Abstract
Aromatic residues are located at structurally important sites of many proteins. Probing their interactions and dynamics can provide important functional insight but is challenging in large proteins. Here, we introduce approaches to characterize dynamics of phenylalanine residues using 1H-detected fast magic-angle spinning (MAS) NMR combined with a tailored isotope-labeling scheme. Our approach yields isolated two-spin systems that are ideally suited for artefact-free dynamics measurements, and allows probing motions effectively without molecular-weight limitations. The application to the TET2 enzyme assembly of ~0.5 MDa size, the currently largest protein assigned by MAS NMR, provides insights into motions occurring on a wide range of time scales (ps-ms). We quantitatively probe ring flip motions, and show the temperature dependence by MAS NMR measurements down to 100 K. Interestingly, favorable line widths are observed down to 100 K, with potential implications for DNP NMR. Furthermore, we report the first 13C R1ρ MAS NMR relaxation-dispersion measurements and detect structural excursions occurring on a microsecond time scale in the entry pore to the catalytic chamber and at a trimer interface that was proposed as exit pore. We show that the labeling scheme with deuteration at ca. 50 kHz MAS provides superior resolution compared to 100 kHz MAS experiments with protonated, uniformly 13C-labeled samples.
Introduction
Many cellular processes such as protein synthesis, chaperoning or enzymatic reactions rely on protein complexes of hundreds of kilodalton in size. Understanding how these machineries function at the atomic scale requires the determination of high-resolution structures, generally by X-ray crystallography or cryo-electron microscopy, as well as the characterization of intramolecular motions. NMR spectroscopy plays a central role in deciphering dynamics at atomic resolution, and thus often providing the crucial link between structure and function.1 While solution-state NMR has been very powerful for many such structure-dynamics-function studies, including the structural characterization of short-lived “excited states”,1,2 it has inherent physical limitations when dealing with large molecules in excess of ca. 50-70 kDa. Their slow overall tumbling in solution results in rapid signal decay, and the low sensitivity and resolution renders atom-specific observation difficult. The use of selective CH3-labeling,3–5 deuteration and methyl-TROSY NMR6 has lifted these protein-size limitations partly, allowing the study of dynamics and interactions of methyl-bearing residues even in proteins as large as 1 MDa.3,7,8 Moieties other than methyls have been thought to be generally undetectable in large proteins by solution-NMR, until recent breakthrough isotope labeling methods allowed observing any kinds of aromatic and aliphatic moieties for a 82 kDa protein malate synthase G (MSG) in solution.9
Aromatic residues have been the subject of much interest since the early days of protein NMR.10–12 They are overrepresented at protein interfaces and play a prominent role in guiding enzyme mechanisms.13 Understanding their mobility can therefore provide key functional information. Characterizing the mobility of buried aromatics, and in particular ring-flip dynamics can reveal ‘breathing’ motions of proteins and thus point towards (local) unfolding events.14 Furthermore, aromatics are important structural probes, as their involvement in hydrophobic core regions implies that they have numerous short-distance contacts that are quite useful for determining protein structures. Therefore, the global fold of the 41-kDa maltodextrin-binding protein (MBP) determined by solution NMR, using the NOEs between the amide and methyl protons, together with the residual dipolar couplings for the polypeptide backbone and hydrogen-bond restraints, resulted in RMSDs larger than 3.8 Å for the side-chain heavy atoms of the N- and C-terminal domains.15,16 On the other hand, the corresponding side-chain RMSDs, obtained exclusively on the basis of the NOEs including aromatic and aliphatic protons other than the methyl moieties, were 2.3 Å for both domains.17,18 These considerations have been at the origin of long-standing interest in aromatics over several decades.11,19–23
The usefulness of solution-NMR studies of aromatics is limited by several factors; first, for large proteins the spectra of aromatics are of low quality. The use of advanced labeling, such as deuteration and introduction of 1H-13C labels with the SAIL scheme17,18,24 or 19F labeling25,26 may extend the limits to several tens of kDa, but slow tumbling necessarily always leads to loss of spectral quality. Second, the overall molecular tumbling represents an inherent obstacle for detecting internal motion. Specifically, internal motions can only be detected when they are either faster than the overall-tumbling correlation time (i.e. ca. 5-30 ns) through spin-relaxation experiments, or slower than ca. 10 μs, where line-broadening effects can be quantified by relaxation dispersion experiments. Consequently, the time scale of ring flips is notoriously difficult to quantify by solution-state NMR studies.20 An additional difficulty for dynamics measurements, and for the ability to even detect and resolve aromatic signals, is the presence of strong scalar couplings between adjacent carbons in uniformly 13C-labeled samples.
Under magic-angle spinning (MAS) NMR conditions, where overall molecular tumbling is absent, atomic-resolution information can be obtained for all backbone and side chain sites, irrespective of the molecular weight, and without an inherent “blind spot” on the time scale of motions, thus resolving both limitations of solution-state NMR. The structure and dynamics of aromatics in peptides and small proteins have been in the focus of many MAS NMR studies over the last decades.21,27–32 The possibility to study samples at very low temperature in MAS NMR furthermore opens avenues for studying fundamental properties of proteins, such as temperature-induced activation of motions.33–35 An inherent challenge for solid-state NMR is related to the presence of dipolar couplings which – although they can be exploited to study dynamics and structure – lead to line broadening, in particular for nuclei with large gyromagnetic ratio (in particular 1H). This problem can be alleviated by faster magic-angle spinning (the term fast evolved with time as the hardware evolved and currently lies around 100 kHz at most), specific isotope labeling, including deuteration, or combinations thereof, as shown herein.
MAS NMR experiments that probe molecular dynamics are commonly based either on spin-relaxation measurements, or on probing the dynamically-averaged anisotropic interactions, most often the dipolar coupling strengths.36 In fully labeled, protonated proteins, the experimental accuracy of both types of approaches is, however, compromised: (i) in relaxation measurements, the presence of multiple spin-spin couplings may lead to an additional apparent spin decay which is due to either proton-driven spin diffusion (for the case of longitudinal relaxation)37–40 or so-called dipolar dephasing41–43, or scalar-coupling-induced signal modulations.44,45 (ii) Even though robust methods for measuring dipolar couplings in fully labeled samples are being actively developed,46–49 the measurement of dipolar coupling tensors with precision remains challenging in the presence of multiple spin-spin couplings and experimental imperfections.50,51 In addition to challenging the precision of dynamics measurements, uniform labeling also compromises spectral resolution, because of the large number of aromatic resonances and the presence of scalar couplings that are difficult to remove. Over the last years, several groups have shown the advantages of combining deuteration and sparse protonation which result to good approximation in two-spin systems, high MAS frequencies (40-100 kHz) and sensitive proton-detected MAS NMR experiments to gain insight into structure and dynamics of the protein backbone43,52–54 and side-chain methyl sites.55,56 Aromatic labeling schemes, with or without deuteration, have been used for solution-NMR or MAS NMR at modest MAS frequencies.24,30,57–61
Herein we demonstrate that specific aromatic labelling24,58 and deuteration with 1H-detected NMR experiments at high MAS frequencies (>40 kHz) allows probing dynamics occurring over many orders of magnitude in time, as exemplified here with the 468 kDa large dodecameric TET2 aminopeptidase TET2 from Pyrococcus horikoshii.62 TET2 forms hollow tetrahedral-shaped particles with a large central cavity (Figure 1A),63 in which peptides of up to ca. 40 amino acids in length64 are degraded to amino acids. Access to this large catalytic chamber is enabled through four ~18 Å-large entry pores on the faces of the tetrahedral structure. How amino acids produced by the catalytic reaction are removed from the cavity is subject to debate.
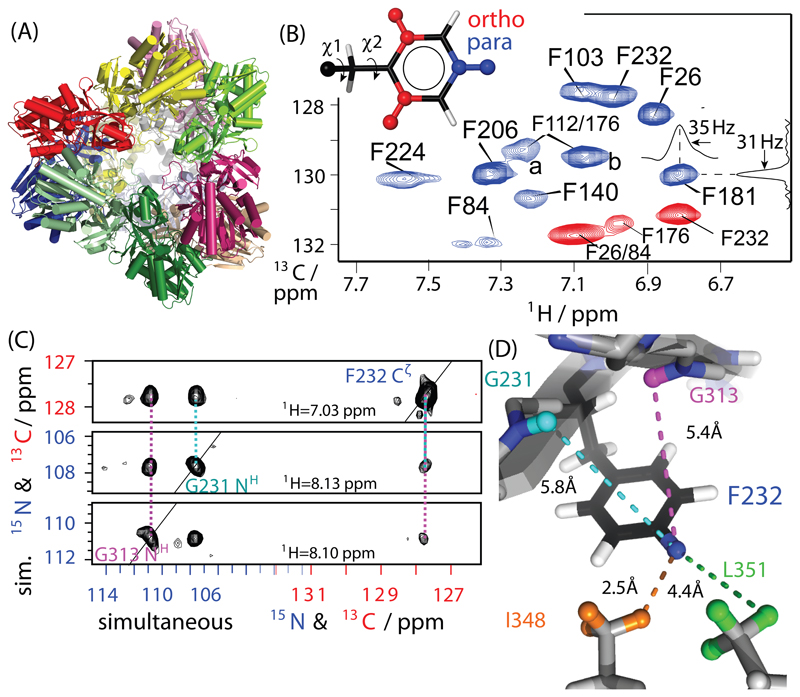
Figure 1.
Proton-detected correlation spectra of the 468 kDa aminopeptidase TET2. (A) Cartoon representation of TET2; different subunits (39 kDa each) are shown in different colors. (B) 1H-13C dipolar-coupling based 2D correlation spectra of para-1H-13C (blue) and ortho-1H-13C (red) Phe-labeled TET2 samples, obtained at 55 kHz MAS and a static magnetic field of 14.1 T. The assignment was obtained from mutagenesis and RFDR experiments. Typical line widths are of the order of ~35 Hz in 1H and ~30 Hz in 13C. (C) Example strips from through-space 1H-1H RFDR TET2 with residue-specific assignments, obtained from a three-dimensional H-(C/N)-(C/N) experiment with simultaneous 13C/15N frequency editing in indirect dimensions, applied to a sample labeled on para-CH sites and Ile, Val, Leu 13CHD2 methyl groups. All pulse sequences used in this study are shown in Figure S2. All detected distance restraints involving para-CH sites and ortho-CH sites are shown in Figures S2 and S3, respectively.
We probe several aspects of phenylalanine dynamics in TET2 with site-specific labeling. (i) Using dipolar-coupling measurements at room temperature, we investigate the ring flip motions, as well as the motion of the Phe ring axis. (ii) Relaxation measurements provide the time scale of these dynamics. A heterogeneity of ring-flip rates is observed, with the fastest flips occurring in the nanosecond range. (iii) By performing 40 kHz MAS NMR experiments at temperatures from 100 K to room temperature, we investigate how the ring flips become activated, and we furthermore report high-quality spectra at low temperature, an interesting finding for dynamic-nuclear polarization (DNP) studies. (iv) We furthermore introduce a 13C R1ρ relaxation-dispersion experiment and show its ability to probe microsecond (μs) mobility; we detect in particular residues at the entry pore and the trimerization interface, which undergo μs motions, and investigate their potential functional relevance by activity measurements with mutants. We furthermore show that the labeling scheme provides superior resolution compared to fully labeled (protonated) samples, even if highest-possible (100 kHz) MAS frequencies are used, and discuss the potential for obtaining structural restraints.
