Abstract
Anti-thymocyte globulin (ATG) use mitigates the risk of graft rejection and graft-versus-host disease (GVHD) after allogeneic hematopoietic cell transplantation (allo-HCT), but ATG overexposure in the setting of lymphopenia negatively affects immune recovery. We hypothesized that standard empiric weight-based dosing of ATG, used to prevent graft rejection in ex vivo CD34-selected allo-HCT, may lead to serious adverse consequences on outcomes in certain patients. We evaluated 304 patients undergoing myeloablative-conditioned ex vivo CD34-selected allo-HCT with HLA matched donors for the treatment of hematologic malignancies. Patients received rabbit ATG at a dose of 2.5 mg/kg/day intravenously on days –3 and/or –2. An ATG dosing cutoff of 450 mg was used for statistical analyses to assess the relationship between ATG and overall survival (OS). Among all patients, median total ATG dose was 360 mg (range, 130 to 510); 279 (92%) received a total dose of ATG ≤450 mg and 25 (8%) received a total dose >450 mg. On the first day of ATG administration (day −3), median absolute lymphocyte count was 0.0 K/μL. For patients who received a total dose of ATG >450 mg or ≤450 mg, the incidences of acute and late-acute GVHD grade 2–4 were statistically similar. At 3 years post-HCT, for patients who received a total dose of ATG >450 mg or ≤450 mg, respectively, non-relapse mortality (NRM) rates were 35% and 18%, P = .029, disease-free survival (DFS) rates were 37% and 61%, P = .003, and OS rates were 40% and 67%, P = .001. Among all patient and HCT characteristics in multivariable analyses, receiving a total dose of ATG >450 mg was associated with an increased risk of NRM (HR 2.9; P = .01), shorter DFS (HR 2.0; P = .03), and inferior (HR 2.1; P = .01). In summary, the use of weight-based ATG at a time of relative lymphopenia prior to ex vivo CD34-selected allo-HCT results in overdosing in heavier patients, leading to higher NRM, and lower DFS and OS. Further pharmacokinetic investigation in this setting is critical to determining the optimal dosing strategy for ATG.
Introduction
Many studies demonstrate that anti-thymocyte globulin (ATG) integrated into conditioning regimens as a method of in vivo T-cell depletion reduces the risks of acute and chronic graft-versus-host disease (GVHD) after conventional and umbilical cord blood allogeneic hematopoietic cell transplantation (allo-HCT).1–6 The efficacy of ATG may come at the cost of increased risk of certain infections and disease-relapse depending on the HCT population studied.1,2,7–10 These variable outcomes associated with ATG are related to its polyclonal and multi-targeted activity, its numerous formulations and dosing schemes, and the heterogeneous allo-HCT settings in which it is used.3
Moreover, the appropriate peri-HCT exposure to ATG will maximize its anti-GVHD properties and minimize undesirable effects. Recent population-based pharmacokinetic (PK) and pharmacodynamic analyses demonstrate that standard empiric weight-based dosing often results in highly variable ATG exposure that has profound effects on immune recovery, infection rates, GVHD, and disease relapse.11–14 A patient’s absolute lymphocyte count (ALC) at the time of ATG administration has emerged as the most important factor in ATG metabolism; thus, the intensity of the conditioning regimen and the timing of ATG dosing relative to allograft infusion must be considered in treatment decisions.14,15
In ex vivo CD34-selected allo-HCT, T-cell depletion markedly reduces acute and chronic GVHD rates while maintaining highly favorable anti-cancer efficacy. In this setting, ATG is incorporated into the conditioning regimen specifically to promote engraftment and reduce the risk of graft rejection.16–22 It is administered to patients who have received the majority of their myeloablative conditioning and are lymphopenic. Given the current standard empiric weight-based dosing and timing of ATG administration in patients undergoing ex vivo CD34-selected allo-HCT, we hypothesized that some patients may be overexposed to ATG, leading to serious adverse consequences on outcomes.
Methods
Study Design
We conducted a retrospective analysis in adult patients who underwent their first allo-HCT and received ex vivo T-cell depleted (TCD) allografts using the CliniMACS CD34 Reagent System (Miltenyi Biotech, Gladbach, Germany) as calcineurin-inhibitor-free GVHD prophylaxis. No further pharmacologic immunosuppression was used after allo-HCT. All patients were treated for acute myeloid leukemia, acute lymphoblastic leukemia, myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPNs), or non-Hodgkin lymphoma at Memorial Sloan Kettering Cancer Center (MSK) between 2006 and 2016. Patients received peripheral blood allografts from 10/10 human leukocyte antigen (HLA)-matched related (MRD) or unrelated (MUD) donors identified by high-resolution DNA sequence-specific oligonucleotide typing for HLA-A, B, C, DRB1, and DQB1 loci. Patients received a pre-HCT myeloablative conditioning regimen at the discretion of the treating physician. The chemotherapy-based conditioning was either a busulfan, melphalan, and fludarabine regimen or a clofarabine, melphalan, and thiotepa regimen. The high-dose total-body irradiation (TBI)-based conditioning (1375 cGy) was either a TBI, thiotepa, and cyclophosphamide regimen or a TBI, thiotepa, and fludarabine regimen. Patients received rabbit ATG (Thymoglobulin [Sanofi, Paris, France]) at a dose of 2.5 mg/kg/day intravenously on days –3 and/or –2. All patients received supportive care including growth factors, prevention of opportunistic infections, and sinusoidal obstruction syndrome prophylaxis according to standard MSK Adult Bone Marrow Transplant Service guidelines. This study was approved by the MSK institutional review board.
Study Endpoints and Statistical Analyses
For the statistical analyses, disease entities were grouped as acute leukemias, MDS, and other hematologic malignancies, which included MPNs, MDS/MPN overlap, or non-Hodgkin lymphoma. Overall survival (OS) and disease-free survival (DFS) were estimated using the Kaplan-Meier method. The cumulative incidence of relapse, non-relapse mortality (NRM), and GVHD were estimated using the cumulative incidence method for competing risks. Relapse and death were considered competing risks for GVHD, as well as, respectively, for NRM and relapse. The associations between patient and HCT characteristics and outcomes were evaluated using Cox proportional hazards regression models. Disease risk was assessed using the validated Disease Risk Index (DRI), and comorbidities were assessed using the Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI).23,24 Cause-specific Cox models were used for relapse, NRM, and GVHD. The final multivariable model for OS was stratified by HCT-CI and DRI to meet the proportional hazards assumption of the model. The significance threshold was set at P < .05.
