Abstract
PURPOSE
There is no consensus on the best choice of an alternative donor (umbilical cord blood [UCB], haploidentical, one-antigen mismatched [7/8]–bone marrow [BM], or 7/8-peripheral blood [PB]) for hematopoietic cell transplantation (HCT) for patients lacking an HLA-matched related or unrelated donor.
METHODS
We report composite end points of graft-versus-host disease (GVHD)–free relapse-free survival (GRFS) and chronic GVHD (cGVHD)–free relapse-free survival (CRFS) in 2,198 patients who underwent UCB (n = 838), haploidentical (n = 159), 7/8-BM (n = 241), or 7/8-PB (n = 960) HCT. All groups were divided by myeloablative conditioning (MAC) intensity or reduced intensity conditioning (RIC), except haploidentical group in which most received RIC. To account for multiple testing, P < .0071 in multivariable analysis and P < .00025 in direct pairwise comparisons were considered statistically significant.
RESULTS
In multivariable analysis, haploidentical group had the best GRFS, CRFS, and overall survival (OS). In the direct pairwise comparison of other groups, among those who received MAC, there was no difference in GRFS or CRFS among UCB, 7/8-BM, and 7/8-PB with serotherapy (alemtuzumab or antithymocyte globulin) groups. In contrast, the 7/8-PB without serotherapy group had significantly inferior GRFS, higher cGVHD, and a trend toward worse CRFS (hazard ratio [HR], 1.38; 95% CI, 1.13 to 1.69; P = .002) than the 7/8-BM group and higher cGVHD and trend toward inferior CRFS (HR, 1.36; 95% CI, 1.14 to 1.63; P = .0006) than the UCB group. Among patients with RIC, all groups had significantly inferior GRFS and CRFS compared with the haploidentical group.
CONCLUSION
Recognizing the limitations of a registry retrospective analysis and the possibility of center selection bias in choosing donors, our data support the use of UCB, 7/8-BM, or 7/8-PB (with serotherapy) grafts for patients undergoing MAC HCT and haploidentical grafts for patients undergoing RIC HCT. The haploidentical group had the best GRFS, CRFS, and OS of all groups.
INTRODUCTION
In the absence of an HLA-matched related or unrelated donor (URD) for hematopoietic cell transplantation (HCT), umbilical cord blood (UCB), haploidentical, single HLA-locus mismatched (7/8)-bone marrow (BM), or 7/8-peripheral blood (PB) represent the most common alternative donor and graft options. Alternative donor HCTs are increasing,1,2 because the probability of finding 8/8 HLA-matched URDs varies widely from 75% among some whites to only 16% among certain blacks.3 Although the ongoing Blood and Marrow Transplant Clinical Trials Network 1101 (ClinicalTrials.gov identifier: NCT01597778) trial is prospectively comparing haploidentical versus UCB HCTs using reduced intensity conditioning (RIC), only a few studies comparing the array of alternative donor options for HCT have been reported.4-11 With the incorporation of novel graft-versus-host disease (GVHD) prophylaxis that includes post-transplantation cyclophosphamide (PTCy), comparisons of contemporary experience with alternative donor choices are further limited.
CONTEXT
Key Objective
This article answers a key question about the best donor or graft source for patients lacking HLA-matched donors.
Knowledge Generated
Patients with a haploidentical donor (using a BM graft and RIC) had the best GVHD-free relapse-free survival (GRFS) and overall survival (OS) compared with those who had a one-antigen mismatched unrelated donor using either a BM or PB graft or those receiving a UCB graft after either RIC or myeloablative conditioning (MAC).
Relevance
Among patients lacking HLA-matched donors, these data support the use of haploidentical BM graft for those receiving RIC and either PB with serotherapy, BM, or UCB grafts for those receiving MAC.
We compared mortality and ongoing GVHD- and relapse-associated morbidity after alternative donor HCT, using data from the Center for International Blood and Marrow Transplant Research (CIBMTR). We evaluated two composite end points: (1) GRFS and (2) chronic GVHD (cGVHD)–free relapse-free survival (CRFS).12 GRFS is defined as the absence of grade 3 to 4 acute GVHD (aGVHD), cGVHD requiring systemic therapy, relapse, or death. CRFS is defined as the absence of cGVHD requiring systemic therapy, relapse, or death. We previously reported that BM grafts from matched sibling donors led to the best GRFS compared with PB grafts from any donor or with UCB,13,14 but we did not compare these with haploidentical donors who were using PTCy. Herein, we analyzed GRFS and CRFS among alternative donor HCTs, including UCB, haploidentical, URD 7/8-BM, or URD 7/8-PB grafts.
MATERIALS AND METHODS
Objectives
The primary objective was to compare GRFS and CRFS among adults (age 18 years or older) with hematologic malignancies who underwent a first alternative donor HCT (excluding HLA-matched sibling or URD). Secondary objectives were to compare events that contributed to GRFS and CRFS among different groups.
