the original data [29]. The data were further processed using generalized logarithm transformation [30],
where a is a constant with a default value of 1. Finally, transformed data was auto-scaled to achieve a zero mean and unit variance for any variable: where xij is the value of the ith metabolite and jth sample, and and si are the mean and standard deviation respectively. The advantage of autoscaling is that all metabolites become equally important [31].
Statistical analysis
Principal component analysis (PCA) for high-dimensional multivariate data was performed on the baseline and 6-month data. The first two principal components (PCs) which capture more data variations than other PCs were examined for their ability to separate groups based on diet (CON vs CPB) or time (0 vs 6 months). A multiple linear regression using each PC as the dependent variable and diet or time as the independent variable, adjusted for sex, age, breed, and body condition score (BCS) was performed and the P-value for the model was obtained. The 6-month data were subjected to partial least-squares discriminant analysis (PLS-DA) to detect key metabolites with the highest Variable Importance in Projection (VIP) scores. A maximum of five components were searched for optimal performance and leave-one-out cross-validation was performed to assess model’s predictive accuracy.
For each metabolite, the effect of diet by time interaction was estimated using a linear mixed-effect model adjusted for age, breed, sex, and BCS. P-values for the interaction terms were obtained and adjusted for multiple testing to control false discovery rate (FDR) [32]. A metabolite was considered significant if FDR was less than 0.05. Fold change (FC) is defined as the ratio of 6 months (t6) over baseline (t0). Because the minimal value in the raw data is far greater than 1, FC ≈ 2(t6−t0) if t6 ≥ t0 or if t6 < t0 such that a negative FC indicates a decrease in value from baseline to 6 months. Spearman’s correlation analysis was performed on the changes from baseline to 6 months between left atrial diameter (LAD) and significant metabolites. A subsequent analysis was performed to assess the correlations between methylpalmitate, margarate, and other significant metabolites. P-values were adjusted for multiple testing. The ratio of omega-6 to omega-3 FAs was determined as the sum of six omega-6 FAs (linoleate (18:2n6), arachidonate (20:4n6), dihomolinoleate (20:2n6), docosadienoate (22:2n6), adrenate (22:4n6), and n6-DPA (22:5n6)) divided by the sum of four omega-3 FAs (linolenate (18:3n3), EPA (20:5n3), DPA (22:5n3), and DHA (22:6n3)). Heatmaps of phospholipids and sphingomyelins were generated using the changes from baseline to 6 months from each dog. Metabolomics data processing and statistical analysis were performed using MetaboAnalyst 3.0, a comprehensive tool suite for metabolomics data analysis [29] and the statistical computing software R (version 3.5.0) [33].
Results
Dietary effects on metabolome
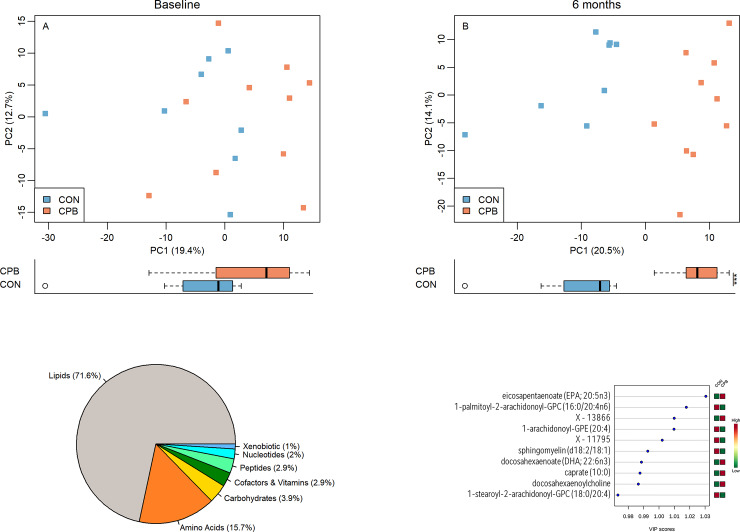
Principal component analysis (PCA) showed no significant clustering at baseline, but clustering by diet along PC1 became evidenced at 6 months (P = 0.19, P = 1.54e-04 respectively, Fig 1A and 1B). The p-values and effects of the adjustment for sex, age, breed, and BCS from the multiple regression models were reported in S3 File. The first two principal components, PC1 and PC2, account for 19.4% and 12.7% of the total data variation at baseline and 20.5% and 14.1% at 6 months respectively. PCA performed to evaluate changes over time found no change in CON dogs but significant time effect along PC1 was observed in CPB dogs ((P = 0.74, P = 3.4e-04 respectively, S1 Fig). No separation was found along PC2 (all P > 0.05).
Fig 1.
(A, B) Principal component analysis (PCA) based on diet effects at baseline (A) and 6 months (B). The percentages of data variation explained by the first two principal components, PC1 and PC2, are indicated on the x and y axes respectively. Distributions of samples along PC1 by diets were plotted below each PCA plot. Blue squares represent CON diet while orange ones represent CPB diet. *P < 0.05; **P < 0.01; ***P < 0.001. (C) Pie chart shows percentage of each metabolite class. (D) Partial least squares discriminant analysis (PLS-DA) identified ten metabolites with the highest Variable Importance in Projection (VIP) scores, which indicates discriminant power between groups.
Linear mixed model analysis identified 112 metabolites that were significant in diet by time interaction (all FDR < 0.05, Table 1). Among 102 known metabolites, 73 were lipids (71.6%) and 16 were amino acids (15.7%) (Fig 1C).
Table 1. Metabolites with significant diet by time interaction.
| Metabolites | Class | Pathway | P | FDR | FC_CON | FC_CPB | KEGG | PUBCHEM | HMDB |
|---|---|---|---|---|---|---|---|---|---|
| carboxyethyl-GABA | AA | Glu Metabolism | 0.0036 | 0.0284 | -1.22 | -1.8 | 2572 | 02201 | |
| Lysine | AA | Lys Metabolism | 0.0023 | 0.0211 | 1.14 | 2.37 | C00047 | 5962 | 00182 |
| 2-aminoadipate | AA | Lys Metabolism | 0.0056 | 0.0378 | -1.46 | 2.32 | C00956 | 469 | 00510 |
| Phenylalanine | AA | Phe and Tyr Metabolism | 0.0015 | 0.0147 | -1.53 | 2.8 | C00079 | 6140 | 00159 |
| N-acetylphenylalanine | AA | Phe and Tyr Metabolism | 0.0009 | 0.0105 | 1.02 | 2.16 | C03519 | 74839 | 00512 |
| 5-bromotryptophan | AA | Try Metabolism | 0.0048 | 0.034 | -1.02 | -1.64 | 96735 | ||
| N-acetylleucine | AA | Leu, Ile and Val Metabolism | 0.007 | 0.0456 | -1.06 | 1.5 | C02710 | 70912 | 11756 |
| S-methylmethionine | AA | Met, Cys, SAM and Tau Metab | 0.0006 | 0.0082 | -1.12 | 2.29 | C05319 | 458 | 38670 |
