Abstract
Background:
Dermatologic adverse events (dAEs) of anticancer therapies may negatively impact dosing and quality of life. While therapy interruption patterns due to dAEs have been studied in hospitalized cancer patients, similar outcomes in outpatient oncodermatology are lacking.
Objectives:
To analyze the therapy interruption patterns, clinico-histopathologic characteristics, and management outcomes of outpatient dermatology consultations for acute dAEs attributed to the most frequently interrupted class of oncologic agents.
Methods:
We performed a retrospective cohort study of all cancer patients who received a same-day outpatient dermatology consultation for acute dAEs at our institution from 1 January to 30 June 2015. Relevant data were abstracted from electronic medical records, including demographics, oncologic history, and explicit recommendations by both the referring clinician and consulting dermatologist on anticancer therapy interruption. Consultations with the most frequently interrupted class of oncologic treatment were characterized according to clinico-histopathologic features, dermatologic management, and clinical outcomes.
Results:
There were 426 same-day outpatient dermatology consultations (median age 59, 60% female, 30% breast cancer), of which 295 (69%) had systemic anticancer therapy administered within 30 days prior. There was weak inter-rater agreement between referring clinicians and consulting dermatologists on interruption of anticancer treatment (n=150, κ = 0.096; 95% CI −0.02–0.21). Seventy-three (25%) consultations involved interruption by the referring clinician, most commonly targeted therapy (24, 33%). Maculopapular rash was commonly observed in 23 consultations with 25 dAEs attributed to targeted agents (48%), and topical corticosteroids were most frequently utilized for management (22, 38%). The majority (83%) of consultations with targeted therapy-induced dAEs responded to dermatologic treatment and 84% resumed oncologic therapy, although three (19%) at a reduced dose. Rash recurred only in two instances (13%).
Conclusions:
A high frequency of positive outcomes in the management of targeted therapy-induced dAEs by outpatient consulting dermatologists and low recurrence of skin toxicity suggests impactful reductions in interruption of anticancer therapy.
Keywords: oncodermatology, consultation, targeted therapy, interruption, toxicity, adverse event
Introduction
According to a status report on the global burden of cancer worldwide, 18.1 million new cancer cases and 9.6 million cancer deaths were estimated in 2018,1 with 10–75% likely developing dermatologic adverse events (dAEs) from therapy.2 Radiation, immunotherapy, targeted, and cytotoxic chemotherapies are known to affect the skin, hair, and nails— thereby negatively impacting quality of life (QoL).3–5 Since the number of cancer patients exposed to novel agents, including targeted and immunotherapy, continues to grow, appropriate management of their dAEs becomes paramount.
Because of the varied presentation of dAEs and their effect on cancer patients’ oncologic treatment outcomes and QoL, the field of oncodermatology has emerged to provide supportive care to cancer patients and survivors.6–12 Clinical diagnoses made by dermatologists can differ from that initially evoked by non-specialists in nearly 50% of the cases;13–20 thus, acute dermatologic conditions arising in cancer patients due to the adverse effects of antineoplastic treatment may be misdiagnosed or under-recognized by non-dermatologists.
While the clinical features and anticancer therapy interruption patterns of consultations for acute dAEs in hospitalized oncology patients is known,21, 22 the characterization of same-day outpatient dermatology consultations is lacking.22, 23 While a recent research letter24 specifically described the impact of dermatology consultations on interruption of oncologic management among hospitalized patients with immune related adverse events, studies that examine the inter-agreement between referring clinicians and consulting dermatologists on recommendations to interrupt anticancer therapy in the outpatient setting have not been reported.
Because therapy-induced dAEs in outpatient oncology may precipitate significant disruption to treatment, an improved understanding of the clinicopathologic characteristics and outcomes of patients with acute dAEs leading to anticancer therapy interruption is critical for implementing a model of therapeutic collaboration between oncologists and dermatologists. Thus, aside from exploring the baseline oncologic characteristics of all outpatient dermatology consultations at our institution, we sought to investigate therapy interruption patterns and impact of dermatologic interventions by consulting dermatologists on acute dAEs attributed to the most frequently interrupted agents.
Materials and Methods
Study Sample
Following approval by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSK protocol 16–410), a retrospective review of all outpatient dermatology consultations in the six-month period from January 1 and June 30, 2015 was conducted. Patients were identified via a same-day consultation request log that was maintained by administrative personnel in the outpatient oncodermatology clinic and cross-referenced using a query of MSK’s health information systems (HIS). Multiple initial consultations for the same patient with different acute dAEs at different times were included. Patients that had no history of malignancy or who did not show for their appointment were excluded (Supplementary Fig. 1). We looked at all consultations in the study period and reviewed them for our exclusion criteria mentioned above resulting in a total of 426 consultations. An additional HIS query was performed to identify and compare the distribution of baseline characteristics of all outpatient visits at MSK compared to those who received a same-day outpatient dermatology consultation in the same time frame.
