The survival of young children with medulloblastoma is worse than that of older children because of the purposeful omission of craniospinal irradiation (CSI) from front-line therapy. This avoidance of CSI, a mainstay of therapy in older children, is justified because adverse effects increase inversely with age, and the youngest suffer irreparable damage to neurocognition, growth, and development. In addition, a significant proportion of this population survives despite the omission of CSI, although it is difficult to predict which patients might be spared the adverse effects of this therapy. To minimize the morbidity of CSI in this population, a series of clinical trials have tried to substitute this modality with other treatment regimens. To distinguish this population from older children, neuro-oncologists call these young patients “infants.” Although the use of this term is incorrect because it includes children older than age 1 year, it has proven useful to identify children in whom the goal is to avoid CSI. Even so, the term infant remains ambiguous because no accepted age cutoff exists, yet it always includes those < 3 years old and sometimes includes those up to 6 years old. Nonetheless, the term has stuck, and “infant” trials have broadly used four categories of treatment: systemic chemotherapy only; systemic chemotherapy with high-dose chemotherapy (HDC) and autologous stem-cell rescue; systemic chemotherapy with intraventricular chemotherapy (IVT); and systemic chemotherapy with primary site irradiation (focal radiotherapy [RT]).
Recently these trials have matured, and results are being published. However, the small population and multiple treatment regimens make results extremely difficult to generalize. Even more confusing is that clinical features such as histology, extent of surgical resection, and presence of metastases also play a role as independent determinants of outcome, further dividing an already small population into smaller groups. To make matters worse, it is now becoming increasingly clear that certain clinical features, such as histologic variants, are not always uniform across different trials, making like-for-like cross-trial comparisons almost impossible.1
Thankfully, contemporary genomic analysis has provided a much deeper understanding of the biology of medulloblastoma. Methods such as DNA methylation profiling have generated more objective and reproducible features that complement the clinical heterogeneity.2 Hence, molecular subgrouping has identified overlapping and uniform characteristics that have made these small studies relatable.
In the article that accompanies this editorial,3 the HIT group from Germany describes 87 patients with nonmetastatic medulloblastoma under 4 years old treated from 2001 to 2011 with a strategy of IVT, systemic chemotherapy, and in some, focal RT (HIT-2000). The authors present the outcome of these patients by clinical features (ie, histology and extent of resection) as well as through the contemporary genomic lens. Thus, although study results are reported as originally conceived, results are also reported in a way that can be compared with other recently published infant medulloblastoma studies with similar analysis.
Infants on HIT-2000 with histologically defined desmoplastic medulloblastoma (DMB) or medulloblastoma with extensive nodularity (MBEN) histology (n = 42) had excellent 5-year progression-free survival (PFS) and overall survival (OS) rates of 93% and 100%, respectively. Infants with classic and/or large-cell/anaplastic (LCA) histology (n = 45) had 5-year PFS and OS rates of 37% and 62%, respectively. This compares with 5-year PFS and OS rates of 53% and 75%, respectively, for DMB/MBEN and 5-year PFS and OS rates of 11% and 51%, respectively, for classic/LCA disease in 81 patients < 5 years old from a St Jude Children’s Research Hospital study (SJYC07).4 Even though the SJYC07 study included patients with metastatic disease, the improved PFS for patients with DMB/MBEN is convincing and suggests an improved outcome.
However, now that other groups have shown that most patients with classic/LCA disease belong to the group 3 or group 4 subgroups and most patients with DMB/MBEN fall into the sonic hedgehog (SHH) subgroup, how do these data relate to the molecular subgroups?1,4 In addition, given that infants with SHH can be divided into two subtypes—SHH-I (also called SHH-β) and SHH-II (also called SHH-γ)—how should these data be interpreted?4,5
Importantly, this study used contemporary genomics to facilitate cross-study comparisons. DNA methylation profiling molecularly categorized patients into SHH-I, SHH-II, group 3, or group 4 and allowed another 71 identically treated patients to be added to the SHH subgroup. The resultant SHH subgroup divided evenly into SHH-I (n = 56; 56%) and SHH-II (n = 43; 43%) in accordance with prior distributions.1,4,5 The outcome was 5-year PFS rates of 73% for SHH-I and 83% for SHH-II, as compared with 22% for SHH-I and 91% for SHH-II in nonmetastatic low-risk patients in the SJYC07 study and 30% for SHH-I and 67% for SHH-II in a similar chemotherapy-only Children’s Oncology Group trial (ACNS1221; Table 1).1,4
TABLE 1.
