Abstract
Novel insect-specific viruses (ISVs) are being discovered in many important vectors due to advances in sequencing technology and a growing awareness of the virome. Several in vitro and in vivo studies indicate that ISVs are capable of modulating pathogenic arboviruses. In addition, there is growing evidence that both vertical and horizonal transmission strategies maintain ISVs in vector populations. As such there is potential to exploit ISVs for stand-alone vector control strategies and deploying them in synergy with other symbiont control approaches such as Wolbachia-mediated control. However, before the applied potential can be realized, a greater understanding of their basic biology is required, including their species range, ability to be maintained and transmitted in native and non-native vector hosts, and the effect of infection on a range of pathogens.
Introduction
Arthropods possess a diverse range of microbes that play a central role in the basic biology of the host. While most research has focused on bacterial symbionts, little is known about viral symbionts. The non-pathogenic, insect-specific viruses (ISVs) of the microbiome vary in phylogeny and are now found in abundance in hematophagous arthropods. Some ISVs are closely related to human and animal pathogens, which has highlighted the rationale for further investigations into ISVs blocking pathogens through prior infections in the vector or as chimeric vaccines in mammals. The pursuit of these goals has neglected to define the most basic biological questions of ISV infection and transmission, as ISVs must employ alternative methods of transmission to arboviruses, because of their restriction in arthropods (Figure 1). An improved understanding of ISV transmission and their interactions with their arthropod hosts, as well as the pathogenic viruses that infect and are vectored by these hosts, may unlock their potential to modulate transmission of arboviruses.
Figure 1.
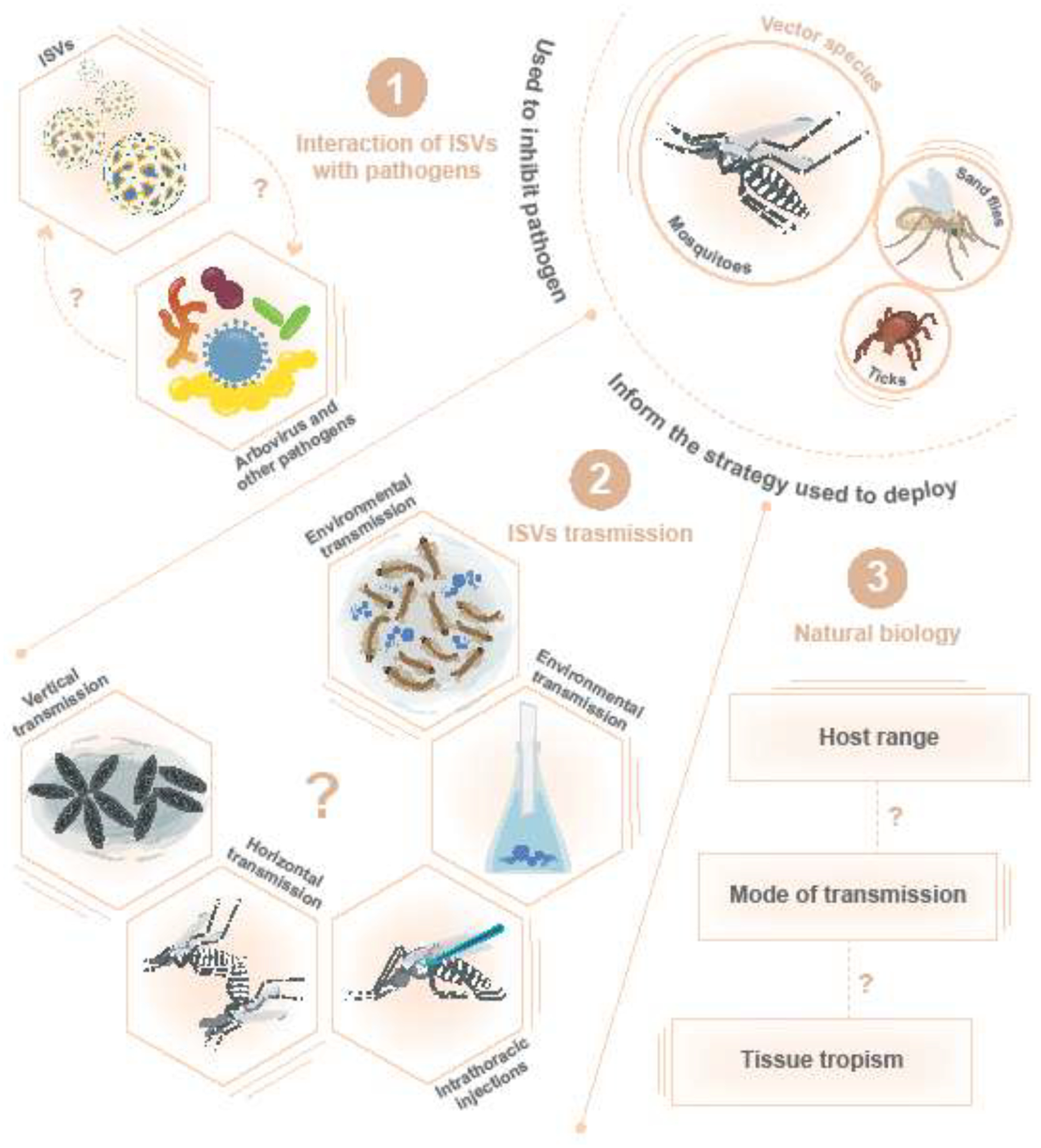
Putative overview of using Insect-specific viruses for vector control. ISVs have been discovered in arthropod vectors. There is evidence that ISVs modulate arbovirus infections. Many modes of transmission may be used in natural and laboratory settings, but aspects of the natural biology of ISVs remain unknown. Combining these attributes will inform methods used for vector control.