Data collection
The distribution of age, sex and primary cancer characteristics were summarized for all outpatient visits at MSK and compared to those observed in patients who received a same-day outpatient oncodermatology consultation for acute dAEs from January 1 to June 30, 2015. Demographics, oncologic history and therapy interruption patterns were abstracted from electronic medical records and analyzed among consultations in patients who received anticancer treatment within 30 days prior to evaluation by consulting dermatologists. To assess inter-rater agreement between referring clinicians and consulting dermatologists on recommendations for anticancer therapy interruption due to acute dAEs, explicit recommendations by both the referring clinician and consulting dermatologist on the initial consultation note was recorded as “yes” or “no.”
Primary cancers were represented according to tumor site or system involvement. Anticancer therapies were subdivided into single-agent therapy and combination therapy regimens. Single agents included cytotoxic chemotherapy (i.e. carboplatin, cyclophosphamide, etc.), endocrine therapy (i.e. anastrozole, leuprolide, etc.), targeted (i.e. kinase inhibitors, EGFR inhibitors, etc.), and immunotherapy (anti-PDL1, anti-CTLA-4). Combination therapy was defined as multi-agent therapy in patients receiving two or more agents, regardless of agent class (i.e. cisplatin + docetaxel [both cytotoxic] = combination; cytotoxic + immune checkpoint inhibitor = combination).
Types of dermatologic conditions diagnosed at all outpatient dermatology consultation visits were abstracted from the consulting dermatologist’s initial assessment note, and summarized according to etiology (i.e. inflammatory reactions, infections, neoplasms, etc.) and severity using the Common Terminology Criteria for Adverse Events (CTCAE) v4.0 scale (grade 1=mild; grade 2=moderate; grade 3=severe).25 All dAEs were included, even when there were multiple conditions diagnosed. The clinical and histopathologic characteristics of acute dAEs explicitly attributed to the most frequently interrupted anticancer agent were presented in table format, including a description of the clinical impression, the suspected anticancer agent, and dermatopathological features.
Dermatologic management and clinical outcomes of acute dAEs attributed to the most frequently interrupted agent was also reported. Outcomes data included presence of a response to dermatologic treatment; quality of the treatment responses (arbitrarily classified as significant [improvement by two or more grades or an improvement to grade 0], moderate [a one-grade improvement], or no improvement [no change or increase in grade]; whether anticancer therapy was resumed following dermatology consultation; recurrence of dAEs following re-challenge; and whether oncologic treatment was permanently discontinued due to skin toxicity.
Data analyses
Descriptive statistics, provided as frequencies and percentages, was used to analyze categorial data. Continuous data was summarized as median values with ranges. Inter-rater agreement between referring physicians and consulting dermatologists was summarized with Cohen’s kappa (κ) coefficient and a corresponding 95% confidence interval (CI). κ ranges from −1.00 (complete disagreement) to 1.00 (complete agreement), with κ = 0 signifying that the observed agreement is no better than a coin flip. Values between 0 and 0.2 can be interpreted as none to slight (weak) agreement, 0.21–0.40 as fair, 0.41– 0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1.00 as almost perfect agreement26. To capture all occurring dAEs, consultations on the same patient were considered independent.
Results
Demographics and oncologic history
Five hundred and four same-day outpatient dermatology consultation requests were screened, resulting in a final cohort of 426 consultations for 412 patients. Two hundred and fifty-four females (60%) and 172 (40%) males met inclusion criteria. The median age was 59 years and most consultations included patients that were diagnosed with solid tumors (342, 80%), particularly breast cancer (127, 30%). The frequency of patients with breast cancer who had a same-day outpatient dermatology consultation for acute dAEs (30%) was higher than that observed in this group for any outpatient visit at MSK during the same study period (23%) (Supplementary Table 1).
Of 426 same-day outpatient dermatology consultations, 295 (69%) had patients who received anticancer therapy dosing no later than 30 days prior to evaluation. Of these 295 consultations, many were requested for patients previously diagnosed with solid tumors (253, 86%), 95 of which (38%) had breast cancer. Two hundred and seven (71%) involved patients receiving single agent anticancer therapy, and 73 (25%) involved interruption of oncologic treatment by referring clinicians due to acute dAEs. Targeted therapy was the most frequently interrupted class of anticancer agents (24, 33%) (Table 1).
Table 1.
