Abstract
Known to occur in widespread outbreaks, epidemic keratoconjunctivitis (EKC) is a severe ocular surface infection with a strong historical association with human adenovirus (HAdV). While the conjunctival manifestations can vary from mild follicular conjunctivitis to hyper-acute, exudative conjunctivitis with formation of conjunctival membranes, EKC is distinct as the only form of adenovirus conjunctivitis in which the cornea is also involved, likely due to specific corneal epithelial tropism of its causative viral agents. The initial development of a punctate or geographic epithelial keratitis may herald the later formation of stromal keratitis, and manifest as subepithelial infiltrates which often persist or recur for months to years after the acute infection has resolved. The chronic keratitis in EKC is associated with foreign body sensation, photophobia, glare, and reduced vision. However, over a century since the first clinical descriptions of EKC, and over 60 years since the first causative agent, human adenovirus type 8, was identified, our understanding of this disorder remains limited. This is underscored by a current lack of effective diagnostic tools and treatments. In part, stasis in our knowledge base has been encouraged by the continued acceptance, and indeed propagation of, inaccurate paradigms pertaining to disease etiology and pathogenesis, particularly with regard to mechanisms of innate and adaptive immunity within the cornea. Owing to its often persistent and medically refractory visual sequelae, reconsideration of key aspects of EKC disease biology is warranted to identify new treatment targets to curb its worldwide socioeconomic burden.
Keywords: Human adenovirus, epidemic keratoconjunctivitis, subepithelial infiltrates, innate immunity, adaptive immunity
1. INTRODUCTION
First described as “superficial punctate keratitis” and “nummular keratitis” in the late 19th century by Austrian physicians Fuchs (Fuchs, 1889) and von Stellwag (Carion von Stellwag, 1889), respectively, epidemic keratoconjunctivitis (EKC) is a highly contagious and potentially visually disabling infection of the conjunctiva and cornea. EKC is most commonly the result of human adenovirus (HAdV) infection, particularly HAdV species D types 8, 37, 53, 54, 56 and 64, and is characterized by a severe acute inflammatory response in these tissues. EKC is initially marked by epiphora and chemosis, conjunctival follicular hyperplasia and exudation, and punctate and/or geographic epithelial keratitis (Rajaiya and Chodosh, 2006). EKC is the most severe form of HAdV-associated conjunctivitis and is the only form to significantly involve the cornea. Its defining feature, or sine qua non, is the formation of delayed-onset, corneal subepithelial infiltrates (SEIs) (Butt and Chodosh, 2006). These cause foreign body sensation, photophobia, glare, and reduced vision, and may persist and/or recur for months to years despite treatment.
The eminent infectious diseases specialist, Ernest Jawetz, first identified HAdV-D8 as the cause of EKC after outbreaks with thousands of cases in shipyard workers of Hawaii and San Francisco in 1941 (Jawetz et al., 1957), leading to characterization of the malady as “shipyard eye”. Jawetz later described his search for an etiology as “replete with mystery” which was made difficult by “myth, fiction and hearsay” (Jawetz, 1959). Here, Jawetz was referring to contradictory and implausible accounts of others claiming to have isolated a viral etiology without definitive and reproducible evidence. Sixty years on, these sentiments are still pertinent. EKC remains relatively misunderstood, and our understanding of its disease biology is affected, to an extent, by continued acceptance of antiquated, erroneous paradigms concerning its key features. The etiologies of EKC, which may include as yet undetected pathogens, have not been completely elucidated. Further, a modem understanding of EKC pathogenesis and its key inflammatory mediators has been undermined by the long-held notion of the cornea as an inert immunological substrate or “immunologic blotter”, an idea still perpetuated within academic circles today. An evidence base is necessary to inform efforts to better define patterns in clinical presentation and disease course, identify feasible treatment targets, and establish guidelines for clinical management. We review current progress and identify future avenues of research to achieve better control of disease transmission, prevent chronic visual complications, and reduce the substantial socioeconomic costs associated with EKC.
2. ETIOLOGY OF EKC
2.1. Human Adenovirus (HAdV): A Short History
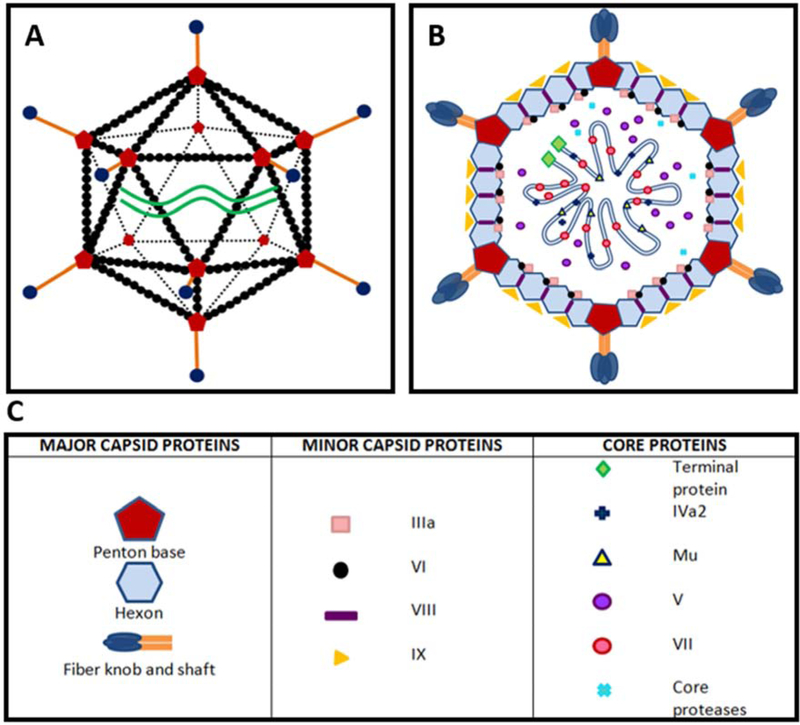
First isolated in 1953 from human adenoids (Rowe et al., 1953), HAdVs belong to the genus Mastadenovirus and family Adenoviridae, and are medium sized (~70–110 nm), double-stranded DNA viruses consisting of a linear genome of approximately 36,000 base pairs (Russell, 2009). They are nonenveloped viruses with an icosahedral capsid composed of 20 triangular faces, 240 hexon trimers, and 12 vertices with each vertex composed of a ring of five penton base proteins with a central protruding trimeric fiber protein and distal “knob” (Fig. 1a). The capsid is reinforced by minor proteins, including Ilia, VI, VIII and IX, which surround a host of core proteins associated with viral DNA, including terminal DNA proteins, IVa2, Mu, V, VII and core proteases (Fig. 1b and 1c). As non-enveloped viruses, HAdVs are highly resistant to environmental desiccation, and remain infectious for up to one month following dissociation from their hosts (Nauheim et al., 1990). They are therefore capable of both direct and indirect modes of transmission. HAdVs exhibit near exclusive species specificity for humans (Ginsberg et al., 1991; Lam et al., 2011), although in experimental models, mice (Chintakuntlawar et al., 2007), rabbits (Gordon et al., 1992) and hamsters (Toth et al., 2008) can all be infected, albeit to a limited degree. HAdVs cause an array of diseases, including mucosa-associated respiratory, gastrointestinal, genitourinary, and ocular surface infections, as well as neurological infections such as meningoencephalitis. In immunocompromised patients, HAdVs are commonly associated with graft-site infections and often lethal disseminated disease, with most cases due to viral reactivation (Hierholzer, 1992; Lion, 2014, 2019; Matthes-Martin et al., 2013; Shields et al.,1985).
Fig. 1.
Ultrastructure and cross-sectional view of human adenovirus. (A) Schematic diagram of the icosahedral ultrastructure of HAdV, composed of20 triangular faces, 240 hexon trimers and 12 vertices. (B) Cross-sectional view through HAdV. (C) Corresponding table of important viral proteins. The HAdV capsid consists of major capsid proteins (hexon, penton, fiber knob and shaft) and minor capsid proteins (Ilia, VI, VIII, and IX). Capsid proteins encapsulate an assortment of core proteins, which include terminal DNA proteins, IVa2, Mu, V, VII and core proteases. The function and location of HAdV core proteins in particular remain a matter of speculation.
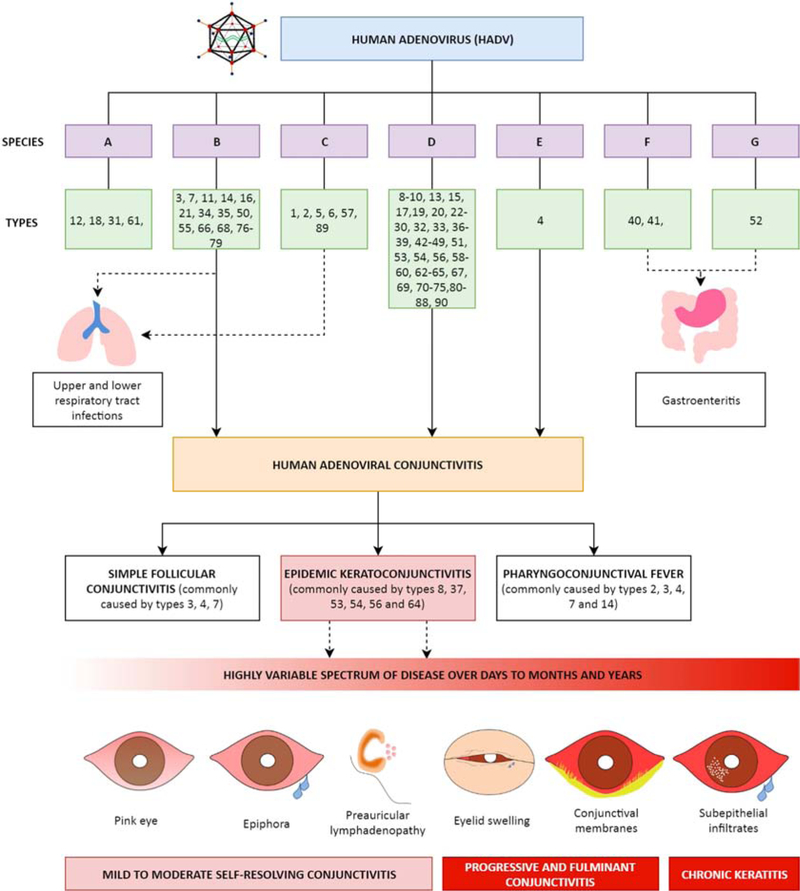
Historically, the initial 51 HAdV types were classified based on serum neutralization and hemagglutination inhibition. Serum neutralization is attributed to the epsilon determinant, an epitope on the hexon protein formed by two hypervariable loops, coded for at the genome level by hypervariable regions 1–6 and 7, respectively. Hemagglutination inhibition is a property of the fiber protein. These methods have now been superseded by whole genome sequencing (WGS) and molecular genotyping, as endorsed by the National Institute of Health GenBank since 2011. Therefore, the term “genotype” now succeeds “serotype” to reflect the method of characterization. As of the writing of this review, there are 7 HAdV species (A-G) and 103 HAdV genotypes in GenBank (Fig. 2 and 3). Proposed new types are screened for approval as “new” types by the Human Adenovirus Working Group, based on either a unique sequence for the penton base, hexon, and/or fiber protein, or by a unique recombination between previously characterized penton base, hexon, and/or fiber gene components (Liu et al., 2012; Seto et al., 2011). New types are generated primarily through homologous recombination during viral replication in the setting of co-infection (Ismail et al., 2018b). The arrival of the HIV pandemic in the mid-late 1980s coincided with the accelerated emergence of new HAdV types, with species D types 43–51 all isolated from AIDS patients (DeJong et al., 1999; Hierholzer et al., 1988; Schnurr and Dondero, 1993). It has been postulated that immunosuppression gives rise to the conditions which favor viral persistence and co-infection, prerequisites to homologous recombination between viruses, but the exact mechanisms for the emergence of so many novel viruses in HIV-infected patients remain elusive (Echavarria, 2008).
Fig. 2.
Overview of HAdV species, types, and associated clinical syndromes. As of the writing of this review, there are seven known species of HAdV (A-G) and 103 genotypes, the latter characterized as unique types either by the presence of novel coding sequences for at least one major capsid protein (penton, hexon, and fiber protein), or by a unique homologous recombination of the major capsid genes. The clinical manifestations of HAdV infection, with a focus on human adenoviral conjunctivitis, are also shown. Note types 91–103 were only recently published, characterized as type D adenoviruses. The sources of these HAdVs are unknown (Ismail et al., 2018a), and their disease associations have yet to be described.
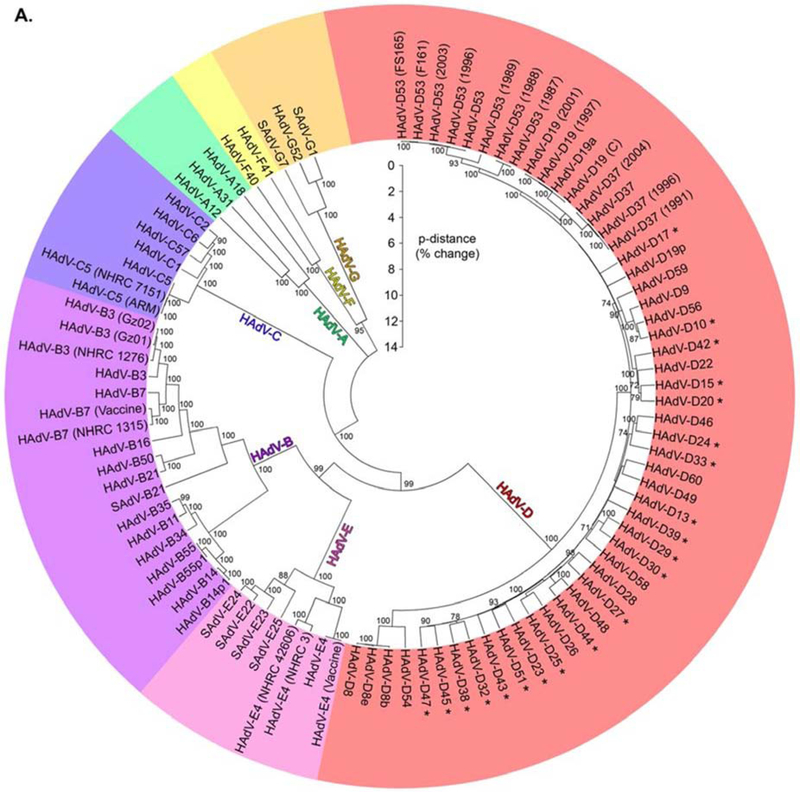
Fig. 3.