| methionine sulfone | AA | Met, Cys, SAM and Tau Metab | 0.0074 | 0.0471 | -1.36 | -3.41 | 69961 | ||
| Cystathionine | AA | Met, Cys, SAM and Tau Metab | 0.0002 | 0.0037 | -1.51 | 3.13 | C02291 | 439258 | 00099 |
| S-methylcysteine | AA | Met, Cys, SAM and Tau Metab | 0.0008 | 0.0101 | 1.52 | 2.92 | 24417 | 02108 | |
| Hypotaurine | AA | Met, Cys, SAM and Tau Metab | 0.0022 | 0.0199 | -1.27 | 1.86 | C00519 | 107812 | 00965 |
| Taurine | AA | Met, Cys, SAM and Tau Metab | 0.0048 | 0.034 | 1.29 | 3.89 | C00245 | 1123 | 00251 |
| Arginine | AA | Urea cycle; Arg and Pro Metab | 0.0042 | 0.0314 | -1.49 | 2.09 | C00062 | 232 | 00517 |
| Citrulline | AA | Urea cycle; Arg and Pro Metab | 0.0028 | 0.0239 | -1.01 | 2.09 | C00327 | 9750 | 00904 |
| 2-aminobutyrate | AA | Glutathione Metabolism | 0.0031 | 0.0255 | 1.29 | 2.69 | C02261 | 439691 | 00650 |
| gamma-glutamyl-alpha-lysine | Peptide | Gamma-glutamyl amino acid | 0.0017 | 0.0163 | 1 | 2.57 | 65254 | ||
| gamma-glutamylphenylalanine | Peptide | Gamma-glutamyl amino acid | 0.0011 | 0.0127 | -1.37 | 2.37 | 111299 | 00594 | |
| Anserine | Peptide | Dipeptide Derivative | 0.0013 | 0.0133 | -2.12 | -1.08 | C01262 | 112072 | 00194 |
| Glycerate | CHO | Glycolysis, Gluconeogenesis & Pyruvate Metabolism | 0.003 | 0.026 | -1.59 | 1.33 | C00258 | 752 | 00139 |
| Ribitol | CHO | Pentose Metabolism | 0.0065 | 0.0436 | -1.51 | 1.62 | C00474 | 6912 | 00508 |
| 2-ketogulonate | CHO | Fructose, Mannose & Galactose Metabolism | 0.004 | 0.031 | -1.09 | 2.26 | C15673 | 50 | 11732 |
| N-acetylneuraminate | CHO | Aminosugar Metabolism | 0.0051 | 0.0352 | 1.6 | 3.44 | C00270 | 439197 | 00230 |
| caprate (10:0)+A25:J39 | Lipid | Medium Chain Fatty Acid | 0 | 0.0002 | 1.03 | 3.01 | C01571 | 2969 | 00511 |
| margarate (17:0) | Lipid | Long Chain Fatty Acid | 0.004 | 0.0304 | 1.17 | -2.31 | 10465 | 02259 | |
| 10-heptadecenoate (17:1n7) | Lipid | Long Chain Fatty Acid | 0.0015 | 0.0147 | 1.3 | -2.49 | 5312435 | 60038 | |
| oleate/vaccenate (18:1) | Lipid | Long Chain Fatty Acid | 0.0043 | 0.0315 | 1.28 | -2.19 | |||
| eicosapentaenoate (EPA; 20:5n3) | Lipid | Polyunsaturated FA (n3 & n6) | 0 | 0 | -1.49 | 3.07 | C06428 | 446284 | 01999 |
| docosapentaenoate (DPA; 22:5n3) | Lipid | Polyunsaturated FA (n3 & n6) | 0.0003 | 0.0046 | -1.34 | 2.71 | C16513 | 6441454 | 06528 |
| docosahexaenoate (DHA; 22:6n3) | Lipid | Polyunsaturated FA (n3 & n6) | 0.0002 | 0.0033 | -1.72 | 2.32 | C06429 | 445580 | 02183 |
| arachidonate (20:4n6) | Lipid | Polyunsaturated FA (n3 & n6) | 0.0021 | 0.0198 | 1.36 | -2.25 | C00219 | 444899 | 01043 |
| adrenate (22:4n6) | Lipid | Polyunsaturated FA (n3 & n6) | 0.0002 | 0.0037 | 1.62 | -2.12 | C16527 | 5497181 | 02226 |
| docosapentaenoate (n6 DPA; 22:5n6) | Lipid | Polyunsaturated FA (n3 & n6) | 0.0004 | 0.006 | 1.97 | -1.89 | C16513 | 6441454 | 01976 |
| mead acid (20:3n9) | Lipid | Polyunsaturated FA (n3 & n6) | 0.0012 | 0.0127 | 1.29 | -2.88 | 5312531 | 10378 | |
| methylpalmitate (15 or 2) | Lipid | FA, Branched | 0.0007 | 0.0082 | 1.1 | -2.76 | 17903417 | ||
| sebacate (decanedioate) | Lipid | FA, Dicarboxylate | 0.0013 | 0.0133 | 1.49 | 2.75 | C08277 | 5192 | 00792 |
| octadecanedioate (C18) | Lipid | FA, Dicarboxylate | 0.0067 | 0.0446 | 1.31 | -1.8 | 70095 | 00782 | |
| Eicosanodioate | Lipid | FA, Dicarboxylate | 0.0022 | 0.0199 | 1 | 2.48 | 75502 | ||
| Docosadioate | Lipid | FA, Dicarboxylate | 0.0002 | 0.0036 | -1.11 | 2.87 | C19625 | 244872 | |
| oleoylcarnitine (C18) | Lipid | FA Metab(Acyl Carnitine) | 0.0038 | 0.0295 | 1.03 | -2.41 | 6441392 | 05065 | |
| adipoylcarnitine (C6-DC) | Lipid | FA Metab(Acyl Carnitine) | 0.0027 | 0.0236 | 1.13 | -1.74 | 71296139 | 61677 | |
| margaroylcarnitine | Lipid | FA Metab(Acyl Carnitine) | 0.0012 | 0.0128 | -1.04 | -2.62 | 06210 | ||
| Deoxycarnitine | Lipid | Carnitine Metab | 0.0002 | 0.0037 | -1.11 | 2.51 | C01181 | 134 | 01161 |
| glycerophosphoethanolamine | Lipid | Phospholipid Metabolism | 0.008 | 0.0486 | 2.15 | -1.23 | C01233 | 123874 | 00114 |
| 1,2-dipalmitoyl-GPC (16:0/16:0) | Lipid | Phospholipid Metabolism | 0 | 0.0006 | -1.85 | 2.54 | D03585 | 452110 | 00564 |
| 1-palmitoyl-2-oleoyl-GPC (16:0/18:1) | Lipid | Phospholipid Metabolism | 0 | 0.0007 | -1.3 | -3.45 | 6436017 | 07972 | |
| 1-palmitoyl-2-linoleoyl-GPC (16:0/18:2) | Lipid | Phospholipid Metabolism | 0 | 0.0003 | 1.02 | -3.16 | 5287971 | 07973 | |
| 1-stearoyl-2-arachidonoyl-GPC (18:0/20:4) | Lipid | Phospholipid Metabolism | 0.0004 | 0.0059 | 1.25 | -2.16 | 16219824 | 08048 | |
| 1-palmitoyl-2-arachidonoyl-GPC (16:0/20:4n6) | Lipid | Phospholipid Metabolism | 0 | 0.0001 | 1.12 | -3.35 | 10747814 | 07982 | |
| 1-linoleoyl-2-linolenoyl-GPC (18:2/18:3) | Lipid | Phospholipid Metabolism | 0 | 0 | -1.34 | 3.3 | 08141 | ||
| 1-palmitoyl-2-palmitoleoyl-GPC (16:0/16:1) | Lipid | Phospholipid Metabolism | 0 | 0.0009 | -1.22 | -3.32 | 07969 | ||
| 1-palmitoyl-2-arachidonoyl-GPE (16:0/20:4) | Lipid | Phospholipid Metabolism | 0.0002 | 0.0035 | 1.01 | -3.65 | 9546800 | 05323 | |
| 1-palmitoyl-2-stearoyl-GPC (16:0/18:0) | Lipid | Phospholipid Metabolism | 0.0078 | 0.0477 | -1.61 | 1.97 | 07970 | ||
| 1-palmitoleoyl-2-linoleoyl-GPC (16:1/18:2) | Lipid | Phospholipid Metabolism | 0.0007 | 0.0084 | 1.28 | -1.94 | 08006 | ||
| 1-linoleoyl-2-arachidonoyl-GPC (18:2/20:4n6) | Lipid | Phospholipid Metabolism | 0.0006 | 0.0082 | -1.25 | 2.22 | 08147 | ||
| docosahexaenoylcholine | Lipid | Phospholipid Metabolism | 0.0027 | 0.0236 | -1.02 | 2.95 | |||
| 1-palmitoyl-2-gamma-linolenoyl-GPC (16:0/18:3n6) | Lipid | Phospholipid Metabolism | 0 | 0.0009 | 1.08 | -3.4 | 07974 | ||
| 1-palmitoyl-GPC (16:0) | Lipid | Lysolipid | 0.0013 | 0.0133 | 1.65 | -2.1 | 86554 | 10382 | |