An ATG dosing cutoff was determined by evaluating multiple methods to assess the relationship between ATG and OS: ROC curves for prediction of survival at fixed time points, smooth estimates of median survival as a functional of ATG dose, martingale residuals plotted from a Cox model including only age against ATG dose, and spline coefficients fitted for ATG in a Cox model providing a smooth fit and highlighting doses at increased risk (Supplemental Material). Based on these analyses, a total ATG dose cutoff of 450 mg (corresponding to patient weights >90 kg) was selected, which included patients at increased risk of all-cause death and an adequate number who had received high ATG doses.
Results
Patient and HCT Characteristics
Patient and HCT characteristics for all evaluated patients are shown in Table 1. A total of 304 patients were evaluated and the median follow-up among survivors was 49 months (range, 8 to 138). Neutrophil engraftment occurred in 302 (99.3%) patients, while 2 (0.7%) patients were unevaluable for engraftment because they died of infection on day 8 and day 10, respectively. Prior to allo-HCT, 295 (97%) patients received 2 infusions of ATG, and 9 (3%) received 1 infusion of ATG. Among all patients, 279 (92%) received a total ATG dose ≤450 mg and 25 (8%) received a total ATG dose >450 mg. The median total ATG dose was 360 mg (range, 130 to 510). The median weight and body mass index (BMI) of patients who received total ATG doses >450 mg or ≤450 mg, respectively, were 107 kg (range, 90.5 to 156.2) vs 76.2 kg (range, 45.3 to 122.3) and BMI 30 (range, 24.8 to 59.2) vs 26.1 (range, 17.5 to 42.8). On day –3, 104 (34%) patients had ALC >0.0 K/μL (range 0.1–0.6). Median ALC on day −3 was 0.0 K/μL for patients receiving chemo-based and TBI-based conditioning.
Table 1.
Patient and Transplantation Characteristics (N = 304)
| Characteristics | n (%) |
|---|---|
| Median Age (range) | 56 (20–73) |
| Female sex | 140 (46) |
| Disease | |
| Acute leukemia | 189 (62) |
| MDS | 77 (25) |
| Other Hematologic Malignancy | 38 (13) |
| DRI | |
| Low | 20 (7) |
| Intermediate | 237 (78) |
| High | 37 (12) |
| Unevaluable | 10 (3) |
| HCT-CI | |
| 0 | 62 (20) |
| 1–2 | 110 (36) |
| >3 | 132 (44) |
| Patient CMV Serostatus | |
| Seropositive | 192 (63) |
| Seronegative | 112 (37) |
| HLA Match | |
| MRD | 132 (43) |
| MUD | 172 (57) |
| Conditioning | |
| Chemo-based | 197 (65) |
| TBI-based | 107 (35) |
| BMI | |
| <25 | 111 (37) |
| 25–30 | 117 (38) |
| >30 | 76 (25) |
| Median Total ATG Dose, mg (range) | 370 (130–510) |
| Total ATG Dose | |
| ≤450 mg | 279 (92) |
| >450 mg | 25 (8) |
| Median ALC on Day −3, K/μL (range) | 0 (0–0.6) |
| ALC on Day −3 | |
| 0 K/μL | 200 (66) |
| 0.1 K/μL | 89 (29) |
| 0.2 K/μL | 8 (2.5) |
| 0.3 K/μL | 4 (1.5) |
| 0.4 K/μL | 2 (0.5) |
| 0.6 K/μL | 1 (0.5) |
Data are reported as n (%) unless otherwise noted. Other hematologic malignancy: myeloproliferative neoplasms (MPNs), MDS/MPN overlap, non-Hodgkin lymphoma. ALC, absolute lymphocyte count; ATG, anti-thymocyte globulin; BMI, body mass index; CMV, cytomegalovirus; DRI, disease risk index; HCT-CI, hematopoietic cell transplantation comorbidity index; HLA, human leukocyte antigen; MDS, myelodysplastic syndromes; MRD, matched related donor; MUD, matched unrelated donor; TBI, total-body irradiation.
Association of Total ATG Dose and Treatment Outcomes
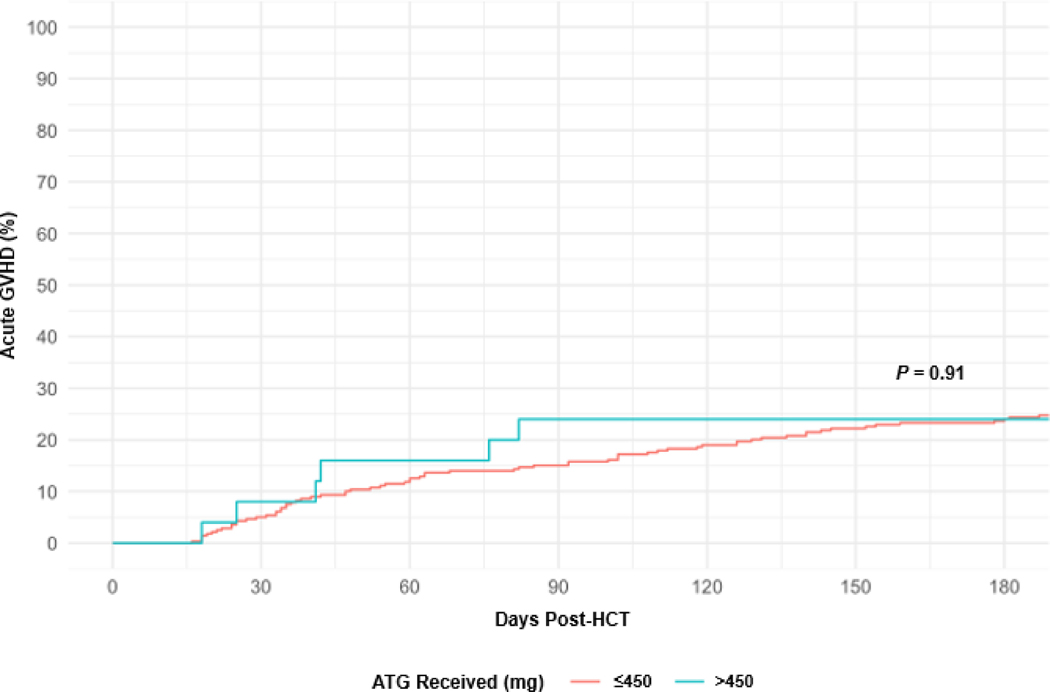
Among all patients, the cumulative incidence of acute and late-acute GVHD grade 2–4 by day 180 was 17% (95% CI, 13% to 21%). For patients who received a total dose of ATG >450 mg or ≤450 mg, the cumulative incidence of acute and late-acute GVHD grade 2–4 at day 100 was, respectively, 24% (95% CI, 10% to 42%) and 16% (95% CI, 12% to 21%), P = .91 (Figure 1). No patient characteristic or HCT characteristics was associated with an increased risk of GVHD in a univariable analysis (data not shown).