Methods
Sample preparation
For selective Phe labelling of TET2 in an otherwise deuterated environment, TET2 was produced by bacterial overexpression in E. coli BL21(DE3) in D2O-based M9 minimal medium. At an OD600 of ca. 0.6, one hour prior to induction of over-expression with 1 mM IPTG, specifically labelled L-phenylalanine (SAIL Technologies Inc.) with 1H-13C spin pairs either at the para-CH or the two ortho-CH sites and otherwise fully 2H,15N labeled, was added at a concentration of 20 mg/L. Prior to addition, the phenylalanine powder was dissolved in a small amount of water with potassium hydroxide. The single-point mutants for assignment were labeled by adding the para-CH labelled ketoacid precursor58 instead of the amino acid (30 mg/L). The incorporation of these (cheaper) compounds is similar to the one of the amino acids. Note that a more conservative mutation (Tyr rather than Ala) may be used for mutagenesis-based Phe assignment; we find that Ala substitution does not lead to structural perturbations as controlled through 2D H-N spectra. Deuterated glucose (2 g/L, not 13C-labeled) and 15N-labelled ammonium chloride (1 g/L) were added as carbon and nitrogen sources. The protein purification was described previously 65. Briefly, it involved heat shock at 85 °C and gel filtration. The purified protein was dissolved in buffer A (20 mM Tris, 50 mM NaCl, pH 7.5) and concentrated to 20 mg/mL using a centrifugal concentration device. To prepare a sample suitable for solid-state NMR, 50% (v/v) of 2-methyl-2,4-pentanediol (MPD) was added, resulting in a white precipitate (possibly nanocrystalline). The samples were filled into 1.3 mm Bruker rotors using an ultracentrifugation device in a SW-32 swinging-bucket rotor at 60000 g for 2 hours.
NMR spectroscopy
All NMR spectra were recorded on Bruker Avance-III spectrometers operating at 14.1 T (600 MHz 1H Larmor frequency; 1.3 mm probe, 55 kHz MAS), with the exception of the low-temperature spectra, collected at 800 MHz 1H Larmor frequency (40 kHz MAS), and the spectra shown in Figure 5, collected at 950 MHz (55 kHz in a probe for 1.3 mm rotors or 103 kHz in a probe for 0.7 mm rotors). Except for the low-temperature experiments, the sample temperature, regulated with a cooling gas flow, was estimated from the 1H bulk water line position relative to the CH resonance of MPD at 4.1 ppm which is almost independent of temperature over the range considered here (see Figure S1).
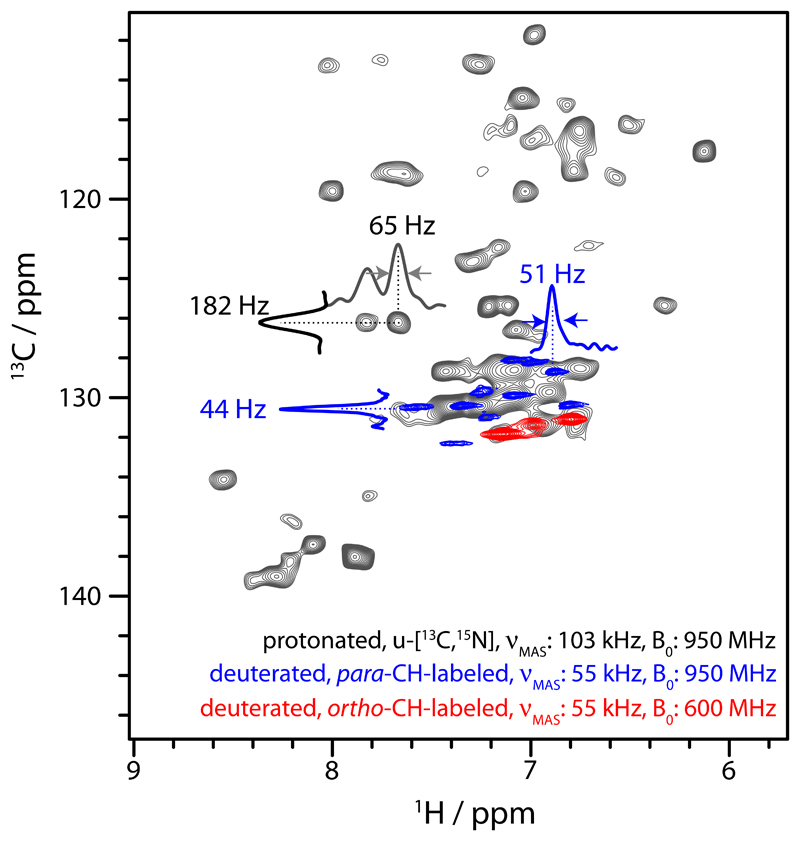
Figure 5.
Comparison of 1H-13C correlation spectra obtained from deuterated, specifically Phe-labelled TET2 at 55 kHz MAS and of protonated, 13C,15N-labelled TET2 at 103 kHz MAS frequency. The simplification of the spectra of the specifically labelled samples are due to narrower line widths, resulting from the removal of 13C-13C scalar couplings and the better suppression of dipolar line broadening due to surrounding proton spins, and the absence of correlations other than those of ortho-CH or para-CH sites. Note that the spectrum at 103 kHz MAS was at an effective sample temperature of ca. 35°C, i.e., ca. 7°C higher than the ones collected at 55 kHz, which likely explains differences in peak positions.
The low-temperature experiments were performed on a widebore 800 MHz Bruker Avance-III spectrometer at CRMN Lyon. Sample cooling was achieved with pre-cooled bearing (2.8 bar), drive and variable-temperature (1200 L/h) gas flows. The reported temperatures are the gas temperatures; we estimated that the 40 kHz MAS rotation leads to an effective sample heating of ca. 10 K.
The spectrum on fully protonated TET2 was obtained at 950 MHz with a 0.7 mm HCN probe (Bruker Biospin). 103 kHz MAS was achieved with 2.66 bar bearing, 3.06 bar drive (ambient temperature) and the sample cooling gas (200 L/h) was at 273 K, leading to an effective sample temperature of ca. 35°C. All experiments employed in this study are based on direct 1H detection with 5 kHz 13C WALTZ decoupling during signal acquisition. All pulse sequences are displayed in Figure S2. All spectra were processed with nmrPipe66 and visualized with CCPNMR67. Quantitative peak integrals in series of 2D spectra (relaxation and REDOR experiments) were extracted with nmr-View (OneMoon Scientific). Integrals from 1D spectra were extracted with in-house written python programs, using the nmr-glue package68 for handling of the processed NMR data. All analyses of relaxation data (mono-exponential fits) and REDOR fitting were performed with in-house written python scripts. The REDOR analysis was similar to previously employed strategies52,56, based on GAMMA69 simulations. All error estimates were obtained from standard Monte Carlo simulations. Briefly, 100-500 “noisy” data sets were generated in which random noise according to 3 times the spectral noise level was added to the back-predicted data points from exponential relaxation fits or from REDOR simulations. These data sets were subsequently fitted, and the reported error margins reflect the standard deviation over these data sets.
Molecular dynamics simulations
All simulations were performed using GROMACS70 MD code using the Amber ff99SBws force field with balanced protein-water interactions 71. Equations of motion were integrated with a time step of 2 fs. All covalent bonds were constrained to their equilibrium values using the LINCS algorithm 72. The electrostatic interactions were calculated by the Particle Mesh Ewald algorithm, and a cutoff of 1.0 nm was used both for Lennard–Jones interaction and for the real-space coulomb contribution. The starting structure was obtained integrating the high-resolution X-ray structure of TET2 (PDB code: 1y0r) with lower resolution cryo-EM data for modelling the flexible loop (120-132), as reported in the PDB entry 6F3K73. The dodecamer was solvated with 77847 TIP4P/200574 water molecules and electro-neutralized with 262 Na+ and 178 Cl- ions in a rhombic dodecahedral box with periodic boundary conditions. After being solvated, the system was energy minimized and then equilibrated while restraining all the protein heavy atoms first in the NVT (T=300 K) ensemble for 1 ns and later in the NPT (P=1 bar, T=300 K) ensemble for 10 ns. Structural restraints on protein atoms were then released for the 1-μs production run in the NPT ensemble (P=1 bar, T=300 K). Constant temperature simulations were performed using by means of a stochastic thermostat 75 and Parrinello-Rahman scheme 76 was used for NPT runs. During all the simulations, the correct conformation of the catalytic site was preserved by artificially restraining the distances between the zinc ions and the coordinating oxygen/nitrogen atoms to their experimental values.
Results
High resolution MAS NMR of ortho- and para-CH Phe sites
TET2, formed by twelve copies of a 39 kDa subunit, is one of the largest systems studied quantitatively by NMR methodologies and to the best of our knowledge the largest system for which near-complete MAS NMR assignments have been achieved73. The large number of aromatic sites represents a challenge for resolving individual sites. For high-resolution aromatic MAS NMR experiments, we labeled Phe residues specifically with an isolated 1H-13C spin pair in an otherwise deuterated environment. The strong dilution of the 1H-1H dipolar-coupling network in such a sample as well as the removal of 13C-13C dipolar and scalar couplings leads to greatly enhanced sensitivity and resolution and facilitates artefact-free analyses of dynamics. Phe labeling at high levels is achieved by addition of suitably-labeled Phe, or its biosynthetic precursor, phenylpyruvate, to a 2H,12C,15N labeled expression medium24,58 (see Methods section for details). We explored two different labeling schemes, where 1H-13C pairs are placed either at the Cζ site (henceforth called para-CH), or the two Cδ sites (ortho-CH; see insert of Figure 1B). The two different moieties are sensitive to different types of Phe motion: the para-CH moiety is insensitive to ring rotations but senses motions of the Phe ring axis, arising as a consequence of χ1 dihedral-angle fluctuations and backbone dynamics; the ortho-CH spin pairs are additionally re-oriented by ring flips (i.e., rotation around the χ2 dihedral, see insert in Figure 1B).10–14
The spectrum of para-CH shows ten well-resolved resonances, as expected for the ten Phe sites in TET2. The ortho-CH labeled sample only features four signals, while the remaining 6 sites are unobservable at temperatures between ca. 0 and 55°C, an observation that we ascribe to ring flips, as investigated further below.
The excellent resolution of these spectra opens the way to site-resolved studies of dynamics. Resonance assignment is necessary for site-specific studies, but far from trivial for a protein of 39 kDa. We have recently achieved near-complete assignment of backbone 1HN, 13C, 15N, as well as Ile-δ1, Leu-δ1, Val-γ1 (ILV) methyl sites.73,77,78 However, we found that coherence transfer into the aromatic rings was inefficient, such that none of the Phe 13C ortho or para carbons had been assigned. We employ here an assignment strategy that combines (i) experiments that probe the spatial proximity between the Phe hydrogens, ILV methyl groups and amide-1H spins, using the known structure63 for assignment of cross-peaks and (ii) a mutagenesis-based assignment approach. For the former approach, we recorded a 3D 1H-1H radiofrequency-driven recoupling79 (RFDR) experiment with two simultaneously edited 13C/15N frequencies and 1H detection, using a deuterated TET2 sample with 1H spins at exchangeable sites (amides), 13CHD2-methyls on ILV residues and para-CH sites. The experiment simultaneously reveals distances between amide, methyl and aromatic sites; for the ortho-CH sample we collected 2D (13C-filtered) and 3D (15N-filtered) versions of the experiment. Examples of through-space contacts of the para-CH sites of F232 with amide sites in the vicinity are shown in Figure 1C, and displayed on the structure in Figure 1D. The full data set for para-CH is shown in Figure S3, and the corresponding data for the ortho-CH assignment are shown in Figure S4.
