Abstract
PURPOSE
To investigate longitudinal associations between physical activity (PA) and neurocognitive problems in adult survivors of childhood cancer.
METHODS
A total of 12,123 5-year survivors diagnosed between 1970 and 1999 (median [range] age at diagnosis, 7 [0-21] years, time since diagnosis at baseline, 16 [6-30] years) and 720 siblings self-reported PA and neurocognitive problems. PA was collected at baseline, and PA and neurocognitive data were obtained 7 (1-12) years and 12 (9-14) years later. PA consistency was defined as any combination of ≥ 75 minutes of vigorous or 150 minutes of moderate activity per week on all surveys. Multiple linear regressions, conducted separately for CNS and non-CNS survivors, identified associations between PA consistency and neurocognitive outcomes (expected mean, 50; standard deviation [SD], 10). Mediating effects of body mass index (BMI) and chronic health conditions (CHCs) were evaluated.
RESULTS
Survivors were less likely than siblings to report consistent PA (28.1% v 33.6%) and more likely to report problems in Task Efficiency (T-scores mean ± SD: siblings, 50.0 ± 0.4; CNS, 61.4 ± 0.4; non-CNS, 53.3 ± 0.3), Emotion Regulation (siblings, 51.4 ± 0.4; CNS, 54.5 ± 0.3; non-CNS 53.4 ± 0.2), and Memory (siblings, 50.8 ± 0.4; CNS, 58.9 ± 0.4; non-CNS, 53.5 ± 0.2; all P < .001). Survivors of CNS cancers (52.8 ± 0.3) also reported poorer Organization than siblings (49.9 ± 0.4; P < .001). After adjusting for age at diagnosis, age at questionnaire, emotional distress, and cancer treatment exposures, consistent PA was associated with fewer neurocognitive problems compared with consistent inactivity for both CNS and non-CNS groups (T-score differences ranging from −7.9 to −2.2) and larger neurocognitive improvements over time (−6.0 to −2.5), all P ≤ .01. BMI and severe CHCs partially mediated the PA-neurocognitive associations, but the mediation effects were small (change in β ≤ 0.4).
CONCLUSION
Adult survivors of childhood cancer who report more consistent PA have fewer neurocognitive problems and larger improvements in these concerns many years after treatment.
INTRODUCTION
Advances in diagnosis and treatment of pediatric cancers have improved survival, leading to a growing population of adult survivors.1 However, cancers and cancer treatments are associated with adverse late effects, including chronic health conditions (CHCs) and neurocognitive deficits.2-5
At least one-third of childhood cancer survivors experience neurocognitive dysfunction that may persist years after completion of therapy.6-13 Survivors may demonstrate deficits in attention, working memory, executive function, processing speed, and/or visuomotor integration and report neurocognitive problems in everyday life.13-20 Impairment severity is associated with specific treatment exposures and can be exacerbated by the development of CHCs.3,21,22
Childhood cancer survivors report lower engagement in physical activity (PA) than healthy controls,23 which is associated with poorer psychosocial well-being, greater somatic symptoms, and higher risk for secondary CHCs.24-27 Notably, PA has been associated with hippocampal neurogenesis in rodents and with neuroimaging indices of brain health and better neurocognitive function in community and clinical populations, including cancer survivors.28,29
In survivors of adult-onset malignancies, higher PA was associated with better neurocognitive outcomes in observational and intervention studies.29 Adult survivors of childhood cancer reporting higher levels of leisure-time PA endorse more positive ratings of neurocognitive function, social function, and health-related quality of life.30 Similarly, a subgroup of survivors from the Childhood Cancer Survivor Study (CCSS) who reported vigorous exercise at baseline reported less psychological burden a median of 7.8 years later, although change in neurocognitive outcome over time was not examined.31 Increased hippocampal volume and white matter fractional anisotropy, as well as improved reaction time, have also been observed in children treated with radiation for brain tumors after a 12-week aerobic exercise intervention.32
The current study examined whether PA is associated with neurocognitive problems and improvements in these problems over time in adult survivors of childhood cancer, using cross-sectional and longitudinal methods, with consideration of PA quantity, intensity, and consistency. Mediating effects of body mass index (BMI) and CHCs were also examined.
METHODS
Participants
This study was conducted within the CCSS, a retrospective cohort study with longitudinal follow-up of childhood cancer survivors treated at 31 institutions in North America. Methodology and design have been previously described33; details are available at the CCSS Web site.34 Methods were approved by a central institutional review board, and informed consent was received from all participants.
Survivors were diagnosed with CNS malignancy, leukemia, Hodgkin lymphoma, non-Hodgkin lymphoma, kidney cancer, neuroblastoma, soft tissue sarcoma, or malignant bone tumor between 1970 and 1999, and were alive at least 5 years after diagnosis. A randomly selected subset identified a sibling closest in age to the survivor, who was asked to participate. Cancer diagnosis and treatment information were obtained from treating institutions through medical record abstraction with a unified protocol. Surveys examining demographic, medical, PA, and psychosocial information35 were sent to participants at recruitment (ie, baseline) and again a median of 7 years later (range, 1-12 years). A subset of survivors treated between 1970 and 1986 completed a second follow-up a median of 12 years later (range, 9-14 years). Neurocognitive data were collected at the two follow-up time points only.