Comparison of SHH-I Versus SHH-II PFS Across Three Recent Infant Medulloblastoma Protocols
This important finding suggests that SHH-I patients markedly benefit from the addition of IVT chemotherapy (ie, methotrexate [MTX]) to systemic chemotherapy. However cross-trial comparison of PFS among the SHH-II subtype does not suggest that SHH-II patients derive the same benefit (Table 1). Collectively, these data divide infant SHH medulloblastoma into SHH-I, which benefits from chemotherapy with IVT-MTX, and SHH-II, which can be cured without IVT-MTX, HDC, or focal RT.
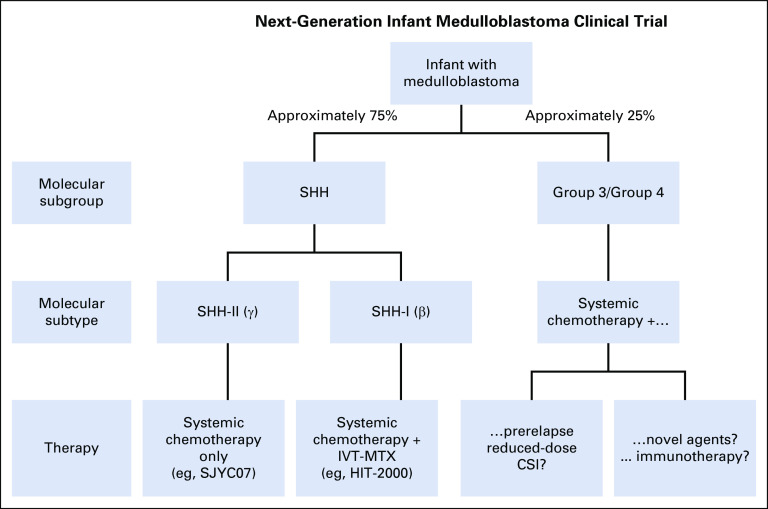
The authors of this study argue against a treatment de-escalation, although we respectfully disagree and propose that this new information might instead prompt investigating a risk-adapted approach whereby SHH-II patients receive a reduced-intensity regimen with systemic chemotherapy only and SHH-I patients receive systemic chemotherapy combined with IVT-MTX (Fig 1). Why expose a young child to more intensive therapy than necessary? Although the authors claim that treatment de-escalation is not necessary because the neurocognitive outcomes of patients on HIT-SKK therapy with IVT-MTX are acceptable, these outcomes are still below average and should not be accepted if the extra treatment is not needed.
FIG 1.
Schematic of a next-generation clinical trial. IVT, intraventricular chemotherapy; MTX, methotrexate; SHH, sonic hedgehog.
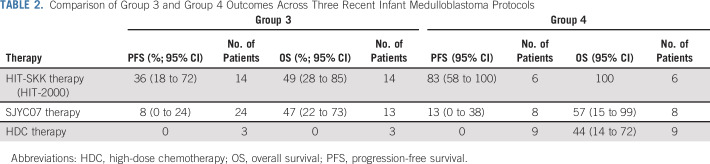
Another important, but disconcerting, finding is that the PFS for patients with group 3 medulloblastoma remains poor. HIT-2000 therapy resulted in a 5-year PFS for group 3 of 36%, as compared with 8% in SJYC07, and no survival benefit of focal RT.4 On the surface, this PFS seems better; however, other studies included patients with metastatic disease, and the wide CIs around the survival rates suggest that these numbers are not significantly different. In all, this represents the third recent publication to describe a poor PFS for group 3, and it signals a desperate need for better therapy (Table 2).4,6 The only consolation is that, as the OS numbers suggest, some patients are treated successfully with salvage therapy, but often only using CSI, which is endeavored to be avoided.
TABLE 2.
Comparison of Group 3 and Group 4 Outcomes Across Three Recent Infant Medulloblastoma Protocols
Interestingly, this study, unlike the other studies before it, shows an encouraging survival rate for group 4 patients (5-year PFS, 83%). Although this is positive, the number of group 4 patients in the infant population is characteristically small (approximately 20% of children < 6 years old and approximately 2% of children < 3 years old).4 In addition, this group included only six nonmetastatic patients, making this finding worthy of follow-up but not actionable. Because older children (> 3 years old) with nonmetastatic group 4 medulloblastoma already attain a ≥ 80% survival with conventional therapy,7 more data are needed before this survival rate is potentially jeopardized.