We complemented these experiments with a mutagenesis approach of para-CH samples. We obtained spectra of three TET2 mutant samples in which individual Phe residues were replaced by Ala, one at a time, and the disappearance of the peak corresponding to the mutated Phe allows straightforward assignment (Figure S3A). Using these approaches, we unambiguously assigned 8 out of 10 para-CH sites. The unambiguously assigned sites include the structurally and functionally most interesting sites located close to the substrate entry pore of TET2 (F84, F140) and trimerization interface (F224) at the apices of the tetrahedral assembly structure.
Phe rings in TET2 have a rigid ring axis
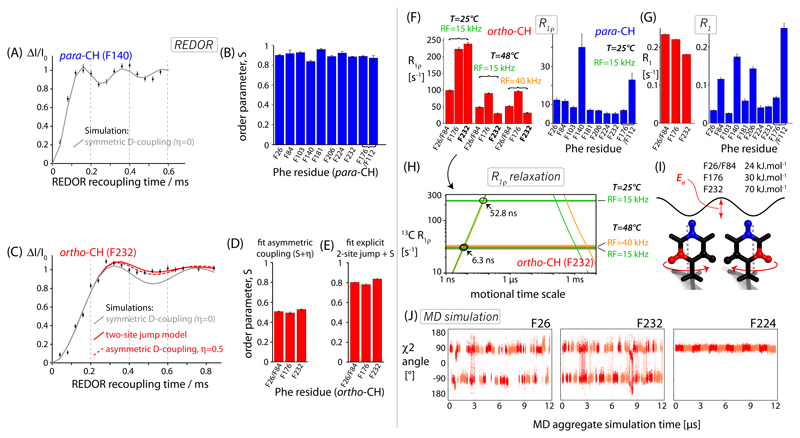
With these assignments at hand, we probed the dynamics of Phe side chains over a wide range of time scales, from picoseconds to milliseconds, using dipolar-coupling and nuclear spin relaxation measurements. The dipolar 1H-13C couplings provide direct information about the amplitude of the motion of the CH bonds, averaged over time scales up to a few hundred μs.36 Comparison of the measured dipolar-coupling tensor parameters compared to the tensor parameters in the absence of motion provides information about the amplitude and geometry of the motion. The amplitude is often expressed as order parameter S (ranging from 1 for rigid sites to 0 for fully flexible sites), as well as the geometry and asymmetry of the motion. The order parameter is obtained from comparing the tensor anisotropy to the rigid-limit value anisotropy δrigid as Motion without axial symmetry (i.e., lower than C3 symmetry) such as two-site ring flips, leads to an asymmetry of the resulting dipolar-coupling tensor,56 and the detection of tensor asymmetry can thus be related to the geometry of the motion. We have recently shown that specific labeling, fast MAS (>50 kHz) and an optimized REDOR experiment allows measuring these tensor parameters at high precision and accuracy.56
Figures 2A,B and S5 show REDOR-based52,80 dipolar-coupling measurements of the para-CH sites. We find high order parameters (S>0.9) for all ten para-CH sites, indicating that the ring axis of all phenylalanines in TET2 undergo only smallscale motions. The good fit of the simulated curves with a single fit parameter, S, and zero tensor asymmetry to the experimental data confirms that the motion can be described by a small-amplitude motion of the axis without significant population of alternative rotamer states around the Cα-Cβ axis. When describing the motion in the framework of the diffusion-in-a-cone model, the opening half-angle of the cone, θ, is 21° at most for the ten Phe sites (S = 0.5. cos θ(1 + cos θ).81,82 This view of little motional freedom of the ring axes in TET2 is in good qualitative agreement with MD simulations described below.
Figure 2.
Sub-microsecond dynamics of Phe residues in TET2 probed at para-CH (blue) and ortho-CH (red) sites using 1H-13C dipolar couplings measured with time-shifted REDOR52,80 (A-E), and 13C relaxation measurements (F-I). (A,C), representative REDOR dephasing curves, and best-fit simulations assuming either an axially symmetric tensor with a single fitted parameter, δ (grey), or a two-parameter fit of a tensor with anisotropy δ and asymmetry η (dashed red in C), or a two-site jump model by 120° (cf. panel I), and an additional fitted order parameter (solid red in C). (B,D,E) Fitted order parameters S; for these calculations we assumed a rigid-limit C-H distance of 1.09 Å.57 All REDOR curves are shown in Figure S5. (F,G) Experimental 13C R1ρ and R1 rate constants. (H) The use of 13C R1ρ rate constants to determine time scales of two-site jump motions, exemplified for ortho-CH site F232. The horizontal lines show the three experimentally measured rate constants, highlighted in bold face in panel (F). Sloped lines represent the theoretical R1ρ rate constants, calculated for the two-site jump model occurring at different exchange rate constants. These theoretical curves have been obtained from numerical spin simulations of the jumps in the GAMMA69 package, as used and described earlier83. Each experimentally determined R1ρ (horizontal lines) intersects the theoretical curve at two different rate constants, either on nanosecond (“left branch”) or micro-millisecond (“right branch”) time scales. Assuming that flips of a particular Phe can be described by one flip rate constant at a given temperature, then only a rate constant on the “fast” branch (ns), can explain all experimental data. This rate constant is shown for two temperatures for the case of F232 in panel (H). (I) Motional model, and estimated activation energy of two-site jump, using the rationale outlined in panel (H) and the two temperatures. Note that the activation energies are rough estimates, based on only two temperatures. (J) MD simulations of ring flips of three representative Phe in TET2. Shown are the time traces of the twelve subunits in the dodecameric assembly. Two colors have been used to differentiate data pertaining to different subunits. The MD data for all Phe sites are shown in Figure S9 (χ2 angle) and Figure S10 (χ1 angle).
Phenylalanine ring flips in TET2
The above-described para-CH REDOR data report only on ring-axis motion (i.e., motions around the side chain χ1 dihedral angle and additional backbone motion), but not on ring flips around χ2. In contrast, the ortho-CH site are sensitive reporters of such motions. The ortho-CH Phe spectrum of TET2 (red in Figure 1B) shows only four cross-peaks, assigned to F26, F84, F176 and F232 (Figure S4). We performed 1H-13C dipolar coupling measurements to probe the motions of these observable ortho-CH sites. Rotations of the Phe ring by a half-turn reorient the two ortho-CH sites by 120°. The dynamically-averaged 1H-13C dipolar-coupling tensor resulting from fast ring flips has a reduced tensor anisotropy (order parameter S=0.625) and it is asymmetric56 (η=0.6; see Figure S6 for illustration of the averaging). The experimental REDOR recoupling data, shown in Figure 2C, are in excellent agreement with the behavior expected for ring flips and small-amplitude local motion: first, the tensor anisotropy is considerably lower than the one of the para-CH sites, as evidenced immediately by the slower buildup of ΔI/I0 (compare the recoupling curves of Figures 2A and 2C). Second, the dipolar-coupling tensors are asymmetric: fits of REDOR curves that assume a symmetric dipolar-coupling tensor (η=0 and individually best-fit δ, grey curves in Figure 2C) lead to systematic deviations from the experimental data. Because the REDOR experiment has a built-in normalization taking into account the relaxation decay,80 such deviations of the experimental data from the simulations can be ascribed to an asymmetric dipolar-coupling tensor due to the two-site jump.56 We used two alternative descriptions of these REDOR data. In a first approach we fitted a general dipolar-coupling tensor with an anisotropy δ and asymmetry η (dashed red line in Figure 2C). The best-fit order parameters for the observed sites are ca. 0.5 (Figure 2D) and the asymmetry is η=0.55. Both values are slightly lower than those expected for jumps between the two ring orientations (S=0.625 and η=0.6), showing that additional motions are present on top of the ring flips, which reduce the dipolar-coupling asymmetry (η<0.6) and anisotropy (S<0.62).
For illustration purposes we also used an alternative description of the problem at hand. We simulated REDOR traces for a spin system undergoing explicit jumps between the two equally populated states, as well as an adjustable order parameter which reflects additional motion such as low-amplitude librations within each of the two ring orientations and motion of the backbone and the Cα-Cβ axis. This two-site jump simulation is also in good agreement with the experimental data when assuming an order parameter of ca. S=0.8 (Figure 2E). This value is slightly lower than the ones detected for the ring axis (i.e., the para-CH order parameters, Figure 2B) and also lower than the typically observed range for Cα bond motion.84 These observations suggest that the ortho-CH sites sense (i) ring flips, (ii) motions of the ring axis, just as the para-CH site does, and (iii) reorientational motion of the ring within each of the two rotamer states. Taken together, the dipolar-coupling data unambiguously establish that the four visible sites undergo ring flips on time scales shorter than ca. 100 μs, the time scale over which dipolar-coupling averaging is effective.36
We used 13C R1ρ relaxation measurements at different temperatures (25, 48°C) and spin-lock radio-frequency (RF) field strengths to estimate the time scales of Phe motion more precisely (Figure 2F,G,H). The R1ρ rate constant depends on the spectral density function describing the underlying motion, in particular the spectral density at frequencies corresponding to sums and differences of MAS frequency and spin-lock frequency (all in the tens of kHz range),36 and it is therefore most sensitive to motions in the range from several nanoseconds to milliseconds (i.e. the inverse of these frequencies). The sloped solid lines in Figure 2H show calculated R1ρ rate constants for the simple 2-site ring-flip motion (obtained in this case by fitting numerical spin simulations of the fate of 13C coherence under a spin-lock RF field and explicit jumps). These curves show that R1ρ is low when the motion is too fast (faster than ns) or too slow (slower than ms), and that the highest R1ρ arises when the motion is ca. 1 μs. As R1ρ depends on spectral density functions at sums and differences of the spectral density function, the R1ρ rate constant is different when a different spin-lock RF field strength is used experimentally, which is shown by orange and green curves in Figure 2H. This difference is only visible when the motion occurs in the μs-ms range, where the rate is close to this frequency difference (νMAS-νRF), i.e. only in the “right branch” of the curve. In addition, when increasing the temperature, the ring flips are expected to be accelerated, which means that R1ρ is either increased (when the motion occurred on the nanosecond time scale) or decreased (if it is in the μs-ms range). Of note, these experiments exploit exclusively relaxation due to the dipolar coupling and 13C CSA; fluctuations of the isotropic chemical shift are disregarded, because the strong spin-lock RF fields (15 kHz at least) suppress any effects that istropic chemical-shift fluctuations may have. (Isotropic chemical-shift fluctuations are explored in Bloch-McConnell-type relaxation-dispersion experiments considered in the last part of this manuscript.)