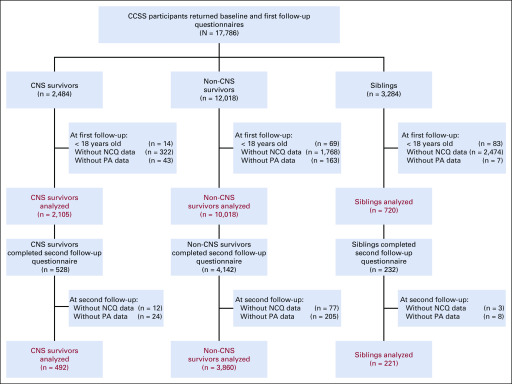
CCSS survivors and siblings were eligible for inclusion in this study if they were ≥ 18 years of age at the time of survey and if they returned both neurocognitive and PA data. Of the 17,786 participants who returned baseline and first follow-up surveys, 12,123 survivors and 720 siblings completed data required for inclusion. A total of 4,352 survivors and 221 siblings completed a second follow-up (Fig 1; comparisons to excluded participants are summarized in the Data Supplement, online only).
FIG 1.
Consort diagram: flow diagram of participants selected from the Childhood Cancer Survivor Study (CCSS). NCQ, Neurocognitive Questionnaire; PA, physical activity.
Measures
Neurocognitive problems and emotional distress.
Participants completed the CCSS-Neurocognitive Questionnaire (NCQ),36,37 a self-report measure of neurocognitive problems in Task Efficiency, Emotional Regulation, Organization, and Memory, and the Brief Symptom Inventory-18 (BSI-18),38 used to adjust for emotional distress when examining associations between PA and neurocognitive problems. The BSI-18 examines symptoms of depression, anxiety, and somatization, and provides a Global Severity Index (GSI).
The sums of item endorsements by participants on each scale were converted to T-scores (mean 50, SD 10), using sibling group data at the same time point; higher scores indicate greater neurocognitive problems or emotional distress. T-scores ≥ 62.82 (ie, ≥ 90th percentile of the sibling distribution) were classified as impaired. For the BSI, these classifications were based on the GSI or any two of the subscales. Longitudinal change of NCQ was calculated by subtracting the NCQ T-score at the first follow-up from the T-score at the second follow-up; negative change scores reflect improvement in neurocognitive problems.
Physical activity.
Participants responded to six questions from the Behavioral Risk Factor Surveillance Survey about PA39 and one question about participation in PA during the past month, as previously described.27 PA was examined three ways: (1) quantity ([#days per week × minutes per day]/60); (2) intensity, based on metabolic equivalents (METs; [#days per week vigorous activity × minutes per day vigorous × 6] + [#days per week moderate activity × minutes per day moderate × 3]/60)40; and (3) consistency (in meeting Centers for Disease Control and Prevention [CDC] criteria of 75 minutes of vigorous activity, 150 minutes of moderate activity, or an equivalent combination of both per week at baseline and follow-up). PA consistency was categorized as consistently active, inconsistently active, and consistently inactive during this period. PA intensity units (MET-h/wk) reflect the participant’s exertion per week.
BMI.
BMI was calculated at follow-up by dividing self‐reported weight (kilograms) by height (meters squared) and categorized as normal (BMI < 25), overweight (BMI 25-30), or obese (BMI ≥ 30).41 For survivors younger than age 20 years, overweight was defined as BMI in the 85-95th percentile, and obesity was defined as BMI ≥ 95th percentile.42
Chronic health conditions.
CHCs were self-reported in the CCSS survey4 and graded according to the Common Terminology Criteria for Adverse Events v 5.0.43 Participants were classified as having a grade 3 or 4 condition with potential association to neurocognitive problems or engagement in PA, with onset before follow-up, including: cardiovascular, respiratory, musculoskeletal, neurologic, hematologic, infectious/immunologic, renal (dialysis), endocrine, vision, and/or secondary malignancy.
Data Analyses
Demographic characteristics were compared between groups using the χ2 test; bootstrapping of families was used to compare survivors and siblings to account for within-family correlations. When the expected count of a cell under the null hypothesis was < 5, Fisher’s exact test was used.
NCQ T-scores and risk of impairment for Task Efficiency, Emotional Regulation, Organization, and Memory were compared between survivor groups and siblings using generalized estimating equations (GEE) with the identity link and Gaussian-distribution variance form (GEE-linear) and GEE with the logit link and binomial-distribution variance form (GEE-logistic),44 respectively, adjusting for sex and age at NCQ. Because CNS survivors typically demonstrate more cognitive deficits and physical limitations relative to non-CNS survivors, CNS and non-CNS survivors were analyzed separately.
NCQ associations with PA consistency were examined using GEE-linear, comparing consistently active and inconsistently active participants with consistently inactive participants. An interaction term was included to compare PA-NCQ associations between survivor and sibling groups. These analyses were repeated with PA intensity and quantity variables. GEE-logistic was used to examine associations of PA consistency with neurocognitive impairment at follow-up. GEE-linear was also used to examine associations of PA consistency with change in NCQ scores across follow-ups. For analyses of change in NCQ scores, consistency in meeting PA criteria was defined by examining all three surveys (baseline and both follow-ups).
Covariates included in these analyses had potential associations with NCQ and/or PA variables: age at survey, age at diagnosis, sex, cranial radiation (yes/no), clinically significant emotional distress (yes/no), and intravenous (IV) and/or intrathecal methotrexate for non-CNS survivors (yes/no). Smoking status (current/previous/never) was considered a potential covariate but was not significantly associated with NCQ and PA variables and was excluded from final analyses. Analyses examining change in NCQ scores were adjusted for initial NCQ scores and time between questionnaires.