Figure 1.
Cumulative Incidence of Acute GVHD by Total ATG Dose.
ATG, anti-thymocyte globulin; GVHD, graft-versus-host disease; HCT, hematopoietic cell transplant;
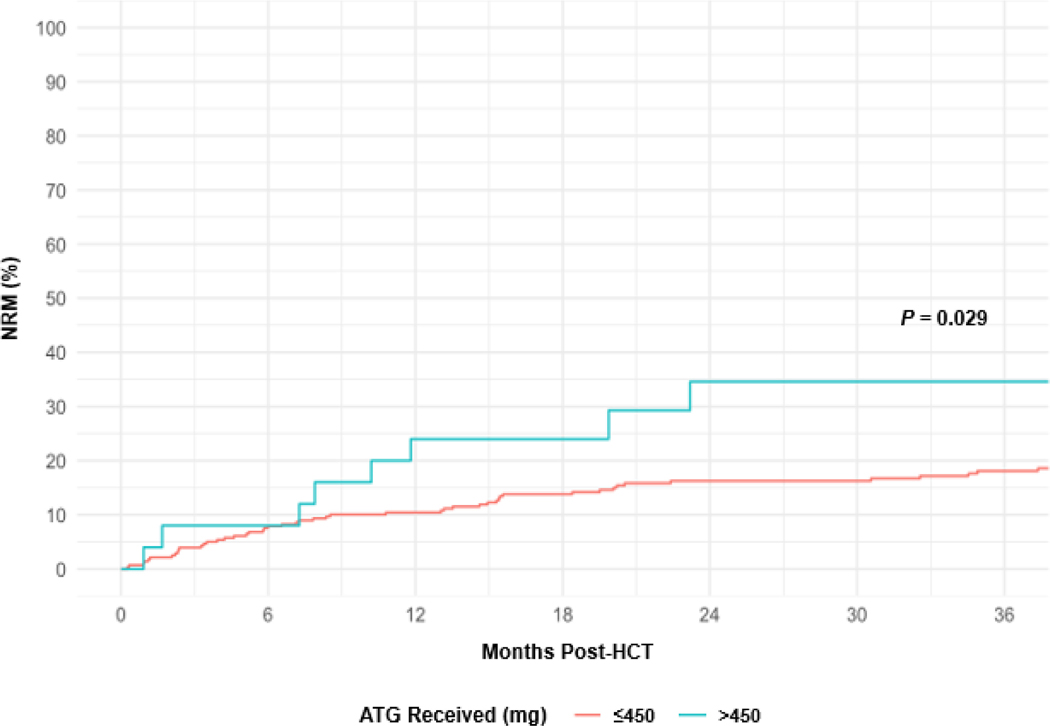
At 1 year and 3 years post-HCT, NRM rates for all patients were 12% (95% CI, 8% to 15%) and 19% (95% CI, 15% to 24%), respectively. For patients who received a total dose of ATG >450 mg or ≤450 mg, NRM rates at 3 years were, respectively, 35% (95% CI, 16% to 55%) and 18% (95% CI, 14% to 23%), P = .029 (Figure 2). Among all patient and HCT characteristics in a univariable analysis, total dose of ATG >450 mg (hazard ratio [HR] 2.5; 95% CI, 1.2 to 5.0; P = .03) and age (HR 1.4; 95% CI, 1.1 to 1.8, per 10 years; P = .006) were associated with an increased risk of NRM (Table 2). In a multivariable analysis, increasing age (HR 1.4; 95% CI, 1.1 to 1.8; P = .001) and total ATG dose >450 mg (HR 2.9; 95% CI, 1.4 to 6.0; P = .01) were associated with increased risk of NRM.
Figure 2.
Cumulative Incidence of NRM by Total ATG Dose.
ATG, anti-thymocyte globulin; HCT, hematopoietic cell transplant; NRM, non-relapse mortality.
Table 2.
Univariable Associations of Patient and HCT Characteristics with NRM
| Variable | Group | Observations | Events | HR (95% CI) | P value |
|---|---|---|---|---|---|
| Total ATG Dose (mg) | <450 | 279 | 52 | Reference | 0.03 |
| >450 | 25 | 9 | 2.5 (1.2–5.0) | ||
| ALC Day −3 | >0 | 104 | 15 | Reference | 0.13 |
| 0 | 200 | 46 | 1.5 (0.9–2.8) | ||
| Sex | Female | 140 | 30 | Reference | 0.81 |
| Male | 164 | 31 | 0.9 (0.6–1.6) | ||
| Disease | Acute leukemia | 189 | 38 | Reference | 0.99 |
| MDS | 77 | 16 | 1.0 (0.6–1.8) | ||
| Other | 38 | 7 | 0.9 (0.4–2.1) | ||
| Conditioning | Chemo-based | 197 | 46 | Reference | 0.07 |
| TBI-based | 107 | 15 | 0.6 (0.3–1.1) | ||
| CMV Serostatus | Negative | 112 | 19 | Reference | 0.15 |
| Positive/Equivocal | 192 | 42 | 1.5 (0.9–2.6) | ||
| HCT-CI | 0 | 62 | 8 | Reference | 0.18 |
| 1–2 | 110 | 25 | 1.9 (0.9–4.3) | ||
| >3 | 132 | 28 | 1.9 (0.9–4.1) | ||
| DRI | Low | 20 | 2 | Reference | 0.44 |
| Intermediate | 237 | 50 | 2.2 (0.5–9.0) | ||
| High | 37 | 6 | 2.5 (0.5–12.2) | ||
| HLA | MRD | 132 | 25 | Reference | 0.61 |
| MUD | 172 | 36 | 1.1 (0.7–1.9) | ||
| BMI | <25 | 111 | 16 | Reference | 0.16 |
| 25–30 | 117 | 28 | 1.8 (0.9–3.3) | ||
| >30 | 76 | 28 | 1.6 (0.8–3.1) | ||
| Age (10 y) | 304 | 61 | 1.4 (1.1–1.8) | 0.006 | |
ALC, absolute lymphocyte count; ATG, anti-thymocyte globulin; BMI, body mass index; CMV, cytomegalovirus; DRI, disease risk index; HLA, human leukocyte antigen; HCT-CI, hematopoietic cell transplantation comorbidity index; MDS, myelodysplastic syndromes; MRD, matched related donor; MUD, matched unrelated donor; TBI, total-body irradiation.