Exploiting the dependency of the observed R1ρ rate constants on RF field and temperature (Figure 2F) allows us to unambiguously establish that the motion of the four ortho-CH sites occurs on the nanosecond time scale, and not on the μs-ms time scales (Figure 2H). We used the ring flip rate constants from the R1ρ measurements to provide a rough estimate of the activation energies of ring flips, which is of the order of ca. 25-70 kJ.mol-1 (Figure 2I). We note that this estimation is based only on two temperatures, and it is, thus, to be considered as a rough approximation, but it is noteworthy that the values are in good agreement with previously reported values from solution-state NMR12,20,85,86 and MAS NMR28,87,88 of peptides and small proteins, which are in the range 37 to 90 kJ.mol-1. As expected from a decreased R1ρ rate constants at increasing temperature (see Figure 2F), the peaks of the four observable Phe sites sharpen up at higher temperature (Figure S8).
The para-CH sites, which are insensitive to ring flip motions and have restricted motion (Figure 2B), have R1ρ rate constants about an order of magnitude lower (~10 s-1) than those of the ortho-CH sites (100-250 s-1), as well as overall significantly lower R1 rate constants (Figure 2F,G).
MD simulations provide a rationale for observed ring flips
As shown from the experimental data reported above, the four visible ortho-CH sites in TET2 are flipping on a nanosecond time scale; however the other six sites are unobservable around room temperature (from ca. 0 to 55°C). The signals of these sites maybe broadened by static disorder or dynamics. Given that all para-CH sites are observed, static disorder of the ortho-CH sites appears somewhat less likely. We speculated that they may be undergoing slower ring flips than the observable phenylalanines: large-amplitude motion on the μs time scale would lead to strong dipolar relaxation; additionally, an isotropic chemical-shift difference between the two rotamer states would induce further line broadening.36. Fast (sub-microsecond) ring flips, in contrast, should lead to more favorable line widths, rendering the peaks observable.
We sought to understand what discriminates the four observable sites from the unobservable ones and turned to all-atom molecular dynamics (MD) simulations of the entire dodecameric 468 kDa particle over a simulation time scale of 1 μs. The simulation of a ~half-megadalton-large particle for time scales extending to microseconds is computationally costly; the explicit simulation of the twelve symmetric subunits improves the sampling statistics considerably, such that we effectively have a longer observation window. Figures 2J and S9 show the time traces and rotamer populations of the χ2 angles of the Phe residues (ring flips), and Figure S10 displays the equivalent data for χ1 dynamics (ring axis dynamics). Along the simulation, three of the four observable Phe rings (F26, F84, F232) undergo rapid ring flips on the tens-of-nanosecond time scale, and the fourth observable residue, F176, undergoes several jumps in our simulation window. The fact that the latter residue undergoes less frequent jumps coincides well with the observation that it has the lowest peak intensity. (High-ns to low-μs motion induces the fastest transverse relaxation; cf. Figure 1H.) Four of the non-observable Phe sites (F103, F112, F140, F224) do not undergo any ring flips along the simulation and another two (F176, F206) undergo only one or two ring flips over the simulation time scale. Taken together, our experimental observations allow quantifying ring flip motions, and the MD simulations provide a rationale for all of the four observed and six unobserved signals. The MD simulation also shows that eight Phe rings in TET2 populate a single rotamer state, and two of them show rare excursions to an alternate conformation, a finding that is in good qualitative agreement with the high order parameters observed for the para-CH sites (Figure 2B).
Ring flips as those observed here and the associated line broadening, may be more generally an explanation for the fact that aromatic ring spin systems are often difficult to detect and assign by MAS NMR.89
High-resolution low-temperature NMR and thermal activation of ring flips
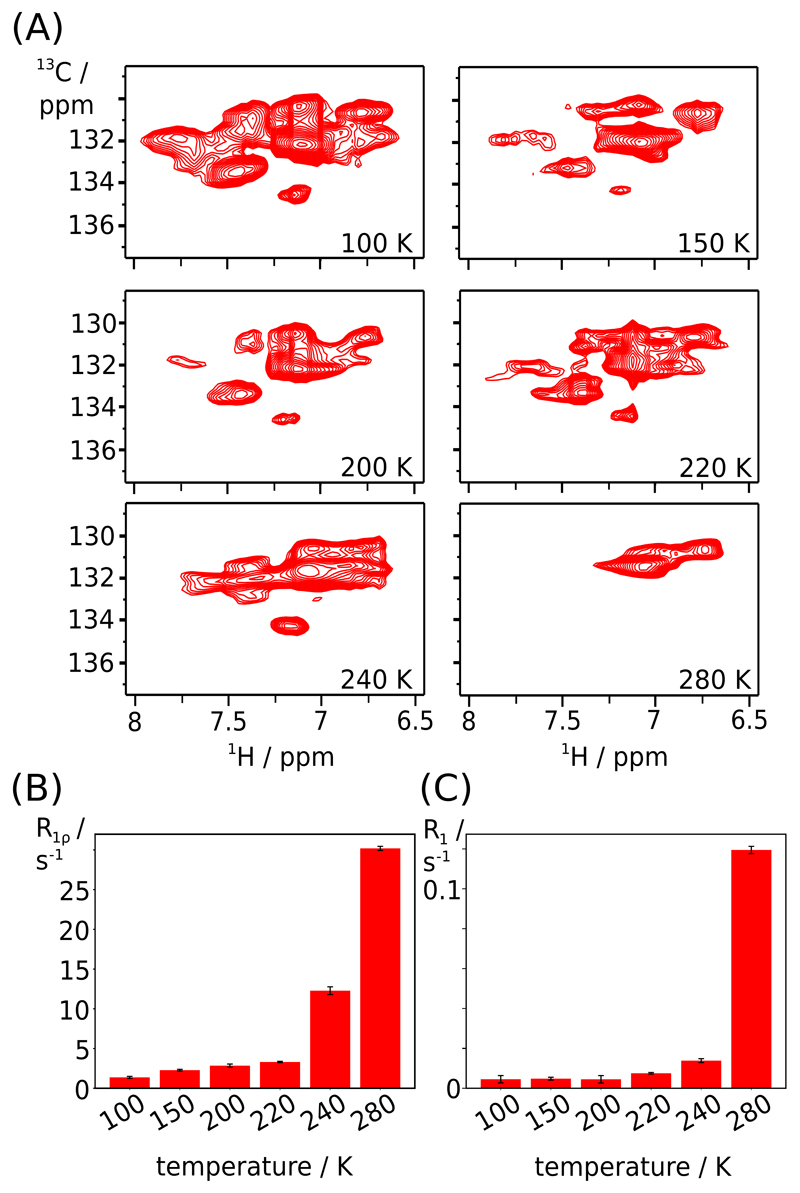
The fact that the ortho-CH sites of six Phe residues are unobservable suggests motions on the μs time scale. We reasoned that higher temperature would accelerate the ring flips such that the associated transverse relaxation rate constant would decrease. However, even at 55°C sample temperature we were unable to detect additional cross-peaks (Figure S7), suggesting that in the accessible temperature range the motion cannot be accelerated sufficiently. We, thus, used a low-temperature MAS NMR setup to cool the sample to 100 K, at a MAS frequency of 40 kHz in a 1.3 mm rotor. Figure 3A shows 1H-13C correlation spectra at six temperatures over this range. While at 280 K only four ortho-CH cross-peaks are seen, the complexity of the spectrum is greatly increased at temperatures of 240 K and below, with at least 10 distinguishable peaks at 100 K. Although we do not have assignments of these peaks, the observation of a large number of peaks strongly suggests that the aromatic ring flips are slowed down sufficiently to shift them out of the μs time window where the motion induces fast transverse relaxation. Remarkably, the line widths remain similar to those at room temperature, all the way down to 100 K. This is in stark contrast to the spectra of amides, which are severely broadened at low temperature, hampering site-specific observation (Figure S11).
Figure 3.
Low-temperature MAS NMR measurements of ortho-CH sites in u-[2H,15N],Phe-ortho-13C1H-labeled TET2. (A) CP-based 1H-13C correlation spectra at temperatures down to 100 K (as indicated) and a MAS frequency of 40 kHz. (B,C) Temperature-dependent 13C R1ρ and R1 relaxation rate constants from one-di-mensional 1H-detected measurements, using the pulse sequence shown in Figure S2D,E.
We used temperature-dependent ortho-CH 13C spin relaxation measurements to study the thermal activation of the Phe ring flips (Figure 3B,C). Systematic analyses of NMR relaxation rate constants have been used previously to obtain activation energies of protein motions from one-dimensional spectra without site-specific resolution.34,35 While in principle the quantitative analysis requires that individual sites are measured at variable temperatures, such an analysis is complicated by the fact that the ortho-CH spectra become complex and overlapped at low temperature, and have not been assigned here. In 1D spectra the relative contributions of different phenylalanine sites to the NMR intensity varies greatly with temperature, as illustrated by the fact that only 4 out of 10 Phe rings contribute at all to the signal at ambient temperature. Interpreting 1D integrals over the entire temperature range, thus, necessarily induces some bias, hampering quantitative analysis. We therefore analyze the data only qualitatively. Both 13C R1 and R1ρ show a biphasic temperature dependence, with very low relaxation rates at low temperatures and a strong increase at temperatures at ca. 220-240 K (R1ρ) or 240-280 K (R1). Qualitatively, this behavior is expected for a model where ring flips and small-amplitude motions – librations within each of the rotamer states – coexist. At low temperatures the ring flips are expected to be very rare and they do not contribute to spin relaxation, such that both R1 and R1ρ are low. The onset of spin flips is observed by increased relaxation; hereby, the raise of R1ρ is observed at lower temperatures than the one of R1. This behavior is expected, because R1ρ senses slower motions (tens-of-ns to ms, see Figure 2H), while sizeable R1 relaxation is induced only when the motions are in the ns range. Our data indicate, qualitatively, that ring flips enter the ms-μs regime at temperatures of ca. 220-240 K, and are accelerated to the ns regime above 240 K. The temperature of this apparent onset of aromatic ring flips is in good agreement with a dynamical transition observed by Mössbauer spectroscopy,90 neutron scattering,91 MD simulations,92 x-ray crystallography93 and NMR.34,94
The remarkably favorable line widths of aromatic sites found at low temperature point out a strong potential of this labeling scheme for dynamic nuclear polarization experiments, where line widths of backbone sites may otherwise become broad (cf. Figure S11).
Relaxation-dispersion experiments reveal short-lived (μs) states at the entry pore and trimer interface
Having studied in detail ring flips and ring-axis motions, occurring primarily on sub-microsecond time scales, we explored the potential of the labeling approach to unravel short-lived alternate conformations by relaxation-dispersion NMR. Many functional processes in proteins, such as enzymatic catalysis95,96 or allosteric signal transduction97,98 are linked to the presence of transient “active” functional states in dynamic equilibrium with the “ground” state. In solution-state NMR, a well-established arsenal of methods for detecting such motions has been developed, in particular relaxation-dispersion (RD) experiments.2,99,100 In MAS NMR, RD methodologies have been introduced only recently.43,101–104 The primary experimental challenge for measuring RD MAS NMR data is the presence of coherent decay mechanisms (dipolar dephasing) which contribute to the observed spin relaxation, thereby complicating the extraction of parameters reflecting dynamics. Deuteration and high MAS frequencies were proposed as solutions for 15N Carr-Purcell-Meiboom-Gill (CPMG) RD101 and 15N R1ρ RD experiments,43,102,103 but to our knowledge these approaches have not been extended to 13C RD experiments.