Phylogenetic analysis of HAdV species and types. A phylogenetic tree of HAdV diversity performed for the first 60 types shows the proportional difference between genornes (Robinson et al., 2013a), reprinted with permission. There are now 103 HAdV types in GenBank.
With reduced expense and greater ease of WGS, HAdV identification at the level of its nucleotide sequence now permits the correct and exact taxonomy of disease causing viruses (Walsh et al., 2010), and WGS has also since been completed for all the original 51 HAdV serotypes (Robinson et al., 20lla). As an example of how WGS has fundamentally changed how we characterize and type HAdVs, genome sequencing has now unequivocally shown that what was previously called HAdV-D19a is actually a recombinant of HAdV-D19, HAdV-D22 and HAdV-D37 (Zhou et al., 2012). Only a small contribution derives from HAdV-D19, specifically the epsilon determinant responsible for its serum neutralization. Now retyped as HAdV-D64, this “Trojan horse” virus appears to the humoral immune system as HAdV-D19, but its genome is comprised almost entirely of the nucleotide sequences of HAdV-D37 with a small contribution from HAdV-D22. HAdV-D64, formerly HAdV-D19a, exhibits corneal epithelial tropism, while the prototype HAdV-D19 does not (Zhou et al., 2012).
2.2. Multiple Etiologies of EKC
The association between HAdV species B, D and E and ocular surface infection is well-established (Fig. 2), with disease manifestations ranging from simple follicular conjunctivitis (types 3, 4, 7) and pharyngoconjunctival fever (types 3, 7, 14) to the more severe EKC (types 8, 37, 53, 54, 56 and 64) (Aoki et al., 2008; Ariga et al., 2004; Hage et al., 2017; Ishiko and Aoki, 2009; Ishiko et al., 2008; Kaneko et al., 2011a; Nilsson et al., 2011; Robinson et al., 2009; Robinson et al., 2011b; Walsh et al., 2009; Zhou et al., 2012). However, the recent isolation of HAdV-85 from two Japanese patients with SEI-associated adenoviral conjunctivitis (Hashimoto et al., 2018) may suggest that we have not yet elucidated all potential etiologies capable of causing EKC, which remains a barrier to our understanding and ability to develop new therapies. Eye care providers and health care facilities are the source of infection in many outbreaks of adenovirus infection in general (Dawson and Darrell, 1963; Sammons et al., 2019), and as discussed above, EKC in particular. However, no index case or source of infection is identified in up to half of affected patients in some case series (Butt and Chodosh, 2006; Mueller and Klauss, 1993). In this context, it has been postulated that in addition to causing acute outbreaks, EKC can also be endemic, in some instances arising from reservoirs found within individual communities, made possible by indirect transmission due to adenoviral survival in the environment (Butt and Chodosh, 2006; Dawson et al., 1963; Dawson et al., 1972; Zweighaft et al., 1977). This would render the adjective “epidemic”, as first proposed by Hogan and Crawford in 1942 (Hogan and Crawford, 1942), as not always true. Secondly, as discussed above, we can draw lessons from the inaccurate ascription ofHAdV-D19 as a prime cause of EKC (Darougar et al., 1977), which unfortunately persists in the current literature.
The most compelling evidence for non-HAdV EKC-associated pathogens comes from a recent analysis of an international randomized controlled trial investigating the administration of auricloscene for keratoconjunctivitis by Lee and colleagues (Lee et al., 2018). They found that within 500 patients thought by the trial investigators to have EKC, 22% were adenoviral negative on quantitative PCR and of these, 36% developed SEIs which were clinically indistinguishable from the PCR-positive cohort. Subsequent WGS utilizing the metagenomics database Kraken (Wood and Salzberg, 2014) on 16 selected PCR-negative patients recovered no HAdV genomic material. Curiously, there was a higher proportion of samples containing torque teno virus (TTV) in these PCR-negative patients when compared to 16 controls. Also of note, of those patients who were PCR-positive for adenovirus, only 81% were PCR-positive for HAdV-D. About 9% of patients were infected with HAdV-E4 and 6% with HAdV-B3, with other HAdV-B viruses accounting for almost all the remainder. These data suggest that as metagenomics-based methods of viral detection improve, other viruses may be implicated in EKC. Known causes of SEIs that can mimic adenoviral keratitis include infection by members of the herpes family, including herpes simplex virus, varicella zoster virus, and Epstein-Barr virus, and also bacterial infections such as chlamydia. Other candidates may be considered, including TTV, which was recently associated with culture-negative endophthalmitis (Lee et al., 2015). RNA viruses which cause conjunctivitis, including the picomaviruses (coxsackie and enterovirus), may also be involved in EKC
3. CLINICAL MANIFESTATIONS OF EKC
Acute EKC remains a clinical diagnosis, characterized by the classical triad of preauricular lymphadenopathy, hyperacute, follicular and sometimes membranous conjunctivitis, and punctate or geographic epithelial keratitis (Chodosh, 2011; Dawson et al., 1972) (Fig. 4). The severity of disease is highly variable following HAdV incubation, usually 5–12 days, and ranges from mild, subclinical ocular surface inflammation to fulminant conjunctivitis characterized by marked photophobia, epiphora, chemosis, and eyelid swelling. The contralateral eye is affected in around 70% of patients (Kimura et al., 2009), albeit usually to a less severe extent. Conjunctival membranes form in 24.1%–60% (Butt and Chodosh, 2006; Dawson et al., 1972) of patients, although there is ongoing misunderstanding regarding the distinction between pseudomembranes and membranes in the literature. The prominent ophthalmologist Duke-Elder distinguished the two by the absence or presence, respectively, of bleeding upon removal (Duke-Elder, 1965a). This appears to be a somewhat arbitrary distinction. Both pseudomembranes and membranes likely exist on a continuum of marked conjunctival inflammatory responses which produce fibrin and neutrophil-rich conjunctival exudates (pseudomembranes). Given time and in the absence of treatment, they may become vascularized (Chintakuntlawar and Chodosh, 2010), and will therefore bleed upon removal (membranes). Bleeding should therefore be expected upon removal when a pseudomembrane is sufficiently severe and not removed or treated before neovascularization has begun. Eventually, untreated conjunctival membranes become incorporated into host tissue, evident as late subconjunctival fibrosis. When membranes are present on adjacent bulbar and tarsal conjunctival surfaces, they can evolve to symblepharon and in the worst cases may contribute to restriction of ocular motility.
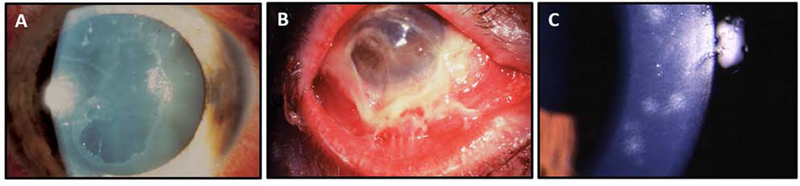
Fig. 4.
Clinical manifestations of EKC. (A) Geographic epithelial keratitis manifesting as a large epithelial defect. (B) Fulminant membranous conjunctivitis with early symblepharon formation. (C) Slit lamp biomicroscopy of corneal SEIs seen in EKC. These are usually <0.5mm and relatively uniform, which may distinguish them from subepithelial keratitis seen in HSV and VZV keratitis in which SEIs tend to be larger and more varied in diameter.
The severity of conjunctivitis in EKC is distinctive, but the hallmark of EKC is corneal involvement, which may begin in the acute stage as punctate or geographic epithelial keratitis (Chodosh et al., 1995; Dawson et al., 1972; Hogan and Crawford, 1942). The epithelial keratitis of EKC resolves within several days of onset, but up to 60% of patients then develop stromal keratitis, manifest by multifocal SEIs, or “nummuli”, typically 14–21 days after symptom onset (Lee et al., 2018). EKC-associated SEIs are typically small, less than 0.5mm, and relatively uniform in size within patients. This can in some cases distinguish them from the nummuli seen in patients with varicella zoster and herpes simplex virus keratitis, in which SEIs tend to vary in size, and often exceed 1 mm in diameter (J Chodosh, personal observation).
Most strongly associated with HAdV-D infection, EKC-associated stromal keratitis can be stubborn and difficult to treat. While SEIs resolve within 5–6 weeks in most cases, there is a significant minority of patients in whom the keratitis will persist or recur for months to years after acute infection (Butt and Chodosh, 2006; Freyler and Sehorst, 1976; Pettit and Holland, 1979). SEIs may be asymptomatic but can also cause corneal aberrations and irregular astigmatism, reduced vision, glare, photophobia, and foreign body sensation (Aydin Kuma et al., 2015). Speculation exists regarding a possible correlation between the severity of conjunctivitis and an increased likelihood of developing SEIs, but existing data is insufficient to draw conclusions (Butt and Chodosh, 2006). Although infection with viruses from HAdV-D is more commonly associated with delayed-onset SEIs, it is now clear they can also develop with infection by other HAdV species (Lee et al., 2018) and with other non-HAdV infections, as discussed above. Regardless, the potential for chronic or relapsing and remitting keratitis in patients with EKC is not consistent with the frequently encountered view that EKC is a trivial, self-limited infection without visually significant sequelae.
4. PATHOGENESIS OF EKC WITH CLINICAL CORRELATIONS
4.1. HAdV Entry into Cells
HAdV corneal infection begins in the corneal epithelium (Chodosh et al., 1995; Dawson et al., 1972; Imre et al., 1963; Maudgal, 1990). Infection requires attachment of the viral fiber knob to a molecule on the cell surface (Huang et al., 1999; Ismail et al., 2016; Louis et al., 1994). Cellular receptors known to bind HAdV fiber knob include coxsackie-adenovirus receptor (CAR)(Bergelson et al., 1997), major histocompatibility complex (MHC) class I (Hong et al., 1997), membrane cofactor protein (CD46) (Gaggar et al., 2003; Segerman et al., 2003a), and sialic acid (Arnberg et al., 2000; Arnberg et al., 2002). Most HAdVs utilize CAR as their primary attachment site, but HAdVs associated with EKC display a preference for CD46 (Wu et al., 2004) and/or sialic acid (Arnberg et al., 2000; Arnberg et al., 2002), more specifically the sialic acid residues on GD1a glycan (Nilsson et al., 2011). HAdV internalization is mediated by a secondary interaction between the amino acid motif, Arg-Gly-Asp (RGD), which is expressed (Segerman et al., 2003b) on a loop protruding from each penton base protein, and host cellular integrins, in particular, αvβl, αvβ3, αvβ5, and α3β1 (Li et al., 2001; Storm et al., 2017; Wickham et al., 1993). Because there are 5 penton base proteins in a ring at the base of each fiber protein, each penton base capsomer binds and aggregates 5 integrin molecules (Chiu et al., 1999), which then undergo a conformational change that activates downstream intracellular signaling.
The subsequent signaling cascade includes activation of numerous downstream messengers such as phosphoinositide 3-kinase (PI3K) and the Rho family GTPases (Li et al., 1998a; Li et al., 1998b). For HAdV-C, these events mediate internalization of virions into endosomes via clathrin-coated pits on the plasma membrane. Dynamin-mediated constriction and subsequent budding of these vesicles allow for their preordained transport to the nucleus where viral replication and gene production occur (Wang et al., 1998). However, viral internalization of HAdV-D differs from that of HAdV-C, and is cell-type specific. For example, HAdV-D37 infection of corneal fibroblasts has been shown to involve caveolin-1 rather than clathrin (Yousuf et al., 2013), suggesting other differences in viral entry and trafficking between HAdV species and/or types, and as shown in other cell types (Teigler et al., 2014). Intracellular signaling for HAdV-D also differs from that of HAdV-C, with Src kinase a critical upstream signaling molecule for EKC viruses (Natarajan et al., 2003), and further involvement of focal adhesion kinase (Natarajan et al., 2002a) and mitogen-activated protein kinases (Rajaiya et al., 2009; Rajaiya et al., 2008; Xiao and Chodosh, 2005) in subsequent downstream events. For HAdV-D associated with EKC, PI3K functions to maintain cell viability sufficient to support viral replication (Rajala et al., 2005), rather than viral internalization (Natarajan et al., 2003).
There are other requisite events in infection of ocular surface cells in vivo beyond receptor binding. Membrane-associated mucins (MAMs) form a tethered interface between epithelium and the external environment. In order to infect mucosal epithelial cells, HAdVs must first pass through a MAM-rich glycocalyx, which forms a physical barrier to infection. On the ocular surface, it has been shown that one MAM in particular, MUC16, plays a dominant role in ocular surface barrier function (Gipson et al., 2014). Remarkably, the EKC pathogen HAdV-D37 induces release of the extracellular domain of MUC16, permitting infection of underlying ocular surface epithelial cells, while the non-EKC inducing HAdV-Dl9 does not (Menon et al., 2016). These data suggest that EKC viruses may have evolved unique means of infection specific to the ocular surface.
4.2. Ocular Surface Inflammation in Acute EKC
Following binding and internalization of HAdVs, a profound but non-specific innate immune response is mounted within infected tissues. Limited evidence suggests this is orchestrated by local dendritic cells, macrophages and natural killer (NK) lymphocytes, which produce a storm of pro-inflammatory cytokines including IL-l, IL-6, IL-8, CCL2, IFN-γ, and TNF-α (Muruve et al., 1999; Yawata et al., 2016), but also by cytokines produced by resident ocular surface epithelial and stromal cells (Chintakuntlawar and Chodosh, 2009; Lee et al., 2019; Mukherjee et al., 2015; Natarajan et al., 2002a; Natarajan et al., 2003; Pennington et al., 2019; Rajaiya et al., 2009; Rajaiya et al., 2008; Rajaiya et al., 2015; Teigler et al., 2014; Xiao and Chodosh, 2005; Yousuf et al., 2016; Yousuf et al., 2013). Increased vascular flow and permeability gives rise to marked conjunctival injection, localized petechial hemorrhages, and frank exudation. HAdVs also induce local immune dysfunction in the conjunctiva. This includes the modulation of mature CD56dim NK populations towards less mature CD56bright NK subsets, amplification of the NK-inhibitory effect of HLA-E found on the surface of conjunctival epithelial cells, and concomitant downregulation of NK-activating ligands (Yawata et al., 2016). The inflammatory response by T-cells and local macrophages leads to the production of fibrin and neutrophil-rich exudates.
These are the very same exudates that form membranes on the bulbar and palpebral surfaces, and can undergo neovascularization. Over time, untreated conjunctival membranes become epithelialized and incorporated into the conjunctiva. Therefore, a prior membranous conjunctivitis may be identified later as subconjunctival fibrosis and/or symblepharon (Chintakuntlawar and Chodosh, 2010).