Insect-specific virus interactions with pathogens
As their name implies, ISVs are only known to replicate in insect (or arthropod) cells but not mammalian cells. A large proportion of ISVs have been discovered through surveillance for pathogenic and emerging viruses, particularly in mosquitoes [1*]. Recent studies have sought to investigate if ISVs affect the transmission of pathogens in mosquitoes through mechanisms like superinfection exclusion, the theory that cells infected with one virus are not susceptible to subsequent infections with similar viruses. Relying on superinfection exclusion necessitates that ISVs maintain a cell tropism to directly block arboviruses at their site of attachment or replication. Experiments have demonstrated that some ISVs can modulate the replication of certain arboviruses in cells and mosquitoes [2–16]. This relationship is poorly understood and phylogeny does not appear to explain the phenotype, as diverse ISVs exert these effects. In addition to directly influencing pathogens, ISVs may indirectly upregulate the immune system either as virions or by integration into the host genome. Endogenous flaviviral elements (EFVEs), many of which appear to be derived from insect-specific flaviviruses (ISFs) and have been hypothesized to play critical functions in arthropod antiviral immunity [17], have introgressed into the genomes of many Aedes and Anopheles mosquitoes [18,19].
A large proportion of ISVs are ISFs, some of which have been shown to impact titers of medically important flaviviruses in mosquitoes or cells [2–12]. These ISFs are either dual-host affiliated (dISFs) that are more closely related to pathogenic arboviruses (dual-host flaviviruses), or from a paraphyletic clade of classical ISFs (cISFs) that group among other viruses restricted to insect hosts [20] (Figure 2). Nhumirim virus (NHUV), a dISF, and Palm Creek virus (PCV), a cISF, have both been shown to decrease titers or transmission of multiple pathogenic flaviviruses in cells or mosquitoes, respectively [2–6]. These interactions appear to be virus family and vector-species dependent, as these ISFs do not reduce titers of alphaviruses in cells [4**,6]. Similarly, a cell fusing agent virus (CFAV) strain isolated from Aedes aegypti mosquitoes reduced dissemination of both dengue virus (DENV) and Zika virus (ZIKV) in Aedes albopictus (C6/36) cell lines and in Ae. aegypti mosquitoes [7**]. In contrast, a CFAV strain derived from an Ae. aegypti cell line (Aag2) [21] has been shown to enhance DENV replication in another Ae. aegypti cell line (Aa20) [8]. Other studies suggest that Culex flavivirus (CxFV) has little effect [9,10,22] or even enhances [11,12] West Nile virus (WNV), DENV, Japanese encephalitis virus, and Rift Valley fever virus transmission. Many of these experiments ignore the specific virus-host pairings [23] and rely on results from cell lines with a defective RNAi response [24]. The diverse outcomes from ISV-arbovirus interactions suggest that more rigorous investigations in vivo of interactions with pathogenic viruses in specific hosts are likely to elucidate both their natural biology and their potential to be exploited in arbovirus transmission-blocking strategies.
Figure 2.

Midpoint-rooted phylogeny of 875–910 nt dual-host flavivirus and insect-specific flavivirus (ISF) NS5 gene sequences. Virus designations followed by GenBank accessions and mosquito species from which they were isolated are indicated. Key interactions of ISFs that inhibit or enhance arbovirus transmission are indicated in green and blue, respectively. The phylogeny was generated using PhyML version 3.0 [61], employing the Akaike information criterion [62] for automatic selection of the general time reversible (GTR) sequence evolution model. Tree topologies were estimated using nearest neighbour interchange (NNI) improvements over 1000 bootstrap replicates. The branch length scale represents substitutions per site.