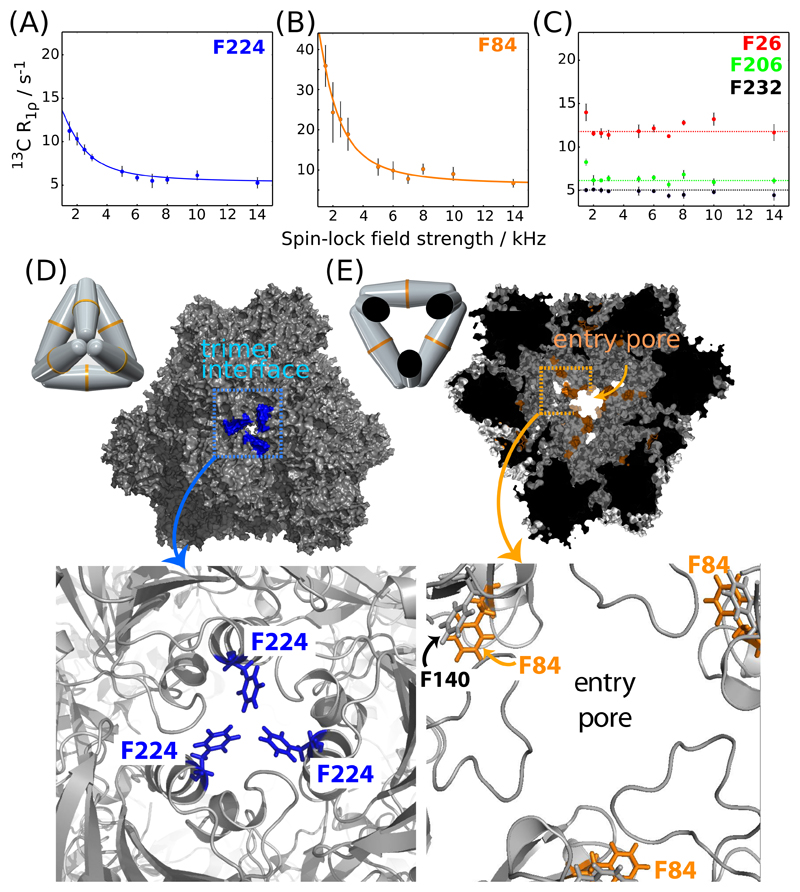
Figure 4 displays 13C R1ρ RD profiles for para-CH sites in TET2. The majority of these sites (7 out of 10) display flat RD profiles over the RF field range (2 to 15 kHz; see Figures 4C and S12). Because μs motion would lead to non-flat RD profiles, if the motion modulates the isotropic chemical shift of the involved sites, these flat curves suggest that these sites do not undergo sizeable motion on the μs time scale. The observation of flat RD profiles for the majority of sites furthermore indicates that dipolar-dephasing contributions to these curves are efficiently suppressed with our approach, even at low RF field strengths. The finding that these artefactual contributions to RD profiles can be suppressed efficiently using deuteration and fast MAS mirrors findings in 15N RD experiments in deuterated proteins.102 Flat RD profiles without motion are required for quantitatively detecting μs motions. Three para-CH sites – F224, F84 and, to lower extent, F140, in immediate vicinity to F84 – show pronounced non-flat RD profiles, revealing μs-ms dynamics. These phenylalanines are located in two structurally and possibly functionally important parts of TET2: F224 is located at the trimer interface and occludes the pore between the neighboring subunits, which has been proposed as a possible product exit from the catalytic chamber.64 F84 is located halfway between the substrate entry gate and the active site (Figure 4D,E). We have fitted a two-site exchange model to the RD data of F84, and of F224 and find an exchange rate constant of ca. 9800 ± 3800 s-1 and 11500 ± 5400 s-1 for these sites, respectively.
Figure 4.
Microsecond dynamics in TET2 probed by 13C Bloch-McConnell R1ρ relaxation dispersion MAS NMR data of para-CH sites (A-C). Sizeable (non-flat) RD profiles for residues F224 (A) and F84 (B) point to microsecond dynamics in the trimerization interface (D) and entry pore (E), respectively. Pictorial representations of the dodecamer in the respective orientations are shown in (D) and (E). The solid lines show numerical fits to a two-site exchange model using the program relax.105 The fitted exchange rate constants kex for F84 and F224 are 9800 ± 3800 s-1 and 11500 ± 5400 s-1 and the exchange parameters ϕex = pApB(2πΔδ)2 are below 1 ppm2 in both cases. All para-CH 13C R1ρ RD profiles are shown in Figure S12.
The above dipolar-coupling measurements (Figure 2B) have shown that these three Phe sites have a rigid axis over time scales up to a few hundred μs, i.e., including the time scale for which these relaxation-dispersion measurements detect motion. However, dipolar-coupling measurements report on the dipolar-coupling tensor averaged over all orientations, such that conformations populated to only a few percent would hardly be detectable in dipolar-coupling measurements. The combination of both views, i.e., the observation of high dipolar-coupling order parameters and pronounced relaxation-dispersion effects suggests that the observed RD profiles are due to exchange with a low-populated state, which might also involve alternate conformations of the backbone and other surrounding side chains. While the functional implications of these states are not within the scope of this study, they may be important for entry of substrates to the catalytic chamber (F84, F140), or product exit via the proposed pore that is occluded in the crystal structure by F224. We performed enzymatic activity experiments to assay the importance of F224 for product release from the cavity; the rationale is that if there is a transiently formed pore at the trimer interface (see Figure 4D), opened by movement of F224, and if this pore enables release of products from the catalytic chamber, as proposed,64 then the replacement of F224 by a very small (Ala) or large (Trp) residue would change the rate of product release. However, we find that the identity of this side chain (Phe, Ala or Trp) has negligible effects on the overall enzymatic activity (Figure S13), indicating either that the product release is not rate-limiting or that the possibly forming transient pore is not involved in release. Current work is directed towards deciphering the functional importance of the conformational-exchange process probed by the phenylalanines.
Discussion
We have demonstrated here the use of a specific Phe labelling scheme for MAS NMR, and shown its power to provide detailed insight into multiple types and time scales of Phe dynamics. Outstanding resolution, with 1H (13C) line widths of the order of ca. 35-50 Hz (1H) and 30-45 Hz (13C) has enabled site-specific assignment of Phe residues in TET2, one of the largest proteins studied to date by MAS NMR, and to our knowledge the largest protein, in terms of subunit size, for which near-complete assignments have been reported. Our study demonstrates that the presented approach can provide quantitative in-depth views of dynamics even in large and complex systems. We find that this specific labelling results in much superior spectral quality compared to fully protonated samples: we collected a 1H-13C correlation spectrum of uniformly 13C,15N-labeled TET2 at a MAS frequency of 100 kHz, which shows significantly larger linewidths than the specifically labeled sample, more cross-peaks and accordingly stronger signal overlap (Figure 5). The deuteration labeling scheme employed here will additionally benefit from higher MAS frequencies, as suggested by recent reports on deuterated, methyl-labeled proteins.106 Additional biosynthetic precursors for labeling other aromatic sites, such as Tyr, His or Trp, are available,24,59,107 and may provide important insight also into more hydrophilic environments, such as membrane pores.108
This study focused on the use of the specific Phe labels for the study of dynamics. The well-isolated 1H-13C spin systems enable several experiments in a quantitative manner, which would be challenging in fully labeled systems because of the abundant dipole-dipole interactions:
-
(i)
relaxation-dispersion MAS NMR experiments, shown here for the first time with 13C spins, probe exchange involving minor conformations exchanging on the μs-ms time scale. The usefulness of the RD technique to characterize in detail states with populations of a few percent has been shown previously for 15N-based experiments in solids,103,104,109,110 and has an impressive track record in solution-state NMR.100,111 Like in solution-NMR, RD experiments would also enable the study of ring flips occurring on this time scale, as long as the two exchanging states have significantly different chemical shifts. In addition to RD experiments that exploit the fluctuation of isotropic chemical shifts, near-rotary resonance RD experiments (NERRD) can detect μs motion even in the absence of chemical-shift exchange.102,103,112
-
(ii)
Dipolar-coupling tensors can be probed with high precision and accuracy using REDOR experiments, including the asymmetry of the dipolar tensor, which provides direct insight into the rotamer jumps, as exemplified here for ring flips. Although none of the ring axes in TET2 undergoes rotamer transitions (around χ1), asymmetric dipolar-coupling tensors would also reveal such jumps, akin to the case of methyl side chains we reported earlier.56
-
(iii)
Finally, the suppression of coherent effects (dipolar dephasing, spin diffusion, J-coupling evolution) allows accurate measurements of nuclear spin relaxation rate constants, additionally shedding light on the time scales of motion. Using such experiments at low temperature, we were able to reveal the onset of Phe ring flips, which enter the μs-ms range at around 200 K.
Conclusion
We have shown here that selective introduction of 1H-13C spin pairs, in the present case into phenylalanine rings, combined with deuteration and 1H-detected MAS NMR provides key insights into various motional processes. Selective protonation of other residue types in otherwise protonated proteins will allow extending this approach in a straightforward manner to, e.g. tyrosine, tryptophane or histidine, opening avenues to study their motions over many time scales at a similar level of detail as presented here. Although not the focus of the present study, the labelling scheme allows the detection of distance restraints to aromatic residues, as highlighted here with 13 distance restraints obtained to amides and methyl groups in TET2. Such restraints in the hydrophobic core of the protein often prove as highly valuable for structure determination.24,113 We are confident that the proposed labeling scheme in combination with high MAS frequencies (currently up to 111 kHz, and to increase within the next years) will prove very useful to study protein structure, interactions and dynamics essentially without limitations on molecular weight, possibly also coupled to DNP enhancement.
Supplementary Material
Details about sample preparation, NMR pulse sequences and data acquisition details, analysis methods, assignment data, REDOR curves, MD trajectories, enzymatic assays of F224 mutants. This material is available free of charge on the ACS Publications website.
Acknowledgment
We thank Bruno Franzetti (IBS Grenoble) for a plasmid encoding the sequence of TET2. This work was supported by the European Research Council (ERC Stg-2012-311318 ProtDyn2Function and ERC-CoG-2010-260887, and FP7-I3-BIO-NMR 261862) and used the platforms of the Grenoble Instruct Center (ISBG; UMS 3518 CNRS-CEA-UJF-EMBL) with support from FRISBI (ANR-10-INSB-05-02) and GRAL (ANR-10-LABX-49-01) within the Grenoble Partnership for Structural Biology (PSB). Financial support from the TGIR-RMN-THC Fr3050 CNRS for conducting the low-temperature measurements at the CRMN Lyon is gratefully acknowledged. A.B. acknowledges the support of the French Agence Nationale de la Recherche (ANR), under grant ANR-14-ACHN-0016. This work was supported in part by MEXT Grants-in-Aid Numbers 2112002 and 26119005 to M.K. and Grants-in-Aid for Young Scientists (B), numbers 23770111 and 25840021 to Y.M.