Therapy interruption patterns among295 same-day outpatient dermatology consultations for acute dermatologic adverse events (dAEs)in patients receiving systemic oncologic treatment within 30 days pre-consultation
| Anticancer therapy interruption by
referring clinician |
||
|---|---|---|
| Yesn = 73 (25) | Non = 222 (75) | |
| Age median [range] | 58 [17–87] | 58 [13–85] |
| Sex (female) | 42 (58) | 136 (61) |
| Race | ||
| White | 43 (59) | 163 (73) |
| Asian | 11 (15) | 17 (8) |
| Black | 9 (12) | 20 (9) |
| Other | 10 (14) | 22 (10) |
| Primary cancer | ||
| Breast | 20 (27) | 75 (34) |
| Gastrointestinal | 13 (18) | 31 (14) |
| Hematologic# | 12 (16) | 30 (14) |
| Lung | 7 (10) | 23 (10) |
| Gynecologic | 6 (8) | 7 (3) |
| Genitourinary | 6 (8) | 25 (11) |
| Soft tissue sarcoma | 3 (4) | 8 (4) |
| Skin | 3 (4) | 7 (3) |
| Neurological | 1 (1) | 5 (2) |
| Head and neck | 0 (0) | 5 (2) |
| Other^ | 2 (3) | 6 (3) |
| Anticancer therapy* | ||
| Targeted## therapy | 24 (33) | 53 (24) |
| Cytotoxic chemotherapy | 22 (30) | 76 (34) |
| Combination therapy | 19 (26) | 67 (30) |
| Immunotherapy | 6 (8) | 10 (5) |
| Other& | 2 (3) | 16 (7) |
Inter-rater agreement on anticancer therapy interruption
Upon review of all medical oncology and dermatology assessment notes that were relevant to the 295 consultations with any systemic anti-cancer therapy administration, we found that only 150 (51%) had explicitly documented, by both the referring clinicians and consulting dermatologist, any recommendations for interruption of anticancer treatment due to dAEs. Whereas there were 44 (29%) consultations for which referring clinicians recommended therapy interruption, consulting dermatologists recommended interruption only 6 (4%) times. Inter-rater agreement was weak, with a κappa (κ)value of 0.096 and a 95% confidence interval of −0.02–0.21 (Table 2).
Table 2.
Inter-rater agreement amongst referring clinicians and consulting dermatologists on anticancer therapy interruptionfor acute dermatologic adverse events (dAEs), n=150 same-day consultationswith recommendations by boththe referring clinician and the consulting dermatologist on whether therapy should be held, kappa (κ) = 0.096; 95% CI-0.02-0.21
| Therapy interruption recommended by consulting dermatologist | |||
|---|---|---|---|
| No | Yes | ||
| Therapy interruption recommended by referring clinician | No | 104 | 2 |
| Yes | 40 | 4 | |
Dermatologic characteristics and management outcomes
Supplementary Table 2 details the conditions diagnosed by consulting dermatologists. There were 736 diagnoses for 426 consultations, indicative of multiple conditions identified during a single encounter. Inflammatory (377, 51%) and infectious (96, 13%) etiologies comprised the most common diagnostic groups. Additional dAEs included xerosis (63, 9%), alopecia and nail disorders (62, 8%).
Of 24 (33%) consultations with targeted therapy interruption due to dAEs (Table 1), consulting dermatologists explicitly attributed 25 dAEs to the patients’ respective agents in 23 consultations for 21 patients. Of these, 23 (92%) dAEs were attributed specifically to kinase-targeted agents, such as erlotinib, panitumumab, etc. Among the two dAEs not thought to be related to kinase inhibitors, the attributed mechanisms of action included drugs with inhibition of protein degradation and transcription regulation.
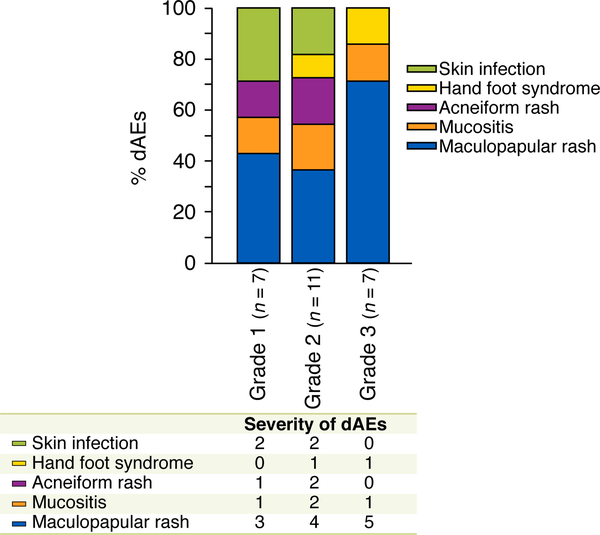
Maculopapular rash (12, 48%) was frequently observed among the 25 dAEs attributed to targeted therapy. While the majority (18, 72%) of dAEs attributed to targeted agents were of mild to moderate (grade ½) in severity, a significant number (7, 28%) were severe. The most common clinical morphology among severe (grade 3) dAEs attributed to targeted therapy was maculopapular rash (5/7, 71%) (Fig. 1).
Figure 1.
Types and severity of 25 acute dAEs in 23 consultations with targeted therapy interruption
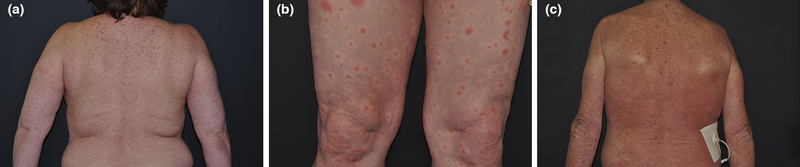
Among all 426, dermatologists performed skin biopsies at 74 consultation visits (17%). Only 4 (17%) dAEs from 23 consultations with targeted therapy interruption were biopsied, all of which involved kinase inhibitors. The results are presented in Table 3. These patients not only consistently exhibited clinical features of maculopapular rash (Figs 2a-c), the histopathologic features of kinase inhibitor-induced reactions were uniformly characterized by mixed pattern perivascular lymphocytic dermatitis with scattered eosinophils. All biopsy results confirmed a drug reaction, some specifying a delayed type hypersensitivity drug reaction (Table 3).