Patient Population
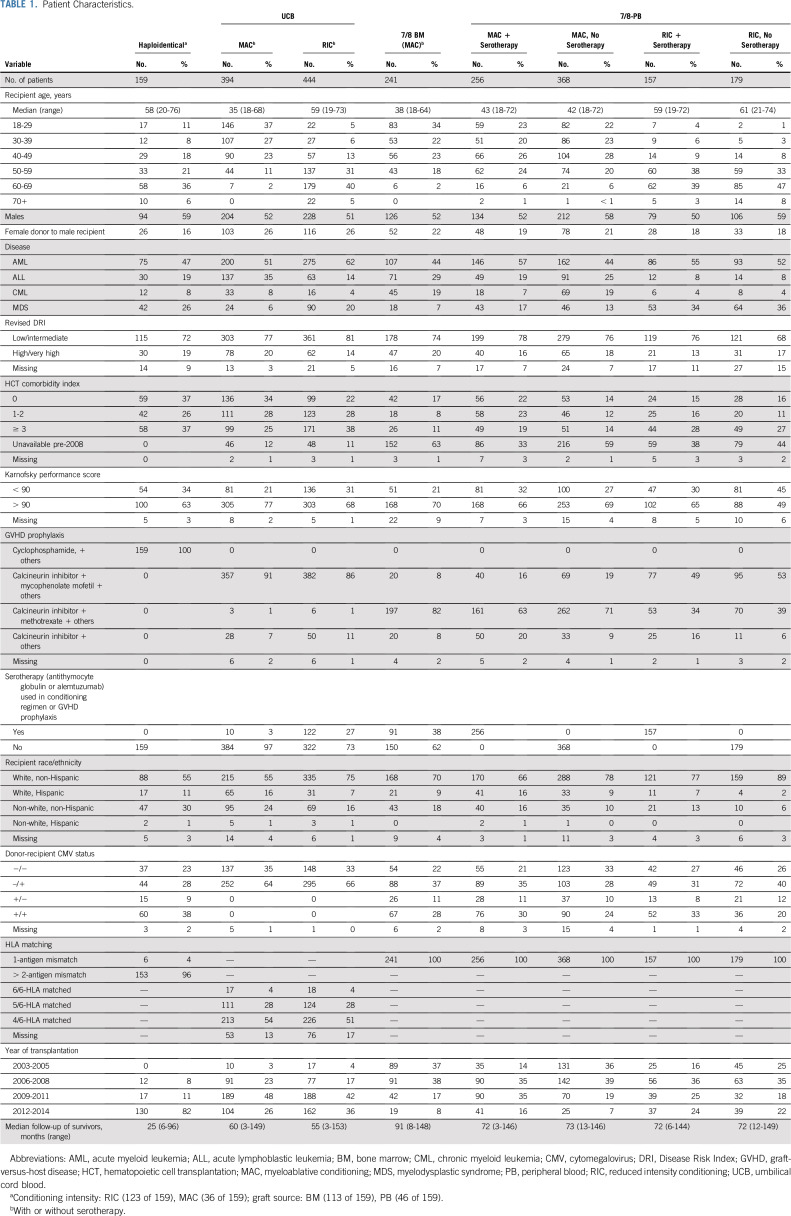
The study population consisted of patients with acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL) in remission, chronic myeloid leukemia, or myelodysplastic syndrome who received an alternative donor HCT from 2003 to 2014. Only haploidentical HCT with PTCy and only those UCB transplantations using fludarabine, cyclophosphamide, and total body irradiation (TBI) conditioning were included. A majority of UCB donors and most 7/8-URDs (64%) had antigen-level HLA data. Given similar outcomes with allele and antigen mismatches in 7/8-URDs,15,16 these were analyzed together. Exclusion criteria were previous autologous or allogeneic HCT or UCB transplantation with any unit having less than a 4/6 HLA match. We also excluded HCT with ex vivo T-cell–depleted or CD34+ selected grafts.
All groups except the haploidentical one were analyzed by conditioning intensity subgroup: MAC versus RIC per standard criteria,17 and by the use of serotherapy with either alemtuzumab or antithymocyte globulin. The haploidentical group was analyzed as a single cohort because the majority (78%) received RIC and BM grafts (71%) and none received serotherapy. The 7/8 BM-RIC groups (with [n = 26] and without [n = 17] serotherapy) were excluded because of small numbers. Both UCB-RIC groups (with [n = 122] and without [n = 322] serotherapy) were combined because no significant differences were noted between the groups in any outcomes tested in pairwise comparisons. Both 7/8 BM-MAC groups (with [n = 91] and without [n = 150] serotherapy) were combined for the same reason. Both UCB-MAC groups (with [n = 10] and without [n = 384] serotherapy) were combined because few patients received serotherapy. Overall, eight groups were compared: haploidentical, UCB-MAC, UCB-RIC, 7/8-BM-MAC, 7/8-PB (MAC, no serotherapy), 7/8-PB (MAC + serotherapy), 7/8-PB (RIC, no serotherapy), and 7/8-PB (RIC + serotherapy).
Definitions and Statistical Analysis
Haploidentical donors were defined as related donors mismatched at one or more HLA-loci. Relapse or progression was defined as the time to recurrence or progression of the underlying malignancy, with death without relapse or progression (nonrelapse mortality [NRM]) treated as a competing risk. Disease-free survival (DFS) was defined as the time from HCT to relapse or progression or death. OS was the time from HCT to death from any cause. aGVHD18 and cGVHD19,20 were diagnosed according to standard criteria, although National Institute of Health criteria21 for cGVHD were not prospectively used in reports to the CIBMTR during most of the study period.
Multivariable analysis was performed using Cox proportional hazards modeling on cause-specific hazards for all outcomes. Because the follow-up period in the haploidentical group was considerably shorter (median, 25 months) than that in other groups, all patients were censored at 3 years after transplantation (75th percentile of the follow-up time for surviving patients in the haploidentical group). All adjusted factors were tested for affirmation of the proportional hazards assumption using a time-dependent covariate approach. No factor violated this assumption, except the main testing variable (donor/graft), which violated the assumption for GRFS, CRFS, and cGVHD. To cope with this violation, we applied a weighted Cox regression approach22,23 for GRFS, CRFS, and cGVHD to compare the average hazards of each donor/graft cohort. A stepwise forward model was built for each outcome by selecting adjusted factors using a threshold of 0.05 for both entry and retention in the model. All variables and categorization as listed in Table 1 were considered in the stepwise model for all outcomes, with P < .05 considered significant for the covariates. The center effect was adjusted via robust sandwich estimates. No two-way interactions between donor/graft and the adjusted clinical variables in the models were detected at a 0.01 significance level. Adjusted plots were created on the basis of the stratified Cox model24 for GRFS, CRFS, OS, and DFS, and a subdistribution hazards model25 was created for cumulative incidence of relapse.
TABLE 1.
Patient Characteristics.
To adjust for multiple testing of the donor/graft variable for several outcomes, P < .0071 (0.05/7) for the donor/graft variable was considered statistically significant in the multivariable regression analysis (while not adjusting for the number of pairwise comparisons of individual donor/graft types to haploidentical types), and P < .00025 (0.0071/ 28) was considered significant for direct pairwise comparisons between multiple subgroups. All P values presented are two-sided. Data were analyzed by using SAS software version 9.4 (SAS Institute, Cary, NC).