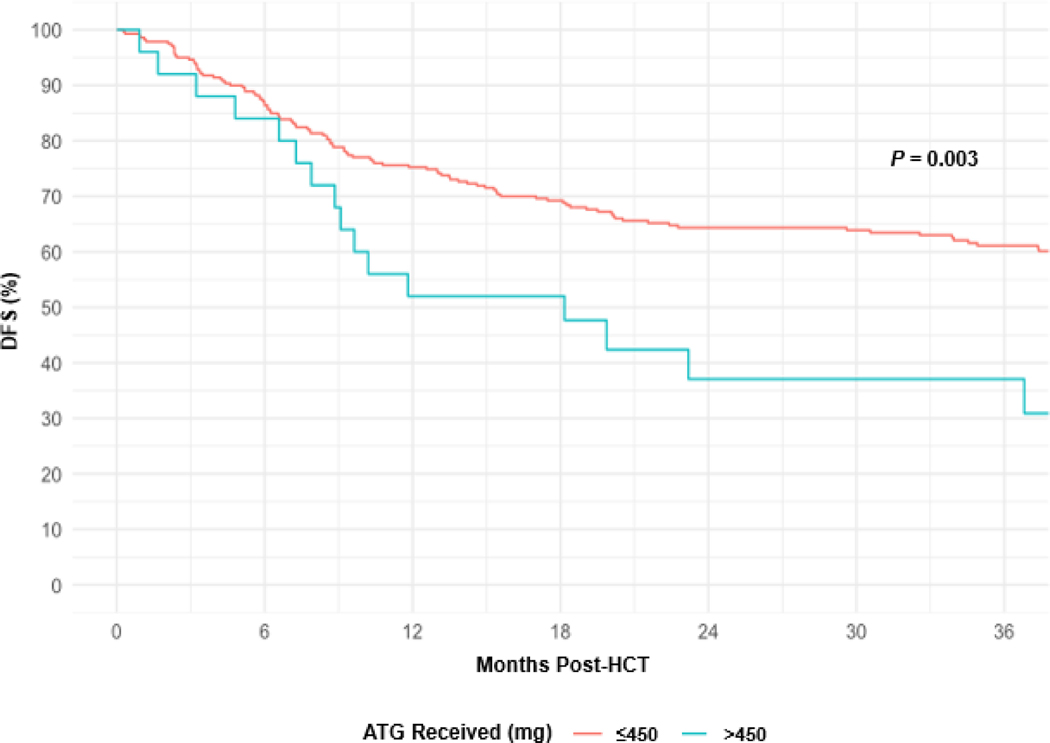
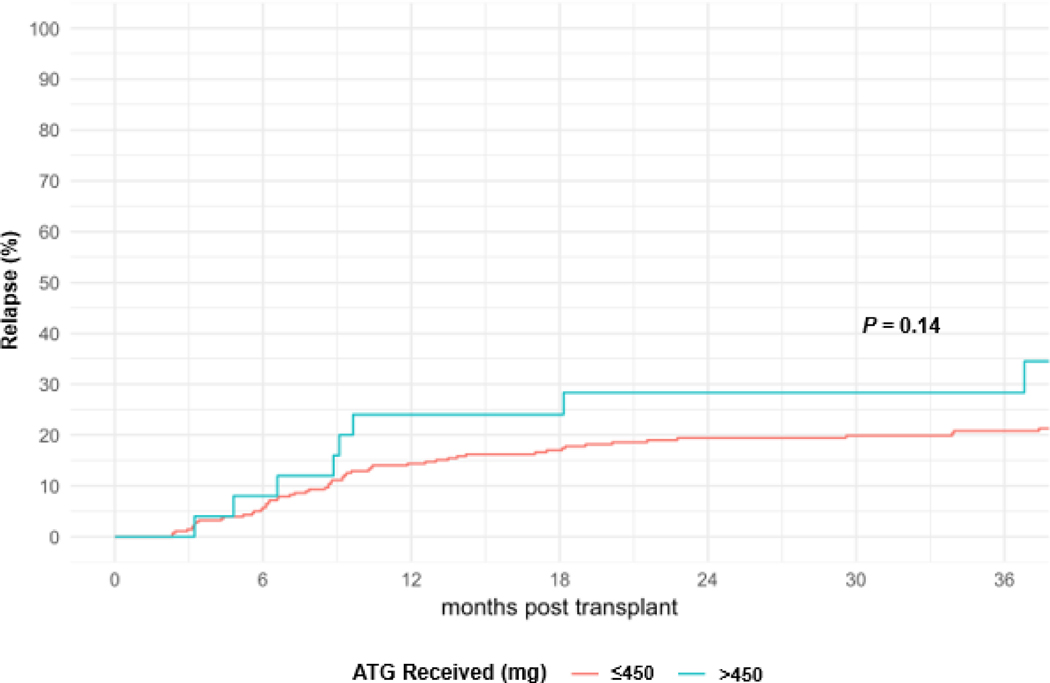
At 1 year and 3 years post-HCT, DFS rates were 73% (95% CI, 68% to 78%) and 59% (95% CI, 53% to 65%), respectively. For patients receiving a total dose of ATG >450 mg or ≤450 mg, DFS rates at 3 years were, respectively, 37% (95% CI, 18% to 56%) and 61% (95% CI, 55% to 67%), P = .003 (Figure 3). Among all patient and HCT characteristics in a univariable analysis, total ATG dose >450 (HR 2.1; 95% CI, 1.3 to 3.5; P = .009), DRI (intermediate HR 1.4; 95% CI, 0.6 to 3.1; and high HR 3.6; 95% CI, 1.5 to 8.6; P < .001), increasing age (HR 1.2; 95% CI, 1.0 to 1.4, per 10 years; P = .03), and patient CMV positive serostatus (HR 1.5; 95% CI, 1.0–2.2, P = .03) were associated with inferior DFS (Table 3). In a multivariable analysis, receiving a total dose of ATG >450 mg (HR 2.0; 95% CI, 1.1 to 3.5; P = .03), DRI (intermediate HR 1.2; 95% CI, 0.5 to 2.8; and high HR 3.2; 95% CI, 1.3 to 7.9; P < .001), increasing age (HR 1.2; 95% CI, 1.0 to 1.4; P = .02), patient CMV positive serostatus (HR 1.6, 95% Cl, 1.1–2.4, P = .0.1) were associated with shorter DFS. At 1 year and 3 years post-HCT, relapse rates were 15% (95% CI, 11% to 19%) and 21% (95% CI, 17% to 26%), respectively. In a univariable analysis, total dose of ATG >450 mg (HR 1.83; 95% CI, 0.9 to 3.8; P = .14) was not associated with an increased risk of relapse (Figure 4).