A mediation analysis was conducted to examine whether BMI and existing CHCs mediated associations of PA consistency and NCQ domains. Associations among these variables and attenuation of the PA-NCQ association with and without these potential mediators were assessed, including covariates described above.
To account for multiple comparisons, P values ≤ .01 were deemed statistically significant for all analyses. Sampling weights were used to account for undersampling of survivors of acute lymphoblastic leukemia across all analyses; weights of 1.21 were applied for participants age 11-20 years at diagnosis and 3.63 for age 1-10 years at diagnosis.
RESULTS
Study Population Characteristics
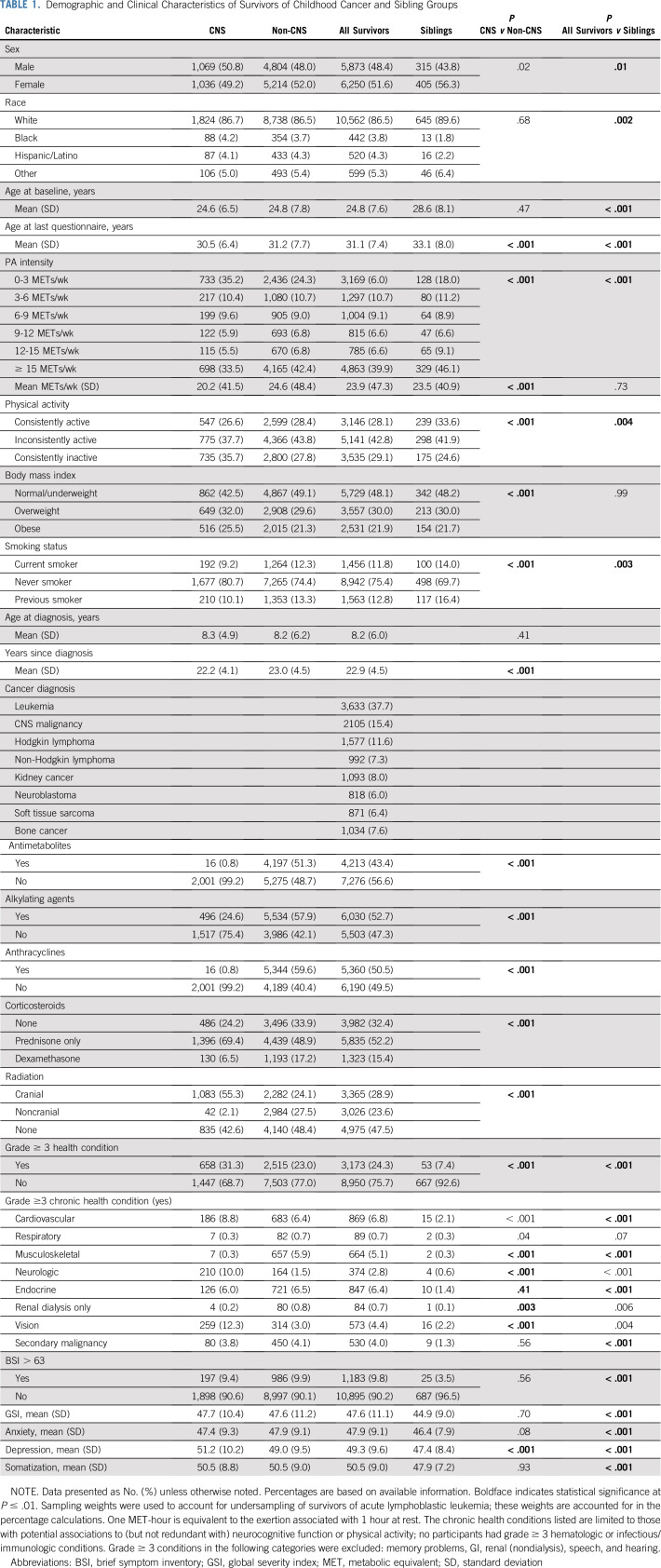
Demographic characteristics are shown in Table 1. Diagnoses included leukemia (37.8%), CNS malignancy (15.5%), Hodgkin lymphoma (11.5%), non-Hodgkin lymphoma (7.3%), kidney cancer (8.0%), neuroblastoma (6.0%), soft tissue sarcoma (6.4%), and bone cancer (7.6%). CNS cancer survivors were less likely than non-CNS cancer survivors to consistently meet CDC activity guidelines and were more likely to self-report high BMI and CHCs and to be nonsmokers (all P < .001). Siblings were more likely than survivors to meet CDC activity guidelines consistently over time (33.6% v 28.1%; P = .003) and to be smokers (P = .003) and less likely to report CHCs or emotional distress (both P < .001).
TABLE 1.
Demographic and Clinical Characteristics of Survivors of Childhood Cancer and Sibling Groups
Neurocognitive Problems
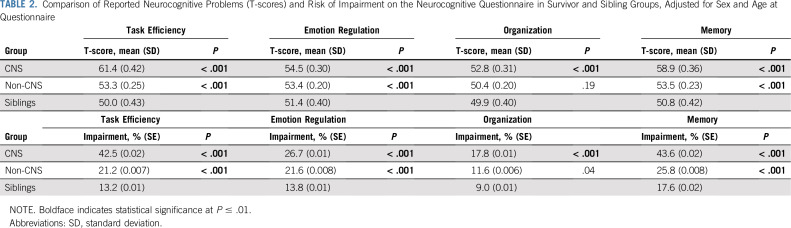
After adjusting for age at survey and sex, survivors of CNS and non-CNS cancers reported more problems with Task Efficiency, Emotion Regulation, and Memory than siblings (Table 2; all P < .001). CNS survivors reported more Organization problems than siblings (P < .001). Similar patterns of deficit were observed for risk of impairment (Table 2), with an approximate two-fold higher risk for CNS survivors compared with non-CNS survivors in Task Efficiency (percentage impaired: CNS, 42.5%; non-CNS, 21.2%) and Memory (percentage impaired: CNS, 43.6%; non-CNS, 25.8%).