4.3. Historical Paradigms Regarding Corneal Responses to Injury
The view of the cornea as an immunologically inert bystander in inflammation following injury persists widely in the published literature, in part due to its erroneous conflation with the notion of corneal immune privilege in the context of transplant biology (Khodadoust and Silverstein, 1972; Streilein et al., 1979), but also due to a general reticence in science and medicine to critically evaluate claims proposed by eminent scholars. The brilliant British ophthalmologist Barrie Jones’ conception of the corneal stroma as an “immunological blotter” (Jones, 1958) following corneal insult continues to be widely cited in reference books, reviews, and original articles (Arcieri et al., 2004; Dosso and Rungger-Brandle, 2008; Duke-Elder, 1965b; Gordon et al., 1996; Jhanji et al., 2015; Laibson et al., 1970; Tullo and Higgins, 1979), and appears to have been canonized in our literature without question. Jones hypothesized that the soaking of adenoviral antigens from the presumed site of infection – the corneal epithelium – into the underlying and inert corneal stroma, culminates in an antibody-antigen reaction in the stroma, giving rise to SEIs (Jones, 1958). While indeed an elegant model, Jones’ theory has never been reproduced experimentally for adenovirus infections. Further, an antigen-antibody precipitate in the cornea typically manifests as a Wessely ring (Wessely, 1911), in which a visible ring in the corneal stroma equates to the zone where concentrations of antibody and antigen are equal. The same phenomenon can be reproduced in vitro and is characterized as an Ouchterlony line (Ouchterlony, 1949). Most importantly, Wessely rings are not part of the clinical presentation of EKC at any stage.
4.4. Innate Immune Responses in EKC
There is now a wealth of experimental evidence to challenge the idea of the cornea as an immunologically apathetic substrate. In vitro studies utilizing a novel 3-dimensional “corneal facsimile” model of the corneal stroma (Chodosh et al., 2000; Rajaiya et al., 2015), essentially a corneal stroma and basement membrane in a test tube, along with development of a mouse model of adenovirus keratitis in which HAdV induces innate immune responses but does not replicate (Chintakuntlawar et al., 2007), have permitted detailed studies of the innate immune responses mediated by infected corneal cells. To create the corneal facsimile, primary human keratocytes are dispersed within a type I collagen matrix, overlaid with an epithelial basement membrane mimic (Matrigel®), and cultured on a tissue culture insert with a pore size large enough to permit passage of leukocytes through the supporting membrane (Fig. 5). After overnight infection with HAdV-D37, and within one hour after placing human peripheral blood leukocytes in the media beneath the insert, the immune cells migrate against gravity through the collagen matrix to form sub-basement membrane foci of neutrophil infiltration, similar to SEIs seen in EKC. Infiltrates form at foci of IL-8 (CXCL8) deposition in the basement membrane, and co-localize to the basement membrane protein heparan sulfate, shown by others to stably bind IL-8 (Frevert et al., 2003) (Fig. 6). Leukocytes do not migrate nor do infiltrates form in corneal facsimiles without the presence of infected keratocytes. Inhibitors of Src and p38 MAP kinase block infiltrate formation (Rajaiya et al., 2015).
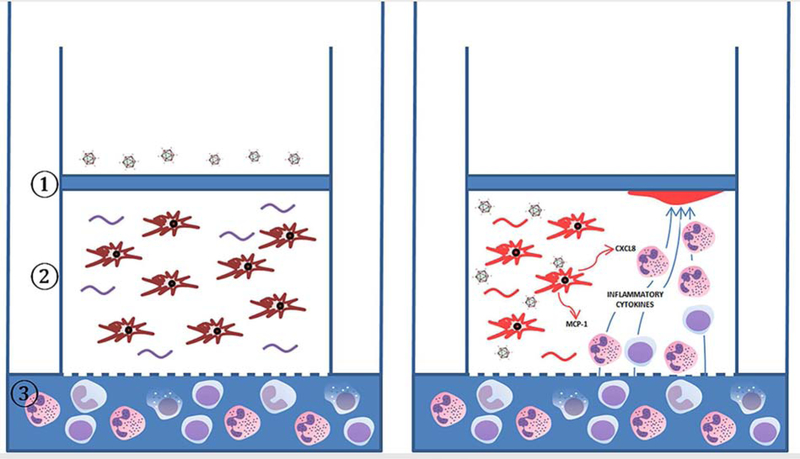
Fig. 5.
Modeling formation of subepithelial infiltrates using the “human corneal facsimile”. (A) The 3-dimensional “corneal facsimile” consists of ① an overlying epithelial basement membrane mimic, Matrigel®, which contains the important molecule heparan sulfate;② primary human keratocytes distributed within a type I collagen matrix, and underlying porous supporting membrane. To model adenovirus keratitis, corneal keratocytes are infected with HAdV-D37 overnight, followed by the placement of human peripheral blood leukocytes in the media beneath the test tube insert, as seen in ③. (B) Remarkably, CD45+ leukocytes migrate against gravity through the matrix to fonn sub-basement membrane foci, similar to those seen in EKC. Localized chemotaxis to the sub-basement membrane is thought to occur due to co-localization of CXCL8 and heparan sulfate.
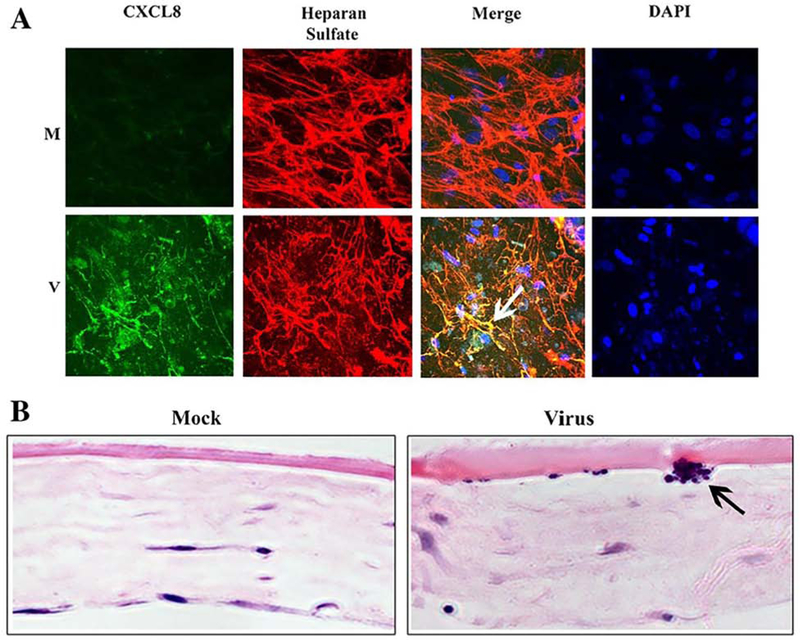
Fig. 6.
Confocal microscopy and histopathology of subepithelial infiltrate formation. (A) Confocal microscopy images of in vitro “corneal facsimiles”, with mock (M, top row) and HAdV-D37-infected facsimiles (V, bottom row), reproduced with permission (Rajaiya et al., 2015). Abundant CXCLS staining in virus-infected facsimiles, with CXCLS and heparan sulfate co-localization to the sub-basement membrane, are seen in the first and third columns, respectively. Staining with 4’, 6-diamidino-2-phenylindole (DAPI), as shown in the fourth column, demonstrated local infiltration of neutrophils to the sub-basement membrane area (white arrow). (B) Hematoxylin and eosin-stained histopathology of mock and infected facsimiles, showing a collection of neutrophils (black arrow) in the sub-basement membrane region only after virus infection.
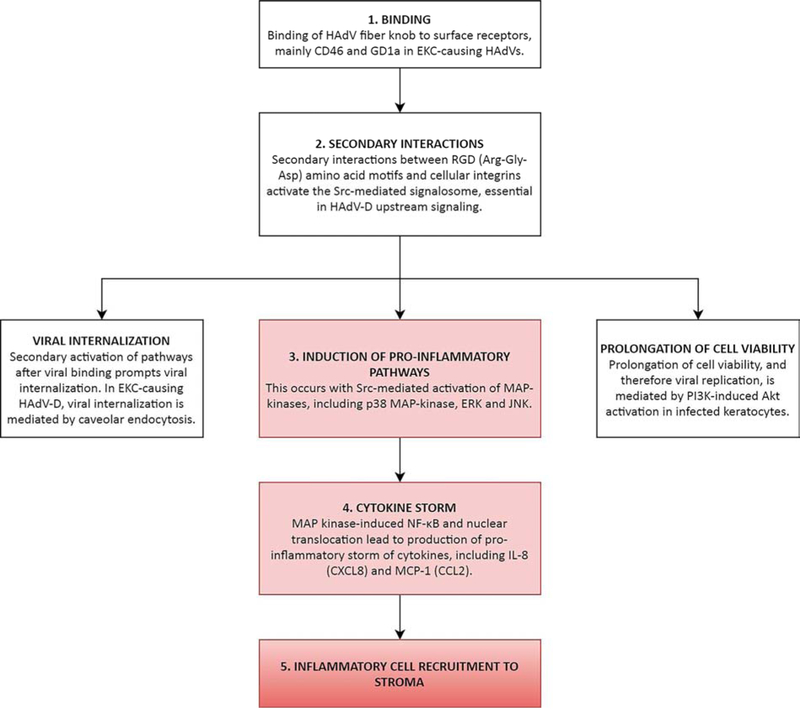
The mouse model of adenovirus keratitis is generated by micro-injection of HAdV-D37 at high titer into the corneal stroma of the C57BL/6j mouse (Chintakuntlawar et al., 2007)(Fig. 7–9). Clinically evident corneal stromal inflammation starts at one day and peaks at four days post-injection, and is characterized by early expression of the mouse IL-8 homologue KC (CXCL1) and delayed expression of MCP-1 (CCL2), which mediate infiltration of neutrophils (Chintakuntlawar and Chodosh, 2009) and then activated monocytes, respectively. Remarkably, at six weeks post infection, long after the acute keratitis has resolved, approximately one-third of mouse eyes develop recurrent SEIs similar to human patients with EKC. In further studies, it was shown that the key virus-associated molecular pattern (VAMP) responsible for acute keratitis is the adenovirus capsid. More specifically, integrin aggregation induced by binding of the multiple (five) penton base RGD loops present on each penton base capsomer (Chiu et al., 1999) induces downstream intracellular signaling requisite for the production of chemokines by infected corneal stromal cells (Chintakuntlawar et al., 2010). In the same mouse model, signaling by interaction of HAdV DNA with toll-like receptor 9 (TLR9) induced IL-6 expression but was by itself insufficient to induce clinically evident keratitis. Thus, HAdV DNA is also a VAMP in the corneal stroma, albeit a less potent inflammatory signal. However, signaling after infection through a combination of TLR2 and TLR9 contributed synergistically to stromal inflammation, and was mediated by MyD88 activation (Zhou et al., 2017). In tum, MyD88 activation contributed to phosphorylation of Src, shown previously to be activated in keratocytes by HAdV-D64 infection. In this experimental system, Src is a key upstream regulator of MAP kinase activation, NFKB phosphorylation and nuclear translocation, and pro-inflammatory gene expression (Natarajan et al., 2003; Rajaiya et al., 2008; Xiao and Chodosh, 2005).
Fig. 7.
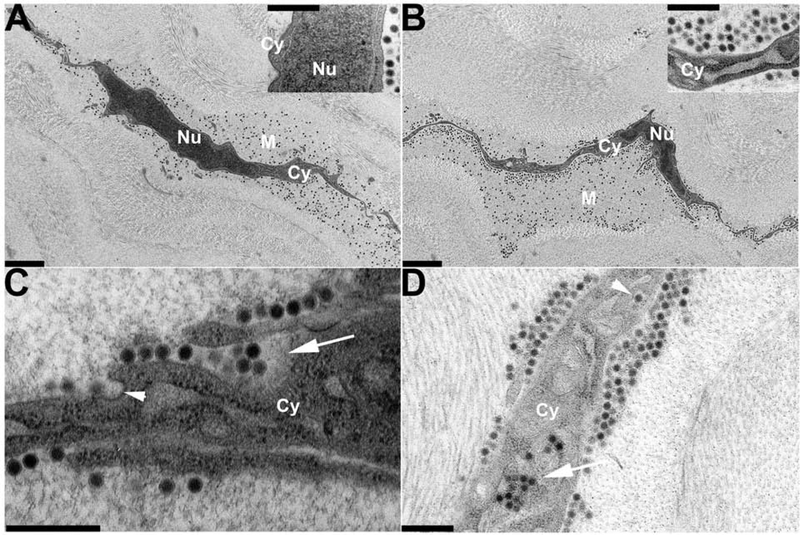
Transmission electron microscopy of early changes in the C57Bl/6j mouse cornea following intra-stromal injection with 1μL (105 tissue culture infectious doses) of HAdV-D37, reprinted with author permission (Mukherjee et al., 2015). Photomicrographs of stromal cells were taken (A) immediately, (B) 30 minutes, (C) 1 hour, and (D) 2 hours following infection. Cy – cytoplasm; Nu – nucleus; M – pericellular matrix. Depending on magnification, viral particles appear either as electron dense black dots. At 1 hour (C), the initial stages of viral entry is mediated by caevolae-dependent endocytosis (arrowhead), while cellular ruffling is seen in response to congregations of multiple viral particles (arrow). Within 2 hours (D), viral internalization within the stromal cell has occurred. Single viruses are internalized via endosomes (top arrowhead), while multiple viruses can be internalized via macropinosomes (bottom arrow). Magnification: (A) and (B) ×3400; (C) ×34000; and insets (A) and (B), and (D) ×19000. Scale bars: 2μm in (A) and (B), and 0.5μm in insets (A) and (B), and (C) and (D). The Association for Research in Vision and Ophthalmology retains copyright of this image.
Fig. 9.