Limited studies have been performed to evaluate pathogen modulation of ISVs from other virus taxa. Eilat virus (EILV), an insect-specific alphavirus, was only able to delay dissemination of chikungunya virus (CHIKV) from the midgut of Ae. aegypti for 3 days [13]. Phasi Charoen-like virus (PCLV), an insect-specific bunyavirus, may be capable of blocking infections of bunya- and flaviviruses, but to see differences between PCLV-infected and uninfected mosquitoes, PCLV was infected at high titers, suggesting the effect may be subtle and not biologically relevant [14,15]. Menghai rhabdovirus and Shinobi tetravirus ISVs, which were isolated from mosquito cells, were also able to inhibit replication of multiple flaviviruses [16]. Despite the evidence mentioned above regarding ISV-pathogen interactions, the majority of ISVs remain unevaluated, including those discovered in ticks, sand flies and midges [25–29].
While there is some experimental evidence indicating that ISVs influence viral pathogens, we have a limited understanding regarding the role of ISV infection on other arthropod-borne pathogens. A survey of the microbiome of Ixodes scapularis identified a positive co-occurrence and correlation between the presence of tick-specific bunyaviruses, South Bay virus and blacklegged tick phlebovirus, and Borrelia burgdorferi, the causative agent of Lyme disease [30*]. These ISVs have been found in ticks across the globe, including areas endemic with Lyme disease [29,31], but experimental infections have not been undertaken to determine if the ISVs promote B. burgdorferi, or vice versa. There are also a range of ISVs that infect Anopheles mosquitoes that transmit Plasmodium [32–37] and phlebotomine sand flies that transmit Leishmania [26–28], warranting further investigations into potential interactions with malaria parasites in these systems.
Maintenance and transmission
The variable prevalence of ISVs in mosquitoes suggests that different types of transmission modes may be maintaining these viruses in vector populations. Many reports have suggested vertical transmission as the mode for maintenance for ISVs in mosquito populations, with a small number of studies providing direct experimental evidence. CFAV, NHUV and Kamiti River virus (KRV), all ISFs, were tested through intrathoracic injection or oral exposure of female mosquitoes for detection of virus in the offspring and to initiate transmission to naïve colonies [3,38–40]. Transmission rates varied greatly from 4% for KRV to 93% for CFAV. When naturally infected hosts were observed, transovarian transmission rates were 100% for both CFAV in Ae. aegypti and CxFV in Culex pipiens [38,39]. The attempt to initiate vertical transmission of CxFV in a naïve laboratory colony of Cx. pipiens was unsuccessful, showing a narrow host range may even be restricted to sub-populations of a species [39]. ISV infections transstadially transmitted from immature life stages to adults have been attributed to vertical transmission from their parents. However, it has not yet been determined whether the aquatic environment during early development also facilitates horizontal transmission of ISVs [33*]. The difference in infection and prevalence may be due to host and viral genetics, which could dictate host range at the species and strain level. In all of these cases, vertical transmission was confirmed by RT-PCR of parents and offspring without taking into account environmental factors, so additional methods are required for the mechanism(s) to be elucidated. Investigations into aphid-borne viruses have used a variety of methods, such as immunofluorescence of dissected reproductive organs and tissues, and binding assays with recombinant viral proteins, to detail the process of transmission with more precision [41*]. Similar studies could provide insights in mosquito-ISV systems.
Recent discoveries in plant arboviruses, viruses that circulate between plants and arthropod host, have demonstrated that infection of either male or female parental insects can result in vertical transmission. Interestingly, this has been attributed to interactions with both structural and non-structural viral proteins [41*–43]. These mechanisms were discovered in plant reoviruses, and reovirus ISVs have also been found in mosquitoes [44]. This family of viruses is non-enveloped, therefore capsid-derived interactions may not be applicable for many ISVs, the majority of which are enveloped. However, ISVs that are not enveloped or have exposed capsid proteins, such as reoviruses, birnaviruses, densoviruses, and negeviruses, may exploit similar mechanisms of vertical transmission in mosquitoes compared to their plant vector relatives. If vertical transmission is not observed, then these ISVs may only require minimal engineering of their capsid proteins to instigate the known mechanism of vertical transmission. For enveloped ISVs, more experiments should be undertaken to investigate the contribution of non-structural proteins in vertical transmission. To pinpoint interactions and replication, future studies may consider using virus isolates expressing visual reporters as a means to track infection through parent and offspring.