TABLE 2.
Comparison of Reported Neurocognitive Problems (T-scores) and Risk of Impairment on the Neurocognitive Questionnaire in Survivor and Sibling Groups, Adjusted for Sex and Age at Questionnaire
Associations With Physical Activity
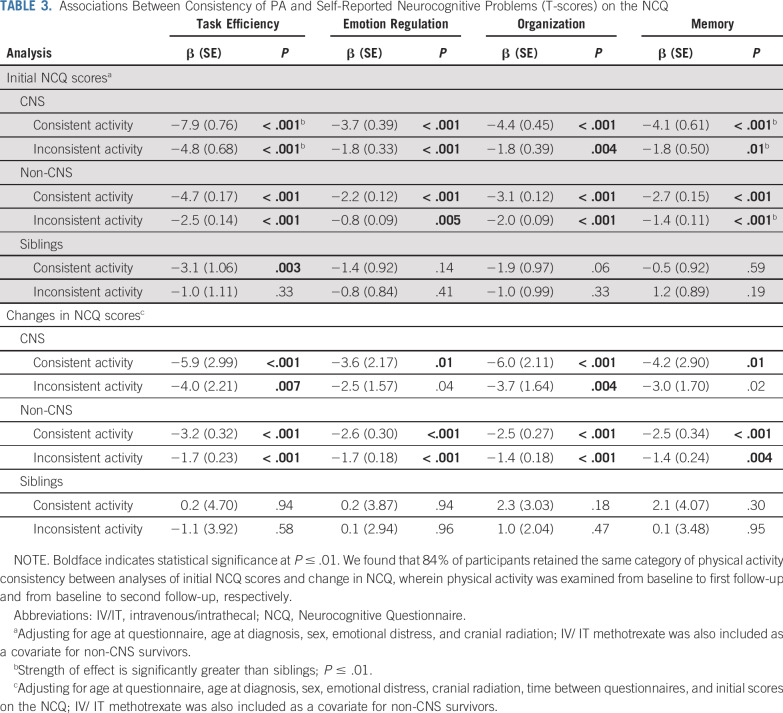
Physically active survivors reported fewer neurocognitive problems across multiple domains compared with consistently inactive survivors (Table 3; consistent PA: T-score differences ranging from −7.9 to −2.2; inconsistent PA: −4.8 to −0.8; P ≤ .01). These associations were replicated for risk of impairment (Data Supplement; consistent PA: odds ratios [ORs], 0.43-0.73; inconsistent PA: ORs, 0.55-0.80; P ≤ .006); however, inconsistent PA was not significantly associated with impairment risk in Emotion Regulation in non-CNS survivors. In siblings, consistent activity was associated with fewer neurocognitive problems relative to consistent inactivity for Task Efficiency only (T-score difference, −3.1; P = .003). Inconsistent activity was not associated with differences in neurocognitive outcomes, and PA consistency did not affect impairment risk in siblings.
TABLE 3.
Associations Between Consistency of PA and Self-Reported Neurocognitive Problems (T-scores) on the NCQ
The magnitude of association of PA consistency with Task Efficiency and Memory problems was significantly larger in CNS survivors than siblings (T-score difference between CNS survivors and siblings for consistent PA, Task Efficiency: β, −4.8; P < .001; Memory: β, −3.6; P = .004; inconsistent PA, Task Efficiency: β, −3.8; P = .004; Memory: β, −3.1; P = .009). An interaction was observed for inconsistent PA and Memory in non-CNS survivors versus siblings (β, −3.1; P = .009). No other interactions reached statistical significance.
Similar trends observed for PA intensity and quantity on neurocognitive problems and risk of impairment were less prominent than for PA consistency (Data Supplement). Higher PA intensity and quantity were associated with fewer neurocognitive problems in Task Efficiency and Organization for both survivor groups (T-score differences ranging from −0.1 to −0.4 per weekly 9 MET-hours; P < .001; and from −0.1 to −0.2 per weekly hour of activity; P < .001). No associations were observed for PA intensity and quantity on neurocognitive problems for siblings.
Change in Neurocognitive Problems Over Time
Consistent PA was associated with improvements in all cognitive domains compared with consistent inactivity, in both survivor groups (Table 3; difference in change in NCQ scores ranging from −6.0 to −2.5; P ≤ .01). Similarly, inconsistent PA was associated with improved Task Efficiency and Organization problems over time compared with consistent inactivity in both survivor groups (difference in change in NCQ scores ranging from −4.0 to −1.4; P ≤ .007) and improved Emotion Regulation and Memory problems in non-CNS survivors (difference in change in NCQ score ranging from −1.7 to −1.4; P < .004). PA consistency was not associated with change in NCQ for siblings (P > .01).