Figure 3.
DFS by Total ATG Dose.
ATG, anti-thymocyte globulin; HCT, hematopoietic cell transplant; DFS, disease-free survival.
Table 3.
Univariable Associations of Patient and HCT Characteristics with Inferior DFS
| Variable | Group | Observations | Events | HR (95% CI) | P value |
|---|---|---|---|---|---|
| Total ATG Dose (mg) | <450 | 279 | 112 | Reference | 0.009 |
| >450 | 25 | 17 | 2.1 (1.3–3.5) | ||
| ALC Day −3 | >0 | 104 | 43 | Reference | 0.95 |
| 0 | 200 | 86 | 1.0 (0.7–1.5) | ||
| Sex | Female | 140 | 56 | Reference | 0.36 |
| Male | 164 | 73 | 1.2 (0.8–1.7) | ||
| Disease | Acute leukemia | 189 | 81 | Reference | 0.86 |
| MDS | 77 | 31 | 0.9 (0.6–1.4) | ||
| Other | 38 | 17 | 1.0 (0.6–1.8) | ||
| Conditioning | Chemo-based | 197 | 84 | Reference | 0.91 |
| TBI-based | 107 | 45 | 1.0 (0.7–1.5) | ||
| CMV Serostatus | Negative | 112 | 40 | Reference | 0.03 |
| Positive/Equivocal | 192 | 89 | 1.5 (1.0–2.2) | ||
| HCT-CI | 0 | 62 | 21 | Reference | 0.12 |
| 1–2 | 110 | 44 | 1.3 (0.8–2.2) | ||
| >3 | 132 | 64 | 1.6 (1.0–2.7) | ||
| DRI | Low | 20 | 6 | Reference | <0.001 |
| Intermediate | 237 | 92 | 1.4 (0.6–3.1) | ||
| High | 37 | 26 | 3.6 (1.5–8.6) | ||
| HLA | MRD | 132 | 62 | Reference | 0.31 |
| MUD | 172 | 67 | 0.8 (0.6–1.2) | ||
| BMI | <25 | 111 | 43 | Reference | 0.54 |
| 25–30 | 117 | 54 | 1.3 (0.8–1.9) | ||
| >30 | 76 | 32 | 1.1 (0.7–1.7) | ||
| Age (10 y) | n/a | 304 | 129 | 1.2 (1.0–1.4) | 0.03 |
ALC, absolute lymphocyte count; ATG, anti-thymocyte globulin; BMI, body mass index; CMV, cytomegalovirus; DFS, disease-free survival; DRI, disease risk index; HCT-CI, hematopoietic cell transplantation comorbidity index; HLA, human leukocyte antigen; MDS, myelodysplastic syndromes; MRD, matched related donor; MUD, matched unrelated donor; TBI, total-body irradiation.
Figure 4.
Cumulative Incidence of Relapse by Total ATG Dose
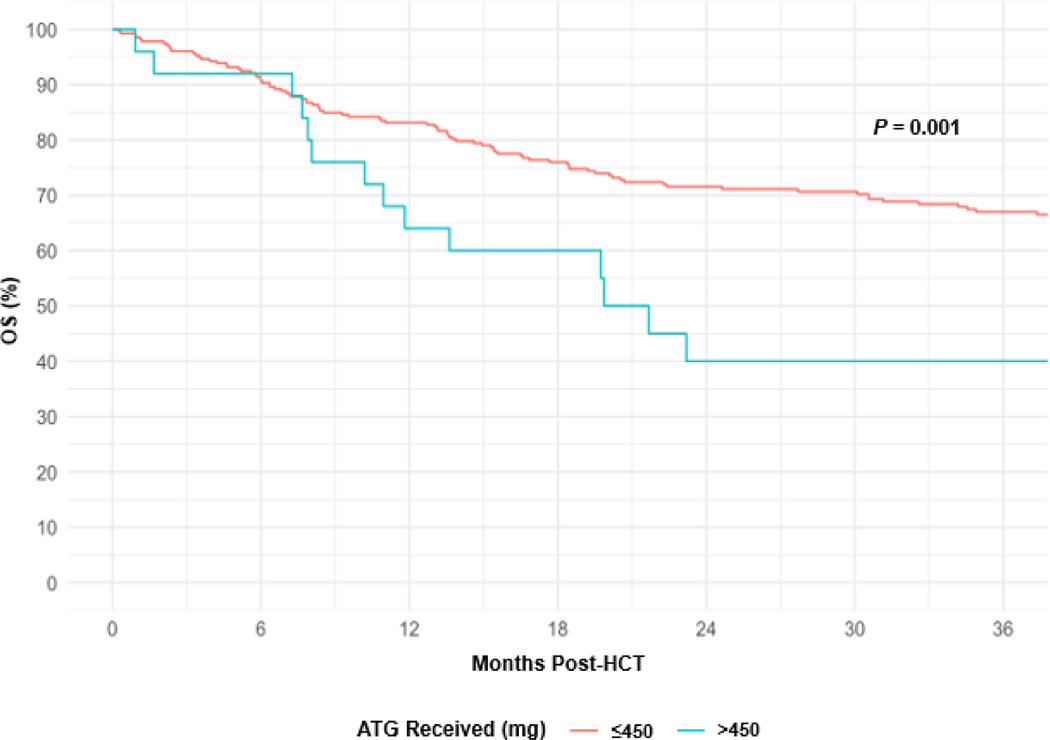
At 1 year and 3 years post-HCT, OS rates were 82% (95% CI, 77% to 86%) and 65% (95% CI, 59% to 70%), respectively. For patients receiving a total dose of ATG >450 mg or ≤450 mg, OS rates at 3 years, respectively, were 40% (95% CI, 20% to 59%) and 67% (95% CI, 61% to 72%), P = .001 (Figure 5). Among all patient and HCT characteristics in a univariable analysis, total ATG dose >450 mg (HR 2.4; 95% CI, 1.4 to 4.1; P = .004), HCT-CI (score 1–2 HR 1.8; 95% CI, 1.0 to 3.4; and score ≥3 HR 2.2; 95% CI, 1.2 to 4.0; P = .02), DRI (intermediate HR 1.9; 95% CI, 0.7 to 5.1; and high HR 4.9; 95% CI, 1.7 to 14.2; P < .001), and increasing age (HR 1.3; 95% CI, 1.1 to 1.5, per 10 years; P = .005) were associated with lower OS (Table 4). In a multivariable analysis stratified by HCT-CI and DRI to ensure proportional hazards, receiving a total dose of ATG >450 mg (HR 2.1; 95% CI, 1.2 to 3.8; P = .01) and increasing age (HR 1.2; 95% CI, 1.0 to 1.5; P = .02) were associated with an increased risk of all-cause death. Of the 279 patients who received a total ATG dose ≤450 mg, 93 (33%) died. Of the 25 patients who received a total ATG dose >450 mg, 16 (64%) died. Table 5 summarizes outcomes and causes of death stratified by total ATG dose.