| 1-linoleoyl-GPC (18:2) | Lipid | Lysolipid | 0.0053 | 0.0367 | 1.38 | -2.34 | C04100 | 11988421 | 10386 |
| 1-arachidonoyl-GPC (20:4) | Lipid | Lysolipid | 0.0002 | 0.0036 | 1.83 | -2.38 | C05208 | 10395 | |
| 1-lignoceroyl-GPC (24:0) | Lipid | Lysolipid | 0.0002 | 0.0037 | -1.13 | 3.17 | 10405 | ||
| 1-oleoyl-GPE (18:1) | Lipid | Lysolipid | 0.0035 | 0.0278 | 1.27 | -2.54 | 9547071 | 11506 | |
| 1-linoleoyl-GPE (18:2) | Lipid | Lysolipid | 0.0002 | 0.0033 | 1.24 | -2.66 | 52925130 | 11507 | |
| 1-arachidonoyl-GPE (20:4) | Lipid | Lysolipid | 0 | 0 | 1.29 | -3.54 | 42607465 | 11517 | |
| 1-arachidonoyl-GPI (20:4) | Lipid | Lysolipid | 0.0028 | 0.0242 | 1.6 | -1.95 | 61690 | ||
| 1-(1-enyl-palmitoyl)-2-palmitoyl-GPC (P-16:0/16:0) | Lipid | Plasmalogen | 0.0006 | 0.0077 | -1.28 | 1.23 | 11146967 | 11206 | |
| 1-(1-enyl-palmitoyl)-2-arachidonoyl-GPE (P-16:0/20:4) | Lipid | Plasmalogen | 0 | 0.0003 | 1.25 | -3.17 | 11352 | ||
| 1-(1-enyl-stearoyl)-2-oleoyl-GPE (P-18:0/18:1) | Lipid | Plasmalogen | 0.0001 | 0.0026 | 1.03 | -2.7 | 11375 | ||
| 1-(1-enyl-palmitoyl)-2-arachidonoyl-GPC (P-16:0/20:4) | Lipid | Plasmalogen | 0 | 0 | 1.15 | -3.43 | 11220 | ||
| 1-(1-enyl-palmitoyl)-2-linoleoyl-GPC (P-16:0/18:2) | Lipid | Plasmalogen | 0 | 0 | -1.02 | -3.07 | 11211 | ||
| 1-(1-enyl-stearoyl)-2-arachidonoyl-GPE (P-18:0/20:4) | Lipid | Plasmalogen | 0 | 0.0004 | 1.17 | -2.59 | 9547058 | 05779 | |
| myristoyl dihydrosphingomyelin (d18:0/14:0) | Lipid | Sphingolipid Metabolism | 0.0039 | 0.03 | -1.53 | 1.62 | 12085 | ||
| palmitoyl SM (d18:1/16:0) | Lipid | Sphingolipid Metabolism | 0.0009 | 0.0111 | -1.24 | -3.14 | 9939941 | ||
| SM (d18:2/14:0, d18:1/14:1) | Lipid | Sphingolipid Metabolism | 0.0001 | 0.0023 | -1.47 | 2.36 | |||
| SM (d18:2/16:0, d18:1/16:1) | Lipid | Sphingolipid Metabolism | 0 | 0.0004 | -1.09 | -3.27 | |||
| SM (d18:1/18:1, d18:2/18:0) | Lipid | Sphingolipid Metabolism | 0 | 0.0001 | -1.34 | -4.1 | 6443882 | 12101 | |
| SM (d18:1/20:0, d16:1/22:0) | Lipid | Sphingolipid Metabolism | 0 | 0.0007 | -1.59 | 2.06 | 12102 | ||
| SM (d18:1/20:1, d18:2/20:0) | Lipid | Sphingolipid Metabolism | 0.0076 | 0.0473 | -1.23 | 1.64 | |||
| SM (d18:1/21:0, d17:1/22:0, d16:1/23:0) | Lipid | Sphingolipid Metabolism | 0.0011 | 0.0127 | -1.57 | 1.97 | |||
| SM (d18:1/22:1, d18:2/22:0, d16:1/24:1) | Lipid | Sphingolipid Metabolism | 0.0001 | 0.0025 | -1.63 | 1.6 | 12104 | ||
| SM (d18:2/23:0, d18:1/23:1, d17:1/24:1) | Lipid | Sphingolipid Metabolism | 0.0029 | 0.0245 | -1.54 | 1.79 | |||
| SM (d18:1/24:1, d18:2/24:0) | Lipid | Sphingolipid Metabolism | 0.0005 | 0.0064 | -1.54 | 1.93 | 12107 | ||
| SM (d18:2/24:1, d18:1/24:2) | Lipid | Sphingolipid Metabolism | 0.0002 | 0.0037 | -1.44 | 1.82 | |||
| N-stearoyl-sphingosine (d18:1/18:0) | Lipid | Sphingolipid Metabolism | 0.0054 | 0.0367 | -1.2 | -3.07 | 5283565 | 04950 | |
| N-stearoyl-sphingadienine (d18:2/18:0) | Lipid | Sphingolipid Metabolism | 0.0069 | 0.045 | -1.23 | -3.21 | |||
| lactosyl-N-nervonoyl-sphingosine (d18:1/24:1) | Lipid | Sphingolipid Metabolism | 0.0013 | 0.0133 | -1.75 | 1.76 | |||
| SM (d18:2/23:1) | Lipid | Sphingolipid Metabolism | 0 | 0 | -1.96 | 2.14 | |||
| SM (d18:2/21:0, d16:2/23:0) | Lipid | Sphingolipid Metabolism | 0 | 0.0001 | -1.63 | 2.27 | |||
| SM (d18:2/24:2) | Lipid | Sphingolipid Metabolism | 0 | 0.0003 | -1.29 | 1.86 | |||
| SM (d18:1/22:2, d18:2/22:1, d16:1/24:2) | Lipid | Sphingolipid Metabolism | 0 | 0.0002 | -1.42 | 1.93 | |||
| SM (d18:0/18:0, d19:0/17:0) | Lipid | Sphingolipid Metabolism | 0.0002 | 0.0033 | -1.32 | -3.54 | 12087 | ||
| SM (d18:2/18:1) | Lipid | Sphingolipid Metabolism | 0 | 0 | -1.11 | -4.62 | |||
| SM (d18:1/19:0, d19:1/18:0) | Lipid | Sphingolipid Metabolism | 0 | 0.0001 | -2.1 | 1.78 | |||
| N-stearoyl-sphinganine (d18:0/18:0) | Lipid | Sphingolipid Metabolism | 0.0076 | 0.0473 | -1.08 | -3.43 | 5283573 | ||
| 7-HOCA | Lipid | Sterol | 0.0003 | 0.0046 | -1.35 | 2.4 | C17337 | 3081085 | 12458 |
| ceramide (d16:1/24:1, d18:1/22:1) | Lipid | Ceramides | 0.0003 | 0.0046 | -1.93 | 2.38 | |||
| AMP | NT | Purine Metab, Adenine containing | 0.0044 | 0.0319 | 1.19 | -2.01 | C00020 | 6083 | 00045 |
| 2'-O-methylcytidine | NT | Pyrimidine Metab, Cytidine containing | 0.0032 | 0.0259 | -2.27 | 1.22 | 150971 | ||
| alpha-CEHC sulfate | CF_Vit | Tocopherol Metabolism | 0 | 0.0006 | -2.81 | 1.26 | |||
| gamma-tocopherol/beta-tocopherol | CF_Vit | Tocopherol Metabolism | 0.0002 | 0.0037 | 1.98 | -2.13 | |||
| retinol (Vitamin A) | CF_Vit | Vitamin A Metabolism | 0.0036 | 0.0284 | -1.09 | 1.56 | C00473 | 445354 | 00305 |
| perfluorooctanesulfonic acid (PFOS) | Xeno | Chemical | 0.0013 | 0.0133 | -2.44 | 1.07 | C18142 | 74483 | 59586 |
| X– 11530 | Unknown | 0.0074 | 0.0471 | -2.27 | 1.04 | ||||
| X– 11538 | Unknown | 0.0014 | 0.0141 | 1.54 | -1.67 | ||||
| X– 11795 | Unknown | 0.0006 | 0.0077 | 4.09 | 2.14 | ||||
| X– 12117 | Unknown | 0.0001 | 0.0033 | 1.11 | 2.6 | ||||
| X– 13866 | Unknown | 0 | 0 | -1.44 | 2.87 | ||||
| X– 16580 | Unknown | 0.0003 | 0.0052 | -1.15 | -3.77 | ||||
| X– 19141 | Unknown | 0.0002 | 0.0033 | 1.14 | 4.14 | ||||
| X– 21785 | Unknown | 0.0011 | 0.0126 | 1.16 | 2.93 | ||||
| X– 23369 | Unknown | 0.0043 | 0.0314 | -1.59 | 1.48 | ||||
| X– 23678 | Unknown | 0.0075 | 0.0471 | 1.17 | -2 |
SM, sphingomyelin; AA, amino acid; CHO, carbohydrate; NT, nucleotide; CF_Vit, cofactors and vitamins; Xeno, xenobiotic; FA, fatty acid; FDR, false discovery rate; FC, fold change; CON, control diet; CPB, cardiac protection blend. KEGG, Kyoto Encyclopedia of Genes and Genomes; PUBCHEM, open chemistry database at NIH; HMDB, Human Metabolome Database. Negative fold changes represent decreases in concentration from baseline to 6 months.