Mediation Analyses
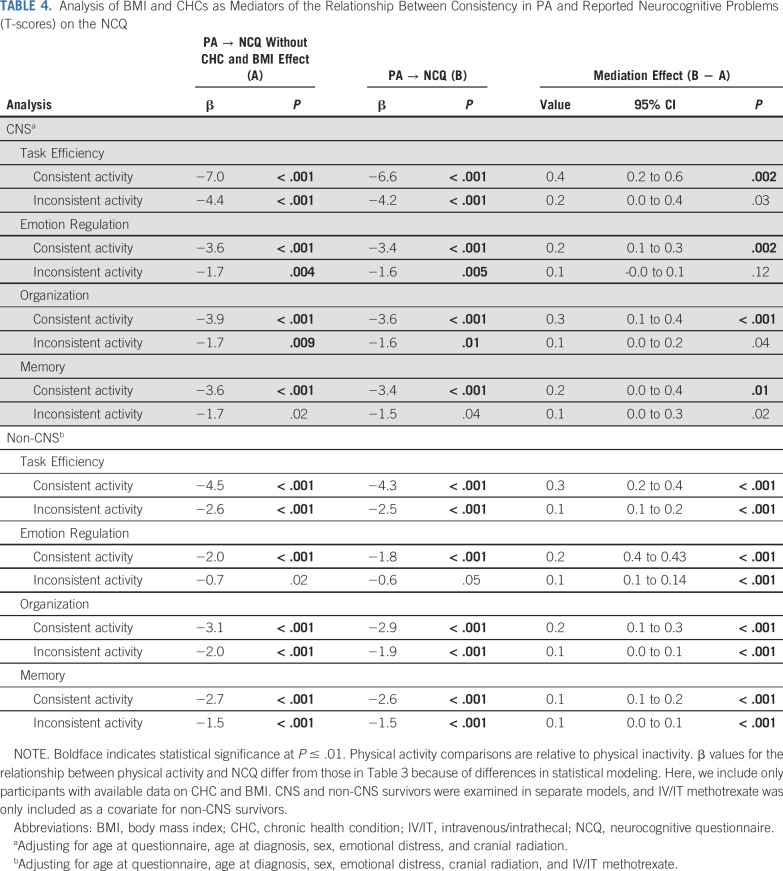
Consistent PA was associated with lower risk of a CHC and lower BMI, relative to consistent inactivity for both survivor groups (all P < .001; Data Supplement). Inconsistent PA was associated with lower risk of a CHC and lower BMI, relative to consistent inactivity, for non-CNS survivors only (P < .001). The presence of a CHC was associated with more reported neurocognitive problems for both survivor groups, except for Memory in the non-CNS group (all P < .001). Higher BMI was associated with more neurocognitive problems among non-CNS survivors (all P ≤ .003) but not for CNS survivors. Adjusting for the presence of a CHC or BMI attenuated associations between NCQ scores and PA consistency, but mediator effects were small (Table 4; change in T-score difference, 0.1-0.4).
TABLE 4.
Analysis of BMI and CHCs as Mediators of the Relationship Between Consistency in PA and Reported Neurocognitive Problems (T-scores) on the NCQ
DISCUSSION
We identified an association of PA with neurocognitive problems many years post treatment, with fewer problems over time for survivors who participated in PA more consistently. Effects were apparent in CNS and non-CNS survivors and across cognitive domains, after adjusting for demographic, clinical, and treatment variables. Many comparisons revealed differences of approximately half a standard deviation between activity groups, typically indicative of clinical significance. BMI or CHCs had minimal impact on this relationship.
PA demonstrated robust associations with self-reported neurocognitive problems, with effects observed across all cognitive domains for both survivor groups. These findings are in line with research demonstrating positive relationships between PA and cognitive outcomes.28,29,32,45,46 Effects were more apparent for PA consistency compared with intensity and quantity and were larger for consistent PA compared with inconsistent PA, suggesting that maintenance of activity over time is important for optimal enhancement of functional outcomes. Moreover, our data support the relevance of CDC recommendations for PA for promotion of healthy cognitive functioning.41,42
Associations between PA and neurocognitive problems were more consistently observed in survivors and were often larger for CNS survivors relative to siblings. Effects may be stronger for survivors, and CNS survivors in particular, given larger variability in neurocognitive problems, perhaps reflecting a greater benefit of PA on functions undergoing change, as previously suggested by meta-analysis.28 Survivors meeting CDC PA guidelines consistently over time reported larger improvements in neurocognitive problems relative to survivors not meeting these criteria. These improvements were observed over 20 years post diagnosis, suggesting continued benefits of PA long after cessation of treatment. Prior intervention research with pediatric brain tumor survivors similarly demonstrated evidencec for positive impacts of PA on cognitive outcomes and indices of brain health an average of 5.25 years post treatment.32
Associations with PA were observed after adjusting for age, sex, emotional distress, and exposure to cranial radiation and/or IV methotrexate, whereas BMI and CHCs accounted for a small portion of the shared variance between PA and neurocognitive outcomes. Individuals with physical limitations may experience greater deficits as a consequence of limited engagement in PA, or those engaging in less PA may experience greater deficits as a consequence of CHCs or higher body adiposity.3,47 However, these effect sizes were small, and mechanisms underlying these associations remain to be elucidated.
Comprehensive reviews have proposed several mechanisms that may underlie the relationship between PA and neurocognitive function, including increases in neurotrophic factors48,49 and dampening of inflammatory processes.29 The neurocognitive sequelae of childhood cancers are believed to be mediated in part by suppression of cell proliferation and increases in neuroinflammation, a consequence of radiation and chemotherapy treatments or cancers directly.50 It is possible that beneficial effects of PA on neurocognitive outcomes are greatest in survivors as a consequence of the overlap in mechanisms of pathologic and neuroplastic change; however, mechanisms remain to be explored.