Figure 5.
OS by Total ATG Dose.
ATG, anti-thymocyte globulin; HCT, hematopoietic cell transplant; OS, overall survival.
Table 4.
Univariable Associations of Patient and HCT Characteristics with OS
| Variable | Group | Observations | Events | HR (95% CI) | P value |
|---|---|---|---|---|---|
| Total ATG Dose (mg) | <450 | 279 | 93 | Reference | 0.004 |
| >450 | 25 | 16 | 2.4 (1.4–4.1) | ||
| ALC Day −3 | >0 | 104 | 37 | Reference | 0.90 |
| 0 | 200 | 72 | 1.2 (0.7–1.2) | ||
| Sex | Female | 140 | 46 | Reference | 0.29 |
| Male | 164 | 63 | 1.23 (0.84–1.8) | ||
| Disease | Acute leukemia | 189 | 70 | Reference | 0.67 |
| MDS | 77 | 27 | 0.92 (0.6–1.4) | ||
| Other | 38 | 12 | 0.77 (0.4–1.4) | ||
| Conditioning | Chemo-based | 197 | 74 | Reference | 0.44 |
| TBI-based | 107 | 35 | 0.85 (0.6–1.3) | ||
| CMV Serostatus | Negative | 112 | 35 | Reference | 0.11 |
| Positive/Equivocal | 192 | 74 | 1.4 (0.9–2.1) | ||
| HCT-CI | 0 | 62 | 14 | Reference | 0.02 |
| 1–2 | 110 | 39 | 1.8 (0.99–3.4) | ||
| >3 | 132 | 56 | 2.2 (1.2–4.0) | ||
| DRI | Low | 20 | 4 | Reference | <0.001 |
| Intermediate | 237 | 77 | 1.9 (0.7–5.1) | ||
| High | 37 | 24 | 4.9 (1.7–14.2) | ||
| HLA | MRD | 132 | 52 | Reference | 0.52 |
| MUD | 172 | 57 | 0.9 (0.6–1.3) | ||
| BMI | <25 | 111 | 31 | Reference | 0.12 |
| 25–30 | 117 | 47 | 1.5 (0.9–2.5) | ||
| >30 | 76 | 31 | 1.5 (0.9–1.5) | ||
| Age (10 y) | n/a | 304 | 109 | 1.3 (1.1–1.5) | 0.005 |
ALC, absolute lymphocyte count; ATG, anti-thymocyte globulin; BMI, body mass index; CMV, cytomegalovirus; DRI, disease risk index; HCT-CI, hematopoietic cell transplantation comorbidity index; HLA, human leukocyte antigen; MDS, myelodysplastic syndromes; MRD, matched related donor; MUD, matched unrelated donor; OS, overall survival; TBI, total-body irradiation.
Table 5.
Patient Outcomes Stratified by ATG Dose
| Variable | Total ATG Dose (mg) | P value | |
|---|---|---|---|
| ≤450 | >450 | ||
| Grade 2–4 GVHD, % | 0.26 | ||
| D100 | 16 | 24 | |
| NRM, % | 0.03 | ||
| 1-year | 10 | 24 | |
| 3-year | 18 | 35 | |
| DFS, % | 0.01 | ||
| 1-year | 75 | 53 | |
| 3-year | 61 | 35 | |
| OS, % | 0.004 | ||
| 1-year | 83 | 59 | |
| 3-year | 67 | 40 | |
| COD, n (%) | |||
| Relapse | 37 (40) | 7 (44) | |
| Infection | 26 (28) | 4 (25) | |
| GVHD | 14 (15) | 3 (19) | |
| Other | 16 (17) | 2 (12) | |
ATG, anti-thymocyte globulin; COD, cause of death; DFS, disease-free survival; GVHD, graft-versus-host disease; NRM, non-relapse mortality.
Discussion
Our data show that while the addition of ATG to myeloablative conditioning prior to ex vivo CD34-selected allo-HCT mitigates the risk of primary graft rejection, the use of weight-based ATG results in overdosing in heavier patients, ultimately leading to higher NRM, and lower DFS and OS.19,25 Patients receiving a total ATG dose >450 mg also appear to have a higher incidence of relapse though this did not meet statistical significance. The association of total ATG dose >450 mg with unfavorable outcomes remained significant even when accounting for important and validated patient and HCT characteristics such as HCT-CI and DRI.23,24 Given that ATG is given based on patients’ actual body weight, it is difficult to separate the effects of these two variables. However, body mass index was not associated with inferior outcomes in our analysis, suggesting that the effect appears to be related to total ATG dose and not weight.
