Abstract
McCune-Albright Syndrome (MAS) is a rare, mosaic disorder presenting along a broad clinical spectrum. Disease arises from somatic activating GNAS mutations, leading to constitutive Gαs activation and ligand-independent signaling of the Gs-coupled protein receptor. The phenotype is largely determined by location and extent of tissues in which the GNAS mutation is expressed, as well as the pathophysiologic effects of Gαs activation within these tissues. Patient present clinically with a variable combination of fibrous dysplasia of bone (FD), café-au-lait skin macules, and hyperfunctioning endocrinopathies. In bone, Gαs leads to impaired differentiation of skeletal stem cells and formation of discrete, expansile FD lesions, resulting in fractures, pain, and functional impairment. A systematic approach to diagnosis and management is critically important to optimize outcomes for patients with FD/MAS. There are no medical therapies capable of altering the disease course in FD, however screening and treatment for endocrinopathies can mitigate some skeletal morbidities. This review summarizes current understanding of MAS pathophysiology, describes the spectrum of clinical features, and includes a detailed discussion of the recommended approach to diagnosis and management
Keywords: fibrous dysplasia, fibroblast growth factor 23, metabolic bone disease, Gαs, mosaicism
Introduction
McCune-Albright Syndrome (MAS) is a rare mosaic disorder that presents along a broad clinical spectrum. MAS was originally described in 1936 as a triad of fibrous dysplasia of bone (FD), café-au-lait skin macules, and precocious puberty (1). However, it is now recognized that the phenotype is far more complex (2). The broad phenotypic spectrum can make MAS a challenging disorder for clinicians. However, taking a systematic approach that considers MAS in its context as a mosaic, multisystemic disorder can provide clarity in the diagnosis and management of this interesting disease.
Pathogenesis
MAS arises from activating mutations in GNAS, which encodes the α-subunit of the Gs G-coupled protein receptor (3). These mutations lead to loss of the α-subunit’s intrinsic GTPase activity, leading to constitutive receptor activation and inappropriate cAMP production. Arg201 is a key component of the GTPase and the most common site of pathogenic mutations (4). >95% of disease causing missense mutations occur equally at the R201H and R201C positions; uncommonly mutations may occur at Q227 and other positions (5).
Mutations in MAS are somatic, occurring early in embryogenesis. The resulting phenotype is determined largely by the extent and location of mutation-bearing tissue. Consistent with mosaic disease, MAS is not inherited, and there are no known cases of vertical transmission. There are no known genetic or environmental risk factors, and disease appears to occur in all ethnic groups (2).
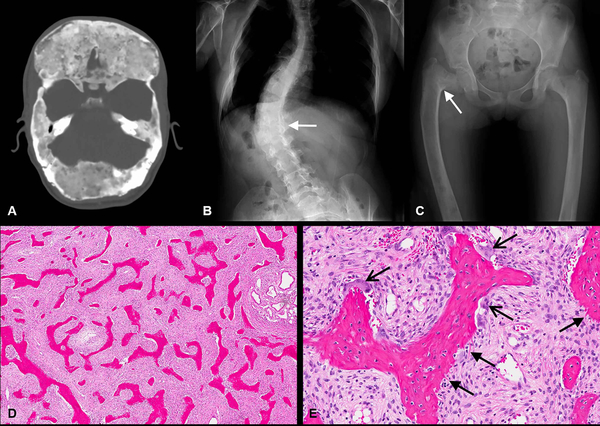
Constitutive, ligand-independent signaling through the LH, FSH, TSH, GHRH, and ACTH receptors results in the hyperfunctioning endocrinopathies characteristic of MAS (3). In bone, constitutive Gαsα activation impairs differentiation of skeletal stem cells, leading to replacement of normal bone and marrow with immature woven bone and fibrotic stroma (6) (Fig 1D&E).
Figure 1. Radiographic and histologic features of fibrous dysplasia (FD).
(A) Axial CT with diffuse skull base FD. Note the characteristic homogeneous, “ground glass” appearance of FD bone. (B) Radiograph of the spine with scoliosis secondary to vertebral FD (white arrow). (C) Femoral FD with a classic varus (“shepherd’s crook”) deformity (white arrow). (D, E) FD histology: H&E stain demonstrating classic curvilinear trabeculae and hypercellular fibrous stroma; (D) low power view and (E) high power view demonstrating signs of increased remodeling including Sharpey fibers (open arrows) and abundant osteoclasts (closed arrows).
Clinical Features
Fibrous Dysplasia
FD ranges from trivial monostotic disease affecting one bone, to debilitating polyostotic disease (7). FD lesions can occur in isolation or in combination with additional features of MAS.
Clinical sequelae of FD in the appendicular skeleton arises due to FD’s tendency to fracture and deform under weight-bearing forces. Patients frequently present to care due to limp or pain (2). The proximal femur is one of the most commonly involved sites and may develop a characteristic coxa vara (“shepherd’s crook”) deformity (8) (Fig 1C). Fracture rates are highest during childhood and adolescence, and steadily decrease into adulthood (9), potentially related to increased FD activity in younger patients (discussed further below).
FD in the axial skeleton can result in scoliosis, which may be progressive and rarely lethal (10) (Fig 1B). Scoliosis is associated with hip malalignment and leg length discrepancy, which contribute to ambulation difficulties (11, 12).
Craniofacial FD typically presents with a slow-growing, painless swelling, which may result in facial asymmetry (2)(Fig 1A). Mild, asymptomatic craniofacial FD may be asymptomatic, and is often found incidentally on imaging studies such as dental radiographs and post-traumatic computed tomography scans (13). In rare and severe cases, patients may experience pain, paresthesia, or functional deficits, such as malocclusion, hearing impairment, and/or visual disturbances (13–16)(Fig 2E). Rarely, compression of the cerebellum and brainstem can develop in patients with skull base FD (17).