Strengths of this work include examination of several PA indices and use of a large North American longitudinal cohort, facilitating evaluation of PA effects while adjusting for relevant covariates. As self-reported neurocognitive problems and PA have shown only moderate correlations with direct measures, results are limited by recall and response bias challenges. Although standardized neurocognitive function rating scales have good ecological validity,51 prospective studies with objective testing are required to elucidate mechanisms involved in cognitive enhancement. Misclassification of PA is also possible, because our definition of PA consistency does not account for activity levels between questionnaire administrations. However, reports in adult populations indicate PA is consistent (measured in 2-year intervals for 10 years) when evaluated by either self-report or accelerometry.52,53 Given advances in technology for remote tracking and mobile Health questionnaire administration, future research could be strengthened by inclusion of more direct assessment of PA. Moreover, concurrent measurement of other activities contributing to cognitive reserve (eg, intellectual, social)54 would help delineate direct effects of PA from overall engagement in enriching activities.
In the current study design, we are unable to establish causality of the relationship between PA and neurocognitive symptoms. Despite the existence of interventional studies with cancer survivors29,32 and proposed mechanisms for PA benefits on neurocognitive outcomes, it has also been found that survivors with neurocognitive complaints are less likely to engage in health behaviors, including PA,55 raising the possibility that the reverse is also true. Improvements in self-reported neurocognitive function may also be related to increased self-efficacy associated with engagement in PA56; however, individuals who perceive better neurocognitive functioning may also experience greater self-efficacy facilitating PA engagement.
It has been well established that childhood cancer survivors are at risk for late neurocognitive problems, with possible implications for attainment of developmental milestones.16,17,57 Continued follow-up and efforts to mitigate these problems are needed to support survivors through adulthood. Our findings highlight the opportunity for and possible benefits of PA for adult survivors of childhood cancer regardless of prior diagnosis and raise the interesting possibility that consistent PA may continue to improve neurocognitive outcomes over time. Additional interventional research with long-term CNS and non-CNS childhood cancer survivors and a randomized clinical trial design are needed to establish directionality and causality of this association, as well as how this relationship behaves over time.
PRIOR PRESENTATION
Presented at the Hospital for Sick Children Late Effects Symposium, Toronto, ON, Canada, September 4, 2018, and at the 2019 meetings of the International Neuropsychological Society, New York, NY, February 20-23, 2019; and the North American Symposium on Late Complications after Childhood Cancer, Atlanta, GA, June 20-22, 2019.
SUPPORT
Supported by National Institutes of Health Grant No. U24-CA55727 (G.T.A.); support to St Jude Children’s Research Hospital from American Lebanese Syrian Associated Charities; the Princess Margaret Cancer Foundation and the Ontario Ministry of Health and Long-Term Care (K.E.); and a Doctoral Studentship from the Canadian Institute of Health Research No. 379609 (E.B.-K.).
AUTHOR CONTRIBUTIONS
Conception and design: Emily Barlow-Krelina, Christine Till, Kirsten K. Ness, Wendy M. Leisenring, Kevin C. Oeffinger, Leslie L. Robison, Gregory T. Armstrong, Kevin R. Krull, Kim Edelstein
Financial support: Gregory T. Armstrong
Administrative support: Gregory T. Armstrong, Kevin R. Krull
Provision of study material or patients: Leslie L. Robison, Gregory T. Armstrong
Collection and assembly of data: Wendy M. Leisenring, Rebecca M. Howell, Leslie L. Robison, Gregory T. Armstrong
Data analysis and interpretation: Emily Barlow-Krelina, Yan Chen, Yutaka Yasui, Christine Till, Todd M. Gibson, Kirsten K. Ness, Wendy M. Leisenring, Paul C. Nathan, Kevin C. Oeffinger, Gregory T. Armstrong, Kevin R. Krull, Kim Edelstein
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Consistent Physical Activity and Future Neurocognitive Problems in Adult Survivors of Childhood Cancers: A Report From the Childhood Cancer Survivor Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kevin R. Krull
Patents, Royalties, Other Intellectual Property: Royalties from Wolters Kluwer
No other potential conflicts of interest were reported.