References
- (1).Mittermaier A, Kay LE. New Tools Provide New Insights in NMR Studies of Protein Dynamics. Science. 2006;312(5771):224–228. doi: 10.1126/science.1124964. [DOI] [PubMed] [Google Scholar]
- (2).Mulder FA, Mittermaier A, Hon B, Dahlquist FW, Kay LE. Studying Excited States of Proteins by NMR Spectroscopy. Nat Struct Biol. 2001;8(11):932–935. doi: 10.1038/nsb1101-932. [DOI] [PubMed] [Google Scholar]
- (3).Sheppard D, Sprangers R, Tugarinov V. Experimental Approaches for NMR Studies of Side-Chain Dynamics in High-Molecular-Weight Proteins. Prog Nucl Magn Reson Spectrosc. 2010;56(1):1–45. doi: 10.1016/j.pnmrs.2009.07.004. [DOI] [PubMed] [Google Scholar]
- (4).Gardner KH. Solution NMR Studies of a 42 KDa Escherichia Coli Maltose Binding Protein/β-Cyclodextrin Complex: Chemica Shift Assignments and Analysis. J Am Chem Soc. 1998;120(45):11738–11748. [Google Scholar]
- (5).Goto NK, Gardner KH, Mueller GA, Willis RC, Kay LE. A Robust and Cost-Effective Method for the Production of Val, Leu, Ile (Delta 1) Methyl-Protonated 15N-, 13C-, 2H-Labeled Proteins. J Biomol NMR. 1999;13(4):369–374. doi: 10.1023/a:1008393201236. [DOI] [PubMed] [Google Scholar]
- (6).Tugarinov V, Hwang PM, Ollerenshaw JE, Kay LE. Cross-Correlated Relaxation Enhanced 1H-13C NMR Spectroscopy of Methyl Groups in Very High Molecular Weight Proteins and Protein Complexes. J Am Chem Soc. 2003;125(34):10420–10428. doi: 10.1021/ja030153x. [DOI] [PubMed] [Google Scholar]
- (7).Mas G, Guan J-Y, Crublet E, Debled EC, Moriscot C, Gans P, Schoehn G, Macek P, Schanda P, Boisbouvier J. Structural Investigation of a Chaperonin in Action Reveals How Nucleotide Binding Regulates the Functional Cycle. Sci Adv. 2018;4:eaau4196. doi: 10.1126/sciadv.aau4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Religa TL, Sprangers R, Kay LE. Dynamic Regulation of Archaeal Proteasome Gate Opening as Studied by TROSY NMR. Science. 2010;328(5974):98–102. doi: 10.1126/science.1184991. [DOI] [PubMed] [Google Scholar]
- (9).Kainosho M, Miyanoiri Y, Takeda M. Experimental Approaches of NMR Spectroscopy. Springer Singapore; Singapore: 2018. Isotope-Aided Methods for Biological NMR Spectroscopy: Past, Present, and Future; pp. 37–61. [Google Scholar]
- (10).Wüthrich K, Wagner G. Internal Motion in Globular Proteins. Trends Biochem Sci. 1977;3(10):227–230. [Google Scholar]
- (11).Campbell ID, Dobson CM, Williams RJP. Proton Magnetic Resonance Studies of the Tyrosine Residues of Hen Lysozyme-Assignment and Detection of Conformational Mobility. Proc R Soc B. 1975;189(1097):503–509. doi: 10.1098/rspb.1975.0070. [DOI] [PubMed] [Google Scholar]
- (12).Campbell ID, Dobson CM, Moore GR, Perkins SJ, Williams RJP. Temperature Dependent Molecular Motion of a Tyrosine Residue of Ferrocytochrome C. FEBS Lett. 1976;70(1):96–100. doi: 10.1016/0014-5793(76)80734-x. [DOI] [PubMed] [Google Scholar]
- (13).Bogan AA, Thorn KS. Anatomy of Hot Spots in Protein Interfaces. J Mol Biol. 1998;280(1):1–9. doi: 10.1006/jmbi.1998.1843. [DOI] [PubMed] [Google Scholar]
- (14).Yang C-J, Takeda M, Terauchi T, Jee J, Kainosho M. Differential Large-Amplitude Breathing Motions in the Interface of FKBP12–Drug Complexes. Biochemistry. 2015;54(47):6983–6995. doi: 10.1021/acs.biochem.5b00820. [DOI] [PubMed] [Google Scholar]
- (15).Gardner KH, Rosen MK, Kay LE. Global Folds of Highly Deuterated, Methyl-Protonated Proteins by Multidimensional NMR. Biochemistry. 1997 doi: 10.1021/bi9624806. [DOI] [PubMed] [Google Scholar]
- (16).Mueller GA, Choy WY, Yang D, Forman-Kay JD, Venters RA, Kay LE. Global Folds of Proteins with Low Densities of NOEs Using Residual Dipolar Couplings: Application to the 370-Residue Maltodextrin-Binding Protein. J Mol Biol. 2000;300(1):197–212. doi: 10.1006/jmbi.2000.3842. [DOI] [PubMed] [Google Scholar]
- (17).Kainosho M, Torizawa T, Iwashita Y, Terauchi T, Mei Ono A, Güntert P. Optimal Isotope Labelling for NMR Protein Structure Determinations. Nature. 2006;440(7080):52–57. doi: 10.1038/nature04525. [DOI] [PubMed] [Google Scholar]
- (18).Kainosho M, Güntert P. SAIL – Stereo-Array Isotope Labeling. Q Rev Biophys. 2009;42(04):247–300. doi: 10.1017/S0033583510000016. [DOI] [PubMed] [Google Scholar]
- (19).Wagner G, DeMarco A, Wüthrich K. Dynamics of the Aromatic Amino Acid Residues in the Globular Conformation of the Basic Pancreatic Trypsin Inhibitor (BPTI). I. 1H NMR Studies. Biophys Struct Mech. 1976;2(2):139–158. doi: 10.1007/BF00863706. [DOI] [PubMed] [Google Scholar]
- (20).Weininger U, Modig K, Akke M. Ring Flips Revisited: 13C Relaxation Dispersion Measurements of Aromatic Side Chain Dynamics and Activation Barriers in Basic Pancreatic Trypsin Inhibitor. Biochemistry. 2014;53(28):4519–4525. doi: 10.1021/bi500462k. [DOI] [PubMed] [Google Scholar]
- (21).Gall CM, Cross TA, DiVerdi JA, Opella SJ. Protein Dynamics by Solid-State NMR: Aromatic Rings of the Coat Protein in Fd Bacteriophage. Proc Natl Acad Sci U S A. 1982;79(1):101–105. doi: 10.1073/pnas.79.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Schaefer J, Stejskal E, McKay R, Dixon WT. Phenylalanine Ring Dynamics by Solid-State 13C NMR. J Magn Reson. 1984;57(1):85–92. [Google Scholar]
- (23).LeMaster DM, Kushlan DM. Dynamical Mapping of E. Coli Thioredoxin via 13C NMR Relaxation Analysis. J Am Chem Soc. 1996;118(39):9255–9264. [Google Scholar]
- (24).Takeda M, Ono AM, Terauchi T, Kainosho M. Application of SAIL Phenylalanine and Tyrosine with Alternative Isotope-Labeling Patterns for Protein Structure Determination. J Biomol NMR. 2010;46(1):45–49. doi: 10.1007/s10858-009-9360-9. [DOI] [PubMed] [Google Scholar]
- (25).Boeszoermenyi A, Chhabra S, Dubey A, Radeva DL, Burdzhiev NT, Chanev CD, Petrov OI, Gelev VM, Zhang M, Anklin C, et al. Aromatic 19F-13C TROSY: A Background-Free Approach to Probe Biomolecular Structure, Function, and Dynamics. Nat Methods. 2019;16(4):333–340. doi: 10.1038/s41592-019-0334-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Sykes BD, Hull WE. Fluorine Nuclear Magnetic Resonance Studies of Proteins. Methods in Enzymology. 1978:270–295. doi: 10.1016/s0076-6879(78)49015-9. [DOI] [PubMed] [Google Scholar]
- (27).Potrzebowski MJ, Paluch P, Pawlak T, Jeziorna A, Trébosc J, Hou G, Vega A, Amoureux J-P, Dracinsky M, Polenova T. Analysis of Local Molecular Motions of Aromatic Sidechains in Proteins by 2D and 3D Fast MAS NMR Spectroscopy and Quantum Mechanical Calculations. Phys Chem Chem Phys. 2015;17:28789–28801. doi: 10.1039/c5cp04475h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Bajaj VS, van der Wel PCA, Griffin RG. Observation of a Low-Temperature, Dynamically Driven Structural Transition in a Polypeptide by Solid-State NMR Spectroscopy. J Am Chem Soc. 2009;131(1):118–128. doi: 10.1021/ja8045926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Opella SJ, Frey MH, Cross TA. Detection of Individual Carbon Resonances in Solid Proteins. J Am Chem Soc. 1979;101(19):5856–5857. [Google Scholar]
- (30).Hong M, Jakes K. Selective and Extensive 13C Labeling of a Membrane Protein for Solid-State NMR Investigations. J Biomol NMR. 1999;14(1):71–74. doi: 10.1023/a:1008334930603. [DOI] [PubMed] [Google Scholar]
- (31).Kinsey RA, Kintanar A, Oldfield E. Dynamics of Amino Acid Side Chains in Membrane Proteins by High Field Solid State Deuterium Nuclear Magnetic Resonance Spectroscopy. Phenylalanine, Tyrosine, and Tryptophan. J Biol Chem. 1981;256(17):9028–9036. [PubMed] [Google Scholar]
- (32).Williams JK, Zhang Y, Schmidt-Rohr K, Hong M. PH-Dependent Conformation, Dynamics, and Aromatic Interaction of the Gating Tryptophan Residue of the Influenza M2 Proton Channel from Solid-State NMR. Biophys J. 2013;104(8):1698–1708. doi: 10.1016/j.bpj.2013.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Vugmeyster L, Ostrovsky D, Ford JJ, Lipton AS. Freezing of Dynamics of a Methyl Group in a Protein Hydrophobic Core at Cryogenic Temperatures by Deuteron NMR Spectroscopy. J Am Chem Soc. 2010;132(12):4038–4039. doi: 10.1021/ja909599k. [DOI] [PubMed] [Google Scholar]
- (34).Lewandowski JR, Halse ME, Blackledge M, Emsley L. Direct Observation of Hierarchical Protein Dynamics. Science. 2015;348(6234):578–581. doi: 10.1126/science.aaa6111. [DOI] [PubMed] [Google Scholar]
- (35).Busi B, Yarava JR, Hofstetter A, Salvi N, Cala-De Paepe D, Lewandowski JR, Blackledge M, Emsley L. Probing Protein Dynamics Using Multifield Variable Temperature NMR Relaxation and Molecular Dynamics Simulation. J Phys Chem B. 2018;122(42):9697–9702. doi: 10.1021/acs.jpcb.8b08578. [DOI] [PubMed] [Google Scholar]
- (36).Schanda P, Ernst M. Studying Dynamics by Magic-Angle Spinning Solid-State NMR Spectroscopy: Principles and Applications to Biomolecules. Prog Nucl Magn Reson Spectr. 2016;96:1–46. doi: 10.1016/j.pnmrs.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Suter D, Ernst RR. Spin Diffusion in Resolved Solid-State NMR Spectra. Phys Rev B. 1985;32(9):5608. doi: 10.1103/physrevb.32.5608. [DOI] [PubMed] [Google Scholar]
- (38).Henrichs PM, Linder M, Hewitt JM. Dynamics of the 13C Spin-exchange Process in Solids: A Theoretical and Experimental Study. J Chem Phys. 1986;85(12):7077–7086. [Google Scholar]
- (39).Grommek A, Meier BH, Ernst M. Distance Information from Proton-Driven Spin Diffusion under MAS. Chem Phys Lett. 2006;427(4–6):404–409. [Google Scholar]