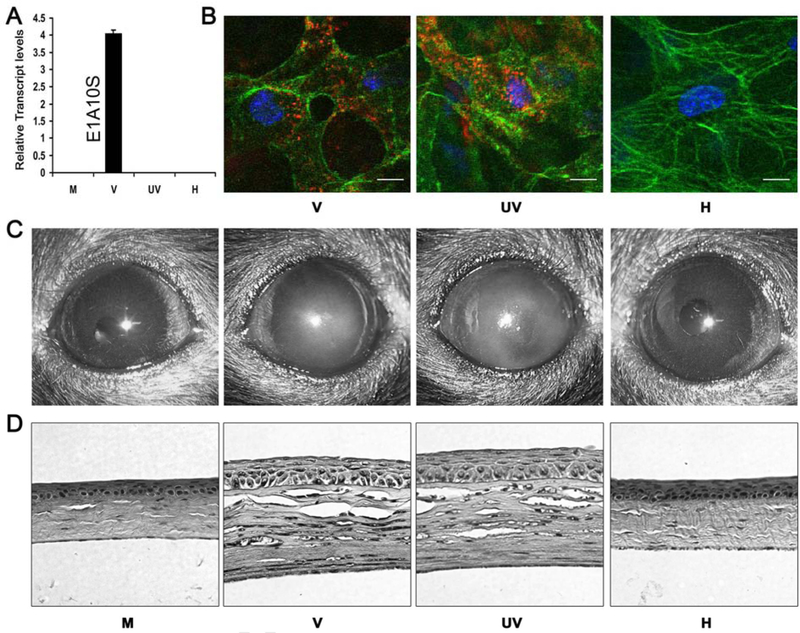
Adenoviral gene expression is not an absolute requirement for adenoviral keratitis. Molecular, cytological, macroscopic and histological evidence that adenoviral gene expression is not essential for the pathogenesis of keratitis, reprinted with author permission (Chintakuntlawar et al., 2010). In this experiment, mock (M), intact (V), UV-inactivated (UV), and heat-inactivated (H) HAdV-D37 was used to infect A549 cells in vitro and C57Bl/6j mouse corneas in vivo to determine if viral gene expression and replication were necessary to induce keratitis. (A) Relative expression of viral transcript ElAlOS in A549 cells, four hours following infection with HAdV-D37, as determined by real-time PCR. Intact virus (V) induced robust ElAlOS expression while mock (M), UV-inactivated (UV) and heat-inactivated (H) virus ElA expression was not detected. (B) To determine if inactivated virus could be internalized, in vivo confocal microscopy of C57Bl/6j mouse corneal stromal cells was performed 90 minutes following infection with Cy3-marked intact (V), UV-inactivated (UV), and heat-inactivated (H) virus. Intact (V) and UV-inactivated (UV) virus gained entry into cells, while heat-inactivated (H) virus did not. Cy3-labeled virus is shown in red; intracellular actin (phalloidin stain) in green; and nuclei (TO-PR03 stain) in blue. Scale: 20μm. (C) Photographs of C57Bl/6j mouse corneas post-infection. Within 24 hours, corneal opacification was demonstrated in mice infected with intact (V) and UV-inactivated (UV) virus. Infection with mock (M) and heat-inactivated (H) virus did not result in opacification. (D) Histopathology of representative mice corneas using hematoxylin and eosin, taken four days post-infection. Localized inflammation and stromal edema were observed in corneas infected with intact (V) and UV-inactivated (UV) virus. Negligible leukocyte infiltration was observed in corneas infected with a control buffer (M) or heat-inactivated (H) virus.
Subsequent corneal stromal infiltration includes both neutrophils and activated monocytes (Chintakuntlawar et al., 2007; Chintakuntlawar and Chodosh, 2009; Chintakuntlawar et al., 2010; Zhou et al., 2017). In human patients, infiltration is driven by the chemokines IL-8 (CXCL-8) and MCP-1 (CCL2) (Chodosh et al., 2000; Natarajan et al., 2002b), requiring activation of MAP kinases, specifically p38 MAP kinase, ERK, and JNK, and leading to NFKB phosphorylation and nuclear translocation (Natarajan et al., 2002a; Natarajan et al., 2003; Rajaiya et al., 2008; Xiao and Chodosh, 2005) (Fig. 10). Infection of the cornea does not occur in isolation from the cytokine storm associated with conjunctival infection, and other pro-inflammatory cytokines, including IL-l, IL-6, TNF-α, CXCLl/KC, CXCL5/LIX, CXCLlO, IFN-α and IFN-β are also involved (Chintakuntlawar and Chodosh, 2009; Lin et al., 2007; Robinson et al., 2013b; Xiao and Chodosh, 2005). In the mouse cornea, the presence of bone marrow derived cells, including cells phenotypically consistent with macrophages and dendritic cells (Brissette-Storkus et al., 2002; Knickelbein et al., 2009) also appears to play a role in keratitis following corneal stromal infection with HAdV-D37 (Ramke et al., 2016). HAdV infection of keratocytes induces a pro-inflammatory cytokine footprint independent of viral gene expression (Chintakuntlawar et al., 2010) or viral replication (Kafri et al., 1998; Natarajan et al., 2003; Trousdale et al., 1995)(Fig. 9). These observations have led to the view that effective treatment of chronic adenovirus keratitis may be best oriented towards immune modulation rather than targeting viral replication.
Fig. 10.
Synthesis of corneal stromal inflammatory responses to HAdV infection. This flowchart synthesizes our current understanding of the events which follow HAdV binding to corneal cells, with emphasis on the induction of pro-inflammatory pathways. In EKC-causing HAdV-D, a secondary interaction between HAdV RGD motifs and cellular integrins activate the Src-mediated signalosome, which is essential in viral internalization, prolongation of cell viability and therefore viral replication, and upregulation of inflammatory cytokine gene expression. The role of the humoral immune system in corneal pathogenesis is likely minor, but has not been investigated.
4.5. Adaptive Immune Responses in EKC
It is known that ocular surface infection by adenovirus, including EKC, causes a rise in the production and circulation of neutralizing antibodies in humans (Hierholzer and Sprague, 1979; Jawetz et al., 1957) and in experimental animal models (Gordon et al., 1992; Trousdale et al., 1995). Serum neutralizing antibodies are directed towards the adenovirus capsid, principally the epsilon determinant of the hexon protein (Crawford-Miksza and Schnurr, 1996). Neutralizing antibodies demonstrate very limited intra-species cross-reactivity, and only when the epsilon determinants of two viruses are closely homologous. For instance, antibodies directed against HAdV-54 are also weakly reactive to HAdV-8 in neutralization assays, leading to previous instances of adenovirus mistyping (Ishiko et al., 2008; Kaneko et al., 2011b). Infection of resident antigen presenting cells within the ocular surface and draining lacrimal system, and subsequent presentation of antigen in local lymphoid tissue and draining lymph nodes to B cells is responsible for the production of type-specific antibodies (Junt et al., 2007), and explains the association of ocular surface infection by HAdVs with conjunctival follicular hyperplasia and preauricular lymphadenopathy (Butt and Chodosh, 2006). Cytotoxic T-cells against HAdV capsid proteins are also induced in draining lymph nodes (Yang et al., 1995), and contribute to the pathology of infection.
5. DIAGNOSIS OF EKC
While most cases of EKC are diagnosed based on the clinical examination alone (Butt and Chodosh, 2006), molecular tests including polymerase chain reaction (PCR) and WGS can be helpful under certain circumstances (Lee et al., 2018). In outbreak settings, epidemiological investigations are now often guided by PCR and/or WGS analysis of samples retrieved from those affected and their physical surroundings (Sammons et al., 2019), providing valuable data related to the types of virus involved, their source, and their modes of transmission. This information can then inform public and/or departmental health measures to limit disease spread (Chodosh, 2019). In contrast, older methods of diagnosis have significant limitations. Viral cultures have limited utility because they typically do not provide results quickly enough to have an impact on management of acute illness or in disease surveillance. Similarly, acute and convalescent serologies are seldom helpful as results only become available when all but the chronic manifestations have resolved.
Point-of-care technologies such as the RPS Adeno Detector (Rapid Pathogen Screening Inc., South Williamsport, PA) and its later iteration, AdenoPlus (RPS ADP; Rapid Pathogen Screening Inc., Sarasota, FL), are FDA-approved for the office-based identification of adenovirus in clinical samples. AdenoPlus is an enzyme-linked immunosorbent assay designed to detect a highly conserved region on the HAdV hexon capsomer, using patient tears, with results obtainable within 10 minutes of sample collection. While a company-funded trial in 2013 reported promising results for the AdenoPlus with sensitivities, specificities, positive and negative predictive values all >85% when compared to cell culture and PCR (Sambursky et al., 2013), its real-life application has yielded less encouraging results. One masked diagnostic accuracy study in the UK involving 121 patients reported a sensitivity and specificity of 39.5% and 95.5%, respectively, when compared to PCR (Kam et al., 2015), while a more recent analysis from the multicenter BAYnovation Study Group found a negative predictive value of only 71% (Lee et al., 2018). Another study of 125 patients at the Mayo Clinic, Rochester, reported a sensitivity and specificity of 50% and 92%, respectively (Holtz et al., 2017). The reasons for discrepancies between these latter results and those of the device manufacturer are unclear, but may include differences in the patient populations sampled or the means of sample collection. Nonetheless, until further clarification, these results may prevent large scale adoption of this technology.
6. MANAGEMENT OF EKC
6.1. Preventing Transmission
Adenovirus can be transmitted in droplets through sneezing or coughing, by eye-hand-eye contact, and through fomites, and remains infectious on porous surfaces for up to 10 days and on nonporous surfaces for over one month (Nauheim et al., 1990). The prior association of EKC with work in naval yards (“shipyard eye”) was actually due to iatrogenic transmission of virus by contaminated tonometers in the offices of eye care providers caring for shipyard workers (Jawetz, 1959; Thygeson, 1949). Therefore, once a presumptive diagnosis of EKC is made, significant attention must be given to preventing spread of the infection to others. Patients should be educated regarding the typical length of viral shedding, typically 10–14 days following the onset of symptoms, and the need to avoid infecting others. The risk of transmission is particularly high when there is tearing or discharge from the affected eye(s), because of the tendency to wipe away tears and discharge with one’s hand, a tissue paper, or towel. These in tum can contaminate anything or anyone they come in contact with. In general, this means discouraging sharing of personal articles including cell phones and computer keyboards. Towels and pillow cases should be washed in soap and hot water and not shared. Touching the infected eye should be avoided as much as possible, and any tissue paper or hand towels used to wipe away tears or ocular discharge should be immediately disposed of or washed. Patients with tearing and/or discharge should, as much as possible, avoid public spaces, health care facilities, schools, and childcare centers. Extreme care must be exercised particularly by healthcare workers and individuals who may have contact with immunocompromised persons, such as neonates, the elderly, and those with serious chronic disease. In addition to being released from work until ocular discharge has ceased, healthcare workers should be counseled not to touch their eye(s) and to wash their hands thoroughly before and after every patient encounter. Disinfection of common surfaces including those in ophthalmology clinics and associated clinical equipment is paramount (Sammons et al., 2019), with the Centers for Disease Control and Prevention (CDC) recommending disinfection with a chlorine-based agent pursuant to equipment compatibility (Rutala et al., 2006).
6.2. Supportive Therapy
Supportive therapy is widely considered the mainstay of EKC management, but typically provides little symptomatic relief and does not fundamentally alter the course of disease. Cool compresses and artificial tears may be helpful, and topical non-steroidal anti-inflammatory agents (Gordon et al., 1998) may reduce discomfort. Sudden worsening in pain and light sensitivity after initial onset of EKC may be the result of a macro-epithelial erosion. In addition, patients should be followed closely for the formation of conjunctival membranes. If present, conjunctival membranes should be gently removed and the eye treated with topical corticosteroids to minimize scar and symblepharon formation.
6.3. Antiviral Therapy
Decades of work have so far failed to identify an effective agent against adenoviral replication that is also well tolerated as an ocular topical preparation. Systemically administered nucleoside analogs, such as acyclovir, its prodrug valacyclovir, and famciclovir, require the intrinsic viral thymidine kinase activity of herpes viruses to be activated (Whitley and Gnann, 1992) and therefore lack activity against adenovirus. However, another nucleoside analog, ganciclovir, has shown variable activity against adenovirus in both cell culture and in animal models (Ibrisimovic et al., 2012; Tollefson et al., 2014; Trousdale et al., 1994; Ying et al., 2014). The putative mechanism of action of ganciclovir against adenovirus involves inhibition of the viral DNA polymerase (Ying et al., 2014). In a retrospective case series of patients with systemic adenovirus infection after hematopoietic stem cell transplantation, the use of intravenous ganciclovir was reported to be associated with improved survival (Bruno et al., 2003). However, commercially available topical ganciclovir 0.15% gel did not improve outcomes in acute EKC in a clinical trial (Yabiku et al., 2011).
In the early 2000s, there was considerable interest in the antiviral cidofovir, a monophosphate nucleotide analogue that, after undergoing cellular phosphorylation, competitively inhibits incorporation of deoxycytidine triphosphate into viral DNA by viral DNA polymerase, and is therefore a broad spectrum inhibitor of viral DNA synthesis for all DNA viruses (DeClercq, 1996; Lea and Bryson, 1996). Cidofovir showed activity in animal models of HAdV-C5 ocular infection (de Oliveira et al., 1996; Kaneko et al., 2004), but unfortunately had a narrow therapeutic window. Ocular topical use of cidofovir 1% for acute EKC was toxic in humans (Hillenkamp et al., 2002), and the drug caused punctal stenosis when applied to the ocular surface of rabbits (Gordon et al., 1994) and in one human case report when used for squamous neoplasia (Sherman et al., 2002). Initial enthusiasm for cidofovir was also dampened by two small randomized controlled trials which showed that it was likely inefficacious when administered in low concentrations (0.2% four times daily), and too toxic to administer at concentrations likely required to prevent SEIs (1% four to ten times daily) (Hillenkamp et al., 2001, 2002). The recent development of the lipid derivative pro-drug of cidofovir, brincidofovir, which is now currently in clinical trials for patients with post-transplant adenoviremia (Hiwarkar et al., 2017) may prompt future studies for potential ocular use. We are also awaiting the publication of results from a randomized phase IIa proof-of-concept trial involving the antiviral agent APD-209, a trisialic acid-containing formulation, in patients with adenoviral EKC (ClinicalTrials.gov Identifier: NCT01977443).
6.4. Topical Immunosuppression and Immunomodulation
Topical corticosteroids are frequently used in EKC, and have been shown to significantly improve the acute signs and symptoms of infection (Wilkins et al., 2011). However, data from experimental infection of rabbits with HAdV-C5 have suggested that use of corticosteroids in the acute phase of ocular adenovirus infection may increase viral shedding (Romanowski et al., 1996; Romanowski et al., 2001, 2002) and reduce the effect of antiviral agents (Romanowski et al., 1997). Limited studies in human patients have shown a reduction in SEI formation when topical corticosteroids were used in the acute phase of EKC (Freyler and Sehorst, 1976; Kocluk et al., 2017; Laibson et al., 1970; Trauzettel-Klosinski et al., 1980). Some of the same studies also reported that SEIs appeared (or re-appeared) when topical corticosteroids were stopped, suggesting that corneal inflammation was delayed but not eliminated by corticosteroid therapy. In our experience, corticosteroids are best reserved in the acute setting for instances of conjunctival membranes and/or significant epithelial keratitis. In the chronic phase, corticosteroids are effective in the treatment of visually symptomatic SEIs, but have well known side effects.