RESULTS
The analysis involved 2,198 patients who had UCB (n = 838), haploidentical (n = 159), 7/8-BM (n = 241), or 7/8-PB grafts (n = 960; Table 1). The most common diagnoses were AML (52%) and ALL (21%). A majority (76%) had low or intermediate revised Disease Risk Index (DRI),26 and about 25% had an HCT comorbidity index (HCT-CI)27 score of 3 or greater. Patients who received MAC were younger (group median age ranged from 35 to 43 years) than those in the RIC cohorts (median age ranged from 59 to 61 years). GVHD prophylaxis differed among groups: mycophenolate mofetil–based regimens were used predominantly in the UCB (88%) and the 7/8-PB-RIC groups (51%), whereas methotrexate-based prophylaxis was used more commonly in the 7/8-BM (82%) and the 7/8-PB-MAC (68%) groups. In the UCB group, a majority of patients (81%) received double-unit grafts; the median pre-cryopreserved dose of total nucleated cells (TNCs) was 5 × 107 per kg (range, 1 to 17 × 107 per kg) and only approximately 10% had a TNC dose of less than 3 × 107 per kg. Most patients in the haploidentical group received BM grafts (71%) and RIC (78%), and a large majority (82%) of the grafts were recent (between 2012 and 2014). Therefore, follow-up of the haploidentical group (median, 25 months) was noticeably shorter than that for other groups (55 to 91 months). In the haploidentical RIC group, the most commonly used regimen was fludarabine, cytarabine, and TBI (n = 95 [77%]; Data Supplement). The Data Supplement also contains the univariable estimates of GRFS, CRFS, aGVHD 3 to 4, cGVHD, relapse, DFS, and OS.
GFRS
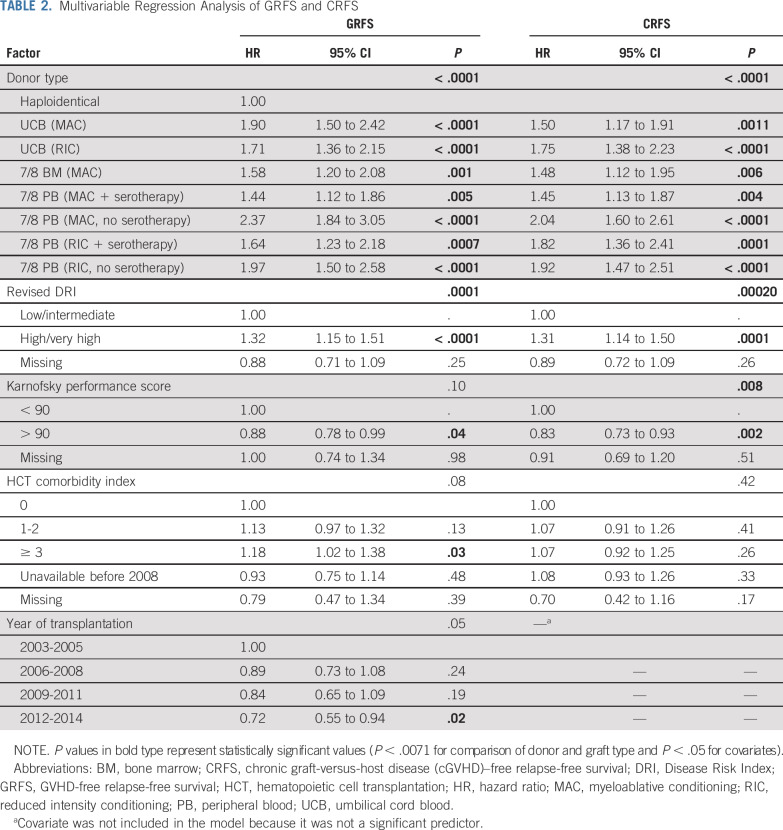
In multivariable analysis, patients with haploidentical grafts had the best GRFS of all the groups (7/8-PB [MAC or RIC, with or without serotherapy]; 7/8-BM-MAC; and UCB [MAC or RIC]) as shown in Figure 1 and detailed in Table 2. Patients with high or very high DRI, Karnofsky performance score (KPS) below 90, and HCT-CI of 3 or greater had significantly inferior GRFS, whereas patients with a recent HCT (2012 to 2014) had superior GRFS. Among the patients with UCB grafts, the degree of HLA matching had no impact on GRFS (Data Supplement); hence, they were not compared with other groups. In the direct pairwise comparisons, among the group of patients who received MAC, we found no differences in GRFS between those who received UCB, 7/8-BM, or 7/8-PB (MAC + serotherapy) grafts, whereas those who received a 7/8-PB (MAC without serotherapy) graft had significantly inferior GRFS compared with those who received a 7/8-BM graft (hazard ratio [HR], 1.50; 95% CI, 1.22 to 1.84; P = .0001) or a 7/8-PB (MAC with serotherapy) graft (HR, 1.65; 95% CI, 1.35 to 2.00; P < .0001; Data Supplement).
FIG 1.
Adjusted graft-versus-host-disease (GVHD)–free relapse-free survival (GRFS) after receiving an HCT with haploidentical, umbilical cord blood (UCB) with myeloablative conditioning (MAC), UCB with reduced intensity conditioning (RIC), 7/8 bone marrow (BM) with MAC, 7/8 peripheral blood (PB) with MAC + serotherapy, 7/8 PB with MAC, 7/8 PB with RIC + serotherapy, or 7/8 PB with RIC grafts. The adjusted factors are the variables listed in the multivariable regression analysis (Table 2).
TABLE 2.
Multivariable Regression Analysis of GRFS and CRFS
CRFS
In multivariable analysis, the haploidentical group had the best CRFS compared with other groups (Fig 2; Table 2). Patients with high or very high DRI and those with a KPS below 90 had inferior CRFS. In the direct pairwise comparisons among the MAC group, we found no differences in CRFS between the UCB, 7/8-BM, and the 7/8-PB (MAC + serotherapy) groups, whereas the 7/8-PB (MAC without serotherapy) group had significantly inferior CRFS compared with the 7/8-PB (MAC with serotherapy) group (HR, 1.41; 95% CI, 1.17 to 1.69; P = .0002) and a trend toward inferior CRFS compared with the UCB group (HR, 1.36; 95% CI, 1.14 to 1.63; P = .0006) and the 7/8-BM group (HR, 1.38; 95% CI, 1.13 to 1.69; P = .002; Data Supplement).
FIG 2.