REFERENCES
- 1. Howlader N, Noone AM, Krapcho M, et al: SEER Cancer Statistics Review, 1975-2013. Bethesda, MD, National Cancer Institute, 2016. [Google Scholar]
- 2.Armenian SH, Robison LL. Childhood cancer survivorship: An update on evolving paradigms for understanding pathogenesis and screening for therapy-related late effects. Curr Opin Pediatr. 2013;25:16–22. doi: 10.1097/MOP.0b013e32835b0b6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung YT, Brinkman TM, Li C, et al. Chronic health conditions and neurocognitive function in aging survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2018;110:411–419. doi: 10.1093/jnci/djx224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 5.Robison LL. Treatment-associated subsequent neoplasms among long-term survivors of childhood cancer: The experience of the Childhood Cancer Survivor Study. Pediatr Radiol. 2009;39(suppl 1):S32–S37. doi: 10.1007/s00247-008-1066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brouwers P, Riccardi R, Fedio P, et al. Long-term neuropsychologic sequelae of childhood leukemia: Correlation with CT brain scan abnormalities. J Pediatr. 1985;106:723–728. doi: 10.1016/s0022-3476(85)80343-7. [DOI] [PubMed] [Google Scholar]
- 7.Butler RW, Haser JK. Neurocognitive effects of treatment for childhood cancer. Ment Retard Dev Disabil Res Rev. 2006;12:184–191. doi: 10.1002/mrdd.20110. [DOI] [PubMed] [Google Scholar]
- 8.Campbell LK, Scaduto M, Sharp W, et al. A meta-analysis of the neurocognitive sequelae of treatment for childhood acute lymphocytic leukemia. Pediatr Blood Cancer. 2007;49:65–73. doi: 10.1002/pbc.20860. [DOI] [PubMed] [Google Scholar]
- 9. doi: 10.1080/13638490310001655528. Mulhern RK, Butler RW: Neurocognitive sequelae of childhood cancers and their treatment. Pediatr Rehabil 7:1-14, 2004; discussion 15-16. [DOI] [PubMed] [Google Scholar]
- 10.Mulhern RK, Merchant TE, Gajjar A, et al. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5:399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 11.Mulhern RK, Fairclough D, Ochs J. A prospective comparison of neuropsychologic performance of children surviving leukemia who received 18-Gy, 24-Gy, or no cranial irradiation. J Clin Oncol. 1991;9:1348–1356. doi: 10.1200/JCO.1991.9.8.1348. [DOI] [PubMed] [Google Scholar]
- 12.Edelstein K, D’agostino N, Bernstein LJ, et al. Long-term neurocognitive outcomes in young adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2011;33:450–458. doi: 10.1097/MPH.0b013e31820d86f2. [DOI] [PubMed] [Google Scholar]
- 13.Janzen LA, Spiegler BJ. Neurodevelopmental sequelae of pediatric acute lymphoblastic leukemia and its treatment. Dev Disabil Res Rev. 2008;14:185–195. doi: 10.1002/ddrr.24. [DOI] [PubMed] [Google Scholar]
- 14.Butler RW, Mulhern RK. Neurocognitive interventions for children and adolescents surviving cancer. J Pediatr Psychol. 2005;30:65–78. doi: 10.1093/jpepsy/jsi017. [DOI] [PubMed] [Google Scholar]
- 15.Cheung YT, Krull KR. Neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia treated on contemporary treatment protocols: A systematic review. Neurosci Biobehav Rev. 2015;53:108–120. doi: 10.1016/j.neubiorev.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: A report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23:705–717. doi: 10.1037/a0016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadan-Lottick NS, Zeltzer LK, Liu Q, et al. Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. J Natl Cancer Inst. 2010;102:881–893. doi: 10.1093/jnci/djq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meadows AT, Gordon J, Massari DJ, et al. Declines in IQ scores and cognitive dysfunctions in children with acute lymphocytic leukaemia treated with cranial irradiation. Lancet. 1981;2:1015–1018. doi: 10.1016/s0140-6736(81)91216-2. [DOI] [PubMed] [Google Scholar]
- 19.Nathan PC, Patel SK, Dilley K, et al. Guidelines for identification of, advocacy for, and intervention in neurocognitive problems in survivors of childhood cancer: A report from the Children’s Oncology Group. Arch Pediatr Adolesc Med. 2007;161:798–806. doi: 10.1001/archpedi.161.8.798. [DOI] [PubMed] [Google Scholar]
- 20.Nazemi KJ, Butler RW. Neuropsychological rehabilitation for survivors of childhood and adolescent brain tumors: A view of the past and a vision for a promising future. J Pediatr Rehabil Med. 2011;4:37–46. doi: 10.3233/PRM-2011-0151. [DOI] [PubMed] [Google Scholar]
- 21.Champaloux SW, Young DR. Childhood chronic health conditions and educational attainment: A social ecological approach. J Adolesc Health. 2015;56:98–105. doi: 10.1016/j.jadohealth.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Vassilaki M, Aakre JA, Cha RH, et al. Multimorbidity and risk of mild cognitive impairment. J Am Geriatr Soc. 2015;63:1783–1790. doi: 10.1111/jgs.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson CL, Stratton K, Leisenring WL, et al. Decline in physical activity level in the Childhood Cancer Survivor Study cohort. Cancer Epidemiol Biomarkers Prev. 2014;23:1619–1627. doi: 10.1158/1055-9965.EPI-14-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox CL, Nolan VG, Leisenring W, et al. Noncancer-related mortality risks in adult survivors of pediatric malignancies: The childhood cancer survivor study. J Cancer Surviv. 2014;8:460–471. doi: 10.1007/s11764-014-0353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones LW, Liu Q, Armstrong GT, et al. Exercise and risk of major cardiovascular events in adult survivors of childhood Hodgkin lymphoma: A report from the childhood cancer survivor study. J Clin Oncol. 2014;32:3643–3650. doi: 10.1200/JCO.2014.56.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keats MR, Culos-Reed SN, Courneya KS, et al. An examination of physical activity behaviors in a sample of adolescent cancer survivors. J Pediatr Oncol Nurs. 2006;23:135–142. doi: 10.1177/1043454206287304. [DOI] [PubMed] [Google Scholar]
- 27.Ness KK, Leisenring WM, Huang S, et al. Predictors of inactive lifestyle among adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2009;115:1984–1994. doi: 10.1002/cncr.24209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hötting K, Röder B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci Biobehav Rev. 2013;37:2243–2257. doi: 10.1016/j.neubiorev.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Zimmer P, Baumann FT, Oberste M, et al. Effects of exercise interventions and physical activity behavior on cancer related cognitive impairments: A systematic review. BioMed Res Int. 2016;2016:1820954. doi: 10.1155/2016/1820954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paxton RJ, Jones LW, Rosoff PM, et al. Associations between leisure-time physical activity and health-related quality of life among adolescent and adult survivors of childhood cancers. Psychooncology. 2010;19:997–1003. doi: 10.1002/pon.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tonorezos ES, Ford JS, Wang L, et al. Impact of exercise on psychological burden in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2019;125:3059–3067. doi: 10.1002/cncr.32173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riggs L, Piscione J, Laughlin S, et al. Exercise training for neural recovery in a restricted sample of pediatric brain tumor survivors: A controlled clinical trial with crossover of training versus no training. Neuro-oncol. 2017;19:440–450. doi: 10.1093/neuonc/now177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. St Jude Children’s Research Hospital: The Childhood Cancer Survivor Study. http://ccss.stjude.org.