Non-steroidal anti-inflammatory agents have not been shown to be useful in the prevention (Gordon et al., 1998) or treatment (J. Chodosh, personal observation) of EKC-associated SEI. The search for alternative steroid-sparing, immunomodulatory agents for EKC has led to investigations of other agents, in particular the calcineurin inhibitors, cyclosporin A and tacrolimus. Calcineurin is a protein phosphatase that activates T lymphocyte responses to cellular injury and infection (Shibasaki et al., 2002). Cyclosporin A and tacrolimus potently inhibit T cell activation, and both have shown promise with acceptably low toxicity in small studies of acute (Asena et al., 2017) and chronic EKC (Berisa Prado et al., 2017; Ghanem et al., 2014; Levinger et al., 2010; Levinger et al., 2014) albeit with varying drug concentrations and dosing. However, the current data on cyclosporin A is mixed. In one small randomized trial for acute EKC, a 1% preparation reduced subjective symptoms but as compared to placebo did not otherwise alter the course of the infection (Hillenkamp et al., 2001). A later, similarly small randomized trial by the same group demonstrated that when given in addition to cidofovir 1%, cyclosporin A was effective in significantly reducing corneal opacities compared to placebo, though severe ocular sutface toxicity was noted and attributed to the cidofovir (Hillenkamp et al., 2002). Indeed, in a rabbit model of ocular adenovirus infection, topical cyclosporine A decreased SEI formation, but led to increased adenoviral replication early in the disease course (Romanowski et al., 2005). For this reason, cyclosporin A use can be recommended only as a steroid-sparing agent for mild recurrences of symptomatic SEI, and to permit tapering of topical corticosteroids in patients with their SEIs suppressed (Jeng and Holsclaw, 2011; Maychuk et al., 2015). The data supporting use oftacrolimus for symptomatic SEIs is stronger. In one study, tacrolimus 0.03% significantly reduced SEIs compared to their degree prior to treatment (Ghanem et al., 2014). Clearly, larger randomized controlled trials are required to further study these agents in EKC, perhaps best suited to patients with recalcitrant SEIs who stand to benefit the most from immunomodulatory therapy.
6.5. Povidone-Iodine Alone or in Combination with Corticosteroid Therapy
Povidone-iodine, a widely used topical antiseptic, inactivates adenoviruses to varying degrees depending on type (Akanuma, 2007; Kawana et al., 1997; Monnerat et al., 2006; Sauerbrei et al., 2004). It has been used successfully as single administration therapy for HAdV conjunctivitis in infants (Ozen Tunay et al., 2015) and thrice daily for two weeks for HAdV conjunctivitis in adults (Yazar et al., 2016). However, burning, stinging, and irritation (chemical conjunctivitis) can occur with even one administration. A combination of povidone-iodine and dexamethasone has shown more promise in the treatment of acute adenoviral conjunctivitis (Kovalyuk et al., 2017; Pelletier et al., 2009), with the results of ongoing phase 3 studies ( NCT02998541 and NCT02998554) expected in the coming years. A recent randomized, controlled, multicenter phase 2 study ( NCT01470664) consisting of 144 RPS AdenoPlus positive patients showed that patients treated with a suspension containing both povidone iodine 0.6% and dexamethasone 0.1% four times daily for 5 days had significantly faster resolution of clinical signs and a more rapid reduction in viral replication than those treated with vehicle (Pepose et al., 2018). It should be noted, however, that the follow-up period for the referenced studies was relatively short (1–2 weeks), and the long-term effect on delayed onset SEIs remains to be determined.
7. FUTURE DIRECTIONS
The first step toward the development of a medical therapy for any illness is a frank assessment of the current state of knowledge about the disorder. EKC represents a common malady and the absence of proven effectiveness for existing treatments characterizes an important unmet need. While cases of viral “pink eye” are seen regularly by eye care providers world-wide, it is humbling how much of the biology and corneal pathogenesis of HAdV remain a mystery, and also disconcerting that there are other, as yet unknown causes (Lee et al., 2018). Furthermore, the history of scholarly works on EKC illustrates how unchallenged scientific dogma can inhibit progress in our understanding and the development of treatments for any disease. As recent work has illustrated, the cornea is quite capable of generating inflammation when injured or infected, and is neither inert nor a passive actor in corneal inflammation. The experimental data we have presented here, including the development of the C57Bl/6j mouse model of adenoviral keratitis, has provided insights that may not have been available in decades past. As the number of adenoviral types continues to increase, so too does the possibility that some will display ocular surface tropism and therefore be important future causes of disease. Furthermore, the rapidly expanding database of whole adenovirus genomes offers hope that mining of genomic data will reveal new insights into ocular tropism (Ismail et al., 2016; Ismail et al., 2018b) and virulence. Future studies on HAdV genomics and pathogenesis, and improved understanding of corneal immunobiology may yet yield new information-based therapies against this common and historically important infection.
Fig. 8.
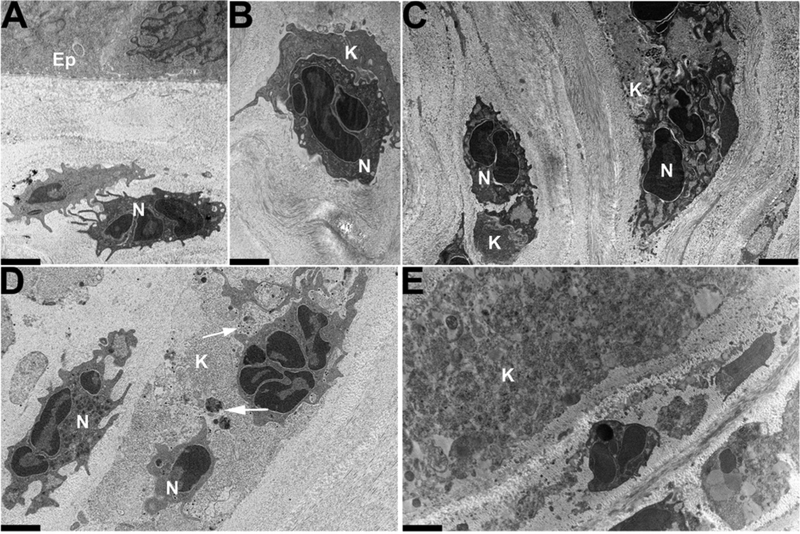
Transmission electron microscopy of later changes in the C57Bl/6j mouse cornea following intra-stromal injection of 1μL (105 tissue cultme infectious doses) of HAdV-D37, reprinted with author permission (Mukherjee et al., 2015). Photomicrographs at a magnification of × 3400 were taken at (A-C) 24 homs, (D) 48 homs and (E) 96 hours following infection. Ep – epithelium; N – neutrophil (polymorphonuclear cell); and K – keratocyte. The inflammatory response following infection is neutrophil-predominant, as shown by (A) stromal infiltration; (B) the beginning of phagocytosis; and (C) eventual phagocytosis. (D) shows whole viral particles (arrows) within stromal cells which now exist amidst aggregations of dense cellular debris. Such debris continues to accumulate and becomes increasingly evident at later time points (E). Scale bars: 2μm. The Association for Research in Vision and Ophthalmology retains copyright of this Image.
ARTICLE HIGHLIGHTS.
Epidemic keratoconjunctivitis (EKC) is a severe ocular surface infection.
EKC causes conjunctivitis of varying severity, but its hallmark is relapsing and remitting corneal inflammation.
Human adenoviruses, particularly species D, are the main pathogens.
Models of adenovirus keratitis suggest corneal cells play a key role in the host inflammatory response.
To date, only topical corticosteroids and tacrolimus appear to alter the course of chronic keratitis following EKC.
ACKNOWLEDGEMENTS
We wish to acknowledge the many students and fellows who contributed to the research cited in this article.
FUNDING
This work was funded by National Institutes of Health grants EY013124, EY021558, and EY014104, a Senior Scientific Investigator Award grant from Research to Prevent Blindness, Inc., New York, NY, and the Massachusetts Lions Eye Research Fund.
LIST OF ABBREVIATIONS
- CAR
coxsackie-adenovirus receptor
- CCL2 C-C
motif chemokine ligand 2 (also monocyte chemoattractant protein 1)
- CXCL1
chemokine ligand 1
- CXCL2
chemokine ligand 2
- CXCL8
chemokine ligand 8
- EKC
epidemic keratoconjunctivitis
- ERK
extracellular signal-regulated kinases
- FDA
Food and Drug Administration
- HAdV
human adenovirus
- HAdV-D
human adenovirus species D
- HLA-E
human leukocyte antigen-E
- HSV
herpes simplex virus
- IL-l
interleukin-1
- IL-6
interleukin-6
- IL-8
interleukin-8
- IFN-α
interferon-α
- IFN-β
interferon-β
- IFN-γ
interferon-γ
- JNK
c-Jun N-terminal kinase pathway
- MAMs
membrane-associated mucins
- MCP-1
monocyte chemoattractant protein 1 (also C-C motif chemokine ligand 2)
- MHC
major histocompatibility complex
- MyD88
myeloid differentiation primary response 88
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NK
natural killer lymphocytes
- PCR
polymerase chain reaction
- PI3K
phosphoinositide 3-kinase
- RGD
tripeptide consisting of arginine, glycine, and aspartate
- Src
proto-oncogene tyrosine-protein kinase Src
- SEIs
subepithelial infiltrates
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor-α
- TTV
torque teno virus
- VAMP
virus-associated molecular pattern
- VZV
varicella zoster virus
- WGS
whole genome sequencing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
Rahul A. Jonas – none to disclose.
Lawson Ung – none to disclose.
Jaya Rajaiya and James Chodosh – work funded by National Institutes of Health grants EY013124, EY021558, and EY014104, a Senior Scientific Investigator Award grant from Research to Prevent Blindness, Inc., New York, NY, and the Massachusetts Lions Eye Research Fund.