Given that the median ALC on day –3 was 0.0 K/μL in patients receiving chemo- and TBI-based conditioning, similar overall outcomes were observed in both groups. This contrasts with a recent exploratory analysis from a randomized phase 3 study of anti-Tlymphocyte globulin (ATLG) versus placebo in patients receiving myeloablative conditioned unmodified MUD allo-HCT, wherein patients receiving TBI-based conditioning with ATLG were more likely to have inferior DFS and OS when compared to patients receiving chemo-based conditioning with ATLG.6 This result appeared to be a consequence of a lower ALC at the time of ATG administration in TBI-conditioned patients compared to chemo-conditioned patients, again highlighting the essential interaction between ALC and ATG.6,26 In our analysis, patients were equally lymphopenic at the time of ATG administration regardless of the conditioning regimen used. Marked lymphopenia effectively removes the metabolic sink required to clear ATG, leaving certain patients vulnerable to prolonged ATG exposure after allograft infusion.3,12 This ATG-ALC interaction may be particularly relevant in our population given the already prolonged recovery of T-cell immunity after ex vivo CD34-selected allo-HCT.25,27–29
It is important to recognize that the PK profile of ATG after ex vivo CD34-selection allo-HCT is unknown. Thus, while patients who received total ATG doses >450 mg had poorer overall outcomes, the precise dose, timing, and exposure of ATG peri-HCT required to mitigate primary graft rejection without negatively affecting other important outcomes requires further inquiry. Interestingly, higher doses of ATG did not significantly influence the incidence of acute GVHD. Given the relative rarity of GVHD after ex vivo CD34-selected allo-HCT compared to conventional allo-HCT, ATG may have limited effect on this outcome.16,17 We also speculate that the polyclonal nature of ATG may result in preferential reduction or preservation of specific immune effector cell subset populations that affect the risk of developing GVHD.3,30
We have previously shown durable engraftment, low rates of opportunistic infections and GVHD, and favorable survival in patients undergoing TCD allo-HCT using a non-ATG-containing conditioning regimen of TBI, thiotepa, and fludarabine.31 However, the use of high-dose TBI is often precluded in an increasingly elderly population because of its association with excessive regimen-related toxicity. Moreover, our results are highly relevant to an ongoing randomized, multicenter phase 3 trial of calcineurin inhibitor-free interventions for prevention of GVHD (PROGRESS II, NCT02345850), in which one experimental arm includes ex vivo CD34-selected allo-HCT using the ATG-containing conditioning regimens studied in our current analysis.4,5,32 The systematic measurement of ATG PK levels in the context of ex vivo CD34-selected allo-HCT is crucial to determine ATG clearance in the setting of marked lymphopenia, and will facilitate the development of personalized dosing that maximizes the anti-rejection and anti-GVHD properties of ATG, while minimizing its adverse effects on immune reconstitution. Ultimately, this strategy may further enhance an already effective transplantation platform.
Supplementary Material
Highlights:
ATG is given in a lympho-depleted state before ex vivo CD34+ selected allo-HCT.
Standard weight-based ATG dosing results in heavier patients receiving high total ATG doses.
High total ATG doses results in higher NRM, and inferior DFS and OS.
Acknowledgement:
Editorial support in the preparation of this paper was provided by Hannah Rice, ELS.
Funding: This research was supported in part by the National Institutes of Health (NIH) award number P01 CA23766 and NIH/National Cancer Institute Cancer Center support grant P30 CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author disclosures: There are no conflicts of interest related to the subject matter of this article. Unless otherwise listed, authors have no disclosures to report. M.Sc. is an ad hoc consultant for McKinsey & Company and a consultant for Angiocrine Bioscience, Inc. V.B. is a consultant for Spectrum Pharmaceuticals, Inc. and Incyte Corporation. G.L.S. receives research funding from Janssen Pharmaceuticals, Inc. and Amgen, Inc. S.T.A received an honorarium in 2015 for Abbott Diagnostics. R.J.O. received royalties following licensure of the EBV-specific T-cell bank by Atara Biotherapeutics and has received research support and consultant fees from Atara Biotherapeutics. J.J.B. is a consultant for Avrobio, Inc., and a consulting member of the DSMB for Magenta Therapeutics, Chimerix, Inc., and Bluebird Bio, Inc. M.P. receives research funding from Incyte Corporation for a clinical trial and is a consultant for Merck. M.P. is on the ad hoc advisory boards of Incyte, Novartis, Nektar Therapeutics, Abbvie, and is on the scientific advisory board for MolMed and NexImmune. M.P. is a DSMB member for Servier and Medigene. S.A.G. receives research funding from Miltenyi Biotec, Takeda Pharmaceutical Co., Celgene Corp., Amgen Inc., Sanofi, Johnson and Johnson, Inc., Actinium Pharmaceuticals, Inc., and is on the Advisory Boards for: Kite Pharmaceuticals, Inc., Celgene Corp., Sanofi, Novartis, Johnson and Johnson, Inc., Amgen Inc., Takeda Pharmaceutical Co., Jazz Pharmaceuticals, Inc., Actinium Pharmaceuticals, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Bacigalupo A, Lamparelli T, Barisione G, et al. Thymoglobulin Prevents Chronic Graft-versus-Host Disease, Chronic Lung Dysfunction, and Late Transplant-Related Mortality: Long-Term Follow-Up of a Randomized Trial in Patients Undergoing Unrelated Donor Transplantation. Biol Blood Marrow Transplant. 2006;12(5). doi: 10.1016/j.bbmt.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 2.Finke J, Schmoor C, Bethge WA, et al. Long-term outcomes after standard graft-versus-host disease prophylaxis with or without anti-human-T-lymphocyte immunoglobulin in haemopoietic cell transplantation from matched unrelated donors: final results of a randomised controlled trial. Lancet Haematol. 2017;4(6):e293–e301. doi: 10.1016/S2352-3026(17)30081-9. [DOI] [PubMed] [Google Scholar]
- 3.Storek J, Mohty M, Boelens JJ. Rabbit Anti-T Cell Globulin in Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2015;21(6):959–970. doi: 10.1016/j.bbmt.2014.11.676. [DOI] [PubMed] [Google Scholar]
- 4.Kröger N, Solano C, Wolschke C, et al. Antilymphocyte Globulin for Prevention of Chronic Graft-versus-Host Disease. N Engl J Med. 2016;374(1):43–53. doi: 10.1056/NEJMoa1506002. [DOI] [PubMed] [Google Scholar]
- 5.Walker I, Panzarella T, Couban S, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: A randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. 2016;17(2):164–713. doi: 10.1016/S1470-2045(15)00462-3. [DOI] [PubMed] [Google Scholar]