Ten metabolites with the highest VIP scores were identified using PLS-DA. Caprate, EPA, DHA and its phosphatidylcholine derivative DHA-PC, and X-13866, a metabolite with unknown identity were higher in CPB dogs than in CON dogs while two glycerophosphatidylcholines, one lysophospholipid, an unknown metabolite X-11795, and one sphingomyelin were lower in CPB dogs (Fig 1D). The values of R2 and Q2, estimates of model’s predictive accuracy, were reported in S3 File.
Omega-6 to omega-3 FA ratio
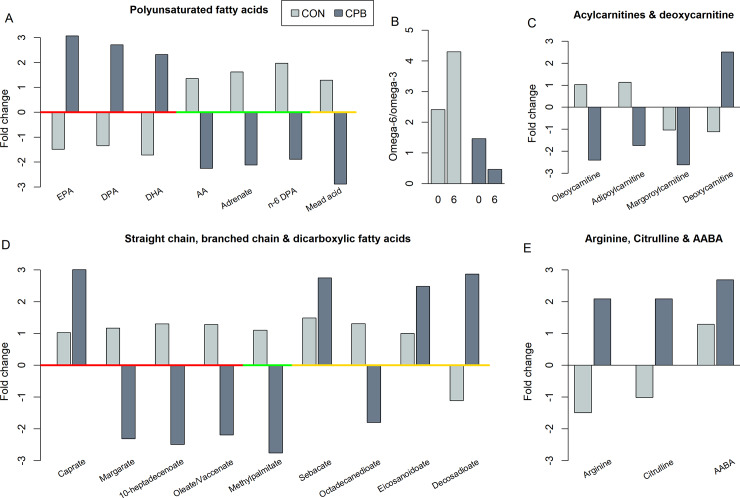
Three omega-3 polyunsaturated fatty acids (PUFAs), eicosapentaenoate (EPA; 20:5n-3), docosapentaenoate (DPA; 22:5n-3), and docosahexaenoate (DHA; 22:6n-3) were increased in CPB dogs but decreased in CON dogs. In contrast, three omega-6 PUFAs, arachidonate (ARA, 20:4n-6), adrenate (22:4n-6), and docosapentaenoate (n6 DPA; 22:5n-6), and one omega-9 PUFA, mead acid (20:3n-9), were decreased over baseline in CPB dogs but increased in CON dogs (Fig 2A). At baseline, the ratio of serum omega-6 to omega-3 was 2.41 for CON dogs and 1.46 for CPB dogs. These ratios changed to 4.30 and 0.46 after 6 months respectively (Fig 2B).
Fig 2.
Fold changes between CPB vs. CON diets in (A) polyunsaturated FAs, (B) ratios of omega-6 to omega-3 FAs, (C) acylcarnitines and deoxycarnitine, (D) straight chain, branched chain, and dicarboxylic FAs, and (E) L-arginine, L-citrulline, and 2-aminobutyric acid (AABA) at baseline (0) and 6 months (6). Red, green, and gold bars along x-axis depict (A) omega-3, -6, and -9 FAs respectively and (D) straight, branched chain, and dicarboxylic FAs respectively. A negative fold change represents a decrease from baseline to 6 months.
Acylcarnitines and deoxycarnitine
Two long chain acyl carnitines, oleoylcarnitine (C18) and margaroylcarnitine (C17), and a short chain dicarboxylic carnitine, adipoylcarnitine (C6-DC), showed large decreases from baseline to 6 months in CPB dogs (FCs = -2.41, -2.62 and -1.74 respectively, all FDR < 0.05. A negative FC indicates a decrease from baseline to 6 months) while little change was found in CON dogs (all FCs < 1.13) (Fig 2C). Two additional acylcarnitines, myristoleoylcarnitine (C14:1) and behenoylcarnitine (C22), were decreased in the CPB dogs although the changes did not reach statistical significance after p value adjustment (both P = 0.03, FDR = 0.12). The concentration of deoxycarnitine, the immediate precursor of carnitine biosynthesis, was significantly elevated in CPB dogs compared to CON dogs (FCs = 2.51 and -1.11 respectively, FDR = 0.004).
MCFAs, LCFAs, BCFAs and DCFAs
Caprate, a 10-carbon MCFA, showed a significant increase from baseline in CPB dogs compared to little change in CON dogs (FDR < 0.001) (Fig 2D). Caprylate, an 8-carbon MCFA, showed a similar trend but the change did not reach statistical significance after p value adjustment (P = 0.025, FDR = 0.11, data not shown). Large decreases were observed in three LCFAs, margarate (17:0), 10-heptadecenoate (17:1n7), and oleate/vaccenate (18:1), and in a branched chain fatty acid (BCFA), the methyl ester of palmitic acid, in CPB dogs while little or no change was found in CON dogs (all FDR < 0.05, Fig 2D). Additionally, four dicarboxylic fatty acids (DCFAs) showed changes: sebacate, eicosanodioate, and decosadioate increased while octadecanedioate (C18) decreased in the CPB dogs (all FDR < 0.05, Fig 2D).
Changes in amino acid profiles
On the pathway level, increases in lysine metabolism, phenylalanine and tyrosine metabolism, branch chain amino acid metabolism, and methionine, cysteine, and taurine metabolism were observed in CPB dogs vs. CON dogs (Table 1). Alpha-aminobutyric acid (AABA), a modulator of glutathione homeostasis in the myocardium [34], was increased by 2.69 fold in CPB dogs compared to a smaller 1.29-fold increase in CON dogs (Fig 2E, FDR = 0.026). Although arginine contents were similar between diets (CON: 1.53% vs. CPB: 1.50%, S1 Table), serum levels of arginine and citrulline were significantly elevated from baseline in CPB dogs (Fig 2E, both FC = 2.09, FDR = 0.031 and 0.024 respectively) but decreased in CON dogs.
Phospholipids
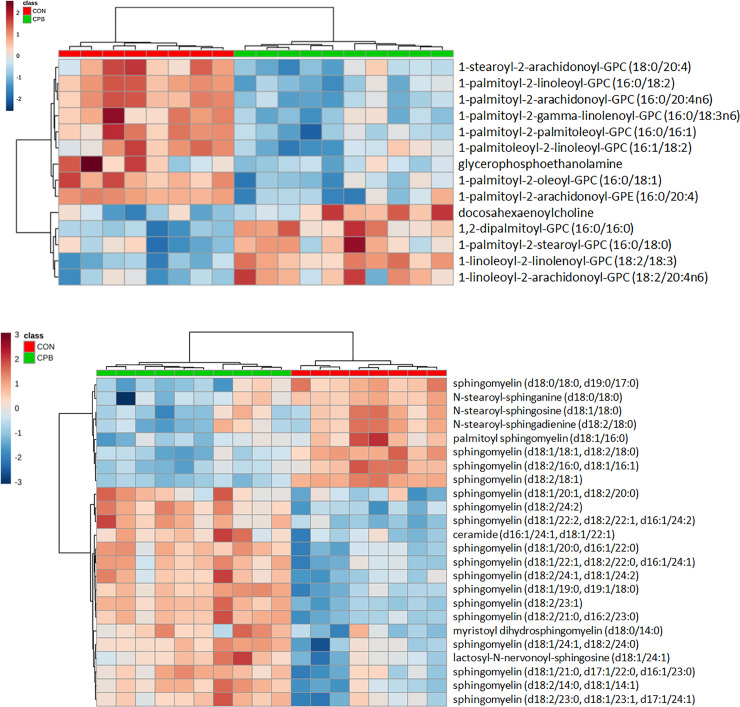
Four glycerophosphatidyl cholines (GPCs) and DHA-choline were increased in CPB group vs. CON group while seven GPCs were decreased (Fig 3A). In addition, glycerophosphoethanolamine and 1-palmitoyl-2-arachidonoyl-glycerophosphatidyl ethanolamine (GPE) (16:0/20:4) were decreased (all FDR < 0.05).
Fig 3.
Heatmaps of changes of phospholipids (A) and sphingomyelins (B) from baseline to 6 months. Legends: diet groups are represented by red (CON) and green (CPB) boxes on the top of the heatmaps; Keys: orange indicates increases while blue indicates decreases from baseline.
Sphingomyelin (SM) is comprised of a phosphatidyl choline or phosphatidyl ethanolamine polar group linked to ceramide, which includes a sphingosine backbone and a FA. Overall, the majority (16/24) of differentially changed SMs were increased in CPB dogs when compared to CON dogs, resulting in an overall increase in SM in the serum (all FDR < 0.05, Fig 3B). SM was further examined based on the saturated LCFA constituents: palmitic acid (SM-16), arachidic acid (SM-20), behenic acid (SM-22), and lignoceric acid (SM-24). SM-16 was decreased by more than 3-fold in CPB dogs while SM-20, SM-22, and SM-24 were increased (Table 2). No significantly altered ceramide with saturated LCFA was found in this study.