REFERENCES
- Akanuma M, 2007. [Evaluation of disinfectant against adenovirus by real-time polymerase chain reaction]. Nippon Ganka Gakkai Zasshi 111, 384–390. [PubMed] [Google Scholar]
- Aoki K, Ishiko H, Konno T, Shimada Y, Hayashi A, Kaneko H, Ohguchi T, Tagawa Y, Ohno S, Yamazaki S, 2008. Epidemic keratoconjunctivitis due to the novel hexon-chimeric-intermediate 22,37/H8 human adenovirus. J Clin Microbial 46, 3259–3269. 10.1128/JCM.02354-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcieri ES, Arcieri RS, Franca ET, Rocha FJ, 2004. Subepithelial infiltrates associated to viral keratoconjunctivitis following photorefractive keratectomy. Eye 18, 1010–1012. 10.1038/sj.eye.6701377. [DOI] [PubMed] [Google Scholar]
- Ariga T, Shimada Y, Ohgami K, Tagawa Y, Ishiko H, Aoki K, Ohno S, 2004. New genome type of adenovirus serotype 4 caused nosocomial infections associated with epidemic conjunctivitis in Japan. J Clin Microbial 42, 3644–3648. 10.1128/JCM.42.8.3644-3648.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnberg N, Edlund K, Kidd AH, Wadell G, 2000. Adenovirus type 37 uses sialic acid as a cellular receptor. J Virol 74, 42–48. [PMC free article] [PubMed] [Google Scholar]
- Arnberg N, Kidd AH, Edlund K, Nilsson J, Pring-Akerblom P, Wadell G, 2002. Adenovirus type 37 binds to cell surface sialic acid through a charge-dependent interaction. Virology 302, 33–43. 10.1006/viro.2002.1503. [DOI] [PubMed] [Google Scholar]
- Asena L, Singar Ozdemir E, Burcu A, Ercan E, Colak M, Altinors DD, 2017. Comparison of clinical outcome with different treatment regimens in acute adenoviral keratoconjunctivitis. Eye 31,781–787. 10.1038/eye.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin Kurna S, Altun A, Oflaz A, Karatay Arsan A, 2015. Evaluation of the impact of persistent subepithelial corneal infiltrations on the visual performance and corneal optical quality after epidemic keratoconjunctivitis. Acta Ophthalmol 93, 377–382. 10.1111/aos.12496. [DOI] [PubMed] [Google Scholar]
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW, 1997. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275, 1320–1323. 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Berisa Prado S, Riestra Ayora AC, Lisa Fernandez C, Chacon Rodriguez M, Merayo-Lloves J, Alfonso Sanchez JF, 2017. Topical Tacrolimus for Corneal Subepithelial Infiltrates Secondary to Adenoviral Keratoconjunctivitis. Cornea 36, 1102–1105. 10.1097/ICO.0000000000001279. [DOI] [PubMed] [Google Scholar]
- Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL, 2002. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci 43, 2264–2271. [PMC free article] [PubMed] [Google Scholar]
- Bruno B, Gooley T, Hackman RC, Davis C, Corey L, Boeckh M, 2003. Adenovirus infection in hematopoietic stem cell transplantation: effect of ganciclovir and impact on survival. Biol Blood Marrow Transplant 9, 341–352. 10.1016/sl083-8791(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Butt AL, Chodosh J, 2006. Adenoviral keratoconjunctivitis in a tertiary care eye clinic. Cornea 25, 199–202. 10.1097/0l.ico.0000170693.13326.fb. [DOI] [PubMed] [Google Scholar]
- Carion von Stellwag K, 1889. A peculiar form of corneal inflammation. Wien Klin Wochenschr 2, 613–614. [Google Scholar]
- Chintakuntlawar AV, Astley R, Chodosh J, 2007. Adenovirus type 37 keratitis in the C57BL/6J mouse. Invest Ophthalmol Vis Sci 48, 781–788. 10.1167/iovs.06-1036. [DOI] [PubMed] [Google Scholar]
- Chintakuntlawar AV, Chodosh J, 2009. Chemokine CXCLl/KC and its receptor CXCR2 are responsible for neutrophil chemotaxis in adenoviral keratitis. J Interferon Cytokine Res 29, 657–666. 10.1089/jir.2009.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintakuntlawar AV, Chodosh J, 2010. Cellular and tissue architecture of conjunctival membranes in epidemic keratoconjunctivitis. Ocul Immunol Inflamm 18, 341–345. 10.3109/09273948.2010.498658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintakuntlawar AV, Zhou X, Rajaiya J, Chodosh J, 2010. Viral capsid is a pathogen-associated molecular pattern in adenovirus keratitis. PLoS Pathog 6, e1000841 10.1371/journal.ppat.1000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CY, Mathias P, Nemerow GR, Stewart PL, 1999. Structure of adenovirus complexed with its internalization receptor, alphavbeta5 integrin. J Virol 73, 6759–6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh J, 2011. Epidemic K eratoconjunctivitis, in: Melki S (Ed.), Atlas of Clinical Wisdom: Cornea, Refractive and External Disease, 1st ed Slack, Thorofare, NJ, pp. 91–96. [Google Scholar]
- Chodosh J, 2019. Neonatal Intensive Care Eye. Ophthalmology 126, 144–145. 10.1016/j.ophtha.2018.08.033. [DOI] [PubMed] [Google Scholar]
- Chodosh J, Astley RA, Butler MG, Kennedy RC, 2000. Adenovirus keratitis: a role for interleukin-8. Invest Ophthalmol Vis Sci 41, 783–789. [PubMed] [Google Scholar]
- Chodosh J, Miller D, Stroop WG, Pflugfelder SC, 1995. Adenovirus epithelial keratitis. Cornea 14, 167–174. [PubMed] [Google Scholar]
- Crawford-Miksza L, Schnurr DP, 1996. Analysis of 15 adenovirus hexon proteins reveals the location and structure of seven hypervariable regions containing serotype-specific residues. J Virol 70, 1836–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darougar S, Quinlan MP, Gibson JA, Jones BR, 1977. Epidemic keratoconjunctivitis and chronic papillary conjunctivitis in London due to adenovirus type 19. Br J Ophthalmol 61, 76–85. 10.1136/bjo.61.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson C, Darrell R, 1963. Infections due to adenovirus type 8 in the United States. I. An outbreak of epidemic keratoconjunctivitis originating in a physician’s office. N Engl J Med 268, 1031–1034. 10.1056/NEJM196305092681901. [DOI] [PubMed] [Google Scholar]
- Dawson C, Darrell R, Hanna L, Jawetz E, 1963. Infections due to adenovirus type 8 in the United States. II. Community-wide infection with adenovirus type 8. N Engl J Med 268, 1034–1037. 10.1056/NEJM196305092681902. [DOI] [PubMed] [Google Scholar]
- Dawson CR, Hanna L, Togni B, 1972. Adenovirus type 8 infections in the United States. IV. Observations on the pathogenesis of lesions in severe eye disease. Arch Ophthalmol 87, 258–268. 10.1001/archopht.1972.01000020260005. [DOI] [PubMed] [Google Scholar]
- DeClercq E, 1996. Therapeutic potential of Cidofovir (HPMPC, Vistide) for the treatment of DNA virus (i.e. herpes-, papova-, pox- and adenovirus) infections. Verh K Acad Geneeskd Belg 58, 19–47; discussion 47–19. [PubMed] [Google Scholar]
- DeJong JC, Wermenbol AG, Verweij-Uijterwaal MW, Slaterus KW, Wertheim-Van Dillen P, Van Doornum GJ, Khoo SH, Hierholzer JC, 1999. Adenoviruses from human immunodeficiency virus-infected individuals, including two strains that represent new candidate serotypes Ad50 and Ad51 of species B1 and D, respectively. J Clin Microbial 37, 3940–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira CB, Stevenson D, LaBree L, McDonnell PJ, Trousdale MD, 1996. Evaluation of Cidofovir (HPMPC, GS-504) against adenovirus type 5 infection in vitro and in a New Zealand rabbit ocular model. Antiviral Res 31, 165–172. 10.1016/0166-3542(95)00962-0. [DOI] [PubMed] [Google Scholar]
- Dosso AA, Rungger-Brandle E, 2008. Clinical course of epidemic keratoconjunctivitis: evaluation by in vivo confocal microscopy. Cornea 27, 263–268. 10.1097/ICO.0b013e31815b7d7d. [DOI] [PubMed] [Google Scholar]
- Duke-Elder S, 1965a. Inflammations of the conjunctiva and associated inflammations of the cornea, in: Duke-Elder S (Ed.), Diseases of the outer eye. C. V. Mosby company, St. Louis, pp.55–56. [Google Scholar]
- Duke-Elder S, 1965b. System of Ophthalmology: Diseases of the Outer Eye, Part I. C.V. Mosby company, St. Louis. [Google Scholar]
- Echavarria M, 2008. Adenoviruses in immunocompromised hosts. Clin Microbial Rev 21, 704–715. 10.1128/CMR.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frevert CW, Kinsella MG, Vathanaprida C, Goodman RB, Baskin DG, Proudfoot A, Wells TN, Wight TN, Martin TR, 2003. Binding ofinterleukin-8 to heparan sulfate and chondroitin sulfate in lung tissue. Am J Respir Cell Mol Biol 28, 464–472. 10.1165/rcmb.2002-00840C. [DOI] [PubMed] [Google Scholar]
- Freyler H, Sehorst W, 1976. [The fate of corneal infiltrations in cases of epidemic keratoconjunctivitis. A follow-up study over two and a half years (author’s transl)]. Wien Klin Wochenschr 88, 341–343. [PubMed] [Google Scholar]
- Fuchs E, 1889. Keratitis punctata superficialis. Wien Klin Wschr 2, 837–843. [Google Scholar]
- Gaggar A, Shayakhmetov DM, Lieber A, 2003. CD46 is a cellular receptor for group Badenoviruses. Nat Med 9, 1408–1412. 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- Ghanem RC, Vargas JP, Ghanem VC, 2014. Tacrolimus for the treatment of subepithelial infiltrates resistant to topical steroids after adenoviral keratoconjunctivitis. Cornea 33, 1210–1213. 10.1097/IC0.0000000000000247. [DOI] [PubMed] [Google Scholar]
- Ginsberg HS, Moldawer LL, Sehgal PB, Redington M, Kilian PL, Chanock RM, Prince GA, 1991. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci US A 88, 1651–1655. 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Tisdale A, Menon BB, 2014. Comparison of the transmembrane mucins MUC1 and MUC16 in epithelial barrier function. PLoS One 9, e100393 10.1371/joumal.pone.0100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon YJ, Aoki K, Kinchington PR, 1996. Adenovirus keratoconjunctivitis, in: Pepose JS, Holland GN, Wilhelmus KR (Eds.), Ocular Infection and Immunity, first ed Mosby, St. Louis, pp. 877–894. [Google Scholar]
- Gordon YJ, Araullo-Cruz T, Romanowski EG, 1998. The effects of topical nonsteroidal anti-inflammatory drugs on adenoviral replication. Arch Ophthalmol 116, 900–905. 10.1001/archopht.116.7.900. [DOI] [PubMed] [Google Scholar]
- Gordon YJ, Romanowski E, Araullo-Cruz T, 1992. An ocular model of adenovirus type 5 infection in the NZ rabbit. Invest Ophthalmol Vis Sci 33, 574–580. [PubMed] [Google Scholar]
- Gordon YJ, Romanowski EG, Araullo-Cruz T, 1994. Topical HPMPC inhibits adenovirus type 5 in the New Zealand rabbit ocular replication model. Invest Ophthalmol Vis Sci 35, 4135–4143. [PubMed] [Google Scholar]
- Hage E, Espelage W, Eckmanns T, Lamson DM, Panto L, Ganzenmueller T, Heim A, 2017. Molecular phylogeny of a novel human adenovirus type 8 strain causing a prolonged, multi-state keratoconjunctivitis epidemic in Germany. Sci Rep 7, 40680 10.1038/srep40680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S, Gonzalez G, Harada S, Oosako H, Hanaoka N, Hinokuma R, Fujimoto T, 2018. Recombinant type Human mastadenovirus D85 associated with epidemic keratoconjunctivitis since 2015 in Japan. J Med Virol 90, 881–889. 10.1002/jmv.25041. [DOI] [PubMed] [Google Scholar]
- Hierholzer JC, 1992. Adenoviruses in the immunocompromised host. Clin Microbial Rev 5, 262–274. 10.1128/cmr.5.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer JC, Sprague JB, 1979. Five-year analysis of adenovirus 8 antibody levels in an industrial community following an outbreak of keratoconjunctivitis. Am J Epidemiol 110, 132–140. 10.1093/oxfordjoumals.aje.a112797. [DOI] [PubMed] [Google Scholar]
- Hierholzer JC, Wigand R, Anderson LJ, Adrian T, Gold JW, 1988. Adenoviruses from patients with AIDS: a plethora of serotypes and a description of five new serotypes of subgenus D (types 43–47). J Infect Dis 158, 804–813. 10.1093/infdis/158.4.804. [DOI] [PubMed] [Google Scholar]
- Hillenkamp J, Reinhard T, Ross RS, Bohringer D, Cartsburg O, Roggendorf M, De Clercq E, Godehardt E, Sundmacher R, 2001. Topical treatment of acute adenoviral keratoconjunctivitis with 0.2% cidofovir and 1% cyclosporine: a controlled clinical pilot study. Arch Ophthalmol 119, 1487–1491. 10.1001/archopht.119.10.1487. [DOI] [PubMed] [Google Scholar]
- Hillenkamp J, Reinhard T, Ross RS, Bohringer D, Cartsburg O, Roggendorf M, De Clercq E, Godehardt E, Sundmacher R, 2002. The effects of cidofovir 1% with and without cyclosporin a 1% as a topical treatment of acute adenoviral keratoconjunctivitis: a controlled clinical pilot study. Ophthalmology 109, 845–850. 10.1016/s0161-6420(02)00992-2. [DOI] [PubMed] [Google Scholar]
- Hiwarkar P, Amrolia P, Sivaprakasam P, Lum SH, Doss H, O’Rafferty C, Petterson T, Patrick K, Silva J, Slatter M, Lawson S, Rao K, Steward C, Gassas A, Veys P, Wynn R, United Kingdom Paediatric Bone Marrow Transplant, G., 2017. Brincidofovir is highly efficacious in controlling adenoviremia in pediatric recipients of hematopoietic cell transplant. Blood 129,2033–2037. 10.1182/blood-2016-11-749721. [DOI] [PubMed] [Google Scholar]
- Hogan MJ, Crawford JW, 1942. Epidemic keratoconjunctivitis: (superficial punctate keratitis, keratitis subepithelialis, keratitis maculosa, keratitis nummularis) with a review of the literature and a report of 125 cases. Am J Ophthalmol 25, 1059–1078. [DOI] [PubMed] [Google Scholar]
- Holtz KK, Townsend KR, Furst JW, Myers JP, Binnicker MJ, Quigg SM, Maxson JA, Espy MJ, 2017. An assessment of the AdenoPlus Point-of-Care test for diagnosing adenoviral conjunctivitis and its effect on antibiotic stewardship. Mayo Clin Proc Innov Qual Outcomes 1, 170–175. 10.1016/j.mayocpiqo.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SS, Karayan L, Toumier J, Curiel DT, Boulanger PA, 1997. Adenovirus type 5 fiber knob binds to MHC class I alpha2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J 16, 2294–2306. 10.1093/emboj/16.9.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Reddy V, Dasgupta N, Nemerow GR, 1999. A single amino acid in the adenovirus type 37 fiber confers binding to human conjunctival cells. J Virol 73, 2798–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrisimovic M, Nagl U, Kneidinger D, Rauch M, Lion T, Klein R, 2012. Targeted expression of herpes simplex virus thymidine kinase in adenovirus-infected cells reduces virus titers upon treatment with ganciclovir in vitro. J Gene Med 14, 3–19. 10.1002/jgm.1638. [DOI] [PubMed] [Google Scholar]
- Imre G, Korchmaros I, Opauszki A, 1963. [On epidemic keratoconjunctivitis]. Orvosi hetilap 104, 353–357. [PubMed] [Google Scholar]