Consequently, the value of this study is that the results can be layered on top of results from other recent publications. This allows neuro-oncologists to develop the next molecularly driven risk-adapted clinical trial by matching the best therapy to each subgroup or subtype (Fig 1). Notably absent, except for a small cohort,6 are data from HDC cohorts, and the community eagerly awaits contemporary analysis on these so that this therapy can be adequately compared.
Nonetheless, before embarking on a major treatment change, it is important to recognize that these studies were not designed to define outcome on the basis of molecular subgroup or subtype and that the sample size in all these studies is small. Thus, caution should be used when basing any treatment recommendation on these results, and it is our strong-held opinion that any treatment change be done on a well-planned and well-monitored clinical trial.
The proverbial elephant in the room is that despite this advancement in genomics and subgrouping, there has been little change in treatment options. Ideally, investigators would like to treat infants with more focused and effective agents that have fewer long-term toxicities and preclude RT. Despite significant efforts to evaluate such agents for front-line clinical trials, investigators have been reluctant to introduce novel approaches because of the historically unpredictable nature of this disease, with some patients doing well and others not. Up-front genomic characterization changes this predictability and presents an opportunity to use an unbiased platform to select patients for arms on prospective clinical trials. The majority of infant medulloblastomas belong to the SHH subgroup. Adequately planning accrual targets for SHH-I and SHH-II subtypes to test treatment hypotheses (eg, IVT and chemotherapy for the SHH-I subgroup v judiciously reduced therapy for the low-risk SHH-II subgroup) is a way forward. Group 3 and a small number of group 4 tumors account for a minor subset of infant medulloblastomas that have a dismal prognosis with current chemotherapy-based treatment paradigms. RT is a successful salvage treatment in approximately half of these patients, but salvage comes at a huge cost. For these rare patients with aggressive biologic disease, novel approaches, such as using prerelapse reduced-dose proton-beam CSI, targeting malignant cells in the neuraxis using radiolabeled monoclonal antibodies, or chimeric antigen receptor T-cell therapy, can now be tried without compromising the survival of patients whose disease is not as aggressive (Fig 1). More results from trials with the appropriate molecular classifications will help lead the way to this next phase.
Footnotes
See accompanying article on page 2028
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Genomics Paves the Way for Better Infant Medulloblastoma Therapy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Giles W. Robinson
Consulting or Advisory Role: Eli Lilly, Genentech
Research Funding: Novartis (Inst), Genentech (Inst)
Amar Gajjar
Research Funding: Genentech (Inst), Kazia Pharmaceutical (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Lafay-Cousin L, Bouffet E, Strother D, et al. Phase II study of nonmetastatic desmoplastic medulloblastoma in children younger than 4 years of age: A report of the Children’s Oncology Group (ACNS1221) J Clin Oncol. 2020;38:223–231. doi: 10.1200/JCO.19.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hovestadt V, Remke M, Kool M, et al. Robust molecular subgrouping and copy-number profiling of medulloblastoma from small amounts of archival tumour material using high-density DNA methylation arrays. Acta Neuropathol. 2013;125:913–916. doi: 10.1007/s00401-013-1126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mynarek M, von Hoff K, Pietsch T, et al. Nonmetastatic medulloblastoma of early childhood: Results from the prospective clinical trial HIT-2000 and an extended validation cohort. J Clin Oncol. 2020;38:2028–2040. doi: 10.1200/JCO.19.03057. [DOI] [PubMed] [Google Scholar]
- 4.Robinson GW, Rudneva VA, Buchhalter I, et al. Risk-adapted therapy for young children with medulloblastoma (SJYC07): Therapeutic and molecular outcomes from a multicentre, phase 2 trial. Lancet Oncol. 2018;19:768–784. doi: 10.1016/S1470-2045(18)30204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavalli FMG, Remke M, Rampasek L, et al. Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell. 2017;31:737–754.e6. doi: 10.1016/j.ccell.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeo KK, Margol AS, Kennedy RJ, et al. Prognostic significance of molecular subgroups of medulloblastoma in young children receiving irradiation-sparing regimens. J Neurooncol. 2019;145:375–383. doi: 10.1007/s11060-019-03307-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramaswamy V, Remke M, Bouffet E, et al. Risk stratification of childhood medulloblastoma in the molecular era: The current consensus. Acta Neuropathol. 2016;131:821–831. doi: 10.1007/s00401-016-1569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]