Adjusted chronic graft-versus-host disease (cGVHD)–free relapse-free survival (CRFS) after receiving an HCT with haploidentical, umbilical cord blood (UCB) with myeloablative conditioning (MAC), UCB with reduced intensity conditioning (RIC), 7/8 bone marrow (BM) with MAC), 7/8 peripheral blood (PB) with MAC + serotherapy, 7/8 PB with MAC, 7/8 PB with RIC + serotherapy, or7/8 PB with RIC grafts. The adjusted factors are the variables listed in the multivariable regression analysis (Table 2)
Component End Points of Grade 3 to 4 aGVHD, cGVHD, and Relapse
aGVHD grade 3 to 4.
In multivariable analysis, we found no differences in the risk of aGVHD between haploidentical, UCB-RIC, and 7/8-PB (RIC + serotherapy) groups. In contrast, aGVHD was significantly higher in all MAC groups (7/8-BM, 7/8-PB, and UCB) and in the 7/8-PB (RIC without serotherapy) group. No other factors influenced the risk of aGVHD grade 3 to 4 (Table 3). In the direct pairwise comparisons of MAC groups, we found no differences between the UCB and 7/8-BM groups, whereas the 7/8-PB without serotherapy group had significantly higher risk of aGVHD compared with the 7/8-PB with serotherapy group (HR, 2.25; 95% CI, 1.60 to 3.18; P < .0001) and a trend toward higher risk of aGVHD than the 7/8-BM group (HR, 1.65; 95% CI, 1.23 to 2.21; P = .0009). Among RIC groups, we found no differences in the risk of aGVHD between the UCB and 7/8-PB + serotherapy groups, whereas the 7/8-PB without serotherapy group had a trend toward higher risk of aGVHD than the UCB group (HR, 1.79; 95% CI, 1.27 to 2.54; P = .001; Data Supplement).
TABLE 3.
Multivariable Regression Analysis of aGVHD, cGVHD Relapse, and Survival
cGVHD.
In multivariable analysis, we found no differences in the risk of cGVHD between haploidentical and UCB-RIC groups, whereas all groups with 7/8-PB (MAC or RIC + serotherapy), UCB-MAC, and 7/8-BM-MAC grafts had significantly higher risk. There were no other significant predictors of cGVHD (Table 3). In the direct pairwise comparisons of MAC groups, we found no difference in the risk of cGVHD between patients with UCB, 7/8-BM, or 7/8-PB (with serotherapy) grafts; however, the 7/8-PB (without serotherapy) group had a significantly higher risk of cGVHD than the UCB group (HR, 1.77; 95% CI, 1.42 to 2.22; P < .0001) compared with both the 7/8-BM group (HR, 1.71; 95% CI, 1.34 to 2.19; P < .0001) and the 7/8-PB with serotherapy group (HR, 1.76; 95% CI, 1.40 to 2.21; P < .0001). Among RIC groups, the 7/8-PB without serotherapy group had a significantly higher risk of cGVHD (HR, 2.74; 95% CI, 2.06 to 3.65; P < .0001). The 7/8-PB + serotherapy group trended toward a higher risk of cGVHD (HR, 1.58; 95% CI, 1.12 to 2.23; P = .009) than the UCB group (Data Supplement).
Relapse.
In multivariable analysis, we found no difference in the risk of relapse among patients with haploidentical, UCB-RIC, and all 7/8-PB (MAC or RIC, with or without serotherapy) grafts, whereas the patients who received UCB-MAC or 7/8-BM-MAC grafts had significantly lower risk than those who received haploidentical grafts (Fig 3A; Table 3). Patients with high or very high DRI and those with KPS below 90 had a significantly higher risk of relapse. In the direct pairwise comparisons of MAC groups, no significant differences were noted. Among RIC groups, the 7/8-PB without serotherapy group (HR, 0.52; 95% CI, 0.32 to 0.74; P = .0008) had a trend toward lower risk of relapse than the UCB group (Data Supplement).
FIG 3.
Adjusted (A) relapse, (B) disease-free survival, and (C) overall survival after receiving an HCT with haploidentical, umbilical cord blood (UCB) with myeloablative conditioning (MAC), UCB with reduced intensity conditioning (RIC), 7/8 bone marrow (BM) with MAC, 7/8 peripheral blood (PB) with MAC + serotherapy, 7/8 PB with MAC, 7/8 PB with RIC + serotherapy, or 7/8 PB with RIC grafts. The adjusted factors are the variables listed in the multivariable regression analysis (Table 3).
DFS.
In multivariable analysis, the UCB-RIC group had significantly inferior DFS, whereas no differences were noted in other groups compared with the haploidentical group (Fig 3B; Table 3). Other factors associated with worse DFS included age older than 50 years, high or very high DRI, and KPS below 90. In the direct pairwise comparisons (excluding the haploidentical group), we found no differences in DFS among groups (Data Supplement).
OS.
In multivariable analysis, the patients who received haploidentical grafts had superior OS compared with all other groups (Fig 3C; Table 3). Age older than 50 years, high or very high DRI, HCT-CI of 3 or greater, and KPS of 90 or lower were significantly associated with poor OS. In the direct pairwise comparisons (excluding the haploidentical group), we found no differences in OS among groups (Data Supplement).
Engraftment
The median time to neutrophil engraftment was 17 days (range, 16 to 18 days) in the haploidentical group, which was comparable to that of the RIC-UCB group (16 days; range, 14 to 18 days) and was marginally longer than that for the 7/8-RIC PB groups (14 to 15 days). Among those who received MAC, it was 24 days (range, 23 to 25 days) in the UCB group, 20 days (range, 18 to 20 days) in the 7/8-BM group, and 13 days in both 7/8-PB groups (Data Supplement). The Data Supplement contains descriptions of events contributing to GRFS and CRFS and lists causes of death.