Severe host restrictions may provide hurdles when trying to colonize diverse species or locations with ISVs but can be advantageous for specific targeting of major vectors and limiting spread to non-target insects. Nonetheless there are a couple of reports of Aedes-specific flaviviruses (AeFVs) being detected in Culex mosquitoes [33*,45], indicating that cross-species infection of ISVs occurs, but mosquito species-specific ISV infections are more likely to persist, as evidenced by mosquito genus- and species-specific ISF clades (Figure 2). Nonetheless, instances of cross-species infection may assist in the transfer of ISVs between species, with subsequent co-evolution [46]. Though the mechanism for such cross-species transmission remains unknown, such findings suggests that there may be secondary modes of transmission through shared environments. Unnatural routes of ISV transmission, such as intrathoracic injections and artificial blood meals, have successfully initiated infection in new host species [10,27]. Although vertical transmission would be advantageous for long-term colonization of vector insects with ISVs that block pathogens, horizontally transmissible ISVs could be used to quickly colonize new mosquito populations.
ISV interactions with other microbial symbionts
It is evident that microbial interactions are ubiquitous in arthropod vectors [32,47–50] and it will be important to determine the extent of interactions between ISVs and other symbionts that are currently being used in vector and pathogen control strategies. Multiple studies have been carried out to define a relationship between ISVs and Wolbachia, an insect symbiont that has been artificially introduced into Ae. aegypti colonies. A survey of field-caught and laboratory-reared mosquitoes evaluated the inevitable and ongoing relationship between the introduced bacteria and the native virome [51**]. The study found that field-caught mosquitoes with Wolbachia had higher titers and rates of ISV infections, and Wolbachia infected mosquitoes reared in the laboratory had higher ISV titers compared to their uninfected counterparts. These results contrast in vitro studies where Wolbachia infections reduce CFAV below detection limits and have no effect on PCLV titers [52–54]. Further investigations are required to determine the interactions of ISVs with other symbionts.
It is possible that ISVs could be used in synergy with Wolbachia population replacement control approaches. In theory, a vertically transmitted ISV could hitchhike with Wolbachia into naïve populations and enhance the anti-viral effect by a distinct mechanism. For example, while Wolbachia does not inhibit the bunyavirus PCLV, PCLV is the only ISV to show inhibition against a negative strand RNA virus, La Crosse virus [14,52,54]. Similarly, it would be interesting to determine if ISVs have played an incidental role in DENV transmission reduction in Wolbachia-colonized mosquitoes in the field.
Other applied uses
Aside from using wild-type ISVs to interfere with pathogens, engineered ISVs may be employed in both insects and mammals to directly prevent or identify infection. Both a diagnostic kit and a vaccine candidate have been developed for CHIKV based on a CHIKV chimera with EILV [55,56]. Chimeric PCV expressing WNV structural proteins replicate in mammalian cells but do not produce infectious virions, offering potential for effective attenuated vaccines for flaviviruses [57]. Paratransgenic approaches using chimeric ISVs may also be useful to interfere with arboviruses either by producing an antiviral molecule, priming the RNAi responses in the vector, producing double-stranded RNAs, or by sequestering receptors required for viral infection.
While the relatedness of ISVs to pathogenic arboviruses may limit applications based on identical structure, such as using an insect-specific alphavirus to mimic pathogenic alphaviruses, other ISV classifications may provide ideal platforms for paratransgenic applications. Mesoniviruses and negeviruses have wide geographic and host ranges, can be grown to high titers in cell culture, and can be genetically tractable [26,27,58–60]. Additionally, these virus groups are strictly insect-specific, negating concerns over reversion or recombination to pathogenic strains.
Conclusions
Gene drives and the use of Wolbachia endosymbionts to manipulate wild populations show promise as valuable tools for reducing pathogen transmission. Adding ISVs to the list of available tools will lead to more effective and synergistic methods to limit transmission rates. Additional layers of pathogen control will also hinder the rise of resistance, a likely consequence of any singular control method.
The potential of ISVs for vector control can only be realized once there is a greater understanding of their basic biology. However, the ability of ISVs to interfere with pathogenic arboviruses and be maintained in vector populations warrants further investigations into these viruses. While genomics studies have been critical for identifying the ubiquitous nature of ISVs, future studies need to build upon these sequencing results and investigate host-microbe, tripartite interactions, and phenotypes in their native and non-native vectors. These basic studies will undoubtedly provide valuable foundation to use ISVs as a tool to combat infectious diseases and offer a platform for vaccines and diagnostics.
Highlights.
ISVs have been found to modulate replication and transmission of arboviruses.
Vertical and horizontal transmission could be exploited for applied strategies.
Similar biology to Wolbachia could be deployed in synergistic control strategies.