- 35. St Jude Children’s Research Hospital: The Childhood Cancer Survivor Study: Questionnaires. https://ccss.stjude.org/tools-and-documents/questionnaires.html.
- 36.Krull KR, Gioia G, Ness KK, et al. Reliability and validity of the Childhood Cancer Survivor Study Neurocognitive Questionnaire. Cancer. 2008;113:2188–2197. doi: 10.1002/cncr.23809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kenzik KM, Huang IC, Brinkman TM, et al. The Childhood Cancer Survivor Study-Neurocognitive Questionnaire (CCSS-NCQ) revised: Item response analysis and concurrent validity. Neuropsychology. 2015;29:31–44. doi: 10.1037/neu0000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Derogatis LR, Savitz KL: The SCL-90-R and Brief Symptom Inventory (BSI) in primary care, in Maruish ME, (ed): Handbook of Psychological Assessment in Primary Care Settings. Mahwah, NJ, Lawrence Erlbaum Associates Publishers, 2000, pp 297-334. [Google Scholar]
- 39.National Center for Chronic Disease Prevention and Health Promotion Behavioral Risk Factor Surveillance System State Questionnaire. 2003 https://www.cdc.gov/brfss/questionnaires/pdf-ques/2003brfss.pdf
- 40.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention About Adult BMI. 2017 https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html
- 42.Centers for Disease Control and Prevention About Child & Teen BMI. 2018 https://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html
- 43. US Department of Health and Human Services: Common Terminology Criteria for Adverse Events v. 5.0. 2017. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf#search=%22ctcae%22.
- 44.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 45.Cox EP, O’Dwyer N, Cook R, et al. Relationship between physical activity and cognitive function in apparently healthy young to middle-aged adults: A systematic review. J Sci Med Sport. 2016;19:616–628. doi: 10.1016/j.jsams.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Singh A, Uijtdewilligen L, Twisk JW, et al. Physical activity and performance at school: A systematic review of the literature including a methodological quality assessment. Arch Pediatr Adolesc Med. 2012;166:49–55. doi: 10.1001/archpediatrics.2011.716. [DOI] [PubMed] [Google Scholar]
- 47.Smith E, Hay P, Campbell L, et al. A review of the association between obesity and cognitive function across the lifespan: Implications for novel approaches to prevention and treatment. Obes Rev. 2011;12:740–755. doi: 10.1111/j.1467-789X.2011.00920.x. [DOI] [PubMed] [Google Scholar]
- 48.Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J Psychiatr Res. 2015;60:56–64. doi: 10.1016/j.jpsychires.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruiz de Almodovar C, Lambrechts D, Mazzone M, et al. Role and therapeutic potential of VEGF in the nervous system. Physiol Rev. 2009;89:607–648. doi: 10.1152/physrev.00031.2008. [DOI] [PubMed] [Google Scholar]
- 50.Rodgers SP, Trevino M, Zawaski JA, et al. Neurogenesis, exercise, and cognitive late effects of pediatric radiotherapy. Neural Plast. 2013;2013:698528. doi: 10.1155/2013/698528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gioia GA, Isquith PK, Roth RM: Behavior rating inventory for executive function, in Kreutzer JS, DeLuca J, Caplan B (eds): Encyclopedia of Clinical Neuropsychology. New York, NY, Springer, 2011, pp 372-376. [Google Scholar]
- 52.Kim Y, Wijndaele K, Sharp SJ, et al. Specific physical activities, sedentary behaviours and sleep as long-term predictors of accelerometer-measured physical activity in 91,648 adults: A prospective cohort study. Int J Behav Nutr Phys Act. 2019;16:41. doi: 10.1186/s12966-019-0802-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith L, Gardner B, Fisher A, et al. Patterns and correlates of physical activity behaviour over 10 years in older adults: Prospective analyses from the English Longitudinal Study of Ageing. BMJ Open. 2015;5:e007423. doi: 10.1136/bmjopen-2014-007423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bittner N, Jockwitz C, Mühleisen TW, et al. Combining lifestyle risks to disentangle brain structure and functional connectivity differences in older adults. Nat Commun. 2019;10:621. doi: 10.1038/s41467-019-08500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krull KR, Annett RD, Pan Z, et al. Neurocognitive functioning and health-related behaviours in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Eur J Cancer. 2011;47:1380–1388. doi: 10.1016/j.ejca.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Phillips SM, Lloyd GR, Awick EA, et al. Relationship between self-reported and objectively measured physical activity and subjective memory impairment in breast cancer survivors: Role of self-efficacy, fatigue and distress. Psychooncology. 2017;26:1390–1399. doi: 10.1002/pon.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kirchhoff AC, Krull KR, Ness KK, et al. Physical, mental, and neurocognitive status and employment outcomes in the childhood cancer survivor study cohort. Cancer Epidemiol Biomarkers Prev. 2011;20:1838–1849. doi: 10.1158/1055-9965.EPI-11-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]