Table 3.
Clinico-histopathologic characteristics ofpatients with acute dAEsand skin biopsy performed during same-day outpatient dermatology consultation with targeted therapy interruption
| Clinical diagnosis | Anticancer therapy | Histopathologic features and diagnosis |
|---|---|---|
| Grade 2 maculopapular rash | BTK inhibitor | Mild interface and superficial dermal perivascular lymphocytic dermatitis with evidence of mild vascular damage, consistent with a drug reaction. |
| Grade 2 maculopapular Fig. 2(a) | EGFR inhibitor | Perivascular lymphocytic dermatitis with scattered eosinophils, consistent with a drug hypersensitivity reaction. |
| Grade 2 maculopapular rash Fig. 2(b) | ERK inhibitor | Interface, perivascular and interstitial lympho-eosinophilic dermatitis with evidence of mild vascular damage, consistent with a drug hypersensitivity reaction. |
| Grade 3 maculopapular rash Fig. 2 (c) | mTOR protein inhibitor | Mixed pattern dermatitis with eosinophils, consistent with a drug reaction. |
Figure 2.
Patients with dAEs attributed to kinase-targeted therapy (a) 43-year-old woman with lung cancer and interruption of treatment with EGFR inhibitor due to grade 2 maculopapular rash (b) 72-year-old man with mesothelioma and interruption of anti-ERK agent due to grade 2 maculopapular rash (c) 67-year-old man with prostate cancer and interruption of mTOR protein targeted therapy due to grade 3 maculopapular rash.
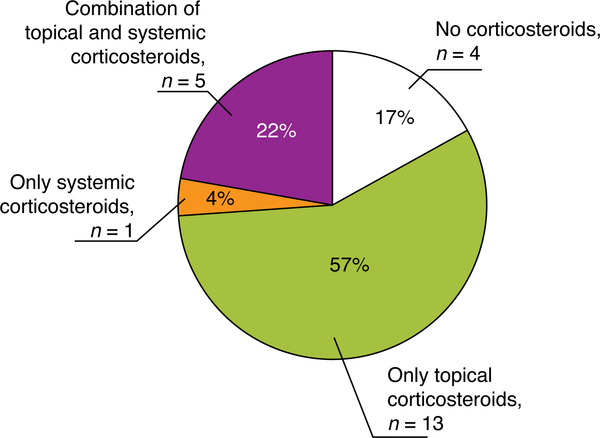
In the 23 consultations for 25 dAEs attributed to targeted therapy interruption, a total of 58 prescriptions were ordered by consulting dermatologists. The most frequently utilized treatments were topical corticosteroids (22, 38%), followed by anti-microbials (16, 28%). Only in 6 (10%) instances were systemic corticosteroids required. We found a higher frequency of topical corticosteroids used alone (57%) than systemic corticosteroids alone (4%) or a combination (22%) (Figure 3).
Figure 3.
Use of corticosteroids for management of 25 acute dAEs in 23 consultations with targeted therapy interruption
Nineteen consultations (83%) resulted in a positive response to dermatologic intervention. Two (9%) of the four patients who did not improve following dermatology consultation were hospitalized. The first was a patient with prostate cancer admitted for worsening mTOR-induced drug hypersensitivity reaction (Fig. 2c); and the second, was a patient with lymphoma who 5 days after starting 40 mg systemic corticosteroids for ibrutinib-induced grade 3 maculopapular rash, developed fever, diaphoresis, lethargy, and headache.
Eight (42%) of the 19 consultations responding to treatment ordered by the consulting dermatologist had a moderate response and 11 (58%) a significant one. Sixteen (84%) of the 19 consultations with a response to dermatologic treatment resumed their targeted anticancer therapy, although three (19%) at a reduced dose. Rash recurred only in two instances (13%). While rash did not recur in 14 (88%) out of the 16 patients who were re-challenged, two (13%) permanently discontinued therapy because of progression of oncologic disease.
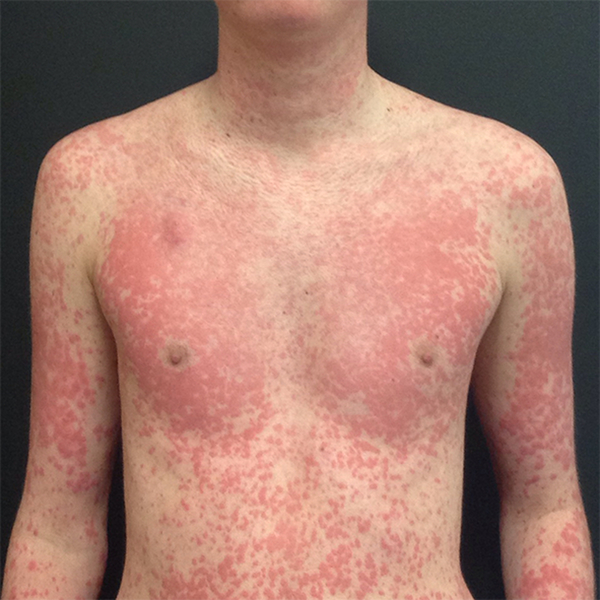
Among the three patients who did not resume therapy, one was a 17-year-old boy with a diagnosis of osteosarcoma and grade 3 maculopapular rash attributed to sorafenib (Fig. 4). Although the patient significantly improved within three days of receiving a high dose (60 mg) prednisone taper, triamcinolone and hydroxyzine, both the primary team and the family decided against resumption of therapy. This was one of the very few patients whose therapy interruption recommendations by the consulting dermatologist was to continue to hold.