Table 2. Sphingomyelin species with long chain saturated fatty acids.
| Metabolite | Species | P-value | FDR | FC_CON | FC_CPB |
|---|---|---|---|---|---|
| palmitoyl SM (d18:1/16:0) | SM-16 | 0.001 | 0.011 | -1.24 | -3.14 |
| SM (d18:2/16:0, d18:1/16:1) | SM-16 | 0.000 | 0.000 | -1.09 | -3.27 |
| SM (d18:1/20:0, d16:1/22:0) | SM-20/22 | 0.000 | 0.001 | -1.59 | 2.06 |
| SM (d18:1/20:1, d18:2/20:0) | SM-20 | 0.008 | 0.047 | -1.23 | 1.64 |
| SM (d18:1/21:0, d17:1/22:0, d16:1/23:0) | SM-21/22/23 | 0.001 | 0.013 | -1.57 | 1.97 |
| SM (d18:1/22:1, d18:2/22:0, d16:1/24:1) | SM-22 | 0.000 | 0.003 | -1.63 | 1.60 |
| SM (d18:2/23:0, d18:1/23:1, d17:1/24:1) | SM-23 | 0.003 | 0.025 | -1.54 | 1.79 |
| SM (d18:1/24:1, d18:2/24:0) | SM-24 | 0.001 | 0.006 | -1.54 | 1.93 |
SM, sphingomyelin; FDR, false discovery rate; FC, fold change; CON, control diet; CPB, cardiac protection blend.
Negative numbers (in green) denote decreases in concentration from baseline to 6 months while positive numbers
(in red) show increases
Plasmalogen is another unique group of phospholipids with a vinyl-ether bond in the 1-acyl position. Three of the identified plasmalogens were GPC while the other three belonged to GPE. The five plasmalogens with omega-6 PUFAs (arachidonoyl and linoleoyl) and omega-9 PUFA (oleoyl) in the 2-acyl position were decreased in CPB group whereas the one with a saturated palmitic acid (16:0) was increased (S2A–S2F Fig).
Lysolipids are derivatives of phospholipids in which 2-acyl groups are removed by hydrolysis. The concentrations of eight lysolipids, including four lysophosphatidyl cholines (LPC), three lysophosphatidyl ethanolamines, and one lysophosphatidyl inositol were altered: while 1-lignoceroyl-GPC (24:0) was increased (FDR = 0.004) in CPB dogs compared with CON dogs, seven others were decreased (all FDR < 0.05) (S2G–S2N Fig), resulting in a net decrease in circulating lysolipids.
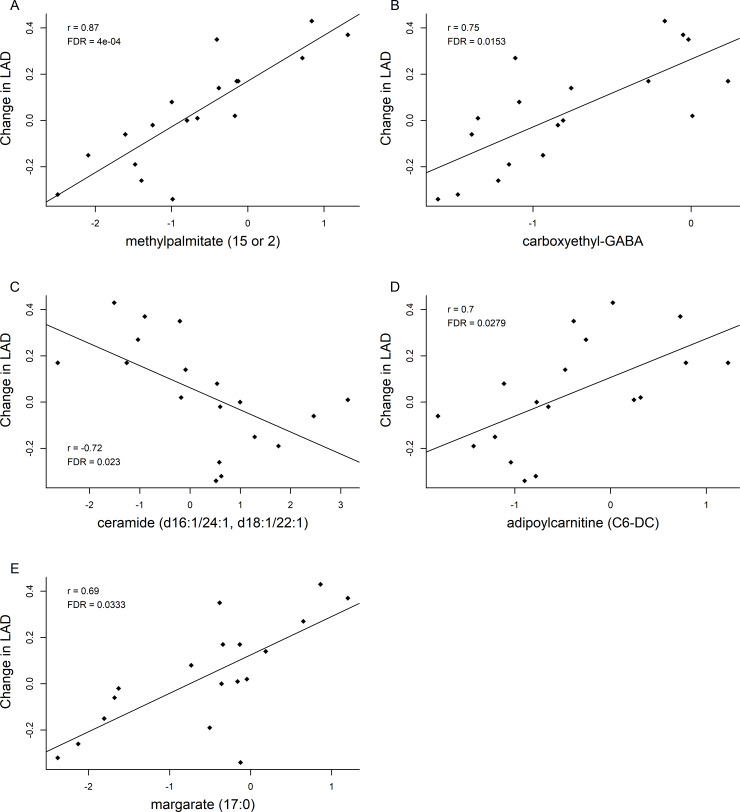
Correlations between LAD and metabolites
The 6-month changes of five metabolites were strongly correlated with changes in LAD (Fig 4, S2 Table). Methylpalmitate, carboxyethyl-GABA, adipoylcarnitine and margarate showed a positive correlation with LAD (FDR < 0.05, r > 0.68 in all cases), while ceramide had a negative correlation (FDR = 0.023, r = -0.72). Based on these results and previous finding that the levels of both methylpalmitate and margarate were decreased in MMVD dogs when compared to healthy dogs [9], the change in both of these 17-carbon FAs were evaluated relative to changes in other significant metabolites. Thirty-three metabolites were correlated with margarate and 57 were correlated with methylpalmitate (all r > 0.50, FDR < 0.05, S3 Table, S4 Table). Among them, 29 were in common (S5 Table), including positive correlations with 10-heptadecenoate (C17:1n7), mead acid, adrenate, and three acylcarnitines (adipoylcarnitine, margaroylcarnitine, and oleoylcarnitine) (S3 Fig, S4 Fig, all r > 0.59). Margarate and methylpalmitate had a nearly perfect correlation with each other (r = 0.91, FDR < 0.0001).
Fig 4.
Scatterplots of changes between left atrial diameter (LAD) and (A) methylpalmitate, (B) carboxyethyl-GABA, (C) ceramide, (D) adipoylcarnitine, and (E) margarate. Correlation coefficients (r) and adjusted p-values (FDR) from Spearman’s correlation analysis are shown in the plots. A linear regression line is also drawn in each plot.
Discussion
To our knowledge, this is the first study to investigate dietary effects on global metabolomic changes in dogs with a cardiac disease. Among the 102 known metabolites with significant diet by time interactions, approximately 72% were lipids and 16% were amino acids. Many of the observed changes were likely due to the differences between diets which differed considerably in their FA compositions. However, clinical benefits from the intervention study suggested a potential association between these diet-induced molecular and metabolic changes and clinical outcomes [17]. PCA analysis showed a clear clustering between diet groups after 6 months while no clustering was observed at baseline.
FAs are the main source of energy for the heart. Thus, lipid modification in the diet could have a primary effect on serum markers relating to cardiac energetics and offer potential benefits in dogs with MMVD by improving fat utilization in cardiac mitochondria. The two diets were similar in total fat content but differed in FA types. These differences were primarily the presence of both MCT and long chain omega-3 FAs in the CPB diet. Increases in serum capric acid (C10) (FDR < 0.001) and caprylic acid (C8) (FDR = 0.11) were seen in the CPB vs CON dogs reflecting this difference. The presence of these MCFAs can improve cardiac energetics and mitochondrial metabolism because MCFAs do not require transporters or carnitine-mediated transport pathway to reach mitochondria for oxidation [18, 19]. Studies have shown that MCTs produced more citric acid cycle intermediates and are more ketogenic than their long chain counterparts [19, 35, 36]. Importantly, MCTs reduced mitochondrial and cytoplasmic ROS in the rat liver [35] and in the heart of spontaneously hypertensive rats [20]. Three omega-3 PUFAs (EPA, DPA, and DHA) were increased in CPB dogs but decreased in CON dogs, as may be expected due to dietary differences. Conversely, three omega-6 PUFAs (arachidonate, adrenate, and n-6 DPA) were increased in CON dogs. Therefore, the ratios of omega-6 to omega-3 were 2.41 and 1.46 for CON and CPB groups at baseline but changed to 4.30 and 0.46 at 6 months respectively. Increases in the ratio of omega-6 to omega-3 are suggested to promote pathogenesis of many diseases including cardiovascular diseases, inflammation, and immune disorders while decreases in the ratio exert suppressive effects [37–39]. To the extent that increased serum MCFAs and omega-3 FAs are hallmarks of their cardiac bioavailability, improved myocardial energetics and protection against oxidative stress and inflammation appear to be facilitated by the CPB.