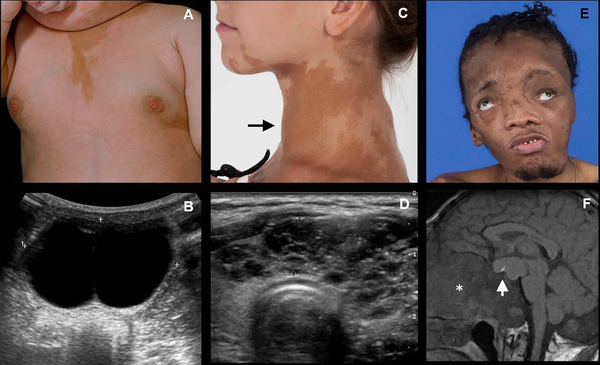
Figure 2. Clinical and radiographic features of McCune-Albright Syndrome (MAS).
(A) An infant with a typical appearing café-au-lait macule and breast budding; (B) corresponding pelvic ultrasound shows a large estrogen-producing ovarian cyst. (C) Adolescent with typical appearing café-au-lait macules affecting her neck and jaw, and a goiter (arrow) consistent with hyperthyroidism; (D) corresponding thyroid ultrasound demonstrating an abnormal, heterogenous cystic gland. (E) Patient with uncontrolled growth hormone excess resulting in macrocephaly and vision loss; (F) T1-weighted MRI with large growth hormone-secreting pituitary adenoma (arrow) and thickened cranial base secondary to fibrous dysplasia.
The natural history of FD includes typical age-related changes in disease progression and activity. In utero skeletal development appears to occur relatively normally, without obvious signs of FD at birth. FD lesions become apparent during early childhood and tend to progress in number and size until final skeletal burden is established by age 15 years (18). In vitro studies suggest that lesions eventually “burn out” during adulthood as the population of mutated skeletal stem cells depletes (19). Age-related changes in FD are also observed radiographically. Appendicular lesions typically have a homogenous, “ground glass” appearance on radiographs, which appear more heterogeneous and sclerotic changes with age (20). Craniofacial lesions tend to develop irregular, radiolucent-appearing areas on computed tomography over time (20).
Overproduction of fibroblast growth factor 23 (FGF23) from mutation-bearing skeletal stem cells is a key feature of FD (21). FGF23 is potent phosphate regulator, acting at the proximal renal tubule to decrease 1-α-hydroxylase activity and increase urinary phosphate excretion. Increased serum FGF23 and renal phosphate wasting are found commonly in patients with FD, however, frank hypophosphatemia is uncommon due to compensatory regulatory mechanisms (22, 23). Hypophosphatemia typically only develops in patients with a high skeletal disease burden and may wax and wane over time (21).
Skin
Café-au-lait macules are often the first clinically apparent manifestations of MAS, presenting at or shortly after birth (2). However, their significance is often noted only in retrospect, after other symptoms have developed. Café-au-lait macules have a characteristic appearance that includes jagged, irregular borders (often described as resembling the “coast of Maine”), and location respecting the midline of the body (5)(Fig 2A&C).
Gonadal involvement
Although MAS gonadal involvement occurs equally in girls and boys, overproduction of sex steroids is far more common in girls. In one large series of patients seen at the NIH, GNAS activation in ovarian tissue resulted in recurrent estrogen-producing cysts in approximately 85% of girls (5, 24)(Fig2A&B). Patients develop acute onset of pubertal signs, including breast development and growth acceleration. Labs show elevated estradiol levels with suppressed gonadotropins, and ultrasonography typically shows uterine enlargement with single or multiple ovarian cysts. Cyst resolution is associated with an acute drop in estradiol, which triggers vaginal bleeding. Between episodes girls are often clinically asymptomatic with undetectable estradiol levels and normal ultrasonographic findings, which may lead to delayed diagnoses. Ovarian torsion is an uncommon complication (25). Autonomous ovarian activity persists into adulthood and is commonly associated with menometorrhagia and fertility effects (25).
GNAS activation in the testes leads to Leydig and Sertoli cell hyperplasia, which presents clinically with macro-orchidism and ultrasonographic abnormalities, including focal masses, diffuse heterogeneity, and microlithiasis (26). Testicular volume is therefore an inaccurate indicator of precocious puberty in boys with MAS. While approximately 85% of boys have testicular involvement, only 15% develop autonomous testosterone production, which presents with clinical signs of androgenization including public and axillary hair, penile enlargement, and growth acceleration (24, 26).
Secondary central puberty may develop as a complication of gonadotropin-independent puberty, typically at a bone age of 9 years or greater.
Thyroid
Thyroid abnormalities have been reported in approximately ~50% of patients with MAS, about half of whom develop frank hyperthyroidism (24, 27)(Fig2C&D). Clinical and ultrasonographic findings include diffuse enlargement, heterogeneity, and discrete cystic and solid nodules (27, 28). Biochemically, GNAS mutations result in constitutive 5’-deiodinase activity, resulting in increased conversion of T4 to T3 and a primary T3 toxicosis (28). Hyperthyroidism typically develops during childhood and persists throughout adulthood.