- 6.Soiffer RJ, Kim HT, McGuirk J, et al. Prospective, randomized, double-blind, phase III clinical trial of anti-T-lymphocyte globulin to assess impact on chronic graft-versus-host disease-free survival in patients undergoing HLA-matched unrelated myeloablative hematopoietic cell transplantation. J Clin Oncol. 2017;35(36):4003–4011. doi: 10.1200/JCO.2017.75.8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Socie G, Schmoor C, Bethge WA, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117(23):6375–6382. doi: 10.1182/blood-2011-01-329821. [DOI] [PubMed] [Google Scholar]
- 9.Soiffer RJ, LeRademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117(25):6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A, Reljic T, Hamadani M, Mohty M, Kharfan-Dabaja MA. Antithymocyte globulin for graft-versus-host disease prophylaxis: an updated systematic review and meta-analysis. Bone Marrow Transplantation. 2018. [DOI] [PubMed] [Google Scholar]
- 11.Admiraal R, van Kesteren C, Jol-van der Zijde CM, et al. Population Pharmacokinetic Modeling of Thymoglobulin® in Children Receiving Allogeneic-Hematopoietic Cell Transplantation: Towards Improved Survival Through Individualized Dosing. Clin Pharmacokinet. 2015;54(4):435–446. doi: 10.1007/s40262-014-0214-6. [DOI] [PubMed] [Google Scholar]
- 12.Admiraal R, Lindemans CA, Van Kesteren C, et al. Excellent T-cell reconstitution and survival depend on low ATG exposure after pediatric cord blood transplantation. Blood. 2016;128(23):2734–2741. doi: 10.1182/blood-2016-06-721936. [DOI] [PubMed] [Google Scholar]
- 13.de Koning C, Admiraal R, Nierkens S, Boelens JJ. Immune reconstitution and outcomes after conditioning with anti-thymocyte-globulin in unrelated cord blood transplantation; the good, the bad, and the ugly. Stem Cell Investig. 2017;4:38–38. doi: 10.21037/sci.2017.05.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Admiraal R, Nierkens S, de Witte MA, et al. Association between anti-thymocyte globulin exposure and survival outcomes in adult unrelated haemopoietic cell transplantation: a multicentre, retrospective, pharmacodynamic cohort analysis. Lancet Haematol. 2017;4(4):e183–e191. doi: 10.1016/S2352-3026(17)30029-7. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy VE, Chen H, Savani BN, et al. Optimizing Antithymocyte Globulin Dosing for Unrelated Donor Allogeneic Hematopoietic Cell Transplantation Based on Recipient Absolute Lymphocyte Count. Biol Blood Marrow Transplant. 2018;24(1):150–155. doi: 10.1016/j.bbmt.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 16.Pasquini MC, Devine S, Mendizabal A, et al. Comparative outcomes of donor graft CD34+ selection. J Clin Oncol. 2012;30(26):3194–3201. doi: 10.1200/JCO.2012.41.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayraktar UD, de Lima M, Saliba RM, et al. Ex vivo T cell-depleted versus unmodified allografts in patients with acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2013;19(6):898–903. doi: 10.1016/j.bbmt.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barba P, Hilden P, Devlin SM, et al. Ex Vivo CD34(+)-Selected T Cell-Depleted Peripheral Blood Stem Cell Grafts for Allogeneic Hematopoietic Stem Cell Transplantation in Acute Leukemia and Myelodysplastic Syndrome Is Associated with Low Incidence of Acute and Chronic Graft-versus-Host Disease and High Treatment Response. Biol Blood Marrow Transplant. 2017;23(3):452–458. doi: 10.1016/j.bbmt.2016.12.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spitzer B, Jakubowski AA, Papadopoulos EB, et al. A Chemotherapy-Only Regimen of Busulfan, Melphalan, and Fludarabine, and Rabbit Antithymocyte Globulin Followed by Allogeneic T-Cell Depleted Hematopoietic Stem Cell Transplantations for the Treatment of Myeloid Malignancies. Biol Blood Marrow Transplant. 2017;23(12):2088–2095. doi: 10.1016/j.bbmt.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kernan NA, Bordignon C, Heller G, et al. Graft failure after T-cell-depleted human leukocyte antigen identical marrow transplants for leukemia: I. Analysis of risk factors and results of secondary transplants. Blood. 1989;74(6):2227–2236. http://www.ncbi.nlm.nih.gov/pubmed/2804361. [PubMed] [Google Scholar]
- 21.Bordignon C, Keever CA, Small TN, et al. Graft failure after T-cell-depleted human leukocyte antigen identical marrow transplants for leukemia: II. In vitro analyses of host effector mechanisms. Blood. 1989. [PubMed] [Google Scholar]
- 22.Devine SM, Carter S, Soiffer RJ, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: Results of the blood and marrow transplant clinical trials network prot. Biol Blood Marrow Transplant. 2011. doi: 10.1016/j.bbmt.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–3671. doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorror ML, Logan BR, Zhu X, et al. Prospective Validation of the Predictive Power of the Hematopoietic Cell Transplantation Comorbidity Index: A Center for International Blood and Marrow Transplant Research Study. Biol Blood Marrow Transplant. 2015;21(8):1479–1487. doi: 10.1016/j.bbmt.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Small TN, Avigan D, Dupont B, et al. Immune reconstitution following T-cell depleted bone marrow transplantation: effect of age and posttransplant graft rejection prophylaxis. BiolBlood Marrow Transplant. 1997;3(1083–8791 (Print)): 65–75. [PubMed] [Google Scholar]
- 26.Admiraal R, Lindemans CA, van Kesteren C, et al. Excellent T-cell reconstitution and survival provided ATG exposure is low or absent after pediatric cord blood transplantation. Blood. 2016;128(23):2734–2742. doi: 10.1182/blood-2016-06-721936. [DOI] [PubMed] [Google Scholar]
- 27.Small TN, Papadopoulos EB, Boulad F, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93(2):467–480. [PubMed] [Google Scholar]
- 28.Wu CJ, Chillemi A, Alyea EP, et al. Reconstitution of T-cell receptor repertoire diversity following T-cell depleted allogeneic bone marrow transplantation is related to hematopoietic chimerism. Blood. 2000;95(1):352–359. [PubMed] [Google Scholar]
- 29.Keever-Taylor CA, Wagner JE, Kernan NA, et al. Comparison of immune recovery in recipients of unmanipulated vs T-cell-depleted grafts from unrelated donors in a multicenter randomized phase II-III trial (T-cell depletion trial). Bone Marrow Transplant. 2010. doi: 10.1038/bmt.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheinberg P, Nunez O, Weinstein B, et al. Horse versus Rabbit Antithymocyte Globulin in Acquired Aplastic Anemia. N Engl J Med. 2011. doi: 10.1056/NEJMoa1103975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jakubowski AA, Small TN, Young JW, et al. T cell-depleted stem-cell transplantation for adults with hematologic malignancies: Sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110(13):4552–4559. doi: 10.1182/blood-2007-06-093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soiffer RJ, Kim HT, McGuirk J, et al. Prospective, randomized, double-blind, phase III clinical trial of anti-T-lymphocyte globulin to assess impact on chronic graft-versus-host disease-free survival in patients undergoing HLA-matched unrelated myeloablative hematopoietic cell transplantation. J Clin Oncol. 2017. doi: 10.1200/JCO.2017.75.8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.