Acylcarnitines are intermediates of FA oxidation. Changes in circulating acylcarnitine concentrations have been used as diagnostic markers for disorders in peroxisomal or mitochondrial oxidation processes [40, 41]. Accumulation of acylcarnitine markers in the circulation likely signifies incomplete fat oxidation [41]. Both succinylcarnitine (C4-DC) and hexanoylcarnitine (C6:0) were increased in MMVD dogs when compared with healthy dogs [9]. In the present study, some carnitine metabolites, e.g., oleycarnitine (18:1), adipoylcarnitine (C6-DC), and margaroylcarnitine (17:0), were decreased in CPB dogs compared to CON dogs, suggesting an improvement in cardiac fat utilization. In addition, deoxycarnitine was increased in CPB dogs. In mammals, L-carnitine is synthesized in liver, brain, and (in humans) kidneys and is released into the circulatory system. The remaining organs are missing the hydroxylase which catalyzes the final conversion from deoxycarnitine to L-carnitine [42, 43]. Heart tissues do not synthesize L-carnitine but can generate deoxycarnitine, which can subsequently be exchanged for L-carnitine from the blood stream [42, 44]. Both in vivo and in vitro evidence demonstrated bidirectional exchanges between carnitine and deoxycarnitine across cardiac sarcolemma [42]. In dogs with MMVD, the myocardiocyte’s ability to synthesize deoxycarnitine may be compromised [9]. Hence, increases in serum deoxycarnitine associated with the CPB diet likely indicate a pathway for the heart to refresh its L-carnitine supply and promote further mitochondrial fat oxidation.
The serum levels of four dicarboxylic fatty acids (DCFAs) differed between diet groups. Sebacate (C10), eicosanodioate (C20:5n-3), and docosadioate (C22:6n-3) were increased in the CPB dogs vs CON dogs while octadecanedioate (C18) showed the opposite. The change in octadecanedioate may be explained by the fact that there was three times more stearic acid (C18:0) in CON diet versus CPB diet (CON: 1.85% vs. CPB: 0.58%, S1 Table). In early studies, DCFAs were found in urine from healthy individuals after administration of MCTs [45]. DCFAs are the products of monocarboxylic ω-oxidation in peroxisomes, which under normal conditions does not appear to be a major pathway. However, this pathway has been proposed as a rescue pathway where mitochondrial dysfunction exists [46]. The results from the present study suggest a potential involvement of MCT-DCFA-ω-oxidation rescue pathway in improving energy metabolism in MMVD dogs with compromised mitochondrial function.
L-arginine concentrations were similar between diets. However, a two-fold increase in serum arginine and citrulline were found in CPB dogs. Nitric oxide (NO) exerts many beneficial roles in the cardiovascular system and protects against hypertension, oxidative stress and cell death [47–50]. Nitric oxide is synthesized by nitric oxide synthase which converts L-arginine to NO and L-citrulline. Supplementation of L-arginine plus L-citrulline have been shown to cause a more rapid increase in NO bioavailability and NO-dependent effects than with each amino acid alone [51]. It was previously noted that, there was an increase of oxidized glutathione but decrease of reduced glutathione in MMVD dogs when compared to healthy dogs [9, 52]. AABA, a modulator for myocardial glutathione homeostasis, was increased 2.7 fold in CPB dogs. AABA activates the intracellular glutathione biosynthesis pathway, rendering protective effects against ROS in murine cardiomyopathy model [34]. Data from the current study suggests that the CPB may also confer NO-dependent protection on the cardiovascular systems and ameliorate oxidative stress via glutathione biosynthesis.
Many of the changes in phospholipids were likely due to the difference in diets relating to FAs. One interesting finding, however, was the decrease in 1-palmitoyl-2-arachidonoyl-GPE (16:0/20:4), in CPB dogs. Elevated phosphatidyl ethanolamine has been previously associated with occlusive arterial disease in human [53]. While not conclusive, these data support the possibility that the decrease in phosphatidyl ethanolamine is associated with improved cardiac function in MMVD-CPB dogs. Lysolipids are the hydrolytic product of phospholipase A1, resulting in respective 1-acyl phospholipids. Plasmalogens belong to a unique group of phospholipids with a vinyl ether bond in the 1-acyl position and commonly a polyunsaturated FA in the 2-acyl position. Plasmalogens constitute 10% of the total phospholipid molecular mass and more than 30% of phospholipids in the adult human heart [54]. In dogs, over 50% of phospholipids in the myocardial sarcolemma are plasmalogens [55]. Both lysolipids and plasmalogens are involved in cellular signaling pathways. In the present study, seven out of eight lysolipids and five out of six plasmalogens were decreased in CPB-fed dogs vs. CON-fed dogs. The biological significance of this, if any, remains to be determined.
Ceramides (Cer) and sphingomyelin (SM) exhibit many biological activities that may influence the pathophysiology of heart failure [56–58]. These properties differ, depending on the LCFA attached. Recent studies suggested causal associations between levels of Cer and SM species with long chain saturated FAs and risk of heart failure. In a meta-analysis with nearly 6000 human participants, the ratio of C24:0 containing ceramide to C16:0 ceramide were inversely associated with coronary heart disease and incident heart failure [56]. Plasma phospholipid with very long chain saturated FAs were associated with lower risk of incident heart failure [57]. In further support of this association, a more recent study with over 4000 participants and a median follow-up of 9.4 years documented that higher levels of Cer-16 and SM-16 with palmitic acid were associated with increased risk of heart failure while higher levels of SM-20, Cer-22, SM-22, and SM-24 were associated with decreased risk of heart failure [58]. Unlike these human studies, Cer with saturated LCFA did not vary within this present study in dogs. However, the levels of SM-16 were lower while levels of SM-20, SM-22, and SM-24 were higher in CPB dogs compared to CON dogs. Hence, while the cardiac diseases in the human studies and this canine study differ, the observed changes in the levels and FA constituents of circulating SMs may suggest markers of cardiac health in dogs and the CPB diet may have thus contributed to a degree of cardiac protection in canine MMVD through these metabolites.
Although even chain FAs represent >99% of the total circulating FAs, there are also detectable amounts of odd-chain FAs in human tissues [59, 60]. Several recent studies have associated plasma odd chain saturated FAs, mainly C15:0 and C17:0, with reduced risk of cardiometabolic diseases [60–63]. Methylpalmitate, a palmitic acid (C16:0) methyl ester, has been associated with anti-inflammatory and anti-oxidative activities, and may possess cardioprotective and antifibrotic effects [64–67]. However, a previous study reported that serum concentrations of margarate and methylpalmitate were increased in dogs with early stage MMVD when compared to healthy dogs [9]. This may suggest a species difference in dogs versus humans. Importantly, in the present study, both margarate and methylpalmitate were decreased in response to the diet intervention yet these decreases were positively associated with reductions in LAD in preclinical MMVD dogs. Because little is known about margarate or methylpalmitate in cardiac health in dogs, more studies from larger cohorts of dogs are warranted.
In summary, untargeted serum metabolomic analysis has identified numerous metabolites that may reflect improved cardiac bioenergetics and fatty acid utilization by cardiac mitochondria in dogs fed the CPB diet. Metabolomic markers also suggest improved cellular redox state and reduced inflammation in MMVD dogs fed the CPB diet. Due to the many similarities between human and canine MMVD, our study may also shed light on metabolic perturbations in human MMVD.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(TIF)
(TIF)
(TIFF)
(TIFF)
(DOCX)
(XLSX)
(DOCX)
Acknowledgments
The authors would like to thank Kristen Lower and Cynthia Steeby for laboratory assistance and Dr. Melanie Barnes for veterinary service. The study was funded by the Nestlé Purina PetCare Company.