Pituitary
GNAS activation in the pituitary results in somatolactroph cell hyperplasia, leading to constitutive growth hormone (GH) and prolactin production in approximately 15% of patients (2, 24). The most common clinical sign is expansion of craniofacial FD, which is particularly sensitive to the effects of GH (29)(Fig2E&F). Symptoms may include progressive macrocephaly, vision loss, and hearing loss, all of which may signal the presence of GH secretion abnormalities, even in the absence of frankly elevated IGF-1 levels (14, 29). Other manifestations include growth acceleration, acromegalic features, and the development of secondary pituitary hormone insufficiencies (30). While growth acceleration and tall stature may suggest the presence of GH excess, this is not a consistent finding in patients with MAS because linear growth is often confounded by skeletal deformities and other endocrinopathies. GH excess is diagnosed by IGF-1, oral glucose tolerance test, and/or overnight GH sampling; most patients also demonstrate concomitant mild elevations in prolactin.
Neonatal Hypercortisolism
Hypercortisolism is one of the rarest and most serious complications of MAS (24). It arises due to GNAS activation in the fetal adrenal gland and presents exclusively during the first year of life (31, 32). Infants are often born small for gestational age and can develop failure to thrive, feeding problems, Cushingoid features, hypertension, respiratory disease, or other signs of illness (33). Symptoms can be insidious and non-specific, which may lead to delayed diagnoses (31). Adrenalectomy may be life-saving for severely affected patients, however medical therapy is an option for patients who are either mildly affected or too unstable for surgery. Hypercortisolism may spontaneously resolve in up to 1/3 of patients, likely due to involution of the fetal adrenal gland (31, 32). Long-term sequelae of neonatal hypercortisolism include neurodevelopmental effects (31) and late-onset adrenal insufficiency after spontaneous resolution (2).
Evaluation and Management
Diagnostic criteria
The diagnosis of MAS is most often made clinically, based on 2 or more characteristic features (Table 1) (2, 34). Mutation detection is variable and depends upon the level of mosaicism in the tissue being tested, and the sensitivity of the technique (2). PCR-based sequencing methods have mutation detection rates of greater than 80% in lesional tissue, and ~20–30% in peripheral blood lymphocytes (35–37). While detection of a pathogenic GNAS mutation may be helpful in establishing the diagnosis, a negative result does not rule out FD/MAS; mutation testing therefore does not typically affect management in patients with established clinical diagnoses.
Table 1.
Diagnostic criteria for fibrous dysplasia/McCune-Albright syndrome1
| • Fibrous dysplasia of bone2 |
| • Café-au-lait skin pigmentation with characteristic features3 |
| • Gonadotropin-independent precocious puberty resulting from recurrent ovarian cysts in girls and autonomous testosterone production in boys |
| • Testicular lesions with or without associated gonadotropin-independent precocious puberty |
| • Thyroid lesions with or without non-autoimmune hyperthyroidism |
| • Growth hormone excess |
| • Neonatal hypercortisolism |
Two or more clinical features are consistent with the diagnosis of FD/MAS.
The presence of an isolated monostotic lesion in the absence of extraskeletal features requires a biopsy for diagnostic certainty.
Hyperpigmentation with irregular borders, distribution reflecting the midline of the body.
Fibrous Dysplasia
By age 5 years, 90% of clinically significant FD can be identified in some form on bone scintigraphy; for this reason, a staging scintigraphy scan at this age is recommended for all patients with known or suspected FD/MAS (18, 34). Depending on the identified areas of involvement, additional imaging with radiographs or computed tomography may be indicated to better characterize individual lesions. No medical treatments have been shown to affect the quality or progression of FD lesions, therefore, clinical management focuses on optimizing function and mitigating risk factors that contribute to skeletal morbidity.
Management of appendicular FD focuses on correcting and preventing deformities and fractures (38). Proximal femoral (“shepherd’s crook”) deformities are particularly challenging, especially in young children where surgical options are limited by small size and skeletal growth (Fig 1C). Techniques commonly used in other conditions, such as curettage and grafting, have limited utility in patients with extensive FD involvement (39). Surgical approaches must therefore be individualized to account for the challenges and needs of each patient (38, 40).
Patients with craniofacial FD require a baseline head computed tomography scan to assess the affected structures (2, 34)(Fig 1A), and should undergo annual evaluations by a neuro-ophthalmologist and otolaryngologist to monitor for vision and hearing impairment (14, 29). Optic neuropathy is uncommon even in patients with FD involving the optic canals; prophylactic optic nerve decompression is therefore not indicated and has been associated with an increased risk of blindness (16, 41). Patients should be monitored for symptoms associated with cranial base deformities such as basilar invagination and Chiari I malformation (17). Consistent dental care should be prioritized for patients with FD affecting the teeth-bearing bones, who can uncommonly develop complications such as periodontal disease and malocclusion (13). Outcomes from craniofacial surgery are often unsatisfactory due to high risk of postoperative FD regrowth, particularly in patients with GH excess (42). Surgical indications should therefore be limited to correction of functional impairment and severe, disabling deformity.
Severe and progressive scoliosis can typically be managed with spinal fusion, which often shows favorable outcomes in FD (12). Regular physiatric care to optimize gait and alignment, including orthoses to correct leg length discrepancies, is an important component of management that may mitigate progression of scoliosis and other deformities (11, 12).
Pain is a common feature in FD, affecting approximately 80% of patients (43). The occurrence and severity of FD pain appear to increase with age, counter to the typical age-related decrease in FD activity (44). Pain levels do not correlate with skeletal disease burden (44). FD pain can be treated medically with oral analgesic medications and supportive measures, and intravenous bisphosphonates may be helpful for patients with persistent, moderate to severe pain (34, 43). Despite their likely analgesic effects, bisphosphonates have not been demonstrated to improve bone quality or prevent FD lesion expansion (45–47). Concerningly, osteonecrosis of the jaw has been reported in association with bisphosphonate treatment in FD (48); therefore, it is recommended that bisphosphonates be limited to treatment of FD-related pain, using the lowest effective dose and interval (34).