Acknowledgements
EIP was supported by the Liverpool School of Tropical Medicine Director’s Catalyst Fund award. JV and JNM were supported by the ANTI-VeC Pump-Priming Award (AV/PP12) sub-awarded by the University of Glasgow from UK government Global Challenges Research Fund (GCRF) Networks in Vector Borne Disease Research funds and icipe institutional funding from the UK’s Department for International Development (DFID), the Swedish International Development Cooperation Agency (SIDA), the Swiss Agency for Development and Cooperation (SDC), and the Kenyan Government. GLH is supported by the NIH (R21AI129507 and R21AI138074), the BBSRC (BB/T001240/1), a John S. Dunn Foundation Collaborative Research Award, and a Royal Society Wolfson Fellowship (RSWF\R1\180013). We would like to thank Manuela Bernardi for preparation of the summary figure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Calisher CH, Higgs S: The discovery of arthropod-specific viruses in hematophagous arthropods: An open door to understanding the mechanisms of arbovirus and arthropod evolution? Annu Rev Entomol 2018, 63:87–103. [DOI] [PubMed] [Google Scholar]; *This review provides a comprehensive list of known ISVs, including a history of their discovery.
- 2.Kenney JL, Solberg OD, Langevin SA, Brault AC: Characterization of a novel insect-specific flavivirus from Brazil: potential for inhibition of infection of arthropod cells with medically important flaviviruses. J Gen Virol 2014, 95:2796–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goenaga S, Kenney JL, Duggal NK, Delorey M, Ebel GD, Zhang B, Levis SC, Enria DA, Brault AC: Potential for co-infection of a mosquito-specific flavivirus, Nhumirim virus, to block West Nile virus transmission in mosquitoes. Viruses 2015, 7:5801–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romo H, Kenney JL, Blitvich BJ, Brault AC: Restriction of Zika virus infection and transmission in Aedes aegypti mediated by an insect-specific flavivirus. Emerg Microbes Infec 2018, 7:181. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This study used NHUV to reduce titer and transmission of flaviviruses in Aedes cells and mosquitoes. These experiments demonstrated that NHUV could interfere with pathogenic virus infections in multiple hosts, building on previous results from WNV in Culex mosquitoes.
- 5.Hall-Mendelin S, McLean BJ, Bielefeldt-Ohmann H, Hobson-Peters J, Hall RA, van den Hurk AF: The insect-specific Palm Creek virus modulates West Nile virus infection in and transmission by Australian mosquitoes. Parasite Vector 2016, 9:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hobson-Peters J, Yam A, Lu J, Setoh Y, May FJ, Kurucz N, Walsh S, Prow NA, Davis SS, Weir R, et al. : A new insect-specific flavivirus from Northern Australia suppresses replication of West Nile Virus and Murray Valley encephalitis virus in co-infected mosquito cells. Plos One 2013, 8:e56534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baidaliuk A, Miot EF, Lequime S, Moltini-Conclois I, Delaigue F, Dabo S, Dickson LB, Aubry F, Merkling SH, Cao-Lormeau V-M, et al. : Cell-fusing agent virus reduces arbovirus dissemination in Aedes aegypti mosquitoes in vivo. J Virol 2019, doi: 10.1128/jvi.00705-19. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This study observed reduced infection of DENV and ZIKV in Ae. aegypti mosquitoes attributed to CFAV infection. CFAV is the most widely documented ISF, often being found in laboratory colonies and cell culture stocks.
- 8.Zhang G, Asad S, Khromykh AA, Asgari S: Cell fusing agent virus and dengue virus mutually interact in Aedes aegypti cell lines. Sci Rep-uk 2017, 7:6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolling BG, Olea-Popelka FJ, Eisen L, Moore CG, Blair CD: Transmission dynamics of an insect-specific flavivirus in a naturally infected Culex pipiens laboratory colony and effects of co-infection on vector competence for West Nile virus. Virology 2012, 427:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talavera S, Birnberg L, Nuñez AI, Muñoz-Muñoz F, Vázquez A, Busquets N: Culex flavivirus infection in a Culex pipiens mosquito colony and its effects on vector competence for Rift Valley fever phlebovirus. Parasite Vector 2018, 11:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kent RJ, Crabtree MB, Miller BR: Transmission of West Nile virus by Culex quinquefasciatus Say infected with Culex flavivirus Izabal. Plos Neglect Trop D 2010, 4:e671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuwata R, Isawa H, Hoshino K, Sasaki T, Kobayashi M, Maeda K, Sawabe K: Analysis of mosquito-borne flavivirus superinfection in Culex tritaeniorhynchus (Diptera: Culicidae) cells persistently infected with Culex flavivirus (Flaviviridae). J Med Entomol 2015, 52:222–229. [DOI] [PubMed] [Google Scholar]