Abbreviations
- CPB
cardiac protection blend
- CON
control diet
- MMVD
myxomatous mitral valve disease
- LAD
left atrial diameter
- FDR
false discovery rate
- PCA
principal component analysis
- MCT
medium chain triglycerides
- FA
fatty acid
- EPA
eicosapentaenoate (20:5n-3)
- DHA
docosahexaenoate (22:6n-3)
- DPA
docosapentaenoate (22:5n-3)
- MCFA
medium chain fatty acid
- LCFA
long chain fatty acid
- BCFA
branched chain fatty acid
- PUFA
polyunsaturated fatty acid
- DCFA
dicarboxylic fatty acid
- SM
sphingomyelin
- Cer
ceramide
- GPC
glycerophosphatidyl choline
- GPE
glycerophosphatidyl ethanolamine
- LPC
lysophosphatidyl choline
- AABA
α-aminobutyric acid or 2-aminobutyric acid
- ROS
reactive oxygen species
- FC
fold change
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was funded by the Nestlé Purina PetCare Company. All authors are employee or consultants of the company. The funder provided support in the form of salaries for author QL and consulting fees for DPL and JEB, and financially supported the research, but did not have any additional role in the study design, data collection and analysis, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res. 2013;113(6):709–24. Epub 2013/08/31. 10.1161/CIRCRESAHA.113.300376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neubauer S. The failing heart—an engine out of fuel. N Engl J Med. 2007;356(11):1140–51. 10.1056/NEJMra063052 . [DOI] [PubMed] [Google Scholar]
- 3.Dorn GW 2nd, Vega RB, Kelly DP. Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev. 2015;29(19):1981–91. 10.1101/gad.269894.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai L, Leone TC, Keller MP, Martin OJ, Broman AT, Nigro J, et al. Energy metabolic reprogramming in the hypertrophied and early stage failing heart: a multisystems approach. Circ Heart Fail. 2014;7(6):1022–31. 10.1161/CIRCHEARTFAILURE.114.001469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lionetti V, Stanley WC, Recchia FA. Modulating fatty acid oxidation in heart failure. Cardiovasc Res. 2011;90(2):202–9. 10.1093/cvr/cvr038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, et al. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation. 2016;133(8):698–705. 10.1161/CIRCULATIONAHA.115.017355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedi KC Jr., Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, et al. Evidence for Intramyocardial Disruption of Lipid Metabolism and Increased Myocardial Ketone Utilization in Advanced Human Heart Failure. Circulation. 2016;133(8):706–16. 10.1161/CIRCULATIONAHA.115.017545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchanan J. Prevalence of cardiovascular disorders In: PR F, D S, NS M, editors. Textbook of canine and feline cardiology. 2nd ed Philadelphia: Saunders; 1999. p. 457–70. [Google Scholar]
- 9.Li Q, Freeman LM, Rush JE, Huggins GS, Kennedy AD, Labuda JA, et al. Veterinary Medicine and Multi-Omics Research for Future Nutrition Targets: Metabolomics and Transcriptomics of the Common Degenerative Mitral Valve Disease in Dogs. OMICS. 2015;19(8):461–70. 10.1089/omi.2015.0057 . [DOI] [PubMed] [Google Scholar]
- 10.Oyama MA, Chittur SV. Genomic expression patterns of mitral valve tissues from dogs with degenerative mitral valve disease. Am J Vet Res. 2006;67(8):1307–18. 10.2460/ajvr.67.8.1307 [DOI] [PubMed] [Google Scholar]
- 11.Lu CC, Liu MM, Culshaw G, Clinton M, Argyle DJ, Corcoran BM. Gene network and canonical pathway analysis in canine myxomatous mitral valve disease: a microarray study. Vet J. 2015;204(1):23–31. 10.1016/j.tvjl.2015.02.021 . [DOI] [PubMed] [Google Scholar]
- 12.Greenhouse DG, Murphy A, Mignatti P, Zavadil J, Galloway AC, Balsam LB. Mitral valve prolapse is associated with altered extracellular matrix gene expression patterns. Gene. 2016;586(1):56–61. 10.1016/j.gene.2016.04.004 . [DOI] [PubMed] [Google Scholar]
- 13.Pedersen HD, Haggstrom J. Mitral valve prolapse in the dog: a model of mitral valve prolapse in man. Cardiovasc Res. 2000;47(2):234–43. 10.1016/s0008-6363(00)00113-9 . [DOI] [PubMed] [Google Scholar]
- 14.Markby GR, Summers KM, MacRae VE, Corcoran BM. Comparative Transcriptomic Profiling and Gene Expression for Myxomatous Mitral Valve Disease in the Dog and Human. Vet Sci. 2017;4(3). 10.3390/vetsci4030034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang L, Wang J, Li R, Fang ZM, Zhu XH, Yi X, et al. Disturbed energy and amino acid metabolism with their diagnostic potential in mitral valve disease revealed by untargeted plasma metabolic profiling. Metabolomics. 2019;15(4):57 10.1007/s11306-019-1518-1 . [DOI] [PubMed] [Google Scholar]
- 16.Oyama MA, Elliott C, Loughran KA, Kossar AP, Castillero E, Levy RJ, et al. Comparative pathology of human and canine myxomatous mitral valve degeneration: 5HT and TGF-beta mechanisms. Cardiovasc Pathol. 2020;46:107196 10.1016/j.carpath.2019.107196 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q, Heaney A, Langenfeld-McCoy N, Boler BV, Laflamme DP. Dietary intervention reduces left atrial enlargement in dogs with early preclinical myxomatous mitral valve disease: a blinded randomized controlled study in 36 dogs. BMC Vet Res. 2019;15(1):425 Epub 2019/11/30. 10.1186/s12917-019-2169-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papamandjaris AA, MacDougall DE, Jones PJ. Medium chain fatty acid metabolism and energy expenditure: obesity treatment implications. Life Sci. 1998;62(14):1203–15. 10.1016/s0024-3205(97)01143-0 . [DOI] [PubMed] [Google Scholar]
- 19.Bach AC, Babayan VK. Medium-chain triglycerides: an update. Am J Clin Nutr. 1982;36(5):950–62. 10.1093/ajcn/36.5.950 . [DOI] [PubMed] [Google Scholar]
- 20.Saifudeen I, Subhadra L, Konnottil R, Nair RR. Metabolic Modulation by Medium-Chain Triglycerides Reduces Oxidative Stress and Ameliorates CD36-Mediated Cardiac Remodeling in Spontaneously Hypertensive Rat in the Initial and Established Stages of Hypertrophy. J Card Fail. 2017;23(3):240–51. 10.1016/j.cardfail.2016.08.001 . [DOI] [PubMed] [Google Scholar]
- 21.Labarthe F, Gelinas R, Des Rosiers C. Medium-chain fatty acids as metabolic therapy in cardiac disease. Cardiovasc Drugs Ther. 2008;22(2):97–106. 10.1007/s10557-008-6084-0 . [DOI] [PubMed] [Google Scholar]
- 22.Kaunitz H. Medium chain triglycerides (MCT) in aging and arteriosclerosis. J Environ Pathol Toxicol Oncol. 1986;6(3–4):115–21. Epub 1986/03/01. . [PubMed] [Google Scholar]
- 23.McGarrah RW, Crown SB, Zhang GF, Shah SH, Newgard CB. Cardiovascular Metabolomics. Circ Res. 2018;122(9):1238–58. Epub 2018/04/28. 10.1161/CIRCRESAHA.117.311002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah SH, Kraus WE, Newgard CB. Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases: form and function. Circulation. 2012;126(9):1110–20. 10.1161/CIRCULATIONAHA.111.060368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah AA, Craig DM, Sebek JK, Haynes C, Stevens RC, Muehlbauer MJ, et al. Metabolic profiles predict adverse events after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2012;143(4):873–8. 10.1016/j.jtcvs.2011.09.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanfear DE, Gibbs JJ, Li J, She R, Petucci C, Culver JA, et al. Targeted Metabolomic Profiling of Plasma and Survival in Heart Failure Patients. JACC Heart Fail. 2017;5(11):823–32. 10.1016/j.jchf.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupte AA, Hamilton DJ, Cordero-Reyes AM, Youker KA, Yin Z, Estep JD, et al. Mechanical unloading promotes myocardial energy recovery in human heart failure. Circ Cardiovasc Genet. 2014;7(3):266–76. 10.1161/CIRCGENETICS.113.000404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ford L, Kennedy AD, Goodman KD, Pappan KL, Evans AM, Miller LAD, et al. Precision of a Clinical Metabolomics Profiling Platform for Use in the Identification of Inborn Errors of Metabolism. J Appl Lab Med. 2020;5(2):342–56. 10.1093/jalm/jfz026 [DOI] [PubMed] [Google Scholar]
- 29.Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Res. 2015;43(W1):W251–7. 10.1093/nar/gkv380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durbin BP, Hardin JS, Hawkins DM, Rocke DM. A variance-stabilizing transformation for gene-expression microarray data. Bioinformatics. 2002;18 Suppl 1:S105–10. 10.1093/bioinformatics/18.suppl_1.s105 . [DOI] [PubMed] [Google Scholar]