- (40).Giraud N, Blackledge M, Böckmann A, Emsley L. The Influence of Nitrogen-15 Proton-Driven Spin Diffusion on the Measurement of Nitrogen-15 Longitudinal Relaxation Times. J Magn Reson. 2007;184(1):51–61. doi: 10.1016/j.jmr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- (41).VanderHart DL, Garroway AN. 13C NMR Rotating Frame Relaxation in a Solid with Strongly Coupled Protons: Polyethylene. J Chem Phys. 1979;71(7):2773–2787. [Google Scholar]
- (42).Akasaka K, Ganapathy S, McDowell CA, Naito A. Spin–Spin and Spin-lattice Contributions to the Rotating Frame Relaxation of 13C in L-alanine. J Chem Phys. 1983;78(6):3567–3572. [Google Scholar]
- (43).Lewandowski JR, Sass HJ, Grzesiek S, Blackledge M, Emsley L. Site-Specific Measurement of Slow Motions in Proteins. J Am Chem Soc. 2011;133(42):16762–16765. doi: 10.1021/ja206815h. [DOI] [PubMed] [Google Scholar]
- (44).Mulder FAA, Akke M. Carbonyl 13C Transverse Relaxation Measurements to Sample Protein Backbone Dynamics. Magn Reson Chem. 2003;41(10):853–865. [Google Scholar]
- (45).Ishima R, Baber J, Louis JM, Torchia DA. Carbonyl Carbon Transverse Relaxation Dispersion Measurements and Ms-Ms Timescale Motion in a Protein Hydrogen Bond Network. J Biomol NMR. 2004;29(2):187–198. doi: 10.1023/B:JNMR.0000019249.50306.5d. [DOI] [PubMed] [Google Scholar]
- (46).Lu X, Zhang H, Lu M, Vega AJ, Hou G, Polenova T. Improving Dipolar Recoupling for Site-Specific Structural and Dynamics Studies in Biosolids NMR: Windowed RN-Symmetry Sequences. Phys Chem Chem Phys. 2016;18(5):4035–4044. doi: 10.1039/c5cp07818k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Hou G, Lu X, Vega AJ, Polenova T. Accurate Measurement of Heteronuclear Dipolar Couplings by Phase-Alternating R- Symmetry (PARS) Sequences in Magic Angle Spinning NMR Spectroscopy Spectroscopy. J Chem Phys. 2014;141(10) doi: 10.1063/1.4894226. 104202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Paluch P, Pawlak T, Amoureux J-P, Potrzebowski MJ. Simple and Accurate Determination of X-H Distances under Ultra-Fast MAS NMR. J Magn Reson. 2013;233:56–63. doi: 10.1016/j.jmr.2013.05.005. [DOI] [PubMed] [Google Scholar]
- (49).Bjerring M, Jain S, Paaske B, Vinther JM, Nielsen NC. Designing Dipolar Recoupling and Decoupling Experiments for Biological Solid-State NMR Using Interleaved Continuous Wave and RF Pulse Irradiation. Acc Chem Res. 2013;46(9):2098–2107. doi: 10.1021/ar300329g. [DOI] [PubMed] [Google Scholar]
- (50).Schanda P, Meier BH, Ernst M. Accurate Measurement of One-Bond H-X Heteronuclear Dipolar Couplings in MAS Solid-State NMR. J Magn Reson. 2011;210(2):246–259. doi: 10.1016/j.jmr.2011.03.015. [DOI] [PubMed] [Google Scholar]
- (51).Chevelkov V, Fink U, Reif B. Accurate Determination of Order Parameters from 1 H, 15 N Dipolar Couplings in MAS Solid-State NMR Experiments. J Am Chem Soc. 2009;131(15):14018–14022. doi: 10.1021/ja902649u. [DOI] [PubMed] [Google Scholar]
- (52).Schanda P, Meier BH, Ernst M. Quantitative Analysis of Protein Backbone Dynamics in Microcrystalline Ubiquitin by Solid-State NMR Spectroscopy. J Am Chem Soc. 2010;132(45):15957–15967. doi: 10.1021/ja100726a. [DOI] [PubMed] [Google Scholar]
- (53).Knight MJ, Pell AJ, Bertini I, Felli IC, Gonnelli L, Pierattelli R, Herrmann T, Emsley L, Pintacuda G. Structure and Backbone Dynamics of a Microcrystalline Metalloprotein by Solid-State NMR. Proc Natl Acad Sci USA. 2012;109(28):11095–11100. doi: 10.1073/pnas.1204515109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Krushelnitsky A, Zinkevich T, Reichert D, Chevelkov V, Reif B. Microsecond Time Scale Mobility in a Solid Protein as Studied by the 15N R1ρ Site-Specific NMR Relaxation Rates. J Am Chem Soc. 2010;132(34):11850–11853. doi: 10.1021/ja103582n. [DOI] [PubMed] [Google Scholar]
- (55).Agarwal V, Xue Y, Reif B, Skrynnikov NR. Protein Side-Chain Dynamics as Observed by Solution- and Solid-State NMR Spectroscopy: A Similarity Revealed. J Am Chem Soc. 2008;130(49):16611–16621. doi: 10.1021/ja804275p. [DOI] [PubMed] [Google Scholar]
- (56).Schanda P, Huber M, Boisbouvier J, Meier BH, Ernst M. Solid-State NMR Measurements of Asymmetric Dipolar Couplings Provide Insight into Protein Side-Chain Motion. Angew Chem Int Ed. 2011;50(46):11005–11009. doi: 10.1002/anie.201103944. [DOI] [PubMed] [Google Scholar]
- (57).Kasinath V, Valentine KG, Wand AJ. A 13C Labeling Strategy Reveals a Range of Aromatic Side Chain Motion in Calmodulin. J Am Chem Soc. 2013;135(26):9560–9563. doi: 10.1021/ja4001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Lichtenecker RJ, Weinhaupl K, Schmid W, Konrat R. α-Ketoacids as Precursors for Phenylalanine and Tyrosine Labelling in Cell-Based Protein Overexpression. J Biomol NMR. 2013;57(4):327–331. doi: 10.1007/s10858-013-9796-9. [DOI] [PubMed] [Google Scholar]
- (59).Schörghuber J, Geist L, Platzer G, Feichtinger M, Bisaccia M, Scheibelberger L, Weber F, Konrat R, Lichtenecker RJ. Late Metabolic Precursors for Selective Aromatic Residue Labeling. J Biomol NMR. 2018;71(3):129–140. doi: 10.1007/s10858-018-0188-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Ohki Sy, Kainosho M. Stable Isotope Labeling Methods for Protein NMR Spectroscopy. Prog Nucl Magn Reson Spectrosc. 2008;53(4):208–226. [Google Scholar]
- (61).Teilum K, Brath U, Lundström P, Akke M. Biosynthetic 13C Labeling of Aromatic Side Chains in Proteins for NMR Relaxation Measurements. J Am Chem Soc. 2006;128(8):2506–2507. doi: 10.1021/ja055660o. [DOI] [PubMed] [Google Scholar]
- (62).Franzetti B, Schoehn G, Hernandez J, Jaquinod M, Ruigrok R, Zaccai G. Tetrahedral Aminopeptidase: A Novel Large Protease Complex from Archaea. EMBO J. 2002;21(9):2132–2138. doi: 10.1093/emboj/21.9.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Borissenko L, Groll M. Crystal Structure of TET Protease Reveals Complementary Protein Degradation Pathways in Prokaryotes. J Mol Biol. 2005;346(5):1207–1219. doi: 10.1016/j.jmb.2004.12.056. [DOI] [PubMed] [Google Scholar]
- (64).Appolaire A, Colombo M, Basbous H, Gabel F, Girard E, Franzetti B. TET Peptidases: A Family of Tetrahedral Complexes Conserved in Prokaryotes. Biochimie. 2015;122:1–9. doi: 10.1016/j.biochi.2015.11.001. [DOI] [PubMed] [Google Scholar]
- (65).Macek P, Kerfah R, Erba EB, Crublet E, Moriscot C, Schoehn G, Amero C, Boisbouvier J. Unraveling Self-Assembly Pathways of the 468-kDa Proteolytic Machine TET2. Sci Adv. 2017;3(4):e1601601. doi: 10.1126/sciadv.1601601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Delaglio F, Grzesiek S, Vuister G, Zhu G, Pfeifer J, Bax A. NMRPIPE - a Multidimensional Spectral Processing System Based on Unix Pipes. J Biomol NMR. 1995;6(3):277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- (67).Vranken WF, Boucher W, Stevens TJ, Fogh RH, Pajon A, Llinas M, Ulrich EL, Markley JL, Ionides J, Laue ED. The CCPN Data Model for NMR Spectroscopy: Development of a Software Pipeline. Proteins. 2005;59(4):687–696. doi: 10.1002/prot.20449. [DOI] [PubMed] [Google Scholar]
- (68).Helmus JJ, Jaroniec CP. Nmrglue: An Open Source Python Package for the Analysis of Multidimensional NMR Data. J Biomol NMR. 2013;55(4):355–367. doi: 10.1007/s10858-013-9718-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Smith S, Levante T, Meier B, Ernst R. Computer Simulations in Magnetic Resonance. An Object-Oriented Programming Approach. J Magn Reson. 1994;106:75–105. [Google Scholar]
- (70).Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX. 2015;1:19–25. [Google Scholar]
- (71).Best RB, Zheng W, Mittal J. Balanced Protein – Water Interactions Improve Properties of Disordered Proteins and Non-Speci Fi c Protein Association. J Chem Theory Comput. 2014;25:(ii). doi: 10.1021/ct500569b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Hess B, Bekker H, Berendsen HJC, Fraaije JGEM. LINCS: A Linear Constraint Solver for Molecular Simulations. J Comput Chem. 1997;18(12):1463–1472. [Google Scholar]
- (73).Gauto D, Estrozi L, Schwieters C, Effantin G, Macek P, Sounier R, Sivertsen AC, Schmidt E, Kerfah R, Mas G, et al. Integrated NMR and Cryo-EM Atomic-Resolution Structure Determination of a Half-Megadalton Enzyme Complex. bioRxiv. 2018 doi: 10.1101/498287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Abascal JLF, Vega C. A General Purpose Model for the Condensed Phases of Water: TIP4P/2005. J Chem Phys. 2005;123(23) doi: 10.1063/1.2121687. 234505. [DOI] [PubMed] [Google Scholar]
- (75).Bussi G, Donadio D, Parrinello M. Canonical Sampling through Velocity Rescaling. J Chem Phys. 2007;126(1) doi: 10.1063/1.2408420. 14101. [DOI] [PubMed] [Google Scholar]
- (76).Parrinello M, Rahman A. Crystal Structure and Pair Potentials: A Molecular-Dynamics Study. Phys Rev Lett. 1980;45(14):1196–1199. [Google Scholar]
- (77).Amero C, Asunción Durá M, Noirclerc-Savoye M, Perollier A, Gallet B, Plevin MJ, Vernet T, Franzetti B, Boisbouvier J. A Systematic Mutagenesis-Driven Strategy for Site-Resolved NMR Studies of Supramolecular Assemblies. J Biomol NMR. 2011;50(3):229–236. doi: 10.1007/s10858-011-9513-5. [DOI] [PubMed] [Google Scholar]
- (78).Mas G, Crublet E, Hamelin O, Gans P, Boisbouvier J. Specific Labeling and Assignment Strategies of Valine Methyl Groups for NMR Studies of High Molecular Weight Proteins. J Biomol NMR. 2013;57(3):251–262. doi: 10.1007/s10858-013-9785-z. [DOI] [PubMed] [Google Scholar]