Evaluation and treatment of MAS endocrinopathies (discussed below) is a key component of management in FD, because uncontrolled endocrinopathies contribute to skeletal morbidity. GH excess fuels craniofacial FD expansion, leading to increased risk for vision loss (29), hearing loss (14), and postoperative FD regrowth (42). Hypophosphatemia and hyperthyroidism increase risk of fractures, deformities, and pain, likely through metabolic effects that further decrease the mechanical stability of FD bone (9, 12, 14, 17). Patients with polyostotic FD should have serum phosphorus levels monitored regularly, with a low threshold for intervention with vitamin D analogues and phosphorus supplementation. Dosing is similar to X-linked hypophosphatemia, a more common disorder of FGF23 excess (49). Burosumab, a monoclonal antibody to FGF23 approved for treatment of X-linked hypophosphatemia, has not yet been studied in FD but holds promise as a potential therapy (50).
Endocrinopathies
All patients with MAS should be evaluated for endocrinopathies with a targeted history and physical exam, review of growth curve, and a bone age examination (2, 34). Gonadal evaluation should include screening testicular ultrasound in all boys, and in girls with findings suggestive of precocious puberty. Treatment should be aimed at mitigating negative effects of precocious puberty on psychosocial development and adult height. Girls can generally be managed with the aromatase inhibitor letrozole, which is effective in decreasing frequency of vaginal bleeding and improving final adult height (51). If letrozole monotherapy is ineffective, estrogen receptor modulators such as tamoxifen and fulvestrant may be considered (52, 53). Boys with precocious puberty are treated with testosterone receptor blockers, such as bicalutamide or spironolactone, used in combination with an aromatase inhibitor to prevent bone age advancement (26). Children may require GnRH agonists therapy in addition to these blocking agents for treatment of secondary central precocious puberty (26, 51). Management of menometorrhagia may include use of progestin-containing intrauterine devices or oral contraceptive pills (25). Pregnancy is likely achievable and safe in women with MAS, however ovarian disease may make conception more challenging, and clinicians should have a low threshold to refer patients desiring pregnancy to fertility specialists (25).
Hyperthyroidism typically responds to anti-thyroidal medications for short-term management. Most patients ultimately elect for definitive treatment, which is ideally achieved through thyroidectomy at an experienced, high-volume surgical center (2). Radioablation is also effective and may be preferred in patients who present surgical risks, or who don’t have access to sufficiently experienced centers. However, clinicians should be aware of the theoretical risk of subtherapeutic radiation exposure to adjacent unaffected tissue given the patchy, mosaic nature of thyroid involvement (27, 34).
Patients with GH excess are typically treated medically with somatostatin analogues, used alone or in combination with GH receptor blockers (34, 54). Because craniofacial FD is particularly susceptible to the effects of GH, aggressive management is preferred, with the goal of decreasing IGF-1 between −2 and +1 standard deviations for children, and as low as possible for adults (2, 34, 54, 55). Hyperprolactinemia in MAS is typically mild, however patients with symptoms such as galactorrhea or central hypogonadism generally respond well to dopamine agonists such as cabergoline. Pituitary involvement is typically diffuse, even if discrete adenomas are visible on magnetic resonance imaging (54, 56). For this reason, surgical treatment typically requires total hypophysectomy, and is generally reserved for patients who are uncontrolled with maximal medical therapy. Surgery is often technically difficult due to FD involvement in the skull base (56). The presence of skull base FD also complicates radiation treatment, which has been associated with sarcomatous transformation in this area and should be reserved for final recourse in patients with severe, disabling disease (34, 54).
Other Manifestations
Gastrointestinal
MAS may be associated with a broad spectrum of gastrointestinal disease. Neonatal cholestasis and hepatitis has been reported in infants (57), and a variety of hepatobiliary abnormalities have been reported in adults, including hepatocellular adenomas, choledochal cysts, and inflammatory adenomas (58, 59). Gastric polyps may be asymptomatic or associated with symptoms of reflux (60, 61). Activating GNAS mutations are known drivers for the development intraductal papillary mucinous neoplasms (IPMNs) (62). These pancreatic cysts have been reported in up to 40% of patients with MAS and have rarely been associated with obstructive pancreatitis and diabetes (58, 60, 63). Gastrointestinal evaluation, including pancreatic imaging, should therefore be considered in all symptomatic patients (2,63). Although IPMNs are considered a potentially pre-malignant lesion in the general population, the risk of pancreatic adenocarcinoma in patients with MAS appears to be low, with only one reported case (64). It is unknown if IPMNs associated with MAS are truly at lower risk compared to the general population, or if the malignant potential of IPMNs is lower than previously thought (65).
Bone marrow and hematologic
Bone marrow failure with pancytopenia and extramedullary hematopoiesis has been rarely reported in patients with severe FD (66, 67). The etiology is not well-established but may be related to reduced marrow capacity in conjunction with splenic sequestration, and patients have clinically improved after splenectomy (66, 67). Platelet dysfunction has also been reported in association with MAS (68), which together with the hypervascularity of FD lesions, may contribute to blood loss with orthopedic procedures.