DISCUSSION
We observed that the group of patients who received haploidentical HCT had the best long-term survival without GRFS or CRFS events compared with all other 7/8-PB (MAC or RIC with or without serotherapy), UCB (MAC or RIC), or 7/8-BM-MAC groups. However, to facilitate the choice of an alternative donor, it is essential to interpret the outcomes in the context of conditioning intensity, a factor that is generally determined by a treating physician on the basis of an individual patient’s status and cannot be controlled in retrospective analyses. We analyzed patients who received haploidentical grafts as a single group because a large majority (123 of 159) received RIC, and about two thirds were age 50 years or older, a group in which MAC and RIC most often yield comparable outcomes (NRM, relapse, DFS, and OS).28
Within the RIC group, patients who received haploidentical grafts had the best GRFS, CRFS, and OS, which supports the use of haploidentical donors over others for patients undergoing RIC-HCT. In rare circumstances in which a haploidentical donor is not available, our data support the use of either 7/8-PB (RIC with or without serotherapy) or UCB-RIC. The use of UCB was associated with a somewhat higher risk of relapse but a somewhat lower risk of aGVHD and a significantly lower risk of cGVHD than 7/8-PB without serotherapy and a slightly lower risk of cGVHD than 7/8-PB + serotherapy, which led to similar GFRS, CRFS, DFS, and OS.
Among the MAC group, we found no differences in GRFS, CRFS, DFS, or OS among the patients who received UCB, 7/8-BM, or 7/8-PB + serotherapy grafts. However, the 7/8-PB without serotherapy group had significantly inferior GRFS compared with the 7/8-BM and 7/8-PB with serotherapy groups, significantly inferior CRFS compared with the 7/8-PB with serotherapy group, a trend toward inferior CRFS compared with the 7/8-BM and UCB groups, significantly higher risk of aGVHD compared with the 7/8-PB with serotherapy group, and significantly higher risk of cGVHD compared with the UCB, 7/8-BM, and 7/8-PB without serotherapy groups. Therefore, these data support the use of UCB, 7/8-BM, or 7/8-PB + serotherapy grafts for patients undergoing MAC-HCT. Conclusions about haploidentical MAC-HCT cannot be made from our analysis.
A randomized trial comparing MAC with RIC in an HLA-matched setting showed lower risk of relapse and improved OS with MAC.29 Because there are no randomized studies on this subject, it is unclear whether the same holds true with mismatched donors, especially given the potentially higher graft-versus-tumor effect in this setting. With the haploidentical group, a CIBMTR study30 showed no difference in relapse but higher NRM and lower OS with MAC than RIC, whereas a study by the European Society for Blood and Marrow Transplantation (EBMT)31 showed higher risk of relapse and poor DFS with RIC. In contrast, conditioning intensity was not associated with survival in some studies with other mismatched URDs.32-34 In our study, MAC UCB and BM grafts, but not PB grafts, were associated with significantly lower risk of relapse than haploidentical grafts (predominantly RIC). In the direct pairwise comparisons of patients who received MAC versus RIC (except in the haploidentical group), we noted a higher risk of relapse with some, but not all RIC groups (Data Supplement), but no difference in GRFS, CRFS, DFS, or OS in any RIC versus MAC group.
Several studies independently compared one alternative donor with another. Outcomes with UCB grafts have been contrasted with those of one-antigen mismatched7,8,11 BM7,35,36 or PB7,36 grafts from URDs and to haploidentical HCT.4,6,9 Haploidentical HCT has been compared with one-antigen mismatched HCT.37 No clear conclusion about the superiority of an alternative donor choice has emerged from these reports. Our study adds to these data by providing direct contemporaneous comparison of multiple alternative donors and graft sources. Moreover, it offers a global perspective of outcomes as assessed by the composite end points GRFS and CRFS in addition to the individual end points.
These comparisons incorporated differences in graft, donor, and GVHD prophylaxis but could not dissect the specific elements driving the outcomes. All haploidentical HCTs included PTCy for GVHD prophylaxis, which was not used in any other groups, although PTCy is now being increasingly used in one-antigen mismatched HCTs.38,39 Most of the haploidentical HCTs (82%) were performed in recent years (2012 to 2014) when few MAC-BM or MAC-PB HCTs were performed. Moreover, haploidentical HCTs included mostly BM and RIC and offered only a limited sample size, which precluded exploration of outcomes in any subsets. A CIBMTR study compared BM (n = 481) to PB (n = 190) grafts for haploidentical HCTs with PTCy in patients with myeloid or lymphoid malignancies. BM grafts were associated with a higher risk of relapse but a significantly lower risk of aGVHD and cGVHD than PB grafts, which translated into superior GRFS with BM grafts.30 In contrast, an EBMT study showed a similar risk of cGVHD, relapse, NRM, DFS, or OS after BM (n = 260) or PB (n = 191) grafts but a significantly higher risk of grade 2 to 4 and grade 3 to 4 aGVHD with PB grafts in patients with ALL or AML who underwent haploidentical HCTs with PTCy.31 In addition, because of the limitation of using registry data, our study could not incorporate allele-level matching for most UCB grafts, which may be of added importance.40-43 Furthermore, we could not directly test the statistical associations of covariates with outcomes because that was not the primary intended aim of the study. Finally, although our analyses were adjusted for any center effect, the possibility of selection bias (in which some centers prefer a particular graft or donor source over another) remains and should be considered while interpreting our results.
This large analysis of HLA-mismatched donors addresses some questions and highlights crucial questions about the choices inherent in selecting an alternative donor. Both GRFS and CRFS are compromised after haploidentical HCT because of the risks of disease relapse, whereas novel strategies to limit graft failure may limit early mortality after UCB or 7/8-BM HCT.
We conclude that, compared with other groups, patients who underwent haploidentical HCT had the best long-term OS with limited morbidity as measured by GRFS and CRFS. With MAC, patients who received 7/8-PB without serotherapy grafts had a higher risk of cGVHD and inferior GRFS and CRFS than patients who received other types of grafts. These data support the use of UCB, 7/8-BM, or 7/8-PB + serotherapy grafts for MAC-HCT. Within the RIC group, patients who received haploidentical grafts had the best GRFS, CRFS, and OS, thus supporting the use of haploidentical BM grafts with PTCy for RIC-HCT. Ongoing comparisons and innovative improvements in care using UCB or haploidentical HCT may further inform graft, donor, and conditioning and treatment choices for those without HLA-matched donors.