- 13.Nasar F, Erasmus JH, Haddow AD, Tesh RB, Weaver SC: Eilat virus induces both homologous and heterologous interference. Virology 2015, 484:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schultz MJ, Frydman HM, Connor JH: Dual Insect specific virus infection limits arbovirus replication in Aedes mosquito cells. Virology 2018, 518:406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fredericks AC, Wallace LE, Russell TA, Davidson AD, Fernandez-Sesma A, Maringer K: Aedes aegypti (Aag2)-derived clonal mosquito cell lines reveal the impact of pre-existing persistent infection with the insect-specific bunyavirus Phasi Charoen-like virus on arbovirus replication. Biorxiv 2019, doi: 10.1101/596205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujita R, Kato F, Kobayashi D, Murota K, Takasaki T, Tajima S, Lim C-K, Saijo M, Isawa H, Sawabe K: Persistent viruses in mosquito cultured cell line suppress multiplication of flaviviruses. Heliyon 2018, 4:e00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houé V, Bonizzoni M, Failloux A-B: Endogenous non-retroviral elements in genomes of Aedes mosquitoes and vector competence. Emerg Microbes Infec 2019, 8:542–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki Y, Frangeul L, Dickson LB, Blanc H, Verdier Y, Vinh J, Lambrechts L, Saleh M-C: Uncovering the repertoire of endogenous flaviviral elements in Aedes mosquito genomes. J Virol 2017, 91:e00571–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lequime S, Lambrechts L: Discovery of flavivirus-derived endogenous viral elements in Anopheles mosquito genomes supports the existence of Anopheles-associated insect-specific flaviviruses. Virus Evol 2017, 3:vew035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blitvich BJ, Firth AE: Insect-specific flaviviruses: A systematic review of their discovery, host range, mode of transmission, superinfection exclusion potential and genomic organization. Viruses 2015, 7:1927–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stollar V, Thomas VL: An agent in the Aedes aegypti cell line (Peleg) which causes fusion of Aedes albopictus cells. Virology 1975, 64:367–377. [DOI] [PubMed] [Google Scholar]
- 22.Newman CM, Krebs BL, Anderson TK, Hamer GL, Ruiz MO, Brawn JD, Brown WM, Kitron UD, Goldberg TL: Culex flavivirus during West Nile virus epidemic and interepidemic years in Chicago, United States. Vector-borne Zoonot 2017, 17:567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longdon B, Hadfield JD, Day JP, Smith SC, McGonigle JE, Cogni R, Cao C, Jiggins FM: The causes and consequences of changes in virulence following pathogen host shifts. Plos Pathog 2015, 11:e1004728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brackney DE, Scott JC, Sagawa F, Woodward JE, Miller NA, Schilkey FD, Mudge J, Wilusz J, Olson KE, Blair CD, et al. : C6/36 Aedes albopictus cells have a dysfunctional antiviral RNA interference response. Plos Neglect Trop D 2010, 4:e856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Modha S, Hughes J, Bianco G, Ferguson HM, Helm B, Tong L, Wilkie GS, Kohl A, Schnettler E: Metaviromics reveals unknown viral diversity in the biting midge Culicoides impunctatus. Viruses 2019, 11:865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nunes M, Contreras-Gutierrez M, Guzman H, Martins LC, Barbirato M, Savit C, Balta V, Uribe S, Vivero R, Suaza J, et al. : Genetic characterization, molecular epidemiology, and phylogenetic relationships of insect-specific viruses in the taxon Negevirus. Virology 2017, 504:152–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasilakis N, Forrester NL, Palacios G, Nasar F, Savji N, Rossi SL, Guzman H, Wood TG, Popov V, Gorchakov R, et al. : Negevirus: a proposed new taxon of insect-specific viruses with wide geographic distribution. Journal of Virology 2013, 87:2475–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moureau G, Ninove L, Izri A, Cook S, Lamballerie X, Charrel RN: Flavivirus RNA in phlebotomine sandflies. Vector-borne Zoonot 2010, 10:195–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tokarz R, Sameroff S, Tagliafierro T, Jain K, Williams SH, Cucura MD, Rochlin I, Monzon J, Carpi G, Tufts D, et al. : Identification of novel viruses in Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis ticks. Msphere 2018, 3:e00614–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cross ST, Kapuscinski ML, Perino J, Maertens BL, Weger-Lucarelli J, Ebel GD, Stenglein MD: co-infection patterns in individual Ixodes scapularis ticks reveal associations between viral, eukaryotic and bacterial microorganisms. Viruses 2018, 10:388. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study identified components of the microbiome of field caught ticks. The results demonstrated a relationship with the occurrence of insect-specific bunyaviruses and B. burgdorferi.