Malignancy
GNAS mutations are weak oncogenes, and in the general population have been associated with thyroid adenomas, pituitary adenomas, and intraductal papillary mucinous neoplasms (69). An increased risk of breast cancer has been identified in patients with MAS, and early screening with mammography is recommended for patients with polyostotic FD and ovarian involvement (70). Malignant transformation of FD has also been reported, particularly in patients exposed to external beam radiation (54, 71). Patients typically present with new-onset pain and/or rapid expansion in a previously inactive lesion, which radiographically may show expansion through the bony cortex. This should be distinguished from fluid-filled aneurysmal bone cysts, which also arise acutely and expand rapidly within FD bone. The prevalence of malignant transformation in FD has not been determined, however it is likely low and has been observed in only 2/250 patients in the NIH cohort (unpublished observation, AMB & MTC). Other malignancies reported in association with MAS include thyroid (72), testicular (26), pancreatic (64), and hepatoblastoma (57).
Future Directions
Identifying treatments capable of altering FD lesion activity is a high priority for clinical and translational research. The recent development of several mouse models represents an important advance that may be used to identify and test novel therapeutic targets (73–75). Another ongoing strategy is high throughput screening to identify molecules with activity at the mutant Gs-receptor (76). Recent evidence suggests denosumab, a monoclonal antibody to receptor activator of nuclear-B kappa ligand, may be effective in treating pain and slowing FD lesion expansion (77, 78). However, concerningly, denosumab discontinuation has been associated with severe, life-threatening hypercalcemia in patients with FD (77), and further research is needed to determine if and how denosumab can be used safely in this population.
Table 2.
Medications commonly used in treatment of McCune-Albright syndrome
| Indication | Medication | Mechanism of action | Potential adverse effects |
|---|---|---|---|
| Precocious puberty | Letrozole | Aromatase inhibitor | Transient transaminemia |
| Tamoxifen | Estrogen receptor modulator | Endometrial hyperplasia | |
| Hyperthyroidism | Methimazole | Thyroperoxidase inhibitor | Hepatotoxicity, agranulocytosis |
| Propylthiouracil | Inhibits thyroperoxidase and 5’-deiodinase | Hepatotoxicity, agranulocytosis | |
| Growth hormone excess | Octreotide; Lanreotide |
Somatostatin analog | Cholelithiasis, hepatotoxicity |
| Pegvisomant | Growth hormone receptor antagonist | Hepatotoxicity | |
| Neonatal hypercortisolism | Metyrapone | 11-beta-hydroxylase inhibitor | Hirsutism, hypertension, hyperkalemia |
| Ketoconazole | Inhibits cortisol synthesis | Hepatotoxicity, hypogonadism | |
| FGF23-mediated hypophosphatemia | Calcitriol; Alfacalcidiol | Increases dietary calcium absorption and renal tubular calcium reabsorption | Hypercalciuria, hypercalcemia |
| Phosphorus | Supplemental | Gastrointestinal effects |
Acknowledgments
Funding Sources: This work was funded by the Intramural Research Program of the National Institute of Dental and Craniofacial Research, National Institutes of Health.
Disclosure Statement: NIDCR receives funding from Amgen, Inc, for an investigator sponsored study of denosumab treatment for fibrous dysplasia.
References
- 1.McCune DJ. Osteitis fibrosa cystica: the case of a nine year old girl who also exhibits precocious puberty, multiple pigmentation of the skin and hyperthyroidism Am J Dis Child. 1936;52:743–7. [Google Scholar]
- 2.Boyce AM, Florenzano P, de Castro LF, Collins MT. Fibrous Dysplasia/McCune-Albright Syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, et al. , editors. GeneReviews((R)). Seattle (WA): University of Washington, Seattle University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle; All rights reserved.; 1993. [Google Scholar]
- 3.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. The New England journal of medicine. 1991;325(24):1688–95. [DOI] [PubMed] [Google Scholar]
- 4.Turan S, Bastepe M. GNAS Spectrum of Disorders. Current osteoporosis reports. 2015;13(3):146–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson C, Collins MT, Boyce AM. Fibrous Dysplasia/McCune-Albright Syndrome: Clinical and Translational Perspectives. Current osteoporosis reports. 2016;14(5):178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riminucci M, Liu B, Corsi A, Shenker A, Spiegel AM, Robey PG, et al. The histopathology of fibrous dysplasia of bone in patients with activating mutations of the Gs alpha gene: site-specific patterns and recurrent histological hallmarks. The Journal of pathology. 1999;187(2):249–58. [DOI] [PubMed] [Google Scholar]
- 7.Boyce AM, Collins MT. Fibrous Dysplasia/McCune-Albright Syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, et al. , editors. GeneReviews((R)): University of Washington, Seattle, Washington.; 1993. [PubMed] [Google Scholar]
- 8.Ippolito E, Farsetti P, Boyce AM, Corsi A, De Maio F, Collins MT. Radiographic classification of coronal plane femoral deformities in polyostotic fibrous dysplasia. Clinical orthopaedics and related research. 2014;472(5):1558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leet AI, Chebli C, Kushner H, Chen CC, Kelly MH, Brillante BA, et al. Fracture incidence in polyostotic fibrous dysplasia and the McCune-Albright syndrome. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2004;19(4):571–7. [DOI] [PubMed] [Google Scholar]
- 10.Berglund JA, Tella SH, Tuthill KF, Kim L, Guthrie LC, Paul SM, et al. Scoliosis in Fibrous Dysplasia/McCune-Albright Syndrome: Factors Associated With Curve Progression and Effects of Bisphosphonates. J Bone Miner Res. 2018;33(9):1641–8. [DOI] [PubMed] [Google Scholar]