Figure 4.
Seventeen-year-old boy with a diagnosis of osteosarcoma and grade 3 maculopapular rash attributed to sorafenib, a tyrosine kinase inhibitor. Consulting dermatologist treated him with high dose (60 mg) systemic corticosteroids, as well as topical triamcinolone and hydroxyzine. Maintenance of therapy hold was also recommended by consulting dermatologist. Patient showed significant improvement within 3 days, but primary team chose not to re-challenge.
Discussion:
Our study population comprised 426 same-day dermatology consultations, a uniquely large number taking place in the outpatient setting at a comprehensive cancer center over six months. Although a similar retrospective study of 459 outpatient oncodermatology visits was carried out in Greece, the patients were examined over a 42-month period.23 Song et al. have also reported on the characteristics of 516 outpatient dermatology consultations; however, those were encountered from 2008 to 2015, and the findings are relevant only to practicing pediatric oncodermatologists.16
Consistent with the most frequently diagnosed cancer among females worldwide, our study reveals that most (30%) patients requiring an outpatient dermatology consultation at our institution were those who with a diagnosis of breast cancer. Our results also compare to the distribution of breast cancer patients among all outpatient visits at MSK between January 1 and June 30, 2015, which shows that this group compared to others (i.e. gastrointestinal, genitourinary, etc.) is also most frequently evaluated in the outpatient setting compared to others (Supplementary Table 1). However, overall, a larger proportion of breast cancer patients were seen by outpatient dermatologist for a same-day consultation than by other services for any other reason, suggesting a high degree of morbidity induced by dAEs attributed to anticancer therapy in this population. Of note, in a study of hospitalized cancer patients with dAEs at our institution during a similar period, patients with hematologic malignancies (47%) were more likely to receive dermatologic consultations than patients with other tumor types, such as breast cancer.21 The ability for close monitoring in the inpatient setting affects clinical decision making regarding both anticancer therapy interruption and dAE-directed dermatologic intervention. Thus, although interrelated, the findings presented in the outpatient setting are not directly generalizable to those described in the inpatient setting.
The weak agreement we found between referring clinicians and consulting dermatologists on interrupting oncologic treatment due to dAEs (κ = 0.096) highlights how delays in reaching an accurate diagnosis may lead to delays in the appropriate management of dAEs. As potentially unfounded interruption or termination of life-saving anticancer therapies may negatively impact disease course, it follows that dermatologists can have a positive impact in the care of cancer patients.
While anticancer therapy interruption patterns have recently been explored in some detail, those have been discussed primarily in the context of immunotherapy,22,24 and not exclusively in the outpatient population.22 Similar to the results reported by Nikolaou et al., we observed a high rate of oncologic treatment modifications with targeted agents due to dAEs.23 Considering how many targeted therapeutic agents are approved by the US Food and Drug (FDA) administration each year,27 including a newly FDA-approved PI3K inhibitor for metastatic breast cancer,28 our study emphasizes the need for more collaboration between dermatologists and oncologists to come up with effective preventive and therapeutic measures.
Overall, we appreciated a predominance of inflammatory conditions (51%), not only in the entire cohort with 426 consultations and 736 dAEs, but also among those with targeted therapy interruption (48%). Since the term “inflammatory” is rather vague and the specific immunologic mechanisms of dAEs to anticancer agents is poorly understood, additional studies are warranted to help us better characterize different types of drug hypersensitivity reactions, including the expression of proinflammatory cytokines and chemokines. 42
It is well known that the dAE profile of immunotherapies11,12 is distinct from targeted agents;34–41 and the histopathologic evidence from the 4 skin biopsies of the patients we encountered with dAEs attributed to kinase-targeted agents uniformly revealed findings consistent with type IV hypersensitivity reactions, which helps us characterize these eruptions beyond a broad non-infectious inflammatory etiology. Unlike one study in outpatient pediatric oncology,16 skin infections were encountered less frequently than inflammatory conditions. Nikolaou et al. reported acneiform rash and perionychias as the most common toxicities implicated in targeted therapy modifications,23 particularly EGFR inhibitors. However, the rash phenotype that we observed in patients receiving kinase-targeted treatment with BTK, mTOR protein, and PI3K inhibitors was maculopapular.