- 31.Vanmechelen B, Laenen L, Vergote V, Maes P: Grotenhout virus, a novel Nairovirus found in Ixodes ricinus in Belgium. Genome Announc 2017, 5:e00288–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nanfack-Minkeu F, Mitri C, Bischoff E, Belda E, Casademont I, Vernick KD: Interaction of RNA viruses of the natural virome with the African malaria vector, Anopheles coluzzii. Sci Rep-uk 2019, 9:6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ajamma Y, Onchuru T, Ouso DO, Omondi D, Masiga DK, Villinger J: Vertical transmission of naturally occurring Bunyamwera and insect-specific flavivirus infections in mosquitoes from islands and mainland shores of Lakes Victoria and Baringo in Kenya. Plos Neglect Trop D 2018, 12:e0006949. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study found diverse ISFs in Aedes and Anopheles mosquitoes that were naturally infected in native aquatic stages, then reared to adults. As Aedes-specific flavivirus was also isolated from Culex mosquitoes, ISFs may have the potential to be transmitted both vertically and horizontally, possibly through shared aquatic environments.
- 34.Villinger J, Mbaya MK, Ouso D, Kipanga PN, Lutomiah J, Masiga DK: Arbovirus and insect specific virus discovery in Kenya by novel six genera multiplex high resolution melting analysis. Mol Ecol Resour 2017, 17:466–480. [DOI] [PubMed] [Google Scholar]
- 35.Öncü C, Brinkmann A, Günay F, Kar S, Öter K, Sarıkaya Y, Nitsche A, Linton Y-M, Alten B, Ergünay K: West Nile virus, Anopheles flavivirus, a novel flavivirus as well as Merida-like rhabdovirus Turkey in field-collected mosquitoes from Thrace and Anatolia. Infect Genetics Evol 2018, 57:36–45. [DOI] [PubMed] [Google Scholar]
- 36.Colmant AM, Hobson-Peters J, Bielefeldt-Ohmann H, van den Hurk AF, Hall-Mendelin S, Chow W, Johansen CA, Fros J, Simmonds P, Watterson D, et al. : A new clade of insect-specific flaviviruses from Australian Anopheles mosquitoes displays species-specific host restriction. Msphere 2017, 2:e00262–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fauver JR, Grubaugh ND, Krajacich BJ, Weger-Lucarelli J, Lakin SM, Fakoli LS, Bolay FK, Diclaro JW, Dabiré K, Foy BD, et al. : West African Anopheles gambiae mosquitoes harbor a taxonomically diverse virome including new insect-specific flaviviruses, mononegaviruses, and totiviruses. Virology 2016, 498:288–299. [DOI] [PubMed] [Google Scholar]
- 38.Guzman H, Thangamani S, Contreras-Gutierrez M, Vasilakis N, Tesh RB: Experimental infection with and maintenance of cell fusing agent virus (Flavivirus) in Aedes aegypti. Am J Tropical Medicine Hyg 2017, 97:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saiyasombat R, Bolling BG, Brault AC, Bartholomay LC, Blitvich BJ: Evidence of efficient transovarial transmission of Culex flavivirus by Culex pipiens (Diptera: Culicidae). J Med Entomol 2011, 48:1031–1038. [DOI] [PubMed] [Google Scholar]
- 40.Lutomiah JJ, Mwandawiro C, Magambo J, Sang RC: Infection and vertical transmission of Kamiti River virus in laboratory bred Aedes aegypti mosquitoes. J Insect Sci 2007, 7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mao Q, Wu W, Liao Z, Li J, Jia D, Zhang X, Chen Q, Chen H, Wei J, Wei T: Viral pathogens hitchhike with insect sperm for paternal transmission. Nat Commun 2019, 10:955. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study determined a mechanism of reovirus vertical transmission in aphid vectors. Many experiments were used to extensively characterize the interaction between virus and host proteins during paternal transmission.