- 11.Paul SM, Gabor LR, Rudzinski S, Giovanni D, Boyce AM, Kelly MR, et al. Disease severity and functional factors associated with walking performance in polyostotic fibrous dysplasia. Bone. 2014;60:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berglund JA, Tella SH, Tuthill KF, Kim L, Guthrie LC, Paul SM, et al. Scoliosis in Fibrous Dysplasia/McCune-Albright Syndrome: Factors Associated with Curve Progression and Effects of Bisphosphonates. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2018. [DOI] [PubMed] [Google Scholar]
- 13.Burke AB, Collins MT, Boyce AM. Fibrous dysplasia of bone: craniofacial and dental implications. Oral diseases. 2017;23(6):697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyce AM, Brewer C, DeKlotz TR, Zalewski CK, King KA, Collins MT, et al. Association of Hearing Loss and Otologic Outcomes With Fibrous Dysplasia. JAMA otolaryngology--head & neck surgery. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeKlotz TR, Kim HJ, Kelly M, Collins MT. Sinonasal disease in polyostotic fibrous dysplasia and McCune-Albright Syndrome. The Laryngoscope. 2013;123(4):823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amit M, Collins MT, FitzGibbon EJ, Butman JA, Fliss DM, Gil Z. Surgery versus watchful waiting in patients with craniofacial fibrous dysplasia--a meta-analysis. PloS one. 2011;6(9):e25179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan KS, Heiss JD, Brown SM, Collins MT, Boyce AM. Chiari I Malformation and Basilar Invagination in Fibrous Dysplasia: Prevalence, Mechanisms, and Clinical Implications. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2018;33(11):1990–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart ES, Kelly MH, Brillante B, Chen CC, Ziran N, Lee JS, et al. Onset, progression, and plateau of skeletal lesions in fibrous dysplasia and the relationship to functional outcome. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007;22(9):1468–74. [DOI] [PubMed] [Google Scholar]
- 19.Kuznetsov SA, Cherman N, Riminucci M, Collins MT, Robey PG, Bianco P. Age-dependent demise of GNAS-mutated skeletal stem cells and “normalization” of fibrous dysplasia of bone. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008;23(11):1731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kushchayeva YS, Kushchayev SV, Glushko TY, Tella SH, Teytelboym OM, Collins MT, et al. Fibrous dysplasia for radiologists: beyond ground glass bone matrix. Insights into imaging. 2018;9(6):1035–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. The Journal of clinical investigation. 2003;112(5):683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins MT, Chebli C, Jones J, Kushner H, Consugar M, Rinaldo P, et al. Renal phosphate wasting in fibrous dysplasia of bone is part of a generalized renal tubular dysfunction similar to that seen in tumor-induced osteomalacia. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2001;16(5):806–13. [DOI] [PubMed] [Google Scholar]
- 23.Bhattacharyya N, Wiench M, Dumitrescu C, Connolly BM, Bugge TH, Patel HV, et al. Mechanism of FGF23 processing in fibrous dysplasia. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012;27(5):1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins MT, Singer FR, Eugster E. McCune-Albright syndrome and the extraskeletal manifestations of fibrous dysplasia. Orphanet journal of rare diseases. 2012;7 Suppl 1:S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyce AM, Casey RK, Ovejero Crespo D, Murdock CM, Estrada A, Guthrie LC, et al. Gynecologic and reproductive outcomes in fibrous dysplasia/McCune-Albright syndrome. Orphanet journal of rare diseases. 2019;14(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyce AM, Chong WH, Shawker TH, Pinto PA, Linehan WM, Bhattacharryya N, et al. Characterization and management of testicular pathology in McCune-Albright syndrome. The Journal of clinical endocrinology and metabolism. 2012;97(9):E1782–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Celi FS, Coppotelli G, Chidakel A, Kelly M, Brillante BA, Shawker T, et al. The role of type 1 and type 2 5’-deiodinase in the pathophysiology of the 3,5,3’-triiodothyronine toxicosis of McCune-Albright syndrome. The Journal of clinical endocrinology and metabolism. 2008;93(6):2383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tessaris D, Corrias A, Matarazzo P, De Sanctis L, Wasniewska M, Messina MF, et al. Thyroid abnormalities in children and adolescents with McCune-Albright syndrome. Hormone research in paediatrics. 2012;78(3):151–7. [DOI] [PubMed] [Google Scholar]
- 29.Boyce AM, Glover M, Kelly MH, Brillante BA, Butman JA, Fitzgibbon EJ, et al. Optic Neuropathy in McCune-Albright Syndrome: Effects of Early Diagnosis and Treatment of Growth Hormone Excess. The Journal of clinical endocrinology and metabolism. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roszko KL, Collins MT, Boyce AM. Mosaic Effects of Growth Hormone on Fibrous Dysplasia of Bone. The New England journal of medicine. 2018;379(20):1964–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown RJ, Kelly MH, Collins MT. Cushing syndrome in the McCune-Albright syndrome. The Journal of clinical endocrinology and metabolism. 2010;95(4):1508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carney JA, Young WF, Stratakis CA. Primary bimorphic adrenocortical disease: cause of hypercortisolism in McCune-Albright syndrome. The American journal of surgical pathology. 2011;35(9):1311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tatsi C, Stratakis CA. Neonatal Cushing Syndrome: A Rare but Potentially Devastating Disease. Clinics in perinatology. 2018;45(1):103–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Javaid MK, Boyce A, Appelman-Dijkstra N, Ong J, Defabianis P, Offiah A, et al. Best practice management guidelines for fibrous dysplasia/McCune-Albright syndrome: a consensus statement from the FD/MAS international consortium. Orphanet journal of rare diseases. 2019;14(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lumbroso S, Paris F, Sultan C. Activating Gsalpha mutations: analysis of 113 patients with signs of McCune-Albright syndrome--a European Collaborative Study. The Journal of clinical endocrinology and metabolism. 2004;89(5):2107–13. [DOI] [PubMed] [Google Scholar]