Our cohort reflects many dAEs with grade 1 and 2 (mild to moderate) severity. Grade 2 dAEs can still be associated with limitations of activities of daily living, and in targeted agents, which were the most frequently interrupted types therapies, we observed a high prevalence of grade 2 dAEs. This highlights the need to develop treatment strategies toward prevention of severe (grade 3) dAEs, which may have profound implications on the QoL of patients being treated for cancer.
We found that most of the prescriptions ordered to manage targeted therapy-induced dAEs were topical steroids (38%), and the majority (83%) of consultations with targeted therapy-induced dAEs responded to dermatologic treatment. We also found that, in most cases, following consultation with an oncodermatologist, it was safe to re-challenge patients with their targeted therapy. With proper assessment and effective management of dAEs by consulting dermatologists in the outpatient oncology setting, most maculopapular rashes induced by targeted agents could be effectively managed with topical corticosteroids. 29 Understandably, dermatologists have been established as superior diagnosticians for cutaneous disorders compared to their nondermatology counterparts;15,16,30–33 therefore, integration of oncodermatologists’ specialized clinical care at a cancer center may reduce anticancer dose alterations, treatment interruptions, and re-hospitalization, whilst maintaining skin-related QoL.
A retrospective data analysis represents a potential limitation to the interpretation of our results. First, it may introduce misclassification bias. Second, because of limited data acquisition from a single institution, we could only comment on those dAEs consulted and evaluated by a dermatologist within a specified period at a single cancer center, leading to limited generalizability. Although we did not compare the outcomes of patients who received a same-day dermatology consultation for acute dAEs with anticancer therapy interruption to those without alteration of oncologic treatment pre-consultation, we found that intervention by a dermatologist may have a clinically significant impact on both dermatologic and oncologic outcomes by reducing the utilization of systemic corticosteroids or permanent therapy interruption due to recurrence of dAEs on re-challenge.
This study included a large number of subjects treated with a variety of solid and hematologic malignancies, which are representative of the outpatient oncologic population with dermatologic events that may benefit from a dermatologic consultation. Also, it provides new evidence around which both oncology health care providers and dermatologists can center their collaborative efforts to mitigate treatment-related dAEs. The findings in this study fill a gap in quantifying the degree of inter-agreement between referring clinicians and consulting dermatologists on the need for anticancer therapy interruption due to acute dAEs. It also provides evidence to support the positive impact that outpatient dermatology consultations may have on reducing permanent targeted therapy interruption, specially targeted agents.
Supplementary Material
Acknowledgements
We would like to thank Dr. Sarah Noor, MD for assisting in the revision of this manuscript.
Funding sources: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research was additionally funded in part by Beca Excelencia, AEDV-Fundación Piel Sana (Dr. Freites-Martinez). The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; or in the preparation, review, or approval of the manuscript.
Common abbreviations:
- dAE
dermatologic adverse event
- QoL
quality of life
- CTCAE
common terminology criteria for adverse events
Footnotes
Original publication: This manuscript contains original unpublished work and is not being submitted for publication elsewhere at the same time.
Ethics: The procedures followed to conduct this research, in accordance with its ethical standards, were reviewed and approved by Memorial Sloan Kettering Cancer Center’s Institutional Review Board, protocol # 16–410.
Conflicts of Interests: Dr. Lacouture has a consultant/speaking role with Legacy Healthcare Services, Adgero Bio Pharmaceuticals, Amryt Pharmaceuticals, Celldex Therapeutics, Debiopharm, Galderma Research and Development, Johnson and Johnson, Novocure Inc, Lindi, Merck Sharp and Dohme Corporation, Helsinn Healthcare SA, Janssen Research & Development LLC, Menlo Therapeutics, Novartis Pharmaceuticals Corporation, F. Hoffmann-La Roche AG, AbbVie Inc, Boehringer Ingelheim Pharma Gmbh & Co. KG, Allergan Inc, Amgen Inc, E.R. Squibb & Sons LLC, EMD Serono Inc, Astrazeneca Pharmaceuticals LP, Genentech Inc, Leo Pharma Inc, Seattle Genetics, Bayer, Manner SAS, Lutris, Pierre Fabre, Paxman Coolers, Adjucare, Dignitana, Biotechspert, Teva Mexico, Parexel, OnQuality Pharmaceuticals Ltd, Novartis, and Our Brain Bank. Dr. Lacouture also receives research funding from Berg, Bristol-Myers Squibb, Lutris, Paxman, Novocure, US Biotest, and Veloce.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Rosen AC, Balagula Y, Raisch DW, Garg V, Nardone B, Larsen N, et al. Life-threatening dermatologic adverse events in oncology. Anticancer Drugs. 2014;25(2):225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen AC, Case EC, Dusza SW, Balagula Y, Gordon J, West DP, et al. Impact of dermatologic adverse events on quality of life in 283 cancer patients: a questionnaire study in a dermatology referral clinic. American journal of clinical dermatology. 2013;14(4):327–33. [DOI] [PubMed] [Google Scholar]