SUPPORT
Supported primarily by Public Health Service Grant/Cooperative Agreement No. 5U24CA076518 from the National Cancer Institute, National Heart, Lung, and Blood Institute, and National Institute of Allergy and Infectious Diseases; Grant/Cooperative Agreement No. 1U24HL138660 from the National Heart, Lung, and Blood Institute and National Cancer Institute; Contract No. HHSH250201700006C from the Health Resources and Services Administration, Department of Health and Human Services; Grants No. N00014‐17‐1‐2388, N00014‐17‐1‐2850, and N00014‐18‐1‐2045 from the Office of Naval Research; and funding from Adaptive Biotechnologies; *Amgen; anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Atara Biotherapeutics; Be the Match Foundation; *bluebird bio; *Bristol-Myers Squibb Oncology; *Celgene; *Chimerix; *CytoSen Therapeutics; Fred Hutchinson Cancer Research Center; Gamida Cell; Gilead Sciences; HistoGenetics; Immucor; *Incyte; Janssen Scientific Affairs; *Jazz Pharmaceuticals; Karius; Karyopharm Therapeutics; *Kite Pharma; Medac; *Mediware; Medical College of Wisconsin; *Merck; *Mesoblast; MesoScale Diagnostics; Millennium Pharmaceuticals, Takeda Oncology; *Miltenyi Biotec; Mundipharma EDO; National Marrow Donor Program; Novartis Pharmaceuticals; Patient-Centered Outcomes Research Institute; *Pfizer; *Pharmacyclics; Predicted Indirectly ReCognizable HLA Epitopes; *Sanofi Genzyme; *Seattle Genetics; Shire; Spectrum Pharmaceuticals; St. Baldrick’s Foundation; Swedish Orphan Biovitrum; *Takeda Oncology; and University of Minnesota, all for the Center for International Blood and Marrow Transplant Research; [*corporate members].
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, the Health Resources and Services Administration, or any other agency of the US government.
AUTHOR CONTRIBUTIONS
Conception and design: Rohtesh S. Mehta, Shernan G. Holtan, Stephen R. Spellman, Joseph Pidala, Hisham Abdel-Azim, Ibrahim Ahmed, Mahmoud Aljurf, Joseph H. Antin, Medhat Askar, Vijaya Raj Bhatt, Lynette Chee, Saurabh Chhabra, Zachariah DeFilipp, James Gajewski, Usama Gergis, Peiman Hematti, Gerhard C. Hildebrandt, Navneet Majhail, David I. Marks, Richard F. Olsson, Miguel Angel Diaz, Olle Ringden, Ayman Saad, Bipin N. Savani, Hélène Schoemans, Takanori Teshima, Baldeep Wirk, Jean A. Yared, Jean Yves-Cahn, Daniel J. Weisdorf
Administrative support: Takanori Teshima
Provision of study materials or patients: Hisham Abdel-Azim, Rodrigo Martino, David I. Marks, Olle Ringden, Jean Yves-Cahn
Collection and assembly of data: Rohtesh S. Mehta, Michael T. Hemmer, Stephen R. Spellman, Mukta Arora, Hisham Abdel-Azim, Mahmoud Aljurf, Joseph H. Antin, Medhat Askar, Attaphol Pawarode, Olle Ringden, Takanori Teshima, Daniel J. Weisdorf
Data analysis and interpretation: Rohtesh S. Mehta, Shernan G. Holtan, Tao Wang, Michael T. Hemmer, Mukta Arora, Daniel R. Couriel, Amin M. Alousi, Joseph Pidala, Hisham Abdel-Azim, Vaibhav Agrawal, A. Samer Al-Homsi, Mahmoud Aljurf, Joseph H. Antin, Medhat Askar, Jeffery J. Auletta, Vijaya Raj Bhatt, Lynette Chee, Saurabh Chhabra, Andrew Daly, Zachariah DeFilipp, James Gajewski, Robert Peter Gale, Peiman Hematti, Gerhard C. Hildebrandt, William J. Hogan, Yoshihiro Inamoto, Rodrigo Martino, Navneet Majhail, Taiga Nishihori, Richard F. Olsson, Miguel Angel Diaz, Tim Prestidge, Hemalatha Rangarajan, Olle Ringden, Ayman Saad, Bipin N. Savani, Hélène Schoemans, Sachiko Seo, Kirk R. Schultz, Melhem Solh, Thomas Spitzer, Jan Storek, Takanori Teshima, Leo Verdonck, Baldeep Wirk, Jean A. Yared, Jean Yves-Cahn, Daniel J. Weisdorf
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Composite GRFS and CRFS Outcomes After Adult Alternative Donor HCT
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Shernan G. Holtan
Consulting or Advisory Role: Incyte, Bristol-Myers Squibb, CSL Behring (Inst), Janssen (Inst)
Mukta Arora
Consulting or Advisory Role: Fate Therapeutics
Research Funding: Syndax Pharmaceuticals (Inst), Kadmon (Inst)
Daniel R. Couriel
Honoraria: Merck
Consulting or Advisory Role: Merck, Seattle Genetics, Fresenius, Incyte
Speakers' Bureau: Seattle Genetics
Research Funding: Mallinckrodt
Travel, Accommodations, Expenses: Merck
Other Relationship: Seattle Genetics
Amin M. Alousi
Honoraria: GENERON, Genentech, Kadmon
A. Samer Al-Homsi
Honoraria: Celyad