- 36.Kalfa N, Philibert P, Audran F, Ecochard A, Hannon T, Lumbroso S, et al. Searching for somatic mutations in McCune-Albright syndrome: a comparative study of the peptidic nucleic acid versus the nested PCR method based on 148 DNA samples. European journal of endocrinology / European Federation of Endocrine Societies. 2006;155(6):839–43. [DOI] [PubMed] [Google Scholar]
- 37.Narumi S, Matsuo K, Ishii T, Tanahashi Y, Hasegawa T. Quantitative and sensitive detection of GNAS mutations causing mccune-albright syndrome with next generation sequencing. PloS one. 2013;8(3):e60525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanton RP IE, Springfield D, Lindaman L, Wientroub S, Leet A. The surgical management of fibrous dysplasia of bone. Orphanet journal of rare diseases. 2012;24 (7): Suppl 1:S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leet AI, Boyce AM, Ibrahim KA, Wientroub S, Kushner H, Collins MT. Bone-Grafting in Polyostotic Fibrous Dysplasia. The Journal of bone and joint surgery American volume. 2016;98(3):211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majoor BCJ, Leithner A, van de Sande MAJ, Appelman-Dijkstra NM, Hamdy NAT, Dijkstra PDS. Individualized approach to the surgical management of fibrous dysplasia of the proximal femur. Orphanet journal of rare diseases. 2018;13(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JS, FitzGibbon E, Butman JA, Dufresne CR, Kushner H, Wientroub S, et al. Normal vision despite narrowing of the optic canal in fibrous dysplasia. The New England journal of medicine. 2002;347(21):1670–6. [DOI] [PubMed] [Google Scholar]
- 42.Boyce AM, Burke A, Cutler Peck C, DuFresne CR, Lee JS, Collins MT. Surgical Management of Polyostotic Craniofacial Fibrous Dysplasia: Long-Term Outcomes and Predictors for Postoperative Regrowth. Plastic and reconstructive surgery. 2016;137(6):1833–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chapurlat RD, Gensburger D, Jimenez-Andrade JM, Ghilardi JR, Kelly M, Mantyh P. Pathophysiology and medical treatment of pain in fibrous dysplasia of bone. Orphanet journal of rare diseases. 2012;7 Suppl 1(Suppl 1):S3-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelly MH, Brillante B, Collins MT. Pain in fibrous dysplasia of bone: age-related changes and the anatomical distribution of skeletal lesions. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2008;19(1):57–63. [DOI] [PubMed] [Google Scholar]
- 45.Boyce AM, Kelly MH, Brillante BA, Kushner H, Wientroub S, Riminucci M, et al. A randomized, double blind, placebo-controlled trial of alendronate treatment for fibrous dysplasia of bone. The Journal of clinical endocrinology and metabolism. 2014;99(11):4133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Florenzano P, Pan KS, Brown SM, Paul SM, Kushner H, Guthrie LC, et al. Age-Related Changes and Effects of Bisphosphonates on Bone Turnover and Disease Progression in Fibrous Dysplasia of Bone. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plotkin H, Rauch F, Zeitlin L, Munns C, Travers R, Glorieux FH. Effect of pamidronate treatment in children with polyostotic fibrous dysplasia of bone. The Journal of clinical endocrinology and metabolism. 2003;88(10):4569–75. [DOI] [PubMed] [Google Scholar]
- 48.Metwally T, Burke A, Tsai JY, Collins MT, Boyce AM. Fibrous Dysplasia and Medication-Related Osteonecrosis of the Jaw. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2016;74(10):1983–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL. A clinician’s guide to X-linked hypophosphatemia. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26(7):1381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carpenter TO, Whyte MP, Imel EA, Boot AM, Hogler W, Linglart A, et al. Burosumab Therapy in Children with X-Linked Hypophosphatemia. The New England journal of medicine. 2018;378(21):1987–98. [DOI] [PubMed] [Google Scholar]
- 51.Estrada A, Boyce AM, Brillante BA, Guthrie LC, Gafni RI, Collins MT. Long-term outcomes of letrozole treatment for precocious puberty in girls with McCune-Albright syndrome. European journal of endocrinology / European Federation of Endocrine Societies. 2016;175(5):477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eugster EA, Rubin SD, Reiter EO, Plourde P, Jou HC, Pescovitz OH. Tamoxifen treatment for precocious puberty in McCune-Albright syndrome: a multicenter trial. The Journal of pediatrics. 2003;143(1):60–6. [DOI] [PubMed] [Google Scholar]
- 53.Sims EK, Garnett S, Guzman F, Paris F, Sultan C, Eugster EA. Fulvestrant treatment of precocious puberty in girls with McCune-Albright syndrome. International journal of pediatric endocrinology. 2012;2012(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salenave S, Boyce AM, Collins MT, Chanson P. Acromegaly and McCune-Albright syndrome. The Journal of clinical endocrinology and metabolism. 2014:jc20133826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tessaris D, Boyce AM, Zacharin M, Matarazzo P, Lala R, De Sanctis L, et al. Growth hormone-Insulin-like growth factor 1 axis hyperactivity on bone fibrous dysplasia in McCune-Albright Syndrome. Clinical endocrinology. 2018;89(1):56–64. [DOI] [PubMed] [Google Scholar]