- 4.Barbu MA, Nitipir C, Voiosu T, Giurcaneanu C. Impact of dermatologic adverse reactions on QOL in oncologic patients: results from a single-center prospective study. Rom J Intern Med. 2018;56(2):96–101. [DOI] [PubMed] [Google Scholar]
- 5.Hackbarth M, Haas N, Fotopoulou C, Lichtenegger W, Sehouli J. Chemotherapy-induced dermatological toxicity: frequencies and impact on quality of life in women’s cancers. Results of a prospective study. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2008;16(3):267–73. [DOI] [PubMed] [Google Scholar]
- 6.Wong RK, Bensadoun RJ, Boers-Doets CB, Bryce J, Chan A, Epstein JB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2013;21(10):2933–48. [DOI] [PubMed] [Google Scholar]
- 7.Lacouture ME, Anadkat MJ, Bensadoun RJ, Bryce J, Chan A, Epstein JB, et al. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2011;19(8):1079–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balagula Y, Rosen ST, Lacouture ME. The emergence of supportive oncodermatology: the study of dermatologic adverse events to cancer therapies. Journal of the American Academy of Dermatology. 2011;65(3):624–35. [DOI] [PubMed] [Google Scholar]
- 9.Sibaud V, Leboeuf NR, Roche H, Belum VR, Gladieff L, Deslandres M, et al. Dermatological adverse events with taxane chemotherapy. European journal of dermatology : EJD. 2016;26(5):427–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sibaud V, Meyer N, Lamant L, Vigarios E, Mazieres J, Delord JP. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Current opinion in oncology. 2016;28(4):254–63. [DOI] [PubMed] [Google Scholar]
- 11.Sibaud V Dermatologic Reactions to Immune Checkpoint Inhibitors : Skin Toxicities and Immunotherapy. American journal of clinical dermatology. 2018;19(3):345–61. [DOI] [PubMed] [Google Scholar]
- 12.Sibaud V, Eid C, Belum VR, Combemale P, Barres B, Lamant L, et al. Oral Lichenoid Reactions associated with anti-PD-1/PD-L1 therapies: clinicopathological findings. Journal of the European Academy of Dermatology and Venereology : JEADV. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maza A, Berbis J, Gaudy-Marqueste C, Morand JJ, Berbis P, Grob JJ, et al. [Evaluation of dermatology consultations in a prospective multicenter study involving a French teaching hospital]. Annales de dermatologie et de venereologie. 2009;136(3):241–8. [DOI] [PubMed] [Google Scholar]
- 14.Tran H, Chen K, Lim AC, Jabbour J, Shumack S. Assessing diagnostic skill in dermatology: a comparison between general practitioners and dermatologists. The Australasian journal of dermatology. 2005;46(4):230–4. [DOI] [PubMed] [Google Scholar]
- 15.Arakaki RY, Strazzula L, Woo E, Kroshinsky D. The impact of dermatology consultation on diagnostic accuracy and antibiotic use among patients with suspected cellulitis seen at outpatient internal medicine offices: a randomized clinical trial. JAMA dermatology. 2014;150(10):1056–61. [DOI] [PubMed] [Google Scholar]
- 16.Song H, Robinson SN, Huang JT. Outpatient dermatology consultation impacts the diagnosis and management of pediatric oncology patients: A retrospective study. Journal of the American Academy of Dermatology. 2017;77(5):879–85. [DOI] [PubMed] [Google Scholar]
- 17.Penate Y, Borrego L, Hernandez N, Islas D. Pediatric dermatology consultations: a retrospective analysis of inpatient consultations referred to the dermatology service. Pediatric dermatology. 2012;29(1):115–8. [DOI] [PubMed] [Google Scholar]
- 18.Connolly DM, Silverstein DI. Dermatology consultations in a tertiary care hospital: A retrospective study of 243 cases. Dermatol Online J. 2015;21(8)13030/qt47m711t2. Published 2015 Aug 15 [PubMed] [Google Scholar]
- 19.Lorente-Lavirgen AI, Bernabeu-Wittel J, Pulpillo-Ruiz A, de la Torre-Garcia JM, Conejo-Mir J. Inpatient dermatology consultation in a Spanish tertiary care hospital: a prospective cohort study. Actas dermo-sifiliograficas. 2013;104(2):148–55. [DOI] [PubMed] [Google Scholar]
- 20.Storan ER, McEvoy MT, Wetter DA, El-Azhary RA, Camilleri MJ, Bridges AG, et al. Experience of a year of adult hospital dermatology consultations. International journal of dermatology. 2015;54(10):1150–6. [DOI] [PubMed] [Google Scholar]