Consulting or Advisory Role: Celyad
Research Funding: Celyad
Travel, Accommodations, Expenses: Celyad
Uncompensated Relationships: Celyad
Joseph H. Antin
Consulting or Advisory Role: Merck, CSL Behring
Medhat Askar
Consulting or Advisory Role: Immucor, CareDx
Jeffery J. Auletta
Consulting or Advisory Role: MORE Health
Vijaya Raj Bhatt
Consulting or Advisory Role: Pfizer, CSL Behring, AbbVie, Incyte, Partner Therapeutics, Agios Pharmaceuticals
Research Funding: Incyte, Tolero Pharmaceuticals, National Marrow Donor Program
Lynette Chee
Travel, Accommodations, Expenses: Amgen
Saurabh Chhabra
Honoraria: Cardinal Health
Consulting or Advisory Role: Takeda
Zachariah DeFilipp
Research Funding: Incyte
James Gajewski
Consulting or Advisory Role: Avalon-GloboCare
Travel, Accommodations, Expenses: Avalon-GloboCare
Robert Peter Gale
Stock and Other Ownership Interests: Celgene
Research Funding: National Institutes of Health Research
Travel, Accommodations, Expenses: Celgene
Usama Gergis
Stock and Other Ownership Interests: Novavax
Honoraria: Incyte, Jazz Pharmaceuticals, Merck, Astellas Pharma
Consulting or Advisory Role: Jazz Pharmaceuticals
Speakers' Bureau: Merck, Astellas Pharma, Incyte, Jazz Pharmaceuticals
Travel, Accommodations, Expenses: Incyte, Jazz Pharmaceuticals, Merck, Astellas Pharma
Other Relationship: Noble Insights
Peiman Hematti
Other Relationship: Cellular Logistics (I)
Gerhard C. Hildebrandt
Stock and Other Ownership Interests: Sangamo Biosciences, AXIM Biotechnologies, Juno Therapeutics, Kite Pharma, Novartis, INSYS Therapeutics, AbbVie, GW Pharmaceuticals, Cardinal Health, Immunomedics, Endocyte, Clovis Oncology, Cellectis, Aetna, CVS Health, Celgene, Bluebird Bio, Bristol-Myers Squibb/Medarex, CRISPR Therapeutics, IDEXX Laboratories, Johnson & Johnson, Pfizer, Procter & Gamble, Vertex Pharmaceuticals, Bayer, Charlotte’s Web Holdings, Aimmune Therapeutics, Medical Properties Trust, CareTrust REIT, ANGI Homeservices, Bayer AG
Consulting or Advisory Role: Pfizer, Kite Pharma, Incyte, Jazz Pharmaceuticals
Research Funding: Takeda, Jazz Pharmaceuticals, Pharmacyclics
Travel, Accommodations, Expenses: Kite Pharma, Incyte, Pfizer, Falk Foundation, Jazz Pharmaceuticals, Astellas Pharma, Incyte
Yoshihiro Inamoto
Honoraria: Kyowa Hakko Kirin, Astellas Pharma, Sumitomo Dainippon Pharma, Novartis
Navneet Majhail
Honoraria: Mallinckrodt
Consulting or Advisory Role: Anthem, Nkarta, Atara Biotherapeutics, Incyte
Travel, Accommodations, Expenses: Atara Biotherapeutics, Incyte, Nkarta, Mallinckrodt
David I. Marks
Consulting or Advisory Role: Amgen, Pfizer, Novartis
Taiga Nishihori
Research Funding: Novartis (Inst), Karyopharm (Inst), Celgene (Inst)
Richard F. Olsson
Employment: AstraZeneca
Attaphol Pawarode
Research Funding: Amgen, Merck
Ayman Saad
Consulting or Advisory Role: Actinium Pharmaceuticals
Research Funding: Fate Therapeutics, Amgen, Kadmon, Orca Health
Bipin N. Savani
Honoraria: Jazz Pharmaceuticals, Therakos, Janssen Pharmaceuticals
Hélène Schoemans
Honoraria: Novartis (Inst), Incyte (Inst), Jazz Pharmaceuticals (Inst), Takeda (Inst)
Consulting or Advisory Role: Incyte (Inst), Janssen (Inst)
Travel, Accommodations, Expenses: Incyte (Inst), AbbVie (Inst), Celgene (Inst)
Kirk R. Schultz
Honoraria: Shire
Consulting or Advisory Role: Meso Scale Discovery, Juno Therapeutics
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Melham Solh
Consulting or Advisory Role: Celgene/Amgen, Daiichi Sankyo
Speakers' Bureau: Amgen, AbbVie, Seattle Genetics
Thomas Spitzer
Honoraria: Janssen
Consulting or Advisory Role: Bluebird Bio, Jazz Pharmaceuticals, ITB-Med
Takanori Teshima
Honoraria: Merck Sharp & Dohme, Kyowa Hakko Kirin, Takeda Pharmaceutical, Pfizer Japan, Bristol-Myers Squibb Japan
Consulting or Advisory Role: Takeda Pharmaceutical, Merck Sharp & Dohme, Sanofi, Novartis Pharma
Research Funding: Kyowa Hakko Kirin, Novartis Pharma, Chugai Pharmaceutical, Astellas Pharma, Teijin Pharma, Fuji Pharma, Nippon Shinyaku
Jean A. Yared
Consulting or Advisory Role: Jazz Pharmaceuticals
Research Funding: Gilead Sciences (Inst)
Jean-Yves Cahn
Travel, Accommodations, Expenses: Genentech, Amgen
Daniel J. Weisdorf
Consulting or Advisory Role: Incyte, Fate Therapeutics, Pharmacyclics
Research Funding: Incyte
No other potential conflicts of interest were reported.