- 56.Vortmeyer AO, Glasker S, Mehta GU, Abu-Asab MS, Smith JH, Zhuang Z, et al. Somatic GNAS Mutation Causes Widespread and Diffuse Pituitary Disease in Acromegalic Patients with McCune-Albright Syndrome. The Journal of clinical endocrinology and metabolism. 2012;97(7):2404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johansen L, Haller W, Thyagarajan M, Kelly D, McKiernan P. Hepatic Lesions Associated With McCune Albright Syndrome. Journal of pediatric gastroenterology and nutrition. 2019;68(4):e54–e7. [DOI] [PubMed] [Google Scholar]
- 58.Gaujoux S, Salenave S, Ronot M, Rangheard AS, Cros J, Belghiti J, et al. Hepatobiliary and Pancreatic neoplasms in patients with McCune-Albright syndrome. The Journal of clinical endocrinology and metabolism. 2014;99(1):E97–101. [DOI] [PubMed] [Google Scholar]
- 59.Nault JC, Fabre M, Couchy G, Pilati C, Jeannot E, Tran Van Nhieu J, et al. GNAS-activating mutations define a rare subgroup of inflammatory liver tumors characterized by STAT3 activation. Journal of hepatology. 2012;56(1):184–91. [DOI] [PubMed] [Google Scholar]
- 60.Wood LD, Noe M, Hackeng W, Brosens LA, Bhaijee F, Debeljak M, et al. Patients with McCune-Albright syndrome have a broad spectrum of abnormalities in the gastrointestinal tract and pancreas. Virchows Archiv : an international journal of pathology. 2017;470(4):391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zacharin M, Bajpai A, Chow CW, Catto-Smith A, Stratakis C, Wong MW, et al. Gastrointestinal polyps in McCune Albright syndrome. Journal of medical genetics. 2011;48(7):458–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Science translational medicine. 2011;3(92):92ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robinson C, Estrada A, Zaheer A, Singh VK, Wolfgang CL, Goggins MG, et al. Clinical and Radiographic Gastrointestinal Abnormalities in McCune-Albright Syndrome. The Journal of clinical endocrinology and metabolism. 2018;103(11):4293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parvanescu A, Cros J, Ronot M, Hentic O, Grybek V, Couvelard A, et al. Lessons from McCune-Albright syndrome-associated intraductal papillary mucinous neoplasms: : GNAS-activating mutations in pancreatic carcinogenesis. JAMA surgery. 2014;149(8):858–62. [DOI] [PubMed] [Google Scholar]
- 65.Rossi RE, Massironi S. Intraductal papillary mucinous neoplasms of the pancreas: a clinical challenge. Expert review of gastroenterology & hepatology. 2018. [DOI] [PubMed] [Google Scholar]
- 66.Robinson C, Boyce AM, Estrada A, Kleiner DE, Mathew R, Stanton R, et al. Bone marrow failure and extramedullary hematopoiesis in McCune-Albright syndrome. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2018;29(1):237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mahdi AJ, Connor P, Thakur I. McCune-Albright syndrome-associated bone marrow failure and extramedullary haematopoeisis secondary to fibrous dysplasia. British journal of haematology. 2017;178(2):179. [DOI] [PubMed] [Google Scholar]
- 68.Bajpai A, Greenway A, Zacharin M. Platelet dysfunction and increased bleeding tendency in McCune-Albright syndrome. The Journal of pediatrics. 2008;153(2):287–9. [DOI] [PubMed] [Google Scholar]
- 69.O’Hayre M, Vazquez-Prado J, Kufareva I, Stawiski EW, Handel TM, Seshagiri S, et al. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nature reviews Cancer. 2013;13(6):412–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Majoor BC, Boyce AM, Bovee JV, Smit VT, Collins MT, Cleton-Jansen AM, et al. Increased Risk of Breast Cancer at a Young Age in Women with Fibrous Dysplasia. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2018;33(1):84–90. [DOI] [PubMed] [Google Scholar]
- 71.Ruggieri P, Sim FH, Bond JR, Unni KK. Malignancies in fibrous dysplasia. Cancer. 1994;73(5):1411–24. [DOI] [PubMed] [Google Scholar]
- 72.Collins MT, Sarlis NJ, Merino MJ, Monroe J, Crawford SE, Krakoff JA, et al. Thyroid carcinoma in the McCune-Albright syndrome: contributory role of activating Gs alpha mutations. The Journal of clinical endocrinology and metabolism. 2003;88(9):4413–7. [DOI] [PubMed] [Google Scholar]
- 73.Zhao X, Deng P, Iglesias-Bartolome R, Amornphimoltham P, Steffen DJ, Jin Y, et al. Expression of an active Galphas mutant in skeletal stem cells is sufficient and necessary for fibrous dysplasia initiation and maintenance. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(3):E428–e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khan SK, Yadav PS, Elliott G, Hu DZ, Xu R, Yang Y. Induced Gnas(R201H) expression from the endogenous Gnas locus causes fibrous dysplasia by up-regulating Wnt/beta-catenin signaling. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(3):E418–e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saggio I, Remoli C, Spica E, Cersosimo S, Sacchetti B, Robey PG, et al. Constitutive expression of Gsalpha(R201C) in mice produces a heritable, direct replica of human fibrous dysplasia bone pathology and demonstrates its natural history. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014;29(11):2357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bhattacharyya N, Hu X, Chen CZ, Mathews Griner LA, Zheng W, Inglese J, et al. A high throughput screening assay system for the identification of small molecule inhibitors of gsp. PloS one. 2014;9(3):e90766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boyce AM, Chong WH, Yao J, Gafni RI, Kelly MH, Chamberlain CE, et al. Denosumab treatment for fibrous dysplasia. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012;27(7):1462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Castro LF, Burke AB, Wang HD, Tsai J, Florenzano P, Pan KS, et al. Activation of RANK/RANKL/OPG Pathway Is Involved in the Pathophysiology of Fibrous Dysplasia and Associated With Disease Burden. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]