- Ishiko H, Aoki K, 2009. Spread of epidemic keratoconjunctivitis due to a novel serotype of human adenovirus in Japan. J Clin Microbial 47, 2678–2679. 10.1128/JCM.r00313-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiko H, Shimada Y, Konno T, Hayashi A, Ohguchi T, Tagawa Y, Aoki K, Ohno S, Yamazaki S, 2008. Novel human adenovirus causing nosocomial epidemic keratoconjunctivitis. J Clin Microbiol 46, 2002–2008. 10.1128/JCM.01835-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AM, Cui T, Dommaraju K, Singh G, Dehghan S, Seto J, Shrivastava S, Fedorova NB, Gupta N, Stockwell TB, Halpin R, Madupu R, Heim A, Kajon AE, Romanowski EG, Kowalski RP, Malathi J, Therese KL, Madhavan HN, Zhang Q, Ferreyra LJ, Jones MS, Rajaiya J, Dyer DW, Chodosh J, Seto D, 2018a. Genomic analysis of a large set of currently-and historically-important human adenovirus pathogens. Emerg Microbes Infect 7, 10 10.1038/s41426-017-0004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AM, Lee JS, Dyer DW, Seto D, Rajaiya J, Chodosh J, 2016. Selection pressure in the human adenovirus fiber knob drives cell specificity in epidemic keratoconjunctivitis. J Virol 90, 9598–9607. 10.1128/NI.01010-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AM, Lee JS, Lee JY, Singh G, Dyer DW, Seto D, Chodosh J, Rajaiya J, 2018b. Adenoviromics: mining the human adenovirus species D genome. Front Microbiol 9, 2178 10.3389/fmicb.2018.02178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawetz E, 1959. The story of shipyard eye. Br Med J 1, 873–876. 10.1136/bmj.1.5126.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawetz E, Thygeson P, Hanna L, Nicholas A, Kimura SJ, 1957. The etiology of epidemic keratoconjunctivitis. Am J Ophthalmol 43, 79–83. 10.1016/0002-9394(57)91483-6. [DOI] [PubMed] [Google Scholar]
- Jeng BH, Holsclaw DS, 2011. Cyclosporine A 1% eye drops for the treatment of subepithelial infiltrates after adenoviral keratoconjunctivitis. Cornea 30, 958–961. 10.1097/ICO.Ob013e31820cd607. [DOI] [PubMed] [Google Scholar]
- Jhanji V, Chan TC, Li EY, Agarwal K, Vajpayee RB, 2015. Adenoviral keratoconjunctivitis. Surv Ophthalmol 60, 435–443. 10.1016/j.survophthal.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Jones BR, 1958. The clinical features of viral keratitis and a concept of their pathogenesis. Proc R Soc Med 51, 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, van Rooijen N, Mempel TR, Whelan SP, von Andrian UH, 2007. Subcapsular sinus macrophages in lymph nodes clear lymph-home viruses and present them to antiviral B cells. Nature 450, 110–114. 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- Kafri T, Morgan D, Krahl T, Sarvetnick N, Sherman L, Verma I, 1998. Cellular immune response to adenoviral vector infected cells does not require de novo viral gene expression: implications for gene therapy. Proc Natl Acad Sci U SA 95, 11377–11382. 10.1073/pnas.95.19.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam KY, Ong HS, Bunce C, Ogunbowale L, Verma S, 2015. Sensitivity and specificity of the AdenoPlus point-of-care system in detecting adenovirus in conjunctivitis patients at an ophthalmic emergency department: a diagnostic accuracy study. Br J Ophthalmol 99, 1186–1189. 10.1136/bjophthalmol-2014-306508. [DOI] [PubMed] [Google Scholar]
- Kaneko H, Aoki K, Ohno S, Ishiko H, Fujimoto T, Kikuchi M, Harada S, Gonzalez G, Koyanagi KO, Watanabe H, Suzutani T, 2011a. Complete genome analysis of a novel intertypic recombinant human adenovirus causing epidemic keratoconjunctivitis in Japan. J Clin Microbial 49, 484–490. 10.1128/JCM.O1044-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko H, Mori S, Suzuki O, Iida T, Shigeta S, Abe M, Ohno S, Aoki K, Suzutani T, 2004. The cotton rat model for adenovirus ocular infection: antiviral activity of cidofovir. Antiviral Res 61, 63–66. 10.1016/j.antiviral.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Kaneko H, Suzutani T, Aoki K, Kitaichi N, Ishida S, Ishiko H, Ohashi T, Okamoto S, Nakagawa H, Hinokuma R, Asato Y, Oniki S, Hashimoto T, Iida T, Ohno S, 2011b. Epidemiological and virological features of epidemic keratoconjunctivitis due to new human adenovirus type 54 in Japan. Br J Ophthalmol 95, 32–36. 10.1136/bjo.2009.178772. [DOI] [PubMed] [Google Scholar]
- Kawana R, Kitamura T, Nakagomi O, Matsumoto I, Arita M, Yoshihara N, Yanagi K, Yamada A, Morita O, Yoshida Y, Furuya Y, Chiba S, 1997. Inactivation of human viruses by povidone-iodine in comparison with other antiseptics. Dermatology 195 Suppl 2, 29–35. 10.1159/000246027. [DOI] [PubMed] [Google Scholar]
- Khodadoust AA, Silverstein AM, 1972. Studies on the nature of the privilege enjoyed by corneal allografts. Invest Ophthalmolll, 137–148. [PubMed] [Google Scholar]
- Kimura R, Migita H, Kadonosono K, Uchio E, 2009. Is it possible to detect the presence of adenovirus in conjunctiva before the onset of conjunctivitis? Acta Ophthalmol 87, 44–47. 10.1111/j.1755-3768.2007.01148.x. [DOI] [PubMed] [Google Scholar]
- Knickelbein JE, Watkins SC, McMenamin PG, Hendricks RL, 2009. Stratification of antigen-presenting cells within the normal cornea. Ophthalmol Eye Dis 1, 45–54. 10.4137/oed.s2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocluk Y, Sukgen EA, Cevher S, Mat E, 2017. Symptomatic treatment of subepithelial infiltrates after viral conjunctivitis: loteprednol or dexamethasone? Ocul Immunol Inflamm 25, 649–653. 10.3109/09273948.2016.1149593. [DOI] [PubMed] [Google Scholar]
- Kovalyuk N, Kaiserman I, Mimouni M, Cohen O, Levartovsky S, Sherbany H, Mandelboim M, 2017. Treatment of adenoviral keratoconjunctivitis with a combination of povidone-iodine 1.0% and dexamethasone 0.1% drops: a clinical prospective controlled randomized study. Acta Ophthalmol 95, e686–e692. 10.1111/aos.13416. [DOI] [PubMed] [Google Scholar]
- Laibson PR, Dhiri S, Oconer J, Ortolan G, 1970. Corneal infiltrates in epidemic keratoconjunctivitis. Response to double-blind corticosteroid therapy. Arch Ophthalmol 84, 36–40. https://doi.org/10.100llarchopht.1970.00990040038010. [DOI] [PubMed] [Google Scholar]
- Lam E, Ramke M, Groos S, Warnecke G, Heim A, 2011. A differentiated porcine bronchial epithelial cell culture model for studying human adenovirus tropism and virulence. J Virol Methods 178, 117–123. 10.1016/j.jviromet.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Lea AP, Bryson HM, 1996. Cidofovir. Drugs 52, 225–230; discussion 231. 10.2165/00003495-199652020-00006. [DOI] [PubMed] [Google Scholar]
- Lee AY, Akileswaran L, Tibbetts MD, Garg SJ, Van Gelder RN, 2015. Identification of torque teno virus in culture-negative endophthalmitis by representational deep DNA sequencing. Ophthalmology 122, 524–530. 10.1016/j.ophtha.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Lee AY, Akileswaran L, Stroman D, Najafi-Tagol K, Kleiboeker S, Chodosh J, Magaret A, Wald A, Van Gelder RN, Group, B.A.S., 2018. Determinants of outcomes of adenoviral keratoconjunctivitis. Ophthalmology 125, 1344–1353. 10.1016/j.ophtha.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Ismail AM, Lee JY, Zhou X, Mateme EC, Chodosh J, Rajaiya J, 2019. Impact of dynamin 2 on adenovirus nuclear entry. Virology 529, 43–56. 10.1016/j.virol.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinger E, Slomovic A, Sansanayudh W, Bahar I, Slomovic AR, 2010. Topical treatment with 1% cyclosporine for subepithelial infiltrates secondary to adenoviral keratoconjunctivitis. Cornea 29, 638–640. 10.1097/ICO.Ob013e3181c33034. [DOI] [PubMed] [Google Scholar]
- Levinger E, Trivizki O, Shachar Y, Levinger S, Verssano D, 2014. Topical 0.03% tacrolimus for subepithelial infiltrates secondary to adenoviral keratoconjunctivitis. Graefes Arch Clin Exp Ophthalmol 252, 811–816. 10.1007/s00417-014-2611-9. [DOI] [PubMed] [Google Scholar]
- Li E, Brown SL, Stupack DG, Puente XS, Cheresh DA, Nemerow GR, 2001. Integrin alpha(v)beta1 is an adenovirus coreceptor. J Virol 75, 5405–5409. 10.1128/NI.75.11.5405-5409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E, Stupack D, Bokoch GM, Nemerow GR, 1998a. Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases. J Virol 72, 8806–8812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E, Stupack D, Klemke R, Cheresh DA, Nemerow GR, 1998b. Adenovirus endocytosis via alpha(v) integrins requires phosphoinositide-3-OH kinase. J Virol 72, 2055–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Carlson E, Diaconu E, Pearlman E, 2007. CXCLl/KC and CXCL5/LIX are selectively produced by corneal fibroblasts and mediate neutrophil infiltration to the corneal stroma in LPS keratitis. J Leukoc Biol 81, 786–792. 10.1189/jlb.0806502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lion T, 2014. Adenovirus infections in immunocompetent and immunocompromised patients. Clin Microbial Rev 27, 441–462. 10.1128/CMR.00116-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lion T, 2019. Adenovirus persistence, reactivation, and clinical management. FEBS Lett. Aug 14 10.1002/1873-3468.13576. [DOI] [PubMed] [Google Scholar]
- Liu EB, Wadford DA, Seto J, Vu M, Hudson NR, Thrasher L, Torres S, Dyer DW, Chodosh J, Seto D, Jones MS, 2012. Computational and serologic analysis of novel and known viruses in species human adenovirus D in which serology and genomics do not correlate. PLoS One 7, e33212 10.1371/journal.pone.0033212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis N, Fender P, Barge A, Kitts P, Chroboczek J, 1994. Cell-binding domain of adenovirus serotype 2 fiber. J Virol 68, 4104–4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes-Martin S, Boztug H, Lion T, 2013. Diagnosis and treatment of adenovirus infection in immunocompromised patients. Expert Rev Anti Infect Ther 11, 1017–1028. 10.1586/14787210.2013.836964. [DOI] [PubMed] [Google Scholar]
- Maudgal PC, 1990. Cytopathology of adenovirus keratitis by replica technique. Br J Ophthalmol 74, 670–675. 10.1136/bjo.74.11.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maychuk DY, Vasil’eva OA, Russu LI, Mezentseva MV, 2015. [Clinical and immunological comparisons of therapeutic regimens for corneal infiltrates secondary to adenoviral keratoconjunctivitis]. Vestn Oftalmol 131, 49–55. 10.17116/oftalma2015131449-55. [DOI] [PubMed] [Google Scholar]
- Menon BB, Zhou X, Spurr-Michaud S, Rajaiya J, Chodosh J, Gipson IK, 2016. Epidemic keratoconjunctivitis-causing adenoviruses induce MUC16 ectodomain release to infect ocular surface epithelial cells. mSphere 1 10.1128/mSphere.00112-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnerat N, Bossart W, Thiel MA, 2006. [Povidone-iodine for treatment of adenoviral conjunctivitis: an in vitro study]. Klin Monbl Augenheilkd 223, 349–352. 10.1055/s-2006-926633. [DOI] [PubMed] [Google Scholar]
- Mueller AJ, Klauss V, 1993. Main sources of infection in 145 cases of epidemic keratoconjunctivitis. Ger J Ophthalmol 2, 224–227. [PubMed] [Google Scholar]
- Mukherjee S, Zhou X, Rajaiya J, Chodosh J, 2015. Ultrastructure of adenovirus keratitis. Invest Ophthalmol Vis Sci 56, 472–477. 10.1167/iovs.14-15635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruve DA, Barnes MJ, Stillman IE, Libermann TA, 1999. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther 10, 965–976. 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- Natarajan K, Ghalayini AJ, Sterling RS, Holbrook RM, Kennedy RC, Chodosh J, 2002a. Activation of focal adhesion kinase in adenovirus-infected human corneal fibroblasts. Invest Ophthalmol Vis Sci 43, 2685–2690. [PubMed] [Google Scholar]
- Natarajan K, Rajala MS, Chodosh J, 2003. Corneal IL-8 expression following adenovirus infection is mediated by c-Src activation in human corneal fibroblasts. J Immunol 170, 6234–6243. 10.4049/jimmuno1.170.12.6234. [DOI] [PubMed] [Google Scholar]
- Natarajan K, Shepard LA, Chodosh J, 2002b. The use of DNA array technology in studies of ocular viral pathogenesis. DNA Cell Biol 21, 483–490. 10.1089/10445490260099782. [DOI] [PubMed] [Google Scholar]
- Nauheim RC, Romanowski EG, Araullo-Cruz T, Kowalski RP, Turgeon PW, Stopak SS, Gordon YJ, 1990. Prolonged recoverability of desiccated adenovirus type 19 from various surfaces. Ophthalmology 97, 1450–1453. 10.1016/sO161-6420(90)32389-8. [DOI] [PubMed] [Google Scholar]
- Nilsson EC, Storm RJ, Bauer J, Johansson SM, Lookene A, Angstrom J, Hedenstrom M, Eriksson TL, Frangsmyr L, Rinaldi S, Willison HJ, Pedrosa Domellof F, Stehle T, Arnberg N, 2011. The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat Med 17, 105–109. 10.1038/nm.2267. [DOI] [PubMed] [Google Scholar]
- Ouchterlony O, 1949. Antigen-antibody reactions in gels. Acta Pathol Microbial Scand 26, 507–515. 10.1111/j.1699-0463.1949.tb00751.x. [DOI] [PubMed] [Google Scholar]
- Ozen Tunay Z, Ozdemir O, Petricli IS, 2015. Povidone iodine in the treatment of adenoviral conjunctivitis in infants. Cutan Ocul Toxicol 34, 12–15. 10.3109/15569527.2014.888077. [DOI] [PubMed] [Google Scholar]
- Pelletier JS, Stewart K, Trattler W, Ritterband DC, Braverman S, Samson CM, Liang B, Capriotti JA, 2009. A combination povidone-iodine 0.4%/dexamethasone 0.1% ophthalmic suspension in the treatment of adenoviral conjunctivitis. Adv Ther 26, 776–783. 10.1007/s12325-009-0062-1. [DOI] [PubMed] [Google Scholar]
- Pennington MR, Saha A, Painter DF, Gavazzi C, Ismail AM, Zhou X, Chodosh J, Rajaiya J, 2019. Disparate entry of adenoviruses dictates differential innate immune responses on the ocular surface. Microorganisms 7 10.3390/microorganisms7090351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepose JS, Ahuja A, Liu W, Narvekar A, Haque R, 2018. Randomized, controlled, phase 2 trial of povidone-iodine/dexamethasone ophthalmic suspension for treatment of adenoviral conjunctivitis. Am J Ophthalmol 194, 7–15. 10.1016/j.ajo.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Pettit TH, Holland GN, 1979. Chronic keratoconjunctivitis associated with ocular adenovirus infection. Am J Ophthalmol 88, 748–751. 10.1016/0002-9394(79)90677-9. [DOI] [PubMed] [Google Scholar]
- Rajaiya J, Chodosh J, 2006. New paradigms in infectious eye disease: adenoviral keratoconjunctivitis. Arch Soc Esp Oftalmol 81, 493–498. [PubMed] [Google Scholar]