REFERENCES
- 1.D’Souza A, Fretham C. Current Uses and Outcomes of Hematopoietic Cell Transplantation (HCT): CIBMTR Summary Slides. 2019 https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx
- 2.Passweg JR, Baldomero H, Bader P, et al. Hematopoietic stem cell transplantation in Europe 2014: more than 40,000 transplants annually. Bone Marrow Transplant. 2016;51:786–792. doi: 10.1038/bmt.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371:339–348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocha V, Aversa F, Labopin M, et al. Outcomes of unrelated cord blood and haploidentical stem cell transplantation in adults with acute leukaemia. Blood. 2005;106 doi: 10.1038/leu.2015.98. (abstr 301) [DOI] [PubMed] [Google Scholar]
- 5.Atsuta Y, Suzuki R, Nagamura-Inoue T, et al. Disease-specific analyses of unrelated cord blood transplantation compared with unrelated bone marrow transplantation in adult patients with acute leukemia. Blood. 2009;113:1631–1638. doi: 10.1182/blood-2008-03-147041. [DOI] [PubMed] [Google Scholar]
- 6.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: Results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: A retrospective analysis. Lancet Oncol. 2010;11:653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 9.Ruggeri A, Labopin M, Sanz G, et al. Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29:1891–1900. doi: 10.1038/leu.2015.98. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi S, Ooi J, Tomonari A, et al. Comparative single-institute analysis of cord blood transplantation from unrelated donors with bone marrow or peripheral blood stem-cell transplants from related donors in adult patients with hematologic malignancies after myeloablative conditioning regimen. Blood. 2007;109:1322–1330. doi: 10.1182/blood-2006-04-020172. [DOI] [PubMed] [Google Scholar]
- 11.Weisdorf D, Eapen M, Ruggeri A, et al. Alternative donor transplantation for older patients with acute myeloid leukemia in first complete remission: A center for International Blood and Marrow Transplant Research-Eurocord analysis. Biol Blood Marrow Transplant. 2014;20:816–822. doi: 10.1016/j.bbmt.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasquini MC, Logan B, Jones RJ, et al. Blood and Marrow Transplant Clinical Trials Network report on the development of novel endpoints and selection of promising approaches for graft-versus-host disease prevention trials. Biol Blood Marrow Transplant. 2018;24:1274–1280. doi: 10.1016/j.bbmt.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–1338. doi: 10.1182/blood-2014-10-609032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta RS, Peffault de Latour R, DeFor TE, et al. Improved graft-versus-host disease-free, relapse-free survival associated with bone marrow as the stem cell source in adults. Haematologica. 2016;101:764–772. doi: 10.3324/haematol.2015.138990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pidala J, Lee SJ, Ahn KW, et al. Nonpermissive HLA-DPB1 mismatch increases mortality after myeloablative unrelated allogeneic hematopoietic cell transplantation. Blood. 2014;124:2596–2606. doi: 10.1182/blood-2014-05-576041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 17.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: Working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 19.Lee SJ, Klein JP, Barrett AJ, et al. Severity of chronic graft-versus-host disease: Association with treatment-related mortality and relapse. Blood. 2002;100:406–414. doi: 10.1182/blood.v100.2.406. [DOI] [PubMed] [Google Scholar]
- 20.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man: A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 21.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401.e1. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schemper M, Wakounig S, Heinze G. The estimation of average hazard ratios by weighted Cox regression. Stat Med. 2009;28:2473–2489. doi: 10.1002/sim.3623. [DOI] [PubMed] [Google Scholar]
- 23.Dunkler D, Ploner M, Schemper M, et al. Weighted Cox regression using the R package coxphw J Stat Softw 841–26.2018. 30450020 [Google Scholar]
- 24.Zhang X, Loberiza FR, Klein JP, et al. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88:95–101. doi: 10.1016/j.cmpb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Zhang MJ. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Methods Programs Biomed. 2011;101:87–93. doi: 10.1016/j.cmpb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–3671. doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solomon SR, Martin AS, Shah NN, et al. T-replete haploidentical cell transplantation using post-transplant cyclophosphamide for acute myeloid leukemia, acute lymphoblastic leukemia and myelodysplastic syndrome: Effect of transplant conditioning regimen intensity on outcomes. Blood. 2018;132 (abstr 1015) [Google Scholar]
- 29.Scott BL, Pasquini MC, Logan BR, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35:1154–1161. doi: 10.1200/JCO.2016.70.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bashey A, Zhang MJ, McCurdy SR, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell-replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2017;35:3002–3009. doi: 10.1200/JCO.2017.72.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruggeri A, Labopin M, Bacigalupo A, et al. Bone marrow versus mobilized peripheral blood stem cells in haploidentical transplants using posttransplantation cyclophosphamide. Cancer. 2018;124:1428–1437. doi: 10.1002/cncr.31228. [DOI] [PubMed] [Google Scholar]
- 32.Mediwake H, Curley C, Butler J, et al. Mismatched unrelated donor allogeneic stem cell transplant for high risk haematological malignancy: A single centre experience. Blood Cancer J. 2017;7:655. doi: 10.1038/s41408-017-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fürst D, Niederwieser D, Bunjes D, et al. Increased age-associated mortality risk in HLA-mismatched hematopoietic stem cell transplantation. Haematologica. 2017;102:796–803. doi: 10.3324/haematol.2016.151340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hauzenberger D, Schaffer M, Ringdén O, et al. Outcome of haematopoietic stem cell transplantation in patients transplanted with matched unrelated donors vs allele-mismatched donors: A single centre study. Tissue Antigens. 2008;72:549–558. doi: 10.1111/j.1399-0039.2008.01148.x. [DOI] [PubMed] [Google Scholar]
- 35.Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 36.Milano F, Gooley T, Wood B, et al. Cord-blood transplantation in patients with minimal residual disease. N Engl J Med. 2016;375:944–953. doi: 10.1056/NEJMoa1602074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaballa S, Ge I, El Fakih R, et al. Results of a 2-arm, phase 2 clinical trial using post-transplantation cyclophosphamide for the prevention of graft-versus-host disease in haploidentical donor and mismatched unrelated donor hematopoietic stem cell transplantation. Cancer. 2016;122:3316–3326. doi: 10.1002/cncr.30180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehta RS, Saliba RM, Chen J, et al. Post-transplantation cyclophosphamide versus conventional graft-versus-host disease prophylaxis in mismatched unrelated donor haematopoietic cell transplantation. Br J Haematol. 2016;173:444–455. doi: 10.1111/bjh.13977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jorge AS, Suárez-Lledó M, Pereira A, et al. Single antigen-mismatched unrelated hematopoietic stem cell transplantation using high-dose post-transplantation cyclophosphamide is a suitable alternative for patients lacking HLA-matched donors. Biol Blood Marrow Transplant. 2018;24:1196–1202. doi: 10.1016/j.bbmt.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 40.Brunstein CG, Cutler CS, DeFor TE, et al. Matching at human leukocyte antigen-C improved the outcomes after double umbilical cord blood transplantation for recipients of two to four of six human leukocyte antigen-matched grafts. Biol Blood Marrow Transplant. 2017;23:126–133. doi: 10.1016/j.bbmt.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oran B, Cao K, Saliba RM, et al. Better allele-level matching improves transplant-related mortality after double cord blood transplantation. Haematologica. 2015;100:1361–1370. doi: 10.3324/haematol.2015.127787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eapen M, Klein JP, Ruggeri A, et al. Impact of allele-level HLA matching on outcomes after myeloablative single unit umbilical cord blood transplantation for hematologic malignancy. Blood. 2014;123:133–140. doi: 10.1182/blood-2013-05-506253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eapen M, Klein JP, Sanz GF, et al. Effect of donor-recipient HLA matching at HLA A, B, C, and DRB1 on outcomes after umbilical-cord blood transplantation for leukaemia and myelodysplastic syndrome: A retrospective analysis. Lancet Oncol. 2011;12:1214–1221. doi: 10.1016/S1470-2045(11)70260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]