- (79).Bennett AE, Rienstra CM, Griffiths JM, Zhen W, Lansbury PT, Griffin RG. Homonuclear Radio Frequency-Driven Recoupling in Rotating Solids. J Chem Phys. 1998 [Google Scholar]
- (80).Gullion T, Schaefer J. Detection of Weak Heteronuclear Dipolar Coupling by Rotational-Echo Double-Resonance Nuclear-Magnetic-Resonance. Adv Magn Reson. 1988;13:57–83. [Google Scholar]
- (81).Lipari G, Szabo A. Effect of Librational Motion on Fluorescence Depolarization and Nuclear Magnetic Resonance Relaxation in Macromolecules and Membranes. Biophys J. 1980;30(3):489–506. doi: 10.1016/S0006-3495(80)85109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Lipari G, Szabo A. Pade Approximants to Correlation Functions for Restricted Rotational Diffusion. J Chem Phys. 1981;75(6):2971–2976. [Google Scholar]
- (83).Marion D, Gauto DF, Ayala I, Giandoreggio-Barranco K, Schanda P. Microsecond Protein Dynamics from Combined Bloch-McConnell and Near-Rotary-Resonance R 1 p Relaxation-Dispersion MAS NMR. ChemPhysChem. 2019;20(2):276–284. doi: 10.1002/cphc.201800935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Sheppard D, Li D-W, Brüschweiler R, Tugarinov V. Deuterium Spin Probes of Backbone Order in Proteins: 2H NMR Relaxation Study of Deuterated Carbon Alpha Sites. J Am Chem Soc. 2009;131(43):15853–15865. doi: 10.1021/ja9063958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Skalicky JJ, Mills JL, Sharma S, Szyperski T. Aromatic Ring-Flipping in Supercooled Water: Implications for NMR-Based Structural Biology of Proteins. J Am Chem Soc. 2001;123(3):388–397. doi: 10.1021/ja003220l. [DOI] [PubMed] [Google Scholar]
- (86).Hattori M, Li H, Yamada H, Akasaka K, Hengstenberg W, Gronwald W, Kalbitzer HR. Infrequent Cavity-Forming Fluctuations in HPr from Staphylococcus Carnosus Revealed by Pressure- and Temperature-Dependent Tyrosine Ring Flips. Prot Sci. 2009;13(12):3104–3114. doi: 10.1110/ps.04877104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Rice DM, Meinwald YC, Scheraga HA, Griffin RG. Tyrosyl Motion in Peptides. Deuterium NMR Line Shapes and Spin-Lattice Relaxation. J Am Chem Soc. 1987;109(6):1636–1640. [Google Scholar]
- (88).Kamihira M, Naito A, Tuzi S, Saitô H. Phenyl Ring Dynamics of Enkephalin Molecules and Behavior of Bound Solvents in the Crystalline States by 2H NMR Spectroscopy. J Phys Chem A. 1999;103(18):3356–3363. [Google Scholar]
- (89).Hiller M, Higman VA, Jehle S, van Rossum B-J, Kühlbrandt W, Oschkinat H. [2, 3-13C]-Labeling of Aromatic Residues Getting a Head Start in the Magic-Angle-Spinning NMR Assignment of Membrane Proteins. J Am Chem Soc. 2008;130(2):408–409. doi: 10.1021/ja077589n. [DOI] [PubMed] [Google Scholar]
- (90).Parak F, Knapp EW, Kucheida D. Protein Dynamics. Mössbauer Spectroscopy on Deoxymyoglobin Crystals. J Mol Biol. 1982;161(1):177–194. doi: 10.1016/0022-2836(82)90285-6. [DOI] [PubMed] [Google Scholar]
- (91).Doster W, Cusack S, Petry W. Dynamical Transition of Myoglobin Revealed by Inelastic Neutron-Scattering. Nature. 1989;337(6209):754–756. doi: 10.1038/337754a0. [DOI] [PubMed] [Google Scholar]
- (92).Smith J, Kuczera K, Karplus M. Dynamics of Myoglobin: Comparison of Simulation Results with Neutron Scattering Spectra. Proc Natl Acad Sci. 2006;87(4):1601–1605. doi: 10.1073/pnas.87.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Tilton RF, Dewan JC, Petsko GA. Effects of Temperature on Protein Structure and Dynamics: X-Ray Crystallographic Studies of the Protein Ribonuclease-A at Nine Different Temperatures from 98 to 320K. Biochemistry. 1992;31(9):2469–2481. doi: 10.1021/bi00124a006. [DOI] [PubMed] [Google Scholar]
- (94).Wood K, Gallat FX, Otten R, Van Heel AJ, Lethier M, Van Eijck L, Moulin M, Haertlein M, Weik M, Mulder FAA. Protein Surface and Core Dynamics Show Concerted Hydration-Dependent Activation. Angew Chem Int Ed. 2013;52(2):665–668. doi: 10.1002/anie.201205898. [DOI] [PubMed] [Google Scholar]
- (95).Loria JP, Berlow RB, Watt ED. Characterization of Enzyme Motions by Solution NMR Relaxation Dispersion. Acc Chem Res. 2008;41(2):214–221. doi: 10.1021/ar700132n. [DOI] [PubMed] [Google Scholar]
- (96).Eisenmesser EZ, Millet O, Labeikovsky W, Korzhnev DM, Wolf-Watz M, Bosco Da, Skalicky JJ, Kay LE, Kern D. Intrinsic Dynamics of an Enzyme Underlies Catalysis. Nature. 2005;438(7064):117–121. doi: 10.1038/nature04105. [DOI] [PubMed] [Google Scholar]
- (97).Shi L, Kay LE. Tracing an Allosteric Pathway Regulating the Activity of the HslV Protease. Proc Natl Acad Sci. 2014;111(6):2140–2145. doi: 10.1073/pnas.1318476111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Brüschweiler S, Schanda P, Kloiber K, Brutscher B, Kontaxis G, Konrat R, Tollinger M. Direct Observation of the Dynamic Process Underlying Allosteric Signed Transmission. J Am Chem Soc. 2009;131(8):3063–3068. doi: 10.1021/ja809947w. [DOI] [PubMed] [Google Scholar]
- (99).Neudecker P, Lundström P, Kay LE. Relaxation Dispersion NMR Spectroscopy as a Tool for Detailed Studies of Protein Folding. Biophys J. 2009;96(6):2045–2054. doi: 10.1016/j.bpj.2008.12.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Palmer AG, Massi F. Characterization of the Dynamics of Biomacromolecules Using Rotating-Frame Spin Relaxation NMR Spectroscopy. Chem Rev. 2006;106(5):1700–1719. doi: 10.1021/cr0404287. [DOI] [PubMed] [Google Scholar]
- (101).Tollinger M, Sivertsen AC, Meier BH, Ernst M, Schanda P. Site-Resolved Measurement of Microsecond-to-Millisecond Conformational-Exchange Processes in Proteins by Solid-State NMR Spectroscopy. J Am Chem Soc. 2012;134(36):14800–14807. doi: 10.1021/ja303591y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Ma P, Haller JD, Zajakala J, Macek P, Sivertsen AC, Willbold D, Boisbouvier J, Schanda P. Probing Transient Conformational States of Proteins by Solid-State R1ρ Relaxation-Dispersion NMR Spectroscopy. Angew Chem Int Ed. 2014;53(17):4312–4317. doi: 10.1002/anie.201311275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Kurauskas V, Izmailov SA, Rogacheva ON, Hessel A, Ayala I, Woodhouse J, Shilova A, Xue Y, Yuwen T, Coquelle N, et al. Slow Conformational Exchange and Overall Rocking Motion in Ubiquitin Protein Crystals. Nat Commun. 2017;8(1):145. doi: 10.1038/s41467-017-00165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Rovó P, Linser R. Microsecond Timescale Protein Dynamics: A Combined Solid-State NMR Approach. ChemPhysChem. 2017;19(1):34–39. doi: 10.1002/cphc.201701238. [DOI] [PubMed] [Google Scholar]
- (105).Morin S, Linnet TE, Lescanne M, Schanda P, Thompson GS, Tollinger M, Teilum K, Gagné S, Marion D, Griesinger C, et al. Relax: The Analysis of Biomolecular Kinetics and Thermodynamics Using NMR Relaxation Dispersion Data. Bioinformatics. 2014;30(15):2219–2220. doi: 10.1093/bioinformatics/btu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Xue K, Sarkar R, Motz C, Asami S, Camargo DCR, Decker V, Wegner S, Tosner Z, Reif B. Limits of Resolution and Sensitivity of Proton Detected MAS Solid-State NMR Experiments at 111 kHz in Deuterated and Protonated Proteins. Sci Rep. 2017;7(1):7444. doi: 10.1038/s41598-017-07253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Miyanoiri Y, Takeda M, Jee J, Ono AM, Okuma K, Terauchi T, Kainosho M. Alternative SAIL-Trp for Robust Aromatic Signal Assignment and Determination of the X2 Conformation by Intra-Residue NOEs. J Biomol NMR. 2011;51(4):425–435. doi: 10.1007/s10858-011-9568-3. [DOI] [PubMed] [Google Scholar]
- (108).Hu F, Luo W, Hong M. Mechanisms of Proton Conduction and Gating in Influenza M2 Proton Channels from Solid-State NMR. Science. 2010;330(6003):505–508. doi: 10.1126/science.1191714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Gauto DF, Hessel A, Rovó P, Kurauskas V, Linser R, Schanda P. Protein Conformational Dynamics Studied by 15N and 1H R1ρ Relaxation Dispersion: Application to Wild-Type and G53A Ubiquitin Crystals. Solid State Nucl Magn Reson. 2017;87:86–95. doi: 10.1016/j.ssnmr.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Le Marchand T, de Rosa M, Salvi N, Sala BM, Andreas LB, Barbet-Massin E, Sormanni P, Barbiroli A, Porcari R, Sousa Mota C, et al. Conformational Dynamics in Crystals Reveal the Molecular Bases for D76N Beta-2 Microglobulin Aggregation Propensity. Nat Commun. 2018;9(1):1658. doi: 10.1038/s41467-018-04078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Xue Y, Kellogg D, Kimsey IJ, Sathyamoorthy B, Stein ZW, McBrairty M, Al-Hashimi HM. Characterizing RNA Excited States Using NMR Relaxation Dispersion. Methods Enzym. 2015;558(1):39–73. doi: 10.1016/bs.mie.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Krushelnitsky A, Zinkevich T, Reif B, Saalwächter K. Slow Motions in Microcrystalline Proteins as Observed by MAS-Dependent 15N Rotating-Frame NMR Relaxation. J Magn Reson. 2014;248:8–12. doi: 10.1016/j.jmr.2014.09.007. [DOI] [PubMed] [Google Scholar]
- (113).Torizawa T, Ono AM, Terauchi T, Kainosho M. NMR Assignment Methods for the Aromatic Ring Resonances of Phenylalanine and Tyrosine Residues in Proteins. J Am Chem Soc. 2005;127(36):12620–12626. doi: 10.1021/ja051386m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.