- 42.Wu W, Huang L, Mao Q, Wei J, Li J, Zhao Y, Zhang Q, Jia D, Wei T: Interaction of viral pathogen with porin channels on the outer membrane of insect bacterial symbionts mediates their joint transovarial transmission. Philosophical Transactions Royal Soc B 2019, 374:20180320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Q, Godfrey K, Liu J, Mao Q, Kuo Y-W, Falk BW: A nonstructural protein responsible for viral spread of a novel insect Reovirus provides a safe channel for biparental virus transmission to progeny. J Virol 2019, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Auguste AJ, Kaelber JT, Fokam EB, Guzman H, Carrington CV, Erasmus JH, Kamgang B, Popov VL, Jakana J, Liu X, et al. : A newly isolated Reovirus has the simplest genomic and structural organization of any Reovirus. J Virol 2015, 89:676–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grisenti M, Vázquez A, Herrero L, Cuevas L, Perez-Pastrana E, Arnoldi D, Rosà R, Capelli G, Tenorio A, Sánchez-Seco M, et al. : Wide detection of Aedes flavivirus in north-eastern Italy – a European hotspot of emerging mosquito-borne diseases. J Gen Virol 2015, 96:420–430. [DOI] [PubMed] [Google Scholar]
- 46.Longdon B, Brockhurst MA, Russell CA, Welch JJ, Jiggins FM: The evolution and genetics of virus host shifts. Plos Pathog 2014, 10:e1004395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hegde S, Khanipov K, Albayrak L, Golovko G, Pimenova M, Saldaña MA, Rojas MM, Hornett EA, Motl GC, Fredregill CL, et al. : Microbiome interaction networks and community structure from laboratory-reared and field-collected Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus mosquito vectors. Front Microbiol 2018, 9:2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hughes GL, Dodson BL, Johnson RM, Murdock CC, Tsujimoto H, Suzuki Y, Patt AA, Cui L, Nossa CW, Barry RM, et al. : Native microbiome impedes vertical transmission of Wolbachia in Anopheles mosquitoes. Proc National Acad Sci 2014, 111:12498–12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamblin SR, White PA, Tanaka MM: Viral niche construction alters hosts and ecosystems at multiple scales. Trends Ecol Evol 2014, 29:594–599. [DOI] [PubMed] [Google Scholar]
- 50.Guégan M, Zouache K, Démichel C, Minard G, Van V, Potier P, Mavingui P, Moro C: The mosquito holobiont: fresh insight into mosquito-microbiota interactions. Microbiome 2018, 6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amuzu HE, Tsyganov K, Koh C, Herbert RI, Powell DR, McGraw EA: Wolbachia enhances insect specific flavivirus infection in Aedes aegypti mosquitoes. Ecol Evol 2018, 8:5441–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study surveyed the virome of laboratory and field-caught mosquitoes in the context of Wolbachia infections. Although Wolbachia is known to interfere with pathogenic arboviruses, mosquitoes with Wolbachia infection had greater rates of ISF infection as well.
- 52.Schnettler E, enu VB, Mottram T, McFarlane M: Wolbachia restricts insect-specific flavivirus infection in Aedes aegypti cells. J Gen Virol 2016, 97:3024–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang G, Etebari K, Asgari S: Wolbachia suppresses cell fusing agent virus in mosquito cells. J Gen Virol 2016, 97:3427–3432. [DOI] [PubMed] [Google Scholar]
- 54.McLean BJ, Dainty KR, Flores HA, O’Neill SL: Differential suppression of persistent insect specific viruses in trans-infected wMel and wMelPop-CLA Aedes-derived mosquito lines. Virology 2019, 527:141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erasmus JH, Auguste AJ, Kaelber JT, Luo H, Rossi SL, Fenton K, Leal G, Kim DY, Chiu W, Wang T, et al. : A chikungunya fever vaccine utilizing an insect-specific virus platform. Nat Med 2016, 23:nm.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Erasmus JH, Needham J, Raychaudhuri S, Diamond MS, Beasley DW, Morkowski S, Salje H, Salas I, Kim D, Frolov I, et al. : Utilization of an Eilat virus-based chimera for serological detection of chikungunya infection. Plos Neglect Trop D 2015, 9:e0004119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Piyasena TB, Newton ND, Hobson-Peters J, Vet LJ, Setoh YX, Bielefeldt-Ohmann H, Khromykh AA, Hall RA: Chimeric viruses of the insect-specific flavivirus Palm Creek with structural proteins of vertebrate-infecting flaviviruses identify barriers to replication of insect-specific flaviviruses in vertebrate cells. J Gen Virology 2019, doi: 10.1099/jgv.0.001326. [DOI] [PubMed] [Google Scholar]
- 58.Vasilakis N, Guzman H, Firth C, Forrester NL, Widen SG, Wood TG, Rossi SL, Ghedin E, Popov V, Blasdell KR, et al. : Mesoniviruses are mosquito-specific viruses with extensive geographic distribution and host range. Virol J 2014, 11:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warrilow D, Watterson D, Hall RA, Davis SS, Weir R, Kurucz N, Whelan P, Allcock R, Hall-Mendelin S, O’Brien CA, et al. : A new species of Mesonivirus from the Northern Territory, Australia. Plos One 2014, 9:e91103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gorchakov R, Tesh R, Weaver S, Nasar F: Generation of an infectious Negev virus cDNA clone. Journal of General Virology 2014, 95:2071–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O: New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Systematic Biol 2010, 59:307–321. [DOI] [PubMed] [Google Scholar]
- 62.Lefort V, Longueville J-E, Gascuel O: SMS: Smart Model Selection in PhyML. Mol Biol Evol 2017, 34:2422–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]