- 21.Phillips GS, Freites-Martinez A, Hsu M, Skripnik Lucas A, Barrios DM, Ciccolini K, et al. Inflammatory dermatoses, infections, and drug eruptions are the most common skin conditions in hospitalized cancer patients. Journal of the American Academy of Dermatology. 2018;78(6):1102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coleman E, Ko C, Dai F, Tomayko MM, Kluger H, Leventhal JS. Inflammatory eruptions associated with immune checkpoint inhibitor therapy: A single-institutional, retrospective analysis with stratification of reactions by toxicity and implications for management. Journal of the American Academy of Dermatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikolaou V, Voudouri D, Tsironis G, Charpidou A, Stamoulis G, Triantafyllopoulou I, et al. Cutaneous toxicities of antineoplastic agents: data from a large cohort of Greek patients. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2019. [DOI] [PubMed] [Google Scholar]
- 24.Chen ST, Molina GE, Lo JA. et al. Dermatology Consultation Reduces Interruption of Oncologic Management Among Hospitalized Patients with Immune-related Adverse Events: A Retrospective Cohort Study [published online ahead of print, 2019 Sep 24] J Am Acad Dermatol. 2019;S0190–9622(19)32770–7 10.1016/j.jaad.2019.09.026 [DOI] [PubMed] [Google Scholar]
- 25.Chen AP, Setser A, Anadkat MJ, Cotliar J, Olsen EA, Garden BC, et al. Grading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events Version 4.0. Journal of the American Academy of Dermatology. 2012;67(5):1025–39. [DOI] [PubMed] [Google Scholar]
- 26.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22(3):276–82. [PMC free article] [PubMed] [Google Scholar]
- 27.Bhullar KS, Lagaron NO, McGowan EM, Parmar I, Jha A, Hubbard BP, et al. Kinase-targeted cancer therapies: progress, challenges and future directions. Mol Cancer. 2018;17(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andre F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. The New England journal of medicine. 2019;380(20):1929–40. [DOI] [PubMed] [Google Scholar]
- 29.Hu JC, Sadeghi P, Pinter-Brown LC, Yashar S, Chiu MW. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. Journal of the American Academy of Dermatology. 2007;56(2):317–26. [DOI] [PubMed] [Google Scholar]
- 30.Davila M, Christenson LJ, Sontheimer RD. Epidemiology and outcomes of dermatology in-patient consultations in a Midwestern U.S. university hospital. Dermatology online journal. 2010;16(2):12. [PubMed] [Google Scholar]
- 31.Falanga V, Schachner LA, Rae V, Ceballos PI, Gonzalez A, Liang G, et al. Dermatologic consultations in the hospital setting. Archives of dermatology. 1994;130(8):1022–5. [PubMed] [Google Scholar]
- 32.Kroshinsky D, Cotliar J, Hughey LC, Shinkai K, Fox LP. Association of Dermatology Consultation With Accuracy of Cutaneous Disorder Diagnoses in Hospitalized Patients: A Multicenter Analysis. JAMA dermatology. 2016;152(4):477–80. [DOI] [PubMed] [Google Scholar]
- 33.Bauer J, Maroon M. Dermatology inpatient consultations: a retrospective study. Journal of the American Academy of Dermatology. 2010;62(3):518–9. [DOI] [PubMed] [Google Scholar]
- 34.Clabbers JMK, Boers-Doets CB, Gelderblom H, Stijnen T, Lacouture ME, van der Hoeven KJM, et al. Xerosis and pruritus as major EGFRI-associated adverse events. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2016;24(2):513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenbaum SE, Wu S, Newman MA, West DP, Kuzel T, Lacouture ME. Dermatological reactions to the multitargeted tyrosine kinase inhibitor sunitinib. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2008;16(6):557–66. [DOI] [PubMed] [Google Scholar]
- 36.Lacouture ME, Desai A, Soltani K, Petronic-Rosic V, Laumann AE, Ratain MJ, et al. Inflammation of actinic keratoses subsequent to therapy with sorafenib, a multitargeted tyrosine-kinase inhibitor. Clinical and experimental dermatology. 2006;31(6):783–5. [DOI] [PubMed] [Google Scholar]
- 37.Drucker AM, Wu S, Busam KJ, Berman E, Amitay-Laish I, Lacouture ME. Rash with the multitargeted kinase inhibitors nilotinib and dasatinib: meta-analysis and clinical characterization. Eur J Haematol. 2013;90(2):142–50. [DOI] [PubMed] [Google Scholar]
- 38.Chu D, Lacouture ME, Weiner E, Wu S. Risk of hand-foot skin reaction with the multitargeted kinase inhibitor sunitinib in patients with renal cell and non-renal cell carcinoma: a meta-analysis. Clin Genitourin Cancer. 2009;7(1):11–9. [DOI] [PubMed] [Google Scholar]
- 39.Chu EY, Wanat KA, Miller CJ, Amaravadi RK, Fecher LA, Brose MS, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. Journal of the American Academy of Dermatology. 2012;67(6):1265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boers-Doets CB, Epstein JB, Raber-Durlacher JE, Ouwerkerk J, Logan RM, Brakenhoff JA, et al. Oral adverse events associated with tyrosine kinase and mammalian target of rapamycin inhibitors in renal cell carcinoma: a structured literature review. The oncologist. 2012;17(1):135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amitay-Laish I, Stemmer SM, Lacouture ME. Adverse cutaneous reactions secondary to tyrosine kinase inhibitors including imatinib mesylate, nilotinib, and dasatinib. Dermatologic therapy. 2011;24(4):386–95. [DOI] [PubMed] [Google Scholar]
- 42.Hawerkamp HC, Kislat A, Gerber PA, Pollet M, Rolfes KM, Soshilov AA, et al. Vemurafenib acts as an aryl hydrocarbon receptor antagonist: Implications for inflammatory cutaneous adverse events. Allergy. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.