- 31.van den Berg RA, Hoefsloot HC, Westerhuis JA, Smilde AK, van der Werf MJ. Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genomics. 2006;7:142 10.1186/1471-2164-7-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B. 1995;57:289–300. [Google Scholar]
- 33.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: 2015. [Google Scholar]
- 34.Irino Y, Toh R, Nagao M, Mori T, Honjo T, Shinohara M, et al. 2-Aminobutyric acid modulates glutathione homeostasis in the myocardium. Sci Rep. 2016;6:36749 10.1038/srep36749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bach A, Phan T, Metais P. Effect of the fatty acid composition of ingested fats on rat liver intermediary metabolism. Horm Metab Res. 1976;8(5):375–9. 10.1055/s-0028-1093617 . [DOI] [PubMed] [Google Scholar]
- 36.Bach A, Schirardin H, Weryha A, Bauer M. Ketogenic response to medium-chain triglyceride load in the rat. J Nutr. 1977;107(10):1863–70. 10.1093/jn/107.10.1863 . [DOI] [PubMed] [Google Scholar]
- 37.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56(8):365–79. Epub 2002/11/22. 10.1016/s0753-3322(02)00253-6 . [DOI] [PubMed] [Google Scholar]
- 38.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood). 2008;233(6):674–88. Epub 2008/04/15. 10.3181/0711-MR-311 . [DOI] [PubMed] [Google Scholar]
- 39.Freeman LM. Beneficial effects of omega-3 fatty acids in cardiovascular disease. J Small Anim Pract. 2010;51(9):462–70. 10.1111/j.1748-5827.2010.00968.x . [DOI] [PubMed] [Google Scholar]
- 40.Shekhawat PS, Matern D, Strauss AW. Fetal fatty acid oxidation disorders, their effect on maternal health and neonatal outcome: impact of expanded newborn screening on their diagnosis and management. Pediatr Res. 2005;57(5 Pt 2):78R–86R. Epub 2005/04/09. 10.1203/01.PDR.0000159631.63843.3E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, et al. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr. 2009;139(6):1073–81. Epub 2009/04/17. 10.3945/jn.108.103754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siliprandi N, Ciman M, Sartorelli L. Myocardial carnitine transport. Basic Res Cardiol. 1987;82 Suppl 1:53–62. Epub 1987/01/01. 10.1007/978-3-662-08390-1_7 . [DOI] [PubMed] [Google Scholar]
- 43.Vaz FM, Wanders RJ. Carnitine biosynthesis in mammals. Biochem J. 2002;361(Pt 3):417–29. Epub 2002/01/23. 10.1042/0264-6021:3610417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sartorelli L, Ciman M, Rizzoli V, Siliprandi N. On the transport mechanisms of carnitine and its derivative in rat heart slices. Ital J Biochem. 1982;31(4):261–8. Epub 1982/07/01. . [PubMed] [Google Scholar]
- 45.Verkade PE, Van Der Lee J. Researches on fat metabolism. II. Biochem J. 1934;28(1):31–40. Epub 1934/01/01. 10.1042/bj0280031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wanders RJ, Komen J, Kemp S. Fatty acid omega-oxidation as a rescue pathway for fatty acid oxidation disorders in humans. FEBS J. 2011;278(2):182–94. Epub 2010/12/16. 10.1111/j.1742-4658.2010.07947.x . [DOI] [PubMed] [Google Scholar]
- 47.Strijdom H, Chamane N, Lochner A. Nitric oxide in the cardiovascular system: a simple molecule with complex actions. Cardiovasc J Afr. 2009;20(5):303–10. Epub 2009/11/13. [PMC free article] [PubMed] [Google Scholar]
- 48.Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26(1–2):33–65. Epub 2005/02/22. 10.1016/j.mam.2004.09.003 . [DOI] [PubMed] [Google Scholar]
- 49.Wink DA, Miranda KM, Espey MG, Pluta RM, Hewett SJ, Colton C, et al. Mechanisms of the antioxidant effects of nitric oxide. Antioxid Redox Signal. 2001;3(2):203–13. Epub 2001/06/09. 10.1089/152308601300185179 . [DOI] [PubMed] [Google Scholar]
- 50.Hermann M, Flammer A, Luscher TF. Nitric oxide in hypertension. J Clin Hypertens (Greenwich). 2006;8(12 Suppl 4):17–29. Epub 2006/12/16. 10.1111/j.1524-6175.2006.06032.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morita M, Hayashi T, Ochiai M, Maeda M, Yamaguchi T, Ina K, et al. Oral supplementation with a combination of L-citrulline and L-arginine rapidly increases plasma L-arginine concentration and enhances NO bioavailability. Biochem Biophys Res Commun. 2014;454(1):53–7. Epub 2014/12/03. 10.1016/j.bbrc.2014.10.029 . [DOI] [PubMed] [Google Scholar]
- 52.Freeman LM, Rush JE, Milbury PE, Blumberg JB. Antioxidant status and biomarkers of oxidative stress in dogs with congestive heart failure. J Vet Intern Med. 2005;19(4):537–41. 10.1892/0891-6640(2005)19[537:asaboo]2.0.co;2 . [DOI] [PubMed] [Google Scholar]
- 53.Kunz F, Stummvoll W. Plasma phosphatidylethanolamine—a better indicator in the predictability of atherosclerotic complications? Atherosclerosis. 1971;13(3):413–25. Epub 1971/05/01. 10.1016/0021-9150(71)90083-9 . [DOI] [PubMed] [Google Scholar]
- 54.Messias MCF, Mecatti GC, Priolli DG, de Oliveira Carvalho P. Plasmalogen lipids: functional mechanism and their involvement in gastrointestinal cancer. Lipids Health Dis. 2018;17(1):41 10.1186/s12944-018-0685-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gross RW. Identification of plasmalogen as the major phospholipid constituent of cardiac sarcoplasmic reticulum. Biochemistry. 1985;24(7):1662–8. 10.1021/bi00328a014 . [DOI] [PubMed] [Google Scholar]
- 56.Peterson LR, Xanthakis V, Duncan MS, Gross S, Friedrich N, Volzke H, et al. Ceramide Remodeling and Risk of Cardiovascular Events and Mortality. J Am Heart Assoc. 2018;7(10). 10.1161/JAHA.117.007931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lemaitre RN, McKnight B, Sotoodehnia N, Fretts AM, Qureshi WT, Song X, et al. Circulating Very Long-Chain Saturated Fatty Acids and Heart Failure: The Cardiovascular Health Study. J Am Heart Assoc. 2018;7(21):e010019 10.1161/JAHA.118.010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lemaitre RN, Jensen PN, Hoofnagle A, McKnight B, Fretts AM, King IB, et al. Plasma Ceramides and Sphingomyelins in Relation to Heart Failure Risk. Circ Heart Fail. 2019;12(7):e005708 Epub 2019/07/13. 10.1161/CIRCHEARTFAILURE.118.005708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47(5):348–80. 10.1016/j.plipres.2008.03.003 . [DOI] [PubMed] [Google Scholar]
- 60.Khaw KT, Friesen MD, Riboli E, Luben R, Wareham N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: the EPIC-Norfolk prospective study. PLoS Med. 2012;9(7):e1001255 10.1371/journal.pmed.1001255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roe CR, Sweetman L, Roe DS, David F, Brunengraber H. Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. J Clin Invest. 2002;110(2):259–69. 10.1172/JCI15311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jacobs S, Schiller K, Jansen E, Fritsche A, Weikert C, di Giuseppe R, et al. Association between erythrocyte membrane fatty acids and biomarkers of dyslipidemia in the EPIC-Potsdam study. Eur J Clin Nutr. 2014;68(4):517–25. 10.1038/ejcn.2014.18 . [DOI] [PubMed] [Google Scholar]
- 63.Jenkins B, West JA, Koulman A. A review of odd-chain fatty acid metabolism and the role of pentadecanoic Acid (c15:0) and heptadecanoic Acid (c17:0) in health and disease. Molecules. 2015;20(2):2425–44. 10.3390/molecules20022425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saeed NM, El-Demerdash E, Abdel-Rahman HM, Algandaby MM, Al-Abbasi FA, Abdel-Naim AB. Anti-inflammatory activity of methyl palmitate and ethyl palmitate in different experimental rat models. Toxicol Appl Pharmacol. 2012;264(1):84–93. 10.1016/j.taap.2012.07.020 . [DOI] [PubMed] [Google Scholar]
- 65.Davoodbasha M, Edachery B, Nooruddin T, Lee SY, Kim JW. An evidence of C16 fatty acid methyl esters extracted from microalga for effective antimicrobial and antioxidant property. Microb Pathog. 2018;115:233–8. 10.1016/j.micpath.2017.12.049 . [DOI] [PubMed] [Google Scholar]
- 66.El-Agamy DS, Elkablawy MA, Abo-Haded HM. Modulation of cyclophosphamide-induced cardiotoxicity by methyl palmitate. Cancer Chemother Pharmacol. 2017;79(2):399–409. 10.1007/s00280-016-3233-1 . [DOI] [PubMed] [Google Scholar]
- 67.Temelli O, Kekilli E, Kizilay A. Postoperative Radiotherapy in Salivary Gland Carcinoma: A Single Institution Experience. Gulf J Oncolog. 2017;1(23):26–32. . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(TIF)
(TIF)
(TIFF)
(TIFF)
(DOCX)
(XLSX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.