- Rajaiya J, Sadeghi N, Chodosh J, 2009. Specific NFkappaB subunit activation and kinetics of cytokine induction in adenoviral keratitis. Mol Vis 15, 2879–2889. [PMC free article] [PubMed] [Google Scholar]
- Rajaiya J, Xiao J, Rajala RV, Chodosh J, 2008. Human adenovirus type 19 infection of corneal cells induces p38 MAPK-dependent interleukin-8 expression. Virol J 5, 17 10.1186/1743-422X-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaiya J, Zhou X, Barequet I, Gilmore MS, Chodosh J, 2015. Novel model of innate immunity in corneal infection. In Vitro Cell Dev Biol Anim 51, 827–834. 10.1007/s11626-015-9910-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala MS, Rajala RV, Astley RA, Butt AL, Chodosh J, 2005. Corneal cell survival in adenovirus type 19 infection requires phosphoinositide 3-kinase/Akt activation. J Virol 79, 12332–12341. 10.1128/NI.79.19.12332-12341.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramke M, Zhou X, Mateme EC, Rajaiya J, Chodosh J, 2016. Resident corneal c-fms(+) macrophages and dendritic cells mediate early cellular infiltration in adenovirus keratitis. Exp Eye Res 147, 144–147. 10.1016/j.exer.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CM, Seto D, Jones MS, Dyer DW, Chodosh J, 2011a. Molecular evolution of human species D adenoviruses. Infect Genet Evol 11, 1208–1217. 10.1016/j.meegid.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CM, Shariati F, Zaitshik J, Gillaspy AF, Dyer DW, Chodosh J, 2009. Human adenovirus type 19: genomic and bioinformatics analysis of a keratoconjunctivitis isolate. Virus Res 139, 122–126. 10.1016/j.virusres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CM, Singh G, Henquell C, Walsh MP, Peigue-Lafeuille H, Seto D, Jones MS, Dyer DW, Chodosh J, 2011b. Computational analysis and identification of an emergent human adenovirus pathogen implicated in a respiratory fatality. Virology 409, 141–147. 10.1016/j.virol.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CM, Singh G, Lee JY, Dehghan S, Rajaiya J, Liu EB, Yousuf MA, Betensky RA, Jones MS, Dyer DW, Seto D, Chodosh J, 2013a. Molecular evolution of human adenoviruses. Sci Rep 3, 1812 10.1038/srep01812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CM, Zhou X, Rajaiya J, Yousuf MA, Singh G, DeSerres JJ, Walsh MP, Wong S, Seto D, Dyer DW, Chodosh J, Jones MS, 2013b. Predicting the next eye pathogen: analysis of a novel adenovirus. MBio 4, e00595–00512. 10.1128/mBio.00595-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanowski EG, Araullo-Cruz T, Gordon YJ, 1997. Topical corticosteroids reverse the antiviral effect of topical cidofovir in the Ad5-inoculated New Zealand rabbit ocular model. Invest Ophthalmol Vis Sci 38, 253–257. [PubMed] [Google Scholar]
- Romanowski EG, Pless P, Yates KA, Gordon YJ, 2005. Topical cyclosporine A inhibits subepithelial immune infiltrates but also promotes viral shedding in experimental adenovirus models. Cornea 24, 86–91. 10.1097/0l.ico.0000127481.23714.b6. [DOI] [PubMed] [Google Scholar]
- Romanowski EG, Roba LA, Wiley L, Araullo-Cruz T, Gordon YJ, 1996. The effects of corticosteroids of adenoviral replication. Arch Ophthalmol 114, 581–585. 10.1001/archopht.l996.01100130573014. [DOI] [PubMed] [Google Scholar]
- Romanowski EG, Yates KA, Gordon YJ, 2001. Short-term treatment With a potent topical corticosteroid of an acute ocular adenoviral infection in the New Zealand white rabbit. Cornea 20, 657–660. 10.1097/00003226-200108000-00020. [DOI] [PubMed] [Google Scholar]
- Romanowski EG, Yates KA, Gordon YJ, 2002. Topical corticosteroids of limited potency promote adenovirus replication in the Ad5/NZW rabbit ocular model. Cornea 21, 289–291. 10.1097/00003226-200204000-00010. [DOI] [PubMed] [Google Scholar]
- Rowe WP, Huebner RJ, Gilmore LK, Parrott RH, Ward TG, 1953. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med 84, 570–573. 10.3181/00379727-84-20714. [DOI] [PubMed] [Google Scholar]
- Russell WC, 2009. Adenoviruses: update on structure and function. J Gen Virol 90, 1–20. 10.1099/vir.0.003087-0. [DOI] [PubMed] [Google Scholar]
- Rutala WA, Peacock JE, Gergen MF, Sobsey MD, Weber DJ, 2006. Efficacy of hospital germicides against adenovirus 8, a common cause of epidemic keratoconjunctivitis in health care facilities. Antimicrob Agents Chemother 50, 1419–1424. 10.1128/AAC.50.4.1419-1424.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambursky R, Trattler W, Tauber S, Starr C, Friedberg M, Boland T, McDonald M, DellaVecchia M, Luchs J, 2013. Sensitivity and specificity of the AdenoPlus test for diagnosing adenoviral conjunctivitis. JAMA Ophthalmol 131, 17–22. 10.1001/2013.jamaophthalmol.513. [DOI] [PubMed] [Google Scholar]
- Sammons JS, Graf EH, Townsend S, Hoegg CL, Smathers SA, Coffin SE, Williams K, Mitchell SL, Nawab U, Munson D, Quinn G, Binenbaum G, 2019. Outbreak of adenovirus in a neonatal intensive care unit: critical importance of equipment cleaning during inpatient ophthalmologic examinations. Ophthalmology 126, 137–143. 10.1016/j.ophtha.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Sauerbrei A, Sehr K, Brandstadt A, Heim A, Reimer K, Wutzler P, 2004. Sensitivity of human adenoviruses to different groups of chemical biocides. J Hosp Infect 57, 59–66. 10.1016/j.jhin.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Schnurr D, Dondero ME, 1993. Two new candidate adenovirus serotypes. Intervirology 36, 79–83. 10.1159/000150325. [DOI] [PubMed] [Google Scholar]
- Segerman A, Arnberg N, Erikson A, Lindman K, Wadell G, 2003a. There are two different species B adenovirus receptors: sBAR, common to species B1 and B2 adenoviruses, and sB2AR, exclusively used by species B2 adenoviruses. J Virol 77, 1157–1162. 10.1128/jvi.77.2.1157-1162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerman A, Atkinson JP, Marttila M, Dennerquist V, Wadell G, Arnberg N, 2003b. Adenovirus type 11 uses CD46 as a cellular receptor. J Virol 77, 9183–9191. 10.1128/jvi.77.17.9183-9191.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto D, Chodosh J, Brister JR, Jones MS, Members of the Adenovirus Research, C., 2011. Using the whole-genome sequence to characterize and name human adenoviruses. J Virol 85, 5701–5702. 10.1128/NI.00354-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman MD, Feldman KA, Farahmand SM, Margolis TP, 2002. Treatment of conjunctival squamous cell carcinoma with topical cidofovir. Am J Ophthalmol 134, 432–433. 10.1016/s0002-9394(02)01569-6. [DOI] [PubMed] [Google Scholar]
- Shibasaki F, Hallin U, Uchino H, 2002. Calcineurin as a multifunctional regulator. J Biochem 131, 1–15. 10.1093/oxfordjournals.jbchem.a003063. [DOI] [PubMed] [Google Scholar]
- Shields AF, Hackman RC, Fife KH, Corey L, Meyers JD, 1985. Adenovirus infections in patients undergoing bone-marrow transplantation. N Engl J Med 312, 529–533. 10.1056/NEJM198502283120901. [DOI] [PubMed] [Google Scholar]
- Storm RJ, Persson BD, Skalman LN, Frangsmyr L, Lindstrom M, Rankin G, Lundmark R, Domellof FP, Arnberg N, 2017. Human adenovirus type 37 uses alphaVbeta1 and alpha3beta1 integrins for infection of human corneal cells. J Virol 91 10.1128/NI.02019-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein JW, Toews GB, Bergstresser PR, 1979. Corneal allografts fail to express Ia antigens. Nature 282, 326–327. 10.1038/282326a0. [DOI] [PubMed] [Google Scholar]
- Teigler JE, Kagan JC, Barouch DH, 2014. Late endosomal trafficking of alternative serotype adenovirus vaccine vectors augments antiviral innate immunity. J Virol 88, 10354–10363. 10.1128/NI.00936-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thygeson P, 1949. The epidemiology of epidemic keratoconjunctivitis. Am J Ophthalmol 32, 951–959. 10.1016/0002-9394(49)91612-8. [DOI] [PubMed] [Google Scholar]
- Tollefson AE, Spencer JP, Ying B, Buller RM, Wold WS, Toth K, 2014. Cidofovir and brincidofovir reduce the pathology caused by systemic infection with human type 5 adenovirus in immunosuppressed Syrian hamsters, while ribavirin is largely ineffective in this model. Antiviral Res 112, 38–46. 10.1016/j.antivira1.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Toth K, Spencer JF, Dhar D, Sagartz JE, Buller RM, Painter GR, Wold WS, 2008. Hexadecyloxypropyl-cidofovir, CMX001, prevents adenovirus-induced mortality in a permissive, immunosuppressed animal model. Proc Natl Acad Sci U S A 105, 7293–7297. 10.1073/pnas.0800200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauzettel-Klosinski S, Sundmacher R, Wigand R, 1980. [The effects of topical steroids in epidemic kerato-conjunctivitis (author’s transl)]. Klin Monbl Augenheilkd 176, 899–906. 10.1055/s-2008-1057579. [DOI] [PubMed] [Google Scholar]
- Trousdale MD, Goldschmidt PL, Nobrega R, 1994. Activity of ganciclovir against human adenovirus type-5 infection in cell culture and cotton rat eyes. Cornea 13, 435–439. 10.1097/00003226-199409000-00011. [DOI] [PubMed] [Google Scholar]
- Trousdale MD, Nobrega R, Wood RL, Stevenson D, dos Santos PM, Klein D, McDonnell PJ, 1995. Studies of adenovirus-induced eye disease in the rabbit model. Invest Ophthalmol Vis Sci 36, 2740–2748. [PubMed] [Google Scholar]
- Tullo AB, Higgins PG, 1979. An outbreak of adenovirus keratoconjunctivitis in Bristol. Br J Ophthalmol 63, 621–626. 10.1136/bjo.63.9.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MP, Chintakuntlawar A, Robinson CM, Madisch I, Harrach B, Hudson NR, Schnurr D, Heim A, Chodosh J, Seto D, Jones MS, 2009. Evidence of molecular evolution driven by recombination events influencing tropism in a novel human adenovirus that causes epidemic keratoconjunctivitis. PLoS One 4, e5635 10.1371/journal.pone.0005635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MP, Seto J, Jones MS, Chodosh J, Xu W, Seto D, 2010. Computational analysis identifies human adenovirus type 55 as are-emergent acute respiratory disease pathogen. J Clin Microbial 48, 991–993. 10.1128/JCM.O1694-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Huang S, Kapoor-Munshi A, Nemerow G, 1998. Adenovirus internalization and infection require dynamin. J Virol 72, 3455–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessely K, 1911. Ueber anaphylaktische erscheinungen an der hornhaut (experimentelle erzeugung einer parenchymatosen keratitis durch artfremdes serum. Munchener Medizinische Wochenschrift 58, 1713. [Google Scholar]
- Whitley RJ, Gnann JW Jr., 1992. Acyclovir: a decade later. N Engl J Med 327, 782–789. 10.1056/NEJM199209103271108. [DOI] [PubMed] [Google Scholar]
- Wickham TJ, Mathias P, Cheresh DA, Nemerow GR, 1993. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73, 309–319. 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- Wilkins MR, Khan S, Bunce C, Khawaja A, Siriwardena D, Larkin DF, 2011. A randomised placebo-controlled trial of topical steroid in presumed viral conjunctivitis. Br J Ophthalmol 95, 1299–1303. 10.1136/bjo.2010.188623. [DOI] [PubMed] [Google Scholar]
- Wood DE, Salzberg SL, 2014. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol 15, R46 10.1186/gb-2014-15-3-r46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu E, Trauger SA, Pache L, Mullen TM, von Seggem DJ, Siuzdak G, Nemerow GR, 2004. Membrane cofactor protein is a receptor for adenoviruses associated with epidemic keratoconjunctivitis. J Virol 78, 3897–3905. 10.1128/jvi.78.8.3897-3905.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Chodosh J, 2005. JNK regulates MCP-1 expression in adenovirus type 19-infected human corneal fibroblasts. Invest Ophthalmol Vis Sci 46, 3777–3782. 10.1167/iovs.05-0724. [DOI] [PubMed] [Google Scholar]
- Yabiku ST, Yabiku MM, Bottos KM, Araujo AL, Freitas D, Belfort R Jr., 2011. [Ganciclovir 0.15% ophthalmic gel in the treatment of adenovirus keratoconjunctivitis]. Arq Bras Oftalmol 74, 417–421. 10.1590/s0004-27492011000600007. [DOI] [PubMed] [Google Scholar]
- Yang Y, Li Q, Ertl HC, Wilson JM, 1995. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol 69, 2004–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawata N, Selva KJ, Liu YC, Tan KP, Lee AW, Siak J, Lan W, Vania M, Arundhati A, Tong L, Li J, Mehta JS, Yawata M, 2016. Dynamic change in natural killer cell type in the human ocular mucosa in situ as means of immune evasion by adenovirus infection. Mucosal Immunol 9, 159–170. 10.1038/mi.2015.47. [DOI] [PubMed] [Google Scholar]
- Yazar H, Yarbag A, Balci M, Teker B, Tanyeri P, 2016. The effects ofpovidone iodine (pH 4.2) on patients with adenoviral conjunctivitis. J Pak Med Assoc 66, 968–970. [PubMed] [Google Scholar]
- Ying B, Tollefson AE, Spencer JP, Balakrishnan L, Dewhurst S, Capella C, Buller RM, Toth K, Wold WS, 2014. Ganciclovir inhibits human adenovirus replication and pathogenicity in permissive immunosuppressed Syrian hamsters. Antimicrob Agents Chemother 58, 7171–7181. 10.1128/AAC.03860-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf MA, Lee JS, Zhou X, Ramke M, Lee JY, Chodosh J, Rajaiya J, 2016. Protein kinase C signaling in adenoviral infection. Biochemistry 55, 5938–5946. 10.1021/acs.biochem.6b00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf MA, Zhou X, Mukherjee S, Chintakuntlawar AV, Lee JY, Ramke M, Chodosh J, Rajaiya J, 2013. Caveolin-1 associated adenovirus entry into human corneal cells. PLoS One 8, e77462 10.1371/joumal.pone.0077462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Ramke M, Chintakuntlawar AV, Lee JY, Rajaiya J, Chodosh J, 2017. Role of MyD88 in adenovirus keratitis. Immunol Cell Biol 95, 108–116. 10.1038/icb.2016.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Robinson CM, Rajaiya J, Dehghan S, Seto D, Jones MS, Dyer DW, Chodosh J, 2012. Analysis of human adenovirus type 19 associated with epidemic keratoconjunctivitis and its reclassification as adenovirus type 64. Invest Ophthalmol Vis Sci 53, 2804–2811. 10.1167/iovs.12-9656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweighaft RM, Hierholzer JC, Bryan JA, 1977. Epidemic keratoconjunctivitis at a Vietnamese refugee camp in Florida. Am J Epidemiol 106, 399–407. 10.1093/oxfordjoumals.aje.a112482. [DOI] [PubMed] [Google Scholar]